
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 03 October 2022
Sec. Health Economics
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1012690
This article is part of the Research Topic From Clinical Trials to Real-World Data Sciences for Value in Health: Access, Utilization, and Quality View all 8 articles
Background: Inappropriate use of antibiotics has become a major driver for the spread of antimicrobial resistance globally, particularly common in China. Antimicrobial stewardship programs are effective in optimizing antimicrobial use and decreasing the emergence of multi-drug-resistant organisms, and the pharmacist has performed a leading role in this program.
Objective: To evaluate the impact of antimicrobial stewardship programs driven by pharmacists on antibiotic consumption and costs and the appropriateness of antibiotic use.
Methods: A single-center retrospective quasi-experimental design was conducted in two independent hepatobiliary surgery wards and two independent respiratory wards in a county-level tertiary general hospital in Jiangsu, China. Each intervention group was served with antimicrobial stewardship programs with prescriptions audit and feedback, antibiotics restriction, education, and training. The propensity score matching method was employed to balance confounding variables between the intervention group and control group, and a difference-in-differences analysis was used to evaluate the impact of antimicrobial stewardship programs. The primary outcome was measured by scores of rationality evaluation of antibiotics.
Results: The DID results demonstrated that the implementation of the antimicrobial stewardship programs was associated with a reduction in the average length of hospital stay (coefficient = −3.234, p = 0.006), DDDs per patient (coefficient = −2.352, p = 0.047), and hospitalization costs (coefficient = −7745.818, p = 0.005) in the hepatobiliary surgery ward, while it was associated with a decrease in DDDs per patient (coefficient = −3.948, p = 0.029), defined daily doses per patient day (coefficient = −0.215, p = 0.048), and antibiotic costs (coefficient = −935.087, p = 0.014) in the respiratory ward. The program was also associated with a decrease in rationality evaluation scores (p < 0.001) in two wards.
Conclusion: The result reveals that the implementation of the antimicrobial stewardship programs is effective in reducing the length of hospital stay, decreasing antibiotics consumption and costs, and improving the appropriateness of antimicrobial use such as decreasing irrational use of cephalosporins, reducing combinations, and improving timely conversion. However, great attention ought to be paid to the improper use of broad-spectrum antibiotics. The government is responsible for providing sustainable formal education for pharmacists, and more funding and staff support to promote antimicrobial stewardship programs.
Overuse of antibiotics has become a major contributor to the emergence and rapid spread of antimicrobial resistance (AMR) (1). As multi-drug resistance has emerged in plenty of species, few antibiotics in the development pipeline make AMR a growing public health crisis on a global level. Unfortunately, it is estimated that 20–50% of prescriptions for antibiotics were inappropriate or unnecessary, and if no action was taken, by 2050 AMR could cause as many as 10 million deaths per year and the economic burden of deaths could reach 100 trillion dollars per year (2, 3).
For several decades, China has experienced an extensive threat of AMR due to antibiotic abuse and was the second largest antimicrobial consumer worldwide (4). In 2011, the National Health and Family Planning Commission (NHFPC) of China put forward the “National Special Stewardship in the Clinical Use of Antibiotics” to improve the use of antibiotics. The campaign made some progress and had positive effects on decreasing the consumption and intensity of antibiotic use (5); however, due to the strong administrative intervention and little attention to attitude and practice on prescriptions of physicians, the trends of AMR had not decreased as expected (5). For instance, the percentage of Escherichia coli resistant to third-generation cephalosporins and quinolones was 59 and 54, respectively, in 2015 (6), which suggested that effective antimicrobial management was urgently needed in China.
Antimicrobial stewardship (AMS) was the foundation of a hospital's antimicrobial stewardship programs (ASPs) and has been successfully implemented in most hospitals in developed countries. The primary goal of the ASPs is to optimize antimicrobial use and patient outcomes while decreasing the spread of multi-resistant infections and reducing adverse effects and some other unintended consequences of antibiotics (7). As an integral part of the antimicrobial stewardship team, pharmacists being active members of the healthcare system have a significant role in implementing strategies and monitoring performance to achieve the primary goals of ASPs. In specific, pharmacists were responsible for developing evidence-based guidelines, reviewing prescriptions and providing feedback, and monitoring antibiotic use and are capable of delivering education and training (8, 9).
Due to the positive effects on abuse and rational use of antibiotics, the Chinese government has attached importance to pharmacist-led antimicrobial stewardship. Only a few studies evaluated the effects of pharmacist-led antimicrobial stewardship in China. A multi-center prospective cohort study showed that pharmacist-driven antimicrobial stewardship had a lower mortality rate and more optimized antimicrobial use in intensive care units (ICUs) (10). However, only the patients in ICUs and pediatric patients were included in the above-mentioned studies. Moreover, the previous studies focused on the effects of ASP in urban hospitals. However, in China, urban–rural disparities exist in antibiotics use and the healthcare services system. Nevertheless, the effects of pharmacist-led antimicrobial stewardship established in county-level tertiary general hospitals in rural areas have not been estimated. Therefore, this study aimed to evaluate the impact of pharmacist-led antimicrobial stewardship on antibiotic consumption and costs and the appropriateness of antibiotic use in internal medicine and surgery departments of a county-level general hospital in Jiangsu, China.
A single-center quasi-experimental study was conducted in Jiangyin, Jiangsu, China, a 2200-bed, teaching, general hospital. The hospital ranked third among the top 100 county hospitals in China in 2020, and it provides comprehensive medical treatment, prevention, healthcare, and rehabilitation care.
The study period was over 20 months, with a pre-intervention phase from March 2018 to October 2018, an ASP implementation phase in January 2019, and an intervention evaluation phase from March 2019 to October 2019. It was conducted in two hepatobiliary surgery wards: one was regarded as an intervention group and the other was the control group, and two respiratory wards: one was an intervention group and the other was the control group. Each intervention group was served with ASPs by an antimicrobial management group, while each control group received standard strategies performed by physicians without pharmacist involvement.
Patients admitted to the hospital with antibiotic treatment were included in the study period. Among them, those who were diagnosed with cholecystectomy and choledocholithotomy in the hepatobiliary surgery ward and with chronic obstructive pulmonary disease (COPD), bronchial asthma, pneumonia, bronchitis, and respiratory failure in the respiratory ward were eligible for the study.
This study was approved by the Ethical Committee of Nanjing Medical University (Grant number: 2020103) and conducted following the Declaration of Helsinki guidelines.
In January 2019, an ASP was introduced in the respiratory ward and hepatobiliary surgery ward of a county-level tertiary general hospital, aiming to improve appropriateness in antibiotic use and reduce antibiotic consumption. The antimicrobial management team was composed of a full-time infectious diseases-trained clinical pharmacist as its core, also with physicians, clinicians, infectious disease specialists, microbiologists, and administrators, and included a leadership group responsible for work deployment and supervision. Generally, an infectious disease physician-led or administration department of nosocomial infection-led antimicrobial stewardship was the main model in hospitals in China, whereas pharmacists played a leading role in AMS in our study for the following reasons: (1) the pharmacist participated in ward rounds per day so that they could communicate with physicians closely, (2) the pharmacists had sufficient knowledge to monitor the drug use of patients and give individual dosing schedules, and (3) the pharmacists were responsible for the education and training of physicians, microbiologists, administrators, and patients. The ASPs were designed by a pharmacist-led antimicrobial team based on the updated Chinese Guidelines for the Clinical Application of Antimicrobial Agents, and multifaceted interventions included:
An audit of antibiotic prescriptions with timely feedback to prescribers was a core strategy in antimicrobial stewardship, which included: (a) a daily review of prescriptions with direct feedback outlining suggestions on potential inappropriate use of antibiotics, rationality for the recommendation, and information on antibiotics-related adverse reaction to prescribers; (b) daily ward rounds with physicians to assess the patients' diagnosis, medication, and microbiological results, and giving advice to the physician to determine the optimal drug treatment; and (c) a discussion on possible recommendations for complex cases with an infectious disease specialist, physicians, and microbiologists.
A defined hospital formulary was established based on the antibiotic classification management policy which classified antibiotics into first-line, second-line, and third-line antibiotics authorization. The interventions on formulary restriction included: (a) limiting the use of third-line antibiotics unless there was a clear indication; (b) giving prescriptive authority to physicians in different positions to prescribe different classes of antibiotics; and (c) incorporating restrictions into computer physician order entry to trigger initial review when the inappropriate rationale for antibiotic selection or physicians without authority for particular antibiotics occurred.
Pharmacists played a significant role in delivering education and training on antimicrobial stewardship to professionals, patients, and members of the public (9, 11). In the proceeding of ASPs implementation, the pharmacists (a) organized seasonal educational meetings with the data sharing of the change in antibiotic resistance, antibiotic consumption, and costs and (b) organized education and training of medical staff on the rational use of antibiotics.
Reward and punishment assessment of the ASPs on antibiotic consumption and costs and appropriate antimicrobial use was performed by pharmacists based on the reported data.
Data were obtained from the hospital information system (HIS) for each inpatient on (1) demographic characteristics: age and gender; (2) clinical information: diagnosis, transition, complications, bacterial culture or not, drug sensitivity test or not, etiology submission or not, procalcitonin value, C-reactive protein value, admission date, and discharge date; (3) antibiotic use: substance name, unit strength, pack size, defined daily dose (DDD), the route of administration, and the date dispensed; and (4) costs: antibiotic costs and hospitalization costs. The HIS was linked with an inpatient database, pharmacy database, and microbiology database, and the data were measured repeatedly by two authors during the study period to assure the data quality and adequacy.
Our primary outcome was measured by scores of rationality evaluation of antibiotics. After hospital discharge, a part of medical records for inpatients in the two groups selected through equidistant sampling was reviewed by two infection specialists, and the phenomena of inappropriate antibiotic use were evaluated based on a scoring system involving items (Table 1). The item was assigned one point if an inappropriate antibiotic use was identified and then multiplied by the corresponding weight calculated through an analytic hierarchy process to obtain final scores.
Secondary outcomes were antibiotic consumption, antibiotic costs, and medical service efficiency. As our study concentrated on the effect of ASP intervention on the appropriate use of antibiotics during the hospitalization period, the discharge medication was not taken into consideration in the study. Antibiotic consumption was measured by DDDs per patient and DDDs per patient per day.
Costs were measured by antibiotic costs, daily costs, and hospitalization costs. Antibiotic costs were computed by the expense for all antibiotics of patients during the study period, while hospitalization costs included costs of all drugs (antibiotics included), examination expenses, surgery expenses, registration fees, etc., and the daily costs were computed by antibiotics costs divided by corresponding DDDs.
Medical service efficiency was measured by the average length of hospital stay (LOS), LOS was calculated by subtracting the admission date from the discharge date, and the average LOS was calculated by the length of stay divided by the number of inpatient hospitalizations.
Propensity score matching (PSM), as a statistical method for dealing with sample selectivity bias, was first proposed by Rosenbaum and Rubin in 1983 (12). The propensity score is defined as the probability of being eligible for ASPs, and a logistic regression was used to estimate propensity scores as a function of a set of covariates that could influence the impact on antibiotic consumption and costs and the likelihood of improving antibiotic use. In this study, the covariates including age, gender, transition, and diagnosis were incorporated into regression and nearest-neighbor matching with one-to-one matches could identify individuals in the control group with similar characteristics as those served with ASPs based on propensity scores. The PSM allowed the selection of the appropriate control group, which helped to solve possible endogenous problems and demonstrate the robustness of the results.
It is widely acknowledged that a difference-in-differences (DID) method is a quasi-experimental technique for policy evaluation. We perform a DID analysis to study the differential impacts of ASPs intervention and construct the following regression model:
where Yit stands for the rationality evaluation, consumption, and costs of antibiotics; the key coefficient β3 is calculated by a DID model and reflects the effect of ASPs intervention; the variable groupirepresents the group dummy variable and groupi=1 indicates the treatment group, whereas groupi=0 indicates the control group, t indicates the month; timetis the time dummy variable where pre-intervention (March 2018–October 2018) is 0, whereas post-intervention (March 2019–October 2019) is 1; the variable timet×groupi denotes the interaction between time and groups; Xit means a series of covariates of resident i at time t; and εitis the residual error.
To estimate the effects of ASPs on the rationality evaluation, consumption, and costs of antibiotics in China, we employed a PSM-DID model rather than a matching method or a DID analysis alone. The combination of two methods can be applied to dealing with relatively weak assumptions to help DID meet common trends on observables (13).
Statistical analyses were conducted using Stata, version 14.0 (Stata Corp., College Station, TX, USA), and P < 0.05 was considered statistically significant. All expenses in this study were measured by CNY.
During the 10-month intervention, Table 2 illustrates that in the hepatobiliary surgery ward, there were 207 and 839 hospitalizations identified in the intervention and control groups, respectively. Most patients were female (564, 53.92%), and there existed a difference in gender between the two groups (p = 0.011). Besides, the transition of patients (p < 0.001) and the diagnosis (p < 0.001) were different between the intervention and control groups. After propensity matching, the sample size decreased to 392 and each group had 196 patients for the study, and the propensity was effective in reducing differences for all covariates.
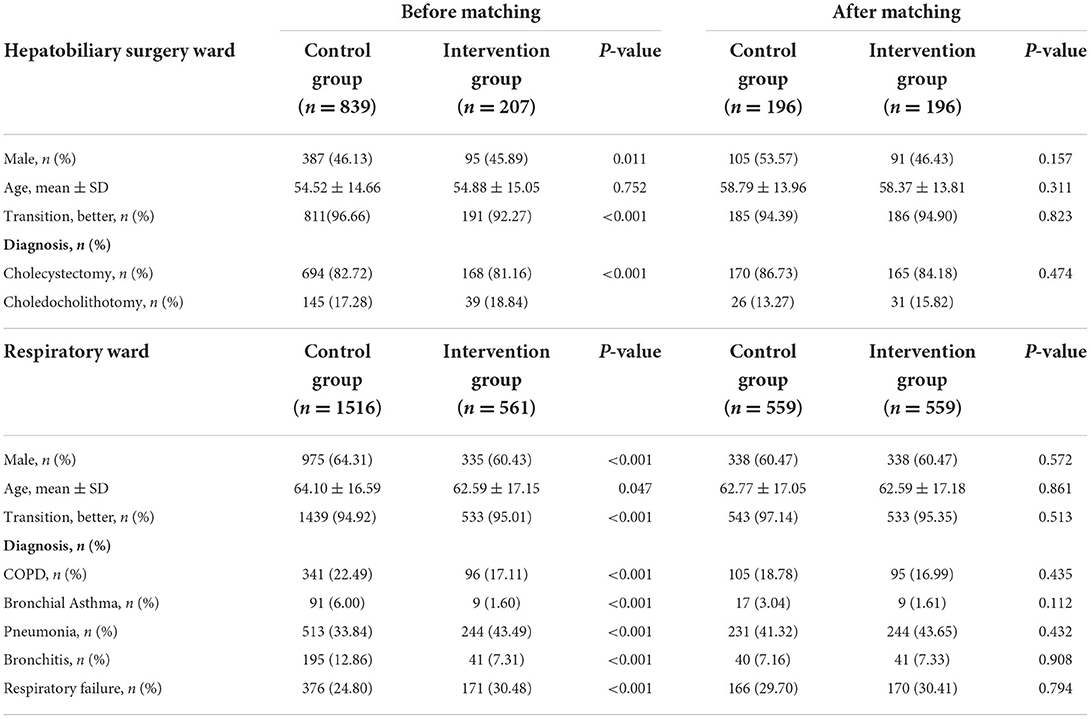
Table 2. Baseline characteristics of patients in the hepatobiliary surgery ward and respiratory ward.
As shown in Table 2, in the respiratory ward there were 564 and 1523 hospitalizations enrolled in the intervention group and control group, respectively. The control group had relatively older patients (64.10 ± 16.59, p = 0.047), and there was a gender difference (p < 0.001), the transition of patients (p < 0.001), and diagnosis (p < 0.001) between the two groups. After propensity matching, the sample size decreased to 1118 and all covariates were well balanced between the two groups.
On average, 1244.55 CNY were spent on antibiotic costs per patient before and after the intervention throughout the study period in the hepatobiliary surgery ward: 964.81 CNY in the intervention group for 6.61DDDs per patient and 1336.33 CNY in the control group for 7.55 DDDs before the intervention and 875.11 CNY in the intervention group for 6.78 DDDs per patient and 1711.17 CNY in the control group for 10.38 DDDs after the intervention. As illustrated in Table 3, the DDDs per patient increased in the two groups and antibiotic costs decreased in the intervention group, but increased in the control group: a 2.57% increase in DDDs and a 9.30% decrease in antibiotic costs in the intervention group, but a 37.48% increase in DDDs and a 28.5% increase in antibiotic costs in the control group. In addition, the DDDs per patient day and daily costs decreased in the two groups: a decrease rate of 5% in DDDs per patient day and a 4.74% decrease in daily costs in the intervention group, but a 7.69% decrease in DDDs per patient day and a 5.96% decrease in daily costs in the control group. After the implementation of ASPs, the average LOS increased by 5.09% and 41.88% in the intervention and control groups, respectively.
Averagely, 2057.63 CNY were spent on antibiotic costs per patient throughout the study period in the respiratory ward: 2620.67 CNY in the intervention group for 18.64 DDDs per patient and 1673.39 CNY in the control group for 14.14 DDDs in the pre-intervention period and 2037.22 CNY in the intervention group for 17.42 DDDs per patient and 2025.02 CNY in the control group for 16.87 DDDs in the post-intervention period. Meanwhile, the DDDs per patient and antibiotic costs decreased in the intervention group, but increased in the control group: a 6.55% decrease in DDDs and a 22.26% decrease in antibiotic costs in the intervention group, but a 19.31% increase in DDDs per patient and a 21.01% increase in antibiotic costs in the control group. In addition, the DDDs per patient day and daily costs decreased in the two groups: a decrease rate of 10.68% in DDDs per patient day and a 19.47% decrease in daily costs in the intervention group, but a 0.53% decrease in DDDs per patient day and a 5.13% decrease in daily costs in the control group. Compared with the pre-intervention period, the average LOS in the post-intervention group declined by 5.38% in the intervention group, but zoomed in the control group.
The results of the effects of ASPs intervention on antibiotic consumption and costs and LOS are illustrated in Table 4. The coefficient evaluation represents the interaction term between time and group variables, indicating the actual effect of the intervention. The results demonstrated that the ASPs intervention contributed to a decrease in LOS, DDDs per patient, and hospitalization costs in the hepatobiliary surgery ward and the coefficients were −3.234 (p = 0.006), −2.352 (p = 0.047), and −7745.818 (p = 0.005), respectively. In addition, the ASPs intervention also had a positive effect on DDDs per patient, DDDs per patient day, and antibiotic costs with coefficients of −3.948 (p = 0.029), −0.215 (p = 0.048), and −935.087 (p = 0.014), respectively, in the respiratory surgery ward. Although the ASPs intervention resulted in a decrease in DDDs per patient day and antibiotic costs in the hepatobiliary surgery ward and a decrease in LOS, daily costs, and hospitalization costs in the respiratory ward, there is no statistical significance in the above variables.
As shown in Table 5, concerning the great volume of medical records and limited time of reviewers, equidistant sampling with an interval of one patient was used and 196 medical records of patients (98 for the intervention group and 98 for the control group) were reviewed and evaluated by infection specialists in the hepatobiliary surgery ward. But, in the respiratory wards, equidistant sampling with an interval of five patients was employed and 186 medical records (93 for the intervention group and 93 for the control group) were reviewed. The results of the scores of rationality evaluation of antibiotics and the estimation of the ASPs intervention are shown in Table 5. On average, in the hepatobiliary surgery ward the rationality evaluation scores decreased by 0.145 in the intervention group, but increased by 0.007 in the control group after the implementation of ASPs. In the respiratory ward, the average rationality evaluation score was 0.316 and decreased to 0.033 in the intervention group after the ASPs, while the scores decreased from 0.209 to 0.196 in the control group. Moreover, the results showed that the ASPs intervention had a positive effect on the rationality evaluation scores in two wards (both p < 0.001), and the declining scores in the respiratory ward were more than those in the hepatobiliary surgery ward.
Figure 1 exhibits the inappropriate use of antibiotics in the hepatobiliary surgery ward. Most cases were identified as inappropriate duration, followed by irrational indication. During the pre-ASP period, 12 cases in the intervention group and 18 cases in the control group were identified as an unnecessary duration for preventive medication, and after the ASPs, the cases of inappropriate duration decreased in the intervention group, but increased in the control group. In addition, the cases with inappropriate indication, choice, and dosing schedule in the intervention group were fewer than the cases in the control group after ASP implementation. However, regarding the dosage item, the cases in the intervention group were more than the cases in the other group. As shown in Figure 2, most cases were identified as inappropriate indications of the use of antibiotics, followed by irrational choice and unnecessary combination use of antibiotics. The cases with irrational use of antibiotics in the intervention group declined dramatically after the ASPs implementation as expected, and those with inappropriate indication decreased from 15 to 2 cases. Except for the conversion item, the cases in the intervention group decreased while having an increasing trend in the control group before and after the intervention. The main inappropriate use of antibiotics in two wards is shown in Figures 3, 4, respectively.
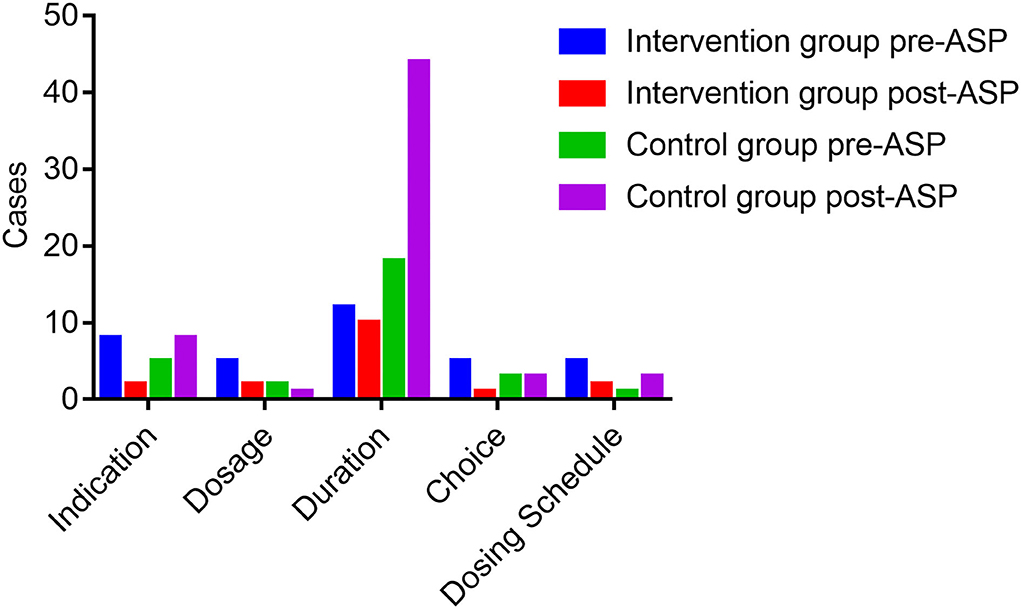
Figure 1. Inappropriate use of antibiotics before and after the ASPs implementation in the hepatobiliary surgery ward. ASPs, antimicrobial stewardship programs.
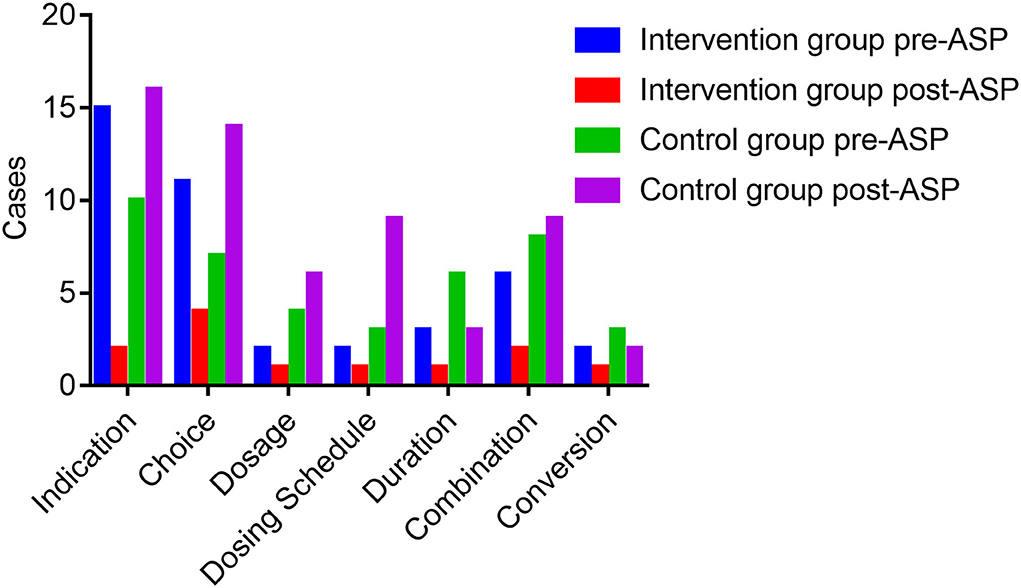
Figure 2. Inappropriate use of antibiotics before and after the ASPs implementation in the respiratory ward. ASPs, antimicrobial stewardship programs.
The previous evidence demonstrated that the temporary absence of pharmacists in the program has resulted in a prolonged duration of therapy, more than the 3-fold number of cases of CDI, and a 27–39% increase in inappropriate use of antimicrobial agents (14). Besides, a study showed that the appropriateness of approvals for restrictive antibiotics performed by infection pharmacists was better than that performed by infection physicians (15). The ASPs in this study were supported by hospital leadership and heavily focused on reviewing prescriptions and restricting antibiotics to affect the antimicrobial prescribing behavior of physicians. Slightly different from other studies, the reward and punishment mechanism which is capable of catalyzing improvement in efforts on rational use of antibiotics (16) was established in this study. After a 10-month ASP, the observed results of reduction in antibiotic consumption and costs and improvement in appropriate use provide additional evidence to support the positive effects of pharmacist-led ASPs in China.
In general, consistent with other findings (17), our result demonstrated the ASPs intervention resulted in a reduction in DDDs per patient and DDDs per patient per day in the respiratory ward and a decrease in DDDs per patient in the hepatobiliary surgery ward, indicating that the ASPs intervention was associated with a reduction in antibiotic consumption. Specifically, compared with the hepatobiliary surgery ward, a larger decrease in antibiotics consumption would be observed in the respiratory ward as the patients admitted to the ward were more likely to have a high rate of nosocomial infection and drug-resistant infection. Regarding antimicrobial costs, we observed a decrease in hospitalization costs in the surgery ward and a 22.26% reduction in antibiotics costs in the respiratory ward. Several reasons can account for the finding: First, the decrease in costs may be associated with a reduction in antimicrobial consumption. Second, as an important part of the antimicrobial stewardship team, microbiologists are responsible for cumulative antimicrobial susceptibility tests for the accuracy of antibiotics, and the shortened duration of empirical treatment due to clear microbiological results was a driver for cost saving. Third, the indicator of the average LOS in this study decreased after the ASPs intervention in the hepatobiliary surgery ward, which was similar to another study in the gastroenterology ward (18). Besides, LOS was a vital influencing factor for hospitalization costs. Meanwhile, the result showed a 4.74 and a 19.47% decline in daily costs after the ASPs implementation in the intervention group, whereas a non-significant decrease was observed through DID analyses in two wards, which suggested the average price of antibiotics used during the study period had not decreased as expected. This may be associated with a common misconception among patients that expensive drugs are more effective than cheap ones (19); a few physicians even were asked to prescribe expensive antibiotics under the pressure of patients.
One beneficial result was that the inappropriate combinations with β-lactams and fluoroquinolones improved in the intervention group after ASP implementation. In common practice, levofloxacin is often combined with cephalosporins or macrolides to treat common respiratory diseases such as chronic bronchitis. But for common infections, the combinations were less effective and an overdose of levofloxacin in the respiratory ward had the potential to result in bacterial resistance. Thus, it is prescribed that levofloxacin should be used only for unexplained infections, and severe infections cannot be improved by single medication, mixed infections, or MDROS infections (20), which contributed to the decrease in cases identified as improper combinations after the ASP intervention. Nevertheless, the high dosage of levofloxacin cannot be reduced for the patients with renal inadequacy in our study.
The other beneficial result demonstrated the reduced consumption and increased rationality of utilization of cephalosporins after the ASP intervention, although the cephalosporins are widely used in clinical departments as a result of the considerable antibacterial activity of them against gram-negative and gram-positive organisms and are consumed in great quantities before the implementation of ASPs. In consistent with the result of a previous study (21), the consumption of cephalosporin compound medicines such as cefoperazone and sulbactam has been reduced through academic education lectures and training courses. Meanwhile, a decrease in the consumption of cephalosporins was observed after the intervention. As the causative agent of surgical infection, the widespread resistance of Escherichia coli to ceftriaxone was revealed in the hepatobiliary surgery ward, which accounted for the reduction in ceftriaxone prescriptions.
Another finding showed that timely conversion from intravenous to oral therapy improved in the intervention group (22). The obstacle to conversion therapy is the deep-rooted misconception that intravenous infusion is more effective than oral drugs (23), which is caused by low Chinese health literacy. In this study, pharmacists addressed that if the oral antibiotics showed excellent oral bioavailability and strong tissue penetration, the efficacy would be comparable to those attained by intravenous infusion.
One interesting finding showed that there was an increase in DDDs per patient and a decrease in antibiotic costs in the hepatobiliary surgery ward after the intervention. On the one hand, the increase in DDDs per patient might be caused by prolonged average LOS. The longer hospital days, which might contribute to more antibiotics, were prescribed. On the other hand, due to the implementation of ASP, low-grade or high-grade physicians were authorized to prescribe different classes of antibiotics, and first-line antibiotics should be recommended as the priority prescription. Therefore, physicians usually prescribe cheaper first-line antibiotics than expensive second-line or third-line antibiotics during the ASPs intervention period.
However, there still existed inappropriate use of broad-spectrum antibiotics, which suggested that the improvement in prescribing behavior of physicians was a tough assignment in antimicrobial management. Theoretically, the antibiotics' effectiveness and resistance development are supposed to be taken into consideration by guidelines when giving medication to patients. Nevertheless, physicians have a different thinking process that they would attach more importance to the effects of antibiotics than the spread of resistance in empirical use (24), which results in the overuse of broad-spectrum antibiotics such as meropenem and biapenem. Meanwhile, the concerns of physicians about the failure of the treatment can also lead to the excessive use of broad-spectrum antibiotics for surgical prophylaxis and frequent switching and combination of antibiotics (18). It is common for some patients without proper indications to require prescriptions for antibiotics (25). For example, although no clinical indication for parenteral therapy, most physicians still would prescribe injectable antibiotics under the pressure from patients and their parents (26).
Although the pharmacists performed the leading role in ASPs, the participation of pharmacists was limited to monitoring antimicrobial use, communicating with physicians and patients on therapy, providing feedback with clear evidence to prescribers, developing medication guidelines, and educating medical staff during the study period, which indicated that the pharmacists played fewer clinical roles than they might otherwise. This may be associated with the limited number of trained pharmacists performing in clinical departments and overburdened pharmacists having difficulty taking various crucial clinical roles (27). Therefore, sustainable formal education and clinical practice ought to be provided for pharmacists to improve their experience in optimizing antimicrobial use and therapy. Additionally, education with different models of reflection on practice and group learning and discussion are introduced to change prescribing behaviors of physicians (28). Last but not least, to maintain the effectiveness of ASPs, more funding and staff support should be provided to overcome the barriers (29).
Our study had several strengths. This study avoided the selection bias as the patients in the control group have similar characteristics to the intervention group through the PSM method, and a DID model has the potential to control the confounding influences of dependent variables and solve the endogeneity problem. However, several limitations should be acknowledged in this study. First, although the PSM was used to balance confounding variables, some unmeasured variables might cause different trends between the intervention and control groups. Second, the respiratory wards and hepatobiliary surgery wards were served by two independent pharmacists and the ASPs strategies were performed in the intervention group in each ward; however, the potential of the influence on the education and training of physicians in the control group was unavoidable. Third, due to a series of strategies included in ASPs, the impact affected by which strategy cannot be accurately assessed. Finally, the impact of clinical outcomes such as mortality, reinfection rate, and resistance rate cannot be estimated as the relevant data were not collected, and we were unable to assess the long-term effects of the ASPs due to the short study period.
This study provides a DID analysis of the impacts of the ASPs involving a pharmacist and reveals that the ASPs are effective in reducing the length of hospital stay, decreasing antibiotics consumption and costs, and improving the appropriateness of antimicrobial use such as decreasing irrational use of cephalosporins, reducing combinations, and improving timely conversion. Nevertheless, it has failed to reduce the daily costs of patients and decrease the improper use of broad-spectrum antibiotics. The government must provide sustainable formal education and clinical practice for pharmacists and more funding and staff support to optimize the structure and promote the construction of ASPs.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
YW, CL, SL, XLL, and XL conceptualized and designed the whole study. YW, CL, SL, XLL, and CZ were responsible for collecting and analyzing the data. YW drafted the initial manuscript. XL took the responsibility for editing. All authors contributed to the critical revision of the manuscript and approved the final version.
This work was supported by the National Natural Science Foundation of China (71673147 and 72074123) and the China Medical Board (Grant no. 17-277).
All authors are grateful for the support of the Department of Information Management of the Affiliated Jiangyin Hospital of Nantong University for providing the original data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The contents are solely the responsibility of the authors and do not reflect the views of the funding bodies or any organization.
1. Bartlett JG. A call to arms: the imperative for antimicrobial stewardship. Clin Infect Dis. (2011) 53:S4–7. doi: 10.1093/cid/cir362
2. O'Neill J. Tackling Drug-Resistant Infections Globally: Final Report Recommendations: The Review on Antimicrobial Resistance. (2016). p. 10–11. Available online at: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf
3. Core Elements of Hospital Antibiotics Stewardship Programs. Centers for Disease Control and Prevention website. Avaialble online at: https://www.cdc.gov/getsmart/healthcare/implementaion/core-elements.html (accessed March 21, 2018) (2018).
4. Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. (2014) 14:742–50. doi: 10.1016/S1473-3099(14)70780-7
5. Ma Z, Zhou YQ, Ma Y, Li K. Evaluation of the effects of Special Rectification on the rational use of antibiotics and reliving bacterial resistance in our hospital. China Pharm. (2015) 26:2780–3. doi: 10.6039/j.issn.1001-0408.2015.20.15
6. China Antimicrobial Resistance Surveillance System. National antimicrobial resistance surveillance report. Chin J Rational Drug Use. (2016) 2016:3–8. doi: 10.3969/j.issn.1672-5433.2016.03.001
7. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. (2016) 111:602–22. doi: 10.1038/ajg.2016.126
8. Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious diseases society of america and the society for healthcare epidemiology of america guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. (2007) 44:159–77. doi: 10.1086/510393
9. Gilchrist M, Wade P, Ashiru-Oredope D, Howard P, Sneddon J, Whitney L, et al. Antimicrobial stewardship from policy to practice: experiences from UK antimicrobial pharmacists. Infect Dis Ther. (2015) 4:51–64. doi: 10.1007/s40121-015-0080-z
10. Li Z, Cheng B, Zhang K, Howard P, Sneddon J, Whitney L, et al. Pharmacist-driven antimicrobial stewardship in intensive care units in East China: a multicenter prospective cohort study. Am J Infect Control. (2017) 45:983–9. doi: 10.1016/j.ajic.2017.02.021
11. Ponto JA. ASHP statement on the pharmacist's role in antimicrobial stewardship and infection prevention and control. Am J Health Syst Pharm. (2010) 67:575–7. doi: 10.2146/sp100001
12. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. (1983) 70:41–55. doi: 10.1093/biomet/70.1.41
13. Sari N, Osman M. The effects of patient education programs on medication use among asthma and COPD patients: a propensity score matching with a difference-in-difference regression approach. BMC Health Serv Res. (2015) 15:332. doi: 10.1186/s12913-015-0998-6
14. Cappelletty D, Jacobs D. Evaluating the impact of a pharmacist's absence from an antimicrobial stewardship team. Am J Health Syst Pharm. (2013) 70:1065–9. doi: 10.2146/ajhp120482
15. Gross R, Morgan AS, Kinky DE, Weiner M, Gibson GA, Fishman NO. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis. (2001) 33:289–95. doi: 10.1086/321880
16. McGee EU, Mason-Callaway AD, Rollins BL. Are We meeting the demand for pharmacist-led antimicrobial stewardship programs during postgraduate training-year 1 (PGY1)? Pharmacy. (2020) 8:91. doi: 10.3390/pharmacy8020091
17. Avdic E, Cushinotto LA, Hughes AH, Hansen AR, Efird LE, Bartlett JG, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. (2012) 54:1581–7. doi: 10.1093/cid/cis242
18. Du Y, Li J, Wang X, Peng X, Wang X, He W, et al. Impact of a multifaceted pharmacist-led intervention on antimicrobial stewardship in a gastroenterology ward: a segmented regression analysis. Front Pharmacol. (2020) 11:442. doi: 10.3389/fphar.2020.00442
19. Wang Y, Zhu Y, Liu X, Xu X, Fang W, Li X. The Effects of county public hospital reform on the consumption and costs of antibiotics: evidence from a quasinatural experiment in Jiangsu, China. Biomed Res Int. (2020) 2020:9262170. doi: 10.1155/2020/9262170
20. Chen L, Wang J. Practice and experience of clinical pharmacist on reasonable application of antibacterials in ICU and respiratory department. Chin J Clin Ration Drug Use. (2017) 10:96–8. doi: 10.15887/j.cnki.13-1389/r.2017.06.046
21. Zhang L, Wang Z, Qian D, Ni J. Effects of changes in health insurance reimbursement level on outpatient service utilization of rural diabetics: evidence from Jiangsu Province, China. BMC Health Serv Res. (2014) 14:185. doi: 10.1186/1472-6963-14-185
22. Vogel F. Intravenous/oral sequential therapy in patients hospitalised with community acquired pneumonia: which patients, when and what agents? Drugs. (2002) 62:309–17. doi: 10.2165/00003495-200262020-00005
23. Reynolds L, McKee M. Factors influencing antibiotic prescribing in China: an exploratory analysis. Health Policy. (2009) 90:32–6. doi: 10.1016/j.healthpol.2008.09.002
24. Metlay JP, Shea JA, Crossette LB, Asch DA. Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients. J Gen Intern Med. (2002) 17:87–94. doi: 10.1046/j.1525-1497.2002.10711.x
25. Ding L, Sun Q, Sun W, Du Y, Li Y, Bian X, et al. Antibiotic use in rural China: a cross-sectional survey of knowledge, attitudes and self-reported practices among caregivers in Shandong province. BMC Inf Dis. (2015) 15:576. doi: 10.1186/s12879-015-1323-z
26. Kutsogiannis J, Alberda C, Gramlich L, Cahill NE, Wang M, Day AG, et al. Early use of supplemental parenteral nutrition in critically ill patients: results of an international multicenter observational study. Crit Care Med. (2011) 39:2691–9. doi: 10.1097/CCM.0b013e3182282a83
27. Fang Y. Pharmacy Practice in China In Fathelrahman AI, Ibrahim MIM, Wertheimer AI, editors. Pharmacy Practice in Developing Countries. Boston: Academic Press (2016), p. 57–78.
28. Stalsby LC, Tamhankar AJ. Understanding and changing human behavior: antibiotic mainstreaming as an approach to facilitate modification of provider and consumer behaviour. Ups J Med Sci. (2014) 119:125–33. doi: 10.3109/03009734.2014.905664
29. Pope SD, Dellit TH, Owens RC, Hooton TM. Results of survey on implementation of Infectious Diseases Society of America and Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Infect Control Hosp Epidemiol. (2009) 30:97–8. doi: 10.1086/592979
Keywords: antibiotics use, antimicrobial stewardship, pharmacist, difference-in-differences, county-level general hospital
Citation: Wang Y, Zhou C, Liu C, Liu S, Liu X and Li X (2022) The impact of pharmacist-led antimicrobial stewardship program on antibiotic use in a county-level tertiary general hospital in China: A retrospective study using difference-in-differences design. Front. Public Health 10:1012690. doi: 10.3389/fpubh.2022.1012690
Received: 05 August 2022; Accepted: 05 September 2022;
Published: 03 October 2022.
Edited by:
Jing Yuan, Fudan University, ChinaReviewed by:
Radica Zivkovic Zaric, University of Kragujevac, SerbiaCopyright © 2022 Wang, Zhou, Liu, Liu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, eGlubGlAbmptdS5lZHUuY24=; Xiaoliang Liu, MzYzMzc3M0BxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.