
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 21 October 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1010172
This article is part of the Research Topic Advances in the Diagnosis and Genomic Research of Surveillance-Response Activities in Emerging, Re-emerging, and Unidentified Infectious Diseases View all 12 articles
 Wen Zeng1†
Wen Zeng1† Ning Liu2†
Ning Liu2† Yuchun Li1†
Yuchun Li1† Ai Gao3†
Ai Gao3† Mengyi Yuan3†
Mengyi Yuan3† Rui Ma3
Rui Ma3 Na Jiang3
Na Jiang3 Dingwei Sun1*
Dingwei Sun1* Guangze Wang1*
Guangze Wang1* Xinyu Feng4,5*
Xinyu Feng4,5*Primaquine, the only licensed antimalarial drug for eradication of Plasmodium vivax and Plasmodium ovale malaria, may cause acute hemolytic anemia in individuals with glucose-6-phosphate dehydrogenase deficiency (G6PDd) during treatment. The different prevalence and distribution patterns of G6PDd in Hainan, the ancient malaria-endemic area, are unclear. This study included 5,622 suspected malaria patients between 2009 and 2011 in 11 counties of Hainan. Glucose-6-phosphate dehydrogenase deficiency prevalence was determined using the fluorescent spot test (FST) and malaria patients was confirmed by a positive light microscopy. The G6PDd prevalence for different ethnic groups, genders, and counties were calculated and compared using χ2-test. Spatial cluster and Spearman rank correlation of G6PDd prevalence and malaria incidence were analyzed. The overall G6PDd prevalence of study population was 7.45%. The G6PDd prevalence of males, Li ethnic minority, and malaria patients was significantly higher than that of females, Han ethnic majority, and non-malarial patients (p < 0.01), respectively. The spatial cluster of G6PDd and malaria located in south-western and central-southern Hainan, respectively, with no significant correlation. The study provides essential information on G6PDd prevalence in ancient malaria-endemic areas of Hainan Province. We also highlight the need for a better understanding of the mechanisms underlying the relationship between G6PDd prevalence and malaria incidence. These findings provide a reference for the safety of the primaquine-based intervention, even after malaria elimination.
The malaria elimination program started in 2010 (1) and succeeded in 2021 in China. Throughout the journey, Hainan Province, the most southern province of China and the most seriously affected malaria-endemic area was always the hot spot for the campaign. Since 2010, no falciparum malaria cases have been reported in the area. However, Plasmodium vivax malaria sustained endemic for another 2 years, presenting a critical barrier to elimination in Hainan Province and other countries planning to eliminate malaria. Notably, P. vivax malaria often occurs with low parasitemia and can be missed under routine surveillance. Furthermore, it has gradually become a consensus that P. vivax malaria can be as debilitating as falciparum malaria. Recently, imported P. vivax malaria has been documented and accounted for more than 80% of reported malaria cases. Therefore, additional measures are still needed to extend the comprehensive response to P. vivax malaria control by reducing P. vivax transmission/relapse, and meanwhile, protecting the most vulnerable populations.
There is increasing use of chemopreventive agents for the eradication of malaria toward malaria-free status. In China, primaquine is the only drug approved by the State Food and Drug Administration (SFDA) of China to prevent malaria relapse. It is one of the critical components of the nationwide malaria elimination program (2). Therefore, a massive primaquine administration was expected and applied in malaria-endemic regions. However, primaquine may cause acute hemolytic anemia in individuals with glucose-6-phosphate dehydrogenase deficiency (G6PDd), a common congenital X-linked hereditary enzyme deficiency widespread across most malaria-endemic countries (3). In order to improve the compliance rate of primaquine for the radical cure of malaria, it is urgent to understand better the distribution pattern of G6PDd in main malaria-endemic areas in China.
Previous studies have revealed a relatively low incidence of G6PDd, about 3–7% in China (3); however, there may still be a large number of people with G6PDd in the country, considering its large population. In addition, G6PDd prevalence varied significantly among ethnic groups (4–6). For example, the G6PDd prevalence of the ethnic minorities is different from that of the Han ethnic majority in Yunnan Province, one of the ancient malaria-endemic areas in China (7, 8). Hainan Province still has a multi-ethnic population, but the G6PDd prevalence of its various ethnic minorities remains unclear.
Generally speaking, G6PDd confers a protective effect against both falciparum malaria and vivax malaria episodes (9), lending to a presumptive consensus that wide geographical distribution of G6PDd in human populations is derived by the epidemic (9). Interestingly, there is ecological overlap between G6PDd and malaria endemic areas, as shown in Africa, southern Europe, the middle east, southeast Asia, and the central and southern Pacific islands (10, 11). Therefore, information on the prevalence of G6PDd is critical to optimize the malaria elimination strategy, especially for radical treatment of P. vivax malaria using primaquine. It is necessary to determine the G6PDd distribution patterns in malaria-endemic areas and further analyze the unveiled relationship between malaria incidence and the G6PDd prevalence.
The present study presented the most recently updated information on G6PDd in Hainan, China. We also analyzed and compared the G6PDd distribution patterns among different counties, ethnic groups, and genders and explored the relationship between the spatial distribution of malaria cases and G6PDd status in the minority area. These results would be beneficial for optimizing existing tools against P. vivax, and deploying more effective measures for protecting the most vulnerable populations.
The study areas were located on the main island of Hainan Province, 18°10′-20°10′ north latitude and 108°37′-111°03′ east longitude, in southern China. This region has a land area of 33,900 km2 with a population of 8.26 million. There are multi-ethnic populations, including Li, Miao, and Hui ethnic minorities living together in the central and southern mountainous areas. The Han ethnic majority distributes throughout the island. The Li ethnic minority is one of the earliest inhabitants and the most populous ethnic minority in Hainan.
This clinical study protocol was reviewed and approved by the Ethics Review Committee of the Hainan Center for Disease Control and Prevention. Each participant provided informed consent (in Chinese) before participating in this study. In most cases, the participants provided their written informed consent. The consent was verbal for patients who could not read or write standard Chinese. In these cases, the research nurse documented the participant's consent in writing, including the contents and methods of information provided to the participant and the date and time of the verbal consent, which was then witnessed and signed by another nurse who was not in the research team. The informed consent record, either written or verbal, was kept in the participant's hospital chart. The Ethics mentioned above Committee reviewed and approved this consent procedure.
We selected 11 malaria-endemic counties (Baisha, Baoting, Changjiang, Dongfang, Ledong, Lingshui, Qionghai, Qiongzhong, Sanya, Wanning, and Wuzhishan) in Hainan according to the reported malaria incidence in the previous 5 years (2004–2009). We selected one comprehensive county-level hospital in each county as the investigation sites. Patients with malaria-like symptoms such as fever, shivering, and perspiring seeking medical service in these 11 hospitals between January 1, 2009 and December 31, 2011 were enrolled. Each participant's demographic and clinical information was collected, including the ethnic group, sex, age, location of residence, clinical symptom, and the routine test.
The peripheral blood sample (5 ml) was obtained from each participant by forearm venipuncture and tested for G6PDd using the fluorescent spot test (FST) method as previously described (12, 13). Briefly, 10 μl of blood sample was added to 100 μl test reagents and incubated at 37°C for 30 min; a spot was made on ordinary filter paper and was permitted to dry; the spots were then visualized under an ultraviolet (UV) light. Spots that showed fluorescence were classified as normal G6PD, and spots that failed to show fluorescence were classified as G6PDd. After being used for G6PDd test, microscopy test, and blood routine test, the remaining blood samples were stored in Hainan CDC for further confirmation assays. The microscopy examined all patients to confirm or rule out a malaria diagnosis.
The G6PDd prevalence for various groups, including different ethnic groups, genders, counties, malaria patients, and patients without malaria, were calculated and compared using the χ2-test. In order to avoid the confounding effects caused by ethnicity in different counties, we calculated the ethnic-standardized G6PD prevalence. The ethnic-standardized G6PDd prevalence of the study area and each county were calculated and compared with their corresponding general G6PDd prevalence using the χ2-test. The malaria incidence of the study participants in each county were calculated.
The G6PDd prevalence of each county were first categorized into three levels, i.e., 0–5.00%, 5.01–10.00%, and 10.01–15.00%, respectively, and then geo-coded and matched to the corresponding polygon on a digital map of Hainan, which was marked with different colors to represent the different levels of the G6PDd prevalence. The spatial autocorrelations of the G6PDd prevalence and ethnic-standardized G6PDd prevalence across the study area was estimated using Moran's I statistic program to determine whether G6PDd was randomly distributed among the counties. The spatial analyses were conducted using the Spatial Analyst Model with ArcGIS 9.2 software (ArcGIS 9.2, Environmental Systems Research Institute, Redlands, CA, USA).
The spatial cluster analyses of malaria incidence and G6PDd prevalence were conducted between 2009 and 2011. In order to detect and compare the counties with a high risk of malaria and G6PDd at different spatial scales, maximum spatial cluster sizes of 20%, 30%, and 40% of the entire population were specified for both malaria incidence and G6PDd prevalence. The cluster window with the highest likelihood ratio (LLR) was the most likely cluster to have the highest risk of malaria or G6PDd. The cluster window with the next to maximum LLR was the secondary likely cluster with the second-highest risk of malaria or G6PDd. The relative risks of malaria or G6PDd within and outside these windows were calculated to evaluate the degree of risk. The cluster analysis was performed using SatScan 7.0.3 (SatScan 7.0.3, Information Management Services Inc., Boston, MA, USA). Clustering was performed using purely spatial and a Poisson model was used during the analysis.
Spearman rank correlation analysis was conducted to detect the relationship between G6PDd prevalence and malaria incidence in the study areas. The index r > 0 denoted a positive correlation, while r < 0 denoted a negative correlation. The correlation was considered significant when P < 0.05.
Among the 5,622 participants in the present study, 3,444 were Han Chinese and 2,178 were Li Chinese, including 3,009 males and 2,613 females, and 670 were malaria patients (including 667 P. vivax malaria patients and 3 Plasmodium falciparum malaria patients) and 4,952 were patients without malaria (Supplementary Table S1). The age of the 5,622 participants ranged from 19 to 72 years old (median = 39 years, IQR 26–52 years). There were 634 (94.63%) malaria patients with a body temperature higher than 37.5°C, 571 (85.22%) with shivering, 595 (88.81%) with perspire, 206 (30.75%) with abdominal pain, 113 (16.87%) with nausea, and 105 (15.67%) with vomiting. The median WBC, RBC, and hemoglobin of the 670 malaria patients were 8.2 × 109/L (4.6–11.3 × 109/L), 4.3 × 1012/L (3.5–5.2 × 1012/L), and 121 g/L (102–139 g/L).
The overall G6PDd prevalence was 7.45% (419/5,622). The G6PDd prevalence of males was significantly higher than that of females (8.91% vs. 5.78%; χ2 = 19.84, P < 0.01, Table 1). The G6PDd prevalence of the Li ethnic minority was significantly higher than that of the Han ethnic majority (12.03% vs. 4.56%; χ2 = 107.96, P < 0.01, Table 1). The G6PDd prevalence of malaria patients was significantly higher than that of patients without malaria (12.39% vs. 6.79%; χ2 = 26.86, P < 0.01, Table 1). The G6PDd prevalence of male patients was significantly higher than that of patients without malaria (12.88% vs. 8.36%; χ2 = 8.07, P < 0.01). After a careful comparative analysis of the subgroups, Li ethnic group, male gender, and G6PDd were at risk of malaria. There was no significant difference between the G6PDd prevalence and the ethnic-standardized G6PDd prevalence of the entire study population (7.45% vs. 8.29%; χ2 = 3.55, P > 0.05).
The G6PDd prevalence varied from 2.96% in Qionghai County to 14.48% in Dongfang County, and the ethnic-standardized G6PDd prevalence varied from 2.11% in Wuzhishan County to 14.82% in Dongfang County (Table 2). There were significant differences in both G6PDd prevalence and ethnic-standardized G6PDd prevalence among the 11 counties (Table 2, χ2 = 121.16, P < 0.01; χ2 = 110.65, P < 0.01). In addition, significantly positive spatial autocorrelations of G6PDd prevalence and ethnic-standardized G6PDd prevalence across the 11 counties [Moran's I = 0.49, Z (I) = 2.62, P < 0.05; Moran's I = 0.53, Z (I) = 2.71, P < 0.05] was observed. However, there was no significant difference between the G6PDd prevalence and the ethnic-standardized G6PDd prevalence in each county. The spatial distribution pattern of the G6PDd prevalence of the 11 counties was heterogeneous (Figure 1).
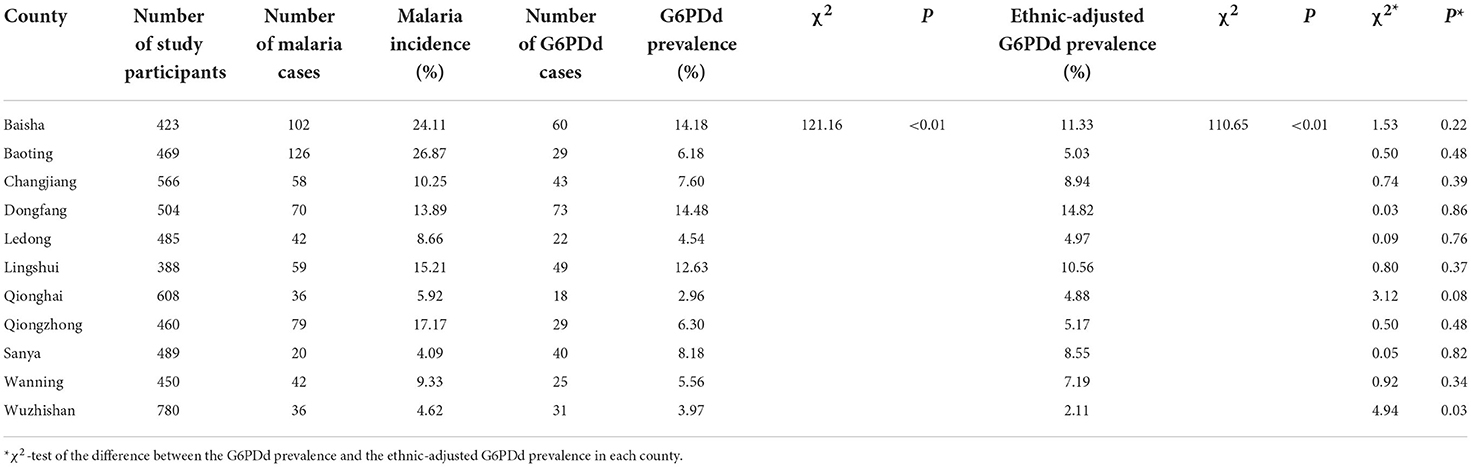
Table 2. The G6PDd prevalence, ethnic-adjusted G6PDd prevalence, and malaria incidence in 11 counties in Hainan, China, 2009–2011.
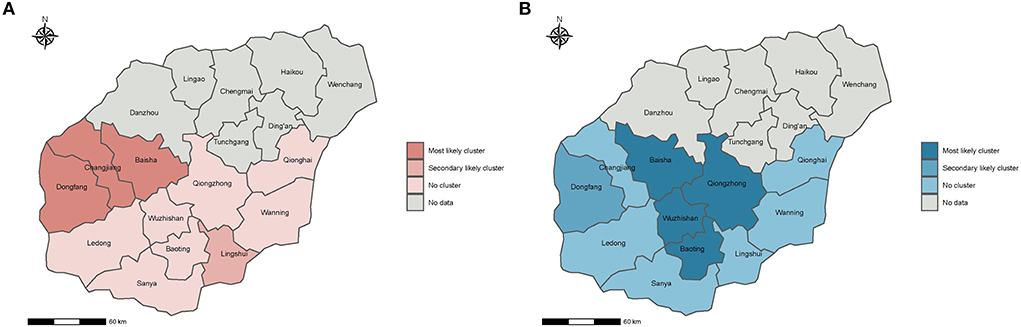
Figure 1. Spatial distribution of clusters with high G6PDd risk and high malaria risk in Hainan, China, 2009–2011. (A,B) is the spatial distribution of clusters with high G6PDd risk and high malaria risk, respectively. The risk of G6PDd and malaria were ordered using the likelihood ratio (LLR). The most likely cluster indicates the area with the highest risk of G6PDd and malaria, and the secondary likely cluster indicates the area with the second highest risk of G6PDd and malaria.
We limit the maximum spatial cluster size to 20% of the population size at risk, three counties constituted the most likely cluster of G6PDd (Baisha Changjiang and Dongfang) and one county constituted the secondary likely cluster (Lingshui) (Figure 1A). When the maximum spatial cluster size was 20% of the population size, four counties constituted the most likely cluster of malaria (Lingshui, Qiongzhong, Wuzhishan, and Baoting), and one county constituted the secondary likely cluster (Baisha) (Figure 1B). These results further indicated that the distribution patterns of G6PDd and malaria were significantly different among the studied counties.
Correlation analysis indicated no significant correlation between the G6PDd prevalence and malaria incidence during 2009–2011 in the study areas (r = 0.46, P > 0.05).
It has been estimated that more than 400 million people are affected by G6PDd in malaria-endemic regions worldwide (10). Moreover, G6PDd prevalence in the ethnic minority areas is most likely underestimated due to no universal access to diagnosis or high expenditure (7, 14–18). So, the actual G6PDd prevalence may need continuous updates leading to additional difficulty in combating malaria (3, 19). The present study demonstrated that the population in Hainan has a significantly higher G6PDd prevalence compared with other areas in China (8, 9, 20).
Though currently, Hainan has eliminated P. vivax malaria, the current undated data on G6PDd prevalence has important implications for treating imported P. vivax malaria and preventing re-transmission in the future.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a common X-linked genetic trait, and thus affects mainly males. Link between G6PDd and gender revealed that the prevalence in males is usually higher than that of females. The G6PD test classifies the heterozygous females as normal because they have both normal and deficient G6PD red blood cells. From the genetic point of view, our result confirmed that the G6PDd prevalence of males is significantly higher than that of females overall (12.03% vs. 4.56%) or at the group level (both in Li ethnic group and Han ethnic group). On the other hand, males are more likely to become infected with malaria because of some social and behavioral characteristics. For example, males are more likely exposed to the environment of Anopheles mosquitoes, and females are more likely to use mosquito nets than males as they tend to protect their young children. Where feasible, particular attention should be paid to the males, especially in the endemic area or where an outbreak happens when massive primaquine is used.
Knowing the G6PDd prevalence is crucial to determining whether primaquine is contraindicated in patients (21, 22). Hainan Province is multi-ethnic with variant genetic backgrounds, while Han and Li are the two main ethnic groups. According to a national survey, the G6PDd prevalence among ethnic groups is usually greater than among ethnic Han Chinese. In the present study, we also found the different G6PDd prevalence among ethnic groups. For example, the G6PDd prevalence (8.91%) of the Li ethnic minority was significantly higher than that of the Han ethnic majority and was also higher than that of other ethnic minority populations reported from other areas in China (8, 20, 23). Li ethnic minority group was the second largest population with unique genetic traits on Hainan Island. However, the G6PDd studies on Hainan Li population are still insufficient because Li populations live in remote or hard-to-reach areas and have limited access to health facilities. Particular attention should be paid to high-risk groups vulnerable to drug reactions, especially when their genetic variants are unknown.
Glucose-6-phosphate dehydrogenase deficiency can provide protection from severe malaria (24). There is significant selective pressure on the G6PD gene in malaria-endemic areas resulting G6PDd prevalence is exceptionally high in some areas of Africa, the Middle East, and South Asia (25). The present study also found that the G6PDd prevalence in malaria patients was more remarkable when compared with that in the non-malaria patient. However, its protective effect on preventing severe malaria needs further evaluation because we did not find such patients in our investigation period. Additionally, the relationship between parasite density and G6PDd status has yet to be explored.
We also found that the distribution patterns of G6PDd were not consistent with the malaria incidence by cluster analysis. These results suggest that the spatial correlation between G6PDd prevalence and malaria incidence is not evident in our study. Similarly, Rosalind et al. established a geostatistical model based on G6PDd prevalence, estimated affected populations, and found that G6PDd was spatially heterogeneous. However, cluster analysis revealed that malaria patients were mainly distributed in the central-southern counties of Hainan, consistent with the epidemic situation during the study period (26). Identification of distribution pattern of G6PD and malaria incidence can provide valuable insights for deliberating the relatedness.
Of note, the present study also has some limitations. Firstly, we only included a small number of participants from 11 counties, which may not reflect the true representative of the overall demographic sample. Secondly, we only focused on the two largest ethnic groups but not include other ethnic minorities. The G6PDd patterns and factors influencing the associations with malaria incidence are needed to examine in a stratified sampling method. Thirdly, the enrolled participants were selected from the hospital instead of the community, which may lead to bias.
In summary, the results of this study represent the actual G6PDd status in malaria-endemic areas of Hainan. The Li ethnic minority had a higher G6PDd prevalence, especially in males and malaria patients. The G6PDd prevalence was not spatially correlated with the malaria incidence. Thus, in order to mitigate the risk of primaquine-induced hemolysis, special attention should be paid to minority populations in Hainan. More efficient and convenient diagnostic testing tools, such as point-of-care field tests, are expected to inform the potential risk of primaquine-associated harm.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
This clinical study protocol was reviewed and approved by the Ethics Review Committee of the Hainan Center for Disease Control and Prevention. Each participant provided informed consent (in Chinese) before participating in this study. In most cases, the participants provided their written informed consent. The consent was verbal for patients who could not read or write standard Chinese. In these cases, the research nurse documented the participant's consent in writing, including the contents and methods of information provided to the participant and the date and time of the verbal consent, which was then witnessed and signed by another nurse who was not in the research team. The informed consent record, either written or verbal, was kept in the participant's hospital chart. The Ethics mentioned above Committee reviewed and approved this consent procedure. The patients/participants provided their written informed consent to participate in this study.
WZ, YL, and GW contributed to the original idea and conceived the paper. GW, NL, AG, MY, RM, and NJ wrote the initial draft of the paper. XF, DS, and GW contributed to the revision of the manuscript. XF reviewed the final version. All authors approved the final manuscript.
This study was supported by the Key Medical Research Project of the Health Department of Hainan Province (2010Qiong-36) and the Hainan Provincial Scientific Research Grant (813251). This study was also supported by Hainan Provincial Basic and Applied Basic Research Program (Natural Science Foundation) for High-level Talents in 2019 (2019RC394).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1010172/full#supplementary-material
1. Chinese Military of Public Health. Plan of Action for the Elimination of Malaria (2010–2020) (2010). Available online at: http://www.nhfpc.gov.cn/zhuzhan/wsbmgz/201304/15a4cc7a40b0452191fe409590ca99d8.shtml (accessed March 26, 2014).
2. Baird JK. Eliminating malaria-all of them. Lancet. (2010) 376:1883–5. doi: 10.1016/S0140-6736(10)61494-8
3. Howes RE, Pie FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. (2012) 9:e1001339. doi: 10.1371/journal.pmed.1001339
4. Phompradit P, Kuesap J, Chaijaroenkul W, Rueangweerayut R, Hongkaew Y, Yamnuan R, et al. Prevalence and distribution of glucose-6-phosphate dehydrogenase (G6PD) variants in Thai and Burmese populations in malaria endemic areas of Thailand. Malar J. (2011) 10:368. doi: 10.1186/1475-2875-10-368
5. Amini F, Ismail E, Zilfalil BA. Prevalence and molecular study of G6PD deficiency in Malaysian Orang Asli. Intern Med J. (2011) 41:351–3. doi: 10.1111/j.1445-5994.2011.02456.x
6. Tantular IS, Matsuoka H, Kasahara Y, Pusarawati S, Kanbe T, Tuda JS, et al. Incidence and mutation analysis of glucose-6-phosphate dehydrogenase deficiency in eastern Indonesian populations. Acta Med Okayama. (2010) 64:367–73. doi: 10.18926/AMO/41322
7. Leslie T, Moiz B, Mohammad N, Amanzai O, Ur Rasheed H, Jan S, et al. Prevalence and molecular basis of glucose-6-phosphate dehydrogenase deficiency in Afghan populations: implications for treatment policy in the region. Malar J. (2013) 12:230. doi: 10.1186/1475-2875-12-230
8. Li H, Zhu J, Che Y, Dao T, Zhao S. Screening of glucose-6-phosphate dehydrogenase (G6PD) deficiency in minority nationalities in high malaria endemic areas of Xishuangbanna prefecture. China Trop Med. (2012). 12:137–48. doi: 10.13604/j.cnki.46-1064/r.2012.02.031
9. Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. (2008) 371:64–74. doi: 10.1016/S0140-6736(08)60073-2
10. Beutler E, Duparc S, G6PD Deficiency Working Group. Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. (2007) 77:779–89. doi: 10.4269/ajtmh.2007.77.779
11. Leslie T, Briceño M, Mayan I, Mohammed N, Klinkenberg E, Sibley CH, et al. The impact of phenotypic and genotypic G6PD deficiency on risk of Plasmodium vivax infection: a case-control study amongst Afghan refugees in Pakistan. PLoS Med. (2010) 7:e1000283. doi: 10.1371/journal.pmed.1000283
12. Beutler E, Mitchell M. Special modifications of the fluorescent screening method for glucose-6-phosphate dehydrogenase deficiency. Blood. (1968) 32:816–8. doi: 10.1182/blood.V32.5.816.816
13. Roca-Feltrer A, Khim N, Kim S, Chy S, Canier L, Kerleguer A, et al. Field trial evaluation of the performances of point-of-care tests for screening G6PD deficiency in Cambodia. PLoS ONE. (2015) 9:e116143. doi: 10.1371/journal.pone.0116143
14. von Fricken ME, Weppelmann TA, Eaton WT, Alam MT, Carter TE, Schick L, et al. Prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency in the Ouest and Sud-Est departments of Haiti. Acta Trop. (2014) 135:62–6. doi: 10.1016/j.actatropica.2014.03.011
15. Khim N, Benedet C, Kim S, Kheng S, Siv S, Leang R, et al. G6PD deficiency in Plasmodium falciparum and Plasmodium vivax malaria-infected Cambodian patients. Malar J. (2013) 12:171. doi: 10.1186/1475-2875-12-171
16. Herdiana H, Fuad A, Asih PB, Zubaedah S, Arisanti RR, Syafruddin D, et al. Progress towards malaria elimination in Sabang Municipality, Aceh, Indonesia. Malar J. (2013) 12:42. doi: 10.1186/1475-2875-12-42
17. Asih PB, Rozi IE, Herdiana, Pratama NR, Hidayati AP, Marantina SS, et al. The baseline distribution of malaria in the initial phase of elimination in Sabang Municipality, Aceh Province, Indonesia. Malar J. (2012) 11:291. doi: 10.1186/1475-2875-11-291
18. Millimono TS, Loua KM, Rath SL, Relvas L, Bento C, Diakite M, et al. High prevalence of hemoglobin disorders and glucose-6-phosphate dehydrogenase (G6PD) deficiency in the Republic of Guinea (West Africa). Hemoglobin. (2012) 36:25–37. doi: 10.3109/03630269.2011.600491
19. Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis. (2009) 42:267–78. doi: 10.1016/j.bcmd.2008.12.005
20. Yao LQ, Zou TB, Wang XT, Quan X, Chen Q, Yang FB, et al. G6PD deficiency among children under 7 years old from Yunnan with unique ethnic m inority origin. Chin J Med Genet. (2013) 30:189–94. doi: 10.3760/cma.j.issn.1003-9406.2013.04.015
21. Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. (1961) 190:372–3. doi: 10.1038/190372a0
22. Beutler E, Yeh M, Fairbanks VF. The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker. Proc Natl Acad Sci USA. (1962) 48:9–16. doi: 10.1073/pnas.48.1.9
23. Hu L, Yang H, Ou C, Zou T, Yao L, Liu J. Yunnan Nujiang Lisu, Clan and Primi, Dulong population children under the age of seven G6PD deficiency investigation. Zhongguo Yousheng Yu Yichuan Zazhi. (2011)19:128–9. doi: 10.13404/j.cnki.cjbhh.2011.12.065
24. Ruwende C, Khoo SC, Snow RW, Yates SN, Kwiatkowski D, Gupta S, et al. Natural selection of hemi-and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature. (1995) 376:246–9. doi: 10.1038/376246a0
25. Xiao D, Long Y, Wang S, Wu K, Xu D, Li H, et al. Epidemic distribution and variation of Plasmodium falciparum and Plasmodium vivax malaria in Hainan, China during 1995–2008. Am J Trop Med Hyg. (2012) 87:646–54. doi: 10.4269/ajtmh.2012.12-0164
Keywords: G6PD, primaquine, vivax malaria, spatial cluster, prevalence
Citation: Zeng W, Liu N, Li Y, Gao A, Yuan M, Ma R, Jiang N, Sun D, Wang G and Feng X (2022) Prevalence of glucose-6-phosphate dehydrogenase deficiency (G6PDd) and clinical implication for safe use of primaquine in malaria-endemic areas of Hainan Province, China. Front. Public Health 10:1010172. doi: 10.3389/fpubh.2022.1010172
Received: 02 August 2022; Accepted: 30 September 2022;
Published: 21 October 2022.
Edited by:
Gisely Melo, Fundação de Medicina Tropical Doutor Heitor Vieira Dourado (FMT-HVD), BrazilReviewed by:
Laila Rowena Albuquerque Barbosa, Heitor Vieira Dourado Tropical Medicine Foundation, BrazilCopyright © 2022 Zeng, Liu, Li, Gao, Yuan, Ma, Jiang, Sun, Wang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyu Feng, ZmVuZ3hpbnl1MjAxM0AxNjMuY29t; Guangze Wang, d2FuZ2d1YW5nemU2M0AxMjYuY29t; Dingwei Sun, c2R3X2JtamNAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.