- 1Division of Infectious Diseases, Weill Cornell Medicine, New York, NY, United States
- 2Mwanza Research Centre, National Institute for Medical Research, Mwanza, Tanzania
- 3Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark
- 4Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, Denmark
- 5Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, Netherlands
- 6Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 7Center for Global Health, Weill Cornell Medical College, New York, NY, United States
- 8Department of Medicine, Weill Bugando School of Medicine, Mwanza, Tanzania
Background: Observational studies in humans have reported a link between schistosome infection and lower adiposity, but this may be explained by socioeconomic and demographic factors, intensity of infection, or common co-infections such as HIV.
Methods: This was a cross-sectional study that investigated the relationship between schistosome infection and adiposity in a large, well-described cohort of Tanzanian adults living with and without HIV. Cross-sectional data were collected among adults living in Mwanza, Tanzania who were enrolled in the Chronic Infections, Co-morbidities and Diabetes in Africa (CICADA) cohort study. Schistosome circulating anodic antigen, secreted by both Schistosoma mansoni and haematobium which are endemic to Tanzania, was quantified from stored samples. Schistosome infection diagnosed by serum circulating anodic antigen levels. The primary outcome was fat mass measured by bioimpedance analysis. Secondary outcomes included fat-free mass, waist circumference, mid-upper arm circumference, and body mass index.
Results: The study enrolled 1,947 adults, of whom 1,923 (98.8%) had serum available for schistosome testing. Of these, 873 (45.4%) had a serum circulating anodic antigen ≥30 pg/mL, indicating schistosome infection. Compared to uninfected individuals, those with schistosome infections had −1.1 kg [95% CI −1.9 to −0.3] lower fat mass after adjusting for age, sex, physical activity, tobacco use, education level, and socioeconomic status. Infected participants also had lower waist circumference, mid-upper arm circumference, and body mass index. Fat-free mass was not different between the two groups. Neither being HIV-infected, nor receiving antiretroviral therapy, modified associations between schistosome infection and adiposity. These associations were also not affected by Schistosoma worm burden.
Conclusions: Schistosome infection was associated with lower fat mass and less central adiposity without a difference in muscle mass, irrespective of confounders, HIV status, or the intensity of schistosome infection. Future studies should adjust for socioeconomic and demographic factors that are associated with schistosome infection and adiposity. Identifying mechanistic pathways by which schistosome infection reduces adiposity while preserving muscle mass could yield new strategies for obesity control and cardiovascular disease prevention.
Introduction
Schistosoma parasites are water-borne helminths that affect over 200 million people worldwide and infection is highly prevalent in fishing communities near Lake Victoria in Tanzania (1, 2). Schistosome infection disproportionately affects the poor, particularly those with limited access to clean water, as individuals become infected when they have physical contact with contaminated water. Once the parasites penetrate through human skin, adult worms lay eggs in the host venules that migrate through host tissue and cause significant, species-dependent morbidity and mortality in various organ systems (1).
Although most research has focused on the deleterious health effects of schistosomes, a few studies have reported beneficial effects of schistosome infection on common measures of obesity, such as body mass index (BMI) (3, 4). However, these studies have not examined whether this reduction in BMI in schistosome-infected adults represents a true reduction in fat mass (FM), they have not adjusted for confounding socioeconomic and demographic factors associated with schistosome infection, and they have not yet explored whether the effect of schistosomes on obesity might be modified by the intensity of infection or common co-infections such as HIV. Given the distinct socio-ecological characteristics of schistosome infection and obesity in Africa, robust assessment of factors associated with schistosome (5, 6) infection, adiposity, or both is essential. A clearer analysis would more accurately characterize the relationship between schistosome infection and adiposity, and possibly lead toward understanding underlying mechanisms.
In this novel study, we hypothesized that schistosome-infected participants would have lower FM than schistosome-uninfected participants, regardless of HIV or antiretroviral therapy (ART) status or intensity of schistosome infection, even after adjusting for significant socioeconomic and demographic factors. To test these hypotheses, we quantified associations between cross-sectional measures of adiposity, including FM, fat-free mass (FFM), waist circumference (WC), mid-upper arm circumference (MUAC), and BMI, while adjusting for characteristics associated with both schistosome infection and adiposity. We also examined the effect modification by HIV and ART.
Methods
Study design, setting, and laboratory testing
We analyzed cross-sectional data collected between October 2016 and November 2017 from adults (age ≥18 years) living in Tanzania who were enrolled in the Chronic Infections, Co-morbidities and Diabetes in Africa (CICADA) study. CICADA examined the risk factors and burden of non-communicable diseases in three groups of participants (HIV-infected, HIV-infected on ART, and HIV-uninfected) and was registered at https://clinicaltrials.gov (NCT03106480). Further details were published previously (7, 8).
Schistosome circulating anodic antigen (CAA), secreted by both Schistosoma mansoni and haematobium, was quantified in stored serum samples using a lateral flow assay (9). Both species are endemic to Tanzania, with S. mansoni predominant. Schistosome infection was defined as CAA ≥30 pg/mL.
Anthropometry and body composition
Participants underwent bioimpedance analysis to calculate FM and FFM using a body composition analyzer (Tanita BC418, Japan). Weight, height, MUAC, and waist and hip circumferences were determined as previously described (7, 8). Physical activity was computed from the WHO STEPS questionnaire and reported in minutes/day (10). Demographic information was collected; socioeconomic status (SES) was calculated using principal component analysis (11).
Ethics statement
Permission to conduct this study was granted by the National Institute for Medical Research in Tanzania and Weill Cornell Medicine in New York. Participants provided written informed consent in Kiswahili.
Data management and statistics
Data were collected using CSPro and analyzed in Stata MP/Version 17 (College Station, Texas). We applied descriptive statistics, using means and standard deviations (SD) for continuous variables and frequencies and percentages for categorical variables. The primary outcome was FM, and secondary outcomes included other measures of adiposity. We used linear and logistic regression to compare characteristics between schistosome-infected and uninfected individuals and measure the effect of schistosome infection intensity on adiposity. We then performed adjusted regression models, accounting for age, sex, physical activity, tobacco use, education level, and SES. We used interaction terms to assess for effect modification by HIV and ART on schistosome infection and adiposity. P-values <0.05 were considered significant.
Results
Among 1,923 participants included, 873 (45.4%) were considered schistosome-infected. The schistosome-infected group was ~1.5 years younger and had a lower frequency of females (Table 1). Distributions of HIV-infected ART naïve, HIV-infected on ART, and HIV-uninfected participants were similar between those with and without schistosome infection. Tuberculosis and malaria infection status and serum C-reactive protein levels were also similar between groups.
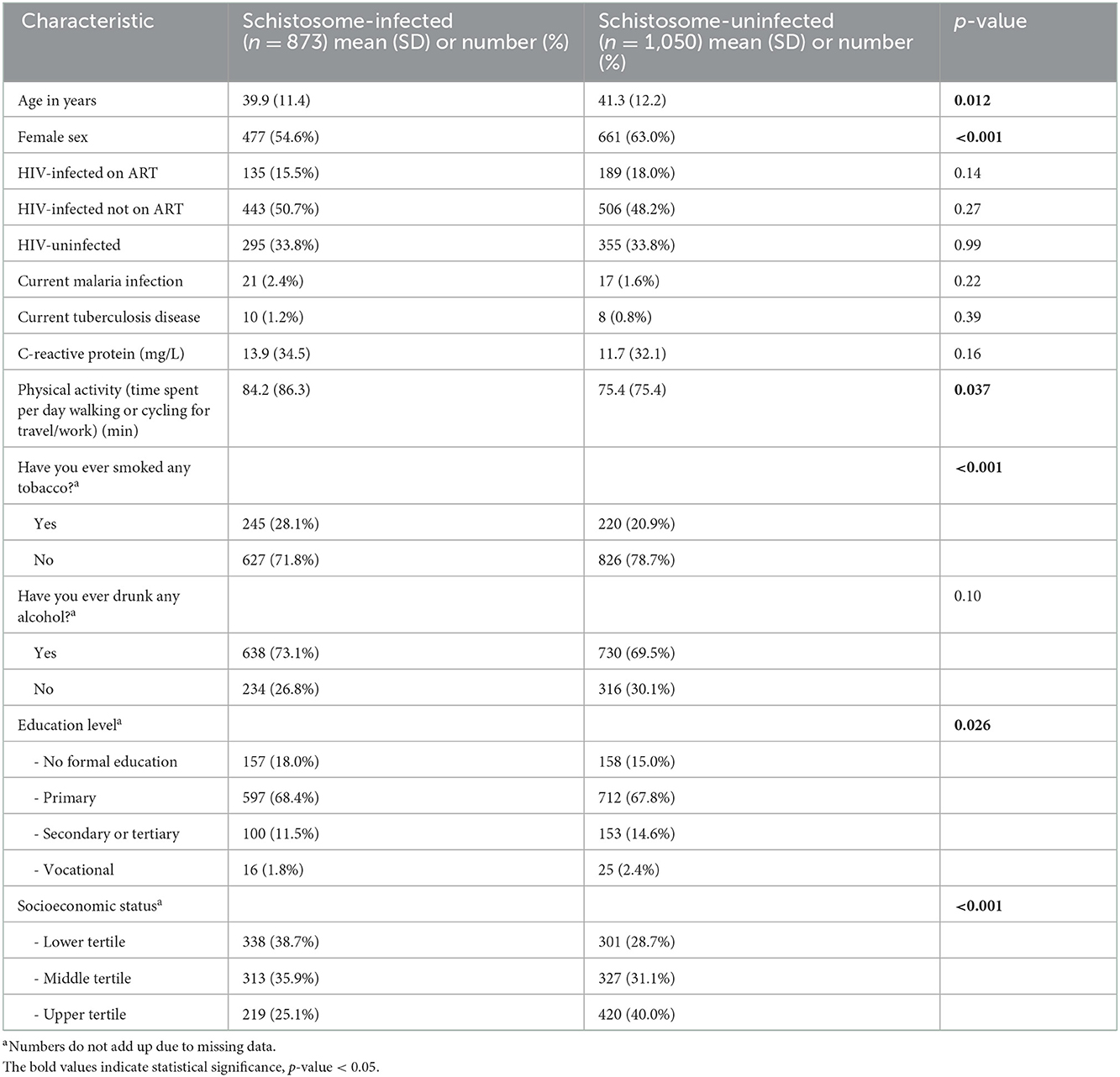
Table 1. Sociodemographic and other characteristics in schistosome-infected vs. uninfected individuals.
Schistosome-infected persons had lower education level and SES, more physical activity, and more tobacco use than their uninfected counterparts, suggesting that these were potential confounders.
Schistosome-infected individuals had significantly lower FM than uninfected individuals (unadjusted regression coefficient: −2.9 kg [−3.8 to −2.1]; p < 0.001, Table 2), as well as lower WC, MUAC, and BMI. Schistosome-infected individuals more frequently had underweight and normal-weight BMI. FFM was not different between those with and without schistosome infection.
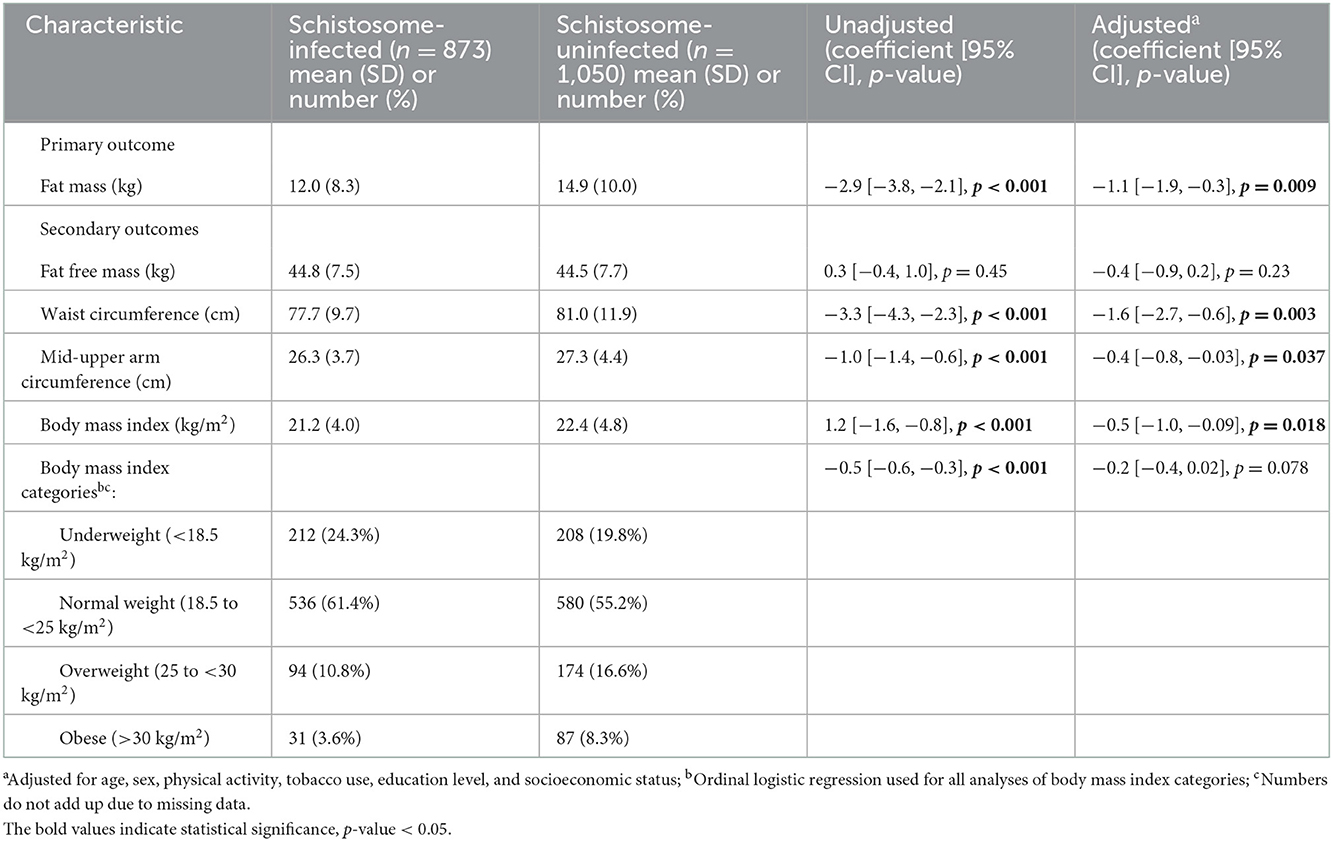
Table 2. Measures of adiposity and body composition in schistosome-infected vs. uninfected individuals.
Adjusted regression models demonstrated that schistosome infection remained inversely associated with FM (adjusted regression coefficient: −1.1 kg [−1.9 to −0.3]; p = 0.009). All other continuous measures of adiposity also remained lower in schistosome-infected individuals after adjustment. Of note, FFM remained similar between the two groups (Figure 1). Further adjustments also showed that CAA level, a well-recognized proxy for Schistosoma worm burden (9), did not change the estimate of adiposity beyond the effect of being schistosome-infected.
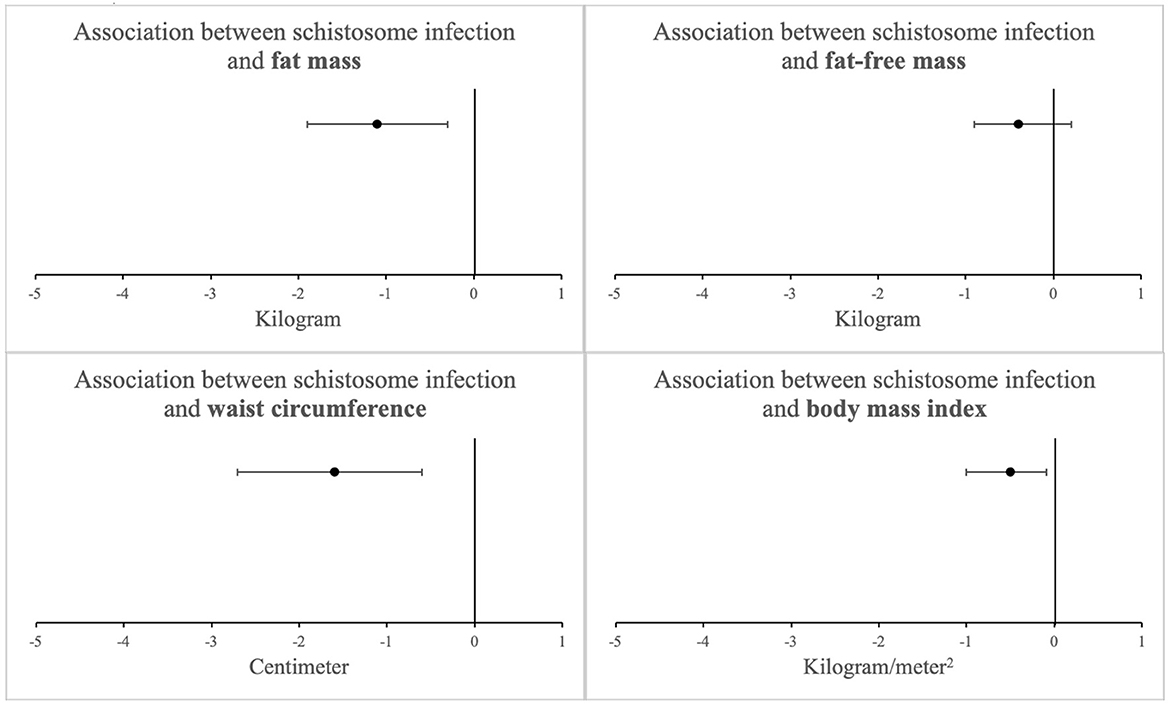
Figure 1. Association between schistosome infection and measures of adiposity among 1923 study participants after adjusting for potential confounders (linear regression coefficients with 95% confidence intervals)a. aAdjusted for age, sex, physical activity, tobacco use, education level, and socioeconomic status, No significant interaction was observed by HIV status (see Table 3).
Additionally, there were no interactions between HIV or ART and schistosome infection with respect to adiposity (p > 0.05) (Table 3).
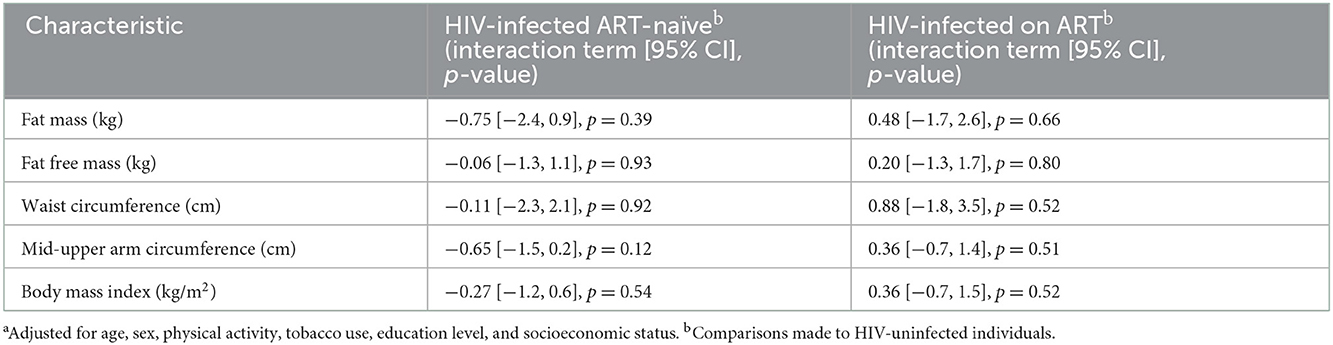
Table 3. Effect modification by HIV status on the association between schistosome infection and measures of adiposity (interaction terms with 95% confidence intervals)a.
Discussion
It is well-known that schistosome infection, which is highly prevalent in sub-Saharan Africa, is associated with less adiposity and that less adiposity is associated with lower cardiovascular disease. We have demonstrated the importance of adjusting for socioeconomic and demographic variables in assessing the relationship between schistosome infection and cardiovascular disease. Adjusting for these variables explained approximately half of the difference in adiposity between schistosome-infected and uninfected study participants. These findings are consistent with experimental mouse models free of confounding, in which schistosome infection was associated with decreased body weight (12).
Even after adjustment, schistosome infection was independently associated with 1 kg lower FM with no effect on muscle mass. Our study confirms and extends prior human and animal studies (3, 4, 12) that have shown decreased BMI and other measures of body composition in people and mice with schistosome infections. Interestingly, higher loads of Schistosoma worm infection did not further magnify the association between infection and adiposity. This suggests that the weight loss associated with schistosome infection is unlike that of other chronic infectious diseases, such as HIV and tuberculosis, which cause wasting of both FM and FFM, and may be driven by alternative metabolic pathways (13, 14). Given known effects of obesity on cardiovascular disease (15), such reduction in adiposity could have an impact on cardiovascular health.
Although the majority of published data indicate a link between schistosome infection and lower adiposity, there are a few exceptions (16). Notably, this study differs from our previous study which used stool eggs to diagnose schistosomiasis (8), which likely underestimated the prevalence of schistosome infection and lowered the study's power to detect differences in adiposity. Antigen testing for schistosome infection has higher sensitivity than egg microscopy, particularly in women and HIV-infected individuals (17).
Our study has strengths and weaknesses. Strengths include a large, well-characterized cohort including many sociodemographic, clinical, and behavioral factors and use of antigens to detect schistosome infection with greater sensitivity. A cross-sectional design limits our ability to infer causality, warranting prospective data to assess the potential causal relationship between schistosome infection and adiposity. Such studies may also consider including cardiovascular outcomes to assess how schistosome infection and adiposity are interrelated and whether they impact non-communicable diseases. Additionally, those studies could consider examining how anthelmintic treatment and the duration of schistosome infection may impact cardiovascular outcomes cross-sectionally and longitudinally.
In conclusion, our study confirms that schistosome infection is associated with lower FM without reduction in FFM and that the potential protective effects of schistosome infection are not explained by confounders, co-infections, or the intensity of schistosome infection. Humans have lived with schistosomes for millennia, and it would therefore not be surprising if coevolution has resulted in beneficial cardiovascular effects of low-level schistosome infection on human health. Control of schistosomiasis globally has been based on large-scale treatment of at-risk population groups, improved sanitation and access to clean water, and control of snails that transmit the parasite (1). Although these policies have reduced the burden of schistosomiasis, infection is still quite prevalent in many tropical and subtropical regions, including Tanzania (1, 2). Our challenge is to identify and harness these beneficial effects while avoiding the harmful effects that result from high schistosome burden.
Data availability statement
The datasets presented in this article are not readily available because data cannot be shared publicly but are available upon request and approval by the Medical Research Coordinating Committee (MRCC) of the National Institute for Medical Research (NIMR) in Tanzania, which requires that data should not be transferred or shared without their permission. For researchers who meet the criteria for access to confidential data, they may use the contact details below to request the data. Requests to access the datasets should be directed to The Secretariat, National Institute for Medical Research, 2448, Baraka Obama Road, P O Box 9653 Dar Es Salaam, Tanzania. ZXRoaWNzQG5pbXIub3IudHo=.
Author contributions
KP: writing—original draft, writing—review and editing, formal analysis, and visualization. GP: conceptualization, funding acquisition, methodology, project administration, validation, and writing—reviewing and editing. DF-J and MO: conceptualization, funding acquisition, methodology, and writing—reviewing and editing. BKa and BKi: project administration, validation, and writing—reviewing and editing. PC and CD: writing—review and editing and validation. HF and SF: conceptualization, funding acquisition, methodology, and writing—reviewing and editing. JD and RP: writing—review and editing, formal analysis, visualization, validation, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Ministry of Foreign Affairs of Denmark and administered by Danida Fellowship Centre (16-P01-TAN). KP was supported by the National Institute of Allergy and Infectious Diseases (NIAID T32 AI007613) and Burroughs Wellcome Fund/American Society of Tropical Medicine & Hygiene Postdoctoral Fellowship in Tropical Infectious Diseases. BKa is supported by a grant from the Fogarty International Center of the US National Institutes of Health (D43 TW009337). RP is supported by the National Heart, Lung, and Blood Institute (R01 HL160332). JD was supported by the National Institute of Allergy and Infectious Diseases (R01 AI 168306). None of the funders had any role in the study design, data collection and analysis, decision to publish results or preparation of the manuscript.
Acknowledgments
We thank all participants in this study and are grateful to the staff of the CICADA project, ART clinics in Mwanza, and NIMR laboratory team for their assistance and collaboration. We also thank Kidola Jeremiah, MD, PhD, for his significant contribution to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author's disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. WHO. Schistosomiasis [Internet]. (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed August 16, 2022).
2. Downs JA, de Dood CJ, Dee HE, McGeehan M, Khan H, Marenga A, et al. Schistosomiasis and human immunodeficiency virus in men in Tanzania. Am J Trop Med Hyg. (2017) 96:856–62. doi: 10.4269/ajtmh.16-0897
3. Wolde M, Berhe N, Medhin G, Chala F, van Die I, Tsegaye A. Inverse associations of schistosoma mansoni infection and metabolic syndromes in humans: a cross-sectional study in Northeast Ethiopia. Microbiol Insights. (2019) 12:1178636119849934. doi: 10.1177/1178636119849934
4. Shen SW, Lu Y, Li F, Shen ZH, Xu M, Yao WF, et al. The potential long-term effect of previous schistosome infection may reduce the risk factors for cardiovascular diseases. Int J Cardiol. (2014) 177:566–8. doi: 10.1016/j.ijcard.2014.08.128
5. Sady H, Al-Mekhlafi HM, Mahdy MAK, Lim YAL, Mahmud R, Surin J. Prevalence and associated factors of Schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis. (2013) 7:e2377. doi: 10.1371/journal.pntd.0002377
6. Halpern MT, Arena LC, Royce RA, Soler RE, Munoz B, Hennessy CM. Neighborhood and individual sociodemographic characteristics associated with disparities in adult obesity and perceptions of the home food environment. Health Equity. (2017) 1:139–49. doi: 10.1089/heq.2017.0010
7. Jeremiah K, Filteau S, Faurholt-Jepsen D, Kitilya B, Kavishe BB, Krogh-Madsen R, et al. Diabetes prevalence by HbA1c and oral glucose tolerance test among HIV-infected and uninfected Tanzanian adults. PLoS One. (2020) 15:e0230723. doi: 10.1371/journal.pone.0230723
8. PrayGod G, Filteau S, Range N, Ramaiya K, Jeremiah K, Rehman AM, et al. The association of Schistosoma and geohelminth infections with β-cell function and insulin resistance among HIV-infected and HIV-uninfected adults: A cross-sectional study in Tanzania. PLoS ONE. (2022) 17:e0262860. doi: 10.1371/journal.pone.0262860
9. Corstjens PLAM, De Dood CJ, Kornelis D, Fat EMTK, Wilson RA, Kariuki TM, et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. (2014) 141:1841–55. doi: 10.1017/S0031182014000626
10. World Health Organization. STEPS Instrument [Internet]. (2022). Available from: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps/instrument (accessed February 9, 2022).
11. Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. (2001) 38:115–32. doi: 10.1353/dem.2001.0003
12. Hussaarts L, García-Tardón N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. (2015) 29:3027–39. doi: 10.1096/fj.14-266239
13. Forrester JE, Spiegelman D, Tchetgen E, Knox TA, Gorbach SL. Weight loss and body-composition changes in men and women infected with HIV. Am J Clin Nutr. (2002) 76:1428–34. doi: 10.1093/ajcn/76.6.1428
14. Mupere E, Malone L, Zalwango S, Okwera A, Nsereko M, Tisch DJ, et al. Wasting among Uganda men with pulmonary tuberculosis is associated with linear regain in lean tissue mass during and after treatment in contrast to women with wasting who regain fat tissue mass: prospective cohort study. BMC Infect Dis. (2014) 14:24. doi: 10.1186/1471-2334-14-24
15. Powell-Wiley TM, Poirier P, Burke LE, Després J-P, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. (2021) 143:e984–1010. doi: 10.1161/CIR.0000000000000973
16. Zinsou JF, Janse JJ, Honpkehedji YY, Dejon-Agobé JC, García-Tardón N, Hoekstra PT, et al. Schistosoma haematobium infection is associated with lower serum cholesterol levels and improved lipid profile in overweight/obese individuals. PLoS Negl Trop Dis. (2020) 14:e0008464. doi: 10.1371/journal.pntd.0008464
Keywords: schistosome infection, adiposity, HIV, antiretroviral therapy, cardiovascular disease
Citation: Pham K, PrayGod G, Faurholt-Jepsen D, Olsen MF, Kavishe B, Kitilya B, Corstjens PLAM, de Dood CJ, Friis H, Filteau S, Downs JA and Peck RN (2023) Association of schistosome infection with adiposity in Tanzania. Front. Public Health 10:1008101. doi: 10.3389/fpubh.2022.1008101
Received: 12 September 2022; Accepted: 01 December 2022;
Published: 04 January 2023.
Edited by:
Monica Catarina Botelho, Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA), PortugalReviewed by:
Aprilianto Eddy Wiria, Kavacare.id, IndonesiaFrancesco Di Gennaro, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), Italy
Copyright © 2023 Pham, PrayGod, Faurholt-Jepsen, Olsen, Kavishe, Kitilya, Corstjens, de Dood, Friis, Filteau, Downs and Peck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khanh Pham,  a2hwOTAwN0BtZWQuY29ybmVsbC5lZHU=
a2hwOTAwN0BtZWQuY29ybmVsbC5lZHU=
†These authors have contributed equally to this work
 Khanh Pham
Khanh Pham George PrayGod
George PrayGod Daniel Faurholt-Jepsen3
Daniel Faurholt-Jepsen3 Brenda Kitilya
Brenda Kitilya Paul L. A. M. Corstjens
Paul L. A. M. Corstjens Suzanne Filteau
Suzanne Filteau Jennifer A. Downs
Jennifer A. Downs