
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 08 December 2022
Sec. Public Health Policy
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1003461
Objectives: A meta-analysis was conducted to examine the effectiveness of HPV self-sampling proposal on cervical cancer screening (CCS) uptake when compared with an invitation to have a clinician to collect the sample. Secondary outcomes were acceptability and preference of self-sampling compared to clinician-collected samples.
Methods: The present systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies examining the CCS uptake comparing self-sampling over invitation to be sampled by an healthcare professional and examining the proportion of women accepting or preferring self-sampling vs. clinician-collected sampling were included. The CCS uptake was also explored according to strategy of self-samplers' distribution, collection device type and screening status. Peters' test and Funnel Plot inspection were used to assess the publication bias. Quality of the studies was assessed through Cochrane Risk of Bias and NIH Quality Assessment tools.
Results: One hundred fifty-four studies were globally identified, and 482,271 women were involved. Self-sampling procedures nearly doubled the probability (RR: 1.8; 95% CI: 1.7–2.0) of CCS uptake when compared with clinician-collected samples. The opt-out (RR: 2.1; 95% CI: 1.9–2.4) and the door-to-door (RR: 1.8; 95% CI: 1.6–2.0) did not statistically significant differ (p = 1.177) in improving the CCS uptake. A higher relative uptake was shown for brushes (RR: 1.6; 95% CI: 1.5–1.7) and swabs (RR: 2.5; 95% CI: 1.9–3.1) over clinician-collected samples. A high between-studies variability in characteristics of sampled women was shown. In all meta-analyses the level of heterogeneity was consistently high (I2 > 95%). Publication bias was unlikely.
Conclusions: Self-sampling has the potential to increase participation of under-screened women in the CCS, in addition to the standard invitation to have a clinician to collect the sample. For small communities door-to-door distribution could be preferred to distribute the self-sampler while; for large communities opt-out strategies should be preferred over opt-in. Since no significant difference in acceptability and preference of device type was demonstrated among women, and swabs and brushes exhibited a potential stronger effect in improving CCS, these devices could be adopted.
Genital infection with human papillomaviruses (HPV) is the most common sexually transmitted infection in the world (1). In some women, HPV infection will persist over time, and if this goes undetected and untreated, it can lead to precancerous cervical lesions and possibly progress to cervical cancer (2). HPV causes about 8.6% of the cancers affecting women worldwide. In absolute terms, about 570, 000 cases/year are estimated, almost all attributable to the HPV16/18 genotypes (3).
The time from HPV infection to cervical cancer will usually take 10–20 years or longer, and leaves great opportunity for screening and early detection (4). Indeed, secondary prevention measures such as cervical cytology (Pap smear), visual inspection with acetic acid or HPV testing, have strongly contributed to the reduction of incidence and mortality of cervical cancer, by identifying those women at high risk (5, 6). However, the adherence to screening programs in some areas of the world remains very low due to the invasiveness of the test and the lack of confidence in its effectiveness. Therefore, it is quite evident that the relevance of this public health issue necessitates innovative early detection approaches (7, 8). HPV testing through self-collected specimens has gained attention for its potential to increase screening participation. Recent systematic reviews have shown that high-risk HPV (hrHPV) testing on self-sampled specimens has a similar accuracy to detect underlying cervical precancer when compared to cytology on clinician-obtained cervical smears and under the condition that validated polymerase chain reaction (PCR)–based HPV assays are used (9, 10). In addition, several systematic reviews of randomized trials in the context of population-based screening programs showed that offering hrHPV self-sampling to never-screened and under-screened women increased participation compared with inviting women to have samples taken by healthcare professionals (HCPs) (11–13).
In recent years, numerous studies have investigated the acceptability of self-sampling methods (10, 14–16). Studies have considered women's attitudes toward self-collection and found that women have a high acceptance of and positive attitudes toward the use of self-collected HPV testing (9–11, 15, 16). Skepticism toward self-sampling has emerged, and it is attributable mainly to the fear of not carrying out a correct self-sampling or toward its underrated diagnostic performance (17, 18). Since the last published meta-analysis (19), several studies have measured the effectiveness of self-sampling in increasing the HPV-screening uptake. Moreover, it remains unclear which type of self-sampler offers a better performance. Therefore, we conducted an updated review and meta-analysis on women's attendance in cervical cancer screening (CCS) comparing self-sampled to clinician-collected specimens was conducted to assess whether the strategy of self-samplers' distribution (direct mailing to home, door-to-door distribution, or availability in clinics/pharmacies) and the type of device (brush, swab, lavage, tampon) and the screening status (never- or under-screneed vs. general population) could act as predictors of CCS uptake. Finally, the overall percentage of women who considered self-sampling to be acceptable and who preferred it over collection performed by healthcare personnel was estimated.
The present systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20). The need for obtaining institutional review board approval or patient informed consent was waived for this study because it is a review of publicly available data.
This study was registered in the International Register of Systematic Reviews (PROSPERO 2021: CRD42021266637) and the protocol is available for download.
Studies were eligible if the following criteria were met: (1) examining the CCS uptake comparing self-sampling over invitation to be sampled by an HCP; (2) reporting enough data to estimate an effect size (Odds- or Risk-Ratio) of CCS uptake; (3) examining the proportion of women accepting or preferring self-sampling vs. clinician-collected sampling; (4) the study population involved women ages 18–70 years both among the general population and among those who were never- or under-screened; (5) the study was in English and published by May, 2022.
The primary outcome was the CCS uptake comparing self-sampling with clinician-collected samples for HPV testing. The CCS uptake was also explored according to strategy of self-samplers' distribution, collection device type and screening status. Self-samplers' distribution strategies evaluated were door-to-door (i.e., self-samplers were directly distributed to women), opt-out (i.e., mailing self-sampling kits directly to women's home addresses) and opt-in (i.e., receiving an invitation to actively order the kit by phone, by ordinary mail, or by picking it up at the pharmacy or local clinics).
Secondary outcomes were acceptability and preference of self-sampling compared to clinician-collected samples. Acceptability was defined as a unique answer (yes/no) to questions like “Did you find self-sampling acceptable?”. Similarly to a previous meta-analysis, the proxy questions “Would you recommend self-sampling to a relative or friend of yours?” or “Would you be willing to use a self-sampler again in the future?” were taken into account (21). Studies in which acceptability was not reported as binary data but measured by a continuous or numerical ordinal variable (e.g., 0–10 scale) were not considered unless an acceptability cut off was established. With regard to the preference outcome, we considered studies in which, after using the self-sampler, women were asked whether they preferred self-sampling or clinician-collected samples for future HPV screening visits.
A detailed bibliographic literature search was conducted until May 2022. Two co-authors (GDG, FL) independently searched PubMed, Web of Science, Scopus, Cochrane Central and Google Scholar combinations of the following keywords/Medical Subject Headings (MeSH) terms: “HPV”, “Human Papillomavirus”, “self-sampler”, “self-sampling”, “self-test”, “self-testing”, “home-based testing”, “community-based test”, “acceptability”, “acceptance”, “willingness”, “uptake”, “participation”, “preference”. Electronic searches were supplemented by manual searches of the reference list of relevant articles. Both observational and randomized studies were searched. Gray literature was not considered.
All articles retrieved from the systematic search were exported to the Mendeley reference manager (www.mendeley.com), wherein duplicates were sought and removed. Three authors (GDG, FL, AT) independently winnowed titles and abstracts of the candidate papers to make a first selection. Full-text of selected papers was read to assess their eligibility in terms of topics of interest and the target population. Disagreements were resolved through discussion with a third author (AB).
Relevant articles were reviewed in full if the study abstract met the inclusion criteria or if an article lacked sufficient information in the abstract to make an inclusion/exclusion judgement, to minimize errors of omission. Figure 1 summarizes the flow diagram of the literature search and the study selection process.
An electronic collection form was used to extract the following information for each study: first author, year of publication, country, type of device (brush, swab, tampon or lavage), screening status (never or under-screened or general population), study design (observational or randomized). Women defined as “never-screened”, “under-screened”, “non-attendee” or “non-responders” to regular screening invitations were classified as “under-screened”. The self-samplers' distribution strategy (i.e., door-to-door, opt-out or opt-in strategy) was also retrieved. Regarding studies on acceptability and preference, information about the setting in which self-sampling occurred (at home or in a clinic) was also extracted.
Study quality was independently assessed by three authors (GDG, FL, AT) through the revised Cochrane Risk of Bias (RoB2). Tools for parallel and cluster-randomized trials or the National Institutes of Health (NIH). Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, depending on the study design (22, 23). The ratings (good, fair or poor methodological quality) assigned by each reviewer were compared and disagreements were discussed between the two reviewers. If consensus was not reached, a third reviewer (AB) arbitrated.
As a primary analysis, the overall CCS uptake were pooled between distribution of self-samplers' and clinician-collected samples, using a DerSimonian and Laird random-effects model (24). Subgroup analyses were successively performed to assess whether differences in the CCS uptake were attributable to the self-samplers' distribution strategy, device type, women's screening status and study design (RCTs vs. observational). Relative Risks (RRs) were reported in the forest plots as measure of the effect size.
Secondary outcomes were analyzed by meta-analysis of proportions. Since outcome proportions were often higher than 80%, the confidence intervals were calculated through Freeman-Tukey double-arcsin transformation, and subsequently retro-transformed to avoid compression of standard errors and consequent biased results. The Wilson method was used to compute 95% Confidence Intervals (CIs). Subgroup analyses were performed to investigate whether brushes, swabs, tampons and lavages were equally accepted and whether the device category influenced the preference of self-sampling vs. outpatient sampling. A further subgroup analysis was performed to estimate the impact of the self-sampling setting (at home or in a clinic) on the acceptability or preference. Cochran's Q test was used to investigate overall differences between subgroups, while pair-wise comparisons (among self-samplers' distribution strategies and device types) were performed by contrasting meta-regression coefficients of models with one predictor only. I-squared consistency index was calculated to assess heterogeneity among studies. Peters' test and Funnel Plot inspection were used to assess the publication bias. To ensure the robustness of the results, subgroup analyses were repeated considering only RCTs. Data were analyzed by the statistical software STATA software, version 16.1 (25).
Databases searches yielded a total of 2, 438 articles, 78 of which were duplicates. Inspection of titles and abstracts resulted in the deletion of 2, 034 articles. A total of 326 full-text articles were retrieved for full review, and 154 articles met the inclusion criteria and were included in the analyses.
Overall, 482,271 women were involved, and all five continents were represented. Fifty-one (33.1%) studies were carried out in low-middle-income countries.
All but one of the RCTs showed a low risk of bias (Table 1). On the contrary, 53 (58.9%) out of 90 quasi-experimental or cross-sectional studies exhibited a fair or low overall quality (Table 2).
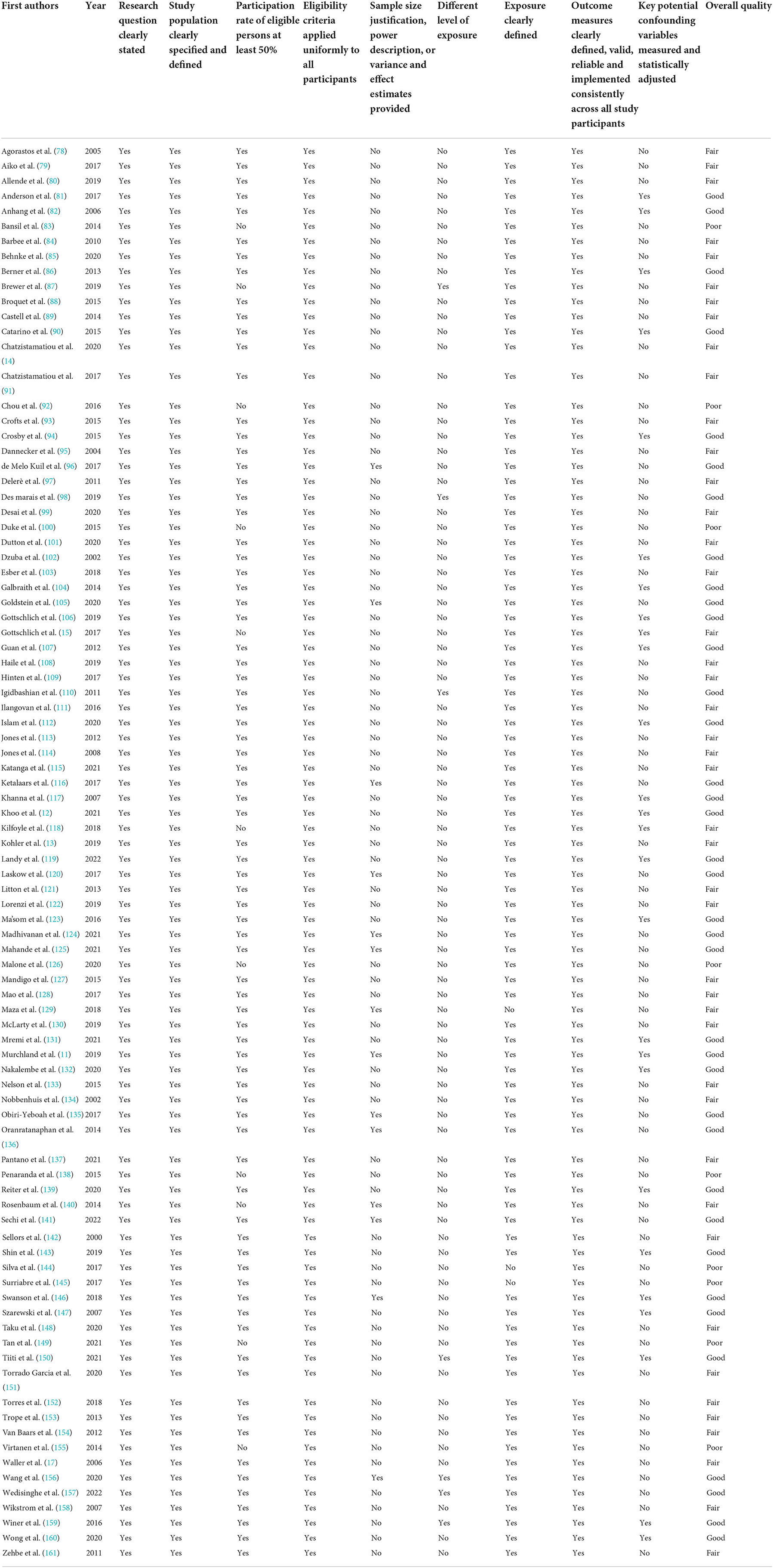
Table 2. Risk of bias of included observational studies assessed by NIH Quality assessment tool for observational cohort and cross-sectional studies.
Forty-nine (31.8%) of studies included measured CCS uptake (Table 3); 46 (93.9%) were RCTs and 3 (5.1%) were quasi-experimental studies. Regarding characteristics of the studied population, 40 studies (81.6%) were focused on under-screened women, while 9 (18.4%) involved the general population. Cervical brushes were used in 21 (42.9%) studies, swabs in 20 (40.8%) studies and lavages in 7 (14.3%) studies. In 3 (6.1%) studies, the type of device was not reported. In 2 (4.1%) studies, both a brush and a lavage were proposed to the participants. In 12 (24.5%) studies self-samplers were directly distributed to women (door-to-door), and the opt-out and opt-in strategies were used in 30 (61.2%) and 10 (20.4%) studies, respectively. In 7 (14.3%) studies both opt-out and opt-in strategies were examined.
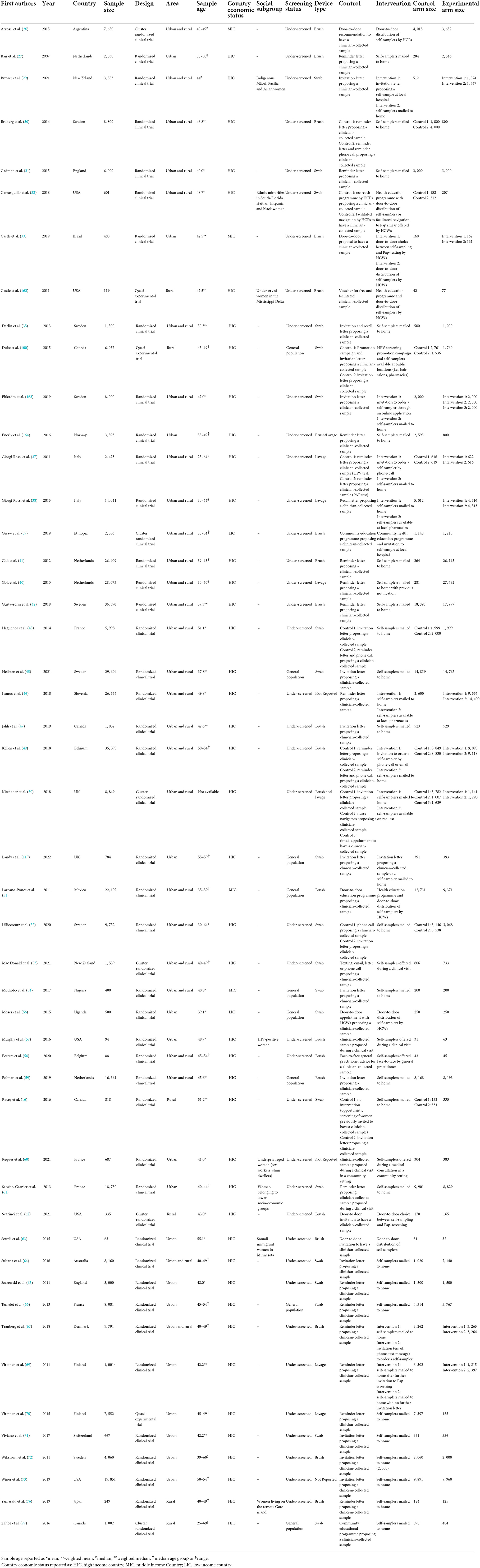
Table 3. Characteristics of the included studies assessing cervical cancer screening (CCS) uptake comparing self-sampling with clinician-collected samples for HPV testing.
Overall, self-sampling procedures nearly doubled the probability (RR: 1.9; 95% CI: 1.8–2.0) of CCS uptake when compared with clinician-collected samples (Figure 2).
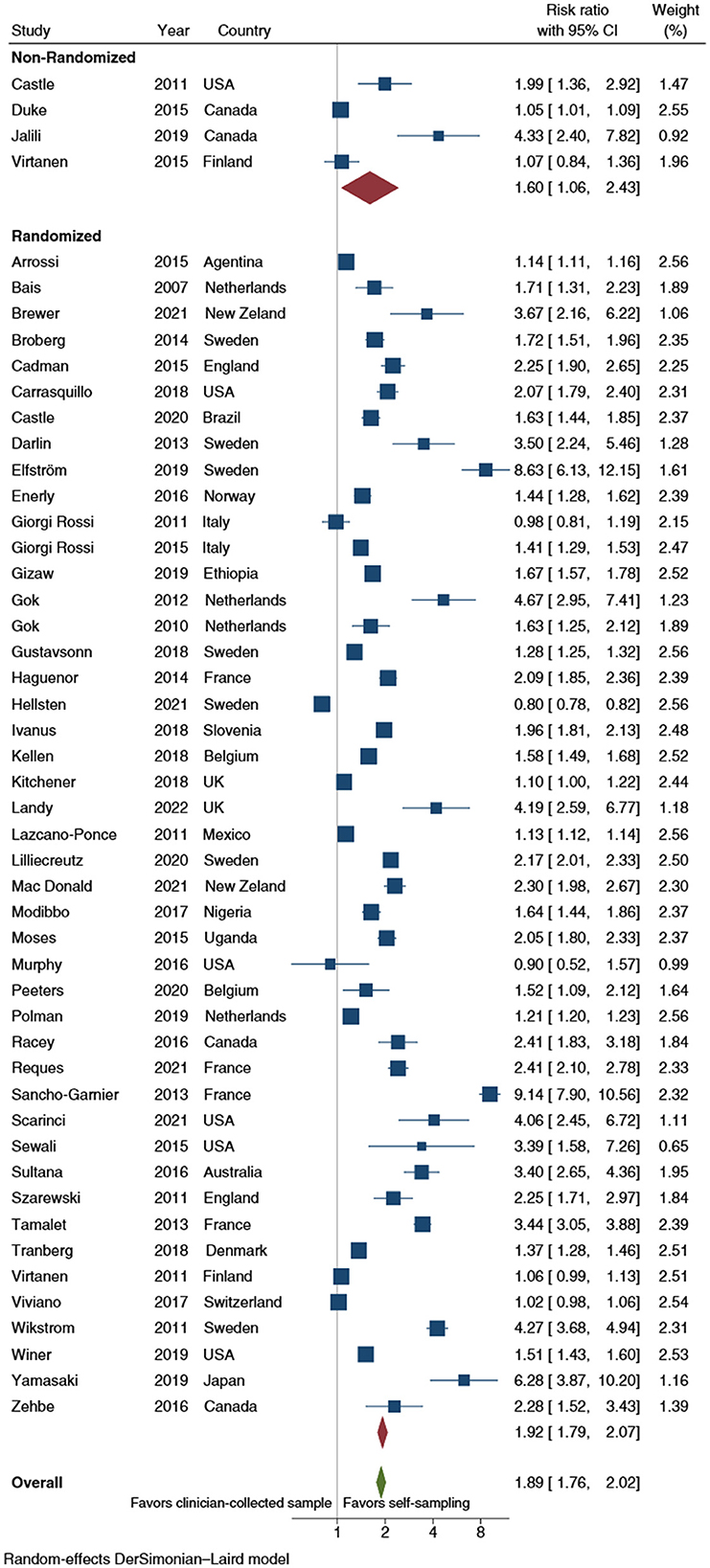
Figure 2. Forest plot comparing cervical cancer screening (CCS) uptake for HPV testing by self-sampling vs. clinician-collected samples, subgrouped by study design (randomized vs. non-randomized). Homogeneity: I2 = 98.9%; Cochrane's Q test for between-group differences: Q = 4,241.88; df = 1; p = 0.399.
With regard to self-sampler distribution strategy, the opt-out (RR: 2.1; 95% CI: 1.9–2.4) and the door-to-door (RR: 1.8; 95% CI: 1.6–2.0) did not statistically significant differ (p = 1.177) in improving the CCS uptake. In contrast, the opt-in (RR: 1.4; 95% CI: 1.2–1.7) showed a significantly lower efficacy than the opt-out strategy (p = 0.001); no statistically significant difference was displayed with respect to door-to-door distribution (p = 0.093) (Figure 3). The pooled analyses restricted to RCTs showed a statistically significant difference in improving CCS uptake between opt-out (RR: 2.2; 95% CI: 2.0–2.5) and door-to-door strategies (RR: 1.7; 95% CI: 1.5–2.0) (p = 0.048) and between the latter and the opt-in strategy (RR: 1.4; 95% CI: 1.1–1.7) (p = 0.048).
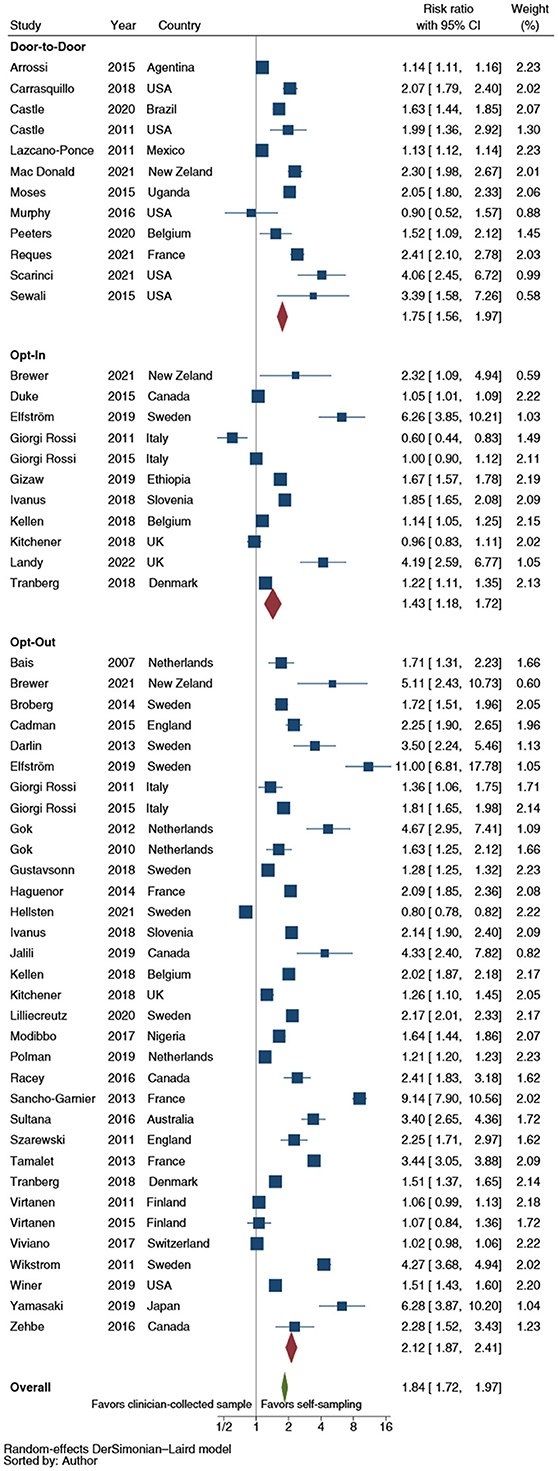
Figure 3. Forest plot comparing cervical cancer screening (CCS) uptake for HPV testing by strategy of self-samplers' distribution vs. clinician-collected samples. Homogeneity (I-squared): 98.8%; Cochrane's Q test for between-group differences: Q = 4,426.36; df = 2; p = 0.02.
Figure 4 showed the RR of CCS uptake for HPV testing by self-sampler type. The results of those analyses showed a higher relative uptake for vaginal lavages (RR: 1.2; 95% CI: 1.1–1.5), brushes (RR: 1.6; 95% CI: 1.5–1.7) and swabs (RR: 2.5; 95% CI: 1.9–3.1) over clinician-collected samples. The analyses compared swabs and brushes and brushes and lavages showed a statistically significant difference (p = 0.004 and p < 0.001, respectively). When the analyses were restricted to RCTs, a pooled RR estimate of 2.7 (95% CI: 2.0–3.7) for swabs, 1.6 (95% CI: 1.5–1.7) for brushes and 1.3 (95% CI: 1.1–1.5) for lavages, were shown. Similarly, both the swabs-brushes (p < 0.001) and the brushes-lavages (p = 0.009) comparisons displayed a statistically significant difference.
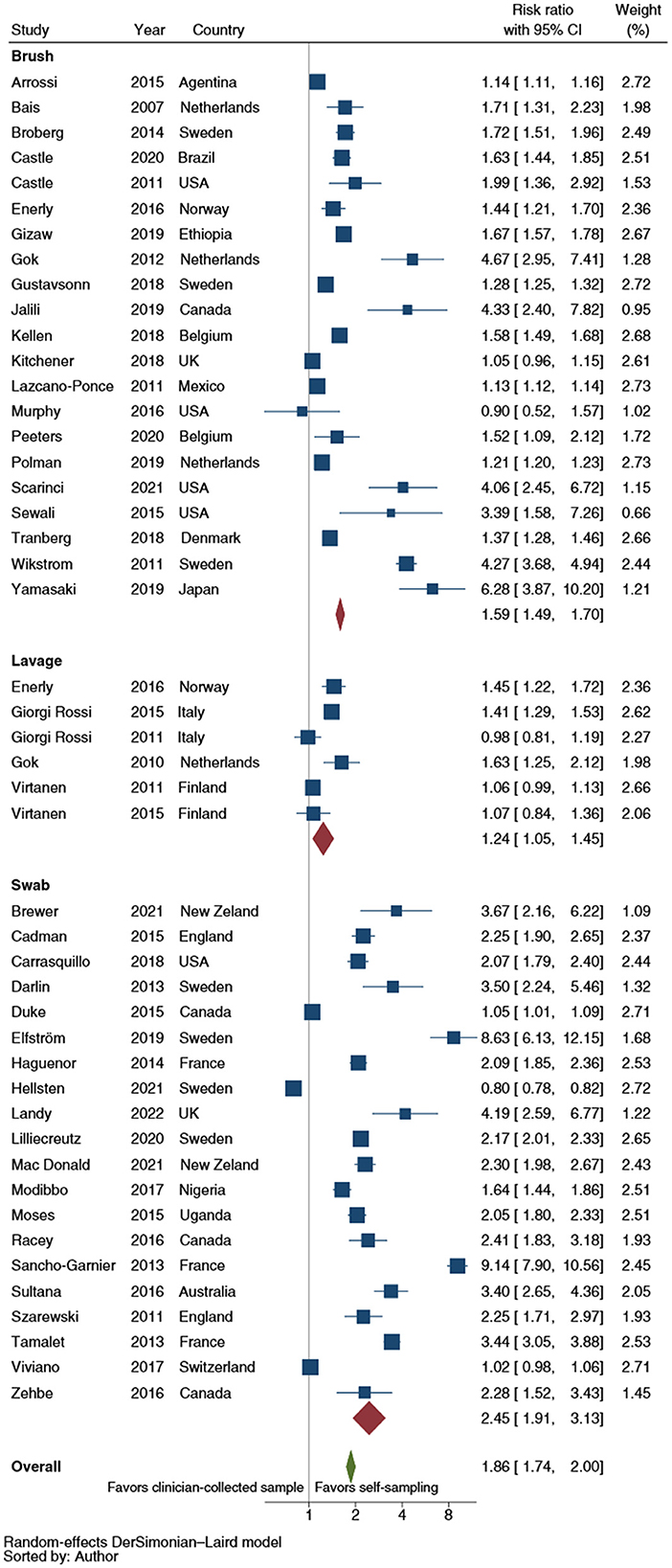
Figure 4. Forest plot comparing cervical cancer screening (CCS) uptake for HPV testing by self-sampler types vs. clinician-collected samples. Homogeneity (I-squared): 98.8%; Cochrane's Q test for between-group differences: Q = 3,904.90; df = 2; p = 0.02.
In the meta-analysis of studies reporting screening status, the overall RR was >1.00 indicating a potential effect of self-sampling in improving CCS uptake both among under-screened women (RR: 2.1; 95% CI: 1.9–2.3) and general population (RR: 1.4; 95% CI: 1.2–1.7) compared to clinician collected samples, and the difference was statistically significant (p < 0.001). Similarly, the efficacy of self-sampling was significantly higher (p = 0.015) when only RCTs were kept in the analysis, in both groups [under-screened women (RR: 2.1; 95% CI: 1.9–2.4) and general population (RR: 1.6; 95% CI: 1.3–1.9)].
The level of heterogeneity was consistently high (I2 > 95%) in the overall and subgroup analyses. Publication bias was unlikely, as suggested by Peters' test (p = 0.06) (Figure 5).
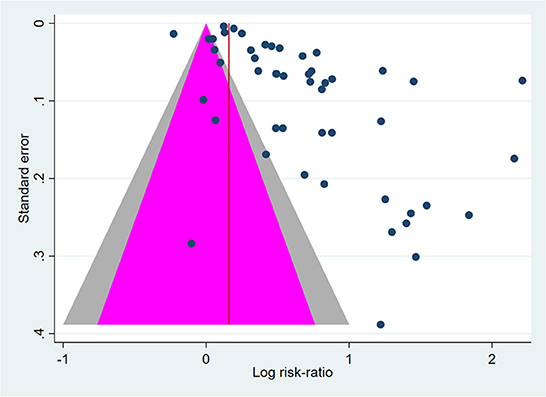
Figure 5. Contour-enhanced funnel plot of cervical cancer screening (CCS) uptake effect size (log odds-ratio) vs. Standard error. Outcome: screening uptake. Pink-area: p > 0.05. Gray area: 0.01 < p < 0.05. Blue dots represent single studies. Peters' test for publication bias: p = 0.060.
Characteristics of the included studies assessing acceptability and preference of self-sampling vs. clinician-collected samples were displayed in Table 4. One-hundred and eight (70.1%) studies measured at least one secondary outcome: 12 (11.1%) of them were RCTs, 68 (63.0%) were cross-sectional studies and 28 (25.9%) had a quasi-experimental design. Seventy-two (66.7%) considered under-screened women, the rest involved the general population. Twenty-eight (25.9%) studies assessed acceptability and in 52 (48.2%) studies women were asked for preference. Both, acceptability and preference, were assessed in 28 (25.9%) studies. In 64 (59.3%) studies self-sampling occurred in a clinical setting, in 39 (36.1%) it occurred at home, and in 4 studies (3.7%) it occurred in both settings. The setting was not reported in one study.
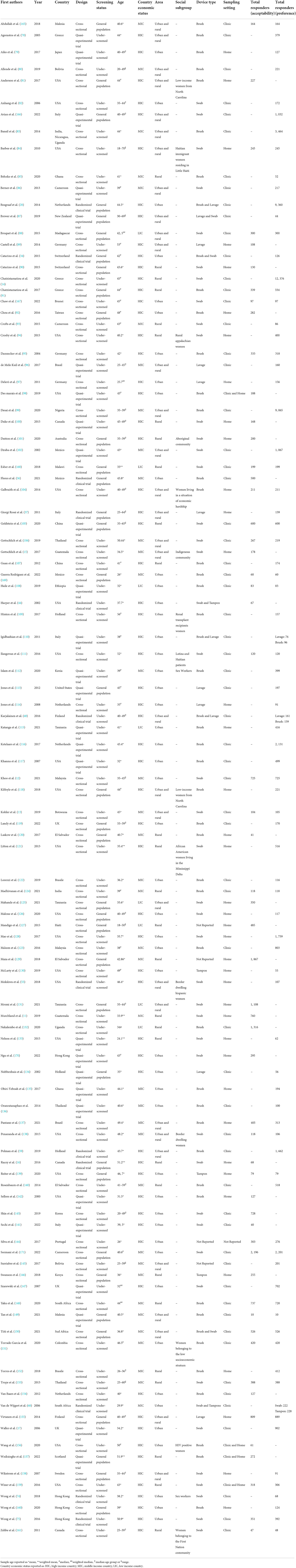
Table 4. Characteristics of the included studies assessing acceptability and preference of self-sampling vs. clinician-collected samples.
Meta-analyses examining the proportion of women who found self-sampling acceptable, showed a very high pooled estimate (95%; 95% CI: 94–97%) (Figure 6). No differences (p = 0.420) were found among acceptability of brushes (93%; 95% CI: 90–96%), swabs (96%; 95% CI: 93–98%), lavages (98%; 95% CI: 95–100%) and tampons (97%; 95% CI: 92–100%). Moreover, the percentage of women who self-reported acceptance of self-sampling at home (96%; 95% CI: 93–98%) overlapped with acceptance of self-sampling in a clinical setting (96%; 95% CI: 94–98%). In all meta-analyses high heterogeneity (I2> 95%) was observed.
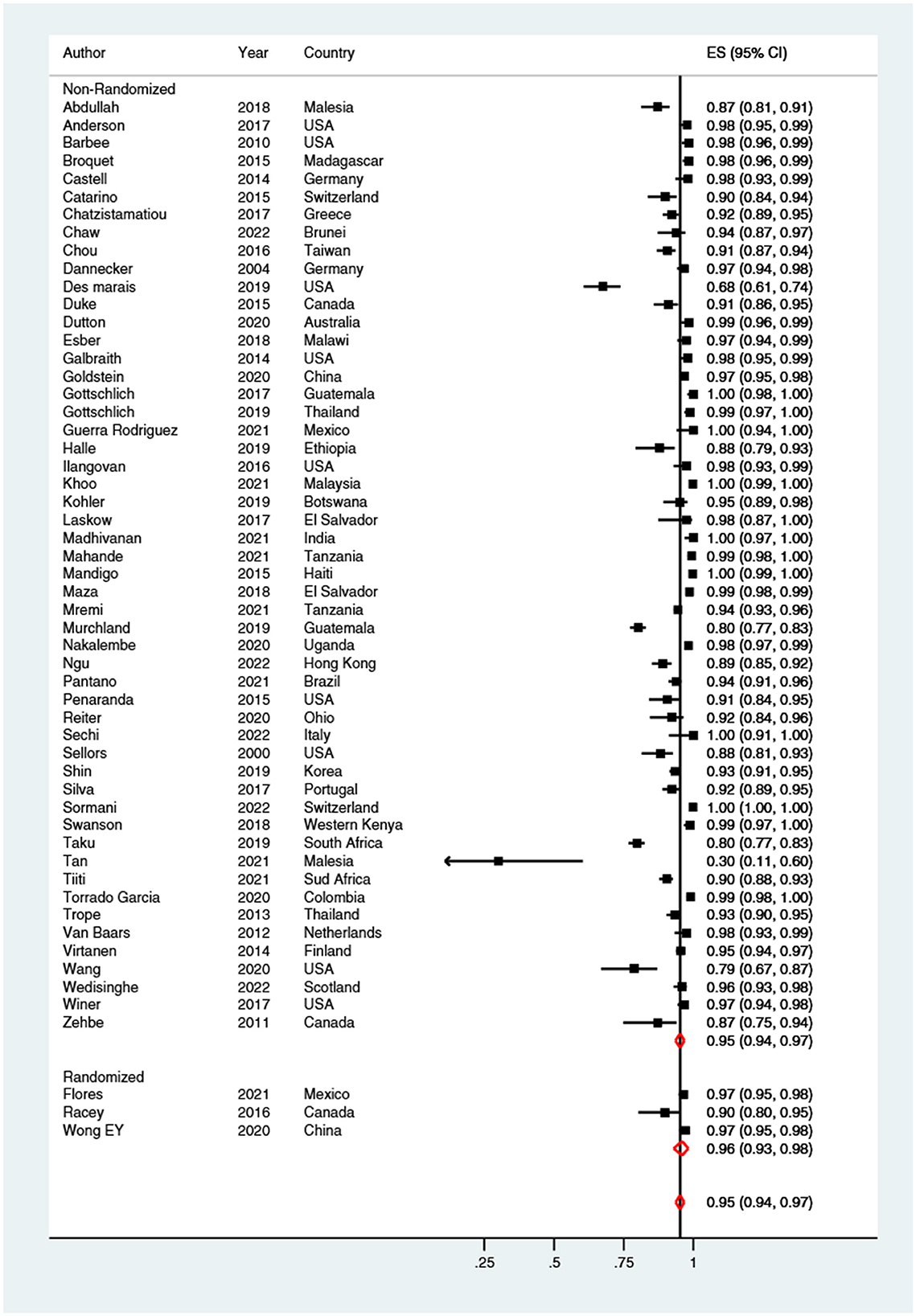
Figure 6. Forest plot of the proportion of women who found self-sampling acceptable. Homogeneity (I-squared): 95.9%; Cochrane's Q test for between-group differences: Q = 1,307.30; df = 54; p < 0.001.
Sixty-six percent (95% CI: 62–70%) of women preferred self-sampling procedures vs. clinician-collected samples (Figure 7). No significant difference (p = 0.850) was shown when brushes (67%; 95% CI: 58–74%), swabs (65%; 95% CI: 59–70%), lavages (68%; 95% CI: 60–76%) and tampons (77%; 95% CI: 31–100%) were compared. Finally, the preference of women for self-sampling was almost equal (p = 0.841) when it was performed at home (66%; 95% CI: 57–74%), or in a clinical setting (67%; 95% CI: 62–71%). The level of heterogeneity was high (I2> 95%).
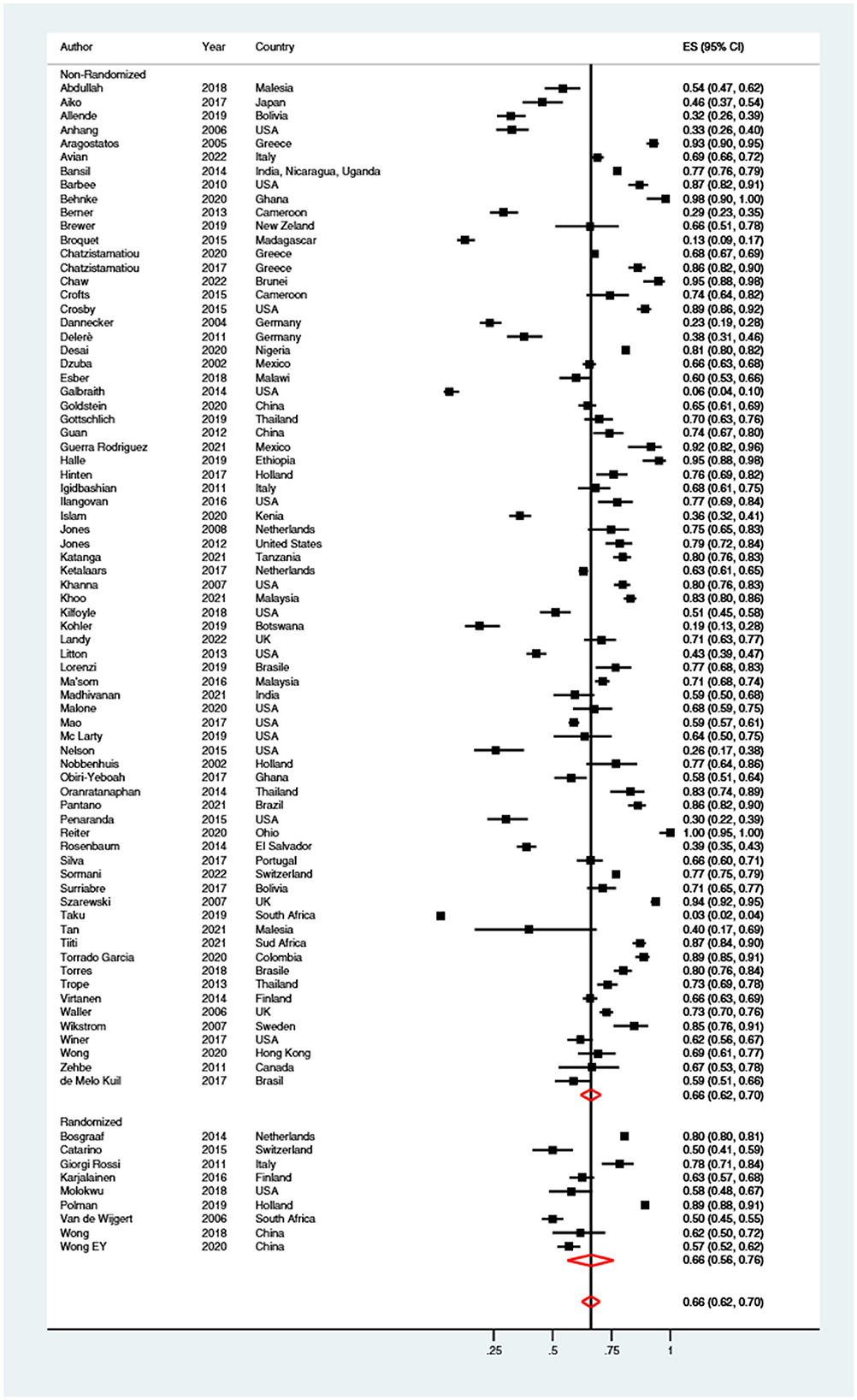
Figure 7. Forest plot of the proportion of women preferring self-sampling over clinician-collected samples. Homogeneity (I-squared): 99.0%; Cochrane's Q test for between-group differences: Q = 7,842.51; df = 81; p < 0.001.
The findings of the present meta-analysis provide a summary of the implementation options of self-sampling for HPV testing. Since the COVID-19 pandemic has had an enormous impact on CCS attendance, self-sampling could offer a unique opportunity for catch-up screening and will play an important role in improving the global coverage of CCS. Indeed, the World Health Organization strongly recommends the use of self-sampling for HPV screening to contribute to reaching a coverage of 70% by 2030 and eliminate HPV correlated diseases in the next decades (172). Considering that for an intervention to be effective it must be broadly accepted, evidence about women's acceptability for CCS comparing self-sampled with clinician-collected specimens is also provided.
The findings of the present meta-analysis showed that self-sampling for HPV testing is an effective tool to reach women in the context of organized CCS programs. Indeed, women were nearly twice as likely to use CCS services through self-sampling as compared with clinician-based sampling. Considering that the option of cervical precancer detection from self-collected samples showed similar clinical accuracy for hrHPV testing as clinician-collected samples (9, 173, 174), this result increases evidence in support of incorporating self-sampling into organized screening programs to better respond to the disruption of CCS programs after the COVID-19 pandemic. Moreover, the meta-analyses split into sub-groups according to dissemination strategies, suggested that a door-to-door approach, in which an HCP visits women at home to inform on CCS and offer a self-sampling HPV test kit, has almost doubled the CCS uptake by seven-fold. However, it has to be pointed out that the door-to-door approach has been mainly investigated in low-resource settings or for reaching under-screened women in high-resource settings. The findings showed an even higher likelihood of attending CCS for the opt-out approach (i.e., mailing of self-collection devices to women's homes without them taking the initiative), compared with controls (i.e., invitation letters sent home, reminding phone calls or suggestions from the HCP to be screened in the local hospital or from a gynecologist). In high-resource settings, research has focused on an alternative invitation scenario (opt-in strategy) in which women request a self-collection kit that is mailed to home or pick it up at pharmacy or clinic. The analyses showed that the opt-in approach reached a high CCS uptake when compared to mailing a reminder letter proposing a clinician-collected samples, although lower than response rates to the opt-out and door-to-door approaches. It should be noted that the opt-in approach has the advantage to be less expensive, especially on a national level. Bring together, these results confirm recent literature. In particular, the meta-analysis by Yeh et al., found that opt-out strategy increased CCS participation (RR: 2.27; 95% CI: 1.89–2.71) (19), and Arbyn et al. found similar results when comparing opt-out self-samplers distribution with a reminder letter/advice from HCP to have a clinician to collect the sample (9).
In the relevant studies, several types of devices to collect exfoliated cells of the cervicovaginal duct for HPV-DNA detection were employed. It should be noted that the distribution of brush- and swab-based devices were associated with significantly higher uptake when compared with invitation to be sampled by a clinician. The latter result deserves attention since, as previously demonstrated, the type of HPV self-sampling device may play an important role in women's acceptability and preference of a CCS strategy (87, 110). The findings of the present meta-analysis highlighted high pooled acceptability and overall preference of self-sampling compared to clinician-based sampling, downsizing potential concerns about self-sampling (e.g., worry of not being able to correctly carry out the sampling), as previously described (17, 175, 176). The finding that especially non-attender women preferred self-sampling to clinician-based sampling for future CCS programs deserves attention, for its potential to increase participation in primary CCS. High acceptability and preference of self-sampling have the potential to improve CCS uptake and its effects on incidence and mortality from cervical cancer. Acceptability of self-sampling demonstrated advantages from both public health and individual patient perspective (177). Proper communication of the self-sampling process to women needs to be realized to address eventual women's concerns and emphasizes that most women are able to successfully obtain an adequate sample or deliver self-sampling by HCPs who can explain the process face-to-face.
In contrast to the findings of Nishimura et al., who documented that swabs were preferred by women when compared with other devices (10) no differences in acceptability regarding the type of self-sampling devices were found.
Contextual factors are essential in real life decision-making: when referring to a small community, offering a door-to-door device could be the most preferable strategy. Differently, when a high number of women have to be reached, mailing the device could represent a cost-effective alternative. Regarding the type of self-sampler device, a pilot investigation could be useful before introducing a large-scale use of self-samplers, as suggested by Arbyn et al. (9). Moreover, elements to consider in order to improve CCS uptake are cultural, religious and socio-economic characteristics of the target community (55, 178, 179). A study carried out on Nigerian women showing that individuals with greater spirituality were less likely to carry out self-sampling (180). Similarly, a systematic review focusing on Islamic women shows that cervical cancer prevention still represents a considerable taboo among them and this can lead to under-screening (181). Further, additional aspects that can interfere with the effectiveness of a self-sampling campaign are the perceived costs and time required for being screened (178, 179, 182). The costs and the need to inform women about the importance of being screened are pivotal among migrants and minorities (183). In the authors' opinion, the use of prepaid and pre-addressed envelopes, the absence of costs for women, the presence of clear and detailed instructions in the self-sampling kits and continuous education about the importance of CCS, could be decisive factors to maximize the uptake.
To the best of our knowledge no recent meta-analysis measuring the effect of self-sampling, across different distribution strategies, type of devices and screening status has been conducted, and the present results could be pivotal to provide practical suggestions for the organization of CCS program. Further strengths consist of the considerable number of subjects included, and the analysis of the recently published results of RCTs.
As above-mentioned, a possible limitation of this meta-analysis is the high heterogeneity, likely attributable to the wide socio-cultural diversity of the samples of women enrolled. Consequently, the results must be interpreted with caution highlighting the need to consider potential factors underlying the success of a self-sampling CCS campaign. Other limitations are the lack of search in the gray literature and the exclusion of all findings reported in languages different than English.
Self-sampling has the potential to increase participation of under-screened women in the CCS, in addition to the standard invitation to have a clinician to collect the sample. For small communities door-to-door distribution could be preferred to distribute the self-sampler; while for large communities opt-out strategies should be preferred over opt-in. Finally, since no significant difference in acceptability and preference of device type was demonstrated among women, and swabs exhibited a potential stronger effect in improving CCS, these devices could be adopted primarily over tampons and lavages.
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author.
FL participated in the conception and design of the study, contributed to the data collection, and wrote the first draft of the article. GD participated in the conception and design of the study, collected the data, performed the data analysis, contributed to analysis interpretation, and wrote the first draft of the article. AT contributed to the data collection and to the data analysis. AB designed the study, was responsible for the data collection and interpretation, wrote the article, and was guarantor for the study. All authors take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CCS, cervical cancer screening; CI, Confidence Interval; HCPs, Healthcare professionals; HPV, Human Papillomavirus; hrHPV, high-risk HPV; RR, Relative Risk; RCT, randomized controlled trial.
1. Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda GA, Zhou Y, et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front Public Health. (2021) 8:552028. doi: 10.3389/fpubh.2020.552028
2. Oyervides-Muñoz MA, Pérez-Maya AA, Rodríguez-Gutiérrez HF, Gómez-Macias GS, Fajardo-Ramírez OR, Treviño V, et al. Understanding the HPV integration and its progression to cervical cancer. Infect Genet Evol. (2018) 61:134–44. doi: 10.1016/j.meegid.2018.03.003
3. De Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int Agency Res Cancer (IARC/WHO). (2017) 141:664–70. doi: 10.1002/ijc.30716
4. Bosch FX, Broker TR, Forman D, Moscicki A-B, Gillison ML, Doorbar J, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. (2013) 31:11–31. doi: 10.1016/j.vaccine.2013.10.003
5. Fisher JW, Brundage SI. The challenge of eliminating cervical cancer in the United States: a story of politics, prudishness, and prevention. Women Heal. (2009) 49:246–61. doi: 10.1080/03630240902915101
6. Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. It has saved thousands of lives, so why change it?' Content analysis of objections to cervical screening programme changes in Australia. BMJ Open. (2018) 8:e019171. doi: 10.1136/bmjopen-2017-019171
7. Gago J, Paolino M, Arrossi S. Factors associated with low adherence to cervical cancer follow-up retest among HPV+/ cytology negative women: a study in programmatic context in a low-income population in Argentina. BMC Cancer. (2019) 19:367. doi: 10.1186/s12885-019-5583-7
8. Limmer K, LoBiondo-Wood G, Dains J. Predictors of cervical cancer screening adherence in the United States: a systematic review. J Adv Pract Oncol. (2014) 5:31–41. doi: 10.6004/jadpro.2014.5.1.2
9. Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. (2018) 363:k4823. doi: 10.1136/bmj.k4823
10. Nishimura H, Yeh PT, Oguntade H, Kennedy CE, Narasimhan M, HPV. self-sampling for cervical cancer screening: a systematic review of values and preferences. BMJ Glob Heal. (2021) 6:e003743. doi: 10.1136/bmjgh-2020-003743
11. Murchland AR, Gottschlich A, Bevilacqua K, Pineda A, Sandoval-Ramírez BA, Alvarez CS, et al. HPV self-sampling acceptability in rural and indigenous communities in Guatemala: a cross-sectional study. BMJ Open. (2019) 9:e029158. doi: 10.1136/bmjopen-2019-029158
12. Khoo SP, Lim WT, Rajasuriar R, Nasir NH, Gravitt P, Woo YL. The acceptability and preference of vaginal self-sampling for human papillomavirus (HPV) testing among a multi-ethnic asian female population. Cancer Prev Res. (2020) 14:105–12. doi: 10.1158/1940-6207.CAPR-20-0280
13. Kohler RE, Elliott T, Monare B, Moshashane N, Ramontshonyana K, Chatterjee P, et al. HPV self-sampling acceptability and preferences among women living with HIV in Botswana. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. (2019) 147:332–8. doi: 10.1002/ijgo.12963
14. Chatzistamatiou K, Vrekoussis T, Tsertanidou A, Moysiadis T, Mouchtaropoulou E, Pasentsis K, et al. Acceptability of self-sampling for human papillomavirus-based cervical cancer screening. J Womens Health. (2020) 29:1447–56. doi: 10.1089/jwh.2019.8258
15. Gottschlich A, Rivera-Andrade A, Grajeda E, Alvarez C, Mendoza Montano C, Meza R. Acceptability of human papillomavirus self-sampling for cervical cancer screening in an indigenous community in guatemala. J Glob Oncol. (2017) 3:444–54. doi: 10.1200/JGO.2016.005629
16. Racey CS, Gesink DC, Burchell AN, Trivers S, Wong T, Rebbapragada A. Randomized Intervention of self-collected sampling for human papillomavirus testing in under-screened rural women: uptake of screening and acceptability. J Womens Health. (2016) 25:489–97. doi: 10.1089/jwh.2015.5348
17. Waller J, McCaffery K, Forrest S, Szarewski A, Cadman L, Austin J, et al. Acceptability of unsupervised HPV self-sampling using written instructions. J Med Screen. (2006) 13:208–13. doi: 10.1177/096914130601300409
18. Fargnoli V, Petignat P, Burton-Jeangros C. To what extent will women accept HPV self-sampling for cervical cancer screening? A qualitative study conducted in Switzerland. Int J Womens Health. (2015) 7:883–8. doi: 10.2147/IJWH.S90772
19. Yeh PT, Kennedy CE, De Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. (2019) 4:e001351. doi: 10.1136/bmjgh-2018-001351
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. The BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
21. Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect. (2017) 93:56–61. doi: 10.1136/sextrans-2016-052609
22. Higgins J, Savović J, Page MJ, Sterne JAC. RoB 2: A revised Cochrane risk-of-bias tool for randomized trials. Br Med J. (2019). doi: 10.1002/9781119536604.ch8
23. National Institute of Health, Bethesda (Maryland, U). Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed November 11, 2022).
24. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
26. Arrossi S, Ramos S, Straw C, Thouyaret L, Orellana L, HPV. testing: a mixed-method approach to understand why women prefer self-collection in a middle-income country. BMC Public Health. (2016) 16:832. doi: 10.1186/s12889-016-3474-2
27. Bais AG, Van Kemenade FJ, Berkhof J, Verheijen RHM, Snijders PJF, Voorhorst F, et al. Human papillomavirus testing on self-sampled cervicovaginal brushes: An effective alternative to protect nonresponders in cervical screening programs. Int J Cancer. (2007) 120:1505–10. doi: 10.1002/ijc.22484
28. Bosgraaf RP, Verhoef VMJ, Massuger LFAG, Siebers AG, Bulten J, De Kuyper-De Ridder GM, et al. Comparative performance of novel self-sampling methods in detecting high-risk human papillomavirus in 30,130 women not attending cervical screening. Int J Cancer. (2015) 136:646–55. doi: 10.1002/ijc.29026
29. Brewer N, Bartholomew K, Grant J, Maxwell A, McPherson G, Wihongi H, et al. Acceptability of human papillomavirus (HPV) self-sampling among never- and under-screened Indigenous and other minority women: a randomised three-arm community trial in Aotearoa New Zealand. Lancet Reg Heal West Pacific. (2021) 16:100265. doi: 10.1016/j.lanwpc.2021.100265
30. Broberg G, Gyrd-Hansen D, Miao Jonasson J, Ryd ML, Holtenman M, Milsom I, et al. Increasing participation in cervical cancer screening: offering a HPV self-test to long-term non-attendees as part of RACOMIP, a Swedish randomized controlled trial. Int J Cancer. (2014) 134:2223–30. doi: 10.1002/ijc.28545
31. Cadman L, Wilkes S, Mansour D, Austin J, Ashdown-Barr L, Edwards R, et al. A randomized controlled trial in non-responders from Newcastle upon Tyne invited to return a self-sample for human papillomavirus testing vs. repeat invitation for cervical screening. J Med Screen. (2015) 22:28–37. doi: 10.1177/0969141314558785
32. Carrasquillo O, Seay J, Amofah A, Pierre L, Alonzo Y, McCann S, et al. HPV Self-sampling for cervical cancer screening among ethnic minority Women in South Florida: a randomized trial. J Gen Int Med. (2018) 33:1077–83. doi: 10.1007/s11606-018-4404-z
33. Castle PE, Silva VRS, Consolaro MEL, Kienen N, Bittencourt L, Pelloso SM, et al. Participation in cervical screening by selfcollection, pap, or a choice of either in Brazil. Cancer Prev Res. (2019) 12:159–70. doi: 10.1158/1940-6207.CAPR-18-0419
34. Catarino R, Vassilakos P, Bilancioni A, Eynde M, Vanden, Meyer-Hamme U, Menoud PA, et al. Randomized comparison of two vaginal self-sampling methods for human papillomavirus detection: dry swab vs. FTA cartridge. PLoS ONE. (2015) 10:e0143644. doi: 10.1371/journal.pone.0143644
35. Darlin L, Borgfeldt C, Forslund O, Hénic E, Hortlund M, Dillner J, et al. Comparison of use of vaginal HPV self-sampling and offering flexible appointments as strategies to reach long-term non-attending women in organized cervical screening. J Clin Virol. (2013) 58:155–60. doi: 10.1016/j.jcv.2013.06.029
36. Aranda Flores CE, Gomez Gutierrez G, Ortiz Leon JM, Cruz Rodriguez D, Sørbye SW. Self-collected vs. clinician-collected cervical samples for the detection of HPV infections by 14-type DNA and 7-type mRNA tests. BMC Infect Dis. (2021) 21:504. doi: 10.1186/s12879-021-06189-2
37. Giorgi Rossi P, Marsili LM, Camilloni L, Iossa A, Lattanzi A, Sani C, et al. The effect of self-sampled HPV testing on participation to cervical cancer screening in Italy: a randomised controlled trial (ISRCTN96071600). Br J Cancer. (2011) 104:248–54. doi: 10.1038/sj.bjc.6606040
38. Giorgi Rossi P, Fortunato C, Barbarino P, Boveri S, Caroli S, Del Mistro A, et al. Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br J Cancer. (2015) 112:667–75. doi: 10.1038/bjc.2015.11
39. Gizaw M, Teka B, Ruddies F, Abebe T, Kaufmann AM, Worku A, et al. Uptake of cervical cancer screening in Ethiopia by self-sampling HPV DNA compared to visual inspection with acetic acid: a cluster randomized trial. Cancer Prev Res. (2019) 12:609–16. doi: 10.1158/1940-6207.CAPR-19-0156
40. Gök M, Heideman DAM, Van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JWM, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: Cohort study. BMJ. (2010) 340:c1040. doi: 10.1136/bmj.c1040
41. Gök M, Van Kemenade FJ, Heideman DAM, Berkhof J, Rozendaal L, Spruyt JWM, et al. Experience with high-risk human papillomavirus testing on vaginal brush-based self-samples of non-attendees of the cervical screening program. Int J Cancer. (2012) 130:1128–35. doi: 10.1002/ijc.26128
42. Gustavsson I, Aarnio R, Berggrund M, Hedlund-Lindberg J, Strand AS, Sanner K, et al. Randomised study shows that repeated selfsampling and HPV test has more than twofold higher detection rate of women with CIN2+ histology than Pap smear cytology. Br J Cancer. (2018) 118:896–904. doi: 10.1038/bjc.2017.485
43. Haguenoer K, Sengchanh S, Gaudy-Graffin C, Boyard J, Fontenay R, Marret H, et al. vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: a randomised trial. Br J Cancer. (2014) 111:2187–96. doi: 10.1038/bjc.2014.510
44. Harper DM, Noll WW, Belloni DR, Cole BF. Randomized clinical trial of PCR-determined human papillomavirus detection methods: Self-sampling vs. clinician-directed-biologic concordance and women's preferences. Am J Obstet Gynecol. (2002) 186:365–73. doi: 10.1067/mob.2002.121076
45. Hellsten C, Ernstson A, Bodelsson G, Forslund O, Borgfeldt C. Equal prevalence of severe cervical dysplasia by HPV self-sampling and by midwife-collected samples for primary HPV screening: a randomised controlled trial. Eur J Cancer Prev. (2021) 30:334–40. doi: 10.1097/CEJ.0000000000000693
46. Ivanus U, Jerman T, Fokter AR, Takac I, Prevodnik VK, Marcec M, et al. Randomised trial of HPV self-sampling among non-attenders in the Slovenian cervical screening programme ZORA: comparing three different screening approaches. Radiol Oncol. (2018) 52:399–412. doi: 10.2478/raon-2018-0036
47. Jalili F, O'Conaill C, Templeton K, Lotocki R, Fischer G, Manning L, et al. Assessing the impact of mailing self-sampling kits for human papillomavirus testing to unscreened non-responder women in Manitoba. Curr Oncol. (2019) 26:167–72. doi: 10.3747/co.26.4575
48. Karjalainen L, Anttila A, Nieminen P, Luostarinen T, Virtanen A. Self-sampling in cervical cancer screening: comparison of a brush-based and a lavage-based cervicovaginal self-sampling device. BMC Cancer. (2016) 16:221. doi: 10.1186/s12885-016-2246-9
49. Kellen E, Benoy I, Vanden Broeck D, Martens P, Bogers JP, Haelens A, et al. A randomized, controlled trial of two strategies of offering the home-based HPV self-sampling test to non- participants in the Flemish cervical cancer screening program. Int J Cancer. (2018) 143:861–68. doi: 10.1002/ijc.31391
50. Kitchener H, Gittins M, Cruickshank M, Moseley C, Fletcher S, Albrow R, et al. A cluster randomized trial of strategies to increase uptake amongst young women invited for their first cervical screen: the strategic trial. J Med Screen. (2018) 25:88–98. doi: 10.1177/0969141317696518
51. Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, Salmerón J, Uribe P, Velasco-Mondragón E, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. (2011) 378:1868–73. doi: 10.1016/S0140-6736(11)61522-5
52. Lilliecreutz C, Karlsson H, Holm ACS. Participation in interventions and recommended follow-up for non-attendees in cervical cancer screening -taking the women's own preferred test method into account—a Swedish randomised controlled trial. PLoS ONE. (2020) 15:e0235202. doi: 10.1371/journal.pone.0235202
53. MacDonald EJ, Geller S, Sibanda N, Stevenson K, Denmead L, Adcock A, et al. Reaching under-screened/never-screened indigenous peoples with human papilloma virus self-testing: a community-based cluster randomised controlled trial. Aust New Zeal J Obstet Gynaecol. (2021) 61:135–41. doi: 10.1111/ajo.13285
54. Modibbo F, Iregbu KC, Okuma J, Leeman A, Kasius A, De Koning M, et al. Randomized trial evaluating self-sampling for HPV DNA based tests for cervical cancer screening in Nigeria. Infect Agent Cancer. (2017) 12:11. doi: 10.1186/s13027-017-0123-z
55. Molokwu JC, Penaranda E, Dwivedi A, Mallawaarachchi I, Shokar N. Effect of educational intervention on self-sampling acceptability and follow-up paps in border dwelling hispanic females. J Low Genit Tract Dis. (2018) 22:295–301. doi: 10.1097/LGT.0000000000000424
56. Moses E, Pedersen HN, Mitchell SM, Sekikubo M, Mwesigwa D, Singer J, et al. Uptake of community-based, self-collected HPV testing vs. visual inspection with acetic acid for cervical cancer screening in Kampala, Uganda: preliminary results of a randomised controlled trial. Trop Med Int Heal. (2015) 20:1355–67. doi: 10.1111/tmi.12549
57. Murphy J, Mark H, Anderson J, Farley J, Allen J. A randomized trial of human papillomavirus self-sampling as an intervention to promote cervical cancer screening among women with HIV. J Low Genit Tract Dis. (2016) 20:139–44. doi: 10.1097/LGT.0000000000000195
58. Peeters E, Cornet K, Devroey D, Arbyn M. Efficacy of strategies to increase participation in cervical cancer screening: GPs offering self-sampling kits for HPV testing vs. recommendations to have a pap smear taken—a randomised controlled trial. Papillomavirus Res. (2020) 9:100201. doi: 10.1016/j.pvr.2020.100194
59. Polman NJ, de Haan Y, Veldhuijzen NJ, Heideman DAM, de Vet HCW, Meijer CJLM, et al. Experience with HPV self-sampling and clinician-based sampling in women attending routine cervical screening in the Netherlands. Prev Med. (2019) 125:5–11. doi: 10.1016/j.ypmed.2019.04.025
60. Reques L, Rolland C, Lallemand A, Lahmidi N, Aranda-Fernández E, Lazzarino A, et al. Comparison of cervical cancer screening by self-sampling papillomavirus test vs. pap-smear in underprivileged women in France. BMC Womens Health. (2021) 21:221. doi: 10.1186/s12905-021-01356-8
61. Sancho-Garnier H, Tamalet C, Halfon P, Leandri FX, Retraite L Le, Djoufelkit K, et al. HPV self-sampling or the Pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France. Int J Cancer. (2013) 133:2681–7. doi: 10.1002/ijc.28283
62. Scarinci IC Li Y, Tucker L, Campos NG, Kim JJ, Peral S, et al. Given a choice between self-sampling at home for HPV testing and standard of care screening at the clinic, what do African American women choose? Findings from a group randomized controlled trial. Prev Med. (2021) 142:106358. doi: 10.1016/j.ypmed.2020.106358
63. Sewali B, Okuyemi KS, Askhir A, Belinson J, Vogel RI, Joseph A, et al. Cervical cancer screening with clinic-based Pap test vs. home HPV test among Somali immigrant women in Minnesota: a pilot randomized controlled trial. Cancer Med. (2015) 4:620–31. doi: 10.1002/cam4.429
64. Sultana F, English DR, Simpson JA, Drennan KT, Mullins R, Brotherton JML, et al. Home-based HPV self-sampling improves participation by never-screened and under-screened women: results from a large randomized trial (iPap) in Australia. Int J Cancer. (2016) 139:281–90. doi: 10.1002/ijc.30031
65. Szarewski A, Cadman L, Mesher D, Austin J, Ashdown-Barr L, Edwards R, et al. HPV self-sampling as an alternative strategy in non-attenders for cervical screening- a randomised controlled trial. Br J Cancer. (2011) 104:915–20. doi: 10.1038/bjc.2011.48
66. Tamalet C, Le Retraite L, Leandri FX, Heid P, Sancho Garnier H, Piana L. Vaginal self-sampling is an adequate means of screening HR-HPV types in women not participating in regular cervical cancer screening. Clin Microbiol Infect. (2013) 19:E44–50. doi: 10.1111/1469-0691.12063
67. Tranberg M, Bech BH, Blaakær J, Jensen JS, Svanholm H, Andersen B. Preventing cervical cancer using HPV self-sampling: Direct mailing of test-kits increases screening participation more than timely opt-in procedures - a randomized controlled trial. BMC Cancer. (2018) 18:273. doi: 10.1186/s12885-018-4165-4
68. Van De Wijgert J, Altini L, Jones H, De Kock A, Young T, Williamson AL, et al. Two methods of self-sampling compared to clinician sampling to detect reproductive tract infections in Gugulethu, South Africa. Sex Transm Dis. (2006) 33:516–23. doi: 10.1097/01.olq.0000204671.62529.1f
69. Virtanen A, Nieminen P, Luostarinen T, Anttila A. Self-sample HPV tests as an intervention for nonattendees of cervical cancer screening in finland: a randomized trial. Cancer Epidemiol Biomark Prev. (2011) 20:1960–9. doi: 10.1158/1055-9965.EPI-11-0307
70. Virtanen A, Anttila A, Luostarinen T, Malila N, Nieminen P. Improving cervical cancer screening attendance in Finland. Int J Cancer. (2015) 136:e677–84. doi: 10.1002/ijc.29176
71. Viviano M, Catarino R, Jeannot E, Boulvain M, Malinverno MU, Vassilakos P, et al. Self-sampling to improve cervical cancer screening coverage in Switzerland: a randomised controlled trial. Br J Cancer. (2017) 116:1382–8. doi: 10.1038/bjc.2017.111
72. Wikström I, Lindell M, Sanner K, Wilander E. Self-sampling and HPV testing or ordinary Pap-smear in women not regularly attending screening: a randomised study. Br J Cancer. (2011) 105:337–9. doi: 10.1038/bjc.2011.236
73. Winer RL, Lin J, Tiro JA, Miglioretti DL, Beatty T, Gao H, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. J Am Med Assoc Netw Open. (2019) 2:e14729. doi: 10.1001/jamanetworkopen.2019.14729
74. Wong ELY, Cheung AWL, Huang F, Chor JSY. Can human papillomavirus DNA self-sampling be an acceptable and reliable option for cervical cancer screening in female sex workers? Cancer Nurs. (2018) 41:45–52. doi: 10.1097/NCC.0000000000000462
75. Wong ELY, Chan PKS, Chor JSY, Cheung AWL, Huang F, Wong SYS. Evaluation of the impact of human papillomavirus DNA self-sampling on the uptake of cervical cancer screening. Cancer Nurs. (2016) 39:E1–11. doi: 10.1097/NCC.0000000000000241
76. Yamasaki M, Abe S, Miura K, Masuzaki H. The effect of self-sampled HPV testing on participation in cervical cancer screening on a remote island. Acta Med Nagasaki. (2019) 62:55–61.
77. Zehbe I, Jackson R, Wood B, Weaver B, Escott N, Severini A, et al. Community-randomised controlled trial embedded in the Anishinaabek cervical cancer screening Study: Human papillomavirus self-sampling vs. Papanicolaou cytology. BMJ Open. (2016) 6:e011754. doi: 10.1136/bmjopen-2016-011754
78. Agorastos T, Dinas K, Lloveras B, Font R, Kornegay JR, Bontis J, et al. Self-sampling vs. physician-sampling for human papillomavirus testing. Int J STD AIDS. (2005) 16:727–9. doi: 10.1258/095646205774763225
79. Aiko KY, Yoko M, Saito OM, Ryoko A, Yasuyo M, Mikiko AS, et al. Accuracy of self-collected human papillomavirus samples from Japanese women with abnormal cervical cytology. J Obstet Gynaecol Res. (2017) 43:710–7. doi: 10.1111/jog.13258
80. Allende G, Surriabre P, Cáceres L, Bellot D, Ovando N, Torrico A, et al. Evaluation of the self-sampling for cervical cancer screening in Bolivia. BMC Public Health. (2019) 19:80. doi: 10.1186/s12889-019-6401-5
81. Anderson C, Breithaupt L, Des Marais A, Rastas C, Richman A, Barclay L, et al. Acceptability and ease of use of mailed HPV self-collection among infrequently screened women in North Carolina. Sex Transm Infect. (2018) 94:131–7. doi: 10.1136/sextrans-2017-053235
82. Anhang R, Nelson JA, Telerant R, Chiasson MA, Wright TCJ. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. J Womens Health. (2005) 14:721–8. doi: 10.1089/jwh.2005.14.721
83. Bansil P, Wittet S, Lim JL, Winkler JL, Paul P, Jeronimo J. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health. (2014) 14:596. doi: 10.1186/1471-2458-14-596
84. Barbee L, Kobetz E, Menard J, Cook N, Blanco J, Barton B, et al. Assessing the acceptability of self-sampling for HPV among Haitian immigrant women: CBPR in action. Cancer Causes Control. (2010) 21:421–31. doi: 10.1007/s10552-009-9474-0
85. Behnke A-L, Krings A, Wormenor CM, Dunyo P, Kaufmann AM, Amuah JE. Female health-care providers' advocacy of self-sampling after participating in a workplace program for cervical cancer screening in Ghana: a mixed-methods study. Glob Health Action. (2020) 13:1838240. doi: 10.1080/16549716.2020.1838240
86. Berner A, Hassel S, Ben, Tebeu PM, Untiet S, Kengne-Fosso G, Navarria1 I, et al. Human papillomavirus self-sampling in Cameroon: women's uncertainties over the reliability of the method are barriers to acceptance. J Low Genit Tract Dis. (2013) 17:235–41. doi: 10.1097/LGT.0b013e31826b7b51
87. Brewer N, Foliaki S, Bromhead C, Viliamu-Amusia I, Pelefoti-Gibson L, Jones T, et al. Acceptability of human papillomavirus self-sampling for cervical-cancer screening in under-screened Māori and Pasifika women: a pilot study. N Z Med J. (2019) 132:21–31.
88. Broquet C, Triboullier D, Untiet S, Schafer S, Petignat P, Vassilakos P. Acceptability of self-collected vaginal samples for HPV testing in an urban and rural population of Madagascar. Afr Health Sci. (2015) 15:755–61. doi: 10.4314/ahs.v15i3.8
89. Castell S, Krause G, Schmitt M, Pawlita M, Deleré Y, Obi N, et al. Feasibility and acceptance of cervicovaginal self-sampling within the German National Cohort (Pretest 2). Bundesgesundheitsblatt Gesundheitsforsch Gesundheitsschutz. (2014) 57:1270–6. doi: 10.1007/s00103-014-2054-9
90. Catarino R, Vassilakos P, Stadali-Ullrich H, Royannez-Drevard I, Guillot C, Petignat P. Feasibility of at-home self-sampling for HPV testing as an appropriate screening strategy for nonparticipants in Switzerland: preliminary results of the depist study. J Low Genit Tract Dis. (2015) 19:27–34. doi: 10.1097/LGT.0000000000000051
91. Chatzistamatiou K, Chatzaki E, Constantinidis T, Nena E, Tsertanidou A, Agorastos T. Self-collected cervicovaginal sampling for site-of-care primary HPV-based cervical cancer screening: a pilot study in a rural underserved Greek population. J Obstet Gynaecol J Inst Obstet Gynaecol. (2017) 37:1059–64. doi: 10.1080/01443615.2017.1323197
92. Chou HH, Huang HJ, Cheng HH, Chang CJ, Yang LY, Huang CC, et al. Self-sampling HPV test in women not undergoing Pap smear for more than 5 years and factors associated with under-screening in Taiwan. J Formos Med Assoc. (2016) 115:1089–96. doi: 10.1016/j.jfma.2015.10.014
93. Crofts V, Flahault E, Tebeu P-M, Untiet S, Fosso GK, Boulvain M, et al. Education efforts may contribute to wider acceptance of human papillomavirus self-sampling. Int J Womens Health. (2015) 7:149–54. doi: 10.2147/IJWH.S56307
94. Crosby RA, Hagensee ME, Vanderpool R, Nelson N, Parrish A, Collins T, et al. Community-based screening for cervical cancer: a feasibility study of rural Appalachian women. Sex Transm Dis. (2015) 42:607–11. doi: 10.1097/OLQ.0000000000000365
95. Dannecker C, Siebert U, Thaler CJ, Kiermeir D, Hepp H, Hillemanns P. Primary cervical cancer screening by self-sampling of human papillomavirus DNA in internal medicine outpatient clinics. Ann Oncol Off J Eur Soc Med Oncol. (2004) 15:863–9. doi: 10.1093/annonc/mdh240
96. de Melo Kuil L, Lorenzi AT, Stein MD, Resende JCP, Antoniazzi M, Longatto-Filho A, et al. The role of self-collection by vaginal lavage for the detection of HPV and high-grade Intraepithelial Neoplasia. Acta Cytol. (2017) 61:425–33. doi: 10.1159/000477331
97. Deleré Y, Schuster M, Vartazarowa E, Hänsel T, Hagemann I, Borchardt S, et al. Cervicovaginal self-sampling is a reliable method for determination of prevalence of human papillomavirus genotypes in women aged 20–30 years. J Clin Microbiol. (2011) 49:3519–22. doi: 10.1128/JCM.01026-11
98. Des Marais AC, Zhao Y, Hobbs MM, Sivaraman V, Barclay L, Brewer NT, et al. Home self-collection by mail to test for human papillomavirus and sexually transmitted infections. Obstet Gynecol. (2018) 132:1412–20. doi: 10.1097/AOG.0000000000002964
99. Desai KT, Ajenifuja KO, Banjo A, Adepiti CA, Novetsky A, Sebag C, et al. Design and feasibility of a novel program of cervical screening in Nigeria: self-sampled HPV testing paired with visual triage. Infect Agent Cancer. (2020) 15:60. doi: 10.1186/s13027-020-00324-5
100. Duke P, Godwin M, Ratnam S, Dawson L, Fontaine D, Lear A, et al. Effect of vaginal self-sampling on cervical cancer screening rates: a community-based study in Newfoundland. BMC Womens Health. (2015) 15:47. doi: 10.1186/s12905-015-0206-1
101. Dutton T, Marjoram J, Burgess S, Montgomery L, Vail A, Callan N, et al. Uptake and acceptability of human papillomavirus self-sampling in rural and remote aboriginal communities: evaluation of a nurse-led community engagement model. BMC Health Serv Res. (2020) 20:398. doi: 10.1186/s12913-020-05214-5
102. Dzuba IG, Díaz EY, Allen B, Leonard YF, Ponce ECL, Shah K V, et al. The acceptibility of self-collected samples for HPV testing vs. The pap test as alternatives in cervical cancer screening. J Women's Heal Gender-Based Med. (2002) 11:265–75. doi: 10.1089/152460902753668466
103. Esber A, Norris A, Jumbe E, Kandodo J, Nampandeni P, Reese PC, et al. Feasibility, validity and acceptability of self-collected samples for human papillomavirus (HPV) testing in rural Malawi. Malawi Med J. (2018) 30:61–6. doi: 10.4314/mmj.v30i2.2
104. Galbraith K V, Gilkey MB, Smith JS, Richman AR, Barclay L, Brewer NT. perceptions of mailed HPV self-testing among women at higher risk for cervical cancer. J Community Health. (2014) 39:849–56. doi: 10.1007/s10900-014-9931-x
105. Goldstein A, Plafker B, Stamper S, Goldstein L, Lipson R, Bedell S, et al. Patient satisfaction with human papillomavirus self-sampling in a cohort of ethnically diverse and rural Women in Yunnan Province, China. J Low Genit Tract Dis. (2020) 24:349–52. doi: 10.1097/LGT.0000000000000560
106. Gottschlich A, Nuntadusit T, Zarins KR, Hada M, Chooson N, Bilheem S, et al. Barriers to cervical cancer screening and acceptability of HPV self-testing: a cross-sectional comparison between ethnic groups in Southern Thailand. BMJ Open. (2019) 9:e031957. doi: 10.1136/bmjopen-2019-031957
107. Guan YY, Castle PE, Wang S, Li B, Feng C, Ci P, et al. A cross-sectional study on the acceptability of self-collection for HPV testing among women in rural China. Sex Transm Infect. (2012) 88:490–4. doi: 10.1136/sextrans-2012-050477
108. Haile EL, Cindy S, Ina B, Belay G, Jean-Pierre VG, Sharon R, et al. HPV testing on vaginal/cervical nurse-assisted self-samples vs. clinician-taken specimens and the HPV prevalence, in Adama Town, Ethiopia. Medicine. (2019) 98:e16970. doi: 10.1097/MD.0000000000016970
109. Hinten F, Hilbrands LB, Meeuwis KA, van Bergen-Verkuyten MC, Slangen BF, van Rossum MM, et al. Improvement of gynecological screening of female renal transplant recipients by self-sampling for human papillomavirus detection. J Low Genit Tract Dis. (2017) 21:33–6. doi: 10.1097/LGT.0000000000000270
110. Igidbashian S, Boveri S, Spolti N, Radice D, Sandri MT, Sideri M. Self-collected human papillomavirus testing acceptability: comparison of two self-sampling modalities. J Womens Health. (2011) 20:397–402. doi: 10.1089/jwh.2010.2189
111. Ilangovan K, Kobetz E, Koru-Sengul T, Marcus EN, Rodriguez B, Alonzo Y, et al. Acceptability and feasibility of human papilloma virus self-sampling for cervical cancer screening. J Womens Health. (2016) 25:944–51. doi: 10.1089/jwh.2015.5469
112. Islam JY, Mutua MM, Kabare E, Manguro G, Hudgens MG, Poole C, et al. High-risk human papillomavirus messenger RNA Testing in wet and dry self-collected specimens for high-grade cervical lesion detection in Mombasa, Kenya. Sex Transm Dis. (2020) 47:464–72. doi: 10.1097/OLQ.0000000000001167
113. Jones HE, Brudney K, Sawo DJ, Lantigua R, Westhoff CL. The acceptability of a self-lavaging device compared to pelvic examination for cervical cancer screening among low-income women. J Women's Health. (2012) 21:1275–81. doi: 10.1089/jwh.2012.3512
114. Jones HE, Wiegerinck MAHM, Nieboer TE, Mol BW, Westhoff CL. Women in the netherlands prefer self-sampling with a novel lavaging device to clinician collection of specimens for cervical cancer screening. Sex Transm Dis. (2008) 35:916–7. doi: 10.1097/OLQ.0b013e3181812cf0
115. Katanga JJ, Rasch V, Manongi R, Pembe AB, Mwaiselage JD, Kjaer SK. Concordance in HPV detection between self-collected and health provider–collected cervicovaginal samples using careHPV in Tanzanian Women. JCO Glob Oncol. (2021). doi: 10.1200/GO.20.00598
116. Ketelaars PJW, Bosgraaf RP, Siebers AG, Massuger LFAG, van der Linden JC, Wauters CAP, et al. High-risk human papillomavirus detection in self-sampling compared to physician-taken smear in a responder population of the Dutch cervical screening: results of the VERA study. Prev Med. (2017) 101:96–101. doi: 10.1016/j.ypmed.2017.05.021
117. Khanna N, Mishra SI, Tian G, Tan MT, Arnold S, Lee C, et al. Human papillomavirus detection in self-collected vaginal specimens and matched clinician-collected cervical specimens. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. (2007) 17:615–22. doi: 10.1111/j.1525-1438.2006.00835.x
118. Kilfoyle KA, Des Marais AC, Ngo MA, Romocki L, Richman AR, Barclay L, et al. Preference for human papillomavirus self-collection and papanicolaou: survey of underscreened women in North Carolina. J Low Genit Tract Dis. (2018) 22:302–10. doi: 10.1097/LGT.0000000000000430
119. Landy R, Hollingworth T, Waller J, Marlow LAV, Rigney J, Round T, et al. Non-speculum sampling approaches for cervical screening in older women: randomised controlled trial. Br J Gen Pract. (2022) 72:e26–33. doi: 10.3399/BJGP.2021.0708
120. Laskow B, Figueroa R, Alfaro KM, Scarinci IC, Conlisk E, Maza M, et al. A pilot study of community-based self-sampling for HPV testing among non-attenders of cervical cancer screening programs in El Salvador. Int J Gynecol Obstet. (2017) 138:194–200. doi: 10.1002/ijgo.12204
121. Litton AG, Castle PE, Partridge EE, Scarinci IC. Cervical cancer screening preferences among African American women in the Mississippi Delta. J Health Care Poor Underserved. (2013) 24:46–55. doi: 10.1353/hpu.2013.0017
122. Lorenzi NPC, Termini L, Longatto Filho A, Tacla M, de Aguiar LM, Beldi MC, et al. Age-related acceptability of vaginal self-sampling in cervical cancer screening at two university hospitals: a pilot cross-sectional study. BMC Public Health. (2019) 19:963. doi: 10.1186/s12889-019-7292-1
123. Ma'som M, Bhoo-Pathy N, Nasir NH, Bellinson J, Subramaniam S, Ma Y, et al. Attitudes and factors affecting acceptability of self-administered cervicovaginal sampling for human papillomavirus (HPV) genotyping as an alternative to Pap testing among multiethnic Malaysian women. BMJ Open. (2016) 6:e011022. doi: 10.1136/bmjopen-2015-
124. Madhivanan P, Nishimura H, Ravi K, Pope B, Coudray M, Arun A, et al. Acceptability and concordance of self- vs. clinician- sampling for HPV testing among Rural. South Indian Women Asian Pac J Cancer Prev. (2021) 22:971–6. doi: 10.31557/APJCP.2021.22.3.971
125. Mahande MJ, Oneko O, Amour C, Pollie M, Smith C, Mboya IB, et al. Feasibility and acceptability of human papillomavirus self-sampling in a semi-urban area in northern Tanzania. Int J Gynecol Obstet. (2021) 154:113–8. doi: 10.1002/ijgo.13579
126. Malone C, Tiro JA, Buist DS, Beatty T, Lin J, Kimbel K, et al. Reactions of women underscreened for cervical cancer who received unsolicited human papillomavirus self-sampling kits. J Med Screen. (2020) 27:146–56. doi: 10.1177/0969141319885994
127. Mandigo M, Frett B, Laurent JR, Bishop I, Raymondville M, Kobetz-Kerman E. Community health workers paired with human papillomavirus self-samplers: a promising method to reduce cervical cancer. Obs Gynecol. (2014) 123:206–10. doi: 10.1097/01.AOG.0000447210.22335.ef
128. Mao C, Kulasingam SL, Whitham HK, Hawes SE, Lin J, Kiviat NB. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health. (2017) 26:609–15. doi: 10.1089/jwh.2016.5965
129. Maza M, Melendez M, Masch R, Alfaro K, Chacon A, Gonzalez E, et al. Acceptability of self-sampling and human papillomavirus testing among non-attenders of cervical cancer screening programs in El Salvador. Prev Med. (2018) 114:149–55. doi: 10.1016/j.ypmed.2018.06.017
130. McLarty JW WDLLS, Hagensee ME, McLarty JW, Williams DL, Loyd S, Hagensee ME. Cervical human papillomavirus testing with two home self-collection methods compared with a standard clinically collected sampling method. Sex Transm Dis. (2019) 46:670–5. doi: 10.1097/OLQ.0000000000001045
131. Mremi A, Linde DS, Mchome B, Mlay J, Schledermann D, Blaakær J, et al. Acceptability and feasibility of self-sampling and follow-up attendance after text message delivery of human papillomavirus results: a cross-sectional study nested in a cohort in rural Tanzania. Acta Obstet Gynecol Scand. (2021) 100:802–10. doi: 10.1111/aogs.14117
132. Nakalembe M, Makanga P, Kambugu A, Laker-Oketta M, Huchko MJ, Martin J, et al. public health approach to cervical cancer screening in Africa through community-based self-administered HPV testing and mobile treatment provision. Cancer Med. (2020) 9:8701–12. doi: 10.1101/2019.12.19.19015446
133. Nelson EJ, Hughes J, Oakes JM, Thyagarajan B, Pankow JS, Kulasingam SL. Human papillomavirus infection in women who submit self-collected vaginal swabs after internet recruitment. J Commun Health. (2015) 40:379–86. doi: 10.1007/s10900-014-9948-1
134. Nobbenhuis MAE, Helmerhorst TJM, van den Brule AJC, Rozendaal L, Jaspars LH, Voorhorst FJ, et al. Primary screening for high risk HPV by home obtained cervicovaginal lavage is an alternative screening tool for unscreened women. J Clin Pathol. (2002) 55:435–9. doi: 10.1136/jcp.55.6.435
135. Obiri-Yeboah D, Adu-Sarkodie Y, Djigma F, Hayfron-Benjamin A, Abdul L, Simpore J, et al. Self-collected vaginal sampling for the detection of genital human papillomavirus (HPV) using careHPV among Ghanaian women. BMC Womens Health. (2017) 17:86. doi: 10.1186/s12905-017-0448-1
136. Oranratanaphan S, Termrungruanglert W, Khemapech N. Acceptability of self-sampling HPV testing among thai women for cervical cancer screening. Asian Pacific J Cancer Prev. (2014) 15:7437–41. doi: 10.7314/APJCP.2014.15.17.7437
137. Pantano N de P, Fregnani JH, Resende JCP, Zeferino LC, Fonseca B de O, Antoniazzi M, et al. Evaluation of human papillomavirus self-collection offered by community health workers at home visits among under-screened women in Brazil. J Med Screen. (2020) 28:163–8. doi: 10.1177/0969141320941056
138. Penaranda E, Molokwu J, Flores S, Byrd T, Brown L, Shokar N. Women's Attitudes toward cervicovaginal self-sampling for high-risk HPV infection on the US-Mexico Border. J Low Genit Tract Dis. (2015) 19:323–8. doi: 10.1097/LGT.0000000000000134
139. Reiter PL, Shoben AB, McDonough D, Ruffin MT, Steinau M, Unger ER, et al. Results of a pilot study of a mail-based human papillomavirus self-testing program for underscreened women from Appalachian Ohio. Sex Transm Dis. (2019) 46:185–90. doi: 10.1097/OLQ.0000000000000944
140. Rosenbaum AJ, Gage JC, Alfaro KM, Ditzian LR, Maza M, Scarinci IC, et al. Acceptability of self-collected vs. provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. (2014) 126:156–60. doi: 10.1016/j.ijgo.2014.02.026
141. Sechi I, Elvezia CC, Martinelli M, Muresu N, Castriciano S, Sotgiu G, et al. Comparison of different self-sampling devices for molecular detection of human papillomavirus (HPV) and other sexually transmitted infections (STIs): a pilot study. Healthc. (2022) 10:459. doi: 10.3390/healthcare10030459
142. Sellors JW, Lorincz AT, Mahony JB, Mielzynska I, Lytwyn A, Roth P, et al. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. C Can Med Assoc J. (2000) 163:513–8.
143. Shin HY, Lee B, Hwang SH, Lee DO, Sung NY, Park JY, et al. Evaluation of satisfaction with three different cervical cancer screening modalities: clinician-collected pap test vs. HPV test by self-sampling vs HPV test by urine sampling. J Gynecol Oncol. (2019) 30:e76. doi: 10.3802/jgo.2019.30.e76
144. Silva J, Cerqueira F, Medeiros R. Acceptability of self-sampling in Portuguese women: The good, the bad or the ugly? Sex Health. (2017) 14:298–300. doi: 10.1071/SH16077
145. Surriabre P, Allende G, Prado M, Cáceres L, Bellot D, Torrico A, et al. Self-sampling for human papillomavirus DNA detection: a preliminary study of compliance and feasibility in BOLIVIA. BMC Womens Health. (2017) 17:135. doi: 10.1186/s12905-017-0490-z
146. Swanson M, Ibrahim S, Blat C, Oketch S, Olwanda E, Maloba M, et al. Evaluating a community-based cervical cancer screening strategy in Western Kenya: a descriptive study. BMC Womens Health. (2018) 18:116. doi: 10.1186/s12905-018-0586-0
147. Szarewski A, Cadman L, Mallett S, Austin J, Londesborough P, Waller J, et al. Human papillomavirus testing by self-sampling: assessment of accuracy in an unsupervised clinical setting. J Med Screen. (2007) 14:34–42. doi: 10.1258/096914107780154486
148. Taku O, Meiring TL, Gustavsson I, Phohlo K, Garcia-Jardon M, Mbulawa ZZA, et al. Acceptability of self- collection for human papillomavirus detection in the Eastern Cape, South Africa. PLoS ONE. (2020) 15:e0241781. doi: 10.1371/journal.pone.0241781
149. Tan CS, Hamzah ND, Ismail ZHF, Jerip AR, Kipli M. Self-sampling in human papillomavirus screening during and post-Covid-19 pandemic. Med J Malaysia. (2021) 76:298–303.
150. Tiiti TA, Mashishi TL, Nkwinika VV, Molefi KA, Benoy I, Bogers J, et al. Evaluation of ilex selfcerv for detection of high-risk human papillomavirus infection in gynecology clinic attendees at a tertiary hospital in south africa. J Clin Med. (2021) 10:4817. doi: 10.3390/jcm10214817
151. Torrado-García LM, Martínez-Vega RA, Rincon-Orozco B. A. Novel strategy for cervical cancer prevention using cervical-vaginal self-collected samples shows high acceptability in women living in low-income conditions from Bucaramanga, Colombia. Int J Womens Health. (2020) 12:1197–204. doi: 10.2147/IJWH.S265130
152. Torres KL, Mariño JM, Pires Rocha DA, de Mello MB, de Melo Farah HH, Reis RDS, et al. Self-sampling coupled to the detection of HPV 16 and 18 E6 protein: a promising option for detection of cervical malignancies in remote areas. PLoS ONE. (2018) 13:e0201262. doi: 10.1371/journal.pone.0201262
153. Trope LA, Chumworathayi B, Blumenthal PD. Feasibility of community-based careHPV for cervical cancer prevention in rural Thailand. J Low Genit Tract Dis. (2013) 17:315–9. doi: 10.1097/LGT.0b013e31826b7b70
154. Van Baars R, Bosgraaf RP, Ter Harmsel BWA, Melchers WJG, Quint WGV, Bekkers RLM. Dry storage and transport of a cervicovaginal self-sample by use of the Evalyn Brush, providing reliable human papillomavirus detection combined with comfort for women. J Clin Microbiol. (2012) 50:3937–43. doi: 10.1128/JCM.01506-12
155. Virtanen A, Nieminen P, Niironen M, Luostarinen T, Anttila A. Self-sampling experiences among non-attendees to cervical screening. Gynecol Oncol. (2014) 135:487–94. doi: 10.1016/j.ygyno.2014.09.019
156. Wang R, Lee K, Gaydos CA, Anderson J, Keller J, Coleman J. Performance and acceptability of self-collected human papillomavirus testing among women living with HIV. Int J Infect Dis. (2020) 99:452–7. doi: 10.1016/j.ijid.2020.07.047
157. Wedisinghe L, Sasieni P, Currie H, Baxter G. The impact of offering multiple cervical screening options to women whose screening was overdue in Dumfries and Galloway, Scotland. Prev Med reports. (2022) 29:101947. doi: 10.1016/j.pmedr.2022.101947
158. Wikström I, Stenvall H, Wilander E. Attitudes to self-sampling of vaginal smear for human papilloma virus analysis among women not attending organized cytological screening. Acta Obstet Gynecol Scand. (2007) 86:720–5. doi: 10.1080/00016340701303747
159. Rachel L, Winer, Angela A, Gonzales, Carolyn J, Noonan, Stephen L, Cherne, Dedra S, Buchwald C, to INCO (CINCO). Assessing acceptability of self-sampling kits, prevalence, and risk factors for human papillomavirus infection in American Indian Women. J Commun Health. (2016) 4:1049–61. doi: 10.1007/s10900-016-0189-3
160. Wong EL-Y, Cheung AW-L, Wong AY-K, Chan PK-S. Acceptability and feasibility of HPV self-sampling as an alternative primary cervical cancer screening in under-screened population groups: a cross-sectional study. Int J Environ Res Public Health. (2020) 17:6245. doi: 10.3390/ijerph17176245
161. Zehbe I, Moeller H, Severini A, Weaver B, Escott N, Bell C, et al. Feasibility of self-sampling and human papillomavirus testing for cervical cancer screening in First nation women from Northwest Ontario, Canada: a pilot study. BMJ Open. (2011) 1:e000030. doi: 10.1136/bmjopen-2010-000030
162. Castle PE, Rausa A, Walls T, Gravitt PE, Partridge EE, Olivo V, et al. Comparative community outreach to increase cervical cancer screening in the Mississippi Delta. Prev Med. (2011) 52:452–5. doi: 10.1016/j.ypmed.2011.03.018
163. Elfström KM, Sundström K, Andersson S, Bzhalava Z, Carlsten Thor A, Gzoul Z, et al. increasing participation in cervical screening by targeting long-term nonattenders: randomized health services study. Int J Cancer. (2019) 145:3033–39. doi: 10.1002/ijc.32374
164. Enerly E, Bonde J, Schee K, Pedersen H, Lönnberg S, Nygård M. Self-sampling for human papillomavirus testing among non-attenders increases attendance to the norwegian cervical cancer screening programme. PLoS ONE. (2016) 11:e0151978. doi: 10.1371/journal.pone.0151978
165. Abdullah NN, Daud S, Wang SM, Mahmud Z, Mohd Kornain NK, Al-Kubaisy W. Human papilloma virus (HPV) self-sampling: do women accept it? J Obstet Gynaecol. (2018) 38:402–7. doi: 10.1080/01443615.2017.1379061
166. Avian A, Clemente N, Mauro E, Isidoro E, Di Napoli M, Dudine S, et al. Clinical validation of full HR-HPV genotyping HPV Selfy assay according to the international guidelines for HPV test requirements for cervical cancer screening on clinician-collected and self-collected samples. J Transl Med. (2022) 20:231. doi: 10.1186/s12967-022-03383-x
167. Chaw L, Lee SHF, Ja'afar NIH, Lim E, Sharbawi R. Reasons for non-attendance to cervical cancer screening and acceptability of HPV selfsampling among Bruneian women: a crosssectional study. PLoS ONE. (2022) 17:e026221. doi: 10.1371/journal.pone.0262213
168. Esber A, McRee A-L, Norris Turner A, Phuka J, Norris A. Factors influencing Malawian women's willingness to self-collect samples for human papillomavirus testing. J Fam Plan Reprod Heal Care. (2017) 43:135–41. doi: 10.1136/jfprhc-2015-101305
169. Rodríguez GMG, Ornelas OAO, Vázquez HMG, Esquivel DSS, Champion JD. Attitude and acceptability of the self-sampling in HPV carrier women. Hisp Heal Care Int. (2022) 20:40–3. doi: 10.1177/15404153211001577
170. Ngu SF, Lau LSK Li J, Wong GYC, Cheung ANY, Ngan HYS, et al. Human papillomavirus self-sampling for primary cervical cancer screening in under-screened women in Hong Kong during the COVID-19 pandemic. Int J Environ Res Public Health. (2022) 19:2650. doi: 10.3390/ijerph19052610
171. Sormani J, Kenfack B, Wisniak A, Datchoua AM, Makajio SL, Schmidt NC, et al. Exploring factors associated with patients who prefer clinician-sampling to HPV self-sampling: a study conducted in a low-resource setting. Int J Environ Res Public Health. (2022) 19:54. doi: 10.20944/preprints202111.0249.v1
172. World Health Organization. WHO Guideline on Self-Care Interventions for Health and Well Being, Vol 156. Geneva: World Health Organization (2021).
173. Verdoodt F, Jentschke M, Hillemanns P, Racey CS, Snijders PJF, Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur J Cancer. (2015) 51:2375–85. doi: 10.1016/j.ejca.2015.07.006
174. Arbyn M, Verdoodt F, Snijders PJF, Verhoef VMJ, Suonio E, Dillner L, et al. Accuracy of human papillomavirus testing on self-collected vs. clinician-collected samples: a meta-analysis. Lancet Oncol. (2014) 15:172–83. doi: 10.1016/S1470-2045(13)70570-9
175. Montealegre JR, Landgren RM, Anderson ML, Hoxhaj S, Williams S, Robinson DJ, et al. Acceptability of self-sample human papillomavirus testing among medically underserved women visiting the emergency department. Gynecol Oncol. (2015) 138:317–22. doi: 10.1016/j.ygyno.2015.05.028
176. Reiter PL, McRee A-L. Cervical cancer screening (Pap testing) behaviours and acceptability of human papillomavirus self-testing among lesbian and bisexual women aged 21-26 years in the USA. J Fam Plan Reprod Heal care. (2015) 41:259–64. doi: 10.1136/jfprhc-2014-101004
177. Lozar T, Nagvekar R, Racheal CR, Mandishora SD, Megan UI, Fitzpatrick B. Cervical cancer screening postpandemic: self-sampling opportunities to accelerate the elimination of cervical cancer. Int J Women's Health. (2021) 13:841–59. doi: 10.2147/IJWH.S288376
178. Elit L, Krzyzanowska M, Saskin R, Barbera L, Razzaq A, Lofters A, et al. Sociodemographic factors associated with cervical cancer screening and follow-up of abnormal results. Can Fam Phys. (2012) 58:e22–31.
179. Eaker S, Adami HO, Sparén P. Reasons women do not attend screening for cervical cancer: a population-based study in Sweden. Prev Med. (2001) 32:361–76. doi: 10.1006/pmed.2001.0844
180. Dareng EO, Jedy-Agba E, Bamisaye P, Isa Modibbo F, Oyeneyin LO, Adewole AS, et al. Influence of spirituality and modesty on acceptance of self-sampling for cervical cancer screening. PLoS One. (2015) 10:e0141679. doi: 10.1371/journal.pone.0141679
181. Amir SM, Idris IB, Yusoff HM. The acceptance of human papillomavirus self-sampling test among Muslim women:a systematic review. Asian Pacific J Cancer Prev. (2022) 23:767–74. doi: 10.31557/APJCP.2022.23.3.767
182. Zehbe I, Wakewich P, King AD, Morrisseau K, Tuck C. Self-administered vs. provider-directed sampling in the Anishinaabek cervical cancer screening study (ACCSS): a qualitative investigation with Canadian first nations wome. BMJ Open. (2017) 7:e017384. doi: 10.1136/bmjopen-2017-017384
Keywords: human papillomavirus, cervical cancer screening, self-sampling, uptake, acceptability, preference, systematic review, meta-analysis
Citation: Di Gennaro G, Licata F, Trovato A and Bianco A (2022) Does self-sampling for human papilloma virus testing have the potential to increase cervical cancer screening? An updated meta-analysis of observational studies and randomized clinical trials. Front. Public Health 10:1003461. doi: 10.3389/fpubh.2022.1003461
Received: 26 July 2022; Accepted: 15 November 2022;
Published: 08 December 2022.
Edited by:
Suneela Garg, University of Delhi, IndiaReviewed by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayCopyright © 2022 Di Gennaro, Licata, Trovato and Bianco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Licata, Zi5saWNhdGFAdW5pY3ouaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.