
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 28 October 2021
Sec. Aging and Public Health
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.753964
This article is part of the Research TopicDiet-Sleep Interaction on Cardiometabolic HealthView all 5 articles
 Chinedu T. Udeh-Momoh1*
Chinedu T. Udeh-Momoh1* Tamlyn Watermeyer2,3
Tamlyn Watermeyer2,3 Shireen Sindi1,4
Shireen Sindi1,4 Parthenia Giannakopoulou1
Parthenia Giannakopoulou1 Catherine E. Robb1
Catherine E. Robb1 Sara Ahmadi-Abhari1
Sara Ahmadi-Abhari1 Bang Zheng1
Bang Zheng1 Amina Waheed1
Amina Waheed1 James McKeand1
James McKeand1 David Salman5,6
David Salman5,6 Thomas Beaney6
Thomas Beaney6 Celeste A. de Jager Loots1
Celeste A. de Jager Loots1 Geraint Price1
Geraint Price1 Christina Atchison7,8
Christina Atchison7,8 Josip Car6,7,9
Josip Car6,7,9 Azeem Majeed6,7
Azeem Majeed6,7 Alison. H. McGregor10
Alison. H. McGregor10 Miia Kivipelto1,4,8,11
Miia Kivipelto1,4,8,11 Helen Ward7,12
Helen Ward7,12 Lefkos T. Middleton1,7
Lefkos T. Middleton1,7Background: Several studies have assessed the impact of COVID-19-related lockdowns on sleep quality across global populations. However, no study to date has specifically assessed at-risk populations, particularly those at highest risk of complications from coronavirus infection deemed “clinically-extremely-vulnerable-(COVID-19CEV)” (as defined by Public Health England).
Methods: In this cross-sectional study, we surveyed 5,558 adults aged ≥50 years (of whom 523 met criteria for COVID-19CEV) during the first pandemic wave that resulted in a nationwide-lockdown (April–June 2020) with assessments of sleep quality (an adapted sleep scale that captured multiple sleep indices before and during the lockdown), health/medical, lifestyle, psychosocial and socio-demographic factors. We examined associations between these variables and sleep quality; and explored interactions of COVID-19CEV status with significant predictors of poor sleep, to identify potential moderating factors.
Results: Thirty-seven percent of participants reported poor sleep quality which was associated with younger age, female sex and multimorbidity. Significant associations with poor sleep included health/medical factors: COVID-19CEV status, higher BMI, arthritis, pulmonary disease, and mental health disorders; and the following lifestyle and psychosocial factors: living alone, higher alcohol consumption, an unhealthy diet and higher depressive and anxiety symptoms. Moderators of the negative relationship between COVID-19CEV status and good sleep quality were marital status, loneliness, anxiety and diet. Within this subgroup, less anxious and less lonely males, as well as females with healthier diets, reported better sleep.
Conclusions: Sleep quality in older adults was compromised during the sudden unprecedented nation-wide lockdown due to distinct modifiable factors. An important contribution of our study is the assessment of a “clinically-extremely-vulnerable” population and the sex differences identified within this group. Male and female older adults deemed COVID-19CEV may benefit from targeted mental health and dietary interventions, respectively. This work extends the available evidence on the notable impact of lack of social interactions during the COVID-19 pandemic on sleep, and provides recommendations toward areas for future work, including research into vulnerability factors impacting sleep disruption and COVID-19-related complications. Study results may inform tailored interventions targeted at modifiable risk factors to promote optimal sleep; additionally, providing empirical data to support health policy development in this area.
The Coronavirus (COVID-19) pandemic still presents unprecedented global challenges. In the UK, the death toll surpassed, as of April 2021, 125,000 individuals (1). Although the UK vaccination programme is underway (2), the social and economic impacts of the pandemic, as well as the public health measures imposed, are likely to persist beyond the programme's success. Understanding the short- and long-term implications of COVID-19 and government-directed social distancing measures on health, psychosocial and lifestyle outcomes for individuals and society remains imperative. This is important for the population sub-set considered by NHS England to be “clinically extremely vulnerable” to COVID-19-related complications (COVID-19 CEV), due to pre-existing morbidity (3). In the UK, these at-risk individuals were encouraged to “shield” during the first lockdown by not leaving their home, except for restricted purposes, such as medical appointments. A large majority of the at-risk population are older adults, mostly women (4). However, men overall show higher COVID-related hospital admissions and death (5), indicating sex/gender-differences in risk.
Sleep quality disturbances have been widely studied as key health consequences of the global pandemic, mostly in younger populations and in those subjected to social distancing measures (6). Individual circumstances may vary as a function of age and, to date, there has been relatively little focus on sleep health during lockdowns in older adults (6). Furthermore, there are no published data (to our knowledge) on the impact of sleep in at-risk (shielding) groups within a UK sample. This is surprising given that sleep quality correlates with health, psychosocial and cognitive outcomes (7) and may accelerate the development of chronic multimorbidity in older adults (8). Furthermore, shielding may engender greater social isolation, which, as we have previously shown, predicted adverse psychosocial outcomes in older adults during the first UK lockdown (9). Social isolation has been associated with poorer sleep quality in this age-group (10), particularly during the current pandemic (6). Other health and lifestyle factors, such as diet (11) and physical activity (12) may also influence sleep parameters in older adults but have received less attention as possible modifiers in several COVID-19 sleep studies, to date.
The aim of this study is to provide a better understanding of COVID-19 restrictions upon sleep quality and its interaction with other pandemic-affected health and lifestyle outcomes in a UK sample of older adults that include “clinically extremely vulnerable” individuals. Greater knowledge of the pandemic implications for such individuals will inform health policies toward personalised interventions for sustained or enhanced health and well-being during current and future periods of restrictions and isolation, as well as into the recovery phase of the pandemic.
Study participants were recruited from the London based Cognitive Health in Ageing Register for Investigational and Observational Trials (CHARIOT; 9) for participation in the CHARIOT COVID-19 Rapid Response (CCRR) Study. The CHARIOT Register was initiated in 2011 through collaborative efforts between GP practises across North and West London and Imperial College London. The register comprises ~40,000 cognitively healthy older adults, aged 50–85 years, at time of recruitment (13).
The CCRR study was initiated at Imperial College London, UK, in early April 2020 less than a month after the sudden government-mandated nationwide lockdown. The study aimed to investigate the impact of the COVID-19 pandemic and associated social distancing measures on the mental and physical health of an older adult population. Data were collected via an online repeated survey administered at 6-weekly intervals, before reducing frequency to 3-monthly from September 2020. This cross-sectional investigation reports on the baseline CCRR study data collected during the first COVID-19 pandemic wave in the UK i.e., April–June 2020 (9).
The exploratory variables and covariates included in the study analyses are described in Table 1. In summary, general demographic data included age, sex (collected as “male,” “female,” or “prefer not to say”), marital status, self-isolation, ethnicity and employment status. Data on whether one was shielding and considered clinically extremely vulnerable (COVID-19CEV; 27) was included as primary exposure.
Reported medical comorbidities were computed into a “number of pre-existing conditions” variable, given notable impact on sleep quality (8), and COVID-19 positivity status at time of survey was captured. Height and weight data were self-reported and used to calculate Body mass index (BMI), categorised according to the standard WHO criteria.
Lifestyle factors included smoking status, alcohol consumption and diet (described in Figure 1). Physical activity level was collected using the International Physical Activity Questionnaire [IPAQ;(14)].
We assessed self-reported loneliness via the question: “During the period of reduced social contact, have you experienced loneliness (felt isolated, with no companions),” with the following responses: “never,” “rarely,” “sometimes,” “often” [obtained from the Centre for Epidemiologic Studies of Depression Scale, for work-free periods (15)]. Depression and anxiety levels were measured via the Hospital Anxiety and Depression Scale (HADS), described as “Normal,” “Borderline,” or “Abnormal” with higher scale scores indicative of greater anxiety and depressive symptoms (16).
The Pittsburgh Sleep Quality Index (PSQI) is one of the most commonly used measures for assessing sleep disturbances in clinical and research settings (17). In the CCRR study, the PSQI was modified to enable assessment of overall sleep quality and individual components before and during the first lockdown period (see Appendix for Supplementary Table 1).
The Adapted-PSQI scale for the CCRR Study (PSQI-CCRR) scale consists of 8 self-reported items to compute four components (subdomains) of sleep latency (SL), sleep duration (SD), habitual sleep efficiency (HSE), and subjective sleep quality (SSQ). Each individual component yields scores ranging from 0 to 3, summed to generate a total score (i.e., global sleep-score) ranging from 0 to 12, with higher scores indicative of worse sleep quality.
Analyses were performed on a sub-sample of participants with complete sleep data, who undertook baseline CCRR assessments in the period from April to June 2020, corresponding to the first wave of the coronavirus pandemic, peak of case numbers, and fatalities and period of first national lockdown (9).
Sample characteristics are described in relation to global sleep quality and poor sleep. Comparisons of global sleep quality scores with study characteristics were made using analysis of variance (ANOVA) or independent t-tests for the continuous measures and chi-squared tests for the categorical variables. Psychometric properties of the Adapted PSQI scale (PSQI-CCRR) were assessed using prescribed tests and indicators (Supplementary Table 2).
Multivariable linear regression analyses adjusting for age, sex and number of pre-existing health conditions were used to independently evaluate associations of global sleep quality during the lockdown with socio-demographic (age, sex, ethnicity, marital/cohabitation status, employment status, COVID-19 positivity and COVID-19CEV status), health/medical (BMI, arthritis, COPD, mental health, neurological conditions, diabetes, high blood pressure and hypercholesterolaemia), lifestyle (alcohol, smoking, diet, and physical activity), psycho-social (loneliness and self-isolating), and mental health (depression and anxiety) factors.
Interactions of COVID-19CEV status with factors significantly associated with sleep quality during lockdown were explored to identify moderators of a hypothesised association of COVID-19CEV status and poor sleep. Sex-stratified analyses of significant moderators of the COVID-19CEV status—sleep quality association were also conducted to investigate potential differences in males and females, and further in relation to sleep quality prior to pandemic-associated lockdown.
All Statistical analyses were performed using STATA (Version 16.1).
Characteristics of the overall CCRR participant population have been previously reported (9). Our analysed sample consisted of CCRR study participants who had completed the sleep quality scale (PSQI-CCRR) from April to June 2020 (Supplementary Table 3: N = 5,558). Of these, 55% were females, 94% white, and cohort mean (± SD) age was 71 ± 7.3 years. During lockdown, the mean (± SD) global PSQI-CCRR score was 3.3 ± 2.6, with the 37% participants who scored >3 categorised as poor sleepers. Most participants were married (68%), not self-isolating (88%) and retired (71%). About 8% of participants had more than two chronic diseases and up to 27% reported feeling lonely often or sometimes (Supplementary Table 2).
Overall, 9% of the cohort were CEV (523 out of 5,558). Comparisons between participants deemed high vs. low risk of COVID-19 complications revealed that those in the COVID-19CEV group were more likely to be older, had higher depression and anxiety scale scores, as well as higher global PSQI-CCRR scores, indicative of poorer sleep quality (Table 1). Similarly, the proportion of COVID-19CEV individuals was higher among males, smokers, those who drank less during lockdown and were retired. Significantly higher numbers of COVID-19CEV participants had low lockdown physical activity, suffered from more than two chronic conditions, and reported unhealthy diets during lockdown (Table 1).
No significant differences were noted across ethnicity, BMI or loneliness groups (Table 1).
Poor sleep quality during lockdown was significantly associated with being in the CEV group (β = 0.35, 95% CI: 0.11–0.58). The association persisted but marginally attenuated, after adjusting for confounders including health and lifestyle determinants of sleep disturbances (β = 0.30, 95% CI: 0.02–0.58) (Table 2). Correspondingly, participants who were not married or co-habiting with a partner as well as those who had higher BMI scores and sufferers of arthritis, COPD or mental health disorders were more likely to report sleeping poorly during the COVID-19 lockdown (see Table 2). Participants who consumed more alcohol (β = 0.35, 95% CI: 0.15–0.55) and had unhealthy diets (β = 0.97, 95% CI: 0.73–1.21) during the early COVID-19 lockdown also reported poorer sleep. Physical activity and smoking status were not significantly associated with sleep quality during the lockdown (Table 2). In relation to psychosocial factors (measures of loneliness and isolation), participants who reported “not ever/rarely” or “sometimes” feeling lonely, as well as those who were not self-isolating were more likely to report sleeping better during the lockdown. On the other hand, anxiety, and depressive symptoms were significantly associated with poorer sleep (β = 0.22, 95% CI: 0.21–0.25 and β = 0.23, 95% CI: 0.21–0.25, respectively). Neither ethnicity, employment status, COVID-19 test positivity nor pre-existing health conditions like diabetes, high blood pressure, asthma, hypercholesterolemia and neurological conditions, were significantly associated with sleep quality (Table 2).
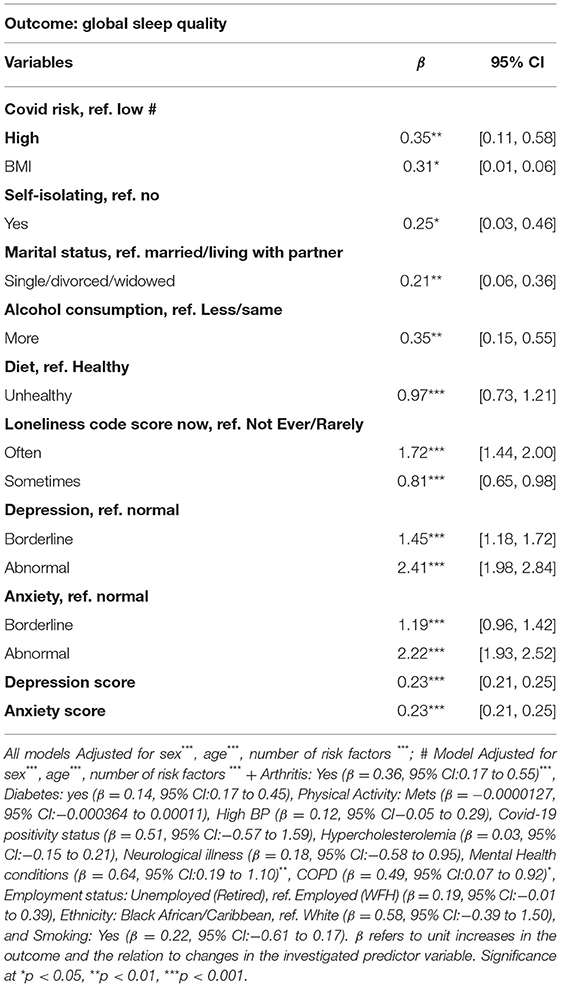
Table 2. Factors associated with sleep quality during the early COVID-19 lockdown (April–June 2020).
Effect-modification of the positive association between COVID-19CEV status and poorer lockdown sleep quality revealed significant interaction effects for marital status, loneliness, anxiety, and diet (Table 3A). Sub-group analyses in relation to COVID-19CEV status revealed that the previously noted significant positive relationship between good sleep quality with having a healthy diet during lockdown, not ever or rarely feeling lonely and being less anxious during lockdown was more pronounced among COVID-19CEV participants (Diet: Low risk β = −0.82, 95% CI: −1.08, −0.57 and High risk β = −1.77, 95% CI: −2.48, −1.06; Loneliness: Low risk β = −0.74, 95% CI: −1.07, −0.42 and High risk β = −2.14, 95% CI: −3.12, −1.15; Anxiety: Low risk β = −0.22, 95% CI: −0.24, −0.20 and High risk β = −0.30, 95% CI: −0.35, −0.23; All P <0.01). The beneficial effect of being married/cohabiting with a partner was only significant among COVID-19CEV participants (β for low risk= −0.15, 95% CI: −0.30, 0.01, p = 0.07; β for high risk = −0.78, CI: −1.31, −0.25, p = 0.004) (Table 3B).
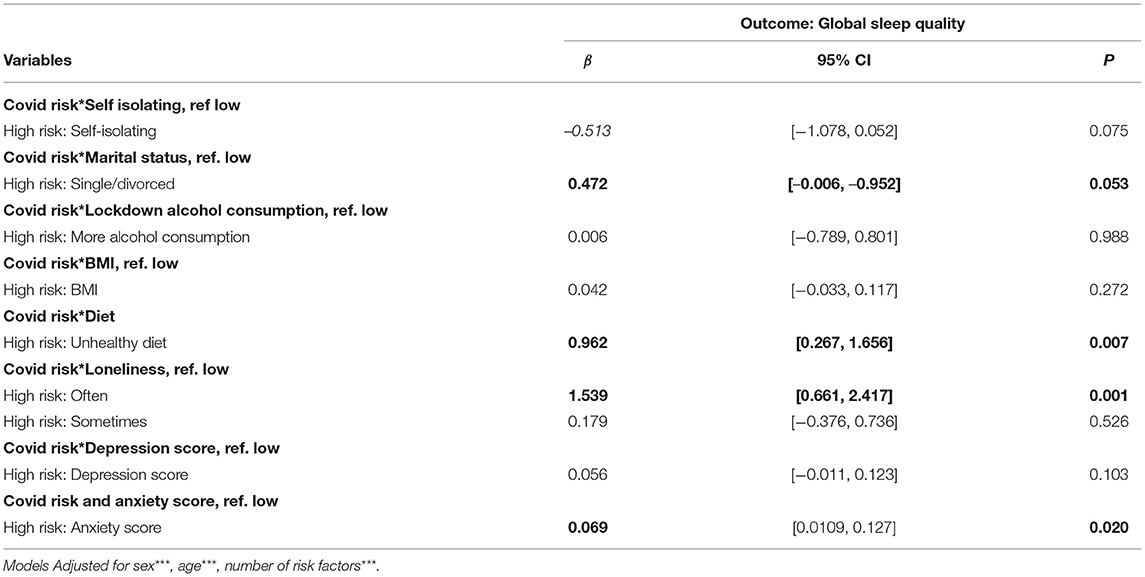
Table 3A. Moderators of the association between COVID-19CEV status and sleep quality during lockdown.
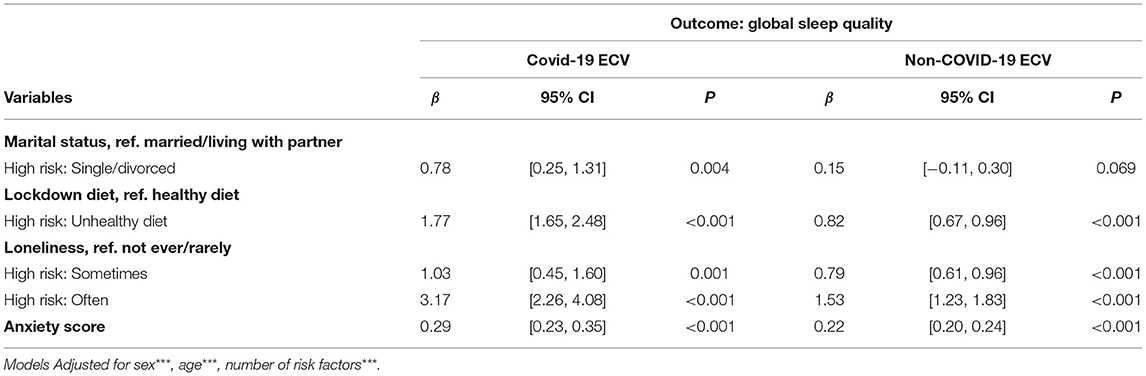
Table 3B. Associations of significant moderators of the COVID-19ECV status—sleep quality relationship, among the COVID-19ECV groups.
Modification by depression and self-isolation status was only marginally significant (p-values for interaction: 0.09 and 0.07 respectively). Conversely, the significant positive association between COVID-19CEV status and worsened sleep quality was not moderated by alcohol consumption during lockdown or BMI (p-value for interaction >0.1) (see Table 3A).
In sex-stratified analyses, comparing interaction effect of COVID-19CEV status and modifiable factors (diet, anxiety, marital status and loneliness) with sleep, reporting that “not ever/rarely” feeling lonely and being less anxious were associated with better sleep quality (β = −2.94, 95% CI: −4.56, −1.37), with the interaction term being statistically significant for COVID-19 CEV males only (β = −0.10, 95%CI: −0.19, −0.02). Furthermore, the moderating effect of being married/cohabiting was only marginally significant in males (β = −0.70, 95%CI: −1.45, −0.04; p = 0.064). On the contrary, the interaction of healthy diet and COVID-19CEV in relation to better sleep quality was only significant among females (β = −1.16, 95% CI: −2.14, −0.19; Table 4).
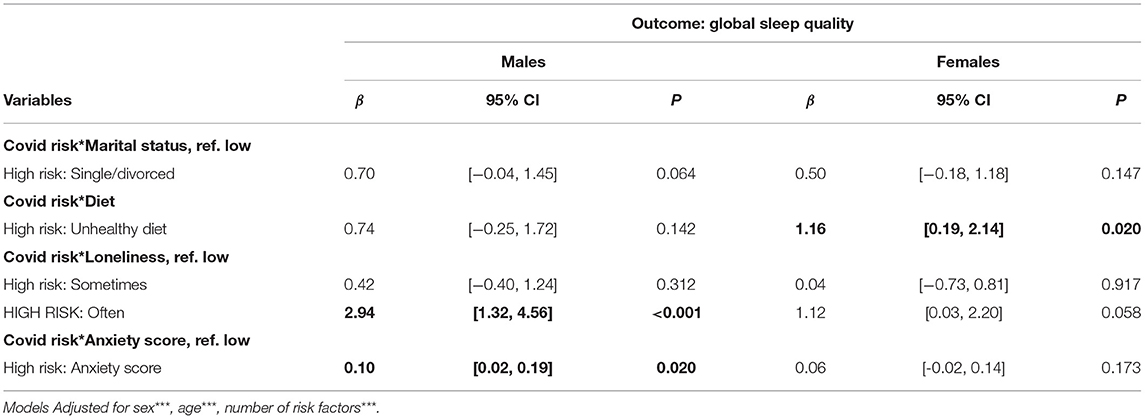
Table 4. Sex-stratified analysis showing modification of relationship between COVID-19CEV status and sleep quality during lockdown, by distinct lifestyle and psychosocial predictors of sleep, in males and females.
The last year has witnessed significant global COVID-19 pandemic-related deaths and disabilities. Yet the impact of the pandemic on sleep quality, a significant risk factor for poor cognitive outcomes and gross morbidities in older adults (7, 8) remains largely unknown, particularly for groups at highest risk of medical complications due to COVID-19.
Here we provide the first systematic investigation of sleep quality among UK older adults during the first pandemic wave that featured a complete unparalleled nation-wide lockdown, with a focus on individuals at highest risk of COVID-19-related complications, defined as “clinically extremely vulnerable” or COVID-19CEV (3).
We found that younger participants, females, and those with multimorbidity had poorer sleep quality during lockdown. In addition, sleep quality during the lockdown was poorer among COVID-19 CEV participants, those with higher BMI, those living alone, and among participants suffering from arthritis, COPD or mental health disorders. Of several lifestyle factors investigated, higher alcohol consumption and unhealthy diet were associated with poor sleep during lockdown, whereas no association was observed with smoking and physical activity, during the first lockdown. Of the psychosocial factors, “never or rarely” feeling lonely, and not self-isolating were associated with better sleep quality, whereas higher levels of anxiety and depressive symptoms were associated with poor sleep.
Factors that attenuated the notable positive association between COVID-19CEV status and poor sleep quality included being married or cohabiting with a partner, having a healthy diet during lockdown, never or rarely feeling lonely and having lower levels of anxiety symptoms. Older adults with good sleep quality prior to lockdown were most likely to report poor sleep during lockdown if they had unhealthy diets (Supplementary Table 4). Also, pre-lockdown good sleep quality was maintained during the lockdown among married or cohabiting participants (Supplementary Table 4). Sub-group analysis by sex showed that “never” or “rarely” feeling lonely and lower anxiety symptoms were associated with better sleep quality, and that the associations were most pronounced for males with COVID-19CEV status. Among COVID19-CEV females, healthier diet promoted better sleep quality (see Figure 2).
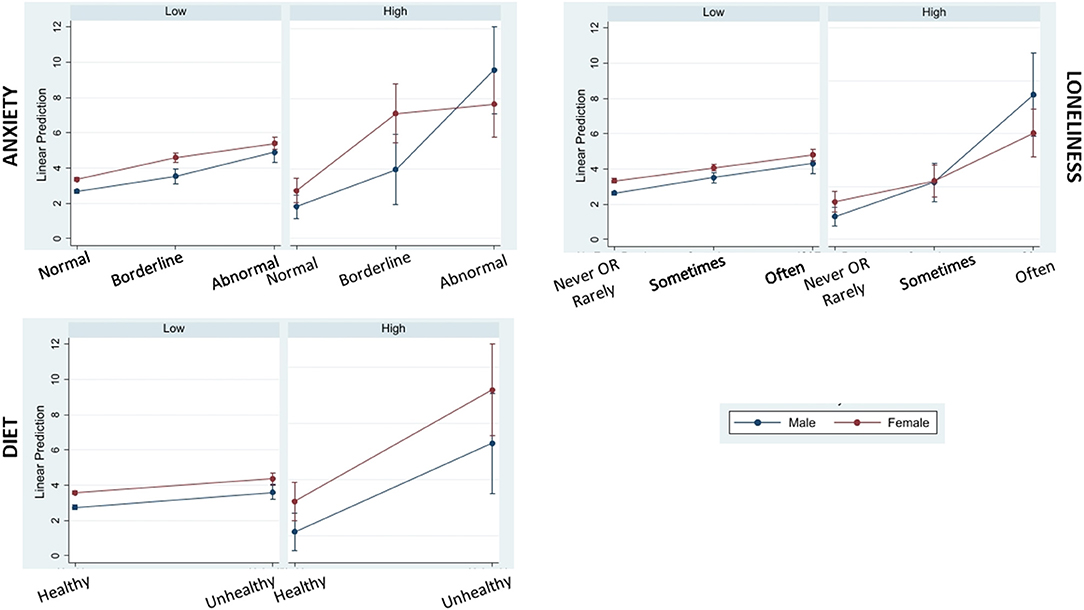
Figure 2. Marginal plots showing interactions of COVID-19 complication risk level (low and high) with significant moderators of poor sleep quality during lockdown (anxiety, loneliness, and diet) by sex (male and female). Higher PSQI-CCRR (sleep scale) scores indicative of poor sleep quality are predicted for males in the Covid High risk category who scored highest in the anxiety component of HADS scale (Abnormal group) and who reported “Often” feeling lonely during the Covid-19 lockdown period. Conversely, higher scores representing poorer sleep quality are predicted for females in the Covid High risk category who had Unhealthy diets during the Covid-19 lockdown period.
Our study supports previous reports, suggesting the development of sleep disturbances during the pandemic among females, those with chronic illnesses, higher stress levels, higher alcohol consumption, loneliness and depressive symptoms (6, 18). We noted that higher alcohol consumption was associated with poor sleep quality; indeed, high alcohol consumption disrupts sleep architecture and compromises sleep quality, though relationships may be bi-directional (19). Findings surrounding the role of a healthy diet are in line with recent evidence suggesting that healthier dietary patterns (e.g., a Mediterranean diet) are associated with better sleep quality (20). Lifestyle factors, such as alcohol consumption and diet, are arguably more amenable to self-management (thus “more” modifiable), compared with psychosocial factors, such as loneliness and mood, during periods of social restrictions. Therefore, results suggest a potential avenue for intervention to improve sleep quality during lockdowns or periods of self-isolation. Although physical activity can improve sleep quality (21), in the current study, due to lockdown measures, the limited variability in exercise levels may has precluded the assessment of such associations. Our results are also consistent with findings showing that individuals with pre-existing health conditions are more likely to experience sleep disturbances during the lockdown (22). Within the co-morbidities recorded in our study, patients with arthritis, COPD or a mental health disorder were more likely to report poor sleep during lockdown, highlighting possible vulnerabilities for these conditions. Our findings are novel in demonstrating that older adults who are at risk of complications related to COVID-19 are particularly susceptible to having poor sleep. Following the introduction of lockdown measures, good sleep quality was noted in high-risk participants who were married or cohabiting, had healthy diet, felt less lonely or anxious during lockdown (Supplementary Table 4). The protective effects of marriage/ cohabiting were previously observed in a younger sample, albeit without consideration of COVID-complications risk-status (22). Like our study, anxiety and depressive symptoms have been associated with worse sleep quality, potentially related to abnormalities of the hypothalamic-pituitary-adrenal axis and resulting hypercortisolism (23). Unhealthy diets have also been associated with poor sleep quality (11). Such results further highlight the adverse effects of social isolation, loneliness and unhealthy lifestyle with regards to their effects on sleep.
An important contribution of our data is the sex differences observed within the COVID-19CEV group, with sleep quality being influenced more by anxiety and loneliness in males and diet in females. The majority of COVID-19 studies report a disproportional impact of the pandemic on mental health (24) and sleep (6) among women. With respect to high-risk status for COVID-19 complications, our findings highlight that sleep may be more disrupted in CEV males reporting symptoms of anxiety and loneliness, suggesting that specific mental health interventions may be appropriate for this group. On the other hand, females in this high-risk group might benefit from dietary interventions promoting a healthier diet. Similar to our findings, but outside of COVID-lockdown, Jaussent et al. found gender differences in sleep/diet associations, with a Mediterranean diet being protective for sleep quality in women only (25). We did not ask participants to endorse which dietary pattern they followed, but instead whether they believed their diet was “healthy or not during lockdown.” Nonetheless, our results support targeted dietary modification in females at risk from COVID-19 complications. Taken together, these findings underscore the importance of understanding the role of moderating factors, such as sex and clinical vulnerability, in planning strategies for tailored public health intervention or promotion policies.
This study is not without limitations. These include the cross-sectional design (specifically addressing the period of the first lockdown), use of self-reported measures that may be vulnerable to participant bias, lack of data on sleep-related medications, and no objective sleep assessment and/or consensus sleep diary that might more accurately assess sleep quality. However, risk of bias due to cognitive impairment that may precipitate measurement error or data inaccuracies was mitigated as the CCRR study included cognitively healthy older adults from the CHARIOT register (13), thus unlikely to have had significant difficulty in completing the survey accurately. Another key limitation is related to selection bias. Collection of data via on-line surveys may have inadvertently excluded individuals beyond the digital divide. Furthermore, we did not assess coping strategies unlike other studies which found that resilience (i.e., increased adaptability to lockdown challenges) moderated the relationship between social isolation and sleep quality (26). Similarly, we did not record personality trait variables, such as emotional stability, intellect and extraversion, which were associated with improved psychosocial and behavioural outcomes during lockdown in the older old (27). Apart from furlough status, we also did not assess adverse life impacts of the pandemic on sleep quality (28). Furthermore, our sample is overwhelmingly white. Sleep differences have been observed between ethnic groups (29) and a recent report suggested that sleep in ethnic minority groups is disproportionately affected by COVID-19 and lockdown restrictions (30). However, their study considered all age groups over 16 years, did not distinguish those with and without “clinically extremely vulnerable” status and had no data on diet and other lifestyle factors. Future research exploring COVID/lockdown restrictions on sleep quality should aim to explore these factors, and interactions thereof, with ethnicity to better inform targeted public health intervention strategies.
Nonetheless, our study has several strengths. We have used a large sample of older adults, including vulnerable participants at high risk for COVID-19-related complications. Our study is rich in individual-level data, including demographic, lifestyle and psychosocial, as well as measurements of both cohabitant status and feelings of loneliness, and the use of well-validated scales. The retrospective assessment of sleep quality prior to lockdown further allowed evaluation of the specific effect of the pandemic on sleep quality.
In conclusion, the initial UK COVID-19 lockdown had an impact on sleep quality among older adults, with several lifestyle and psychosocial factors playing an important role, that differ by COVID-19CEV status and sex. Importantly, most factors assessed in this study are modifiable and could be targeted to improve sleep among older adults, including those at risk for COVID-19-related complications; through health promotion, psychosocial support, and clinical interventions such as cognitive behavioural therapy for insomnia (CBTi) and sleep hygiene. Within the at-risk group, males and females might benefit from targeted mental health and dietary interventions, respectively. Our findings may inform future interventional research and tailor policy to address poor sleep in older adults and those deemed “clinically extremely vulnerable.” Future work could expand on our research by assessing other vulnerability factors to sleep disruption and COVID-19-related complications, such as ethnicity and polypharmacy. Our next step will examine the longer-term impact of the distinct pandemic waves on sleep quality across distinct domains.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by data collected as in this study are anonymized and kept strictly confidential in accordance with the UK General Data Protection Regulations (2016). The CCRR study was ethically approved by the Imperial College London Joint Research Compliance Office (20IC5942) and by the Health Research Authority (16/EM/0213). The patients/participants provided their written informed consent to participate in this study.
CU-M conceived the sleep project, developed the sleep survey, conducted the analysis, and wrote the draught manuscript, with TW, SS, PG, and CR. SA-A and BZ advised on statistical methods. AW and JM supported with data cleaning and data management activities. CU-M, PG, CR, SA-A, CdJL, DS, TB, GP, CA, AM, AHM, HW, and LM developed the CCRR Study and are Study Investigators, with HW as Principal Investigator. All authors were involved in the interpretation of the results, as well as review and editing of the manuscript.
This study was sponsored by Imperial College London and partly funded by the Imperial College Healthcare Trust, NIHR Biomedical Research Centre.
LM reports clinical trial grants from Janssen R&D, Novartis and Takeda outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the CCRR Study research team, study participants and study sponsor—Imperial College London. This study was also supported by the Imperial College Healthcare NHS Trust—Biomedical Research centre (ICHT-BRC). TB and AM are supported by the NIHR Applied Research Collaboration (ARC) NW London. The views expressed in this article are those of the authors and not necessarily those of the ICHT-BRC, NIHR or the Department of Health and Social Care.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.753964/full#supplementary-material
1. Office of National Statistics. Deaths Due to COVID-19–Interactive Map 2021. (2021). Available online at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/articles/deathsduetocovid19interactivemap/2021-02-25 (accessed: May 18, 2021).
2. Majeed A, Molokhia M. Vaccinating the UK against covid-19. BMJ. (2020) 371:m4654. doi: 10.1136/bmj.m4654
3. Public Health England. Guidance on Shielding and Protecting People Who Are Clinically Extremely Vulnerable From COVID-19 202. (2021). Available online at: https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19#cev (accessed: May 18, 2021).
4. National Health Service. Coronavirus Shielded Patient List open data set, England 2021. (2021). Available online at: https://digital.nhs.uk/dashboards/shielded-patient-list-open-data-set (accessed: May 18, 2021).
5. Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. (2020) 11:6317. doi: 10.1038/s41467-020-19741-6
6. Jahrami H, BaHammam AS, Bragazzi NL, Saif Z, Faris M, Vitiello MV. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. (2021) 17:299–313. doi: 10.5664/jcsm.8930
7. Sindi S, Kåreholt I, Johansson L, Skoog J, Sjöberg L, Wang X, et al. Sleep disturbances and dementia risk: a multicenter study. Alzheimers Dement. (2018) 14:1235–42. doi: 10.1016/j.jalz.2018.05.012
8. Sindi S, Pérez LM, Vetrano DL, Triolo F, Kåreholt I, Sjöberg L, et al. Sleep disturbances and the speed of multimorbidity development in old age: results from a longitudinal population-based study. BMC Med. (2020) 18:382. doi: 10.1186/s12916-020-01846-w
9. Robb CE, de Jager CA, Ahmadi-Abhari S, Giannakopoulou P, Udeh-Momoh C, McKeand J, et al. Associations of social isolation with anxiety and depression during the early COVID-19 pandemic: a survey of older adults in London, UK. Front Psychiatry. (2020) 11:591120. doi: 10.3389/fpsyt.2020.591120
10. Yu B, Steptoe A, Niu K, Ku PW, Chen J, et al. Prospective associations of social isolation and loneliness with poor sleep quality in older adults. Qual Life Res. (2018) 27:683–91. doi: 10.1007/s11136-017-1752-9
11. Campanini MZ, Guallar-Castillón P, Rodríguez-Artalejo F, Lopez-Garcia E. Mediterranean diet and changes in sleep duration and indicators of sleep quality in older adults. Sleep. (2017) 40:zsw083. doi: 10.1093/sleep/zsw083
12. Wang F, Boros S. The effect of physical activity on sleep quality: a systematic review. Eur J Physiother. (2021) 23:11–18. doi: 10.1080/21679169.2019.1623314
13. Larsen ME, Curry L, Mastellos N, Robb C, Car J, Middleton LT. Development of the CHARIOT research register for the prevention of Alzheimer's dementia and other late onset neurodegenerative diseases. PLoS ONE. (2015) 10:e0141806. doi: 10.1371/journal.pone.0141806
14. Cleland C, Ferguson S, Ellis G, Hunter RF. Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol. (2018) 18:176. doi: 10.1186/s12874-018-0642-3
15. Carleton RN, Thibodeau MA, Teale MJ, Welch PG, Abrams MP, Robinson T, et al. The center for epidemiologic studies depression scale: a review with a theoretical and empirical examination of item content and factor structure. PLoS ONE. (2013) 8:e58067. doi: 10.1371/journal.pone.0058067
16. Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J. (1986) 292:344. doi: 10.1136/bmj.292.6516.344
17. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. (2016) 25:52–73. doi: 10.1016/j.smrv.2015.01.009
18. Robillard R, Dion K, Pennestri MH, Solomonova E, Lee E, Saad M, et al. Profiles of sleep changes during the COVID-19 pandemic: demographic, behavioural and psychological factors. J Sleep Res. (2021) 30:e13231. doi: 10.1111/jsr.13231
19. He S, Hasler BP, Chakravorty S. Alcohol and sleep-related problems. Curr Opin Psychol. (2019) 30:117–122. doi: 10.1016/j.copsyc.2019.03.007
20. Binks HE, Vincent G, Gupta C, Irwin C, Khalesi S. Effects of diet on sleep: a narrative review. Nutrients. (2020) 12:936. doi: 10.3390/nu12040936
21. Vanderlinden J, Boen F, van Uffelen JGZ. Effects of physical activity programs on sleep outcomes in older adults: a systematic review. Int J Behav Nutr Phys Act. (2020) 17:11. doi: 10.1186/s12966-020-0913-3
22. Gualano MR, Lo Moro G, Voglino G, Bert F, Siliquini R. Effects of Covid-19 lockdown on mental health and sleep disturbances in Italy. Int J Environ Res Public Health. (2020) 17:4779. doi: 10.3390/ijerph17134779
23. Tafet GE, Nemeroff CB. Pharmacological treatment of anxiety disorders: the role of the HPA axis. Front Psychiatry. (2020) 11:443. doi: 10.3389/fpsyt.2020.00443
24. Xiong J, Lipsitz O, Nasri F, Lui LMW, Gill H, Phan L, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. (2020) 277:55–64. doi: 10.1016/j.jad.2020.08.001
25. Jaussent I, Dauvilliers Y, Ancelin ML, Dartigues JF. Insomnia symptoms in older adults: associated factors and gender differences. Am J Geriatr Psychiatry. (2011) 19:88–97. doi: 10.1097/JGP.0b013e3181e049b6
26. Grossman ES, Hoffman YSG, Palgi Y, Shrira A. COVID-19 related loneliness and sleep problems in older adults: worries and resilience as potential moderators. Pers Individ Dif. (2021) 168:110371. doi: 10.1016/j.paid.2020.110371
27. Okely JA, Corley J, Welstead M, Taylor AM, Page D, Skarabela B, et al. Change in physical activity, sleep quality, and psychosocial variables during COVID-19 lockdown: evidence from the lothian birth cohort 1936. Int J Environ Res Public Health. (2021) 18:210. doi: 10.3390/ijerph18010210
28. Gao C, Scullin MK. Sleep health early in the coronavirus disease 2019 (COVID-19) outbreak in the United States: integrating longitudinal, cross-sectional, and retrospective recall data. Sleep Med. (2020) 73:1–10. doi: 10.1016/j.sleep.2020.06.032
29. Petrov ME, Lichstein KL. Differences in sleep between black and white adults: an update and future directions. Sleep Med. (2016) 18:74–81. doi: 10.1016/j.sleep.2015.01.011
Keywords: sleep quality, COVID-19 lockdown, clinically extremely vulnerable older adults, modifiable factors, sex differences
Citation: Udeh-Momoh CT, Watermeyer T, Sindi S, Giannakopoulou P, Robb CE, Ahmadi-Abhari S, Zheng B, Waheed A, McKeand J, Salman D, Beaney T, de Jager Loots CA, Price G, Atchison C, Car J, Majeed A, McGregor AH, Kivipelto M, Ward H and Middleton LT (2021) Health, Lifestyle, and Psycho-Social Determinants of Poor Sleep Quality During the Early Phase of the COVID-19 Pandemic: A Focus on UK Older Adults Deemed Clinically Extremely Vulnerable. Front. Public Health 9:753964. doi: 10.3389/fpubh.2021.753964
Received: 05 August 2021; Accepted: 22 September 2021;
Published: 28 October 2021.
Edited by:
Steven A. Cohen, University of Rhode Island, United StatesReviewed by:
Leland Waters, Virginia Commonwealth University, United StatesCopyright © 2021 Udeh-Momoh, Watermeyer, Sindi, Giannakopoulou, Robb, Ahmadi-Abhari, Zheng, Waheed, McKeand, Salman, Beaney, de Jager Loots, Price, Atchison, Car, Majeed, McGregor, Kivipelto, Ward and Middleton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chinedu T. Udeh-Momoh, Yy51ZGVoQGltcGVyaWFsLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.