
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health, 27 October 2021
Sec. Public Health Policy
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.750122
This article is part of the Research TopicThe Role of Healthcare Delivery, Payment & Policy Innovations in Decreasing the Global Burden of Chronic DiseaseView all 11 articles
Background: The benefits of prevention are widely recognized; ranging from avoiding disease onset to substantially reducing disease burden, which is especially relevant considering the increasing prevalence of chronic diseases. However, its delivery has encountered numerous obstacles in healthcare. While healthcare professionals play an important role in stimulating prevention, their behaviors can be influenced by incentives related to reimbursement schemes.
Purpose: The purpose of this research is to obtain a detailed description and explanation of how reimbursement schemes specifically impact primary, secondary, tertiary, and quaternary prevention.
Methods: Our study takes a mixed-methods approach. Based on a rapid review of the literature, we include and assess 27 studies. Moreover, we conducted semi-structured interviews with eight Dutch healthcare professionals and two representatives of insurance companies, to obtain a deeper understanding of healthcare professionals' behaviors in response to incentives.
Results: Nor fee-for-service (FFS) nor salary can be unambiguously linked to higher or lower provision of preventive services. However, results suggest that FFS's widely reported incentive to increase production might work in favor of preventive services such as immunizations but provide less incentives for chronic disease management. Salary's incentive toward prevention will be (partially) determined by provider-organization's characteristics and reimbursement. Pay-for-performance (P4P) is not always necessarily translated into better health outcomes, effective prevention, or adequate chronic disease management. P4P is considered disruptive by professionals and our results expose how it can lead professionals to resort to (over)medicalization in order to achieve targets. Relatively new forms of reimbursement such as population-based payment may incentivize professionals to adapt the delivery of care to facilitate the delivery of some forms of prevention.
Conclusion: There is not one reimbursement scheme that will stimulate all levels of prevention. Certain types of reimbursement work well for certain types of preventive care services. A volume incentive could be beneficial for prevention activities that are easy to specify. Population-based capitation can help promote preventive activities that require efforts that are not incentivized under other reimbursements, for instance activities that are not easily specified, such as providing education on lifestyle factors related to a patient's (chronic) disease.
Healthcare prevention, ranging from regular dental cleaning to collective initiatives to promote a healthier lifestyle, is one of the most important pillars of public health (1). Major gains in health can be accomplished through prevention (2). Moreover, prevention has the potential to substantially reduce disease's economic burden (3), especially in the current environment of growing chronic illness (4). Healthcare prevention focuses on promoting and protecting people's health by ensuring they receive care that conforms to their needs and stage of disease (5). While primary, secondary, and tertiary prevention focus on delivering care to avoid disease onset, allow early diagnose and reduce disease impact, respectively (6), quaternary prevention aims to protect patients from receiving redundant, unnecessary care (7). Healthcare professionals face the challenge of having to promptly assess a patient's need for preventive interventions (8, 9).
This crucial deliberation could, however, be disrupted by incentives in reimbursement systems (10). In healthcare systems with a purchaser-provider split, third-party funders such as insurers or the government can pay professionals for the services provided based on different types of reimbursement schemes (11). Reimbursement schemes can vary on many aspects, such as unit of payment (e.g., per service, per patient, or per day), payment amount, or timing (11). Different combinations of these characteristics are argued to create different (financial) incentives that promote or hinder professionals' behavior in everyday practice, e.g., providing less or more services than necessary (10, 11). As for pay-for-performance (P4P), one of its reported perverse incentives is that it might focus providers' attention to what is being measured, and consequently marginalize other quality criteria that are not being rewarded (12).
All-in-all, reimbursements have a well-documented reputation for incentivizing unwanted behavior. However, in the same train of thought, well-designed reimbursement schemes may allow the possibility to incentivize the behavior we do want, i.e., to focus professionals on prevention. Therefore, reimbursement schemes may play an important role in supporting meaningful prevention (13).
Much research has been devoted to investigating how various reimbursement schemes (and their respective incentives) impact healthcare delivery (11, 14–16). However, the existing body of literature still lacks comprehensive research on the impact of reimbursement schemes on professionals' behavior on all four levels of prevention. This study addresses this gap by incorporating evidence from both a rapid review and original empirical research, to address our research question: How do different types of reimbursement schemes in healthcare affect healthcare professionals' behavior in terms of the delivery of prevention?
To address our research question, we use a mixed-methods approach (17). We conduct both a rapid literature review for a broad overview of the literature and semi-structured interviews with healthcare professionals for more in-depth insights. In this section, we present the research methods.
We review the literature using a rapid review methodology. With more widely established systematic reviews, time and resource consumption may pose as barriers for its use in strategic decision making and health policy formulation. Rapid reviews are known for providing information on a specific research topic within a limited timeframe, applying systematic review methodology with explicitly stated shortcuts whilst maintaining rigorous methodology (18). The shortcuts applied to tailor our rapid review are specifically stated as the use of one database and one main reviewer. As progress toward universal health coverage should be informed by timely evidence, rapid reviews are an efficient approach to producing relevant evidence often to support decision-makers and strengthen health policies (19).
For our review, studies were systematically identified using the online database PubMed. Only English language scientific articles were considered for which full text was available, published from 2010 up until April 25th 2020, using the Pubmed “Humans” filter. Different search strings were tested using five previously identified relevant papers. To ensure that the relevant studies were included, the final search string was achieved based on the results of this testing. The search string can be found in the Supplementary Material.
The inclusion and exclusion criteria were defined a priori. All types of academic primary research studies (empirical studies and conceptual works) were considered. Editorials, systematic reviews, and studies reporting and/or commenting on data from other studies were excluded. The population of interest is healthcare professionals, broadly defined as qualified medical professionals who deliver primary, secondary, and/or tertiary healthcare services. Therefore, the search string contains various terms that are used to describe different types of healthcare professionals. As previously described, it is expected that reimbursement schemes and payment models induce a variety of behaviors in professionals, thus impacting the delivery of prevention. The phenomenon of interest comprises reimbursement schemes' effect on primary, secondary, tertiary and/or quaternary prevention. Professionals' behaviors expressed in process and/or outcome measures were included as long as it pertained to primary, secondary, tertiary and/or quaternary prevention. Studies that do not define the specific type of reimbursement under analysis were excluded, as well as studies that analyze the effect of reimbursement on prevention in combination with other interventions without isolating the effect of reimbursement. For example, a study from Kalwij et al. (20) examines the impact of financial incentives combined with practice-based support (audits and feedback) on performance and on screening behavior, instead of the isolated effect of financial incentives. This led to its exclusion during full text screening.
Our rapid review's search string yielded 3,591 papers from PubMed. Figure 1 illustrates the inclusion and exclusion process. One author (ES) screened titles and abstracts. This resulted in 75 papers eligible for full-text screening. Full-text screening led to the exclusion of 48 studies due to reasons such as type of reimbursement is not specified, or link to prevention is not clear. Each step in this process was discussed within the author team, before as well as during execution of each step. A total of 27 conceptual or empirical papers published between January 2010 and April 2020 were included for the qualitative synthesis, all related to the effect of reimbursement of healthcare professionals on delivery of prevention.
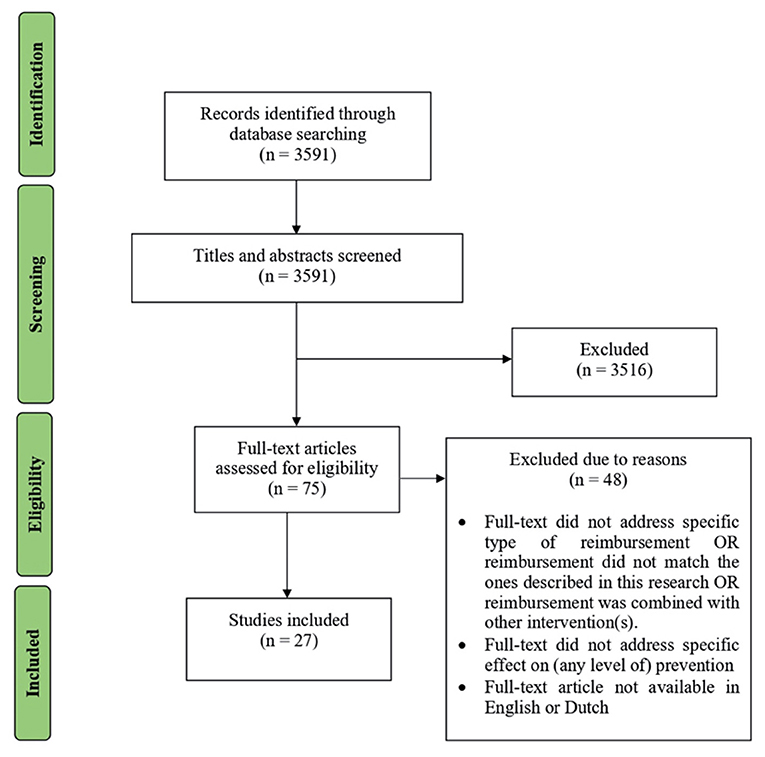
Figure 1. Flow diagram of inclusion and exclusion process based on PRISMA flow diagram (21).
Two authors (HE and ER) provided feedback and assisted in calibrating eligibility criteria, screening and selecting papers, and cross-checking data extraction. The relevant data from all included studies were extracted and collected in an Excel spreadsheet. These results were posteriorly synthesized according to the type of reimbursement and prevention level(s) addressed and analyzed in terms of the relationships between reimbursement types and preventive behaviors.
We conducted semi-structured interviews to further our understanding of the subject matter. We chose this qualitative method to collect in-depth data and capture meanings and perceptions people attribute to a certain phenomenon (22). In conjunction with the rapid nature of our research and limited by COVID-19 restrictions, our number of interview participants is limited. In total, ten semi-structured in-depth interviews were conducted with eight Dutch healthcare professionals—consisting of four general practitioners (GP) and four physical therapists (PT)—as well as two members of the prevention and purchasing departments of Dutch insurance companies (representing the payers in the Dutch healthcare system). An overview of respondents' characteristics can be found in the Supplementary Material.
In the Netherlands, GP practices are reimbursed through a 3-segment funding model. In the first segment, GPs are reimbursed through a mix of capitation and FFS for primary care activities provided. The second segment consists of funding through episode-based payments where GPs receive a fixed fee for every patient for which they provide multidisciplinary care such as diabetes care and other selected chronic diseases. In segment 3, GPs and insurance companies have the opportunity to negotiate additional P4P contracts and GPs become eligible to receive a bonus for reaching certain outcomes (at practice level) pertaining to the care delivered in segments 1 and 2.
Physical therapy in the Netherlands is reimbursed under FFS, in other words, paid according to the number of physical therapy sessions. Practices are free to make specific agreements with purchasers regarding delivery of care, volume or outcomes in exchange for financial rewards. Professionals under different types of reimbursement were purposely selected and answers were analyzed according to the type of reimbursement.
All semi-structured interviews were held between March and April 2020 and were conducted via telephone due to the COVID-19 outbreak. Interviews were recorded and transcribed verbatim in Dutch and the quotes used in this manuscript were posteriorly translated to English. Interviews were coded and analyzed using software ATLAS.ti, version 8.4.4. The interview transcripts were analyzed first using open coding and subsequently using axial coding to integrate codes into categories and identify relationships between categories. Examples of codes include “Obstacles for prevention,” “Efforts made toward prevention,” “Perceptions about own role in prevention” and “Strategies to mitigate overmedicalization.” The code group list can be found in the Supplementary Material.
As previously mentioned, qualitative research allows for the collection of in-depth data such as perspectives and perceptions; elements that would be much more difficult to obtain from quantitative data (22). However, these essential elements that provide an extra dimension and enrich findings of qualitative research are also the subject of controversies regarding quality and trustworthiness of its results. Therefore, besides the reliance on multiple research methods, we have incorporated other strategies in this research to enhance its validity and reliability. Concerning the empirical part of this research, interviews were recorded to ensure descriptive validity and increase reliability. The use of in-depth open interviews helps mitigate interpretation bias and consequently increase internal validity. Interviews were transcribed verbatim and transcripts were made available to increase internal reliability. The use of a topic list to guide interviews helped mitigate researcher bias regarding assumptions or beliefs that might otherwise have compromised validity. The topic list can be found in the Appendix. The interviewing process as well as data collection, analysis and interpretation steps were discussed between the authors and are described in detail allowing for a well-documented audit-trail of materials and processes. Regarding our rapid review, we tested different search strings in order to find the search string that yields as many relevant studies and thus achieve higher sensitivity.
In this section, the research findings are presented. We present the rapid review's findings organized by type of reimbursement scheme and complement these with relevant findings from the semi-structured interviews.
The rapid review yielded 27 studies; their respective characteristics are presented in more detail in Table 1. Another table with more detailed information about the included studies can be found in the Supplementary Material. The reviewed studies were executed in a variety of countries and delivery models. More than half (14 out of 27) in a country with a National Health Insurance (NHI) model: eight in Canada (27, 29, 35, 36, 38, 40, 41, 46), five in Taiwan (24, 25, 31, 37, 44), and one in Rwanda (28). A further five in a country with a Beveridge model: four from the UK (34, 39, 43, 48) and one from Italy (32). Four studies were executed in the US, with their focus varying from publicly funded safety-net community health centers (30), Medicaid-focused managed care (26), a commercial health plan (23) to a cross-sector study (45). Two studies in a country with a Bismarck model: one in Estonia (42) and one in France (49). One study was executed in Mozambique and concerned a donor-sponsored program (47). Finally, one study (33) analyzed data from 14 different European countries, including countries with a Bismarck model (such as The Netherlands) and countries with a Beveridge model (such as Sweden).
As presented in Table 1, a total of 20 studies focus on the relationship between P4P bonuses and prevention (23, 24, 26, 28, 30–32, 34, 35, 37–40, 42–44, 46–49). Seven papers study P4P incentives awarded at practice level (26, 28, 34, 39, 43, 47, 48), from which four studies pertain to the Quality and Outcome Framework (QOF) P4P program in the UK, where incentives represent up to 25% of annual income (34, 39, 43, 48). In one study, an extra bonus is awarded to practices that, besides achieving the targets, also manage to do this within a short period of time (26).
The remaining 13 studies on P4P consider bonuses directed at individual professionals (23, 24, 30–32, 35, 37, 38, 40, 42, 44, 46, 49), from which four studies specify that incentives represent between 2 and 4% of professional's income (23, 30, 35, 42) and 1 < 10% (40). Two studies (31, 44) consider a program where an additional bonus (on top of P4P for achieving targets) is awarded to professionals who rank in the top 25%.
From all 20 studies considering P4P, eight studies do not further specify bonus characteristics besides bonus amount and if directed at practice or at individual level (24, 28, 32, 37, 38, 46, 47, 49).
From the remaining seven studies that do not address P4P (25, 27, 29, 33, 36, 41, 45), four studies compare the impact of multiple payment models (FFS vs. salary vs. capitation) (27, 33, 41), or different blends of FFS, capitation and incentives (36), on professionals' behavior toward prevention. One study evaluates the effect of (different levels of) capitated payments (45), another studies a mix of per-diem reimbursement with FFS as an alternative to pure FFS (29), and one other study compares episode-based payments to FFS (25). In these seven studies, FFS reimbursement is used as the benchmark against which other payment models are compared with respect to one or more outcome measures that capture preventive behaviors.
From the 27 studies, the majority (n=16) pertains exclusively to the preventive behaviors of primary care professionals/practices (27, 30, 32, 34–36, 38–43, 45, 46, 48, 49), while the remaining 11 studies pertain to either a hospital setting (25, 29), multiple settings (23, 24, 31, 33, 37, 44) or do not specify the setting (26, 28, 47).
A total of 12 studies focus on chronic disease management (23, 24, 31, 32, 34, 36–39, 41, 44, 48). While 11 further studies consider preventive care such as screenings (28, 35, 36, 46, 49), immunizations (26, 42) or both (27, 30, 33, 40). The two studies pertaining to hospital care in general (25, 29) are labeled under secondary and tertiary prevention. Two studies focus on activities that correspond to primary, secondary and tertiary levels of prevention (45, 47). From the 27 included studies only one study explicitly addresses quaternary prevention (43).
Both per-diem (29) and episode-based payment (25) are only considered by one study each. Neither of these studies, nor our own semi-structured interviews yielded conclusive support for the claim that these types of reimbursement impacted prevention. Echevin and Fortin (29) observe that adding a per-diem fee in 14 departments at a hospital in Quebec (Canada) increased the average length of stay but had ultimately no impact on the delivery of preventive care. Besides this, none of our interviewees is reimbursed on a per-diem basis, therefore no original empirical evidence was collected on this reimbursement through the interviews of our study. Concerning episode-based reimbursement, Cheng et al. (25) conclude that the effect of DRG (diagnosis-related group) payment in 486 Taiwanese hospitals had no significant impact on healthcare preventable adverse outcomes after discharge. As for empirical findings, our interviewed GPs have experience with episode-based reimbursement in primary care, specifically for chronic disease management. Although interviewed GPs are positive about these programs, the difference in type of episode-based reimbursement (hospital vs. primary care) makes it challenging to draw reliable conclusions on this type of reimbursement.
The remainder of the section focusses on the relation between levels of prevention and the better-documented reimbursement systems: FFS (including multiple payment models FFS vs. salary vs. capitation or different blends of FFS) and P4P, respectively. We wish to stress that the included studies vary greatly in design, which affects the extent to which the results may be interpreted as causal or correlative. Clearly, we do not claim that e.g., “the results of cross-sectional studies are by definition correlative” or that “(quasi-)experiments always facilitate causal conclusions”. The causal nature of the results is not always clear to the reader of these studies, us as reviewers included. To inform our analyses, we make mention of study designs in our synthesis of prior research and our own primary research below.
Most of the included papers in our review describe FFS-based reimbursement, sometimes in combination with capitation and/or salary-based reimbursement. First, we discuss our findings on FFS vs. salary vs. capitation on primary and secondary prevention.
In a cross-sectional study, Jusot et al. (33) report that FFS is associated with a higher delivery of primary and secondary preventive services (specifically, immunization, and screenings) compared to salary and capitation, suggesting that under FFS, professionals have incentives to increase service volume. These results are coherent with what interview respondents report about FFS incentives. One of the interviewed healthcare purchasing specialists believes that preventive activities that are reimbursed through FFS, such as immunizations, will be stimulated under this reimbursement scheme. Two professionals under FFS acknowledge the incentive to increase production of the reimbursed service as this will lead to increased revenue and cited that when preventive activities, such as patient education, are not reimbursable through a fee, this will act as a disincentive for that type of prevention.
On the other hand, in a longitudinal study and a cross-sectional study respectively, Kiran et al. (36) and Dahrouge et al. (27) find no statistically significant differences between these payment models pertaining to screening (27, 36) and immunizations (27). Both studies suggest that the practice's structure (number of enrolled patients per full-time equivalent GP) and organizational factors (such as working with electronic reminders or team-based care) could be stronger determinants for the delivery of preventive services. Accordingly, lack of time was recurrently mentioned during our interviews (by both salaried professionals and professional under FFS) as a reason for not addressing prevention. Two salaried respondents (one GP and one PT) believe that their provider organization plays a crucial role in stimulating prevention at practice-level by making the necessary resources available for professionals to be able to focus their efforts on prevention, such as extending the length of consultations. Salaried respondents claim they would be open to invest more time in prevention, but the perceived pressure from the provider organization (reimbursed under FFS) to generate revenue is hampering prevention, as one PT illustrates: “I think it depends on your employer and their vision [.] and whether or not they want to stimulate certain things [.] The fact that my schedule is overloaded is because [employers] have certain ideas on how they want to organize things making them less flexible [.] and this will ultimately compromise quality of care”. Consistent with this statement, two professionals under FFS demonstrate no desire to increase consultation length (as the reimbursed service pertains to a consultation with a predefined length) nor regard this as an important enough obstacle for prevention that needs to be overcome. GPs reimbursed under a mix of capitation and FFS regard the responsibility placed on them for providing primary prevention as unrealistic. The GP does not think it is feasible to extend consultation length and spend (more) time addressing prevention during consultations, suggesting that in order to stimulate prevention in healthcare, other entities such as the municipal health services should be made responsible for addressing prevention. This way, GPs can focus on curative tasks and not patient education: “As a GP I would really like to apply my medical knowledge and since obesity is a big social problem, I think I would be seeing people all day long and discussing how we are going to tackle someone's obesity. Well, I don't think I would want to do that, no.”
On the other hand, an interviewed GP recently changed reimbursement from the mixed capitation and FFS to a population-based capitated payment regarding the first segment of GP care. According to this GP, this shift removed the incentive for (over)production. As the provider no longer profits from providing more consultations, this led this GP to extend the consultations' length: “Now I know what I earn per quarter; it no longer depends on how often I see my patients. So, I choose to take more time for my patients because I don't have to see thirty patients a day to earn my living […]. What we notice is that we no longer, or less often, have to book double appointments […]. And that we have just enough time to approach [prevention]. At first, I was skeptical about it, because you feel that you are losing money by not being able to claim your consultations. But if I compare my practice's finances with those from practices under the traditional reimbursement, I realize that we are definitely not in a bad position financially.”
Concerning disease management, Kiran et al. (36) found that GPs' reimbursement with a greater percentage of FFS presented the lowest improvements in diabetes management while reimbursement mostly composed out of capitated payments achieved the largest improvements in diabetes care. With reference to our own empirical research, one respondent PT believes that FFS hinders prevention by reimbursing professionals for every service provided but with no further incentive to avoid disease development, explore potential risk factors that might be the underlying reason for the patient's health complaint or prevent deterioration of a health condition. Similar to Kiran et al. (36), Liddy et al. (41) observe in a cross-sectional study that practices under FFS showed the greatest gaps in adherence to evidence-based guidelines pertaining to cardiovascular disease care. Capitation and salary were similar to each other in results; While salaried GPs scored significantly higher on glucose level control, capitation was linked to increased weight management and smoking cessation drug prescription when compared to FFS and salary. Pearson's et al. (45) cross-sectional study established that GPs for whom >75% of reimbursement consisted of capitation relative to FFS were three times more likely to provide patient education. In sum, salary, and even more so capitation, rather than FFS, appear to be related to better disease management.
Our rapid review yielded no results on quaternary prevention under FFS, salary nor capitation. Nevertheless, our interviews suggest that overmedicalization is still prevalent in healthcare. When asked about overmedicalization, respondents under these three reimbursement schemes believe that it is mostly driven by patient demand, not by reimbursement, and claim they run responsible practices as overprovision might have consequences for the patient's health and healthcare expenditure: “We are always critical about what is necessary, what is medically indicated.” However, all professionals acknowledge that in some circumstances they might (partially) give in to patients' demands, as a salaried GP illustrates: “I also try to negotiate a little bit [.] but yeah, I'm not saying I don't do it. Because you also have a future with that patient, in your doctor-patient relationship. [.] I never give [prescription for blood test] without explaining very clearly what you can and what you cannot get with it [.] Because some things just have consequences [.] and then you enter into an unnecessary medicalization process.”
In the remainder of this section, we present our findings on P4P-based reimbursement in relation to the levels of prevention. First, we discuss primary and secondary prevention.
In an interrupted times series study, Chien et al. (26) examines the effect of a piece-rate P4P bonus for full and timely childhood immunization. Results show that immunization within the P4P program increased at a significantly higher rate than the comparison group. Similarly, Merilind et al. (42) reveals that GPs under P4P achieved the target of 90% coverage rate for all vaccinations while the comparison group only achieved the target rate for one vaccination. Both studies suggest that P4P schemes with a bonus specifically for immunizations can improve immunization rates. Pendrith et al. (46) observed that FFS on its own provided low incentives for the delivery of cancer screening and that a P4P bonus for achieving 60% or 80% screening rates for three types of cancer combined with FFS lead to an increased provision of these screenings. The combination of capitation and the same P4P bonus also presented higher screening rates in comparison to FFS, leading authors to suggest that adding these P4P incentives was associated with higher cancer screening rates. Conversely to these findings, Kiran et al. (35) observed that despite the increase in billing for self-reported provision of cancer screenings leading to larger expenditures there was little or no significant increase in cancer screening rates after the introduction of P4P bonuses for the achievement of different targets in screening rates. Similarly, two other studies observed that cancer screening rates (30) and immunization rates (30, 40) did not suffer significant changes after implementation of a P4P bonus among GPs for the achievement of targets pertaining to the delivery of these preventive services. Both studies hypothesize that these incentives representing 3–4% (30) and <10% of annual income (40) might have been too small to induce the desired changes in practice. Besides this, authors suggest that other aspects such as provider training (30) and lack of provider reminder systems (35) could impact performance. Li et al. (40) question P4P's effect on the quality of (preventive) care and suggest that the introduction of five different indicators simultaneously might decrease the likelihood of physician's response to any of them. Further research on why and how P4P's design features can help increase professionals' response is required (40).
Different studies provide different interpretations of how P4P's components influence professionals' performance. De Walque's et al. (28) results reveal that P4P higher incentives (US$4.59) had a greater and significant impact on indicators such as couples HIV testing, compared to lower incentives (US$0.92) for individual HIV testing. The latter shows little or no significant effect on professionals' performance. In line with De Walque et al. (28), Sicsic and Franc's (49) study observes little impact of a P4P program among French GPs on breast cancer screening rates, concluding that the “low-powered” financial incentive (maximum €245/target) did not have enough leverage to stimulate providers. Contrastingly, Rajkotia et al. (47) propose that practices are not necessarily more responsive to more profitable indicators (such as the survival rate after treatment of HIV-infected children with a $11.20 reward) than to less profitable ones ($4.20), but instead prioritize targets that can be achieved with a lower level of effort, in this case the number of HIV-infected pregnant women initiating antiretroviral therapy ($10 reward) or the number of family planning consultations given to HIV-infected women ($5 reward). Taken together, these studies suggest that both bonus magnitude (28, 30, 35, 40, 49) and required effort (30, 47) are important components in a P4P scheme. Our interviews did not produce results pertaining to the effect of P4P on primary and secondary prevention.
Regarding the effect of P4P on disease management, two studies reveal that Taiwanese diabetes mellitus (DM) patients of P4P-enrolled GPs had higher continuity of care and lower mortality rates (44) and were more likely to receive the P4P-rewarded guideline-recommended DM examinations than patients treated by non-P4P GPs, as presented in a cross-sectional study by Lai and Hou (37). Two longitudinal cohort studies on cardiovascular disease management (23) and diabetes management (32) observe that financially incentivizing disease management check-ups and treatments also resulted in fewer (avoidable) hospitalizations. Chen et al. (23) suggest that P4P success might be due to an easily achievable target concerning the percentage of patients receiving improved quality of care and reaching positive health outcomes for cardiovascular disease. The low baseline rate for improvement (42%) stimulated participation in the P4P program. Hsieh et al. (31) observe that rewarding professionals for process indicators (e.g., control visits and cholesterol and glucose testing) led to no difference in the number of visits nor tests performed. When outcome indicators rewarding improvement in clinical levels (e.g., cholesterol levels) were added to the P4P program, quality of care improved.
Serumaga's et al. (48) interrupted time series study found that rewarding blood pressure control and drug prescription for hypertension disease management did not increase the delivery of these services in a clinically or statistically significant manner, nor were there changes in mortality rates or other hypertension related adverse outcomes. The authors suggest that blood pressure control had already improved before the implementation of P4P and that P4P targets might have been set too low for significant change to take place. Similarly, the results of both Lee et al. (39) and Chen et al. (24) show that the delivery of secondary and tertiary preventive services did not significantly vary between the P4P and non-P4P groups. Chen et al. (24) cited the small bonus size ($3-$30/service/per patient) and low provider participation as probable explanations for low behavioral change in the delivery of three guideline-recommended preventive services and test for Hepatitis B and Hepatitis C patients. Lee et al. (39) stated that future P4P programs rewarding blood pressure and cholesterol level controls should include achievable but at the same time challenging targets to create enough leverage among different practices.
Two of our interviewed GPs believe that performance targets set by the healthcare purchaser limit their professional autonomy and control the way care is delivered without taking other factors into consideration: “The healthcare purchaser imposes targets on me that I must meet, which may not be feasible at all for a great part of my patients because I have many elderly people or immigrants, for example, and then I have a problem.” Another GP adds: “If I prescribe expensive medication once, it is probably because there is medical necessity, which is never accepted (by the purchaser), because then there are again twenty-six conditions that it must meet.” The interviewees also believe these targets are set primarily in order to reduce healthcare costs and not to increase quality.
In a P4P program financially rewarding providers for conducting all recommended diabetes management actions, LeBlanc et al. (38) conclude that although patients of GPs claiming the bonus received more glucose tests per year and had consequently better GP follow-up, this was ultimately not translated into lower glucose levels for those patients relative to the comparison group. Similarly, Karunaratne et al. (34) prospective longitudinal cohort study examines the management of chronic kidney disease in primary care before and after implementation of P4P bonuses for initial and ongoing management actions such as blood pressure measurement and control. Contrary to Serumaga's et al. (48) findings, in this case blood pressure measurement and prescription medication significantly increased as did costs associated with increased prescribing. However, Karunaratne et al. (34) cannot establish if these events consequently resulted in improved health outcomes, delayed disease progression or decreased mortality. Similarly, in our empirical research, two respondent GPs question the quality improvement incentive P4P programs aims to induce and raised some concerns regarding such incentives of P4P: “You can also ask yourself whether that [P4P] really improves quality, because you mainly measure whether people have been seen [by the doctor], but whether you will take action or do something with those [blood test] results is the real question.”
Norman's et al. (43) interview study with GPs is the only study in our research that explicitly investigates P4P's impact on quaternary prevention. GPs under P4P revealed experiencing an incentive to reduce any risk factor and prophylactically treat patients as otherwise it might disturb P4P target achievement. Also, Norman et al. (43) report that GPs acknowledged opting to medicate patients rather than trying non-pharmacological approaches simply to be able to timely achieve P4P targets, and additionally acknowledged that even when indicators went against GPs' inner values, they still complied and strived to achieve targets. These reported incentives are acknowledged by two of our own respondent GPs who disagree with the implementation of P4P and worry that money may become the incentive for action, adding: “Because then we will do things because you get money for it and not because you actually want to work that way”.
Our research collected empirical evidence on the relationships between different reimbursement schemes and four levels of prevention. Most of our results relate to P4P, FFS, capitation and salary. We also obtained results on both per-diem and episode-based payment, however neither the rapid review nor our own empirical research could confirm the impact of these types of reimbursements on prevention. Quaternary prevention was addressed by one study only. The integration of findings from both the rapid review and our original empirical research allows us to draw the following main discussion points regarding reimbursement schemes and prevention.
The findings of our rapid review on the impact of salary-based reimbursement are ambiguous, associating salary to both higher and lower delivery of preventive services compared to FFS and capitation (33, 41). In previous literature, while Gosden et al. (50) claims salaried professionals want to minimize their personal efforts, Kane et al. (4) proposes that these professionals could be incentivized to engage in preventive care more than professionals reimbursed under FFS. An obstacle experienced by salaried respondents are the incentives from the employer organization and not making the necessary resources available to stimulate prevention, suggesting that the reimbursement of the provider organization or practice might interfere with salaried professionals' behavior toward prevention. Therefore, it should be taken into consideration that these different interactions between the professional and the provider organization can align or misalign incentives which might impact prevention in practice.
Concerning FFS, although ambiguous, results suggested that its widely reported incentive to increase production might work in favor of preventive services such as immunizations, eye exams and screening for cancer (33). The corollary is that non-reimbursed activities, longer consultations or less consultations might evoke feelings of loss for these professionals, according to empirical findings. In fact, interviewees acknowledged the fact that when prevention is not as widely reimbursed in fees this poses as an obstacle for the provision of preventive services. More than a decade ago, Ellis and Miller (14) already proposed that activities not reimbursed through fees (such as preventive counseling) can be neglected under FFS. Results also suggest that FFS restricts the delivery of care to predefined standards and does not allow the flexibility to organize care delivery differently, as some forms of prevention might require. Therefore, FFS might work for some forms of prevention, in particular when the prevention activity can be specified as reimbursable under FFS (such as immunizations), but it can be questioned whether it will stimulate professionals to address prevention when the preventive activity requires efforts/services that are not reimbursed through a fee.
Both PTs and GPs state that, as the patient's medical complaint receives primary attention during consultations and sometimes the consultation is even too short to address the complaint in detail, there is usually not enough time left to address risk factors and address secondary and tertiary prevention. However, opinions of GPs under population-based capitated payment contrast with those of GPs under traditional capitation and FFS reimbursement. A GP under traditional reimbursement considers the responsibility to deliver preventive services by GPs to be unrealistic and therefore does not feel increasing consultation length to be necessary. Instead, the responsibility for prevention should be placed elsewhere. A focus on revenue and profit might be a reason to increase the number of consultations per day and no desire to extend consultation length. On the other hand, GPs under population-based capitation report having altered the delivery of care to facilitate the delivery of prevention through extending consultation length. This is due to a bigger focus on prevention as a cost reduction strategy from capitation incentives and a reduced volume incentive with the elimination of FFS.
Although respondents show that they are aware of the importance of quaternary prevention, they acknowledge that overmedicalization still persists in healthcare due to patients' demands. In case of time constraints, professionals might be likely to resort to unnecessary prescriptions or referrals in detriment of providing important information, comprehensively discussing alternative approaches that mitigate overmedicalization, compromising quaternary prevention.
Both our rapid review and our interviews' ambiguous findings question P4P's promise to improve healthcare quality through better prevention. Studies show that the achievement of targets for performing disease management examinations is not necessarily translated into better patient outcomes or effective secondary and tertiary prevention (34, 38). Similarly, Flodgren et al. (51) already concluded in their systematic review that P4P effectively managed to change professional's practice, however no effect on patient outcomes is subsequently observed. Empirical results also report how P4P's metrics might steer professional's behavior and how this might conflict with what is best for the patient. The risk of losing a bonus might trigger professionals to circumvent factors standing in the way of achieving targets. The results of our rapid review underscore the importance of different P4P design features in stimulating behavior. However, the role of bonus size and level of effort in responsiveness to an indicator are discordantly described. While Gavagan et al. (30), Li et al. (40), De Walque et al. (28), Chen et al. (24), and Sicsic and Franc (49) cite the importance of bonus size, Rajkotia et al. (47) claim that the level of effort necessary to reach a target is the most important determinant of behavior. Serumaga et al. (48) and Chen et al. (23) claim that targets should be low enough to motivate professionals while Chen et al. (24) and Lee et al. (39) report that, in order to make P4P cost-effective, targets should not be set too low and easy to achieve. On the other hand, Hsieh et al. (31) claim that only when incentives for outcome indicators are added to the P4P program, improvements in quality of care are observed. Taken together, these studies suggest that bonus magnitude, required effort and type of indicator are important components in a P4P scheme. Even though our research did not further investigate the role of these components, our results suggest this should be considered as it most likely will influence a reimbursement's effectiveness toward prevention.
Regarding quaternary prevention, Norman et al. (43) show how P4P can lead professionals to resort to (over)medicalization to achieve targets, compromising this level of prevention. Karunaratne et al. (34) show that P4P leads to a rise in medication prescription and costs related to increased prescription with no further improvement in health outcomes. In line with these results, interviewees are critical about P4P and worry that money may become the incentive for action, and this may “crowd out” intrinsic motivation to deliver efficiency and quality in healthcare. While P4P is promoted by purchasers, it is considered disruptive by professionals, who suggest it might trigger different unintended behaviors in professionals that ultimately hinder effective prevention at different levels.
Both per-diem and episode-based payment are only considered by one study each and neither can claim these reimbursement schemes impact prevention. None of our interviewees has experience with per-diem reimbursement. Interviewees stated that primary care episode-based payment creates incentives to better organize disease management and actively monitor patients so as to avoid complications from which the provider could incur additional costs. This type of episode-based payment is a form of prospective reimbursement, with revenues known upfront and hence there is a strong incentive for cost avoidance.
The benefits of preventive health services are widely recognized. However, delivery of prevention services encounters numerous obstacles in healthcare. It is argued that reimbursement schemes play an important role in both hindering and stimulating the provision of healthcare services (13). Nevertheless, there has been little focus on how reimbursement schemes could specifically contribute to the delivery of preventive health services.
Our research provides insights into how different types of reimbursement (e.g., fee-for-service, or pay-for-performance) impact healthcare professionals' behavior; stimulating or hindering their efforts to address prevention. We distinguish between four levels of prevention, ranging from avoiding disease onset, allowing early diagnose and reducing disease impact to protecting patients from receiving redundant, unnecessary care. We find that not one ideal reimbursement scheme exists, providing incentives that stimulate (or hinder) prevention at all its levels. There are, however, certain types of reimbursements that work well for certain types of preventive care services. For example, the volume incentive from FFS could be beneficial for some levels of prevention when clearly specified preventive actions are concerned (such as immunization, as an example of primary prevention or screenings as an example of secondary prevention). On the other hand, population based capitated reimbursement might facilitate the delivery of some forms of prevention that are more difficult to specify as a reimbursable service, or for which lack of time poses as an obstacle under other reimbursement schemes, allowing the flexibility to alter the delivery of care. We also discuss P4P, as this is prominent in both our literature review as well as amongst our interviewees. As our study empirically reported, P4P's incentives might have unintended consequences for professionals' intrinsic motivation. P4P's criteria for medication prescription is an example of how what is being measured and therefore reimbursed for could influence a professional's practice away from what they initially intended. Additionally, the achievement of P4P's targets does not always imply better health outcomes (34, 38). Besides this, our study also describes how the pressure to (timely) achieve targets arising from this type of reimbursement can lead professionals to resort to (over)medicalization and discard other approaches that could better fit the patient's needs, compromising quaternary prevention (43).
The strength of our study lies in the incorporation of evidence from both a rapid review of the literature and interviews with professionals to help consolidate results and achieve a more comprehensive explanation of how reimbursement schemes can affect prevention. Furthermore, this research raises awareness on overmedicalization by contemplating quaternary prevention as the fourth level of prevention, contributing with a more overarching definition of prevention. Although efforts were made to mitigate bias, this research has a few limitations which will be outlined in this section. These limitations could provide additional guidance for future research.
First regarding the rapid review. Despite efforts to test and strengthen our search strategies, it is entirely possible that studies to prevention have unintentionally been omitted, due to them not being described with the term “prevention.” Quaternary prevention is still a relatively new concept and therefore strategies to reduce medical overuse might not be perceived and labeled as (a level of) prevention. In addition, despite the advantages of rapid review methodology, drawbacks must be acknowledged, i.e., the process of data collection, selection and analysis was primarily performed by one reviewer. This may have compromised the study selection procedure and consequently the reliability of results. Also, unlike more traditional systematic literature reviews, rapid reviews rely on narrative analysis and synthesis rather than meta-analysis of the included studies (18). Although we argue that our rapid review fits our research aim well, a more traditional systematic approach would be better suited for more detailed and quantitatively sophisticated meta-analysis of the effectiveness of payment models in relation to e.g., case mix of patients. Meta-analysis would also allow for further assessment of the quality of the results and the level of evidence provided by the included studies.
As addressed by some studies included in our review, the effectiveness of any reimbursement scheme is likely to be affected by the generosity of the payment and not solely the type of reimbursement. However, not all studies from our rapid review disclose the reimbursed amounts in analogous ways, making it impossible to compare payment generosity and draw conclusions on this matter. Therefore, we did not investigate this matter in our review which could have provided additional and valuable insights to our research. We recognize this as a limitation of our study.
We acknowledge that there may be various other factors that interact with reimbursement scheme to impact delivery of prevention services. These can be factors related to the healthcare delivery model of a country, the level of investment in healthcare in a country, social, economic or cultural differences between countries, or differences between countries or regions in support structures offered to healthcare professionals other than reimbursement. We summarize the evidence at a relatively high level of abstraction, between reimbursement scheme and delivery of prevention services only, and cannot account for all differences between countries. Moreover, our rapid review is skewed toward studies executed in OECD countries, more specifically North America, and countries with a National Health Insurance (NHI) model. Jusot et al. (33) have shown, in their 14-country study, that factors related to reimbursement were most strongly related to utilization of preventive services, with other system-level factors, like capacity or structure, playing a lesser role. This lends credibility to our assumption that reimbursement scheme is a very important factor, even if it may interact with other factors. Still, looking at reimbursement separately is a limitation, and we recommend future research to also study interactions with other factors.
Concerning the empirical research, interviews were planned to be conducted face-to-face, however, due to local restrictions regarding the COVID-19 outbreak at the time of this study's data collection, interviews had to be conducted via telephone. Furthermore, some of the initially targeted respondents had to cancel their participation. The initial intention was to conceptualize a “theoretical sample” by means of literature review and subsequently select respondents based on that theoretical sample. Both research setting and respondents had to be rearranged in a short period of time and respondents had to be recruited through “convenience sampling” and “snowball sampling” (52), limiting the opportunities to draw a varied sample of respondents as initially intended. Medical specialists and patients, who could have added valuable insights, were not interviewed. Due to the qualitative research design and convenience sampling of a limited number of respondents, this research could lack representativeness and external validity.
Neither the rapid review nor the empirical research provided ample insights on all relevant reimbursement schemes. On the one hand because our sample did not include respondents who experienced all types of reimbursement, and secondly because the rapid review identified relatively many studies on certain reimbursement schemes but less so on others. Future research should target also other respondent samples for a more comprehensive understanding of how reimbursement may affect prevention. Besides this, most respondents were paid under a mix of reimbursements which makes it difficult to assess their isolated effect and can compromise results.
The fact that healthcare professionals' behaviors might be stimulated or hindered by incentives from different types of reimbursement schemes could be regarded as in conflict with the oath of ethics concerning non-maleficence. During the interviews it was noticeable that some respondents were more hesitant to talk about possible incentives altering their behavior in terms of under- and/or overprovision of care and might have held back valuable information.
Finally, our rapid review identified only one study on quaternary prevention and more research is needed on this level of prevention and how different reimbursement schemes impact quaternary prevention.
EZ conceived the idea, performed the review and interviews, and wrote the first draft of the manuscript. ER and HE assisted the rapid review and contributed to the data interpretation process. All authors wrote and edited the manuscript and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the participants in this study. An earlier version of this paper has been presented at the HSMO ScienceClub at Erasmus University Rotterdam. We thank the participants for their constructive comments. We would especially like to thank Dr. Jeroen van Wijngaarden, Pieter Vandekerckhove, and Dr. Sandra Sülz for their constructive and insightful feedback on the manuscript leading to the improvement of our work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.750122/full#supplementary-material
1. Batarseh FA, Ghassib I, Chong D, Su PH. Preventive healthcare policies in the US: solutions for disease management using big data analytics. J Big Data. (2020) 7:38. doi: 10.1186/s40537-020-00315-8
2. Outwater AH, Leshabari SC, Nolte E. Disease prevention: an overview. In: Quah SR, editor. International Encyclopedia of Public Health. 2nd ed. Oxford: Academic Press (2017). p. 338–49. doi: 10.1016/B978-0-12-803678-5.00117-X
3. Jansen JAMJL. Health promotion and disease prevention can substantially reduce the total economic burden of diabetes in the Netherlands. Neth J Med. (2017) 75:263–64.
4. Kane RL, Johnson PE, Town RJ, Butler M. Economic incentives for preventive care. Evid Rep Technol Assess. (2004) 101:1–7. doi: 10.1037/e439682005-001
5. Jenkins CD. Building Better Health: A Handbook of Behavioral Change. Washington, DC: Pan American Health Organization (2003).
6. Tulchinsky TH, Varavikova EA. Chapter 2 - expanding the concept of public health. In: Tulchinsky TH, Varavikova EA. The New Public Health. 3rd ed. San Diego, CA: Academic Press (2014). p. 43–90. doi: 10.1016/B978-0-12-415766-8.00002-1
7. Alber K, Kuehlein T, Schedlbauer A, Schaffer S. Medical overuse and quaternary prevention in primary care - a qualitative study with general practitioners. BMC Fam Pract. (2017) 18:99. doi: 10.1186/s12875-017-0667-4
8. Gérvas J, Starfield B, Heath I. Is clinical prevention better than cure? Lancet. (2008) 372:1997–9. doi: 10.1016/S0140-6736(08)61843-7
9. Gérvas J. Quaternary prevention in the elderly. Rev Esp Geriatr Gerontol. (2012) 47:266–9. doi: 10.1016/j.regg.2012.07.001
10. Emons W. Incentive-Compatible reimbursement schemes for physicians. J Inst Theor Econ. (2013) 169:605–20. doi: 10.1628/093245613X671869
11. Scott A, Sivey P, Ait Ouakrim D, Willenberg L, Naccarella L, Furler J, et al. The effect of financial incentives on the quality of health care provided by primary care physicians. Cochrane Database Syst Rev. (2011) 9:CD008451. doi: 10.1002/14651858.CD008451.pub2
12. Cattel D, Eijkenaar F, Schut FT. Value-based provider payment: towards a theoretically preferred design. Health Econ Policy Law. (2020) 15:94–112. doi: 10.1017/S1744133118000397
13. WorldEconomicForum. Laying the foundation for health system transformation. In: Value in Healthcare. Geneva: World Economic Forum (2017).
14. Ellis RP, Miller MM. Provider payment methods incentives. In: HK Heggenhougen, editor. International Encyclopedia of Public Health. Oxford: Academic Press (2008). p. 395–402. doi: 10.1016/B978-012373960-5.00173-8
15. Conrad DA. The theory of value-based payment incentives and their application to health care. Health Serv Res. (2015) 50 (Suppl. 2):2057–89. doi: 10.1111/1475-6773.12408
16. Phipps-Taylor M, Shortell SM. More than money: motivating physician behavior change in accountable care organizations. Milbank Q. (2016) 94:832–61. doi: 10.1111/1468-0009.12230
17. Fetters MD, Molina-Azorin JF. The journal of mixed methods research starts a new decade:principles for bringing in the new and divesting of the old language of the field. J Mix Methods Res. (2017) 11:3–10. doi: 10.1177/1558689816682092
19. Langlois EV, Straus SE, Antony J, King VJ, Tricco AC. Using rapid reviews to strengthen health policy and systems and progress towards universal health coverage. BMJ Glob Health. (2019) 4:e001178. doi: 10.1136/bmjgh-2018-001178
20. Kalwij S, French S, Mugezi R, Baraitser P. Using educational outreach and a financial incentive to increase general practices' contribution to chlamydia screening in South-East London 2003–2011. BMC Public Health. (2012) 12:802. doi: 10.1186/1471-2458-12-802
21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
22. Langley A, Abdallah C. Templates turns in qualitative studies of strategy management. In: Bergh DD, Ketchen DJ, editors. Building Methodological Bridges. Bingley: Emerald Group Publishing Limited. (2011). p. 201–35.
23. Chen JY, Tian H, Juarez DT, Yermilov I, Braithwaite RS, Hodges KA, et al. Does pay for performance improve cardiovascular care in a “real-world” setting? Am J Med Qual. (2011) 26:340–8. doi: 10.1177/1062860611398303
24. Chen HJ, Huang N, Chen LS, Chou J, Li CP, Wu CY, et al. Does pay-for-performance program increase providers adherence to guidelines for managing hepatitis b and hepatitis C virus infection in Taiwan? PLoS ONE. (2016) 11:e0161002. doi: 10.1371/journal.pone.0161002
25. Cheng SH, Chen CC, Tsai SL. The impacts of DRG-based payments on health care provider behaviors under a universal coverage system: a population-based study. Health Policy. (2012) 107:202–8. doi: 10.1016/j.healthpol.2012.03.021
26. Chien AT, Li Z, Rosenthal MB. Improving timely childhood immunizations through pay for performance in medicaid-managed care. Health Serv Res. (2010) 45 (6 Pt. 2):1934–47. doi: 10.1111/j.1475-6773.2010.01168.x
27. Dahrouge S, Hogg W, Tuna M, Russell G, Devlin RA, Tugwell P, et al. Age equity in different models of primary care practice in Ontario. Can Fam Phys. (2011) 57:1300–9.
28. De Walque D, Gertler PJ, Bautista-Arredondo S, Kwan A, Vermeersch C, de Dieu Bizimana J, et al. Using provider performance incentives to increase HIV testing and counseling services in Rwanda. J Health Econ. (2015) 40:1–9. doi: 10.1016/j.jhealeco.2014.12.001
29. Echevin D, Fortin B. Physician payment mechanisms, hospital length of stay and risk of readmission: evidence from a natural experiment. J Health Econ. (2014) 36:112–24. doi: 10.1016/j.jhealeco.2014.03.008
30. Gavagan TF, Du H, Saver BG, Adams GJ, Graham DM, McCray R, et al. Effect of financial incentives on improvement in medical quality indicators for primary care. J Am Board Fam Med. (2010) 23:622–31. doi: 10.3122/jabfm.2010.05.070187
31. Hsieh HM, Shin SJ, Tsai SL, Chiu HC. Effectiveness of pay-for-performance incentive designs on diabetes care. Med Care. (2016) 54:1063–9. doi: 10.1097/MLR.0000000000000609
32. Iezzi E, Lippi Bruni M, Ugolini C. The role of GP's compensation schemes in diabetes care: evidence from panel data. J Health Econ. (2014) 34:104–20. doi: 10.1016/j.jhealeco.2014.01.002
33. Jusot F, Or Z, Sirven N. Variations in preventive care utilisation in Europe. Eur J Ageing. (2012) 9:15–25. doi: 10.1007/s10433-011-0201-9. Erratum in: Eur J Ageing. (2011) 9:93–4.
34. Karunaratne K, Stevens P, Irving J, Hobbs H, Kilbride H, Kingston R, et al. The impact of pay for performance on the control of blood pressure in people with chronic kidney disease stage 3-5. Nephrol Dial Transplant. (2013) 28:2107–16. doi: 10.1093/ndt/gft093
35. Kiran T, Victor JC, Kopp A, Shah BR, Glazier RH. The relationship between primary care models and processes of diabetes care in Ontario. Can J Diabetes. (2014) 38:172–8. doi: 10.1016/j.jcjd.2014.01.015
36. Kiran T, Kopp A, Moineddin R, Glazier RH. Longitudinal evaluation of physician payment reform and team-based care for chronic disease management and prevention. CMAJ. (2015) 187:E494–502. doi: 10.1503/cmaj.150579
37. Lai CL, Hou YH. The association of clinical guideline adherence and pay-for-performance among patients with diabetes. J Chin Med Assoc. (2013) 76:102–7. doi: 10.1016/j.jcma.2012.06.024
38. LeBlanc E, Bélanger M, Thibault V, Babin L, Greene B, Halpine S, et al. Influence of a pay-for-performance program on glycemic control in patients living with diabetes by family physicians in a Canadian province. Can J Diabetes. (2017) 41:190–6. doi: 10.1016/j.jcjd.2016.09.008
39. Lee JT, Netuveli G, Majeed A, Millett C. The effects of pay for performance on disparities in stroke, hypertension, and coronary heart disease management: interrupted time series study. PLoS ONE. (2011) 6:e27236. doi: 10.1371/journal.pone.0027236
40. Li J, Hurley J, DeCicca P, Buckley G. Physician response to pay-for-performance: evidence from a natural experiment. Health Econ. (2014) 23:962–78. doi: 10.1002/hec.2971
41. Liddy C, Singh J, Hogg W, Dahrouge S, Taljaard M. Comparison of primary care models in the prevention of cardiovascular disease - a cross sectional study. BMC Fam Pract. (2011) 12:114. doi: 10.1186/1471-2296-12-114
42. Merilind E, Salupere R, Västra K, Kalda R. The influence of performance-based payment on childhood immunisation coverage. Health Policy. (2015) 119:770–7. doi: 10.1016/j.healthpol.2015.01.015
43. Norman AH, Russell AJ, Macnaughton J. The payment for performance model and its influence on British general practitioners' principles and practice. Cad Saude Public. (2014) 30:55–67. doi: 10.1590/0102-311X00149912
44. Pan CC, Kung PT, Chiu LT, Liao YP, Tsai WC. Patients with diabetes in pay-for-performance programs have better physician continuity of care and survival. Am J Manag Care. (2017) 23:e57–66.
45. Pearson WS, King DE, Richards C. Capitated payments to primary care providers and the delivery of patient education. J Am Board Fam Med. (2013) 26:350–5. doi: 10.3122/jabfm.2013.04.120301
46. Pendrith C, Thind A, Zaric GS, Sarma S. Financial incentives and cervical cancer screening participation in ontario's primary care practice models. Healthc Policy. (2016) 12:116–28. doi: 10.12927/hcpol.2016.24758
47. Rajkotia Y, Zang O, Nguimkeu P, Gergen J, Djurovic I, Vaz P, et al. The effect of a performance-based financing program on HIV and maternal/child health services in mozambique-an impact evaluation. Health Policy Plan. (2017) 32:1386–96. doi: 10.1093/heapol/czx106
48. Serumaga B, Ross-Degnan D, Avery AJ, Elliott RA, Majumdar SR, Zhang F, et al. Effect of pay for performance on the management and outcomes of hypertension in the United Kingdom: interrupted time series study. BMJ. (2011) 342:d108. doi: 10.1136/bmj.d108
49. Sicsic J, Franc C. Impact assessment of a pay-for-performance program on breast cancer screening in France using micro data. Eur J Health Econ. (2017) 18:609–21. doi: 10.1007/s10198-016-0813-2
50. Gosden T, Forland F, Kristiansen I, Sutton M, Leese B, Giuffrida A, et al. Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database Syst Rev. (2000) 10:CD002215. doi: 10.1002/14651858.CD002215
51. Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev. (2011) 2011:CD009255. doi: 10.1002/14651858.CD009255
52. Robinson JC. Theory practice in the design of physician payment incentives. Milbank Q. (2001) 79:149–77. doi: 10.1111/1468-0009.00202
Introduction
• Thank respondent for their availability
• Briefly describe the purpose and logistics of this interview
• Ask for permission to record interview for the purpose of transcription
• Start the interview by first collecting the respondent's professional information.
Views on prevention
• Professional's definition of prevention
• Prevention in (daily) practice
• Roles in the provision of prevention
• Prevention and the patient
Reimbursement and prevention
• Reimbursement of preventive services
• Preventive care programs
• Encouragements/obstacles for the delivery of preventive services
• Healthcare purchaser's role
Quaternary prevention/Medicalization
• Benefits/risks of prevention for the patient
• Overmedicalization in healthcare
• Causes for overmedicalization
• Healthcare professionals and overmedicalization
• Strategies to mitigate overmedicalization
Conclusion
• Guarantee respondent's anonymity
• Ask permission to use quotes
• Thank the respondent.
Keywords: prevention, reimbursement, incentives, primary prevention, secondary prevention, tertiary prevention, quaternary prevention, rapid review methods
Citation: Zwaagstra Salvado E, van Elten HJ and van Raaij EM (2021) The Linkages Between Reimbursement and Prevention: A Mixed-Methods Approach. Front. Public Health 9:750122. doi: 10.3389/fpubh.2021.750122
Received: 30 July 2021; Accepted: 29 September 2021;
Published: 27 October 2021.
Edited by:
Steven W. Howard, Saint Louis University, United StatesReviewed by:
Jason Scott Turner, Rush University Medical Center, United StatesCopyright © 2021 Zwaagstra Salvado, van Elten and van Raaij. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hilco J. van Elten, dmFuZWx0ZW5AZXNocG0uZXVyLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.