- 1Medical Center for Digestive Diseases, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Key Lab of Holistic Integrative Enterology, Nanjing Medical University, Nanjing, China
Objective: To explore the attitudes and views of patients with inflammatory bowel disease (IBD) on COVID-19 vaccination.
Methods: An online interview questionnaire concerning the acceptance or hesitancy toward vaccination for COVID-19 was designed and 543 patients with IBD in China were invited to complete the structured self-administered anonymous questionnaire.
Results: Of all the participants, 50.7% were indecisive about the vaccination and only 16.0% opted for it. Vaccination hesitancy was significantly associated with women and those without medical or biomedical backgrounds. The acceptance of COVID-19 vaccination was higher in participants with no history of immune-modifying therapies, especially in those without immunosuppressants. Participants who considered vaccination critically important to self-health or the health of others were more likely to choose immediately or later vaccination. Safety and potential adverse reactions, personal hypoimmunity, efficacy, and reliability of COVID-19 vaccines were the top three concerns of the participants that were independent of their willingness for vaccination.
Conclusions: This study discloses the presence of hesitancy for COVID-19 vaccination in patients with IBD. Further studies are warranted to evaluate the efficacy and safety of COVID-19 vaccines in IBD individuals, with a specific focus on the impact of immune-modifying therapies. Health education and recommendation from authoritative sources may facilitate COVID-19 vaccination efforts.
Introduction
The COVID-19 pandemic has claimed the lives of more than 4.45 million people worldwide (accessed August 26, 2021) (1), and led to substantial concerns for inflammatory bowel disease (IBD) patients due to the presence of dysbiosis and immunocompromised status, despite studies demonstrating similar risk and mortality between patients with IBD and the general population (2). The pandemic significantly affected the daily lives of patients with IBD compared with non-IBD patients (3). The fear of SARS-CoV-2 infection in patients with IBD is more pronounced, especially in those taking immunosuppressants (3, 4). IBD treatments involve many immunosuppressive medications controlling an overactive immune response to induce and maintain clinical remission (5). The long-term systemic corticosteroid (especially prednisone > 20 mg/day), sulfasalazine/mesalamine and thiopurine usage is associated with an increased risk of severe COVID-19, whereas tumor necrosis factor (TNF) antagonist therapy is not (6, 7). The management of IBD during the COVID-19 pandemic has been a challenge for clinicians and patients. Based on the suggestions of experts, IBD therapies other than prednisone should be continued to sustain remission in patients not infected with SARS-CoV-2 (5, 8). However, prednisone, thiopurines, methotrexate, and tofacitinib should be stopped if patients with IBD test positive for SARS-CoV-2 or develop COVID-19 (5, 8, 9). The use of biologics should be comprehensive, based on the severity of COVID-19 and IBD (5, 8).
Efforts have been made to prevent patients with IBD from COVID-19, including the management and treatment guidelines for patients with IBD (8, 9) and the development of related studies (10, 11). The development of safe and effective vaccines is the best way to mitigate against the risk of COVID-19 and prevent the situation from worsening. Despite the promising effectiveness and safety of the candidate vaccines, immunocompromised patients or patients on immunosuppressant medications were excluded from these studies (12–14), thus creating concerns regarding the safety and generalizability of outcomes for IBD individuals (15). Furthermore, patients with IBD on immune-modifying therapies have a higher risk of suboptimal vaccine response and unknown safety (16–18).
China began its COVID-19 vaccination campaign on December 19, 2020, for people at a higher risk of infection, such as the personnel responsible for the inspection and quarantine of the imported or exported cold-chain goods (products or materials that are required to be stored at a specific low temperature), the medical and health workers, and the transportation personnel. Since January 09, 2021, the campaign was extended to the general public unless they were in an active or uncontrolled stage of chronic disease, having acute disease, pregnant, allergic to vaccine components, and having other contraindications evaluated by experts (http://www.gov.cn). At the time of the survey, one type of inactivated viral vaccine was available for Chinese and four other vaccines (two inactivated vaccines, one recombinant protein vaccine, and one adenovirus vector vaccine) were in Phase III clinical trial. Internationally, IBD populations are suggested to use SARS-CoV-2 vaccines as soon as possible regardless of immune-modifying therapies (19, 20). However, in China, at present, there are no official recommendations about whether or not patients with IBD could take the vaccination. It is mainly based on their wish after seeking medical advice from their physicians or other authoritative resources. Therefore, we designed this questionnaire to investigate the attitudes and views of individuals with IBD on COVID-19 vaccines with the aim of achieving a better understanding of vaccine acceptance and/or hesitancy in this particular population.
Materials and Methods
Study Design, Setting, and Participants
This study was a questionnaire survey conducted among patients with IBD in China about their attitudes and views on COVID-19 vaccination. The questionnaire was reviewed by experts in IBD and pilot-tested in 10 patients with IBD to verify the readability and importance of content. The electronic questionnaire was created using Wen Juan Xing (an online survey tool by Changsha Ranxing Information Technology Co., Ltd., Hunan, China). It was then broadcasted on several WeChat (a social media application operated by Tencent Co., Ltd., Shenzhen, China) groups, where a subset of patients with IBD gathered from all over the country. Patients completed the questionnaire independently and voluntarily in the context of anonymity and free of charge. Each participant had only one chance to answer the questionnaire, and they could submit it only after responding to all the required questions, as prompted by the electronic system. Parents can assist their children or adolescents if they were unable to complete the survey independently. This study was approved by the institutional ethical review committee at the Second Affiliated Hospital of Nanjing Medical University ([2020]KY106).
Questionnaire Design
A self-administered questionnaire was designed based on the literature review and clinical experience of the specialists in the management of patients with IBD during the COVID-19 epidemic. It consisted of 21 questions focusing on the views and attitudes of patients with IBD on COVID-19 vaccines. Besides demographic information of the participants, characteristics of IBD, current IBD treatments, and impact of COVID-19 pandemic on patients with IBD were also covered in the questionnaire as the potential factors influencing their attitudes toward vaccination. The choice of answers to the attitudes toward COVID-19 vaccination included “Yes,” “Not right away, but later,” “Undecided,” and “No.” A multiple-choice question was set for investigating reasons for COVID-19 vaccination. Participants who chose “Yes” or “No” were required to select the three main reasons for their respective choices. While those who chose “Not right away, but later” or “Undecided” had to respond to both the questions (see the questionnaire in Supplementary File 1).
Statistical Analysis
Data collection and statistical analysis were carried out using the SPSS software system (SPSS for Windows, Version 23.0, IBM Corp, Armonk, United States). The electronic questionnaires were exported directly and were checked by two researchers independently to prevent vulnerabilities in the web system. Continuous variables were presented as median (interquartile range) for abnormal distribution and analyzed by Kruskal–Wallis H test. Categorical data were described as numbers (percentages) and tested by Chi-square analysis or Fisher's exact test. Cramer's V is an index that provides the correlation strength of classified variables (“0.1–0.3”: weak correlation, “0.3–0.5”: moderate correlation, and “≥0.5”: strong correlation). Pairwise comparison of Chi-square test was used to determine the differences between groups (P values were adjusted according to Bonferroni method), and Binary Logistic Regression was used for multivariate analysis of the screened factors. P < 0.05 and adjusted p < 0.05/(time of comparisons) were considered statistically significant for all the analyses.
Results
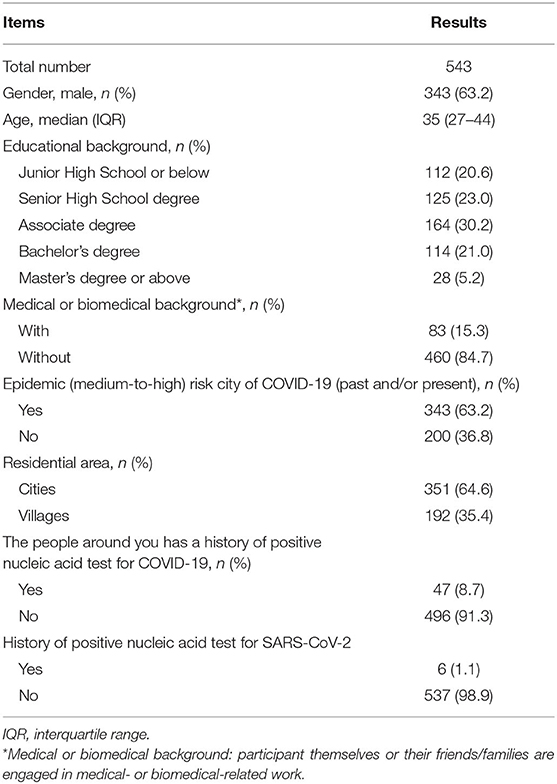
From December 31, 2020, to January 3, 2021, a total of 543 electronic questionnaires were returned. We confirmed that all of them were complete, standardized, and qualified for analysis (543/543), with an effective rate of 100%. Our questionnaire covered 106 cities in 26 provinces of China. Areas with COVID-19 cases or new confirmed COVID-19 cases within 14 days were considered as a medium- to high-risk areas of COVID-19 based on the criteria set by the government (http://www.gov.cn). In our study, 63.2% of participants were from the area with medium-to-high COVID-19 risk in the past and/or at present. Less than one in six (15.3 %) patients or their family members had medical or biomedical backgrounds. Table 1 demonstrates the demographics of participants and their exposure to COVID-19.
Characteristics of Patients and IBD Medications
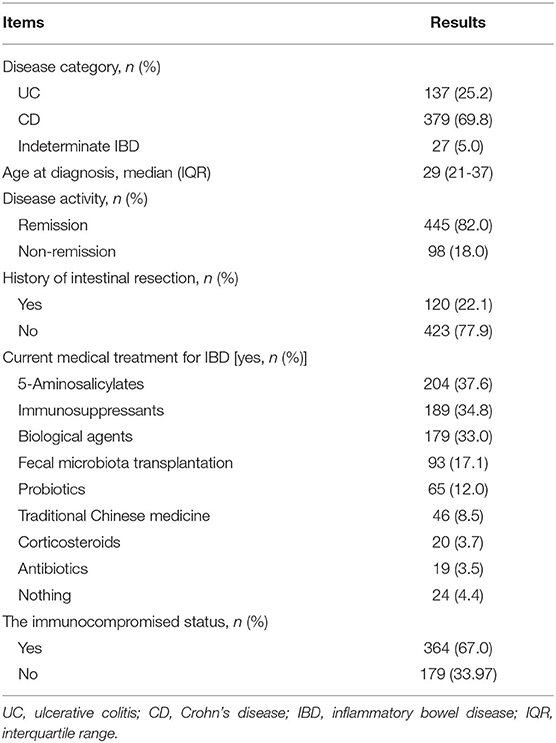
More than half of the respondents had Crohn's disease (69.8%) and the remainder had ulcerative colitis (25.2%) or indeterminate IBD (5.0%). There were 82.0% of participants in remission. 5-Aminosalicylates were the most commonly used medications (37.6%), followed by immunosuppressants (34.8%) and biological agents (33.0%). Patients with IBD who were taking biological agents, immunosuppressants, or corticosteroids (alone or in combination) for long-term were regarded as those in the immunocompromised status (67.0%). The demographics and characteristics of the patients are detailed in Table 2.
Attitudes and Views of Patients With IBD on SARS-CoV-2 Vaccination
Figure 1A shows the attitudes of patients with IBD toward SARS-CoV-2 vaccination. Half of the participants were indecisive about vaccination (50.7%), which was consistent with the finding that 61.5% of them had no idea about what type of vaccine they would prefer to receive (Figure 1B). Those who had a clear attitude toward either being vaccinated or not accounted for 16.0 and 12.7%, respectively. The inactivated COVID-19 vaccine was the most popular of the four types of vaccines (26.3%). In the present study, most of the respondents agreed to SARS-CoV-2 vaccination as a vital measure for self-health (79.2%) or the health of others (85.6%).
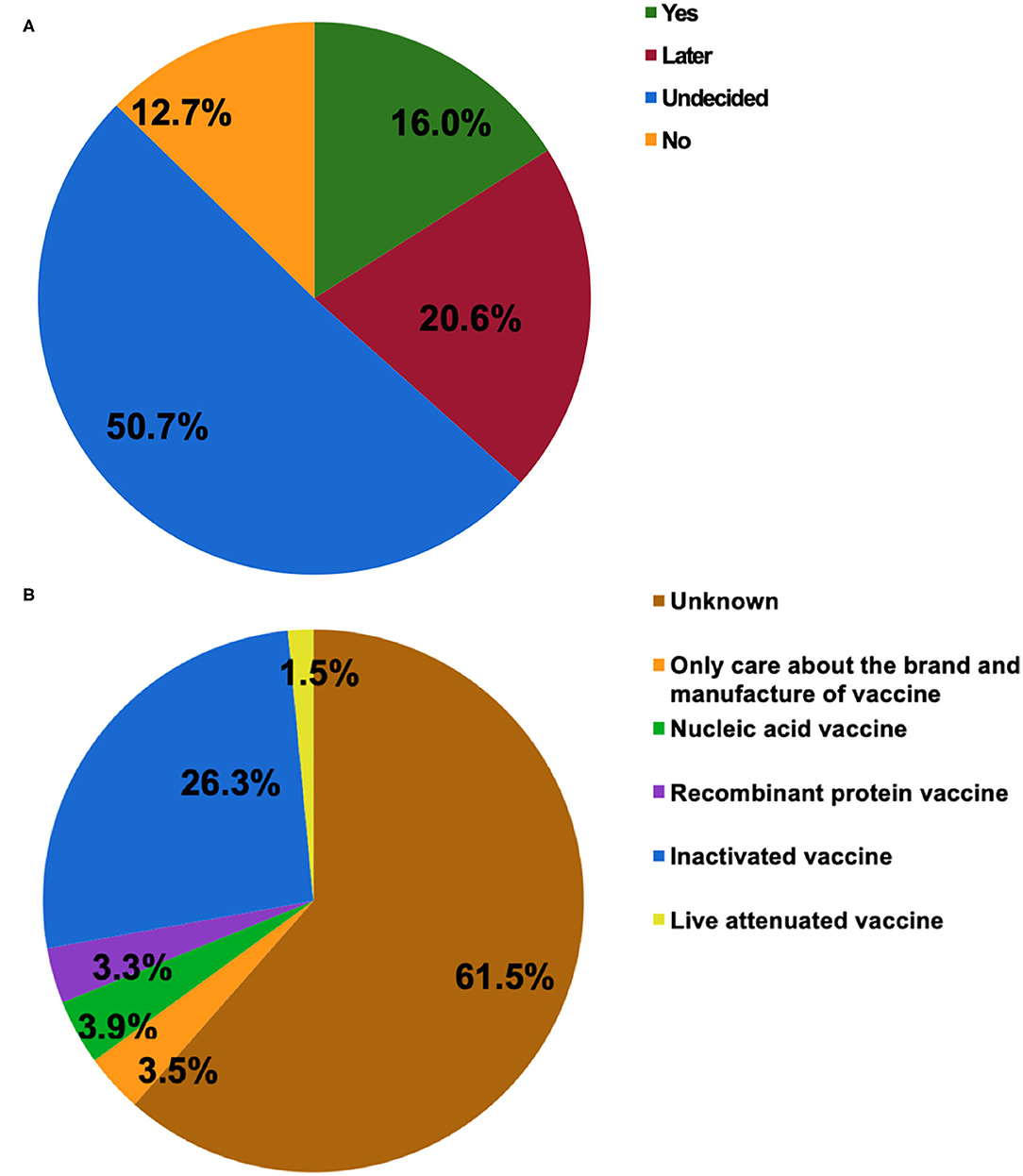
Figure 1. The attitudes of patients with inflammatory bowel disease (IBD) toward COVID-19 vaccination (A). The type of COVID-19 vaccines that participants would prefer to receive (B).
Five factors (gender, medical or biomedical background, immunocompromised status, immunosuppressant usage, and the importance of vaccination for the health of others) were found to have a weak association with the attitude of individuals with IBD toward SARS-CoV-2 vaccination (Chi-square test, p < 0.035, Cramer's V = 0.181, 0.132, 0.126, 0.132, and 0.297 respectively). The importance of SARS-CoV-2 vaccination for self-health had a moderate relationship with the attitudes of participants toward vaccination (Chi-square test, p < 0.001, Cramer's V = 0.359). No other significant difference was found based on disease category, disease activity, treatment with fecal microbiota transplantation, and other parameters (Tables 1, 2).
Pairwise comparison of the Chi-square test was used to determine the differences between groups (Figure 2). The adjusted p < 0.0083 was considered statistically significant. Men showed a higher rate of choosing “Yes” or “Not right away, but later” in comparison with women who tended more toward choosing “Undecided” (Figure 2A, p = 0.001 and p = 0.001, respectively). Participants with medical or biomedical background had a higher rate of acceptance for the vaccination, while those without it were more likely to choose “Undecided” (Figure 2B, p = 0.004). Immunocompromised patients or patients on immunosuppressants tended toward not getting the vaccination (Figures 2C,D, p < 0.006). Overall, participants who considered vaccination critically important to self-health or the health of others chose to get vaccinated immediately or later, although 50.7% of patients were undecided about the SARS-CoV-2 vaccination. However, people who did not recognize the role of SARS-CoV-2 vaccines had a higher rate of not getting vaccinated (Figures 2C,D, p < 0.003). Table 3 shows the result of Binary Logistic Analysis between different groups.
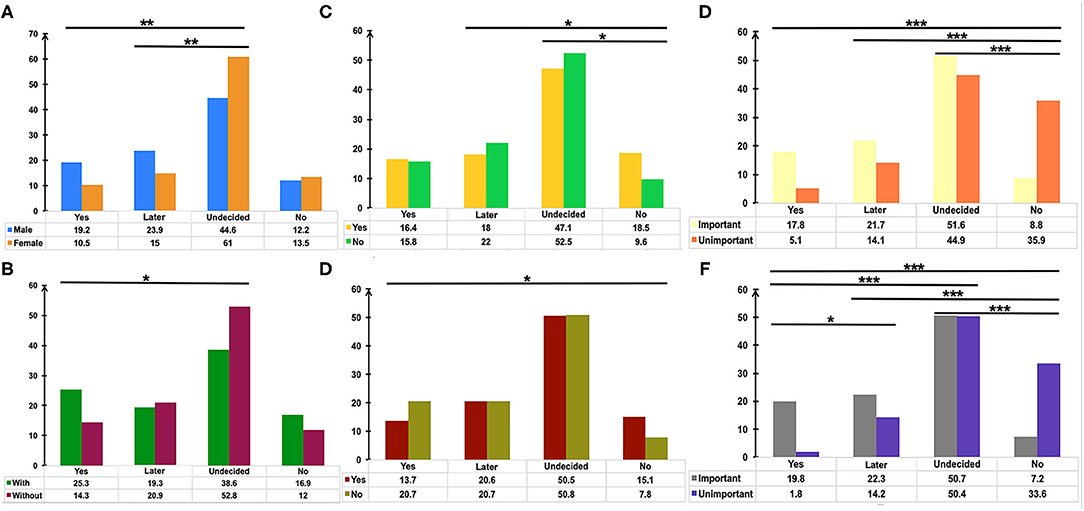
Figure 2. The potential factors influencing the attitudes of patients with IBD toward COVID-19 vaccination. Pairwise comparison of Chi-square test was used to determine the differences of gender (A), medical or biomedical background (B), immunosuppressants usage (C), immunocompromised status (D), the importance of vaccination for the health of others (E), the importance of vaccination for self-health (F) between groups. P values were adjusted according to the Bonferroni method. *p < 0.0083, **p <0.0017, ***p < 0.00017.
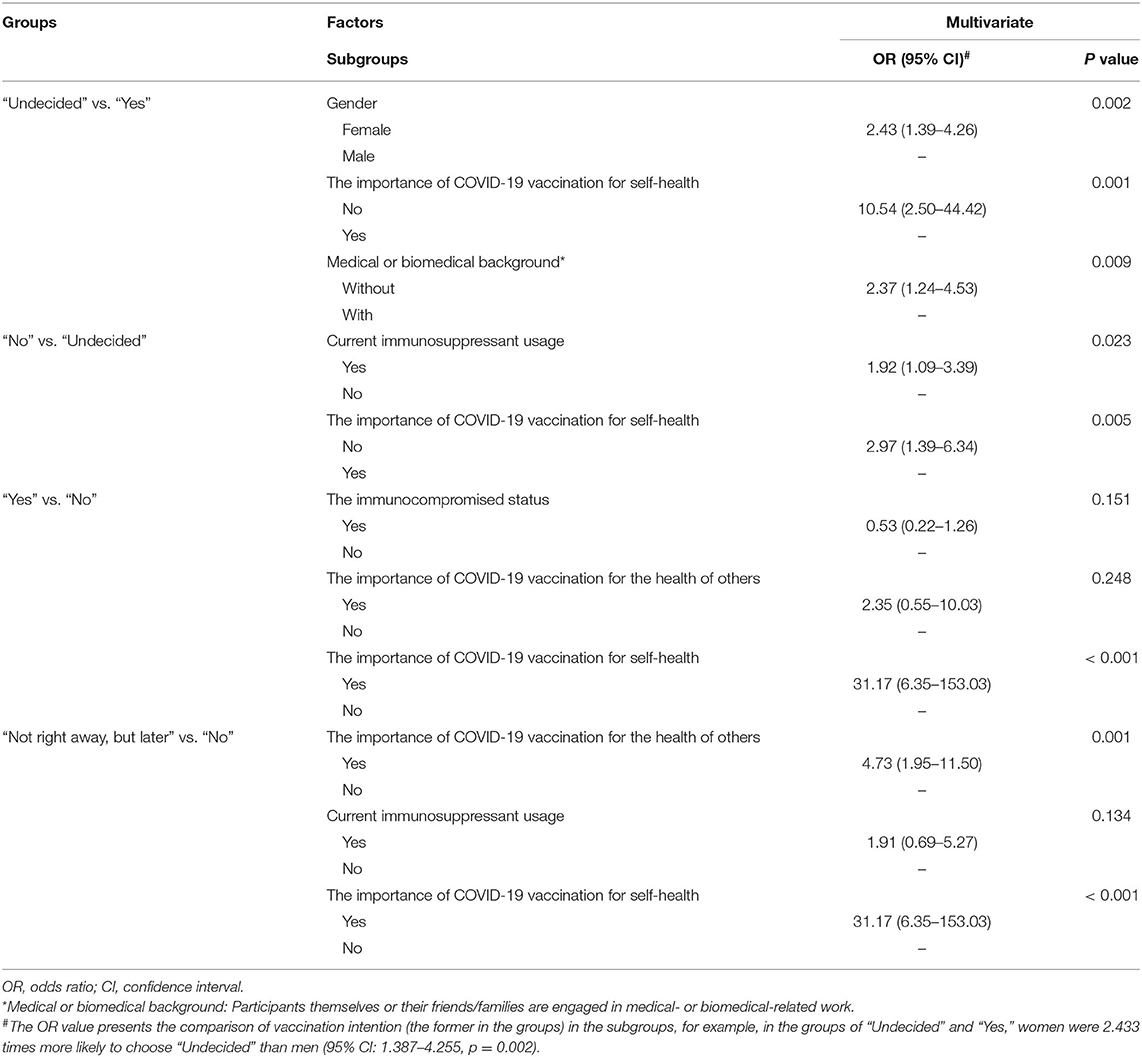
Table 3. The independent factors impacting the attitude of patients toward SARS-CoV-2 vaccination, identified by Binary Logistic Analysis.
Reasons Affecting Attitudes Toward SARS-CoV-2 Vaccination
Figure 3 depicts the major reasons that influence the willingness for SARS-CoV-2 vaccination. Safety and adverse reactions, personal hypoimmunity, and efficacy and validity of COVID-19 vaccines were the top three concerns of patients that were independent of their willingness toward vaccination (Figure 3B). Notably, the national initiatives and recommendations from attending physicians were also the main reasons for some patients getting vaccinated.
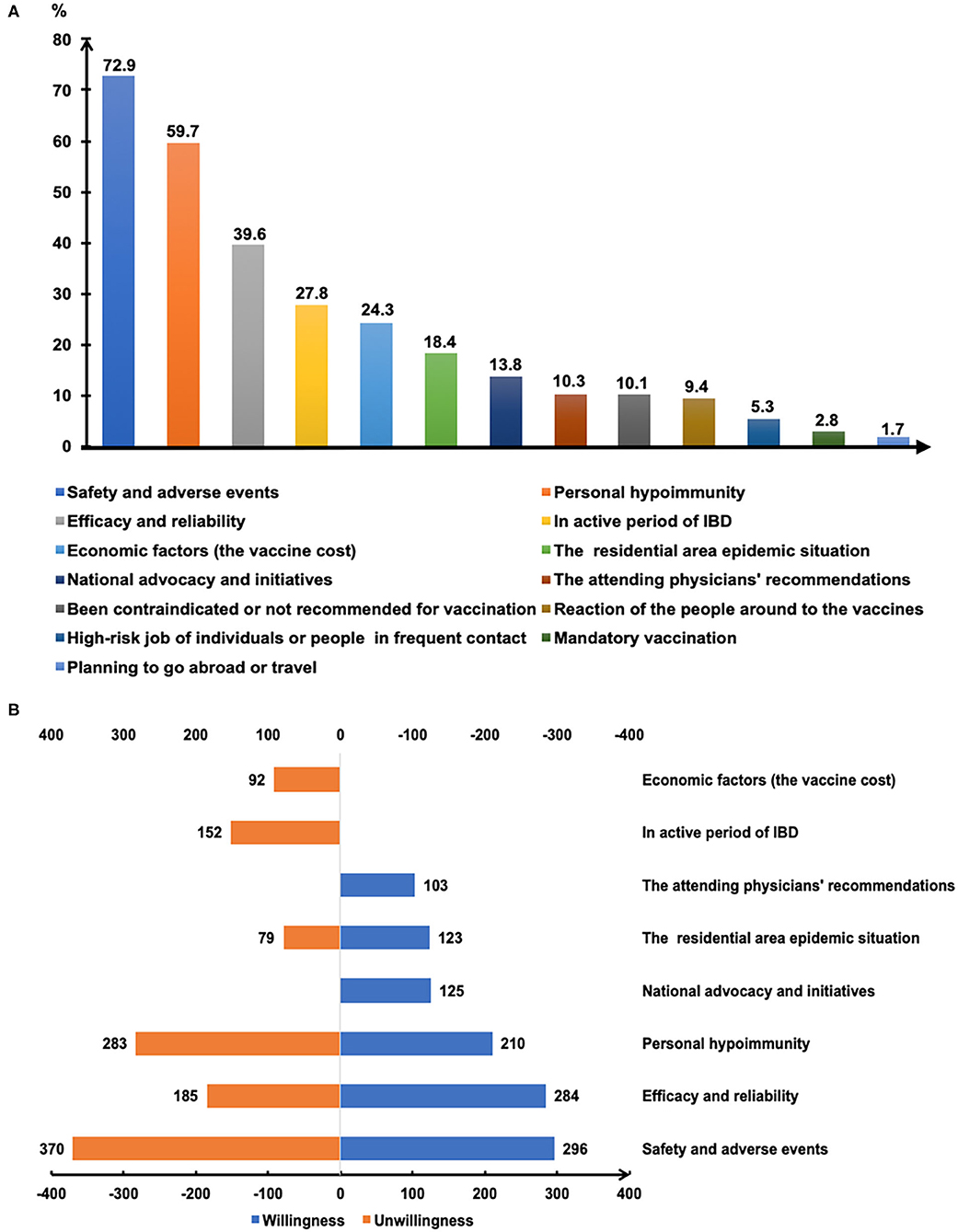
Figure 3. The reasons affecting COVID-19 vaccination of patients with IBD (A). The main reasons for getting or not getting COVID-19 vaccinated (B).
Discussion
The COVID-19 pandemic has brought great trouble and concerns for patients with IBD. In our present survey, 63.2% of patients came from the COVID-19 risk area and 1.1% of those patients had a positive history of SARS-CoV-2 infection (Table 1). Safe and effective vaccines are expected by health authorities and the medical community as the most powerful weapon against the COVID-19 pandemic (21), and nearly 80.0% of respondents in our study also recognized that SARS-CoV-2 vaccination was critical to the self-health and health of others.
Willingness to be vaccinated matters greatly, for the reason that a sufficient vaccination rate in a population is indispensable before herd immunity is achieved. The published expert consensus and opinions recommend vaccinating all IBD populations as soon as they are able to receive SARS-CoV-2 vaccines (15, 19, 20). In the present study, COVID-19 vaccination hesitancy existed in 50.7% of patients with IBD and merely 16.0% of participants opted for vaccination (Figure 1). The attitude of the participants towards the COVID-19 vaccine was significantly more negative than that of the general population in China and the patients with IBD from other countries (22–24). It was reported that 67.1% of the general population in China were willing to accept the COVID-19 vaccination during the same period (22). Furthermore, the rate of vaccination intent among IBD participants in our present study was lower than that among the IBD population in Boston (80.9%) (23) and Italy (80.3%) (24). Similarly, a lower rate of intent for hepatitis B (18.8%) and varicella vaccines (6.6%) was observed among patients with IBD in China (25). The potential reasons could be the insufficient knowledge about vaccination and many concerns about vaccine safety in this specific population, as reflected in our study and other reports (16–18, 23).
Gender was found to be one of the determinants of attitude towards COVID-19 vaccination in this study (Figure 2A), in agreement with several other surveys (26, 27). The reasons for vaccination hesitancy in the female group needs to be further explored in the future, for concerns regarding reproductive impact, personality trait, and others. Patients with IBD with medical or biomedical background had a higher rate of immediately getting vaccinated (Figure 2B), and 21.7% of the respondents considered the recommendations of their attending physicians as one of the three main reasons for getting vaccinated (Figure 3B). An anonymous cross-sectional survey conducted among Chinese adults reported that the recommendation of the doctor was a contributing factor in the decision-making for vaccination (27). As recommended by clinical practice guidelines for vaccination of the immunosuppressed host (28), IBD specialists should share the responsibility of communicating with their patients about the recommendations and appropriate vaccinations amidst the COVID-19 pandemic.
Most vaccines are broadly recommended for patients with IBD, including the COVID-19 vaccines (19). However, the vaccine hesitancy and unwillingness are particularly pronounced among patients with IBD mainly due to the unpredictable outcome amidst disease activity and immunocompromised status, together with the blunted immunogenicity of some vaccines found in the immune-modifying therapies (16–18, 23, 29). Similarly, we demonstrated that the acceptance of COVID-19 vaccination was lower in participants being treated with immune-modifying therapies, especially in those using immunosuppressants (Figures 2C,D). Moreover, previous clinical studies of COVID-19 vaccines have excluded patients with immune conditions (12–14), thus heightening the concerns of patients with IBD about the safety and generalizability of the outcomes (15). We found that safety and adverse events, personal hypoimmunity, as well as efficacy and validity of COVID-19 vaccines are the top three concerns of patients with IBD for COVID-19 vaccination (Figure 3B). This phenomenon was commonly shared in the IBD populations from other countries (23). Therefore, the expert panel suggested that patients with IBD receiving SARS-CoV-2 vaccination should be referred to prospective registries tracking the amplitude and duration of immune responses across different vaccine platforms (19).
Although half of the patients with IBD were undecided about SARS-CoV-2 vaccination, participants who realized the vital importance of vaccination to self-health or the health of others had a higher intention for immediate or later vaccination when compared to those who did not fully appreciate its importance (Figures 2E,F). This result highlights the role of health education (23, 25) and recommendations from authoritative sources, such as government and IBD specialists, in removing some barriers along the process of vaccination (Figure 3B).
The vaccine candidates can be grouped into protein subunit vaccines, nucleic acid vaccines, whole-inactivated viral vaccines, live attenuated viral vaccines, and others (30). In China, more than 177.7 million doses of vaccines have been administrated to the general population (accessed August 6, 2021) (1). In general, the patients with IBD can safely receive the inactivated vaccines for vaccine-preventable diseases irrespective of immune conditions (19, 29), which may be the reason that the inactivated COVID-19 vaccine was the most popular of the four types of vaccines (Figure 1B). In reality, the types of SARS-CoV-2 vaccines including the inactivated vaccine, messenger RNA vaccines, replication-incompetent vector vaccines, and recombinant vaccines are considered safe for patients with IBD (19). The BNT162b2, ChAdOx1 nCoV-19, and mRNA-1273 vaccines have received regulatory approval in the United Kingdom, which applies to patients with IBD, indicating that an immunosuppressed state is not a contraindication (20). Even so, more research should be carried out highlighting the safety and efficacy of COVID-19 vaccines in this particular population.
This study has some limitations. First, we conducted the survey via an electronic questionnaire. Thus, although the disadvantages of the paper questionnaire, such as skipped questions or the time-consuming task of uploading the collected data, were avoided, the data of patients who could not operate electronic devices were lost. Second, response bias inherent to the online survey and an abbreviated study period may have led to an inaccurate understanding of vaccination intent. Finally, our survey results did not completely represent the attitude or the concerns of IBD populations on COVID-19 vaccination from other countries, due to different situations of the pandemic globally.
In conclusion, this study discloses COVID-19 vaccination hesitancy and paucity of knowledge about the COVID-19 vaccines in the IBD population. Further studies evaluating the efficacy and safety of various COVID-19 vaccines in IBD individuals, with a specific focus on the impact of immune-modifying therapies and serologic responses, should be carried out to alleviate the concerns of such patients. Health education and recommendation from authoritative sources, such as government and IBD specialists, may facilitate COVID-19 vaccination efforts.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
This study was approved by the Second Affiliated Hospital of Nanjing Medical University institutional ethical review board ([2020]KY106).
Author Contributions
FZ, BC, GJ, XW, and JL designed the study. XW did the statistical analysis. XW and JL drafted the manuscript. HB edited the manuscript for professional English. QD, BC, and GJ were involved in the revision of the manuscript. All authors have read and approved the final version of this manuscript.
Funding
This study was supported by the publicly donated Intestine Initiative Foundation; the National Natural Science Foundation of China (81600417); and the 789 Outstanding Talent Program of SAHNMU (789ZYR20200802250).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate Jianlin Bai for his guidance in statistical analysis. We also appreciate Richard Shi and Cicilia Marcella for kindly revising the manuscript for language proficiency. The authors would like to thank all the participants for their time and effort.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.731578/full#supplementary-material
References
1. Global real-time data on the COVID-19 pandemic. Available online at: https://covid19.who.int/ (accessed August 26, 2021).
2. Taxonera C, Sagastagoitia I, Alba C, Manas N, Olivares D, Rey E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. (2020) 52:276–83. doi: 10.1111/apt.15804
3. Grunert PC, Reuken PA, Stallhofer J, Teich N, Stallmach A. Inflammatory bowel disease in the COVID-19 pandemic - the patients' perspective. J Crohns Colitis. (2020) 14:1702–8. doi: 10.1093/ecco-jcc/jjaa126
4. D'Amico F, Rahier J-F, Leone S, Peyrin-Biroulet L, Danese S. Views of patients with inflammatory bowel disease on the COVID-19 pandemic: a global survey. Lancet Gastroenterol Hepato. (2020) 5:631–2. doi: 10.1016/s2468-1253(20)30151-5
5. Rubin DT, Abreu MT, Rai V, Siegel CA. International organization for the study of inflammatory bowel D. Management of patients with crohn's disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an international meeting. Gastroenterology. (2020) 159:6–13 e16. doi: 10.1053/j.gastro.2020.04.002
6. Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. (2021) 70:725–32. doi: 10.1136/gutjnl-2020-322539
7. Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. (2020) 159:481–91 e483. doi: 10.1053/j.gastro.2020.05.032
8. Rubin DT, Feuerstein JD, Wang AY, Cohen RD, AGA. Clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. (2020) 159:350–7. doi: 10.1053/j.gastro.2020.04.012
9. Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol. (2020) 17:253–5. doi: 10.1038/s41575-020-0294-8
10. Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. (2021) 70:698–706. doi: 10.1136/gutjnl-2020-323020
11. Gupta S, Parker J, Smits S, Underwood J, Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces - a rapid review. Colorectal Dis. (2020) 22:611–20. doi: 10.1111/codi.15138
12. Walsh EE, Frenck RW. Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. (2020) 383:2439–50. doi: 10.1056/NEJMoa2027906
13. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtmacccccc n A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
14. Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. (2020) 396:1979–93. doi: 10.1016/s0140-6736(20)32466-1
15. Kumar A, Quraishi MN, Segal JP, Raine T, Brookes MJ. COVID-19 vaccinations in patients with inflammatory bowel disease. Lancet Gastroenterol Hepatol. (2020) 5:965–6. doi: 10.1016/S2468-1253(20)30295-8
16. Andrisani G, Frasca D, Romero M, Armuzzi A, Felice C, Marzo M, et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-alpha agents: effects of combined therapy with immunosuppressants. J Crohns Colitis. (2013) 7:301–7. doi: 10.1016/j.crohns.2012.05.011
17. deBruyn JC, Hilsden R, Fonseca K, Russell ML, Kaplan GG, Vanderkooi O, et al. Immunogenicity and safety of influenza vaccination in children with inflammatory bowel disease. Inflamm Bowel Dis. (2012) 18:25–33. doi: 10.1002/ibd.21706
18. Fiorino G, Peyrin-Biroulet L, Naccarato P, Szabo H, Sociale OR, Vetrano S, et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. (2012) 18:1042–7. doi: 10.1002/ibd.21800
19. Siegel CA, Melmed GY, McGovern DP, Rai V, Krammer F, Rubin DT, et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. (2021) 70:635–40. doi: 10.1136/gutjnl-2020-324000
20. Alexander JL, Moran GW, Gaya DR, Raine T, Hart A, Kennedy NA, et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol Hepatol. (2021) 6:218–24. doi: 10.1016/S2468-1253(21)00024-8
21. Sewell HF, Agius RM, Kendrick D, Stewart M. Covid-19 vaccines: delivering protective immunity. BMJ. (2020) 371:m4838. doi: 10.1136/bmj.m4838
22. Wang C, Han B, Zhao T, Liu H, Liu B, Chen L, et al. Vaccination willingness, vaccine hesitancy, and estimated coverage at the first round of COVID-19 vaccination in China: A national cross-sectional study. Vaccine. (2021) 39:2833–42. doi: 10.1016/j.vaccine.2021.04.020
23. Dalal RS, McClure E, Marcus J, Winter RW, Hamilton MJ, Allegretti JR. COVID-19 Vaccination intent and perceptions among patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2021) 19:1730–2.e2. doi: 10.1016/j.cgh.2021.02.004
24. Costantino A, Noviello D, Conforti FS, Aloi M, Armuzzi A, Bossa F, et al. COVID-19 vaccination willingness and hesitancy in patients with inflammatory bowel diseases: analysis of determinants in a national survey of the Italian IBD patients' association. Inflamm Bowel Dis. (2021) izab172. doi: 10.1093/ibd/izab172
25. Feng S, Lin S, Ma L, Xu S, Chen Y. Insufficient knowledge and vaccination practice of inflammatory bowel disease patients in the People's Republic of China. Patient Prefer Adherence. (2020) 14:1513–21. doi: 10.2147/PPA.S265346
26. Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. The Lancet Public Health. (2021). doi: 10.1016/s2468-2667(21)00012-8
27. Wang J, Jing R, Lai X, Zhang H, Lyu Y, Knoll MD, et al. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines (Basel). (2020) 8:482. doi: 10.3390/vaccines8030482
28. Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. (2014) 58:309–18. doi: 10.1093/cid/cit816
29. Caldera F, Ley D, Hayney MS, Farraye FA. Optimizing immunization strategies in patients with IBD. Inflamm Bowel Dis. (2021) 27:123–33. doi: 10.1093/ibd/izaa055
Keywords: inflammatory bowel disease, SARS-CoV-2 vaccination, perspective, willingness, immunocompromised population
Citation: Wu X, Lin J, Buch H, Ding Q, Zhang F, Cui B and Ji G (2021) The COVID-19 Vaccination Hesitancy Among the People With Inflammatory Bowel Disease in China: A Questionnaire Study. Front. Public Health 9:731578. doi: 10.3389/fpubh.2021.731578
Received: 28 June 2021; Accepted: 06 September 2021;
Published: 11 October 2021.
Edited by:
Shisan Bao, The University of Sydney, AustraliaReviewed by:
Marisa Silvia Castro, Institute of Studies on Humoral Immunity (IDEHU), ArgentinaOana Sandulescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2021 Wu, Lin, Buch, Ding, Zhang, Cui and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bota Cui, Y3VpYm90YSYjeDAwMDQwO25qbXUuZWR1LmNu; Guozhong Ji, amd6emwmI3gwMDA0MDsxNjMuY29t
†These authors have contributed equally to this work
 Xia Wu
Xia Wu Jue Lin1,2†
Jue Lin1,2† Faming Zhang
Faming Zhang Bota Cui
Bota Cui