
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 19 August 2021
Sec. Digital Public Health
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.726140
This article is part of the Research Topic Data-Enabled Intelligence for Medical Technology Innovation View all 18 articles
Objective: Previous studies discussing phenotypic and temporal heterogeneity of knee osteoarthritis (KOA) separately have fatal limitations that either clustering patients with similar severity or assuming all knees have a single common progression pattern, which are unreliable. This study tried to uncover more reliable information on phenotypic and temporal heterogeneity of KOA.
Design: Data were from Osteoarthritis Initiative database. Six hundred and seventy-eight unilateral knees that have greater Kellgren and Lawrence (KL) grade than the contralateral knees at baseline and in all follow-up 48 months were included. Measurements of biomarkers at baseline were chosen. Subtype and Stage Inference model (SuStaIn) was applied as a subtype-progression model to identify subtypes, subtype biomarker progress sequences and stages of KOA.
Results: This study identified three subtypes which account for 15, 61, and 24% of knees, respectively. Each subtype has distinct subtype biomarker progress sequence. For knees with KL grade 0/1, 2, 3, and 4, they have different distributions on stage and 26, 53, 89, and 95% of them are strongly assigned to subtypes. When assessing whether a knee has KL (grade ≥ 2), subtypes and stages from subtypes-progression model (SuStaIn) are significantly better fitting than those from subtypes-only (mixture of Gaussians) (likelihood ratio = 105.59, p = 2.2 × 10−16) or stages-only (SuStaIn where setting c = 1) (likelihood ratio = 58.04, p = 2.57 × 10−14) model. Stages in subtypes-progression model has greater β than stages-only model. Subtypes from subtypes-progression model have no statistical significance.
Conclusions: For subtypes-progression model, stages contain more complete temporal information and subtypes are closer to real OA subtypes.
Knee osteoarthritis (KOA) is recognized as a complex condition with different clinical characteristics (1–3). Most of previous studies only discussed phenotypic or temporal heterogeneity of KOA, which referred as a subtypes-only models or stages-only models.
Subtypes-only models cluster knees together into subtypes based on the similarity of the biomarker measurements (1, 4). The limitation is that those models could result in clusters of patients with similar osteoarthritis (OA) severity, which would not represent true OA subtypes. Stages-only models are always built based on regression model (5–8). The inherent assumption is that all knees have a same single common progression pattern. But disease progressions in most cases are complex and knees have phenotypic heterogeneity. Therefore, stages identified based on the above assumption have limited reliability. Some researchers tried to investigate subtypes and temporal heterogeneity together (2, 9). However, they discussed the distinct subtypes and OA severity scores separately. The above-mentioned limitations could not be avoided in previous studies.
KOA is a chronic progressive disease and has a long course. The ideally long-term frequent follow-up data are difficult to obtain. In this study, we use cross-sectional data to research the characteristics of KOA progression and phenotypic heterogeneity. There are three basic assumptions for this study. (1) Cross-sectional data contain a certain amount of temporal information. Knees have different changes in biomarker measurement, which implies the disease stage that they belong to. (2) Cross-sectional data consists of subjects at all the periods of the whole disease course. It can be roughly affirmed by the knees distributed in all the KL grades. The first two assumptions make it possible that researchers can reconstruct the trajectory of disease progression with cross-sectional data. (3) Knees from different subtypes have different trajectories of biomarker progression. Thus, the optimal number of biomarkers progression trajectories that maximizes the data likelihood represents the optimal number of subtypes.
New machine learning and deep learning methods (10–12) are brought into medical research. The recently introduced Subtype and Stage Inference model (SuStaIn) is an unsupervised machine-learning technique (13) and learns distributions of biomarker values from the data. SuStaIn calculates the optimal subtype biomarker progress sequences and the optimal number of sequences to maximize the data likelihood. The number of subtypes is represented by the optimal number of sequences. The subtype biomarker progress sequence stands for the order of the biomarker changed as disease progresses for a particular group of knees. With the subtype biomarker progress sequences, knees can be assigned into a certain subtype and progression stage. Therefore, we used SuStaIn as the subtypes-progression model to uncover phenotypic and temporal heterogeneity of KOA simultaneously. And finally, we identified 3 KOA subtypes, rebuilt the subtype biomarker progress sequences and assign each individual to a most probable subtype and stage.
The data were from Osteoarthritis Initiative (OAI) database (https://nda.nih.gov/oai). OAI is a large multi-center, 10-year prospective observational cohort study. The original OAI participant recruitment and data collection process have obtained ethical approval and informed consent. No specific ethical approval was required for this study.
Previous study shows that risk of KOA increased with the incidence of contralateral knee OA (3). For the two knees of each subject which afflicted with KOA earlier and later, there may be different risk factors and disease progression patterns. As limited by available number of knees, we decided to discuss a single condition that the unilateral knees have greater Kellgren and Lawrence (KL) grade than the contralateral knees at baseline and in all the follow-up 48 months. The exclusion criteria are knees (1) having no KL grade assessment at baseline or in any follow-up visit, (2) having no complete data of radiograph and MRI image assessment at baseline. Finally, our study population includes 678 eligible knees with different KOA severity.
Obviously, to construct the subtype biomarker progress sequences, the data should cover the whole disease course of KOA. Ideally, it should contain complete biomarkers' measurements in the whole disease course of patients. However, the course of KOA, just like other chronic diseases, takes a period of decades. Following up for the whole disease course is impossible. Study the disease trajectories with only cross-sectional data is necessary. Then, we only chose the measurements of biomarkers at baseline for each knee. Because knees' KOA severity increases over time and the number of knees with mild severity reduces at follow-up time points. KOA severity of study group ranges more widely at baseline than any follow-up time points.
Candidate biomarkers used in this study were the OA symptoms which were obtained through questionnaires [Western Ontario and McMaster Universities (WOMAC) pain score], quantitative radiographic readings [Joint Space Width (JSW)], quantitative MRI measures of cartilage thickness, and semi-quantitative radiographic readings (osteophytes and sclerosis, per anatomical compartment for the tibia and femur).
To simplify the model, increase the clinical utility and improve the generalization ability the most, we used the backward deletion to select the fewest biomarkers, which maximized the data likelihood (14). All the biomarkers were used to reconstruct KOA subtypes progression models shown in Table 1 and Figure 2A.
The study roadmap is shown in Figure 1. We included 678 knees in the study population. Measurements of biomarkers at baseline were chosen. Three models (SuStaIn model, mixture of Gaussians model and SuStaIn model where setting c = 1) were fitted to the study population and formulated the subtypes-progression model, subtypes-only model, and stages-only model, respectively. The subtypes, subtype biomarker progress sequences and stages from the subtypes-progression model were described and assessed. Finally, we compared the subtypes and stages among the three models.
In this study, we used SuStaIn as the subtypes-progression model to identify KOA subtypes, rebuilt the subtype biomarker progress sequences and assigned an individual to a most possible subtype and stage. SuStaIn defined four important concepts. (1) Biomarker event. A biomarker event is a new change in symptom or structural lesion as KOA progression advances. Each biomarker event corresponds to a switching from a z-score to another. (2) Subtype. knees from different subtypes have different trajectories of biomarker progression. The number of biomarkers progression trajectories can be discovered in a disease represents the number of all subtypes it contains. (3) Subtype biomarker progress sequence. Each disease subtype has a particular progress course. The course of each disease subtype progression can be depicted by the order in which the biomarker events occur as each disease subtype progresses, which is called as subtype biomarker progress sequence. (4) Stage. Each disease subtype progression advances from 0-th stage to S-th stage. S is the number of all biomarker events. The i-th stage that a knee belongs to is defined as a specific state that the previous i events of the sequence have occurred. Occurrence of an event indicates that the disease advances in a biomarker and disease progression switches from a stage to the next.
SuStaIn is an unsupervised machine-learning technique and doesn't rely on a priori staging or subtype. SuStaIn is a mixture of linear z-score models. It describes the subtype biomarker progress sequence as a linear z-score model, which is an improved model of the original event-based model (EBM) (15, 16). In EBM, each event represents the switch of a biomarker from a normal to an abnormal level. SuStaIn reformulates the events to make them correspond to the continuous linear accumulation and each event of a biomarker represents change from one z-score to another. SuStaIn simultaneously optimizes the number of subtypes, subtype biomarker progress sequence, and the posterior distributions of both. What's more, SuStaIn estimates the probability of assignment to a most probable subtype and stage, respectively, for each knee. The most likely biomarker event sequences are ones that maximizes the data likelihood. The optimal number of subtype biomarker progress sequences that maximize the data likelihood represent the optimal number of subtypes. We fitted SuStaIn with python (version 3.7). The source code of SuStaIn can be acquired on https://github.com/EuroPOND/pySuStaIn.
Every biomarker measurement was expressed as a z-score relative to the control group. Since this, the corresponding z-score can describe the abnormal degree of each biomarker measurement from study population relative to control group. Inclusion criterion for the OAI control group are (1) no pain, aching or stiffness in both knee in the past year; (2) no radiograph OA; (3) no eligibility risk factors; (4) age ≤ 70 years. This study had an additional exclusion criterion: incomplete data on radiographic and MRI image assessment at baseline.
With KOA progress, medial minimum JSW and mean cartilage thickness decrease and their z-scores became negative. We took the negative value of the z-scores for convenience, so that all the z-scores would increase as KOA severity increasing.
A biomarker event is a new change in symptom or structural lesion as KOA progression advances. Each biomarker event corresponds to a switching from a z-score to another. The z-score events of each biomarker were the most important input. For each biomarker, the z-score events initially include z-scores of 1, 2, 3, and 5. However, some z-score events had low occurrence frequency in the disease progression, thus fewer than 10 knees had greater than that z-score. To simplify the model, we excluded those z-score events. Finally, 32 z-score events were included from the 13 biomarkers.
The maximum z-score means the final stage of the progression. If the maximum z-score event was 1, 2, 3, and 5, the input “maximum z-score” was set to be 2, 3, 5, and 7, respectively. Detail of z-score of each biomarker is shown in Table 1.
We used SuStaIn as the subtypes-progression model to identify the KOA subtypes, subtype biomarker progress sequences and the probability of assignment to a most probable subtype and stage, respectively, for each knee.
We used 10-fold cross-validation to gain the optimal number of subtypes by the Cross-Validation Information Criterion (CVIC) (16). The CVIC was defined as CVIC = −2 × log [P (X|M)], where P (X|M) is the probability of the data X for a particular subtypes-progression model M.
We tested the differences between the subtypes for a specific biomarker with R (version 3.6.3). The logistic regression was used for binary measurements and a general linear model for continuous or ordered categorical measurements. Then we used post-hoc analysis with a SNK test adjustment to test for which subtypes the measurements were different.
We measured the strength of assignment to one of the subtypes in KOA subtypes progression. A strong assignment was defined as that the maximum probability of assigning to a particular subtype is 1.5 times greater than any other two subtypes.
We surveyed the consistency of KL grade and stages from KOA subtypes progression model. KL grade represented the temporal state of knees with KOA roughly. We grouped the knees by KL grade and estimated the probability of assignment to a stage.
We used SuStaIn as the subtypes-progression model to identify the KOA subtypes, subtype biomarker progress sequences, the probability of assignment to a most probable subtype, and stage, respectively, for each knee. The subtypes-only model and stages-only model can also assign knees to a subtype or stage, respectively. The subtype or stage that a knee is assigned reflects the phenotypic or temporal information from our models.
We formulated subtypes-only and stages-only models as close as possible to the subtypes-progression model. Thus, subtypes and stages from the three models (subtypes-only model and stages-only model, subtypes-progression model) are comparable.
In this study, the subtypes-only model was fitted to OA (KL grade ≥2) with a mixture of Gaussians model. The stages-only model was formulated by SuStaIn model, setting the subtype number to 1.
To compare the subtypes and stages from the three models, we put forward a task that separates the knees with/without doubtful KOA (KL grade 0/1) from those with KOA (KL grade ≥2). This task can test the ability of the stages from the three models that separates the knees in time-perspective. The more benefit the stages contribute to the task, the more temporal information are included in the stages. However, if the subtypes have more contributions to the task, it has negative meaning. It indicates that those subtypes include temporal heterogeneity and tend to cluster the knees with similar KOA severity and don't represent true KOA subtypes.
We used logistic regression to compare the subtypes and stages from the three models by the task of separating no or doubtful KOA (KL grade 0/1) from KOA (KL grade ≥2). The input variables were stages and subtypes from the three models and demographic factors: gender, age, BMI and injury. Likelihood ratio comparison between two models was used to assess the goodness of fit of them (17). Statistical significance was set as P < 0.05. All were analyzed with R (version 3.6.3).
There were 678 unilateral knees included, which are from subjects with average age 62.15 years old, 55.01% female, average BMI of 23.1 and 20.65% injury in this study. KL grade 0/1, 2, 3, and 4 accounted for 7.47, 34.22, 40.56, and 17.85%, respectively. 140 knees have injury which was defined as ever injured badly enough to limit ability to walk for at least 2 days.
As is shown in Figure 2, the subtypes-progression model identified three subtypes. Each subtype had different clinical symptoms and structural lesions at different progression stages. We termed three subtypes as early pain, structural lesions concurrence pain and late pain, which account for 15, 61, and 24% of the knees. Early pain subtype could be described as a mild subtype. Serious pain occurs at first stages and osteophytes follows. Not until the latter half stages, other structural lesions occur. In structural lesions concurrence pain, serious pain also occurs at first stages. but all the structural lesions appear and progress soon and almost distribute in almost all the rest stages. Late pain subtype is very similar to the former, but the pain hides till the last stages and structural lesions occur at very early stages.
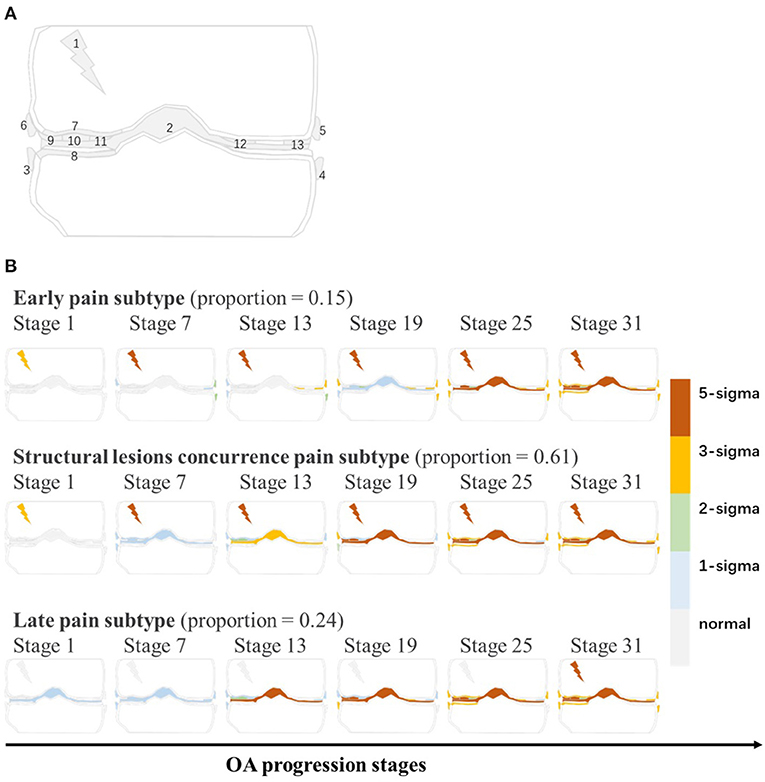
Figure 2. (A) The graphical representation of all biomarkers. Gray line is coronal sketch map of knee joint. Numbers in this figure match the order numbers of biomarkers in Table 1. (B) KOA subtypes and subtype biomarker progress sequences identified by subtypes-progression model. Proportion of knees assigned to each subtype are shown. Each row represents an biomarkers changes order as KOA stages advance. Biomarkers from different stages reach different z-scores relative to control group. At each stage color in each region indicates level of severity of pain or lesions: gray is unaffected; blue is mildly affected (z-score of 1), and so on.
All subtypes contained knees of the entire range of the KL grade, except for the early pain subtype on KL grade 0/1 (Table 2). Table 2 shows that early pain subtype had the highest proportion of female (65%) than other two subtypes. BMI in late pain subtype was significantly lowest. Age, injury proportion and gender proportion had no significant difference between the three subtypes.
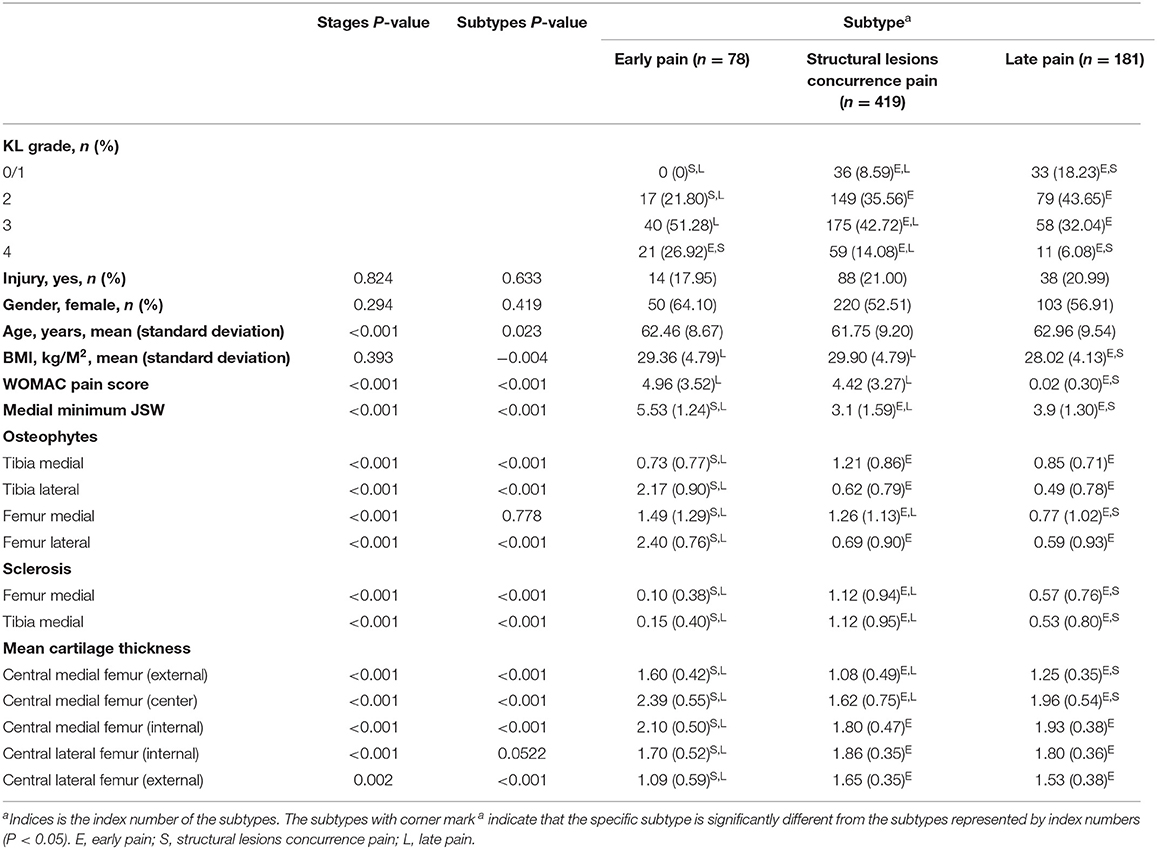
Table 2. Baseline demographics and risk factors for three subtypes and differences between the measurements of all biomarkers used for subtype definitions.
After correction for stages, all biomarkers except osteophytes femur medial compartment showed significant differences between the subtypes. Post-hoc analysis showed that early pain subtype was mainly different with others. Early pain subtype had more changes in WOMAC pain score. What's more, structural lesions between the lateral and medial compartment were different. More severe lesions occurred in osteophytes from tibia lateral compartment and mean cartilage thickness in lateral femur compartment. Less changes occurred in medial minimum JSW, sclerosis and mean cartilage thickness in central medial subregion. Further, the characteristics between the other subtypes were different. Late pain subtype had slighter change in most structural lesions (WOMAC pain score, medial minimum JSW, osteophytes, sclerosis, and in mean cartilage thickness of central lateral femur subregion).
As is shown in Figure 3, for OA knees with KL grade 0/1, 2, 3, and 4, the strong assignment to subtypes were 26, 53, 89, and 95%. With disease progress, the strength of assignment in KOA subtypes increased. Moreover, even at early disease stages (KL grade 0/1), 26% of the knees were strongly assigned to a subtype.
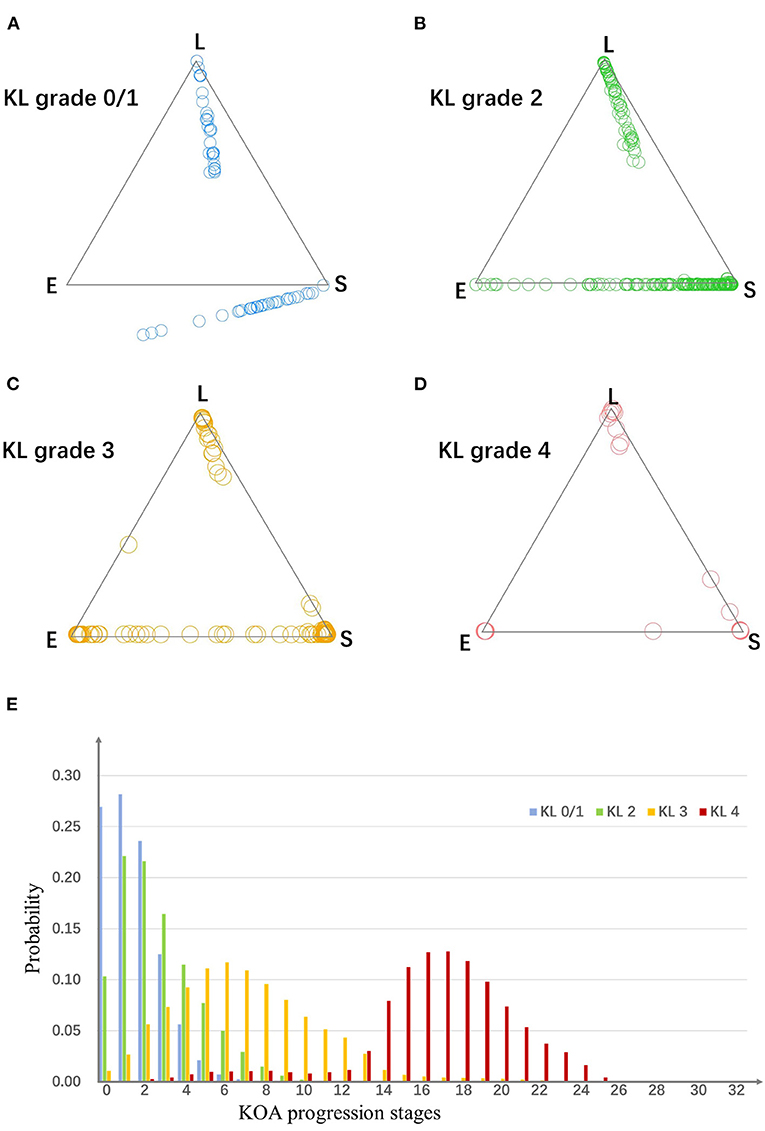
Figure 3. (A–D) The assign ability of subtypes and stages. Plots in four scatter plots indicate knees from four KL grade groups. For a triangle scatter plot, each corner indicates a probability of 1 of assigning to a particular subtype, and 0 for the other two subtypes; the center point of the triangle indicates a probability of 1/3 of assigning to each subtype. (E) The probability of knees from each KL grade group belong to each stage. E, early pain; S, structural lesions concurrence pain; L, late pain.
To evaluate the assignment to a particular stage, we estimated the probability knees from each KL grade belonging to each of the stages. As is shown in Figure 3, the knees from different KL grade had different distributions in the stages.
We used logistic regression model to separate knees with/without doubtful KOA (KL grade 0/1) from those with KOA (KL grade ≥2). Table 3 shows that stages (p = 1.08 × 10−8) and subtypes (p = 0.332) from subtypes-progression model had significant hazards.
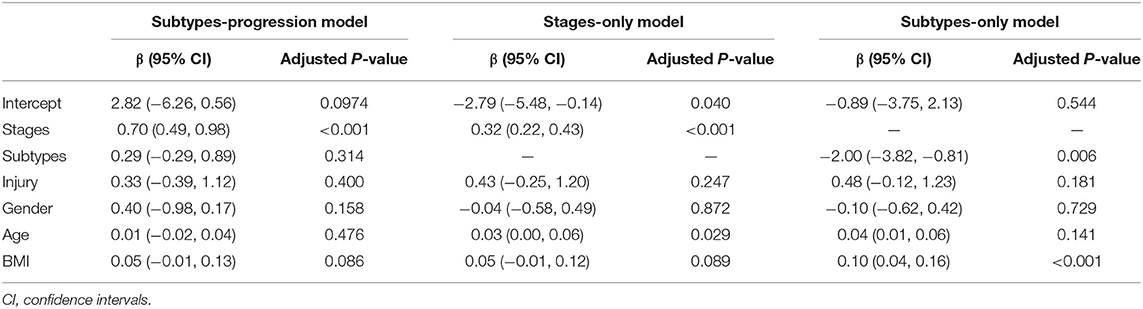
Table 3. Results for comparing the subtypes and stages from subtypes-progression model with those from subtypes-only and stages-only with logistic regression model by separating knees with no or doubtful KOA (KL grade 0/1) from those with KOA (KL grade ≥2).
We compared subtypes-progression model, subtypes-only and stages-only models with likelihood ratio tests. Subtypes-progression model was significantly better fit than subtypes-only (likelihood ratio = 105.59, p = 2.2 × 10−16) and stages-only (likelihood ratio = 58.04, p = 2.57 × 10−14) models. A likelihood ratio of above 1 shows that, for distinguish knees with no or doubtful KOA (KL grade 0/1) from those with OA (KL grade ≥2), the subtypes and stages of KOA subtypes progression model provided a significantly better fit than a subtypes-only or stages-only model.
Table 3 shows that the stages in subtypes-progression model had greater β than stages-only model. Rather than subtypes-only model, the subtypes from subtypes-progression model had no statistical significance.
Some studies tried to describe the temporal or phenotypic heterogeneity for KOA. However, those studies that only explain the temporal progression based on the assumption that all the knees came from a single disease progress sequence (1, 4). As KOA is a disease with complex clinical characteristics, this assumption may not hold. Some studies only discussed about phenotypic heterogeneity and don't account for temporal heterogeneity (5–8). Subtypes identified by those studies tend to cluster the knees with similar OA severity, which don't represent true OA subtypes. In our study, we used SuStaIn as a subtypes-progression model to study the temporal or phenotypic heterogeneity simultaneously and constructed a reliable picture of how the lesions spread from a distinct region over the rest of the knee in each subtype.
Because of the limitation of available number of knees, we only discuss a single condition that the unilateral knees have greater KL grade than the contralateral knees at both baseline and all the follow-up 48 months. We fitted the SuStaIn model to 678 knees to identify KOA subtypes and subtype biomarker progress sequences. The biomarkers included the WOMAC pain score, medial minimum JSW, quantitative MRI measures of cartilage thickness, and semi-quantitative radiographic readings (osteophytes and sclerosis, per compartment for the tibia and femur). The subtypes-progression model identified three subtypes and each subtype had its distinct biomarker progress sequence. Three subtypes had different characteristics and were termed as early pain, structural lesions concurrence pain and late pain. The severity of all biomarker increased with greater stages.
Our results are agreed with previous studies, such as bigger pain sore is always associated with severer osteophytes (18–23), and narrower medial minimum JSW is always associated with thinner mean cartilage thickness (24–28).
Between lateral and medial subregion of each subtype, the changes in mean cartilage thickness existed inconsistency. Early pain subtype had significantly slightest changes in medial subregion and significantly greatest changes in lateral subregion. Structural lesions concurrence pain subtype had opposite changes. Late pain subtype had medium changes in both subregion. Some studies found that greater BMI is associated with incident medial tibiofemoral OA (3) and more serious changes in lateral subregion in mean cartilage thickness, which is consistent with our results.
The existence of phenotypic heterogeneity of KOA is proved by our study. When we measured the strength of a knee's assignment to a given subtype, it showed strong assignment of KOA patients to the subtypes. Therefore, explaining heterogeneity in this study about KOA progression is very necessary.
The subtypes and stages we identified have power to separate the knees with phenotypic or temporal heterogeneity. At no or doubtful KOA (KL grade 0/1), many knees gather around the vertices of the triangles. It shows the subtypes are so effective that have the ability of identifying knees even in very early stages. Besides, the stages are certified to have the power to separate the knees with different disease severity. The probabilities of knees with each KL grade belonging to each of the stages show that the distribution of the stages differ between KL grades.
The subtypes and stages from subtypes-progression model performed significantly better than subtypes-only and stages-only models. We used logistic regression to compare the subtypes and stages from the three models by the task of separating no or doubtful KOA (KL grade 0/1) from KOA (KL grade ≥2). With the temporal task of separating early disease stages (KL grade 0/1) from OA (KL grade ≥2), it shows that subtypes and stages from subtypes-progression model are close to true KOA subtypes and stages. Bigger regression coefficient of stages from subtypes-progression model shows that they contain more complete temporal information. Furthermore, rather than those from subtypes-only model, subtypes from subtypes-progression model have no contribution to this temporal task. It suggests that the subtypes from subtypes-progression model contain hardly any temporal heterogeneity and are close to true OA subtypes.
The subtypes and stages identified by this study have clinical practice value. First, with our subtypes-progression model, each knee can be assigned into certain subtype, suggesting particular disease characteristics. So that, we can help doctor to identify the significant features of each knee and make more proper treatment plan. Second, whole KOA progress course is divided into more detailed stages. They offer a mini scale for doctor to learn disease progress statue of a knee. What's more, the biomarker progress sequence can show the progress pathway for each knee. The doctors and patients can foresee their subsequent biomarker changes and estimate if a knee maintain same stage over a period of time. It has a significant meaning in chronic disease management. And finally, the study method can also be expanded to carry out the research of other chronic disease.
There are some limitations in this study. As being confined to the quantity of the knees available, the study population only included the knee that afflicted with KOA earlier and had greater KL grade for every knee. So our results may only act as a reference for the knee with more serious OA condition of the subject. In addition, the biomarkers only contain the pain score and image assessment data. In future work, we can study other groups of knees that afflicted with KOA later and remain smaller KL grade or alternately having smaller KL grade, and try to analyze more categories of biomarkers, e.g., biochemical biomarker measurements from serum and urine samples, so that we can learn the KOA progression in wider range.
The subtypes-progression model identifies three subtypes and each subtype has its distinct biomarker progress sequence. There exists phenotypic heterogeneity of KOA, bigger pain sore is always associated with severer osteophytes and the changes in mean cartilage thickness exist inconsistency between lateral and medial subregions of each subtype. The subtypes and stages from subtypes-progression model have power to separate the knees with phenotypic or temporal heterogeneity and perform significantly better than those from stages-only model and subtypes-only model. In a word, with subtypes-progression model, stages contain more complete temporal information and subtypes are close to real OA subtypes.
Publicly available datasets were analyzed in this study. This data can be found here: https://nda.nih.gov/oai/.
The original OAI participant recruitment and data collection process have obtained ethical approval and informed consent. No specific ethical approval was required for this study.
ML participated in the design of the study, analysis and interpretation of the data, and drafting of the article. LL participated in analysis of data, manuscript preparation, and critical revision of the article for important intellectual content. JL participated in the design of the study and analysis of the data. LP participated in analysis and interpretation of the data. XZ participated in design of the study and critical revision of the article for important content. XL participated in interpretation of the data and drafting of the article. All authors read and approved the final manuscript.
This work was supported by the Center of Excellence-International Collaboration Initiative Grant (No. 139170052) and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC18010). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors declare that this study received funding from the Center of Excellence-International Collaboration Initiative Grant (No. 139170052) and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC18010). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors gratefully acknowledge the support of the OAI for providing data for this work.
1. Waarsing JH, Bierma-Zeinstra SM, Weinans H. Latent class cluster analysis shows four distinct subtypes of knee OA: data from the osteoarthritis initiative. Osteoarthritis Cartilage. (2012) 20:S177–8. doi: 10.1016/j.joca.2012.02.272
2. Waarsing JH, Bierma-Zeinstra SM, Weinans H. Distinct subtypes of knee osteoarthritis: data from the osteoarthritis initiative. Rheumatology (Oxford). (2015) 54:1650–8. doi: 10.1093/rheumatology/kev100
3. Wei J, Gross D, Lane NE, Lu N, Wang M, Zeng C, et al. Risk factor heterogeneity for medial and lateral compartment knee osteoarthritis: analysis of two prospective cohorts. Osteoarthritis Cartilage. (2019) 27:603–10. doi: 10.1016/j.joca.2018.12.013
4. Kittelson AJ, Stevens-Lapsley JE, Schmiege SJ. Determination of pain phenotypes in knee osteoarthritis: a latent class analysis using data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). (2016) 68:612–20. doi: 10.1002/acr.22734
5. Cibere J, Sayre EC, Guermazi A, Nicolaou S, Kopec JA, Esdaile JM, et al. Natural history of cartilage damage and osteoarthritis progression on magnetic resonance imaging in a population-based cohort with knee pain. Osteoarthritis Cartilage. (2011) 19:683–8. doi: 10.1016/j.joca.2011.02.008
6. Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the foundation for the national institutes of health osteoarthritis biomarkers consortium. Arthritis Rheumatol. (2016) 68:2422–31. doi: 10.1002/art.39731
7. de Lange-Brokaar BJ, Bijsterbosch J, Kornaat PR, Yusuf E, Ioan-Facsinay A, Zuurmond AM, et al. Radiographic progression of knee osteoarthritis is associated with MRI abnormalities in both the patellofemoral and tibiofemoral joint. Osteoarthritis Cartilage. (2016) 24:473–79. doi: 10.1016/j.joca.2015.09.021
8. Yoo JJ, Kim DH, Kim HA. Risk factors for progression of radiographic knee osteoarthritis in elderly community residents in Korea. BMC Musculoskelet Disord. (2018) 19:80. doi: 10.1186/s12891-018-1999-5
9. Halilaj E, Le Y, Hicks JL, Hastie TJ, Delp SL. Modeling and predicting osteoarthritis progression: data from the osteoarthritis initiative. Osteoarthritis Cartilage. (2018) 26:1643–50. doi: 10.1016/j.joca.2018.08.003
10. Zeng N, Wang Z, Liu W, Zhang H, Hone K, Liu X. A dynamic neighborhood-based switching particle swarm optimization algorithm. IEEE Trans Cybern. (2020) 1:1–12. doi: 10.1109/TCYB.2020.3029748
11. Zeng N, Wang Z, Zineddin B, Li Y, Du M, Xiao L, et al. Image-based quantitative analysis of gold immunochromatographic strip via cellular neural network approach. Med Imaging. (2014) 33:1129–36. doi: 10.1109/TMI.2014.2305394
12. Zeng N, Wang Z, Zhang H, Kim K-E, Li Y, Liu X. An improved particle filter with a novel hybrid proposal distribution for quantitative analysis of gold immunochromatographic strips. IEEE Trans Nanotechnol. (2019) 18:819–29. doi: 10.1109/TNANO.2019.2932271
13. Young AL, Marinescu RV, Oxtoby NP, Bocchetta M, Yong K, Firth NC, et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun. (2018) 9:4273. doi: 10.1038/s41467-018-05892-0
14. Abe S. Modified Backward Feature Selection by Cross Validation. Bruges: European Symposium on Esann (2005). p. 163–8.
15. Fonteijn HM, Modat M, Clarkson MJ, Barnes J, Lehmann M, Hobbs NZ, et al. An event-based model for disease progression and its application in familial Alzheimer's disease and Huntington's disease. NeuroImage. (2012) 60:1880–9. doi: 10.1016/j.neuroimage.2012.01.062
16. Gelman A, Hwang J, Vehtari A. Understanding predictive information criteria for Bayesian models. Stat Comput. (2013) 24:997–1016. doi: 10.1007/s11222-013-9416-2
17. Royle JA, Dorazio RM. 2 - Essentials of statistical inference. In: Royle JA, Dorazio RM, editors. Hierarchical Modeling and Inference in Ecology. Academic Press: San Diego (2009). p. 27–82.
18. Aylward S, Hadjiiski LM, Galván-Tejada JI, Celaya-Padilla JM, Martínez-Torteya A, Rodriguez-Rojas J, et al. Wide association study of radiological features that predict future knee OA pain: data from the OAI. In: Medical Imaging 2014: Computer-Aided Diagnosis. (2014). p. 93539.
19. Javaid MK, Kiran A, Guermazi A, Kwoh CK, Zaim S, Carbone L, et al. Individual magnetic resonance imaging and radiographic features of knee osteoarthritis in subjects with unilateral knee pain: the health, aging, and body composition study. Arthritis Rheum. (2012) 64:3246–55. doi: 10.1002/art.34594
20. Javaid MK, Lynch JA, Tolstykh I, Guermazi A, Roemer F, Aliabadi P, et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: the most study. Osteoarthritis Cartilage. (2010) 18:323–8. doi: 10.1016/j.joca.2009.11.002
21. Sayre EC, Guermazi A, Esdaile JM, Kopec JA, Singer J, Thorne A, et al. Associations between MRI features versus knee pain severity and progression: data from the Vancouver longitudinal study of early knee osteoarthritis. PLoS ONE. (2017) 12:e0176833. doi: 10.1371/journal.pone.0176833
22. Niethammer TR, Valentin S, Gülecyüz MF, Roßbach BP, Ficklscherer A, Pietschmann MF, et al. Bone marrow edema in the knee and its influence on clinical outcome after matrix-based autologous chondrocyte implantation. Am J Sports Med. (2015) 43:1172–9. doi: 10.1177/0363546515573935
23. Zhai G, Cicuttini F, Ding C, Scott F, Garnero P, Jones G. Correlates of knee pain in younger subjects. Clin Rheumatol. (2007) 26:75–80. doi: 10.1007/s10067-006-0248-8
24. Eckstein F, Maschek S, Roemer FW, Duda GN, Sharma L, Wirth W. Cartilage loss in radiographically normal knees depends on radiographic status of the contralateral knee - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. (2019) 27:273–7. doi: 10.1016/j.joca.2018.10.006
25. Eckstein F, Wirth W, Hunter DJ, Guermazi A, Kwoh CK, Nelson DR, et al. Magnitude and regional distribution of cartilage loss associated with grades of joint space narrowing in radiographic osteoarthritis - data from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage. (2010) 18:760–8. doi: 10.1016/j.joca.2009.12.009
26. Pelletier J-P, Raynauld J-P, Berthiaume M-J, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. (2007) 9:R74. doi: 10.1186/ar2272
27. Wirth W, Buck R, Nevitt M, Le Graverand MPH, Benichou O, Dreher D, et al. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography - data from the OA initiative. Osteoarthritis Cartilage. (2011) 19:689–99. doi: 10.1016/j.joca.2011.02.011
28. Wirth W, Maschek S, Roemer FW, Sharma L, Duda GN, Eckstein F. Radiographically normal knees with contralateral joint space narrowing display greater change in cartilage transverse relaxation time than those with normal contralateral knees: a model of early OA? - data from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage. (2019) 27:1663–8. doi: 10.1016/j.joca.2019.06.013
Keywords: osteoarthritis, phenotype, subtype, progression, trajectories
Citation: Li M, Lan L, Luo J, Peng L, Li X and Zhou X (2021) Identifying the Phenotypic and Temporal Heterogeneity of Knee Osteoarthritis: Data From the Osteoarthritis Initiative. Front. Public Health 9:726140. doi: 10.3389/fpubh.2021.726140
Received: 16 June 2021; Accepted: 16 July 2021;
Published: 19 August 2021.
Edited by:
Yonghong Peng, Manchester Metropolitan University, United KingdomReviewed by:
Haiyan Yu, Chongqing University of Posts and Telecommunications, ChinaCopyright © 2021 Li, Lan, Luo, Peng, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengjiao Li, bGltZW5nY2hpYW85MUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.