- 1Department of Public Health, School of Health Policy and Management, Nanjing Medical University, Nanjing, China
- 2Department of Clinical Pharmacy, School of Pharmacy, Nanjing Medical University, Nanjing, China
- 3Jiangsu Medicine Information Institute, Nanjing, China
- 4Center for Global Health, Nanjing Medical University, Nanjing, China
Objective: The study aimed to evaluate the impact of the National Health Insurance Coverage (NHIC) policy on the utilisation and accessibility of innovative anti-cancer medicines in Nanjing, China.
Methods: We used the adjusted World Health Organisation and Health Action International methodology to calculate the price and availability of 15 innovative anti-cancer medicines included in the National Health Insurance drug list in 20 tertiary hospitals and six secondary hospitals in Nanjing before and after NHIC policy implementation. Interrupted time-series regression was used to analyse the changes in the utilisation of the study medicines.
Results: The price reduction rates of innovative anti-cancer medicines ranged between 34 and 65%. The mean availability rate was 27.44% before policy implementation and increased to 47.33% after policy implementation. The utilisation of anti-cancer medicines suddenly increased with a slope of 33.19–2,628.39 when the policy was implemented. Moreover, the usage rate of bevacizumab, bortezomib, and apatinib significantly increased (p < 0.001, p = 0.009, and p < 0.001, respectively) after policy implementation. With regard to price reduction and medical insurance reimbursement, the medicines became more affordable after policy implementation (0.06–1.90 times the per capita annual disposable income for urban patients and 0.13–4.46 times the per capita annual disposable income for rural patients).
Conclusion: The NHIC policy, which was released by the central government, effectively improved the utilisation and affordability of innovative anti-cancer medicines. However, the availability of innovative anti-cancer medicines in hospitals remained low and the utilisation of innovative anti-cancer medicines was affected by some factors, including the incidence of cancer, limitation of indications within the insurance program, and the rational use of innovative anti-cancer medicines. It is necessary to improve relevant supporting policies to promote the affordability of patients. The government should speed up the process of price negotiation to include more innovative anti-cancer medicines in the medical insurance coverage, consider including both medical examinations and adjuvant chemotherapy in the medical insurance, and increase investment in health care.
Introduction
Cancer has been a major health issue worldwide and is one of the most serious health problems in China. Based on the data from the National Central Cancer Registry in 2019, 2.929 million patients were newly diagnosed with cancer and 2.338 million deaths were reported (1). Cancer accounts for 25% of all cases and deaths worldwide (2). Population growth and increased life expectancy are among the important reasons for the high morbidity of cancer (2).
To address the increase in the risk of cancer, the pharmaceutical industry has focused on the research and development of novel anti-cancer medicines worldwide. In recent years, a number of innovative anti-cancer medicines were approved for marketing every year (3), such as molecular targeted therapy drugs (osimertinib), immunotherapy drugs, programmed cell death protein (PD)-1 inhibitors, and PD ligand 1 inhibitors. Innovative anti-cancer medicines play an important role in the treatment of malignant tumours, bringing hope to patients with cancer who have a higher mortality risk. Moreover, innovative anti-cancer medicines can improve the quality of life; some patients may even achieve normal lives (4). Opinions of the State Council on the Implementing the Healthy China Action clearly stated that by 2022 and 2030, the overall 5-year survival rates of cancer should be >43.3% and >46.6%, respectively (5). Actively advocating for cancer prevention and promoting early diagnosis and treatment, supplemented by the early use of suitable innovative anticancer medicines, can help patients receive better treatment, thereby improving the overall five-year survival rate (6). However, due to patent protection and technology monopoly of innovative drugs, the cost of innovative anti-cancer medicines is usually higher than the catastrophic health expenditure, which imposes a financial burden on patients and hinders access to these medicines (7, 8).
In some developing countries, the financing healthcare systems rely mainly on out-of-pocket payment (OOP), which increases the risk of financial catastrophe in many patients with cancer and their families (9). In China, the high cost of cancer treatment has become a major issue in the area of social security. An empirical study conducted by the National Cancer Centre in 2016 showed that the treatment cost per patient with cancer in China was US$9,739, while the average household income in the same year was US$8,607 (10). Another study in inner Mongolia found that the annual direct medical cost for cancer reached CNY 86,100 after the basic medical insurance reimbursement, which exceeded the “catastrophic health expenditure” defined by the World Health Organisation (WHO) and imposed a heavy economic and mental burden on patients and their families (11). Furthermore, in China, a 1-month supply of Gleevec costs > CNY 23,000, which is not covered by health insurance in most cities in China. A patient with leukaemia named Yong Lu borrowed the idea from the Oscar-winning film Dallas Buyers Club and smuggled unapproved, India-made drugs for himself and for >1,000 other patients to obtain medicines at a relatively affordable price. However, any unapproved medications are illegal in China (12). Patients often give up treatment because of financial burden, putting their physical and mental health at risk (13).
In low- and middle-income countries (LMICs), poor availability and high expenditure are among the greatest obstacles to cancer treatment (14). Even in developed countries such as the United States, 29% of cancer survivors reported at least one cancer-related financial problem (15). Statistics have shown that during the median survival period, most cancer patients spend >US$150,000. In fact, only a few patients used innovative anti-cancer medicines. In 2017, 87% of these drugs were used in < 1,000 patients nationwide (16). It is important to guarantee the availability and affordability of anti-cancer medicines (17) to measure whether patients can receive medical treatment at an affordable price (18).
The 2011 Political Declaration of the High-Level Meeting of the United Nations General Assembly on the Prevention and Control of Non-Communicable Diseases declared that the global burden and threat of non-communicable diseases including cancer have caused a great social and economic burden. It highlighted the primary role of governments and the responsibilities they need to undertake (19). Thus, to improve the rational use of drugs and promote accessibility and affordability of innovative anti-cancer medicines, the Chinese government implemented a series of policies such as the National Health Insurance Coverage (NHIC) policy for innovative medicines.
For instance, Yang et al. calculated the financial burden of patients with human epidermal growth factor receptor 2-positive breast cancer who used trastuzumab after the implementation of special insurance drug coverage in Jiangsu province. The results showed that the total medical cost had dropped significantly compared to the price before policy implementation. Moreover, the compensation ratio of reimbursement increased from 23.44 to 72.81% after policy implementation (20). Tian et al. evaluated the affordability of three anti-cancer targeted drugs (gefitinib, trastuzumab, and sunitinib) used in urban and rural residents of Hubei province using the WHO and the Health Action International (HAI) standard survey method. The affordability of the three drugs increased from 64 to 74% after applying a 50% discount and the reimbursement (21). On the other hand, Diao et al. used an interrupted time-series (ITS) design to evaluate the changes in the utilisation of targeted anti-cancer medicines covered by the provincial government health insurance program from 2013 to 2016 in 69 hospitals in Hangzhou, Zhejiang province. The results showed that the inclusion of expensive targeted anti-cancer medicines in government health insurance coverage notably increased the utilisation of these medicines and improved the affordability of patients. However, the financial burden of patients remained high (22).
In April 2017, the Ministry of Human Resources and Social Security of the People's Republic of China initiated the centralised strategic price negotiation with pharmaceutical companies, which gave full play to the advantage of “group purchase” in terms of exchange quantity and price. After the price negotiation, the prices of innovative medicines were reduced and some new medicines were included in the National Insurance Medicine List. Moreover, the pharmaceutical enterprises obtained the market share under the premise of ensuring their business profits (23, 24). A total of 44 new medicines were included in the price negotiation, and a reduction in the price of 36 medicines was successfully negotiated. On average, the price of innovative medicines was reduced by 44%. Among the 36 new medicines, 15 innovative anti-cancer medicines were included in the “National Basic Medical Insurance, Work-Related Injury Insurance and Childbirth Insurance Medicine List (Category B)” (25), which was an important step toward facilitating access to expensive anti-cancer medicines (26).
The NHIC policy has been implemented in Nanjing, Jiangsu province since September 1, 2017. The 15 negotiated innovative anti-cancer medicines were listed in the “Provincial Medical Institution Drugs Centralised Procurement Online and Supervision Platform,” along with the medical insurance payment standards. With this, all public medical institutions in Nanjing could purchase the innovative anti-cancer medicines on the centralised platform. Furthermore, to reduce the financial burden of patients with cancer, the local health insurance policy reimbursed 70–90% of the cost of the 15 innovative anti-cancer medicines in Nanjing.
However, there was little evidence for government about the changes in the availability, utilisation, and affordability of these 15 anti-cancer medicines after policy implementation. Thus, in this study, we tried to explore whether the NHIC policy had a positive impact on the price, availability, utilisation, and affordability of anti-cancer medicines in Nanjing City.
Using the WHO/HAI methodology, the study aimed to analyse the impact of the NHIC policy on the availability and affordability of negotiated innovative anti-cancer medicines in 20 tertiary hospitals and six secondary hospitals in Nanjing City, the capital city of Jiangsu province. Furthermore, an ITS analysis was used to analyse the changes in the utilisation of the study medicines.
Materials and Methods
Study Design
The study was performed in Nanjing City from January 2016 to December 2018. Nanjing is the capital of Jiangsu province. It has 11 municipal districts and a population of >6.8 million permanent residents. The gross domestic product was CNY 1.17 trillion in 2017 (27). The NHIC policy was implemented on September 1, 2017, in Nanjing, where cancer is ranked as the second leading cause of death (28).
Sample and Data Source
We analysed the monthly sales data of the surveyed anti-cancer medicines from January 2016 to December 2018, which were obtained from the Jiangsu Medicine Information Institute. As primary hospitals are not qualified to prescribe most anti-cancer medicines, they were not included among the study hospitals. Moreover, tertiary and secondary hospitals that did not provide oncotherapy services were also excluded due to the lack of tumour therapy services. Hence, 20 tertiary hospitals and six secondary hospitals, including an oncology department, were included in this study. A total of 15 innovative anti-cancer medicines were evaluated in this survey. The essential features of the 15 drugs are presented in Table 1.
Date Analysis
Price
Price was expressed as the median defined daily dose cost (DDDc). A higher DDDc indicated that the medicine was more expensive. We calculated the DDDc by dividing the total amount of medicine into Defined Daily Doses (DDDs) before and after policy implementation.
Availability
Availability of innovative anti-cancer medicines was expressed as the proportion of all survey hospitals that had the study medicines within the time period. Furthermore, we compared the availability of these medicines between tertiary and secondary hospitals before and after policy implementation. The following criteria were used to describe the level of availability (18, 29, 30):
• Absent: 0%, the medicine was not available in any surveyed hospital;
• Very low: <30%, the medicine was available hardly in surveyed hospitals;
• Low: 30–50%, the medicine was available in few surveyed hospitals;
• Fairly high: 50–80%, the medicine was available in many surveyed hospitals;
• High: >80%, the medicine had a good availability and was available in most surveyed hospitals.
Utilisation
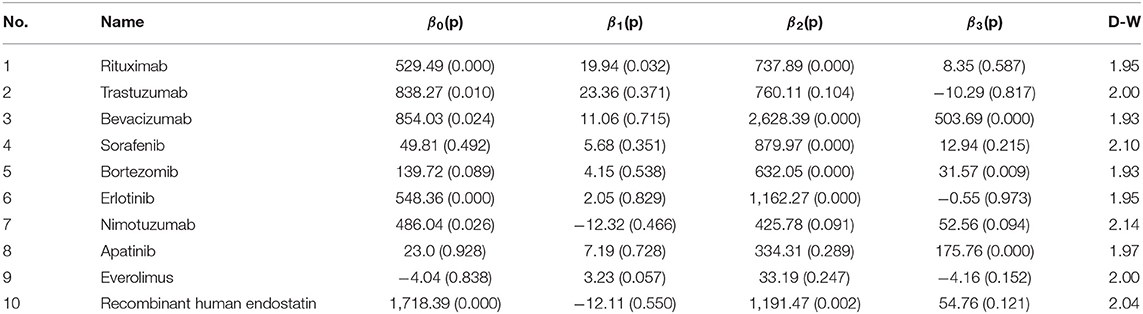
Utilisation was expressed as monthly DDDs; the greater the DDDs, the greater frequency of using the medicine. The monthly DDDs were calculated by dividing the monthly sales data in volume into the DDD. As there was no standard DDD for anti-cancer medicines, we accessed the medicine DDD information based on the daily dose and its main indication from the authoritative medicine specification database. ITS regression analysis was used to analyse the changes in the utilisation of anti-cancer medicines within 36 months. When it was difficult or impossible to find a control group, the ITS model considered a quasi-experimental design to analyse the longitudinal effects of the interventions. The ITS model could evaluate whether policy intervention had a transient or long-term impact (31). The NHIC policy was implemented on September 1, 2017. Hence, there were 20 months before intervention and 16 months after intervention. To perform independent tests, utilisation was divided into three parts—(i) the slope of the utilisation before policy implementation, (ii) the change in the level of policy intervention, and (iii) the slope after policy implementation. The following ITS model formula was used:
Yt is the monthly utilisation measured at each T time point. T is the time since the study was initiated (T = 1, 2, 3 . 36). D is the dummy variable for the two time periods before and after policy implementation (D = 0 represents the time period before policy implementation and D = 1 represents the time period after policy implementation). P is the time point after policy intervention (P = 0 indicates before policy intervention and P = 1, 2, 3, 16 indicates after policy intervention). β0 is the intercept (which refers to the outcome when the time is 0 at the baseline level), β1 is the slope of the baseline, β2 is the level of changes in the intervention, β3 is the trend change of the outcome caused by the policy intervention, β1 + β3 is the slope after the intervention, and ε is the error term (32). The ITS model is presented in Figure 1.
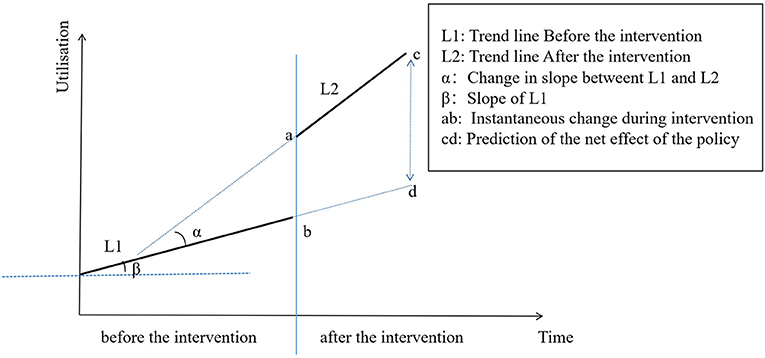
Figure 1. Graphic illustration of the interrupted time-series model and measurement of the policy effect based on trend lines for data points before and after policy implementation.
The Durbin–Watson test was used to test the first-order autocorrelation of the data (33). It is extremely possible that the observations are independent. The feasible generalised least square method was used to modify the first-order autocorrelation errors if needed (34–36). All analyses were performed using STATA v.14 software (STATA Corporation, College Station, TX, USA), and a p = 0.05 was considered significant.
Affordability
Using the WHO/HAI affordability method, the affordability of patients was estimated using the minimum daily income of unskilled government workers by determining the daily income required to purchase the selected courses of treatment for a common acute or chronic condition. Generally, if the outcome was <1, the medicine was affordable for patients. If the outcome was >1, the patients could not afford the medicines. In consideration of the long-term treatment and heavy financial burden of anti-cancer medicines, a study in Hangzhou, Zhejiang province calculated the affordability as the number of average per capita disposable annual income needed to pay for the OOP expenditure of the study medicines based on the standard treatment guideline of a certain regimen used in patients within a particular duration (22). In this study, we assessed the duration of medicine treatment based on the median progression-free survival (mPFS). For trastuzumab, the 1-year mPFS was evaluate based on the treatment guideline (37–48).
OOP expenditure = the total cost of medicine × (1–proportion of reimbursement)
The proportion of reimbursement was based on each drug OOP ratio, defined by the Nanjing Medical Insurance Bureau.
Availability of patients = OOP expenditure of the medicine for mPFS treatment course/per capita annual disposable income
Data on the per capita annual disposable income were obtained from the 2018 and 2019 Nanjing Statistical Yearbook (27, 49).
Before policy implementation, some innovative anti-cancer medicines were included in the patient assistance program (PAP) of pharmaceutical companies. In China, PAP refers to the donation of drugs or funds by pharmaceutical companies to charitable organisations or other third-party, non-profit organisations and is initiated by these organisations to help patients with specific conditions. Patients with low income or a guaranteed minimum income as defined by the local government are included in the PAP. The eligible patients receive a defined free dose of innovative anti-cancer medicines after making an OOP of a defined dose. The six innovative anti-cancer medicines included in the PAP are shown in Table 2.
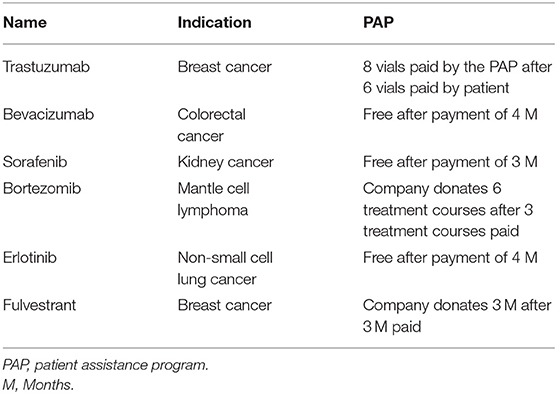
Table 2. Patient assistance program (PAP) of pharmaceutical companies to increase access to six innovative anti-cancer medicines.
Results
Changes in the Price of Anti-cancer Medicines
Table 3 shows the changes in the DDDc of the study anti-cancer medicines. The rate of change shows the impact of the NHIC policy on the DDDc. As a result of the national price negotiation, the DDDc significantly decreased, varying from 34 to 65%. The price reduction rate was between 34.39 (recombinant human endostatin) and 65.45% (trastuzumab), and mean reduction rate was 50.1%.
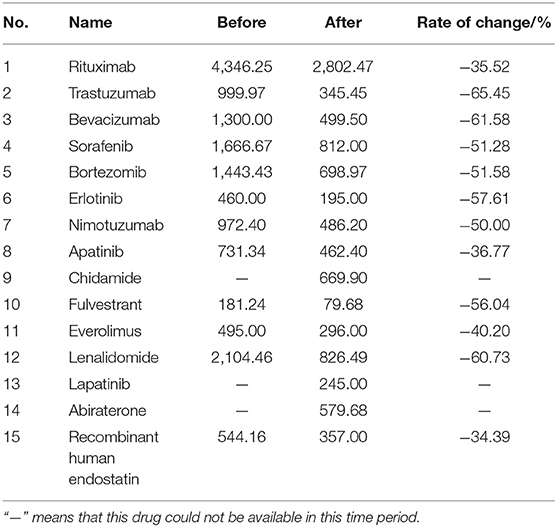
Table 3. Prices of 15 innovative anti-cancer medicines before and after policy implementation (CNY).
Changes in the Availability of Anti-cancer Medicines
As shown in Table 4, some anti-cancer medicines were unavailable before policy implementation. The mean availability of the studied anti-cancer medicines improved after policy implementation of medical insurance coverage (32.67 vs. 48.67% in tertiary hospitals; 10 vs. 25.56% in secondary hospitals). The availability of these medicines in tertiary hospitals was higher than that in secondary hospitals.
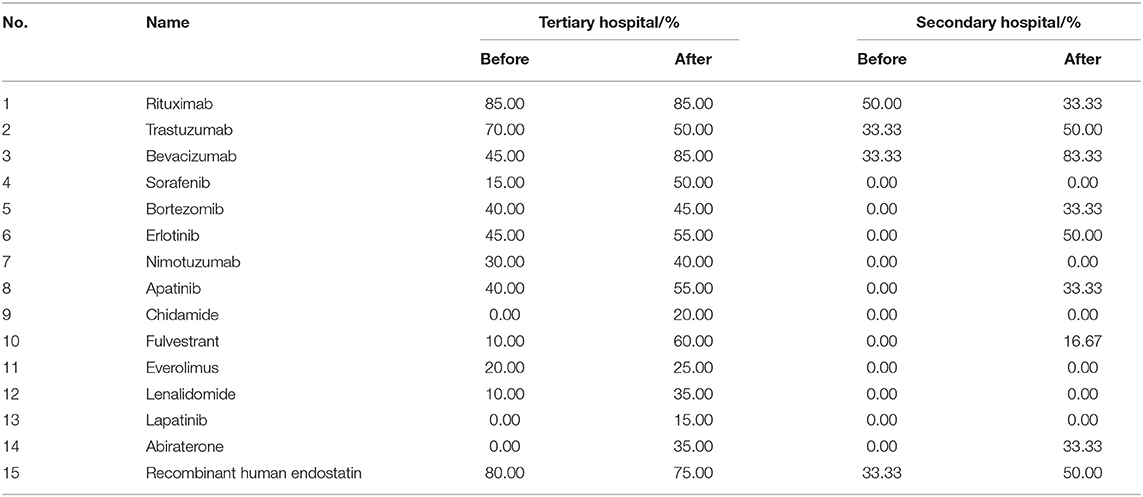
Table 4. Availability of 15 innovative anti-cancer medicines before and after policy implementation.
Changes in the Level of Utilisation
Chidamide, fulvestrant, lapatinib, lenalidomide, lapatinib, and abiraterone were excluded as these medicines had no purchase records at most time points.
Figure 2 shows the scatter plots of the observed utilisation of the study medicines. The implementation period of the policy intervention was considered September 1, 2017, and the time series was divided into two parts. Based on the results shown in Table 5, the utilisation of rituximab increased significantly before policy implementation (p < 0.001). When the NHIC policy was implemented, the utilisation rate suddenly increased [range: 33.19 (p = 0.247, everolimus) to 2,628.39 (p < 0.001, bevacizumab)]. The utilisation rate of bevacizumab, bortezomib, and apatinib significantly increased (p < 0.05), while that of trastuzumab, erlotinib, and everolimus decreased, with no significant difference (p = 0.817, p = 0.973, and p = 0.152, respectively). An upward trend was observed in the utilisation rate of nimotuzumab and recombinant human endostatin.
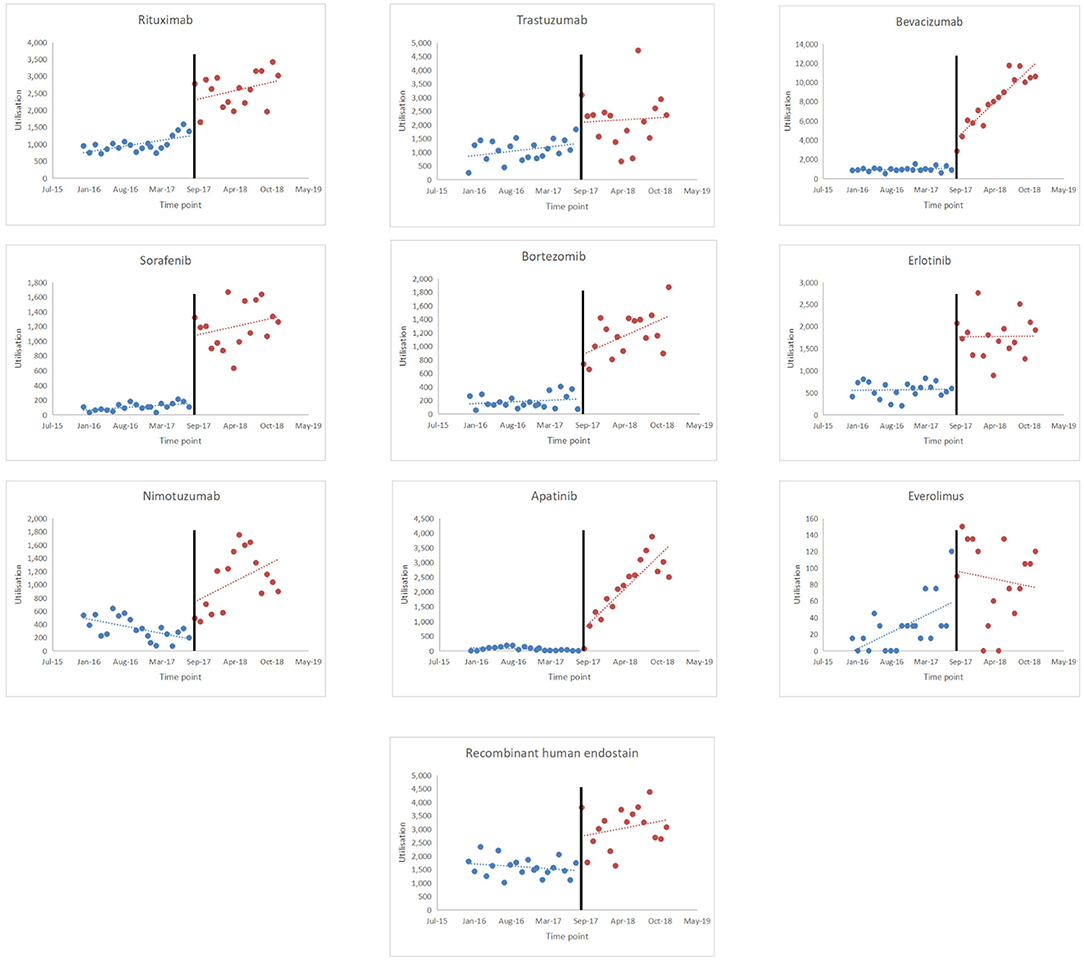
Figure 2. Results of the regression analysis of the utilisation of study drugs before and after policy implementation shown in a scatter plot.
Changes in Affordability of Patients
To compare the affordability of the studied anti-cancer medicines before and after medical reimbursement, we excluded chidamide, lapatinib, and abiraterone because they had zero purchase data before policy implementation. Among the remaining 12 drugs, one indication for each drug was selected to calculate the affordability. Table 6 shows the affordability during the mPFS treatment course of urban and rural patients before and after policy implementation. Before policy implementation, the affordability rates of patients were 0.64–16.02 times higher than per capita annual disposable income for urban patients and 1.50–37.76 times higher than per capita annual disposable income for rural patients. With the PAP, only fulvestrant could be affordable. After policy implementation, the affordability was obviously improved (0.06–1.90 times higher than that of per capita annual disposable income for urban patients and 0.13–4.46 times higher than that of per capita annual disposable income for rural patients). Therefore, most of the studied anti-cancer medicines could be affordable for patients.
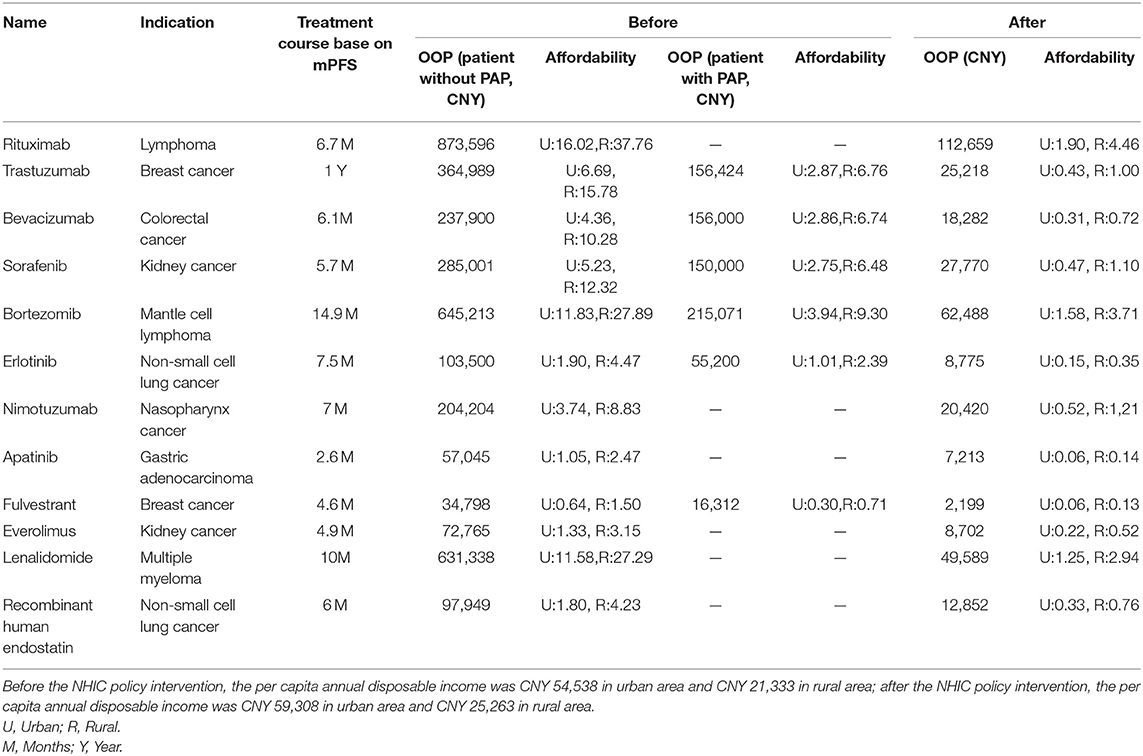
Table 6. The change of patient affordability for mPFS treatment course before and after the NHIC policy.
Discussion
Decrease in the Price of Innovative Anti-cancer Medicines
The high cost of medical treatment has been a long-term problem in China. Expensive anti-cancer medicines prevent patients, especially low-income individuals, from seeking medical treatment. Thus, reducing the price of medicines is needed.
The NHIC policy stipulates that the governments should use their bargaining power to effectively reduce the high price of innovative anti-cancer medicines. The price of trastuzumab has decreased by 65.45% (from a daily cost of CNY 999.97 to CNY 345.45) after a series of price negotiations. The policy has significantly reduced the economic burden on patients. However, the discovery and research of innovative medicines are complex and lengthy processes. Pharmaceutical companies invest a lot of talent and financial resources in drug research and development; therefore, it is important to protect innovative profits. The centralised strategic price negotiation underpinned by evidence from Health Technology Assessment (HTA) panels were drawn from a pool of pharmacoeconomists and health insurance auditors to guarantee corporate innovation profits. Pharmaceutical companies that take part in price negotiations could then obtain benefits, such as bulk sales, after their drugs were included in the medical insurance catalogue list. The government vigorously supported the development and production of innovative medicines under the guidance of the Health China Strategy and the policy established by an innovative country. Thus, enterprises should break through technical problems and continue to promote the research and development of innovative drugs under the guidance of the spirit of innovation. The NHIC policy benefits people, and pharmaceutical companies have received widespread attention from society because of this. Entering the medical insurance catalogue is a positive incentive for pharmaceutical manufacturers to speed up research and development. The price drop of innovative anti-cancer medicines is also conducive to relieving pressure on medical insurance funds.
The government has implemented a “zero-tariff” policy to ensure accessibility and promote the price reduction of imported innovative anti-cancer medicines. However, the number of anti-cancer medicines in China is relatively lower than that in other countries, with most anti-cancer medicines in the country being generic drugs, because of patent protection and technology monopolies. Hence, the government should increase the subsidy for domestic pharmaceutical companies, cultivate pharmaceutical talents, and speed up the approval process of innovative drugs to embrace the innovative enthusiasm of enterprise in China.
Positive Effects of the NHIC Policy on the Availability of the Studied Drugs
The availability of innovative anti-cancer medicines is an important index to evaluate whether patients could access medical treatment. This study found that patients had more chances to access the studied anti-cancer medicines in tertiary hospitals than in secondary hospitals throughout the study period. This could be due to the fact that patients in China are more inclined to seek medical treatment in tertiary hospitals when they have serious illnesses, such as cancer. However, an increase in the number of patients with cancer will greatly increase the resource demand. The mean availability of the studied anti-cancer medicines was 27.44%, implying that these drugs were hardly available in the surveyed hospitals before the NHIC policy was implemented, and this low availability may be caused by various factors. One factor is the medication expenditures related to the availability of these drugs in hospitals in LMICs (50). The 15 negotiated innovative anti-cancer medicines were all originator brands, and the prices of these drugs were higher than those of other common medicines even after the price negotiation. The high cost might be related to the low availability of these medicines in hospitals. According to the study of Zhu et al. in Jiangsu province, health service providers make higher profits from more costly pharmaceuticals because of the markup ratio prescribed by the government. The “zero-tariff” policy achieved full promotion in the public hospitals in Jiangsu province by the end of 2015. However, for hospitals, capital occupation costs, management costs, and damage costs are expensive, which are likely to reduce the revenue of hospitals without the mark-up ratio for high-value drugs. Therefore, due to financial constraints, secondary hospitals were unable to supply the innovative anti-cancer medicines (18). Furthermore, a study conducted in Pakistan showed that government hospitals experienced medicine shortage or medicine unavailability more frequently compared to private hospitals (17). In addition, some of the negotiated drugs lack real-world data, and similar alternative medicines are being sold in hospitals; thus, the purchase enthusiasm was not high.
After policy implementation, the mean availability increased to 43.33%, which means that the 15 innovative anti-cancer medicines became available in a few of the surveyed hospitals. Thus, the NHIC policy had a positive impact on the availability of medications in the hospital. However, drug availability is still low, which might be associated with the control of medical insurance cost. The control of medical insurance cost is an effective way to control the growth of medical expenses by allocating the medical insurance budget to all medical institutions and covering all medical services. Once the medical insurance costs of the hospital exceed the budget, however, the hospital needs to pay the excess cost. As the price of innovative anti-cancer medicines remained high, the hospitals were not motivated to pay the bill.
Hospitals should insist on the rational use of innovative anti-cancer medicines to avoid unnecessary medical expenses. Moreover, the government should release a supporting policy to separate the cost of innovative anti-cancer medicines and innovate the health insurance payment of negotiated drugs to facilitate the availability of innovative anti-cancer medicines in hospitals.
Factors Associated With Changes in Utilisation
The epidemic characteristics of cancer affect the utilisation of drugs. Lung cancer and breast cancer have a high incidence in China; the baseline levels of anti-cancer treatment drugs such as trastuzumab, bevacizumab, and recombinant human endostatin were high. The monthly utilisation of 10 of the study medicines suddenly increased in September 2017. One possible explanation is that new patients and their doctors waited for the implementation of the policy to use much cheaper drugs. The utilisation of innovative anti-cancer medicines increased at different levels after the policy was implemented. The most important reason was the price reduction, which may have affected the purchasing behaviour of patients. Many factors were associated with the changes in utilisation. For example, the remarkable increase in the utilisation of bevacizumab and bortezomib may be related to the cancellation of the PAP, which possibly led patients to buy more drugs at their own expense. The utilisation of apatinib for gastric adenocarcinoma significantly also increased, mainly because gastric adenocarcinoma had a high incidence in China. Furthermore, there was no obvious upward trend in the utilisation of other innovative anti-cancer medicines, which may be associated with the limitation of indications by the insurance program. For example, only eight treatment courses of rituximab, which is used to treat relapsed or drug-resistant follicular central lymphoma and non-Hodgkin's lymphoma, are covered by medical insurance. In Nanjing, patients with cancer can purchase innovative anti-cancer medicines with a prescription from a physician at the specialty pharmacy.
This study analysed the utilisation of innovative anti-cancer medicines in hospitals but not in retail pharmacies. Therefore, this study cannot completely reflect the utilisation of innovative anti-cancer medicines in Nanjing. In addition, the off-label drug use of innovative anti-cancer medicines has been previously reported. Standardised rational medication in hospitals has improved with the development of guidelines for the clinical diagnosis and treatment of cancers. This improvement may affect the utilisation of innovative anti-cancer medicines.
Including innovative anti-cancer medicines in the medical insurance implies an increase in insurance expenditures in the future and calls for a more careful monitoring of drug use. The government should improve the dynamic adjustment mechanism of the medical insurance catalogue to guarantee the accessibility of patients to innovative drugs and reduce the burden of medical insurance funds.
Improvement in the Affordability of Innovative Anti-cancer Medicines
Before the implementation of the NHIC policy, the OOP expenditures on the 12 innovative anti-cancer medicines for the mPFS treatment course were much higher than the per capita annual disposable income per capita. Even when the PAP relieved the economic burden of six innovative anti-cancer medicines to some degree, the high cost of innovative anti-cancer medicines may impoverish more households and lead to more premature cancer deaths (51). Therefore, the drug price reduction is closely related to winning the battle against poverty. Drug price reduction and reimbursement effectively alleviated most of the financial burden on patients. With these, patients can purchase innovative anti-cancer medicines, which can effectively improve the quality and length of life.
However, although the NHIC policy improved drug affordability, the supporting policies that help reduce the financial burden of innovative anti-cancer medicines were inadequate. For example, the PAP was cancelled after the NHIC policy was implemented. However, the affordability problem was even more serious for rural patients than for urban patients, especially low-income patients, during the study period. Hence, the government should increase tax incentives for medicines and encourage companies to implement more assistance projects to promote the PAP and improve the medical security system of China. Moreover, patients need to undergo a number of medical examinations and standard tests for cancer before being diagnosed. They also need to use adjuvant chemotherapy drugs for treatment. However, the costs of medical examinations and adjuvant chemotherapy are a tremendous financial burden.
Policies should focus on addressing the inequity in the affordability of innovative anti-cancer medicines between urban and rural areas. The government should also consider including medical examinations and adjuvant chemotherapy in the medical insurance coverage and reducing the ratio of individual payments. Setting the reimbursement ratio based on the actual conditions of patients is a better measure; the reimbursement ratio of rural patients should be higher than that of urban patients. Moreover, the government should speed up the progress of medical insurance negotiations to include more innovative anti-cancer medicines in medical insurance to reduce the financial burden of more patients with cancer. With the increase in the incidence of cancer, the government should increase investment in health. Early screening, detection, diagnosis, and treatment are of great importance to protect the health of the people.
Our study has several strengths. This study is one of the few quantitative studies that provide evidence on the accessibility of the 15 negotiated innovative anti-cancer medicines. The study findings will serve as a basis for policymakers in the government to optimise and improve supporting policies. This study selected 36 months of data before and after the policy was implemented to comprehensively evaluate the long-term effects of the NHIC policy. The ITS model was used to better reflect the impact of policy interventions and the later stages of intervention. Nevertheless, this study has some limitations. First, we underestimated the availability and utilisation of anti-cancer medicines because private hospitals and pharmacies were not included in this study. Second, this study lacked a control group to explore other factors besides policy implementation that resulted in changes in drug utilisation. Third, this study was only conducted in Nanjing, Jiangsu province. More municipalities and provinces should be included to increase the representativeness of the findings. Medication equity is one of the purposes of the NHIC policy. Changes in medication equity after policy implementation may need to be analysed in future studies.
Conclusion
This study revealed that the NHIC policy effectively reduced the price and improved the availability and utilisation of 15 negotiated innovative anti-cancer medicines. The NHIC policy reduced the economic burden of patients with cancer. However, the availability of these innovative anti-cancer medicines remains low. The utilisation rate of innovative anti-cancer medicines is affected by many factors, which include the incidence of cancer, limitation of indications within the insurance program, and the rational use of innovative anti-cancer medicines. Improving supporting policies is necessary to promote the use of innovative anti-cancer medicines, and reducing the financial burden of patients is closely related to winning the battle against poverty. The government should introduce more policies to reduce the financial burden of patients and improve the health level of people. In addition, more innovative anti-cancer medicines should be included in the national price negotiation. The government should consider including medical examinations and adjuvant chemotherapy in the medical insurance and increase investment in health.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
WF, XX, and XL initiated the study concept. WF conducted data analysis and wrote the first draft of the manuscript. XX and YZ collected the drugs clinical trials data. HD led the data collection. XL and LS contributed to the data analysis and interpretation of the data and revised the draft of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 72074123), the Soft Science Project funded by Jiangsu Science and Technology Department (No. BR2020043).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful for the support of Jiangsu Medicine Information Institute. We also appreciate all participants who contributed to the data collection and analysis in this study. We would like to thank Editage (www.editage.com) for English language editing.
References
1. Sun KX, Zheng RS, Zhang SW, Zeng HM, Zou XN, Chen R, et al. Report of cancer incidence and mortality in different areas of China, 2015. China Cancer. (2019) 28:1–11. doi: 10.11735/j.issn.1004-0242.2019.01.A001
2. Chen ZN. The frontier and innovation of biomedicine in the new era. China Food & Drug Administration Magazine. (2019) 11:8–13. doi: 10.3969/j.issn.1673-5390.2019.11
3. Wilson A, Cohen J. Patient access to new cancer drugs in the United States and Australia. Value in Health. (2011) 14:944–52. doi: 10.1016/j.jval.2011.05.004
4. Zhou YP, Wang HG, Hu QH, Zhou RS, Cheng BS, Kang FH, et al. Accessibility of high-cost anti-cancer medicines in China. Chinese J Evidence-Based Med. (2017) 17:862–8. doi: 10.7507/1672-2531.201703028
5. THE STATA COUNCIL. Opinions of the State Council on implementing the Health China Action. (2019). Available online at: http://www.gov.cn/zhengce/content/2019-07/15/content_5409492.htm (accessed: December 24, 2020).
6. Li Y. Don't let innovative anti-cancer drugs become a mirror image, experts call for improving access to innovative drugs. Pharmaceutical Economic News. (2020). Available online at: //kns.cnki.net/kcms/detail/detail.aspx?DBCode=CCND&DBName=CCNDLAST2020&fileName=YYJJ202010220060 (accessed January 1, 2021).
7. Kantarjian HM, Fojo T, Mathisen M, Leonard A. Zwelling. Cancer drugs in the United States: Justum Pretium—the just price. J Clini Oncol. (2013) 31:3600–4. doi: 10.1200/JCO.2013.49.1845
8. Robinson JC, Howell S. Specialty pharmaceuticals: policy initiatives to improve assessment, pricing, prescription, and use. Health Aff. (2014) 33:1745–50. doi: 10.1377/hlthaff.2014.0498
9. Saluja R, Arciero VS, Cheng S, McDonald E, Wong WWL, Cheung MC, et al. Examining trends in cost and clinical benefit of novel anticancer drugs over time. J Oncol Pract. (2018) 14:e280–94. doi: 10.1200/JOP.17.00058
10. Huang HY, Shi JF, Guo LW, Bai YN, Liao XZ, Liu GX, et al. Expenditure and financial burden for common cancers in China: a hospital-based multicentre cross-sectional study. Lancet. (2016) 388:S10. doi: 10.1016/S0140-6736(16)31937-7
11. Wang L. The exploration of medical insurance in precise poverty alleviation in inner Mongolia Bringing tumor targeting drugs into the payment of m4edical insurance. Chinese Health Insurance. (2016) 48–50. doi: 10.369/j.issn.1674-3830.2016.3.009
12. China Daily USA. China Society. Cancer patients look to India for lifesaving drugs. (2015). Available online at: http://usa.chinadaily.com.cn/china/2015-01/28/content_19426279.htm (accessed: January 1, 2021).
13. Kale HP, Carroll NV. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. (2016) 122:283–289. doi: 10.1002/cncr.29808
14. Knaul F, Frenk J, Shulman L. Closing the Cancer Divide: A Blueprint to Expand Access in Low and Middle Income Countries. In: Social Science Research Network. Boston, MA, USA: Harvard Global Equity Initiative (2011).
15. Kent EE, Forsythe LP, Yabroff KR, Weaver KE, de Moor JS, Rodriguez JL, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care?. Cancer. (2013) 119:3710–3717. doi: 10.1002/cncr.28262
16. Ji J. Special report on consumption upgrading series (ii): Access to anti-cancer medicines is increasing. Modern Commercial Banking. (2018) 21:51–8. (in chinese).
17. Sarwar MR, Iftikhar S, Saqib A. Availability of anticancer medicines in public and private sectors, and their affordability by low, middle and high-income class patients in Pakistan. BMC Cancer. (2018) 18:14. doi: 10.1186/s12885-017-3980-3
18. Zhu Y, Wang Y, Sun X, Li X. Availability, price and affordability of anticancer medicines: evidence from two cross-sectional surveys in the Jiangsu Province, China. Int J Environ Res Public Health. (2019) 16:3728. doi: 10.3390/ijerph16193728
19. Bollyky TJ. Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases. (2011). Available online at: https://www.un.org/en/ga/president/68/pdf/calendar/20140711-ncd.pdf (accessed January 1, 2021).
20. Yang S, Wang Y, Xu W, Du ZZ, Jiang XS, Du WW. Research on the evaluation of special drug policy in Changzhou, Jiangsu: taking HER-2 positive breast cancer as the example. China Health Economics. (2017) 36:43–5. doi: 10.7664/CHE20170311
21. Tian MY, Cui D, Zhang YX, Yin X, Fang X, Hu JL. Affordability evaluation for 3 kinds of anti-cancer medicines targeted drugs: Taking Hubei province as an example. China Pharmacy. (2017) 28:2746–9. doi: 10.6039/j.issn.1001-0408.2017.20.03
22. Diao Y, Qian J, Liu Y, Zhou Y, Wang Y, Ma H, et al. How government insurance coverage changed the utilisation and affordability of expensive targeted anti-cancer medicines in China: an interrupted time-series study. J Glob Health. (2019) 9:020702. doi: 10.7189/jogh.09.020603
23. Si L, Xu LZ, Chen MS, Jan S. Using strategic price negotiations to contain costs and expand access to medicines in China. BMJ Glob Health. (2020) 5:e002256. doi: 10.1136/bmjgh-2019-002256
24. Morgan S, Daw J, Thomson P. International best practices for negotiating ‘reimbursement contracts' with price rebates from pharmaceutical companies. Health Aff(Millwood). (2013) 32:771–7. doi: 10.1377/hlthaff.2012.1268
25. Jiangsu province department of human resources and social security. Notice of Jiangsu provincial department of human resources and society on the inclusion of 36 drugs into list of basic medical insurance, industrial injury insurance and maternity insurance. (2017). Available online at: http://jsrlzyshbz.jiangsu.gov.cn/art/2017/8/4/art_77281_9077851.html (accessed: May 31, 2020).
26. Gao L, Wang X. Healthcare Supply Chain Network Coordination Through Medical Insurance Strategies with Reference Price Effect. Int J Environ Res Public Health. (2019) 16:3479. doi: 10.3390/ijerph16183479
27. Nanjing Bureau of Statistics. China. Nanjing Statistical Yearbook 2018. (2019). Available online at: http://tjj.nanjing.gov.cn/ (accessed: May 30, 2020).
28. Nanjing Municipal Health Commission. A brief analysis of the causes of deaths from illnesses and injuries of Nanjing residents in 2017. (2018). Available online at: http://wjw.nanjing.gov.cn/njswshjhsywyh/201810/t20181023_643194.html (accessed: May 30, 2020).
29. Yang H, Dib HH, Zhu MM, Qi G, Zhang XP. Prices, availability and affordability of essential medicines in rural areas of Hubei Province, China. Health Policy Plan. (2010) 25:219–229. doi: 10.1093/heapol/czp056
30. Gong SW, Cai HB, Ding YF, Li WJ, Juan X, Peng JL, et al. The availability, price and affordability of antidiabetic drugs in Hubei province, China. Health Policy Plan. (2018) 33:937–947. doi: 10.1093/heapol/czy076
31. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. (2002) 27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x
32. Wang Y, Zhu Y, Shi H, Sun X, Chen N, Li X. The effect of the full coverage of essential medicines policy on utilization and accessibility of primary healthcare service for rural seniors: a time series study in Qidong, China. Int J Environ Res Public Health. (2019) 16:4316. doi: 10.3390/ijerph16224316
33. Crosbie J. Interrupted time-series analysis with brief single-subject data. J Consult Clin Psychol. (1993) 61:966–74. doi: 10.1037/0022-006X.61.6.966
34. Orcutt G. Application of least squares regression to relationships containing auto-correlated error terms. J Am Stat Assoc. (1949) (245):32–61. doi: 10.1080/01621459.1949.10483290
35. Choi CY, Hu L, Ogaki M. Robust estimation for structural spurious regressions and a Hausman-type cointegration test. J Econometric. (2008) 142:327–51. doi: 10.1016/j.jeconom.2007.06.003
36. Ali S, Liu Y, Ishaq M, Shah T, Abdullah, Ilyas A, Din IU. Climate Change and Its Impact on the Yield of Major Food Crops: Evidence from Pakistan. Foods. (2017) 6: E39. doi: 10.3390/foods6060039
37. Zinzani PL, Khuageva NK, Wang H, Garicochea B, Walewski J, Van Hoof A, et al. Bortezomib plus rituximab versus rituximab in patients with high-risk, relapsed, rituximab-naïve or rituximab-sensitive follicular lymphoma: subgroup analysis of a randomized phase 3 trial. J Hematol Oncol. (2012) 5:67. doi: 10.1186/1756-8722-5-67
38. Professional committee of breast cancer. Chinese anti-cancer association. Guidelines and norms for the diagnosis and treatment of breast cancer in Chinese anti-cancer association. Chinese Oncology. (2015) 25:692-754. (in chinese).
39. Yin CX, Ma G, Rong YM, Kong PF, Yang Q, Jiang C, et al. The efficacy of bevacizumab in different line chemotherapy for chinese patients with metastatic colorectal cancer. J Cancer. (2016) 7:1901–6. doi: 10.7150/jca.15802
40. Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. (2013) 14:552–562. doi: 10.1016/S1470-2045(13)70093-7
41. Orlowski RZ, Nagler A, Sonneveld P, Bladé J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. (2007) 25:3892–3901. doi: 10.1200/JCO.2006.10.5460
42. Scagliotti GV, Shuster D, Orlov S, von Pawel J, Shepherd FA, Ross JS, et al. Tivantinib in Combination with Erlotinib versus Erlotinib Alone for EGFR-Mutant NSCLC: An Exploratory Analysis of the Phase 3 MARQUEE Study. J Thorac Oncol. (2018) 13:849–854. doi: 10.1016/j.jtho.2017.12.009
43. Zhao C, Miao J, Shen G, Li J, Shi M, Zhang N, et al. Anti-epidermal growth factor receptor (EGFR) monoclonal antibody combined with cisplatin and 5-fluorouracil in patients with metastatic nasopharyngeal carcinoma after radical radiotherapy: a multicentre, open-label, phase II clinical trial. Ann Oncol. (2019) 30:637–643. doi: 10.1093/annonc/mdz020
44. Li J, Qin SK, Xu JM, Xiong JP, Wu CP, Bai YX, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. (2016) 34:1448–1454. doi: 10.1200/JCO.2015.63.5995
45. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. (2016) 17:425–439. doi: 10.1016/S1470-2045(15)00613-0
46. Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. (2010) 116:4256–4265. doi: 10.1002/cncr.25219
47. Kim K, Kim SJ, Voelter V, Suh C, Yoon SS, Lee JJ, et al. Lenalidomide with dexamethasone treatment for relapsed/refractory myeloma patients in Korea-experience from 110 patients. Ann Hematol. (2014) 93:113–21. doi: 10.1007/s00277-013-1893-z
48. Wang Z, Zhang H, Zhou C, Long X, Guan R, Yang N, et al. Real-world outcomes of various regimens of recombinant human endostatin combined with chemotherapy in non-driver gene mutation advanced non-small cell lung cancer. Cancer Med. (2019) 8:1434–1441. doi: 10.1002/cam4.2014
49. Nanjing Bureau of Statistics. China. Nanjing Statistical Yearbook 2019. (2020). Available online at: http://tjj.nanjing.gov.cn/ (accessed May 30, 2020).
50. Kolasani BP, Malathi DC, Ponnaluri RR. Variation of Cost among Anti-cancer Drugs Available in Indian Market. J Clin Diagn Res. (2016) 10:FC17–FC20. doi: 10.7860/JCDR/2016/22384.8918
Keywords: innovative anti-cancer medicines, national health insurance coverage, utilisation, affordability, interrupted time series
Citation: Fang W, Xu X, Zhu Y, Dai H, Shang L and Li X (2021) Impact of the National Health Insurance Coverage Policy on the Utilisation and Accessibility of Innovative Anti-cancer Medicines in China: An Interrupted Time-Series Study. Front. Public Health 9:714127. doi: 10.3389/fpubh.2021.714127
Received: 24 May 2021; Accepted: 06 July 2021;
Published: 06 August 2021.
Edited by:
Kevin Lu, University of South Carolina, United StatesReviewed by:
Chunsong Yang, Sichuan University, ChinaYumei Zhu, China Pharmaceutical University, China
Copyright © 2021 Fang, Xu, Zhu, Dai, Shang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, eGlubGlAbmptdS5lZHUuY24=; Linlin Shang, U0xMX1pZMTIzQDE2My5jb20=
 Wenqing Fang
Wenqing Fang Xinglu Xu2
Xinglu Xu2 Yulei Zhu
Yulei Zhu Xin Li
Xin Li