
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 05 November 2021
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.710209
Most cervical cancers were closely associated with human papillomavirus (HPV) infections. Therefore, understanding the ecological diversity of HPV prevalence and genotype distribution among various populations in different geographical regions was essential for optimizing HPV vaccination and maximizing the vaccination effects. A total of 12,053 patient data from the three-level hospitals in Hengyang city were retrospectively analyzed. In this study, the HPV prevalence was 10.16% overall, and the multiple-type infection rate was 1.83%. The HR-HPV infection rate was 8.52%. The top six HPV genotypes were as follows in descending order: HPV16, HPV58, HPV52, HPV39, HPV51, and HPV53. The HPV prevalence in the group above 60 years old was the most, and their HR-HPV infection rate corresponded to the most too. The infection rates of HPV and HR-HPV among outpatients were both lower than those among the hospitalized-patients, respectively. Among the hospitalized-patients, the infection rates of HPV and HR-HPV among the 50–60 years group were the most in both. The HR-HPV ratio-in-positive among HPV-positive patients with the histopathologic examination was higher than that among those patients without. Among 52 HPV-positive patients with cervical squamous carcinoma, the ratio-in-positive of HPV16 was 61.54%. This study demonstrated that the HPV prevalence varied with age among women from Hengyang district of Hunan province in China and showed that HPV16, HPV58, HPV52, HPV39, HPV51, and HPV53 genotypes were more popularly distributed in this region, which could provide the experimental basis for Chinese public health measures on cervical cancer prevention.
Human papillomavirus (HPV) is a spherically non-enveloped and double-stranded DNA virus and prefers to infect the epithelial cells of the skin and mucous membrane. HPV has more than 200 genotypes, and its infection is widespread in the crowd (1). According to the disease risk caused by HPVs, they were divided into low-risk HPV (LR-HPV) genotype and high-risk HPV (HR-HPV) genotype. Nearly all cervical cancers and also a large proportion of epithelial cancers including some vulvar, vaginal, anal, penile, and oropharyngeal cancers were caused by HPVs (2). Cervical cancer had become the second leading cause of women's death globally, especially in developing countries according to the reports of the International Agency for Cancer Research (3, 4). In China, there were more and more new cases of cervical cancer (5, 6), suggesting that cervical cancer had put a heavy burden on Chinese women, where HPV-related infections were the primary cause of their morbidity and mortality (3). At present, the potential long-term solution for preventing cervical cancer in developing countries is vaccination against HPV. The bivalent HPV vaccine (Cervarix) against HPV16/18, the tetravalent vaccine (Gardasil) against HPV6/11/16/18, and the 9-valent HPV vaccine (2nd Gardasil) against HPV 6/11/16/18/31/33/45/52/58 can be possible to prevent most cervical cancers related their corresponding HPV genotype. However, these HPV vaccines were not therapeutic but prophylactic to cervical cancer and had dissatisfied effects on married women. Moreover, these vaccines were not only expensive but also scarce for Chinese women. Meanwhile, those vaccines were introduced to Chinese women lately, result in unclear long term effects and even may not fit them very well. Therefore, the HPV infection of Chinese women should not be neglected still.
The cervical cancer susceptibility can be affected mainly by HPV infection and varied with some other factors, such as HPV awareness and toll-like receptor gene polymorphisms (7–9). It was reported that due to the nation, socioeconomic status, age, ethnicity, and geographic location of populations were different, the HPV prevalence was substantially varied with different populations (10, 11), and the HPV genotype distribution also differed in various regions worldwide. Therefore, prevention and control measures for HPV infections should be implemented according to their local situation (6). Understanding the ecological diversity of HPV prevalence and its genotype distribution among various populations in different geographical regions of China is essential to optimize HPV vaccination and maximize its effects on Chinese women.
Hengyang city has 7.337 million permanent residents from over 40 nationalities and is the deputy center of Hunan province in China, which also is the second-largest city in this province. Therefore, the HPV prevalence and HPV genotype distribution of this city are significant for Chinese epidemiological data, and it can provide important advice for Chinese public health measures on cervical cancer prevention.
In this study, the data of 12,053 women from the tertiary hospitals in Hengyang city between July 2018 and July 2019 were retrospectively analyzed. HPV testing was used as a routine examination for those suspected gynecologic patients from better-off families. HPV positive was identified by PCR, and HPV PCR-Flow fluorescence assay was used for HPV genotyping. 27 HPV genotypes were tested, including 17 HR-HPV genotypes (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 26, 53, and 82) and 10 LR-HPV genotypes (HPV 6, 11, 40, 42, 43, 44, 55, 61, 81, and 83).
The prevalence of HPV infection, presence of single or multiple HPV infections, and HPV genotypes, as well as, their corresponding 95% CIs were estimated with binomial distribution analysis. The Chi-square test was performed to compare the differences in the infection rates (the positive amount occupied among its corresponding whole population) of HPV or HR-HPV and the ratio-in-positive (the positive amount occupied among its corresponding positive population) of HR-HPV or LR-HPV among various patients. A p-value of 0.05 was considered statistically significant. The statistical data analysis was performed by SPSS version 13.0 (SPSS, IBM, USA).
A total of 1,224 women were positive for HPV infection in 12,053 women, among which 1,003 were positive for a single genotype infection and 221 for multiple infections (Figure 1). It was reported that HPV prevalence in women worldwide had significant regional variations too, for example in 2012 Africa had the highest HPV prevalence (24%), followed by Eastern Europe (21%), Latin American (16%), and South-Eastern Asia (14%) in turn (12). In this study, the HPV infection rate was about 10.16% (1,224/12,053) overall, which was comparable with those reported in Zhejiang Province (13.3%) (13) in China, and those reported in other countries, such as in Guatemala (13%) (14) and in Iran, its neighboring countries, and Persian Gulf area (0.62–25%) (15), but was lower than those reported in some regions of China, such as Shenzhen City (15.9%) (16), the northwestern Yunnan Province (18.4%) (17), southern Yunnan Province (14.7%) (18), Yangcheng County (14.8%) (19), urban Tianjin (14.71%) (20), Shenyang City (16.8%) (21), south Taiwan (19.3%) (22), and those reported in other countries, such as Denmark, Norway, and Sweden in 2006–2008 (36.5%), and in 2012–2013 (34.5%) (23). The different HPV prevalence from Hengyang populations may be because that these various investigated people were from different geographical regions, from different nations, and with different ethnicity, lifestyle, and urbanization.
In the current study, 221 women were positive for multiple infections, among which a total of 163 patients were with double infections, 36 patients with triple infections, and 12 patients with quadruple infections, and there were patients even with septuple or sextuple infections (Figure 1). As shown in Figure 1, the multiple-genotype infection rate was 1.83% (221/12,053), which was similar to that in Beijing (1.5%) from 2014 to 2015 (24), but lower than those in Yunnan Province (4.2%) (17, 25). The high multiple-infection rate maybe because the diseases were more complicated or the illness more serious, which was in accordance with the report that multiple infections could elevate the risk of cervical cancer (26). Therefore, routine screening for HPV is very necessary for the health of women.
From 12,053 patients, 27 different HPV genotypes were identified. As shown in Figure 2, 1,027 women were infected by at least one type of HR-HPV, that is, HR-HPV infection rate was 8.52% (1,027/12,053), which was also a little lower than that found among women of Sichuan province (12.6%) (27), those among Han women from Mojiang county of Yunnan Province (12.6%) (28), and among the rural and urban women of southern Yunnan Province in China (11.4%) (17), and it was comparable with those reported from Beijing City (9.9%) (29), Tibet City (7.0%) (30), or Zhejiang Province (10.2%) (13) in China.
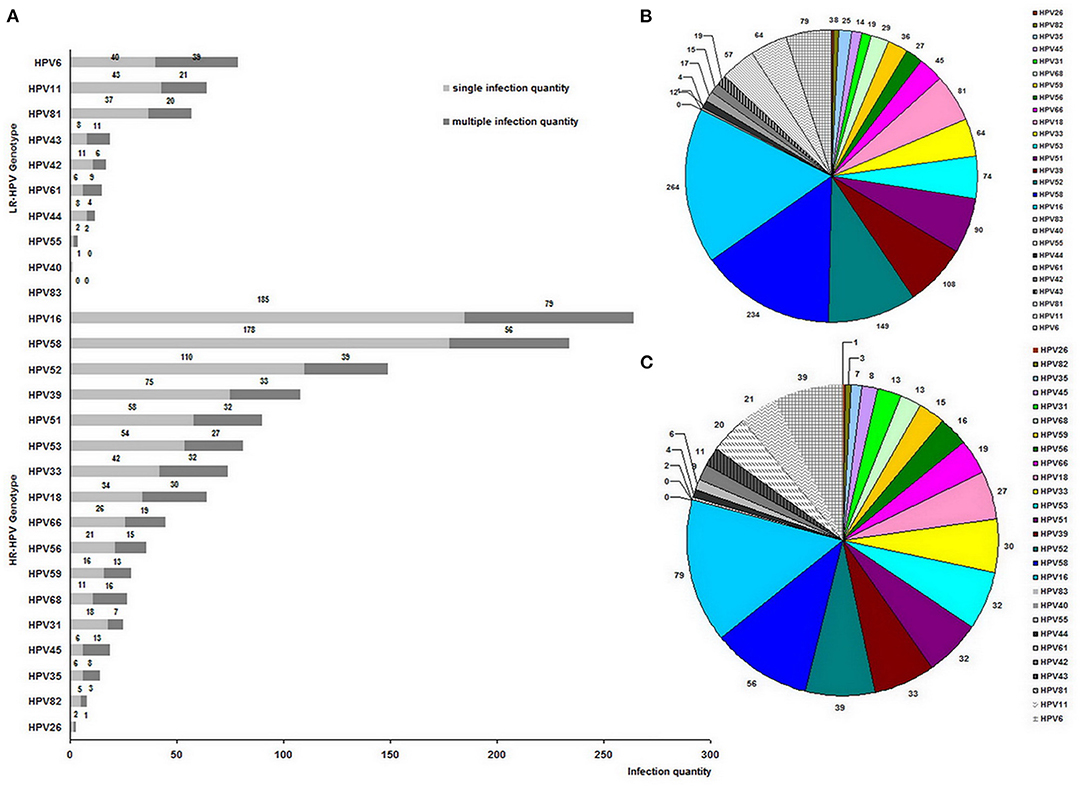
Figure 2. Genotype distribution of 1,538 clinical HPV strains among 1,224 HPV-positive women (A), and genotype proportion of 1,538 clinical HPV strains (B), and 535 clinical HPV strains from multiple infections (C).
The distribution of the remaining top five genotypes was as follows in descending order: HPV58, HPV52, HPV39, HPV51, and HPV53, which differed from HPV prevalence in Guatemala, where the top six genotypes were HPV16, HPV18, HPV39, HPV58, HPV52, and HPV45 (14). Furthermore, this result about HPV genotype distribution was also different in that HPV52 had the most frequency followed by HPV39 and HPV68 among the urban population of Yunnan Province and some previous reports in China or other Asian countries (31, 32). However, this result was mainly consistent with the investigation in the Wuhan City of Hubei Province, where the most common genotype was HPV 52 followed by HPV 16, 58, 39, and 51 (33), and in agreement with the results in the rural population of Yunnan Province (18) and in Shanxi Province of China (34), where the relatively high prevalence of HPV16, HPV58, and HPV52 infections were reported, confirming that HPV58 and HPV52 were also responsible for HPV infections in Asia.
Among 12,053 women, HPV16 was the most frequent genotype with an infection rate of 2.19% (264/12,053). Moreover, the HPV16 positive patients occupied 21.57% among 1,224 HPV positive patients, that is, the ratio-in-positive of HPV16 was 21.57% (264/1,224), which was near to that (22%) in Guatemala (14), indicating that HPV16 was the most frequent as the general in all continents of the world. However, it could be seen that a slightly inferior ratio-in-positive (19.12%) was from HPV58, suggesting that HPV58 was the same major infected-genotype as HPV16 in China. In the Western country or South American continents, such as in Guatemala (14), HPV18 occupied the second frequency, but in Asian countries, HPV58 and HPV52 were more prevalent than HPV18 (35, 36). The variation of genotype distribution in Asia may be due to the different geographic regions, the diverse gene interactions, and various immune levels of patients (35). Therefore, the introduced HPV vaccine from the West does not fit Chinese women very well, suggesting that the study on the native therapeutic vaccine is still important for Chinese researchers.
The amount of all clinical HPV strains found from 1,224 positive women was 1,538, among which 535 clinical HPV strains were from 221 women with multiple infections. As shown in Figure 2B, HR-HPVs occupied 82.57% (1,270/1,538) and were more popular than exclusive LR-HPVs (17.42%, 268/1,538), which was the same as among some population from Yunnan Province (17, 18), Shanxi Province (19), Taiwan Province (22) in China and some Amerindians (37). Moreover, the infections by HR-HPV were more popular than those by exclusively LR-HPV among these women as Amerindians (37). Also, the cervical abnormalities were consistent with their high HPV infection prevalence. Furthermore, among the multiple infection genotypes, HPV16 was detected most frequently, and HR-HPVs were more popular than LR-HPVs (Figure 2C).
Although the proportion of outpatients was 66.42% (8,005/12,053) more than that (33.58%, 4,048/12,053) of hospitalized-patients, their HPV infection rate (8.96%, 717/8,005) was lower than that (12.52%, 507/4,048) of hospitalized-patients (p < 0.01) (Figure 3A), the same as their multiple HPV infection rate (1.17%, 94/8,005; 3.14%, 127/4,048) (Figure 3B). It may be due to the fact that the hospitalized patients had severe or more complicated illnesses than outpatients, which may be explained by the fact that HPV infection, especially multiple HPV infections, would worsen some gynecologic related diseases or cause some severe diseases that occurred while suggesting that it was urgent to develop the therapeutic vaccine for HPV infection.
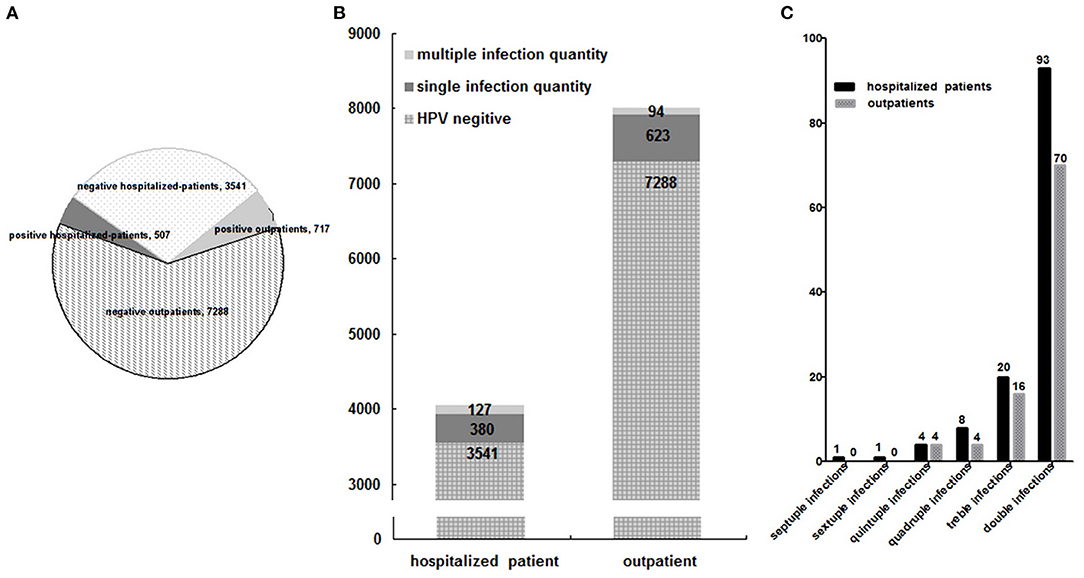
Figure 3. Group distribution of HPV infection among 12,053 patients (A), and of multiple HPV infections among 12,053 patients (B), and between outpatients and hospitalized-patients (C).
The difference of multiple-infection distribution between the outpatients and hospitalized-patients was not statistically significant (p > 0.05) (Figure 3C).
As shown in Table 1, the ages of 12,053 patients were focused are from 21 to 50 years, which occupied 93.19% (11,232/12,053), and HPV-positive patients from 21 to 50 years correspondingly occupied 85.13% (1,042/1,224). Furthermore, the HPV infection rates of patients from above 60 years old (26.32%, 50/190) and below 21 years old (23.21%,13/56) were the most and second highest (p < 0.01), respectively, followed by that of 50–60 years group (20.70%, 119/575). The HPV infection rates of the most and the second prevalent group were slightly higher than those in Guatemala (younger women <30 years, corresponding to 22% and older women ≥60 years, corresponding to 15%) (14). Therefore, this age-specific prevalence curve did not well agree with that conducted in highly developed countries (38). The HPV infection rate of the youngest group (below 21 years) was high and different from that of Xishuang Banna (17), but in line with the data reported in some previous studies (30), suggesting that the higher urbanization may make the young women exposed to HPV infection more easily, which to a large extent may be ascribed to the early sexual activity. Moreover, it indicated that the HPV infection incidence showed a greater trend in the younger population and should not be neglected, indicating that the young people should improve their HPV-related knowledge more deeply for the prevention and intervention for cervical cancer (39).
Among all HPV infections, HR-HPV infection was identified as the infection with at least one HR-HPV genotype, and its infection rate was 8.52% (1,027/12,053). The group above 60 years old with the highest HPV infection rate (26.32%, 50/190) had the highest HR-HPV infection rate (22.63%, 43/190), which was obviously higher than that (7.4%) in Beijing from 2014 to 2015. It may be due to that the investigated population in Hengyang was from gynecologic patients and was different from the population that received free screening in Beijing (24). On the contrary, the 50–60 years group had the third most infection rate in HPV (20.70%, 119/575) but the lowest in HR-HPV (17.39%, 100/575) (p < 0.01).
In this study, the ratio-in-positive of HR-HPV (83.91%, 1,027/1,224) was higher than that of LR-HPV (16.09%, 197/1,224) among 1,224 HPV-positive patients. Although HR-HPV ratio-in-positive of the group above 60 years old (86%, 43/50) and below 20 years group (69.23%, 9/13) were the most or least, respectively, the difference between them (p > 0.05) or among those of all age groups (p > 0.05) had no statistical significance (Figure 4).
As seen from Table 2, among 4,048 hospitalized-patients, 507 patients were detected with HPV infections, among which 421 patients were infected with HR-HPVs and the other with only LR-HPVs. In the hospitalized-patients, the infection rate (10.40%, 421/4,048) and ratio-in-positive (83.04%, 421/507) of HR-HPV was more higher than those of only LR-HPV infection (2.12%, 86/4,048; 16.96%, 86/507), respectively. The difference of HR-HPV ratio-in-positive between the hospitalized-patients (83.04%, 421/507) and outpatients (84.52%, 606/717) was not statistically significant (p > 0.05). However, the HR-HPV infection rate (10.40%, 421/4,048) of the hospitalized-patients was higher than that of the outpatients (7.57%, 606/8,005) (p < 0.01) and even the average level (8.52%, 1,027/12,053), showing that the diseases related with HR-HPV infection would be more severe than those infected by LR-HPV only, thereby indicating the importance of the therapeutic vaccine.
The investigated population focused was 20–50 years old (93.19%, 11,232/12,053), so the same as the hospitalized-patients (93.82%, 3,798/4,048). Moreover, the patients of this age bracket were the most (82.45%, 418/507) among the 507 HPV-infected hospitalized-patients. Among 4,048 hospitalized-patients, the HPV infection rate (42%, 63/150) and HR-HPV infection rate (34%, 51/150) of 50–60 years patients were both the most (p < 0.01, p < 0.01), followed by those of above 60 years patients (26.58%, 21/79; 24.05%, 19/79). The high prevalence of HPV and HR-HPV among above 50 patients was in accordance with the situation that the cervical cancers are likely to happen in 50–70 years old patients, and might be attributable to their decreased immunity for clearing HPV infections, suggesting that the older people were the high-risk population for HPV.
Among 4,048 hospitalized-patients, although the patients above 60 years old had the highest HR-HPV ratio-in-positive (90.48%, 19/21) and those below 21 years had the least (80%, 4/5), they had an obvious difference in HR-HPV ratio-in-positive (p > 0.05), and that difference among all age groups had no statistical significance (p > 0.05) (Figure 5).
Among 507 hospitalized-patients with HPV infection, 284 patients had been performed with histopathologic examination according to their illness, but the other 223 patients did not need examination. Among the 284 HPV-infected hospitalized-patients with the histopathologic examination, 70 patients were with cervical carcinoma or its precancerous lesions, 157 with uterine fibroid or its related lesions, and 57 with the pelvic neoplasm or its related lesions. Among 507 HPV-infected hospitalized-patients, the HR-HPV ratio-in-positive of 284 patients with the histopathologic examination was 90.49% (257/284) and higher than that (78.03%, 174/223) of 223 patients without (p < 0.01) or even the average level (83.04%, 421/507) (p < 0.05), which confirmed that HR-HPV can make the disease severe and even result in organ damage.
Among 284 HPV-infected hospitalized-patients with the histopathologic examination, the patients with cervical carcinoma or its precancerous lesions had the highest HR-HPV ratio-in-positive (95.71%, 67/70) (p < 0.05) (Figure 6), suggesting that cervical carcinoma or its precancerous lesions had a close relationship with HR-HPV virus.
As shown in Figure 6, among 70 HPV infected patients with cervical lesions or carcinoma, 52 patients were with cervical squamous carcinoma, and HR-HPV infection occupied 98.57% (69/70). Among the above 52 patients, the other top six genotypes were HPV52, HPV39, HPV51, HPV58, HPV18, and HPV53 (Figure 7), which was mainly consistent with the frequency of HPV16, HPV58, HPV52, HPV39, HPV51, and HPV53 infection in the whole population, and this was similar with that in Beijing from 2014 to 2015 (24). It was worth mentioning that there was one patient with HPV6 single infection among cervical carcinoma patients. Among the above patients, the HPV16 ratio-in-positive (61.54%) was comparable to that in Beijing from 2014 to 2015 (58.54%) (24) and was obviously more than that among esophageal carcinoma samples (45.24%) (40). Moreover, HPV16 infections occupied 61.54% (32/52) among cervical cancer samples, indicating that the HPV16 virus had played a key role in the development of cervical squamous carcinoma. It was worth mentioning that in this current study there was one cervical carcinoma patient with HPV 6 single infection, suggesting that the role of LR-HPV in cervical squamous carcinoma could not be neglected.
On the basis of the study on the awareness and knowledge levels on HPV (41), this study demonstrated that HPV prevalence and its genotype distribution in Hengyang city of Hunan province in China were mainly in accordance with the present situation of HPV prevalence in China. The controlled variations may occur due to the different populations in different socioeconomic and ethnic environments with different lifestyles and living standards from the different geographical regions. This study indicated that the HPV prevention and control strategies need to be carried out urgently for Hengyang city of Hunan in China, and it also highlighted the importance of HPV53 and HPV39 in China, which can guide the further study for the next generation of HPV vaccines.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The human data in present study were collected in line with the Helsinki Declaration and was approved by the Ethical Review Committees of University of South China.
SYT analyzed and interpreted the patient data and was a major contributor in writing the manuscript. YQL, YH, and HYS also analyzed and interpreted the patient data collaboratively. YPW and YMW performed the collection of data. All authors read and approved the final manuscript.
The authors acknowledge the grants of the Provincial Natural Science Foundation of Hunan Province (2021JJ50014), Scientific Research Project of Hunan Provincial Health Commission (202105012137), Municipal Science project of Hengyang City in Hunan Province (202002042122), National Natural Science Foundation of China (81402169), Educational Committee Foundation of Hunan Province (19C1616), Hunan Provincial Key Laboratory for Special Pathogens Prevention and Control (2014-5) and Construct Program of the Double First-Class discipline of University of South China (2020SYL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology. (2013) 445:2–10. doi: 10.1016/j.virol.2013.04.023
2. Pandey D, Solleti V, Jain G, Das A, Shama Prasada K, Acharya S, et al. Human Papillomavirus (HPV) infection in early pregnancy: prevalence and implications. Infect Dis Obstet Gynecol. (2019) 2019:4376902. doi: 10.1155/2019/4376902
3. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. (2010) 127:2893–917. doi: 10.1002/ijc.25516
4. Flores-Pulido JJ, Martínez-Correa M. Cervical cancer and human papillomavirus. A glance from a family medical viewpoint. Rev Med Inst Mex Seguro Soc. (2015) 53:S162–4.
5. Shi JF, Canfell K, Lew JB, Qiao YL. The burden of cervical cancer in China: synthesis of the evidence. Int J Cancer. (2012) 130:641–52. doi: 10.1002/ijc.26042
6. Gu XY, Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, et al. Incidence and mortality of cervical cancer in China, 2014. Zhonghua Zhong Liu Za Zhi. (2018) 40:241–6. doi: 10.3760/cma.j.issn.0253-3766.2018.04.001
7. Pandey S, Chandravati. Human Papillomavirus-mediated cervical cancer awareness and Gardasil vaccination: a pilot survey among North Indian women. J Community Health. (2013) 38:907–10. doi: 10.1007/s10900-013-9697-6
8. Pandey S, Mittal RD, Srivastava M, Srivastava K, Singh S, Srivastava S, et al. Impact of toll-like receptors [TLR] 2 (-196 to−174 del) and TLR 4 (Asp299Gly, Thr399Ile) in cervical cancer susceptibility in North Indian women. Gynecol Oncol. (2009) 114:501–5. doi: 10.1016/j.ygyno.2009.05.032
9. Pandey S, Mittal B, Srivastava M, Singh S, Srivastava K, Lal P, et al. Evaluation of Toll-like receptors 3 (c.1377C/T) and 9 (G2848A) gene polymorphisms in cervical cancer susceptibility. Mol Biol Rep. (2011) 38:4715–21. doi: 10.1007/s11033-010-0607-z
10. Li J, Kang LN, Qiao YL. Review of the cervical cancer disease burden in mainland China. Asian Pac J Cancer Prev. (2010) 12:1149–53.
11. Nakagawa M, Spencer HJ, Coleman HN, Greenfield WW. Distribution of human papillomavirus (HPV) types and anti-HPV T-cell immune responses among different racial/ethnic groups in Central Arkansas. J Ark Med Soc. (2013) 109:160–3.
12. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. (2012) 30:F12–23. doi: 10.1016/j.vaccine.2012.07.055
13. Ye J, Cheng X, Chen X, Ye F, Lu W, Xie X. Prevalence and risk profile of cervical Human papillomavirus infection in Zhejiang Province, southeast China: a population-based study. Virol J. (2010) 7:66. doi: 10.1186/1743-422X-7-66
14. Lou H, Gharzouzi E, Guerra SP, Domgue JF, Sawitzke J, Villagran G, et al. Low-cost HPV testing and the prevalence of cervical infection in asymptomatic populations in Guatemala. BMC Cancer. (2018) 18:562. doi: 10.1186/s12885-018-4438-y
15. Sohrabi A, Hajia M. Cervical cancer and genital infections: assessment of performance and validation in human papillomavirus genotyping assays in Iran, its neighbouring Countries and Persian Gulf Area. Iran J Pathol. (2017) 12:35–44. doi: 10.30699/ijp.2017.24229
16. Liu ZH, Lin W, Wang YY, Wu B, Yuan SX, Yao JL, et al. Risk stratification of type-specific human papillomavirus for cervical precancers: evidence from a cross-sectional study in Shenzhen. Zhonghua Zhong Liu Za Zhi. (2018) 40:757–63. doi: 10.3760/cma.j.issn.0253-3766.2018.10.007
17. Baloch Z, Yuan T, Wang B, Tai W, Feng Y, Liu Y, et al. Ethnic and geographic variations in HPV prevalence and genotype distribution in North-Western Yunnan, China. J Med Virol. (2016) 88:532–40. doi: 10.1002/jmv.24352
18. Baloch Z, Yuan T, Yindi S, Feng Y, Tai W, Liu Y, et al. Prevalence of genital human papillomavirus among rural and urban populations in southern Yunnan province, China. Braz J Med Biol Res. (2016) 49:e5254. doi: 10.1590/1414-431x20165254
19. Dai M, Bao YP, Li N, Clifford GM, Vaccarella S, Snijders PJ, et al. Human papillomavirus infection in Shanxi Province, People's Republic of China: a population-based study. Br J Cancer. (2006) 95:96–101. doi: 10.1038/sj.bjc.6603208
20. Chen X, Wallin KL, Duan M, Gharizadeh B, Zheng B, Qu P. Prevalence and genotype distribution of cervical human papillomavirus (HPV) among women in urban Tianjin, China. J Med Virol. (2015) 87:1966–72. doi: 10.1002/jmv.24248
21. Li LK, Dai M, Clifford GM, Yao WQ, Arslan A, Li N, et al. Human papillomavirus infection in Shenyang City, People's Republic of China: A population-based study. Br J Cancer. (2006) 95:1593–7. doi: 10.1038/sj.bjc.6603450
22. Lin H, Ma YY, Moh JS, Ou YC, Shen SY, Changchien CC. High prevalence of genital human papillomavirus type 52 and 58 infection in women attending gynecologic practitioners in South Taiwan. Gynecol Oncol. (2006) 101:40–5. doi: 10.1016/j.ygyno.2005.09.028
23. Dillner J, Nygård M, Munk C, Hortlund M, Hansen BT, Lagheden C, et al. Decline of HPV infections in Scandinavian cervical screening populations after introduction of HPV vaccination programs. Vaccine. (2018) 36:3820–29. doi: 10.1016/j.vaccine.2018.05.019
24. Shen J, Gao LL, Zhang Y, Han LL, Wang JD. Prevalence of high-risk HPV and its distribution in cervical precancerous lesions among 35-64 years old women who received cervical cancer screening in Beijing. Zhonghua Yu Fang Yi Xue Za Zhi. (2018) 52:493–97. doi: 10.3760/cma.j.issn.0253-9624.2018.05.008
25. Sun LL, Jin Q, Li H, Zhou XR, Song ZQ, Cheng XM, et al. Population-based study on the prevalence of and risk factors for human papillomavirus infection in Qujing of Yunnan province, Southwest China. Virol J. (2012) 9:153. doi: 10.1186/1743-422X-9-153
26. Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. (2005) 191:1796–07. doi: 10.1086/428850
27. He L, He J. Distribution of high-risk HPV types among women in Sichuan province, China: a cross-sectional study. BMC Infect Dis. (2019) 19:390. doi: 10.1186/s12879-019-4038-8
28. Baloch Z, Yue L, Yuan T, Feng Y, Tai W, Liu Y, et al. Status of human papillomavirus infection in the ethnic population in Yunnan Province, China. Biomed Res Int. (2015) 2015:314815. doi: 10.1155/2015/314815
29. Li C, Wu M, Wang J, Zhang S, Zhu L, Pan J, et al. A population based study on the risks of cervical lesion and human papillomavirus infection among women in Beijing, People's Republic of China. Cancer Epidemiol Biomarkers Prev. (2010) 19:2655–64. doi: 10.1158/1055-9965.EPI-10-0212
30. Jin Q, Shen K, Li H, Zhou XR, Huang HF, Leng JH, et al. Prevalence of human papillomavirus infection in women in Tibet Autonomous Region of China. Zhonghua Fu Chan Ke Za Zhi. (2009) 44:898–902. doi: 10.3760/cma.j.issn.0529-567x.2009.12.005
31. Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. (2005) 366:991–98. doi: 10.1016/S0140-6736(05)67069-9
32. Yamasaki K, Miura K, Shimada T, Miura S, Abe S, Murakami M, et al. Epidemiology of human papillomavirus genotypes in pregnant Japanese women. J Hum Genet. (2011) 56:313–15. doi: 10.1038/jhg.2011.11
33. Xiang F, Guan Q, Liu X, Xiao H, Xia Q, Liu X, et al. Distribution characteristics of different human papillomavirus genotypes in women in Wuhan, China. J Clin Lab Anal. (2018) 2018:e22581. doi: 10.1002/jcla.22581
34. Xu H, FH, Gao XH, Hu SY, Chen JF, Liu ZH, et al. Cost-effectiveness analysis on the once-in-a-lifetime cervical cancer screening program for women living in rural and urban areas of China. Zhonghua Liu Xing Bing Xue Za Zhi. (2013) 34:399–03. doi: 10.3760/cma.j.issn.0254-6450.2013.04.021
35. de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. (2007) 7:453–59. doi: 10.1016/S1473-3099(07)70158-5
36. Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. (2008) 26:K1–16. doi: 10.1016/j.vaccine.2008.05.064
37. Vargas-Robles D, Magris M, Morales N, de Koning MNC, Rodríguez I, Nieves T, et al. High rate of infection by only oncogenic human papillomavirus in amerindians. mSphere. (2018) 3:e00176–18. doi: 10.1128/mSphere.00176-18
38. Franceschi S, Herrero R, Clifford GM, Snijders PJ, Arslan A, Anh PT, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. (2006) 119:2677–84. doi: 10.1002/ijc.22241
39. Preston SM, Darrow WW. Improving human papillomavirus-related knowledge and attitudes among ethnically diverse young adults. Health Equity. (2019) 3:254–63. doi: 10.1089/heq.2018.0091
40. Li S, Shen H, Li J, Hou X, Zhang K, Li J. Prevalence of the integration status for human papillomavirus 16 in esophageal carcinoma samples. Turk J Gastroenterol. (2018) 29:157–63. doi: 10.1186/s13027-017-0164-3
Keywords: human papillomavirus, prevalence, genotype, distribution, cervical cancer
Citation: Tang SY, Liao YQ, Hu Y, Shen HY, Wan YP and Wu YM (2021) HPV Prevalence and Genotype Distribution Among Women From Hengyang District of Hunan Province, China. Front. Public Health 9:710209. doi: 10.3389/fpubh.2021.710209
Received: 05 July 2021; Accepted: 19 August 2021;
Published: 05 November 2021.
Edited by:
Hongcui Cao, Zhejiang University, ChinaReviewed by:
Zulqarnain Baloch, Kunming University of Science and Technology, ChinaCopyright © 2021 Tang, Liao, Hu, Shen, Wan and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-ping Wan, d2FueXkxOTkxQGFsaXl1bi5jb20=; Yi-mou Wu, d3V5aW1vdUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.