
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 05 July 2021
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.691163
Respiratory viral infections are the leading cause of morbidity and mortality in the world; however, there are several groups of viruses that are insufficiently routinely sought for, and can thus be considered neglected from a diagnostic and clinical standpoint. Timely detection of seasonality of certain respiratory viruses (e.g., enveloped viruses such as seasonal coronaviruses) in the local context can aid substantially in targeted and cost-effective utilization of viral diagnostic approaches. For the other, non-enveloped and year-round viruses (i.e., rhinovirus, adenovirus, and bocavirus), a continuous virological diagnosis needs to be implemented in clinical laboratories to more effectively address the aetiology of respiratory infections, and assess the overall impact of these viruses on disease burden. While the coronavirus disease 2019 (COVID-19) pandemic is still actively unfolding, we aimed to emphasize the persistent role of seasonal coronaviruses, rhinoviruses, adenoviruses and bocaviruses in the aetiology of respiratory infections. Consequently, this paper concentrates on the burden and epidemiological trends of aforementioned viral groups on a global level, but also provides a snapshot of their prevalence patterns in Croatia in order to underscore the potential implications of viral seasonality. An overall global prevalence in respiratory tract infections was found to be between 0.5 and 18.4% for seasonal coronaviruses, between 13 and 59% for rhinoviruses, between 1 and 36% for human adenoviruses, and between 1 and 56.8% for human bocaviruses. A Croatian dataset on patients with respiratory tract infection and younger than 18 years of age has revealed a fairly high prevalence of rhinoviruses (33.4%), with much lower prevalence of adenoviruses (15.6%), seasonal coronaviruses (7.1%), and bocaviruses (5.3%). These insights represent a relevant discussion point in the context of the COVID-19 pandemic where the testing of non-SARS-CoV-2 viruses has been limited in many settings, making the monitoring of disease burden associated with other respiratory viruses rather difficult.
Respiratory tract infections (RTIs) represent a major public health matter in developed and developing countries alike, being responsible for about 19% of all deaths among children younger than 5 years of age, as well as 8.2% of disability and premature mortality (1–4). According to the World Health Organization (WHO), RTIs are actually placed first when we measure burden of disease by using disability-adjusted life-years (DALYs). Furthermore, lower RTIs are the third leading cause of death in the world overall (4). They are predominantly caused by viruses, and although our focus is primarily on human respiratory syncytial virus (RSV) in children and influenza viruses in adults, there is an underappreciated burden of RTIs caused by several viral groups, further compounded by the ongoing pandemic of coronavirus disease 2019 (COVID-19). There are various reasons why many respiratory viruses are not routinely sought for, and can thus be considered neglected from diagnostic and clinical standpoint. Moreover, due to the current lack of vaccines for many neglected respiratory viruses, which may change in not so distant future, a better grasp of their prevalence (especially in children), distribution and seasonality is indispensable for effective prevention, control, and treatment endeavours (5). This review aims to provide an outline of four viral groups (seasonal coronaviruses, rhinoviruses, adenoviruses, and bocaviruses), concentrating on epidemiological trends worldwide and estimating/summarizing the overall infection burden, and also to present a snapshot of their prevalence patterns in a large sample of Croatian children with RTIs, evaluating viral seasonality as well.
Epidemiological understanding of seasonal coronaviruses (sCoVs) is currently incomplete in many settings around the world, primarily due to the fact that these viruses are not a part of standard diagnostic armamentarium, or testing is guided by specific clinical case definitions (6). It is well-established that sCoVs are endemically found in co-circulation with other prevalent respiratory viruses, which is a principal reason why co-infections are commonly observed (7–9). Furthermore, the habitual occurrence of sCoVs during periods characterized by high influenza activity underscores the significance of their consideration within the context of viral respiratory infections (9–11). Likewise, during the COVID-19 pandemic, co-circulating viruses may be responsible for symptoms that resemble the clinical presentation of COVID-19, which then poses a problem on how to quickly establish a correct diagnosis in settings without the capacity for multiplex testing (9).
Four sCoV strains can give rise to cold symptoms in human individuals and are responsible for 15–30% of respiratory infections every year: 229E-CoV, NL63-CoV, OC43-CoV, and HKU1-CoV (12, 13). Regardless of the fact that they utilized different host receptors for cell entry, all sCoVs express the spike glycoprotein (which protrudes from the surface of the virions), with high homology pattern between 229E-CoV and NL63-CoV (14). This spike glycoprotein is comprised of two subunits: S1 harbours the receptor-binding domain responsible for binding to cell surface receptors, while S2 is pivotal for mediating the fusion of viral and host membranes and subsequent cell entry (15). It has to be noted that, in comparison to S1 that is more variable, certain portions of the S2 subunit represent the most conserved part of the molecular structure among both sCoVs and zoonotic coronaviruses (including SARS-CoV-2) (12, 16).
The increased availability of reverse-transcriptase polymerase chain reaction (RT-PCR) enabled much easier detection of the infection for each of the four aforementioned sCoVs (17). Nonetheless, as we gained more valuable data from population-based studies that utilized molecular methods, there was still not much attention paid to these viruses and their possible role in various clinical presentations, possible because very little was known regarding their exact role in respiratory illnesses (15, 18, 19). And while recent studies on hospitalized patients have confirmed the important role of sCoVs and their global circulation (20), we still lack pertinent data on the frequency and seasonality in the broader community context (21).
However, a recent systematic review by Park et al. (22) revealed a consistent winter peak of sCoV incidence in the northern hemisphere (akin to other respiratory viruses), with moderate decrease during the summer months. It has to be noted that there is a substantial variation in the percentage of sCoVs infections in different epidemiological studies that appraised patients with acute respiratory infections. Heimdal et al. (23) followed Norwegian children hospitalized with respiratory infection for 9 years, and revealed that sCoVs were implicated in 9.1% of the episodes. Among different coronaviruses, OC43-CoV was the most commonly found, while 229E-CoV was the rarest, with most of the infections occurring during winter months (23).
For some time now we know that, in contrast to the observed situation with COVID-19, infection rates of sCoVs are much higher in children and adolescents. This is especially evident in a recent cohort study from Michigan in United States, that demonstrated the highest infection rates of sCoVs in children younger than 5 years of age (18 per 100 person-years) when compared to older age groups (7–11 per 100 person-years) (18). Children have little or no pre-existing immunity against sCoVs, and they are often in close contact in nurseries and childcare settings (12). Other interesting finding is that seasonal sCoVs infections in younger individuals may actually act as a protecting factor from symptomatic/severe SARS-CoV-2 infections by limiting viral infection, since large amounts of cross-reactive antibodies between sCoVs and SARS-CoV-2 have been found in this age group (16).
Some other studies point toward the important role of sCoVs in younger age groups. A cross-sectional, prospective study on a convenience sample of 1,404 Mexican children with community-acquired pneumonia demonstrated the presence of sCoVs in 2.2% of all the samples (24). Conversely, the prevalence was much larger in paediatric patients with cancer in a three-year retrospective study from Turkey, where sCoVs were the third most prevalent viral group (after rhinovirses and parainfluenza viruses) with the prevalence of 14.8% (25). In a recent big study from Scotland, the prevalence of sCoVs was 4.0% among all the tested patients (i.e., 74 519 of them), contributing to 10.7% of all respiratory virus detections (11). In the latter study the most prevalent detection was OC43-CoV, while the prevalence of HKU1-CoV was very low (i.e., 0.3% overall).
In a study from Russia on 1560 children with upper or lower respiratory infection, sCoVs were found in 0.8% of positive samples (26). On the other hand, a large retrospective observational study from Moscow found sCoVs in 2.6–6.1% of individuals with acute respiratory infection (ARI) between January 2016 and March 2020 (i.e., prior to SARS-CoV-2 pandemic), with winter-spring seasonal activity pattern and peak levels in December (27).
Interestingly, a recent study has found that sCoVs activity in the temperate regions of China seems to be less seasonal, with high activity observed in the summer, autumn and winter (28). Moreover, a large retrospective study from Beijing showed that sCoVs are present in 1% of clinical samples from adults with ARI (29), while a prominent 12-month prospective study from Hong Kong demonstrated that a total prevalence of sCoVs in patients with ARI was 2.1% (with HCoV-NL63 showing a highest positivity rate of 1.3%) (30).
Many of these studies highlight the potential of interactions and co-infections between sCoVs and other respiratory viruses. An in-depth analysis by Nickbakhsh et al. (11) validates positive interactions at the sCoV type level. On the other hand, the evidence of immunological cross-protection between different human coronaviruses is lacking, with inconsistent reports of antigenic cross-reactivity demonstrated by some studies (31, 32), but not confirmed by other ones (33). Genetic relatedness of coronaviruses enables the cross-reactivity at the genus level (34), but more general cross-reactivity between 229E-CoV and OC43-CoV has also been described (35).
In any case, serological surveys on the population level will be indispensable for establishing true sCoV infection burden and the respective age distribution, as well as appraising the prospect of coveted cross-protective immunity. Findings from a recent study by Fischer et al. endorse the use of national influenza surveillance systems for sCoV early detection and monitoring, which will enable a an enhanced estimation of the burden of disease, and also aid in tracking emerging coronaviruses such as SARS-CoV-2 (20).
Rhinoviruses are the most common cause of ARI in all age groups (36–39), which poses a significant burden for the health care system, as well as a substantial economic loss caused by absenteeism (40). Although they lead in the aetiology of ARI, they have been neglected for years for a number of reasons: (i) they were seen as rare (or improbable) causative agents of lower respiratory tract infections (LRTIs) since their replication is difficult at temperatures above 33°C; (ii) because they cause self-limiting upper respiratory tract infections (URTIs) in healthy individuals, do not cause hospitalization and, consequently, do not burden the hospital system; (iii) until the development of molecular detection methods the laboratory diagnosis of these viruses was relatively slow, therefore clinically irrelevant and expensive, and; (iv) to date there is no commercially available effective specific antiviral therapy or vaccine specifically targeting rhinovirus (41). The increased availability of molecular detection methods, together with the fact that rhinoviruses can replicate efficiently at lower airway temperatures (42), allowed rhinovirus infections to be viewed from a different vantage point.
Rhinoviruses are estimated to cause more than half of URTIs usually presenting as common cold syndrome; however, during the last two decades, numerous studies have revealed their role as leading causes of LRTIs (38, 39, 43, 44). A recent prospective study conducted in 11 European countries has shown that in adults presenting to primary care with LRTI, the most common viral pathogens detected were human rhinoviruses (20.1%) (45). Another recent study conducted on hospitalized children with ARI in Croatia also revealed rhinovirus as the most frequently detected virus, diagnosed in 33.4% patients; 60.4% as monoinfection, and 39.6 % as co-infection with other respiratory viruses (46). More than half of children infected with rhinovirus (55.8%) presented with LRTI (46).
The high prevalence of rhinoviruses each year is not surprising, given the enormous diversity of these viruses as a result of a large number of serotypes/genotypes. After overcoming the infection, serotype-specific humoral immunity important for preventing the infection is induced, with little or insignificant cross-neutralization among serotypes (47, 48); hence, infection with different serotype can frequently occur. Rhinoviruses antigenic diversity is also major obstacle in vaccine development (49).
Currently, ~170 genotypes (50) are classified into three species: Rhinovirus A (RV-A), Rhinovirus B (RV-B), and Rhinovirus C (RV-C) under the Enterovirus genus. Current classification is based on capsid region sequences analysis (VP4/VP2 or VP1), since sequencing the 5′ untranslated region (5′UTR) cannot discriminate between all rhinovirus species (particularly RV-A and RV-C) (30).
In addition to relevant differences in genetic sequence, there are several phenotypic key differences between species. Depending on the species, rhinoviruses attach to the different host cell receptor: most HRV-A and HRV-B attach to the intercellular adhesion molecule (ICAM)-1, the others alternatively bind to low density lipoprotein receptor (LDL-R), whereas RV-C utilizes human cadherin-related family member 3 (CDHR3) (51). Furthermore, RV-A and RV-B can be cultured in cell cultures while RV-C cannot be grown, which is why the latter species was discovered much later than RV-A and RV-B (52).
The currently unresolved issue that is the focus of scientific interest is whether a particular species of rhinovirus causes a more severe illness compared to another species. In any case, the data is controversial. Some studies have recorded an association of severe disease with HRV-C (53), and to a lesser extent with HRV-A (54), while other research groups have not corroborated this association (55). The complexity of these observations is emphasized by the finding that the relationship between species and disease severity also depends in some extent on age, i.e., it is frequently noted that RV-C tends to cause more severe disease in children (56) and HRV-A in adults (54). Most studies are however in agreement that RV-B tends to occur sporadically and often in asymptomatic patients (46, 54, 57).
In an already mentioned study from Russia that included children with URTIs or LRTIs, rhinovirus was found in 15.1% of positive samples (26). Likewise, a study in adults demonstrated the presence of rhinovirus in 15.5% of patients with positive microbiology results (58). There was a high prevalence of rhinovirus infection in paediatric patients with ARI (59), while a large study from the CAP-China Network revealed its presence in 1.8% of adult patients with community-acquired pneumonia—although its potential role in the pathogenesis is still not clear (60). In addition, a recent meta-analysis did not find any significant difference in the prevalence between children of different age groups, or those with severe disease in comparison to asymptomatic ones (61). Consequently, until the true role of rhinovirus in more severe disease presentations is established, it is hard to make any steadfast recommendations regarding surveillance and clinical approach.
Since the discovery of rhinovirus, many longitudinal studies have shown that rhinoviruses can be detected throughout the year (62), with the awareness that in countries with temperate climate they occur more frequently in autumn and spring (63). Recent studies from Croatia have also shown this pattern of rhinovirus prevalence with peak of rhinovirus prevalence detected during autumn and spring, while influenza viruses, respiratory syncytial virus, and metapneumovirus predominate in the winter (46, 64). However, some studies indicate that severe rhinovirus infection are more common in the winter (65), possibly due to a weaker induction of interferon at low temperatures, resulting in less efficient antiviral defence response of infected cells (66). There are also differences in the occurrence of individual species, i.e., some studies have observed that HRV-C usually occurs in winter months (46, 67).
Human adenovirus (HAdV) is associated with a wide range of illnesses, ranging from common cold to more serious conditions—including serious acute respiratory infections, gastroenteritis, conjunctivitis, haemorrhagic cystitis or meningoencephalitis, which are often underreported (68). Its paramount role as an agent of RTIs is especially pertinent for children between 1 and 5 years of age (69, 70), primarily due to their immature immune system (71). The vast majority of cases are asymptomatic and self-limited (72); nonetheless, the clinical spectrum is broad, and dissemination or pneumonia can be fatal, both in immunocompetent and immunocompromised patients (73, 74).
The virus is spread via aerosolized droplets, direct inoculation to the conjunctiva, exposure to infected tissue/blood, as well as via faecal-oral route (72). There is also a possibility of viral acquisition from exogenous sources (e.g., pillows, linens, lockers, guns) or viral reactivation (75). It is important to note that AdV RTIs normally occur all-year-round (62), unless there is an epidemic outbreak (70, 76). In such cases connections between certain periods of the year and the incidence of AdV RTIs can be drawn.
For example, one study finds that in Brazil the adenovirus is more active during the rainy season with higher air humidity (77). Another study demonstrated that HAdV circulated year-round, with higher frequency during winter and early spring; increases in the average monthly temperature were associated with decreases in HAdV infections (70). Other studies have found HAdVs throughout the year, with a notably higher prevalence in summer (78).
In a majority of studies predominant clinical symptoms in the HAdV-infected children were fever, cough and pneumonia (68)—however, clinical presentation alone is usually not sufficient for establishing a correct diagnosis. As most cases of acute respiratory infection show similar symptoms regardless of the causative viral agent, correct diagnosis usually relies on laboratory confirmation (78). When multiplex PCR is used as a diagnostic tool in RTI, the rate of adenoviral infections is between 20 and 36% (69, 79, 80). Positive adenovirus PCR accompanied with a higher viral load is associated with more severe symptoms and worse prognosis, which is when the early use of cidofovir may improve the outcome (78).
Moreover, viral–bacterial co-infections frequently occur, and a number of research studies underscore the potential risk of synergistic presentation during the co-infection process with respiratory viruses and bacteria, resulting in longer hospital stays and higher morbidity (81). These co-infection patterns dominate among children when compared to the adults; more specifically, they account for 35% of cases among paediatric patients, and only 5.8% among adult ones (80). Also, it has to be taken into account that a substantial mortality burden can be attributed to secondary bacterial infections from Streptococcus pneumoniae or Staphylococcus aureus (81).
Diverse clinical presentations of adenoviral infections can be epidemiologically linked to various genotypes (81). These genotypes show diverse tissue tropisms that are linked with the manifestation of infection. For example, HAdV species B (which includes HAdV-3, 7, 11, 14, 16, 21, 50 and 55), species C (which includes HAdV-1, 2, 5 and 6) and species E (which includes HAdV-4) are predominantly related to respiratory diseases (82, 83) and, thus, of interest for researchers and clinicians alike. A large retrospective cohort study from Monroe Carell Jr. Children's Hospital at Vanderbilt revealed that HAdV species C and HAdV species B were the most frequent ones, with notable differences in clinical manifestations and outcomes (84).
Other recent studies have shown that the three predominant genotypes in children younger than 5 years of age were HAdV-3, HAdV-7 and HAdV-2 (85). The patients infected with the HAdV-2 genotype were also accompanied with leucocytosis. Furthermore, two genotypes have a much higher chance of infecting young children as well as causing more severe symptoms, and those are HAdV-1 and HAdV-2 (86). A study from Switzerland singled out HAdV8 as a predominant genotype causing HAdV infection among young adults, middle-aged and elderly, and HAdV1-3 as predominant genotypes causing HAdV infection among young children (68). In a study from Kuwait, HAdV C1, C2, C5, B3, and B7 were recognized as the main types identified in patients with severe respiratory infection (87).
Furthermore, in a large study from Russia that analysed respiratory tract samples of 4,731 patients, HAdV infection has been detected in 6.9% of diseased children and 2.9% of adults year-long, with a notable peak in October-December (88). Another study demonstrated the presence of HAdV in 3.5% of positive samples in Russian children with RTI (26). In children that were hospitalized due to severe ARI in Beijing and Shanghai, the prevalence of HAdV was 13.7% (89). A very recent study from Macao on hospitalized children with ARI showed even higher prevalence of HAdV of 15.8%, with infection peaks in summer and winter (90).
Nearly all adenovirus infections with high morbidity and severe symptoms in children are associated with HAdV-7 instead of HAdV-3 (91). One notable example is China, where HAdV-7 is one of the predominant genotypes, accounting for 26.9% of all adenoviral infections (and also responsible for a myriad of outbreaks) (83, 92). Still, all these type-specific studies of adenoviral infections come with certain limitations, as we are not dealing with only a mechanistic process of infection, but also a panoply of host and environmental factors.
Since it has been initially discovered 16 years ago in nasopharyngeal samples of children with ARI, it quickly became evident that human bocavirus (HBoV)—and more specifically HBoV type 1 (HBoV 1)—can be considered an important respiratory pathogen (93, 94). Not much later, three other bocaviruses have been discovered and consequently named HBoV 2-4; nevertheless, their role in clinical disease remains somewhat controversial. Even from the more fundamental perspective, some investigators hypothesize that HBoV infection under clinical conditions may depend on helper viruses, such as herpesviruses, or that HBoV replicates utilizing a mechanism atypical for parvoviruses (95). These insights could at least partially contribute to understanding the high burden of co-detection of HBoV with other viruses.
HBoV is classified into Parvoviridae family and Bocaparvovirus genus, with two species that infect humans: Primate bocaparvovirus 1 that contains HBoV 1 and HBoV 3, and Primate bocaparvovirus 2 containing HBoV 2 and HBoV 4 (93, 96). Akin to other parvoviruses, HBoV is most likely transmitted via droplets and aerosol, while the total global prevalence of HBoV was estimated at 6.3% (93). Seroepidemiological studies revealed that, by the age of six, 90–100% of children have circulating antibodies against at least one of the four human bocaviruses (97). Later, HBoV1 IgG antibody concentrations remain high during adulthood, probably because of the “immunity boost” caused by circulating HBoV1, or by an infection with related HBoV2, HBoV3, or both (93, 97). Primary bocavirus infections mostly arise between 6 and 24 months of age, which is later than RSV infections, but earlier in childhood than influenza (97).
When country-specific data is considered, a Croatian study revealed a high rate of HBoV1 among infants and small children with lower respiratory tract infection that required hospitalization (i.e., 23.1% of those with proven viral aetiology) (98), while the other study showed that two thirds of HBoV positive patients were between 1 and 3 years of age (99). In Egypt, the prevalence of HBoV in nasopharyngeal swabs taken from children with acute respiratory tract infection was 9.3% (100). Furthermore, HBoV was detected in 1.9% of the patients in Kuwait, with a peak incidence among children <1 year of age, as well as the predominance of HBoV-1 genotype (101). In a Belgian study, HBoV was detected in 5.7% of children with a median age of 10.6 months (102).
A recent study from Russia found HBoV in 5.8% of positive samples in children with RTI (26). On the other hand, the prevalence can be substantially higher in children hospitalized with severe ARI, as demonstrated by a recent study on hospitalized children in Beijing and Shanghai (i.e., encompassing both northern and southern China), where the prevalence was 19.1% (89).
In any case, it is evident that the role of HBoV 1 as a respiratory pathogen is rather well-established, so nowadays the virus is generally acknowledged as an important player in both upper and lower acute respiratory disease. In children, the virus is linked to rhinitis, acute otitis media, pneumonia, bronchiolitis, and asthma exacerbations (97). One large meta-analysis showed that HBoV 1 is the third most common viral agent detected in children with bronchiolitis (103), while other investigators placed HBoV as the third most common virus in children suffering from wheezing with prevalence of 8.1%, following rhinoviruses and respiratory syncytial virus (104). Due to its non-enveloped nature, HBoV is resistant to disinfectants and detergents, and should be considered as a possible nosocomial pathogen (which is especially pertinent for immunocompromised children) (105).
A few case reports have been published describing extrapulmonary manifestations in children infected with HBoV 1-3, such as encephalitis, hepatitis and myocarditis (97, 106–108); however, clinical presentation in other age groups remains an open question. One study showed that HBoV 1 can also be found in immunocompetent adults with respiratory infection, where it can be associated with a high incidence of pneumonia—especially in elderly individuals and patients with nosocomial infections (109). On the other hand, a study among adult patients with severe pneumonia necessitating ICU admission demonstrated that monoinfection with HBoV 1 is not common finding, i.e., there is a significant burden of co-infections with other well-established respiratory pathogen (110).
This co-infection pattern with other respiratory pathogens may arise due to the prolonged shedding and possible persistence, especially in children. It is well-established that asymptomatic children can shed virus more than 1 month and in prolonged duration up to 1 year (111). However, even in respiratory samples containing actively transcribing HBoV1, other viruses have been detected in almost 60% of the cases (97). Moreover, a recent study from Italy showed co-infection of HBoV in 51.7% of patients (112). The true significance of such co-detection or co-infection is yet to be determined, which hampers our attempts to address true pathogenic potential of bocavirus (113).
The diagnostic approach is another hurdle. Due to the difficulties in replicating the virus in cell cultures, and the fact that the serology of bocavirus is complicated by the phenomenon known as the “original antigenic sin,” the diagnosis of HBoV infection is almost exclusively based on molecular detection methods (93, 97, 100). Most laboratories currently use in-house PCR and real-time PCR assays targeting the NP-1, NS-1 or VP1/2 gene, but other nucleic acid-based detection methods for the diagnosis of HBoV have also been described (114). However, clinical value of PCR detection is low due to prolonged shedding and common co-detection with other viruses. Therefore, the other diagnostic strategies are needed. Albeit a point-of-care test detecting viral antigen has been used (115), additional evaluation of this test was not pursued.
The aforedescribed global overview is indispensable for placing any local data into the appropriate context, especially taking into account global prevalence ranges informed by the detailed literature review (Table 1). As the ongoing COVID-19 pandemic hampered many regular epidemiological endeavours, data gathered just prior to its emergence will be increasingly used to inform our further epidemiological approaches in this field. Consequently, a recent study conducted between May 2017 to April 2019, on 590 individuals younger than 18 years of age (median age 1.75 years; range 7–17), may be used as an informative blueprint. This epidemiological study utilized a multiplex RT-PCR for the detection of 15 respiratory viruses in nasopharyngeal and pharyngeal flocked swabs (46).
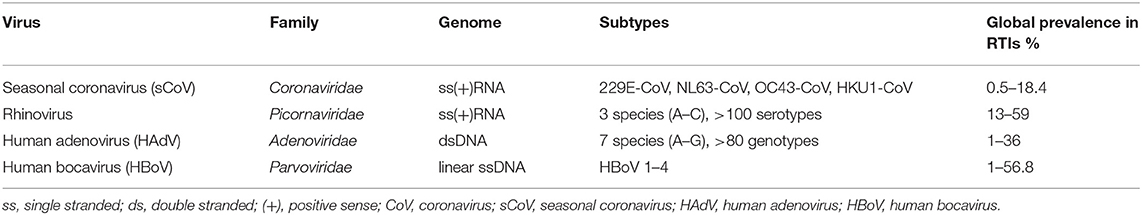
Table 1. Taxonomy and global prevalence of four viral groups (seasonal coronaviruses, rhinoviruses, adenoviruses and bocaviruses) in individuals with respiratory tract infections (RTIs).
The male to female ratio in the study was 1.42:1; furthermore, the upper respiratory tract infection has been established in 46.9% patients, while lower respiratory tract infection was found in 53.1% of patients. Pursuing a comprehensive panel of respiratory viruses on this population had both epidemiological and clinical merit, as the results of virology testing were sent to the attending physicians once per week. In a total of 76.4% of patients there was a proven viral infection; 69.8% of positive patients had a monoinfection with a single virus, while 30.2% of them harboured two or more viruses synchronously (46).
In any case, this epidemiological analysis (covering north-western and central part of Croatia) revealed that rhinoviruses were the most commonly detected group with a prevalence of 33.4%. Adenoviruses were the third most common group (after respiratory syncytial virus, prevalence of 15.6%), seasonal coronaviruses were on the sixth place (following influenza viruses and parainfluenza viruses, prevalence of 7.1%), and bocaviruses were placed immediately after seasonal coronaviruses (prevalence of 5.3%) (46). Hence, albeit overlooked, the clinical importance of these viruses is becoming increasingly evident.
As a follow-up to this review, we also argue that there is a need to always take into account the seasonality of neglected (or unappreciated) respiratory infections. This is the reason why we decided to present here novel graphical data on temporal distribution of seasonal coronavirus, rhinovirus, adenovirus and bocavirus positive cases in these 590 hospitalized children within a 2-year period (Figure 1). Although the prevalence is expectedly the highest in the winter period, it is clearly visible that seasonality of different viral groups may differ. Also, from the graphical data it is clear that the most common seasonal/endemic coronavirus is HCoV-OC43 (Figure 1).
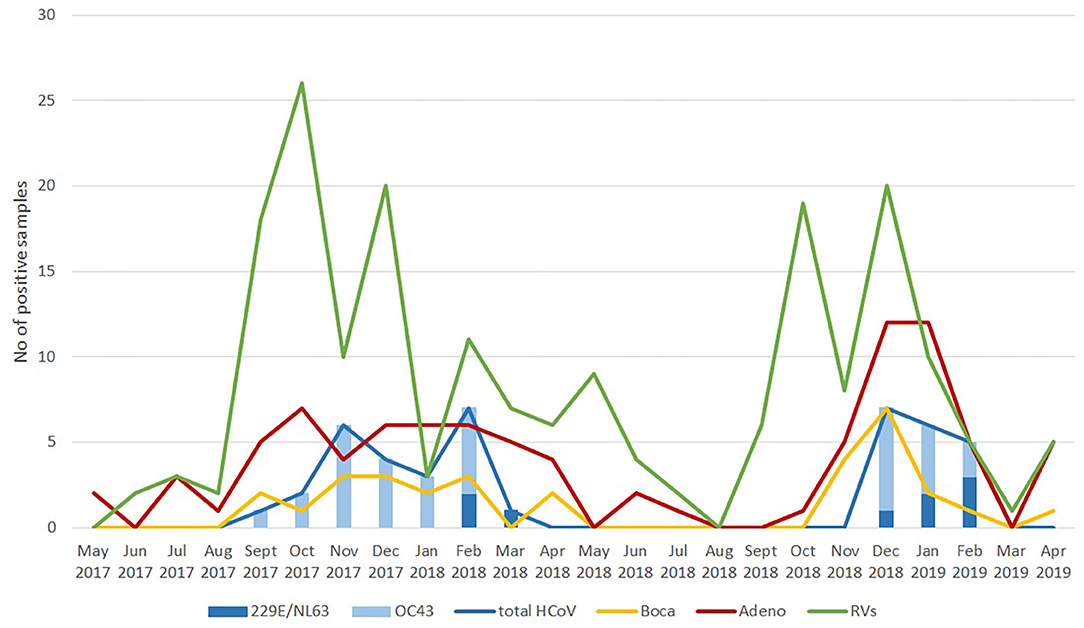
Figure 1. Temporal distribution of cases positive for seasonal coronaviruses, rhinoviruses, adenoviruses, and bocaviruses in hospitalized Croatian children with acute respiratory infection from May 2017 to April 2019.
Timely detection of seasonality of some respiratory viruses (enveloped viruses such as seasonal coronaviruses) in the local context can help the targeted and cost-effective use of viral diagnostics. For the other, non-enveloped, year-round viruses (i.e., rhinovirus, adenovirus and bocavirus), continuous virological diagnosis needs to be implemented in clinical laboratories to more effectively address the aetiology of respiratory infections and assess the impact of these viruses on disease burden. As these viral groups still represent a significant global infectious burden, they should not be neglected—even when all research and clinical efforts seem to be devoted to COVID-19. In conclusion, appraising the exact prevalence of (often neglected) respiratory viruses is indispensable for adequate prevention, control and therapeutic approaches to RTIs.
SL-S and TM conceptualized and designed the manuscript. SL-S, TM, IL, and MM conducted epidemiological investigation and data curation. SL-S, TM, IL, and MM interpreted the data and prepared the draft of the manuscript. SL-S and TM created visualizations. JV supervised the project, and JV and SL-S critically reviewed the draft. Project administration was done by SL-S. All authors have read and agreed to the published version of the manuscript.
This work has been fully supported by Croatian Science Foundation under the project No. IP-2016-06-7556 titled New and neglected respiratory viruses in vulnerable groups of patients (principal investigator SL-S). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. (2002) 2:25–32. doi: 10.1016/S1473-3099(01)00170-0
2. Morikawa S, Hiroi S, Kase T. Detection of respiratory viruses in gargle specimens of healthy children. J Clin Virol. (2015) 64:59–63. doi: 10.1016/j.jcv.2015.01.006
3. Ge X, Guo Y, Chen J, Hu R, Feng X. Epidemiology and seasonality of respiratory viruses detected from children with respiratory tract infections in Wuxi, East China. Med Sci Monit. (2018) 24:1856–62. doi: 10.12659/MSM.908483
4. Avendaño Carvajal L, Perret Pérez C. Epidemiology of Respiratory Infections. In Bertrand P, Sánchez I, editors. Pediatric Respiratory Diseases. Cham: Springer International Publishing. (2020) p. 263–72.
5. Ma X, Conrad T, Alchikh M, Reiche J, Schweiger B, Rath B. Can we distinguish respiratory viral infections based on clinical features? A prospective pediatric cohort compared to systematic literature review. Rev Med Virol. (2018) 28:e1997. doi: 10.1002/rmv.1997
6. Alchikh M, Conrad T, Hoppe C, Ma X, Broberg E, Penttinen P, et al. Are we missing respiratory viral infections in infants and children? Comparison of a hospital-based quality management system with standard of care. Clin Microbiol Infect. (2019) 25:380.e9–16. doi: 10.1016/j.cmi.2018.05.023
7. Greer RM, McErlean P, Arden KE, Faux CE, Nitsche A, Lambert SB, et al. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol. (2009) 45:10–5. doi: 10.1016/j.jcv.2009.03.008
8. Gaunt ER, Hardie A, Claas ECJ, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. (2010) 48:2940–7. doi: 10.1128/JCM.00636-10
9. Nickbakhsh S, Thorburn F, Von Wissmann B, McMenamin J, Gunson RN, Murcia PR. Extensive multiplex PCR diagnostics reveal new insights into the epidemiology of viral respiratory infections. Epidemiol Infect. (2016) 144:2064–76. doi: 10.1017/S0950268816000339
10. Nickbakhsh S, Mair C, Matthews L, Reeve R, Johnson PCD, Thorburn F, et al. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci USA. (2019) 116:27142–50. doi: 10.1073/pnas.1911083116
11. Nickbakhsh S, Ho A, Marques DFP, McMenamin J, Gunson RN, Murcia PR. Epidemiology of seasonal coronaviruses: establishing the context for the emergence of coronavirus disease 2019. J Infect Dis. (2020) 222:17–25. doi: 10.1093/infdis/jiaa185
12. Guthmiller JJ, Wilson PC. Remembering seasonal coronaviruses. Science. (2020) 370:1272–3. doi: 10.1126/science.abf4860
13. Audi A, AlIbrahim M, Kaddoura M, Hijazi G, Yassine HM, Zaraket H. Seasonality of respiratory viral infections: will COVID-19 follow suit? Front Public Health. (2020) 8:567184. doi: 10.3389/fpubh.2020.567184
14. Penner RC. Conserved high free energy sites in human coronavirus spike glycoprotein backbones. J Comput Biol. (2020) 27:1622–30. doi: 10.1089/cmb.2020.0193
15. Artika IM, Dewantari AK, Wiyatno A. Molecular biology of coronaviruses: current knowledge. Heliyon. (2020) 6:e04743. doi: 10.1016/j.heliyon.2020.e04743
16. Ng KW, Faulkner N, Cornish GH, Rosa A, Harvey R, Hussain S, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. (2020) 370:1339–43. doi: 10.1126/science.abe1107
17. Vijgen L, Moës E, Keyaerts E, Li S, Van Ranst M A. pancoronavirus RT-PCR assay for detection of all known coronaviruses. Methods Mol Biol. (2008) 454:3–12. doi: 10.1007/978-1-59745-181-9_1
18. Monto AS, DeJonge PM, Callear AP, Bazzi LA, Capriola SB, Malosh RE, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in michigan. J Infect Dis. (2020) 222:9–16. doi: 10.1093/infdis/jiaa161
19. Jiang C, Yao X, Zhao Y, Wu J, Huang P, Pan C, et al. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microbes Infect. (2020) 22:236–44. doi: 10.1016/j.micinf.2020.05.005
20. Fischer N, Dauby N, Bossuyt N, Reynders M, Gérard M, Lacor P, et al. Monitoring of human coronaviruses in Belgian primary care and hospitals, 2015-20: a surveillance study. Lancet Microbe. (2021) 2:e105–14. doi: 10.1016/S2666-5247(20)30221-4
21. Tang JW, Lam TT, Zaraket H, Lipkin WI, Drews SJ, Hatchette TF, et al. INSPIRE investigators. Global epidemiology of non-influenza RNA respiratory viruses: data gaps and a growing need for surveillance. Lancet Infect Dis. (2017) 17:e320–6. doi: 10.1016/S1473-3099(17)30238-4
22. Park S, Lee Y, Michelow IC, Choe YJ. Global seasonality of human coronaviruses: a systematic review. Open Forum Infect Dis. (2020) 7:ofaa443. doi: 10.1093/ofid/ofaa443
23. Heimdal I, Moe N, Krokstad S, Christensen A, Skanke LH, Nordbø SA, et al. Human coronavirus in hospitalized children with respiratory tract infections: a 9-year population-based study from Norway. J Infect Dis. (2019) 219:1198–206. doi: 10.1093/infdis/jiy646
24. Wong-Chew RM, García-León ML, Noyola DE, Perez Gonzalez LF, Gaitan Meza J, Vilaseñor-Sierra A. Respiratory viruses detected in Mexican children younger than 5 years old with community-acquired pneumonia: a national multicenter study. Int J Infect Dis. (2017) 62:32–8. doi: 10.1016/j.ijid.2017.06.020
25. Aydin Köker S, Demirag B, Tahta N, Bayram N, Oymak Y, Karapinar TH, et al. A 3-year retrospective study of the epidemiology of acute respiratory viral infections in pediatric patients with cancer undergoing chemotherapy. J Pediatr Hematol Oncol. (2019) 41:e242–6. doi: 10.1097/MPH.0000000000001418
26. Kurskaya O, Ryabichenko T, Leonova N, Shi W, Bi H, Sharshov K, et al. Viral etiology of acute respiratory infections in hospitalized children in Novosibirsk City, Russia (2013 - 2017). PLoS One. (2018) 13:e0200117. doi: 10.1371/journal.pone.0200117
27. Yatsyshina SB, Mamoshina MV, Shipulina OY, Podkolzin AT, Akimkin VG. Analysis of human coronaviruses circulation. Vopr Virusol. (2020) 65:267–76. doi: 10.36233/0507-4088-2020-65-5-3
28. Li Y, Wang X, Nair H. Global seasonality of human seasonal coronaviruses: a clue for postpandemic circulating season of severe acute respiratory syndrome coronavirus 2? J Infect Dis. (2020) 222:1090–7. doi: 10.1093/infdis/jiaa436
29. Ren L, Gonzalez R, Xu J, Xiao Y, Li Y, Zhou H, et al. Prevalence of human coronaviruses in adults with acute respiratory tract infections in Beijing, China. J Med Virol. (2011) 83:291–7. doi: 10.1002/jmv.21956
30. Lau SK, Woo PC, Yip CC, Tse H, Tsoi HW, Cheng VC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. (2006) 44:2063–71. doi: 10.1128/JCM.02614-05
31. Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N Engl J Med. (2003) 348:1953–66. doi: 10.1056/NEJMoa030781
32. Sun ZF, Meng XJ. Antigenic Cross-Reactivity between the Nucleocapsid Protein of Severe Acute Respiratory Syndrome (SARS) coronavirus and polyclonal antisera of antigenic group I animal coronaviruses: implication for SARS diagnosis. J Clin Microbiol. (2004) 42:2351–2. doi: 10.1128/JCM.42.5.2351-2352.2004
33. Peiris J, Lai S, Poon L, Guan Y, Yam L, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. (2003) 361:1319–25. doi: 10.1016/S0140-6736(03)13077-2
34. Lehmann C, Wolf H, Xu J, Zhao Q, Shao Y, Motz M, et al. Line immunoassay utilizing recombinant nucleocapsid proteins for detection of antibodies to human coronaviruses. Diagn Microbiol Infect Dis. (2008) 61:40–8. doi: 10.1016/j.diagmicrobio.2007.12.002
35. Chan KH, Cheng VCC, Woo PCY, Lau SKP, Poon LLM, Guan Y, et al. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin Diagn Lab Immunol. (2005) 12:1317–21. doi: 10.1128/CDLI.12.11.1317-1321.2005
36. Mäkelä MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimäki M, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. (1998) 36:539–42. doi: 10.1128/JCM.36.2.539-542.1998
37. Cox DW, Le Souëf PN. Rhinovirus and the developing lung. Paediatr Respir Rev. (2014) 15:268–74. doi: 10.1016/j.prrv.2014.03.002
38. Walter JM, Wunderink RG. Severe respiratory viral infections. Infect Dis Clin North Am. (2017) 31:455–74. doi: 10.1016/j.idc.2017.05.004
39. Kwiyolecha E, Groendahl B, Okamo B, Kayange N, Manyama F, Kidenya BR, et al. Patterns of viral pathogens causing upper respiratory tract infections among symptomatic children in Mwanza, Tanzania. Sci Rep. (2020) 10:18490. doi: 10.1038/s41598-020-74555-2
40. Bertino JS. Cost burden of viral respiratory infections: issues for formulary decision makers. Am J Med. (2002) 112:42–9. doi: 10.1016/S0002-9343(01)01063-4
41. To KKW, Yip CCY, Yuen K-Y. Rhinovirus – From bench to bedside. J Formos Med Assoc. (2017) 116:496–504. doi: 10.1016/j.jfma.2017.04.009
42. Papadopoulos N, Sanderson G, Hunter J, Johnston S. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. (1999) 58:100–4. doi: 10.1002/(sici)1096-9071(199905)58:1<100::aid-jmv16>3.0.co;2-d
43. Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, et al. Rhinoviruses infect the lower airways. J Infect Dis. (2000) 181:1875–84. doi: 10.1086/315513
44. Esposito S, Daleno C, Prunotto G, Scala A, Tagliabue C, Borzani I, et al. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses: respiratory viruses in pediatric pneumonia. Influenza Other Respir Viruses. (2013) 7:18–26. doi: 10.1111/j.1750-2659.2012.00340.x
45. Ieven M, Coenen S, Loens K, Lammens C, Coenjaerts F, Vanderstraeten A, et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. (2018) 24:1158–63. doi: 10.1016/j.cmi.2018.02.004
46. Ljubin-Sternak S, Meštrović T, Ivković-Jureković I, Kolarić B, Slović A, Forčić D, et al. The emerging role of rhinoviruses in lower respiratory tract infections in children – clinical and molecular epidemiological study from Croatia, 2017–2019. Front Microbiol. (2019) 10:2737. doi: 10.3389/fmicb.2019.02737
47. Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. (2013) 26:135–62. doi: 10.1128/CMR.00077-12
48. Glanville N, McLean GR, Guy B, Lecouturier V, Berry C, Girerd Y, et al. Cross-serotype immunity induced by immunization with a conserved rhinovirus capsid protein. PLoS Pathog. (2013) 9:e1003669. doi: 10.1371/journal.ppat.1003669
49. Makris S, Johnston S. Recent advances in understanding rhinovirus immunity. F1000Res. (2018) 7:1537. doi: 10.12688/f1000research.15337.1
50. Royston L, Tapparel C. Rhinoviruses and respiratory enteroviruses: not as simple as ABC. Viruses. (2016) 8:16. doi: 10.3390/v8010016
51. Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci USA. (2015) 112:5485–90. doi: 10.1073/pnas.1421178112
52. Lau SKP, Yip CCY, Tsoi H-W, Lee RA, So L-Y, Lau Y-L, et al. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. (2007) 45:3655–64. doi: 10.1128/JCM.01254-07
53. Turunen R, Jartti T, Bochkov YA, Gern JE, Vuorinen T. Rhinovirus species and clinical characteristics in the first wheezing episode in children: rhinovirus species and the first wheezing episode. J Med Virol. (2016) 88:2059–68. doi: 10.1002/jmv.24587
54. Zlateva KT, van Rijn AL, Simmonds P, Coenjaerts FEJ, van Loon AM, Verheij TJM, et al. Molecular epidemiology and clinical impact of rhinovirus infections in adults during three epidemic seasons in 11 European countries (2007–2010). Thorax. (2020) 75:882–90. doi: 10.1136/thoraxjnl-2019-214317
55. Hasegawa K, Jartti T, Bochkov YA, Gern JE, Mansbach JM, Piedra PA, et al. Rhinovirus species in children with severe bronchiolitis: multicenter cohort studies in the United States and Finland. Pediatr Infect Dis J. (2019) 38:e59–62. doi: 10.1097/INF.0000000000002141
56. Erkkola R, Turunen R, Räisänen K, Waris M, Vuorinen T, Laine M. Rhinovirus C is associated with severe wheezing and febrile respiratory illness in young children. Pediatr Infect Dis J. (2020) 39:283–6. doi: 10.1097/INF.0000000000002570
57. Richter J, Nikolaou E, Panayiotou C, Tryfonos C, Koliou M, Christodoulou C. Molecular epidemiology of rhinoviruses in Cyprus over three consecutive seasons. Epidemiol Infect. (2015) 143:1876–83. doi: 10.1017/S0950268814002933
58. Zakharenkov IA, Rachina SA, Dekhnich NN, Kozlov RS, Sinopalnikov AI, Ivanchik NV, et al. Etiology of severe community-acquired pneumonia in adults: results of the first Russian multicenter study. Ter Arkh. (2020) 92:36–42. doi: 10.26442/00403660.2020.01.000491
59. Cai XY, Wang Q, Lin GY, Cai ZW, Lin CX, Chen PZ, et al. Respiratory virus infections among children in South China. J Med Virol. (2014) 86:1249–55. doi: 10.1002/jmv.23931
60. Zhou F, Wang Y, Liu Y, Liu X, Gu L, Zhang X, et al. Disease severity and clinical outcomes of community-acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from the CAP-China Network. Eur Respir J. (2019) 54:1802406. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A4212
61. Baillie VL, Olwagen CP, Madhi SA. Review on clinical and molecular epidemiology of human rhinovirus-associated lower respiratory tract infections in African and Southeast Asian children. Pediatr Infect Dis J. (2018) 37:e185–94. doi: 10.1097/INF.0000000000001897
62. Price RHM, Graham C, Ramalingam S. Association between viral seasonality and meteorological factors. Sci Rep. (2019) 9:929. doi: 10.1038/s41598-018-37481-y
63. Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol. (2006) 78:644–50. doi: 10.1002/jmv.20588
64. Civljak R, Tot T, Falsey AR, Huljev E, Vraneš J, Ljubin-Sternak S. Viral pathogens associated with acute respiratory illness in hospitalized adults and elderly from Zagreb, Croatia, 2016 to 2018. J Med Virol. (2019) 91:1202–9. doi: 10.1002/jmv.25437
65. Lee W-M, Lemanske RF, Evans MD, Vang F, Pappas T, Gangnon R, et al. Human Rhinovirus Species and Season of Infection Determine Illness Severity. Am J Respir Crit Care Med. (2012) 186:886–91. doi: 10.1164/rccm.201202-0330OC
66. Foxman EF, Storer JA, Fitzgerald ME, Wasik BR, Hou L, Zhao H, et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci USA. (2015) 112:827–32. doi: 10.1073/pnas.1411030112
67. Linder JE, Kraft DC, Mohamed Y, Lu Z, Heil L, Tollefson S, et al. Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol. (2013) 131:69–77.e6. doi: 10.1016/j.jaci.2012.09.033
68. Akello JO, Kamgang R, Barbani MT, Suter-Riniker F, Leib SL, Ramette A. Epidemiology of human adenoviruses: a 20-year retrospective observational study in hospitalized patients in Bern, Switzerland. Clin Epidemiol. (2020) 12:353–66. doi: 10.2147/CLEP.S246352
69. De Conto F, Conversano F, Medici MC, Ferraglia F, Pinardi F, Arcangeletti MC, et al. Epidemiology of human respiratory viruses in children with acute respiratory tract infection in a 3-year hospital-based survey in Northern Italy. Diagn Microbiol Infect Dis. (2019) 94:260–7. doi: 10.1016/j.diagmicrobio.2019.01.008
70. Pscheidt VM, Gregianini TS, Martins LG, da Veiga ABG. Epidemiology of human adenovirus associated with respiratory infection in southern Brazil. Rev Med Virol. (2020) e2189. doi: 10.1002/rmv.2189
71. Leen AM, Rooney CM. Adenovirus as an emerging pathogen in immunocompromised patients. Br J Haematol. (2005) 128:135–44. doi: 10.1111/j.1365-2141.2004.05218.x
72. Lynch J, Kajon A. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med. (2016) 37:586–602. doi: 10.1055/s-0036-1584923
73. Lim LM, Woo YY, de Bruyne JA, Nathan AM, Kee SY, Chan YF, et al. Epidemiology, clinical presentation and respiratory sequelae of adenovirus pneumonia in children in Kuala Lumpur, Malaysia. PLoS ONE. (2018) 13:e0205795. doi: 10.1371/journal.pone.0205795
74. Shachor-Meyouhas Y, Hadash A, Kra-Oz Z, Shafran E, Szwarcwort-Cohen M, Kassis I. Adenovirus respiratory infection among immunocompetent patients in a pediatric intensive care unit during 10-year period: co-morbidity is common. Isr Med Assoc J. (2019) 21:595–8.
75. Yao L, Wang C, Wei T, Wang H, Ma F, Zheng L. Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017–2018. Virol J. (2019) 16:78. doi: 10.1186/s12985-019-1185-x
76. Binder AM, Biggs HM, Haynes AK, Chommanard C, Lu X, Erdman DD, et al. Human adenovirus surveillance — United States, 2003–2016. MMWR Morb Mortal Wkly Rep. (2017) 66:1039–42. doi: 10.15585/mmwr.mm6639a2
77. Castro IA, Costa LDC, Oliveira ACR, Souza M, das Dôres de Paula Cardoso D, Camargos PAM, et al. Circulation profile of respiratory viruses in symptomatic and asymptomatic children from Midwest Brazil. Braz J Microbiol. (2020) 51:1729–35. doi: 10.1007/s42770-020-00368-0
78. Li Y, Liang Y, Ling Y, Duan M, Pan L, Chen Z. The spectrum of viral pathogens in children with severe acute lower respiratory tract infection: a 3-year prospective study in the pediatric intensive care unit. J Med Virol. (2019) 91:1633–42. doi: 10.1002/jmv.25502
79. Barnadas C, Schmidt DJ, Fischer TK, Fonager J. Molecular epidemiology of human adenovirus infections in Denmark, 2011–2016. J Clin Virol. (2018) 104:16–22. doi: 10.1016/j.jcv.2018.04.012
80. Mandelia Y, Procop GW, Richter SS, Worley S, Liu W, Esper F. Dynamics and predisposition of respiratory viral co-infections in children and adults. Clin Microbiol Infect. (2020) 27:631.e1–6. doi: 10.1016/j.cmi.2020.05.042
81. Jung J, Seo E, Yoo RN, Sung H, Lee J. Clinical significance of viral–bacterial codetection among young children with respiratory tract infections: findings of RSV, influenza, adenoviral infections. Medicine. (2020) 99:e18504. doi: 10.1097/MD.0000000000018504
82. Wold WSM, Ison MG. “Adenovirus”. In: Knipe DM and Howley PM, editors. Fields Virology. 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins (2013). p. 1732–67.
83. Duan YL, Zhu Y, Xu BP Li CC, Chen AH, Deng L, Bao YX, et al. Multicenter study of human adenovirus infection in pediatric community-acquired pneumonia in China. Zhonghua Er Ke Za Zhi. (2019) 57:27–32. doi: 10.3760/cma.j.issn.0578-1310.2019.01.008
84. Probst V, Datyner EK, Haddadin Z, Rankin DA, Hamdan L, Rahman HK, et al. Human adenovirus species in children with acute respiratory illnesses. J Clin Virol. (2021) 134:104716. doi: 10.1016/j.jcv.2020.104716
85. Lin M-R, Yang S-L, Gong Y-N, Kuo C-C, Chiu C-H, Chen C-J, et al. Clinical and molecular features of adenovirus type 2, 3, and 7 infections in children in an outbreak in Taiwan, 2011. Clin Microbiol Infect. (2017) 23:110–6. doi: 10.1016/j.cmi.2016.11.004
86. Ji T, Liu Y, Li Y, Khan SA, Zhou Q, Chen B, et al. Molecular typing and genomic characteristic of human adenoviruses in Datong, Northern China. J Med Virol. (2020) 92:3111–8. doi: 10.1002/jmv.26203
87. Chehadeh W, Al-Adwani A, John SE, Al-Dhufairi S, Al-Dousari H, Alkhaledi M, et al. Adenovirus types associated with severe respiratory diseases: a retrospective 4-year study in Kuwait. J Med Virol. (2018) 90:1033–9. doi: 10.1002/jmv.25059
88. Yatsyshina SB, Ageeva MR, Vorobieva NS, Valdokhina AV, Elkina MA, Gorelov AV, et al. Adenoviruses in the etiological structure of acute respiratory viral infection in Moscow in 2004–2014. Zh Mikrobiol Epidemiol Immunobiol. (2015) 5:50–7.
89. Zhao Y, Lu R, Shen J, Xie Z, Liu G, Tan W. Comparison of viral and epidemiological profiles of hospitalized children with severe acute respiratory infection in Beijing and Shanghai, China. BMC Infect Dis. (2019) 19:729. doi: 10.1186/s12879-019-4385-5
90. Lei C, Yang L, Lou CT, Yang F, SiTou KI, Hu H, et al. Viral etiology and epidemiology of pediatric patients hospitalized for acute respiratory tract infections in Macao: a retrospective study from 2014 to 2017. BMC Infect Dis. (2021) 21:306. doi: 10.1186/s12879-021-05996-x
91. Fu Y, Tang Z, Ye Z, Mo S, Tian X, Ni K, et al. Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect Dis. (2019) 19:36. doi: 10.1186/s12879-018-3651-2
92. Duan Y, Li C, Deng L, An S, Zhu Y, Wang W, et al. Genetic analysis of human adenovirus type 7 strains circulating in different parts of China. Virol Sin. (2021) 1–11. doi: 10.1007/s12250-020-00334-y
93. Guido M, Tumolo MR, Verri T, Romano A, Serio F, De Giorgi M, et al. Human bocavirus: current knowledge and future challenges. World J Gastroenterol. (2016) 22:8684. doi: 10.3748/wjg.v22.i39.8684
94. Kahn J. Human bocavirus: clinical significance and implications. Curr Opin Pediatr. (2008) 20:62–6. doi: 10.1097/MOP.0b013e3282f3f518
95. Streiter M, Malecki M, Prokop A, Schildgen V, Lüsebrink J, Guggemos A, et al. Does human bocavirus infection depend on helper viruses? A challenging case report. Virol J. (2011) 8:417. doi: 10.1186/1743-422X-8-417
96. International Committee on Taxonomy of Viruses (ICTV). Virus Taxonomy: 2017. (2019). Available online at: https://talk.ictvonline.org/taxonomy (accessed April 4, 2021).
97. Christensen A, Kesti O, Elenius V, Eskola AL, Døllner H, Altunbulakli C, et al. Human bocaviruses and paediatric infections. Lancet Child Adolesc Health. (2019) 3:418–26. doi: 10.1016/S2352-4642(19)30057-4
98. Ljubin-Sternak S, Meštrović T, Ivković-Jureković I, Tešović G, Mlinarić-Galinović G, Lukšić I, et al. High detection rates of human bocavirus in infants and small children with lower respiratory tract infection from croatia. Clin Lab. (2019) 65:101–7. doi: 10.7754/Clin.Lab.2018.180702
99. Ljubin-Sternak S, Marijan T, Ivković-Jureković I, Cepin-Bogović J, Gagro A, Vraneš J. Etiology and clinical characteristics of single and multiple respiratory virus infections diagnosed in croatian children in two respiratory seasons. J Pathog. (2016) 2016:1–8. doi: 10.1155/2016/2168780
100. Abozahra R, Abdelhamid SM, Khairy K, Baraka K. Detection and phylogenetic analysis of Human bocavirus in children diagnosed with acute respiratory tract infection. J Med Microbiol. (2020) 69:1197–202. doi: 10.1099/jmm.0.001243
101. Madi NM, Al-Adwani A. Human bocavirus (HBoV) in Kuwait: molecular epidemiology and clinical outcome of the virus among patients with respiratory diseases. J Med Microbiol. (2020) 69:1005–12. doi: 10.1099/jmm.0.001219
102. Verbeke V, Reynders M, Floré K, Vandewal W, Debulpaep S, Sauer K, et al. Human bocavirus infection in Belgian children with respiratory tract disease. Arch Virol. (2019) 164:2919–30. doi: 10.1007/s00705-019-04396-6
103. Kenmoe S, Kengne-Nde C, Ebogo-Belobo JT, Mbaga DS, Fatawou Modiyinji A, Njouom R. Systematic review and meta-analysis of the prevalence of common respiratory viruses in children <2 years with bronchiolitis in the pre-COVID-19 pandemic era. PLoS ONE. (2020) 15:e0242302. doi: 10.1371/journal.pone.0242302
104. Kengne–Nde C, Kenmoe S, Modiyinji AF, Njouom R. Prevalence of respiratory viruses using polymerase chain reaction in children with wheezing, a systematic review and meta–analysis. PLoS ONE. (2020) 15:e0243735. doi: 10.1371/journal.pone.0243735
105. Kobayashi H, Shinjoh M, Sudo K, Kato S, Morozumi M, Koinuma G, et al. Nosocomial infection by human bocavirus and human rhinovirus among paediatric patients with respiratory risks. J Hosp Infect. (2019) 103:341–8. doi: 10.1016/j.jhin.2019.05.002
106. Brebion A, Vanlieferinghen P, Déchelotte P, Boutry M, Peigue-Lafeuille H, Henquell C. Fatal subacute myocarditis associated with human bocavirus 2 in a 13-month-old child. J Clin Microbiol. (2014) 52:1006–8. doi: 10.1128/JCM.03013-13
107. Akturk H, Sik G, Salman N, Sutcu M, Tatli B, Ciblak MA, et al. Atypical presentation of human bocavirus: severe respiratory tract infection complicated with encephalopathy. J Med Virol. (2015) 87:1831–8. doi: 10.1002/jmv.24263
108. Haytoglu Z, Canan O. Bocavirus Viremia and hepatitis in an immunocompetent child. Balkan Med J. (2017) 34:281–3. doi: 10.4274/balkanmedj.2015.1492
109. Lee HN, Koo HJ, Kim SH, Choi S-H, Sung H, Do K-H. Human bocavirus infection in adults: clinical features and radiological findings. Korean J Radiol. (2019) 20:1226. doi: 10.3348/kjr.2018.0634
110. Choi S-H, Huh JW, Hong S-B, Jung J, Kim MJ, Chong YP, et al. Severe human bocavirus–associated pneumonia in adults at a referral hospital, Seoul, South Korea. Emerg Infect Dis. (2021) 27:226–8. doi: 10.3201/eid2701.202061
111. Eşki A, Öztürk GK, Gülen F, Çiçek C, Demir E. Risk factors for influenza virus related severe lower respiratory tract infection in children. Pediatr Infect Dis J. (2019) 38:1090–5. doi: 10.1097/INF.0000000000002447
112. Petrarca L, Nenna R, Frassanito A, Pierangeli A, Di Mattia G, Scagnolari C, et al. Human bocavirus in children hospitalized for acute respiratory tract infection in Rome. World J Pediatr. (2020) 16:293–8. doi: 10.1007/s12519-019-00324-5
113. Lin CY, Hwang D, Chiu NC, Weng LC, Liu HF, Mu JJ, et al. Increased Detection of Viruses in Children with Respiratory Tract Infection Using PCR. Int J Environ Res Public Health. (2020) 17:564. doi: 10.3390/ijerph17020564
114. Lu Y, Li DD, Jin Y, Duan ZJ A. review of detection methods for human bocaviruses. Bing Du Xue Bao. (2014) 30:298–302.
Keywords: respiratory tract infections, seasonal coronavirus, rhinovirus, adenovirus, bocavirus, Croatia
Citation: Ljubin-Sternak S, Meštrović T, Lukšić I, Mijač M and Vraneš J (2021) Seasonal Coronaviruses and Other Neglected Respiratory Viruses: A Global Perspective and a Local Snapshot. Front. Public Health 9:691163. doi: 10.3389/fpubh.2021.691163
Received: 05 April 2021; Accepted: 04 June 2021;
Published: 05 July 2021.
Edited by:
Yousef Saleh Khader, Jordan University of Science and Technology, JordanReviewed by:
Hashaam Akhtar, Yusra Institute of Pharmaceutical Sciences Islamabad, PakistanCopyright © 2021 Ljubin-Sternak, Meštrović, Lukšić, Mijač and Vraneš. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomislav Meštrović, dG9taXNsYXYubWVzdHJvdmljQGdtYWlsLmNvbQ==; dG1lc3Ryb3ZpY0B1bmluLmhy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.