
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 22 July 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.679853
This article is part of the Research TopicInfectious Disease Surveillance: Applying Cooperative Research to Recent Outbreaks including COVID-19View all 50 articles
 Can Chen†
Can Chen† Zhou Guan†
Zhou Guan† Chenyang Huang†
Chenyang Huang† Daixi Jiang
Daixi Jiang Xiaoxiao Liu
Xiaoxiao Liu Yuqing Zhou
Yuqing Zhou Danying Yan
Danying Yan Xiaobao Zhang
Xiaobao Zhang Yiyi Zhou
Yiyi Zhou Cheng Ding
Cheng Ding Lei Lan
Lei Lan Yushi Lin
Yushi Lin Jie Wu*
Jie Wu* Lanjuan Li*
Lanjuan Li* Shigui Yang*
Shigui Yang*Background: The incidence of other infectious diarrhea (OID) ranked second in class C notifiable disease in China. It has posed a great threat to public health of all age groups. The aim of this study was to investigate the epidemiological trends and hotspots of OID in mainland China.
Materials and Methods: Incidence and mortality data for OID stratified by date, age and region from 2004 to 2017 was extracted from the data-center of China public health science. Joinpoint regression and space-time analyses were performed to explore the epidemiological trends and hotspots of OID.
Results: The average annual incidence of OID was 60.64/100,000 and it showed an increased trend in the mainland China especially after 2006 (APC = 4.12, 95 CI%: 2.06–6.21). Children of 0–4 year age group accounts for 60.00% (5,820,897/11,414,247) of all cases and its incidence continuously increased though 2004–2017 (APC = 6.65, 95 CI%: 4.39–8.96). The first-level spatial and temporal aggregation areas were located in Beijing and Tianjin, with the gathering time from 2005/1/1 to 2011/12/31 (RR = 5.52, LLR = 572893.59, P < 0.001). The secondary spatial and temporal aggregation areas covered Guangdong, Guangxi, Hainan and Guizhou from 2011/1/1 to 2017/12/31 (RR = 1.98, LLR = 242292.72, P < 0.001). OID of Tianjin and Beijing presented a decreased trend since 2006. However, the incidence of OID in Guangdong, Guangxi, Hainan and Guizhou showed increased trends through 2004–2017.
Conclusion: Our study showed that OID showed a constantly increasing trend and brought considerable burden in China especially in the 0–4 age group. The high-risk periods and clusters of regions for OID were identified, which will help government develop disease-specific and location-specific interventive measures.
Infectious diarrhea is one of the most common infectious diseases around the world and acts as an important indicator to regional hygiene, food safety and public health (1). In 2010, the diarrhoeal disease ranked second in the global burden of infectious diseases and in 2015, about 2.3 billion people had experienced diarrhea worldwide (2). It was also a major cause of malnutrition and mortality among children under 5 in developing countries (3). China is one of 15 countries with a high disease burden of pneumonia and diarrhea (4). In China, other infectious diarrhea (OID) was defined as infectious diarrhea other than cholera, dysentery, typhoid/paratyphoid fever (5). Due to the wide spectrum of pathogens and lack of effective vaccine protection, many regions presented high incidences (6). In 2017, the incidence of OID ranked second in class C notifiable diseases in China (7). It has posed a great threat to public health of all age groups, especially to infants and young children, which therefore caused a heavy economic burden in China.
Although many researches has pictured the epidemiological features of OID in China, the majority of these studies were based on the region-specific level (8, 9). Therefore, in order to further describe the overall epidemic characteristics and trends of OID, we systematically analyzed the reported cases of OID from 31 provinces in China. Meanwhile, we then performed the space-time analyses to identify the hotspots of OID. The results of this study would provide a theoretical basis for OID prevention and control in China.
The incidence and mortality data for infectious diarrhea from 2004 to 2017 were obtained from the data-center of China public health science, which is the main data center of the National Population Health Science data sharing platform in China and covered a population of about 1.3 billion people from 31 provinces and regions in mainland China (10).
The definition of OID cases in our study followed the criteria issued by the Ministry of Health of the People's Republic of China. In each level medical institution, once a OID case identified, clinicians complete a standard case report card for infectious diseases. The field investigations were performed using a standardized form.
To assess the epidemiological trends and hotspots of OID in mainland China, the data of OID including the number of cases and deaths, the incidence and mortality were stratified by date (month and year), age and region.
We used joinpoint regression model to examine incidence trends of OID from 2004 to 2017. The annual percentage changes (APCs) with their 95% confidence interval (CI) were obtained for each trend segment (11). We used Z test to assess whether APCs was significant (P < 0.05), and the trends were further described as increased or decreased when the APCs were positive or negative, respectively. While, the trends were considered as stable when the APCs values were not significant (P ≥ 0.05).
The seasonal decomposition analysis was based on the seasonal trend decomposition using SEATS (Seasonal Extraction in ARIMA Time Series) (12), which filters the trend and seasonal component from the time series data and decomposes into three components: trend (T), seasonal (S), and remainder or random (R). The equation can be described as follows. Yt = Tt + St + Rt (12). In this study, Yt is the number of OID cases. t is time in the unit of month.
Spatial autocorrelation refers to the potential interdependence of some variables between observed data in the same distribution area. The global Moran's I and local Moran's I were used to measure spatial autocorrelation (13). The global Moran's I ranging from −1 to 1 was used to detect the degree of spatial autocorrelation of research object from the whole region (14). The local Moran's I was used to explore the spatial position of clustering. According to the results of local Moran's I, it presented four categories results including high-high cluster, low-low cluster, high-low cluster, and low-high cluster (15). Z test was used to assess the significant difference.
The retrospective spatiotemporal scan statistic based on the discrete Poisson model was applied to detect the space-time cluster of OID in China (16). The dynamic space-time two-dimensional cylinder scanning window was constructed to scan geographic units and time within the study area. The null hypothesis presumed that the window area and outside areas have the same relative risk (RR) of incidence. The actual and theoretical incidence numbers inside and outside the scanning window were used to calculate the log likelihood ratio (LLR). The cluster was classified according to the LLR value (e.g. secondary cluster 1, 2) (17). Monte Carlo simulation was used to evaluate statistical significance. We used Microsoft Excel 2016 for data extraction, sorting, and cleaning, and R (version 3.2.3), and SatScan (version 9.5), Joinpoint (version 4.8.0.1) for further data analysis.
A total of 11,414,247 OID cases were reported in mainland China from Jan 1, 2004 to Dec 31, 2017. The average annual incidence was 60.64/100,000, from 2004 (31.70/100,000) to the 2017 (93.10/100,000), (Figure 1A). The incidence of OID presented an increased trend in mainland China especially after 2006 (APC = 4.12, 95CI%: 2.06–6.21, P < 0.05), (Figure 1B). Before 2013, two peaks of OID showed up during June to August and September to November, but it turned to the June to August and December to February (Figures 1C,D).
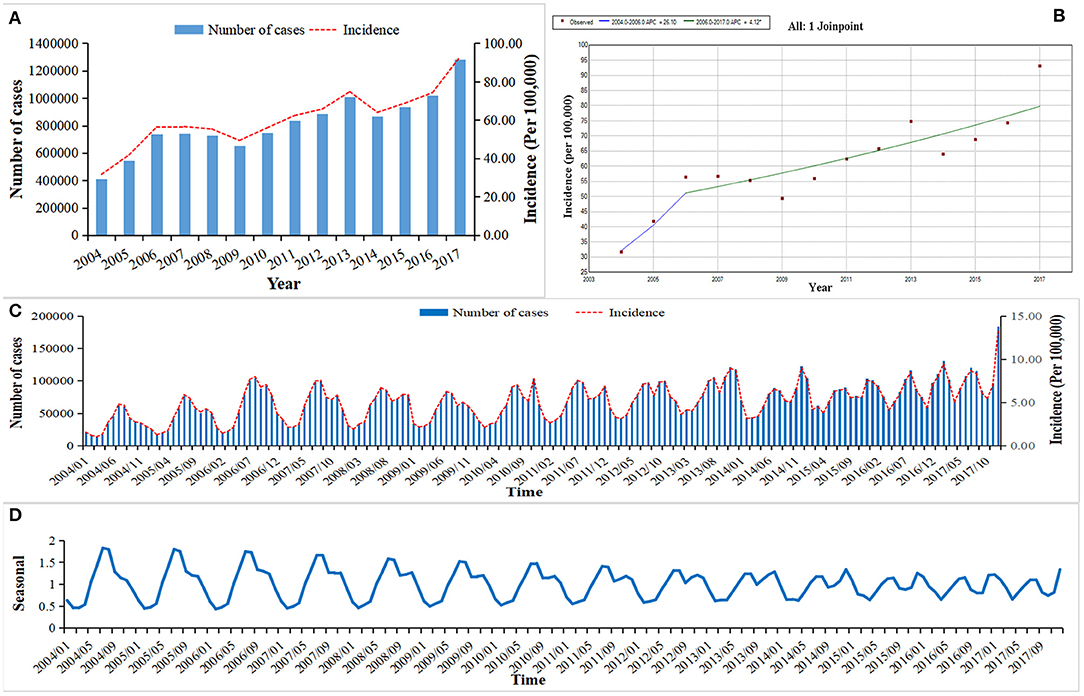
Figure 1. The incidence and trend of other infectious diarrhea in mainland China from 2004 to 2017. (A) The annual incidence of OID from 2004 to 2017. (B) The Joinpoint analysis of OID from 2004 to 2017. (C) The monthly incidence of OID from 2004 to 2017. (D) Seasonal decomposition analysis of OID from 2004 to 2017.
The age group of 0- years showed the highest average annual incidence of 1318.10/100,000. Children in 0–4 years group were most at-risk group infected with OID, accounting for 60.00% (5,820,897/11,414,247) of all cases. With the increase of age, the incidences showed a downward trend, and after the four-year age group, the incidences were relatively stable (Figure 2A). The results of Joinpoint analysis showed that the incidence of 0–4 age group continuously increased through 2004–2017 (APC = 6.65, 95CI%: 4.39–8.96, P < 0.05) (Figure 2B).
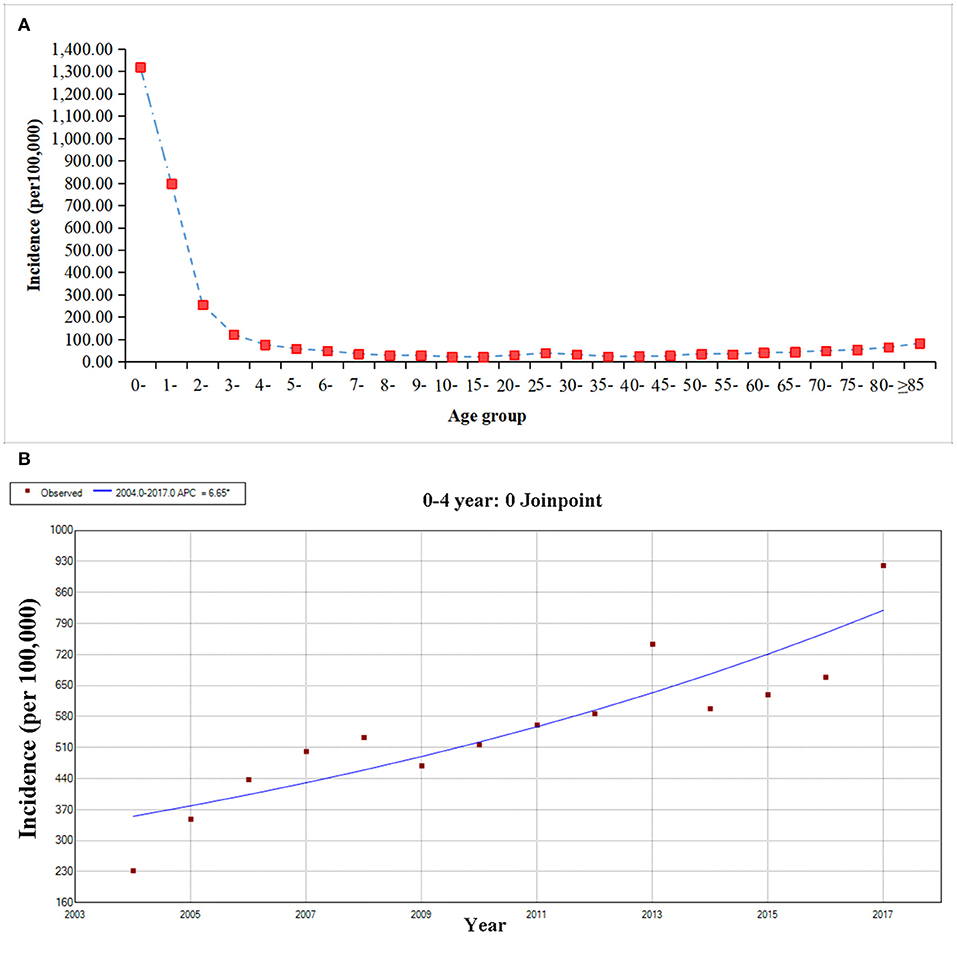
Figure 2. The age group distribution and trend of other infectious diarrhea from 2004 to 2017. (A) The age group distribution of OID. (B) The Joinpoint analysis of OID in the age of 0–4 year.
During 2004–2017, The top three incidence regions of OID were Tianjin (289.33/100,000), Beijing (253.67/100,000) and Zhejiang province (200.34/100,000). The OID was higher in the eastern China (83.65/100,000) than central (43.79/100,000) and western China (47.95/100,000), (Figure 4A, Supplementary Table 1). The global spatial autocorrelation analysis results demonstrated a positive correlation during the 2004-2017 (Supplementary Table 2). Then, local spatial autocorrelation at the provincial and autonomous levels were further analyzed. The high-high aggregation areas of OID were found in Tianjin and Beijing from 2004–2015. The low-low aggregation areas were found in Heilongjiang and Jilin from 2004 to 2017 (Figure 3).
Two spatial and temporal aggregation areas were revealed according to spatial and temporal aggregation analyses. The first-level spatial and temporal aggregation areas were distributed in Beijing and Tianjin, with the gathering time in 2005/1/1 to 2011/12/31. The actual number of cases reported in the regions was 658,479, which was much higher than that of the number of expected cases, that is, 125,134 (RR = 5.52, LLR = 572893.59, P < 0.001). The secondary spatial and temporal aggregation areas covered four provinces from 2011/1/1 to 2017/12/31. The areas included Guangdong, Guangxi, Hainan and Guizhou. The actual number of cases reported in the region was 1,396,809, but the number of expected cases was 749,523 (RR = 1.98, LLR = 242,292.72, P < 0.001), (Figure 4B).
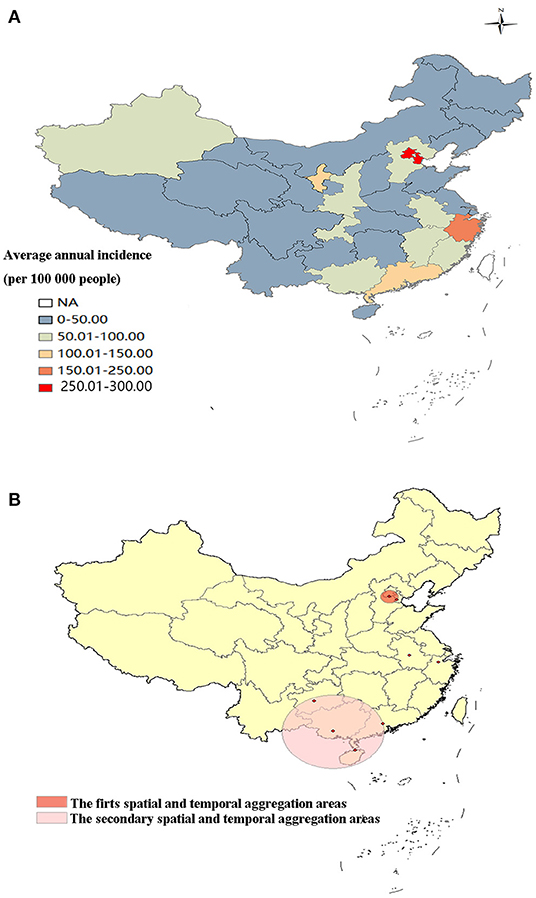
Figure 4. Spatial and temporal aggregation of other infectious diarrhea. (A) Geographical distribution of OID in mainland China. (B) Spatial and temporal aggregation of OID from 2004 to 2017.
The results of Joinpoint analysis by areas showed that OID showed an increasing trend in most provinces (21/31) (Supplementary Table 1). In Tianjin and Beijing, the OID presented a decreasing trend since 2006. However, the incidence of OID in Guangdong, Guangxi, Hainan and Guizhou showed an increased trend throughout 2004–2017 (Figure 5).

Figure 5. The trends of other infectious diarrhea in hotspots. (A) The Joinpoint analysis of OID in Beijing from 2004 to 2017. (B) The Joinpoint analysis of OID in Tianjin from 2004 to 2017. (C) The Joinpoint analysis of OID in Guangdong from 2004 to 2017. (D) The Joinpoint analysis of OID in Guangxi from 2004 to 2017. (E) The Joinpoint analysis of OID in Hainan from 2004 to 2017. (F) The Joinpoint analysis of OID in Guizhou from 2004 to 2017.
In this study, we investigated the epidemic trend and distribution characteristics of 11,414,247 OID cases in mainland China during a 14-year time period. Our results demonstrated that OID presented a constantly increased trend in mainland China and in 0–4 age group from 2004 to 2017. Two levels hotspots were found. Our findings will provide scientific evidence to policy maker to better prevent and control OID in the following stage.
The expanding of pathogenic spectrum, especially viral pathogens, has promoted the prevalence of OID and caused increasing outbreaks and public health emergencies which usually brought a large number of cases (18). In recent years, as the development of infectious disease surveillance and reporting system, the OID cases were more likely to be detected and reported. The prevalence of OID shifted from summer and autumn peaks to summer and winter peaks since 2013. This might attribute to the increased incidence of viral infectious diarrhea such as rotavirus and norovirus which clustered in winter (19). In 2005–2019, the four most common pathogens of OID in China were rotavirus (85.74%), adenovirus (4.28%), salmonella (3.58%) and norovirus (2.82%) (20). Since 2013, norovirus was reported as the dominant pathogeny in OID outbreaks in China, and rotavirus also increased rapidly in children under 5 years of age (21, 22). The OID incidence of 0–4 years age group showed the increased trends in China. On the one hand, once children are infected, the symptoms are relatively severe due to immature immune system (23). Additionally, parents are more likely to seek medical treatments for their children and then the cases were more likely to be reported. On the other hand, as proportion of viral pathogens in OID increased, children under 5 years old were more vulnerable (24).
In previous studies, diarrhea was found presenting a specific temporal and spatial distribution which usually clustered spatially in different geographical locations (25–27). Several factors including sociodemographic variables, personal hygiene, and environmental and climatic changing were considered to be associated with the incidence of diarrhea (28, 29). Spatiotemporal aggregation of OID in China could be divided into two stages. The first hotspots were detected in the Beijing-Tianjin through 2005–2011. The two sites are both developed areas in China. In the early phase of development and urbanization, it attracted larger floating population characterized by low immune systems, poor living environments and living conditions, and poor health and knowledge of epidemic prevention measures which might cause OID to spread easily (30, 31). As the improvement of the environment and the management of mobile population, risks of infection by various pathogens would reduce. In the Joinpoint regression, the incidence of OID in Beijing-Tianjin decreased since 2006. The second hotspots was identified in Guangdong, Guangxi, Hainan and Guizhou through 2011–2017. The OID in those region showed an increased trend through 2004–2017. In weng's study, public health emergencies caused by OID were found mainly clustered in the Guangdong, Guangxi and Fujian provinces (18). South China is closer to the equator with a subtropical monsoon and tropical monsoon climate, which are characterized by high humidity, temperature, rainfall, and wind speed. And this also facilitated OID transmission (31). Furthermore, residents of coastal areas usually have the habit of eating raw seafood, which also contains a wide variety of pathogens such as rotavirus, norovirus, and vibrio parahaemolyticus (32, 33). Our results show that the incidences of OID in China has a clear population, seasonal and regional distribution. The comprehensive prevention and control measures should be implemented to reduce the incidences of OID before the epidemic peaks. The specific hotspots, highly risk groups should be considered as the priority of prevention and control. At the same time, the monitoring of pathogens should be conducted to further clarify the epidemic characteristics of OID in each jurisdiction. For some pathogens such as rotavirus could be prevented by vaccines. The knowledge should be strengthened to improve the coverage of vaccines, especially in children (34).
Our study demonstrated that the incidence of OID continuously increased in mainland China especially in 0–4 years age group. The seasonal peak of OID prevalence shifted from summer and autumn to summer and winter since 2013. Currently, Guangdong, Guangxi, Hainan and Guizhou were identified as the hotspots of OID in mainland China. The high-risk periods and clusters of regions for the OID were identified which will help governments to develop disease-specific and location-specific intervention measures.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
SY, LJL, and JW: designed the study. CC, ZG, CH, DJ, XL, YZ, DY, XZ, YZ, and YL: collected data. CC, ZG, and CH: analyzed data and interpreted data and wrote the report. CD and LL: checked the data and results. SY: revised the report from preliminary draft to submission. All authors have read and approved the manuscript.
This study was supported by grants from the National Natural Science Foundation of China (Grant Numbers: 81672005, U1611264, 81001271, and 81721091) and the Mega-Project of National Science and Technology for the 12th and 13th Five-Year Plan of China (Grant Numbers: 2018ZX10715-014-002 and 2014ZX10004008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.679853/full#supplementary-material
1. Parker M, Unaka N. Diagnosis and management of infectious Diarrhea. JAMA pediatrics. (2018) 172:775–6. doi: 10.1001/jamapediatrics.2018.1172
2. Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1545–602. doi: 10.1016/S0140-6736(16)31678-6
3. Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. (2004) 363:641–53. doi: 10.1016/S0140-6736(04)15599-2
4. International Vaccine Access. Pneumonia and Diarrhea Progress Report. Available online at: https://www.jhsph.edu/ivac/resources/pdpr/ (accessed March 1, 2021)
5. Liu HX, Zhang J. Analysis of reported infectious diarrhea (other than cholera, dysentery, typhoid and paratyphoid) in China in 2011. Zhonghua Yu Fang Yi Xue Za Zhi. (2013) 47:328–32 doi: 10.3760/cma.j.issn.0253-9624.2013.04.009
6. Zhang P, Zhang J. Surveillance on other infectious diarrheal diseases in China from 2014 to 2015. Zhonghua Liu Xing Bing Xue Za Zhi. (2017) 38:424–30. doi: 10.3760/cma.j.issn.0254-6450.2017.04.003
7. China Centers for Disease Control and Prevention (2018). Available online at: http://www.nhc.gov.cn/jkj/s3578/201802/de926bdb046749abb7b0a8e23d929104.shtml (accessed March 1, 2021)
8. Wang H, Di B, Zhang T, Lu Y, Chen C, Wang D, et al. Association of meteorological factors with infectious diarrhea incidence in Guangzhou, southern China: a time-series study (2006–2017). Sci Total Environ. (2019) 672:7–15. doi: 10.1016/j.scitotenv.2019.03.330
9. Wang WQ, Liu D, Zhao B, Fu HQ, Zhang ZK, Yu JX, et al. Epidemiological and etiological surveillance on infectious diarrhea in Pudong New Area, Shanghai, 2013–2017. Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:417–22. doi: 10.3760/cma.j.issn.0254-6450.2020.03.026
10. Data-center of China public health science. Available online at: http://www.phsciencedata.cn/Share/index.jsp (accessed March 01, 2021).
11. Liu X, Jiang J, Yu C, Wang Y, Sun Y, Tang J, et al. Secular trends in incidence and mortality of bladder cancer in China, 1990–2017: a joinpoint and age-period-cohort analysis. Cancer Epidemiol. (2019) 61:95–103. doi: 10.1016/j.canep.2019.05.011
12. Dagum EB, Bianconcini S. Seasonal Adjustment Methods and Real Time Trend-Cycle Estimation. Cham: Springer. (2016). doi: 10.1007/978-3-319-31822-6
13. Thompson ES, Saveyn P, Declercq M, Meert J, Guida V, Eads CD, et al. Characterisation of heterogeneity and spatial autocorrelation in phase separating mixtures using Moran's I. J Colloid Interface Sci. (2018) 513:180–7. doi: 10.1016/j.jcis.2017.10.115
14. Sun S, Fu C, Cong J, Li Y, Xie S, Wang P. Epidemiological features and trends of influenza incidence in mainland China: a population-based surveillance study from 2005 to 2015. Int J Infect Dis. (2019) 89:12–20. doi: 10.1016/j.ijid.2019.08.028
15. Parra-Amaya ME, Puerta-Yepes ME, Lizarralde-Bejarano DP, Arboleda-Sánchez S. Early detection for dengue using local indicator of spatial association (LISA) analysis. Diseases. (2016) 4:16. doi: 10.3390/diseases4020016
16. Edens C, Alden NB, Danila RN, Fill MA, Gacek P, Muse A, et al. Multistate analysis of prospective Legionnaires' disease cluster detection using SaTScan, 2011–2015. PLoS ONE. (2019) 14:e0217632. doi: 10.1371/journal.pone.0217632
17. Li H, Li H, Ding Z, Hu Z, Chen F, Wang K, et al. Spatial statistical analysis of Coronavirus Disease 2019 (Covid-19) in China. Geospat Health. (2020) 15:11–8. doi: 10.4081/gh.2020.867
18. Weng X, Wang Z, Ren J, Zhang Y, Yu L, Wang R. Surveillance for public health emergencies caused by infectious diarrhea other than cholera, dysentery, typhoid and paratyphoid in China, 2014–2016. Dis Surveill. (2019) 34:565–70. doi: 10.3784/j.issn.1003-9961.2019.06.020
19. Li W, Xiang W, Li C, Xu J, Zhou D, Shang S. Molecular epidemiology of rotavirus A and adenovirus among children with acute diarrhea in Hangzhou, China. Gut Pathog. (2020) 12:19. doi: 10.1186/s13099-020-00359-4
20. Luo HM. Epidemiological characteristics and changing trends of other infectious diarrhoeal diseases in China from 2005 to 2019. China CDC. (2020). doi: 10.27511/d.cnki.gzyyy.2020.000117
21. Luo HM, Ran L, Yao LY, Wang LP. Epidemiological analysis of cases of rotavirus diarrhea under 5 years of age in China 2005–2018. Chin J Prev Med. (2020) 2:181–6. doi: 10.3760/cma.j.issn.0253-9624.2020.02.013
22. Liao QH, Lu R, Jin M, Yuan J, Ma HL, Ban HQ. Technical guide for investigation and prevention and control of norovirus infections (version 2015). Chin J Viral Dis. (2015) 5:448–58. doi: 10.16505/j.2095-0136.2015.06.003
23. Shah MP, Hall AJ. Norovirus illnesses in children and adolescents. Infect Dis Clin North Am. (2018) 32:103–18. doi: 10.1016/j.idc.2017.11.004
24. Florez ID, Niño-Serna LF, Beltrán-Arroyave CP. Acute infectious diarrhea and gastroenteritis in children. Curr Infect Dis Rep. (2020) 22:4. doi: 10.1007/s11908-020-0713-6
25. Chaikaew N, Tripathi NK, Souris M. Exploring spatial patterns and hotspots of diarrhea in Chiang Mai, Thailand. Int J Health Geogr. (2009) 8:36. doi: 10.1186/1476-072X-8-36
26. Phung D, Huang C, Rutherford S, Chu C, Wang X, Nguyen M, et al. Temporal and spatial patterns of diarrhoea in the Mekong Delta area, Vietnam. Epidemiol Infect. (2015) 143:3488–97. doi: 10.1017/S0950268815000709
27. Hao Y, Zhang N, Wu J, Su B, Gong L, Ma W, et al. Identifying infectious diarrhea hot spots and associated socioeconomic factors in Anhui province, China. Am J Trop Med Hyg. (2019) 101:549–54. doi: 10.4269/ajtmh.19-0161
28. Chowdhury FR, Ibrahim QSU, Bari MS, Alam MMJ, Dunachie SJ, Rodriguez-Morales AJ, et al. The association between temperature, rainfall and humidity with common climate-sensitive infectious diseases in Bangladesh. PLoS ONE. (2018) 13:e0199579. doi: 10.1371/journal.pone.0199579
29. Ding Z, Zhai Y, Wu C, Wu H, Lu Q, Lin J, et al. Infectious diarrheal disease caused by contaminated well water in Chinese schools: a systematic review and meta-analysis. J Epidemiol. (2017) 27:274–81. doi: 10.1016/j.je.2016.07.006
30. Tong MX, Hansen A, Hanson-Easey S, Cameron S, Xiang J, Liu Q, et al. Infectious diseases, urbanization and climate change: challenges in future China. Int J Environ Res Public Health. (2015) 12:11025–36. doi: 10.3390/ijerph120911025
31. Mao Y, Zhang N, Zhu B, Liu J, He R. A descriptive analysis of the Spatio-temporal distribution of intestinal infectious diseases in China. BMC Infect Dis. (2019) 19:766. doi: 10.1186/s12879-019-4400-x
32. Chen C, Wu B, Zhang H, Li KF, Liu R, Wang HL, et al. Molecular evolution of GII.P17-GII.17 norovirus associated with sporadic acute gastroenteritis cases during 2013–2018 in Zhoushan Islands, China. Virus Genes. (2020) 56:279–87. doi: 10.1007/s11262-020-01744-6
33. Chen C, Yan JB, Wang HL, Li P, Li KF, Wu B, et al. Molecular epidemiology and spatiotemporal dynamics of norovirus associated with sporadic acute gastroenteritis during 2013–2017, Zhoushan Islands, China. PLoS ONE. (2018) 13:e0200911. doi: 10.1371/journal.pone.0200911
Keywords: other infectious diarrhea, epidemiological trends, hotspots, joinpoint regression, space-time analyses
Citation: Chen C, Guan Z, Huang C, Jiang D, Liu X, Zhou Y, Yan D, Zhang X, Zhou Y, Ding C, Lan L, Lin Y, Wu J, Li L and Yang S (2021) Epidemiological Trends and Hotspots of Other Infectious Diarrhea (OID) in Mainland China: A Population-Based Surveillance Study From 2004 to 2017. Front. Public Health 9:679853. doi: 10.3389/fpubh.2021.679853
Received: 12 March 2021; Accepted: 03 June 2021;
Published: 22 July 2021.
Edited by:
Roger Hewson, Public Health England, United KingdomReviewed by:
Jimin Sun, Zhejiang Center for Disease Control and Prevention, ChinaCopyright © 2021 Chen, Guan, Huang, Jiang, Liu, Zhou, Yan, Zhang, Zhou, Ding, Lan, Lin, Wu, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigui Yang, eWFuZ3NoaWd1aUB6anUuZWR1LmNu; Lanjuan Li, bGpsaUB6anUuZWR1LmNu; Jie Wu, MTU5NTUxMTg0NzlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.