
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 25 June 2021
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.661674
 Homa Hajjaran1*†
Homa Hajjaran1*† Reza Saberi2,3*†
Reza Saberi2,3*† Alireza Borjian1†
Alireza Borjian1† Mahdi Fakhar2†
Mahdi Fakhar2† Seyed Abdollah Hosseini2†
Seyed Abdollah Hosseini2† Sajjad Ghodrati1
Sajjad Ghodrati1 Mehdi Mohebali1†
Mehdi Mohebali1†Leishmaniasis is one of the most common vector-borne parasitic diseases in Iran. Leishmania species identification is necessary for epidemiological aspects, precise prognosis, control and treatment of the disease. We systematically searched all the studies, reports, and documentation related to species identification and geographical distribution of causative agents of cutaneous (CL), mucosal (ML), and visceral leishmaniasis (VL) using DNA-based molecular diagnostic techniques in Iran. International databases including PubMed, ScienceDirect, Embase, Google Scholar, Scopus, and Web of Science were systemically searched for English articles and Iran's databases including SID, IranMedex and Magiran were searched for Persian reports and articles. Searches were performed from 1999 to 2019 (20 years). The current review was conducted using the keywords: cutaneous leishmaniasis, visceral leishmaniasis, Leishmania species, Human, Molecular, PCR, and Iran. The study quality was evaluated using the NOS checklist. This meta-analysis procedure was accomplished using STATA, version 2.7.9. Of the 3,426 records identified in the initial search, 154 articles met inclusion criteria and qualified for the systematic review and meta-analysis. In subgroup analysis, the pooled frequency of causative agents of CL isolates was 67.3% (95% CI: 59.51–74.67%) for L. major and 32.1% (95% CI: 24.72–39.87%) for L. tropica. In addition, the pooled frequency of causative agents of VL isolates was 97.1% (95% CI: 94.6–98.8%) for L. infantum and 2.9% (95% CI: 1.12–5.37%) for L. tropica. The findings of this study showed that the main causative agents of CL and VL in Iran are L. major and L. infantum, respectively. Moreover, kinetoplast DNA (kDNA) and internal transcriber spacer (ITS) were the most used markers for identifying Leishmania species. The current study provides valuable data to encourage and direct researchers as well as public health managers in the comprehensive leishmaniasis control and prevention planning in Iran.
Leishmaniasis is a neglected tropical disease (NTD) caused by the Leishmania parasites, which are transmitted by the bite of sand flies (1). There are four clinical forms of the disease: cutaneous leishmaniasis (CL), visceral leishmaniasis (VL), and mucocutaneous leishmaniasis (MCL) and mucosal leishmaniasis (ML) (2). Despite universal scientific community efforts to reduce cases of human leishmaniasis, numerous cases of such devastating disease are still reported worldwide (3). The disease currently affects 12 million people with 350 million people are living in regions with a high risk of infection. World Health Organization (WHO) estimates the annual global incidence of 0.7–1.2 million cases of CL and 0.1–0.4 million cases of VL (4). At present, the majority (about 90%) of CL cases occur in eight countries mainly including Asian and South American countries (4). Moreover, more than 90% of global cases of VL had been reported from seven countries mainly including African and South American countries (4, 5). In Iran, CL is the most common form of the disease and recent reports estimates >20,000 annual cases (6), but VL has been reported sporadically, with about 100–300 new serologically positive cases of VL reported annually (7).
Species discrimination is important, because of differences among the Leishmania species in levels of virulence and responses to the various chemical drugs (8, 9). As a result, distinguishing Leishmania spp. is critical for accurate diagnosis and appropriate treatment (9). Morphological identification of Leishmania species is not possible, but a variety of DNA-based molecular diagnostic techniques, including restriction fragment length polymorphism (RFLP), nested-PCR methods as well as high-resolution melting analysis PCR (HRM-PCR) have been reported for identification of Leishmania on different taxonomical levels (genus and species) (10). According to our literature review, several target markers were used to identify Leishmania species, including minicircle kinetoplastic DNA, heat shock protein 70 gene, N-acetylglucosamine-1-phosphate transferase (nagt) gene, and internal transcription spacer (ITS1 & 2).
There are several studies regarding the identification of Leishmania species causing CL and VL in Iran. The aim of this systematic review and meta-analysis was therefore to define the geographical distribution of Leishmania spp. among human populations as well as exploring molecular markers used for identifying Leishmania spp. in this population throughout two decades ago (1999–2019) in Iran.
This systematic study was achieved according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (11). The present study was carried out to estimate the species identification and geographical distribution of causative agents of CL and VL cases in Iran. A search in literature was carried out via the nine English and Persian databases, including PubMed, Embase, Google Scholar, Science Direct, Scopus, Web of Science and SID, IranMedex and Magiran up to Sep 2019, respectively. The current review was conducted by the Medical Subject Headings (MeSH) terms including: “Cutaneous leishmaniasis”, “Visceral leishmaniasis,” “Leishmania”, “Species”, “Human,” “Molecular”, “PCR”, and “Iran”, alone or combined together with “OR” or/and “AND” operators.
Initially, the titles and abstracts of searched articles were screened for eligibility by two authors independently, and those that did not describe identification of Leishmania species were removed. Data on the identification of Leishmania spp., were extracted from studies according to the following including criteria: (a) peer-reviewed original research, (b) papers studies that surveyed identification of Leishmania species using various polymerase chain reaction techniques, (c) studies published in English or Persian during 1999–2019 and (d) full-text articles were available. Additionally, the exclusion criteria were as follows; (a) duplicated data, (b) review studies, and (c) studies on animal reservoirs.
Out of the retrieved papers, 154 papers were eligible for inclusion in this study. Required data were collected based on the first author, publication year, province, total sample, positive number, Leishmania spp., types of clinical manifestation, diagnostic methods, marker genetic used and quality assessment. Three independent authors extracted the above details carefully.
In the current study, the Newcastle-Ottawa Scale was used to evaluate the quality of studies. NOS score ranged from 0 and 7 [low quality, (1 and 2), moderate quality, (3–5), and high quality (6 and 7)] (12).
This meta-analysis was completed using STATA software, as comprehensive meta-analysis software (http://statsdirect.com). The heterogeneity index was assessed using standard Cochran's Q- and I-squared statistics, with the random effects estimate they imply. Egger's test was used to assess potential publication bias. A p < 0.05 (≤0.05) is statistically significant.
Records retrieved in the mentioned electronic databases based on preparatory search strategies of nine databases yielded 3,426 papers; after removal of duplication papers, 2,244 papers were extracted. In the next step, using the abstract screening based on the inclusion/exclusion criteria, 1,683 other articles were excluded. Following that, 561 full-text articles were screened, of which 154 were found to be eligible for systematic review and meta-analysis. Figure 1 summarizes the flow chart presenting the study design process. The baseline characteristics of all included studies are tabulated in Tables 1, 2.
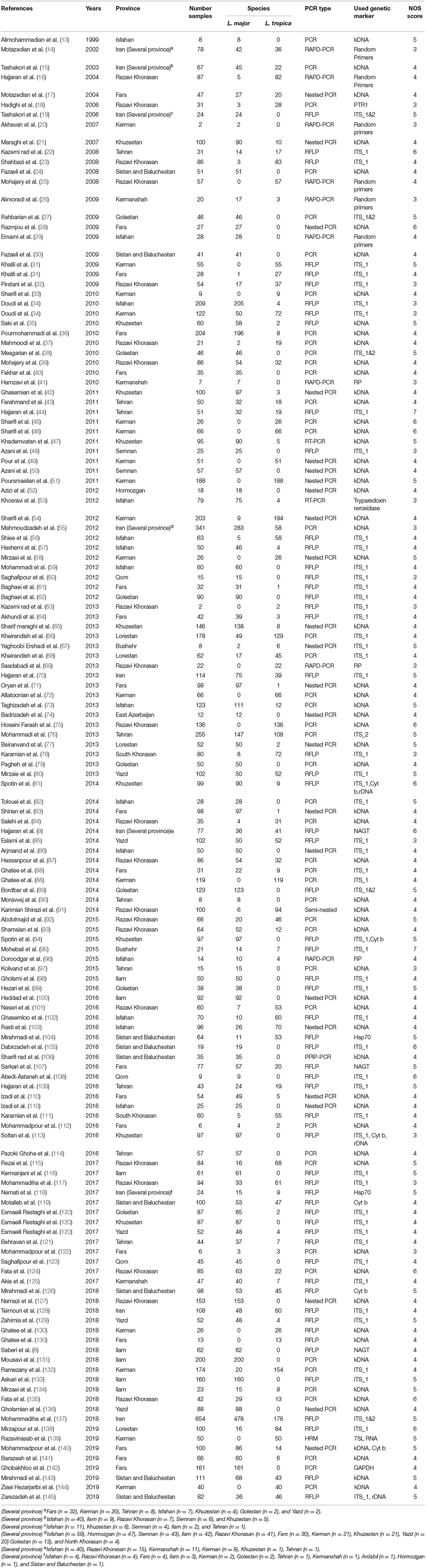
Table 1. Baseline characteristics of the Leishmania species identification from CL cases in the systematic review and meta-analysis from 1999 to 2019.
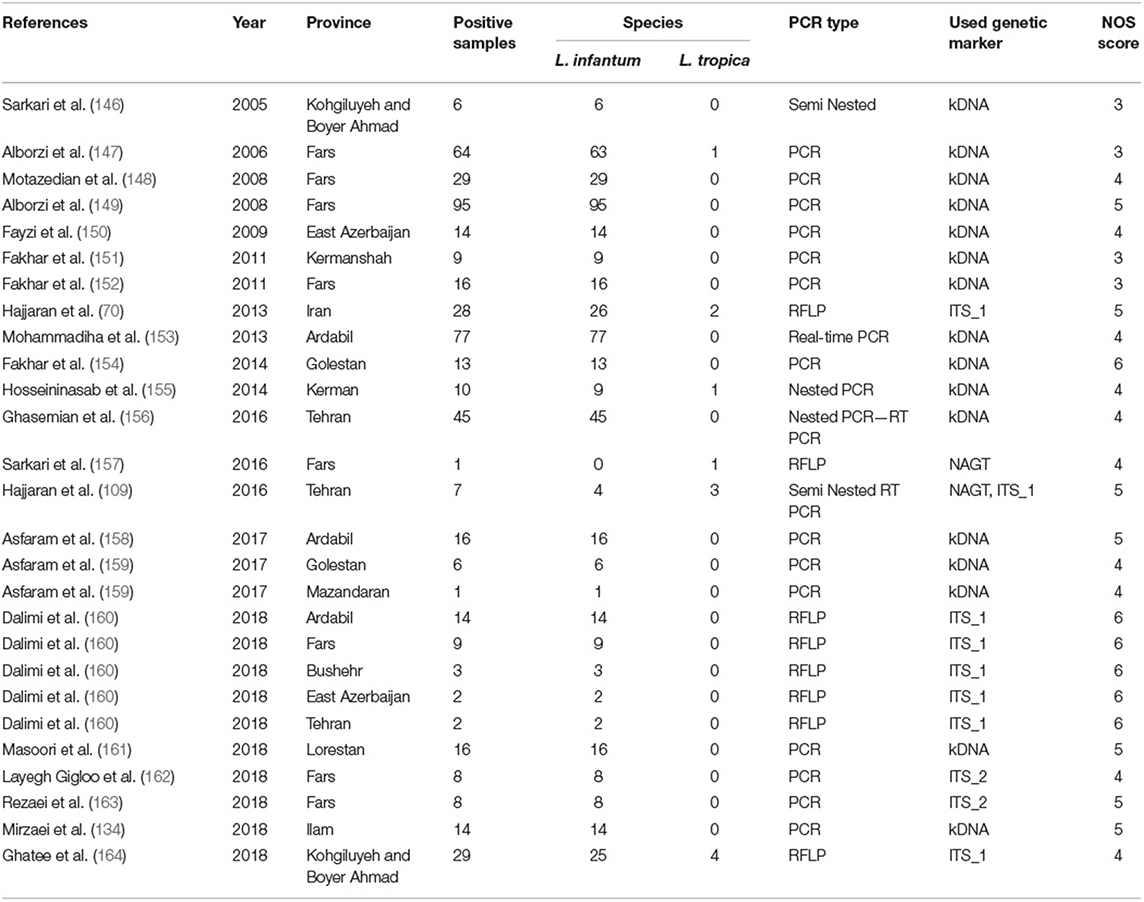
Table 2. Baseline characteristics of the Leishmania species identification from the VL cases in the systematic review and meta-analysis from 2005 to 2019.
In total, 10,586 CL isolates were identified, with two causatives of ZCL (L. major, n = 6,714) and ACL (L. tropica, n = 3,872) being reported in 19 provinces across Iran (Fars, Khuzestan, Isfahan, Golestan, Ilam, Razavi Khorasan, Kerman, Sistan & Balochistan, Tehran, Yazd, Hormozgan, Semnan. Most of the L. major isolates belonged to Fars (n = 992), Khuzestan (n = 844), and Isfahan (n = 687) provinces in the southern and central regions of Iran. In addition, the majority of L.tropica isolates belonged to Kerman (n = 1,142), Razavi Khorasan (n = 949) in the east, and Lorestan (n = 260) in the west (Figure 2). In contrast, 542 isolates for VL cases were determined in 11 provinces (Fars, Ardabil, Tehran, Kohgiluyeh and Boyer-Ahmad, Golestan, Ilam, Lorestan, East-Azerbaijan, Boushehr, Kerman, and Mazandaran), with the majority of VL isolates belonging to Fars (n = 230) in southwestern Iran and Ardabil (n = 107) in northwestern Iran (Figure 3).
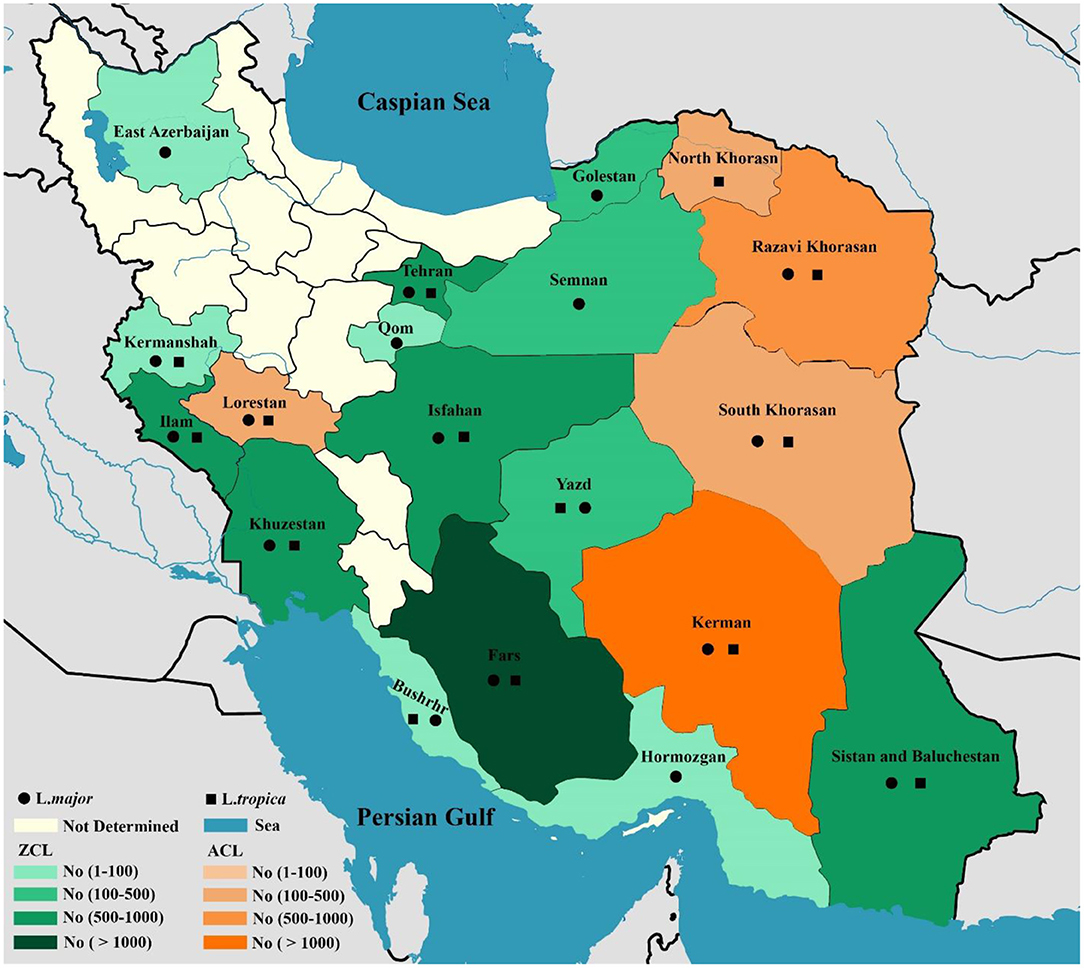
Figure 2. Map of distribution of Leishmania spp. causing ZCL and ACL using molecular methods in different geographical areas of Iran.
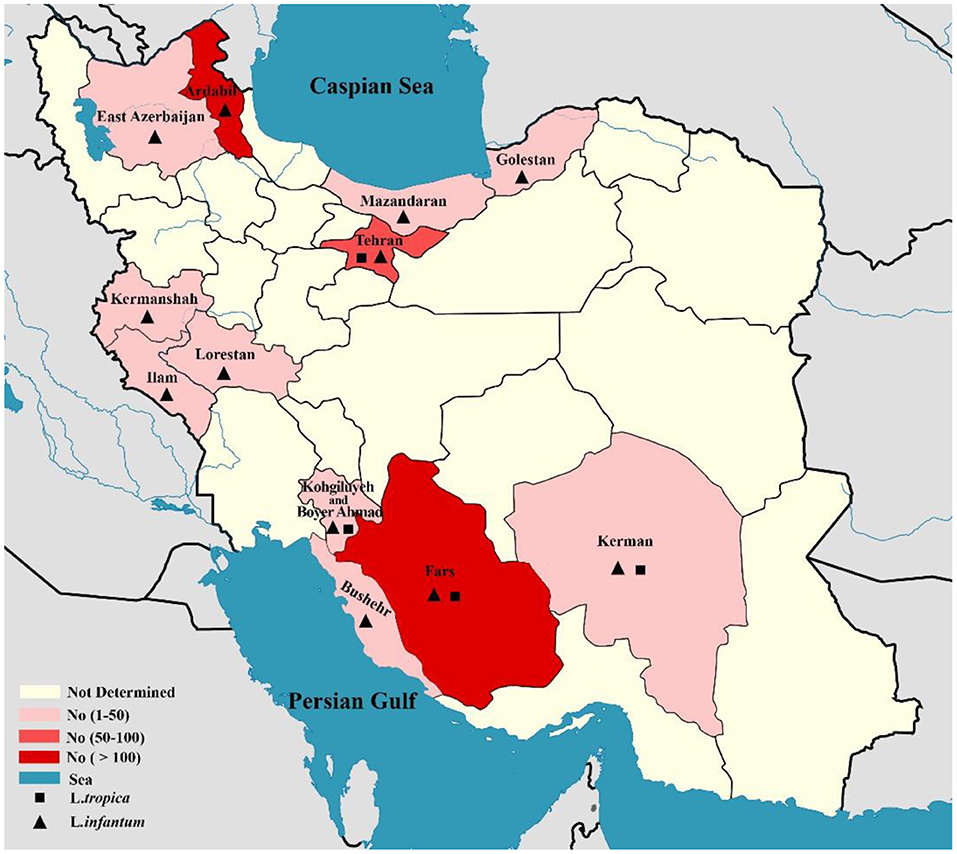
Figure 3. Map of distribution of Leishmania spp. causing of visceral leishmaniasis using molecular methods in different geographical areas of Iran.
According to the literature review, nine genetic markers (kinetoplast DNA, internal transcribed spacer region-1 and 2, cytochrome b, heat shock protein 70, N -acetylglucosamine-1-phosphate transferase, pteridine reductase 1, tryparedoxin peroxidase, Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) and 7SL RNA) were used for identification Leishmania species that the most species were identified with kinetoplast DNA (n = 5,592) and ITS markers (n = 4,544). It should be noted that some species of Leishmania were identified by Random amplified polymorphic deoxyribonucleic acid analysis by PCR (RAPD-PCR) method using random primers. The used molecular methods of Leishmania species identification in most studies were nested PCR and PCR-RFLP.
In subgroup analysis, the pooled frequency of causative agents of CL isolates was 67.3% (95% CI: 59.51–74.67%) for L. major and 32.1% (95% CI: 24.72–39.87%) for L. tropica (Table 3 and Supplementary Figures 1, 2). Also of note, the 14 and four isolates were identified as L. infantum and L. turanica as causative agents of CL and ZCL cases, respectively (14, 22, 36, 47, 74, 89, 120, 122).
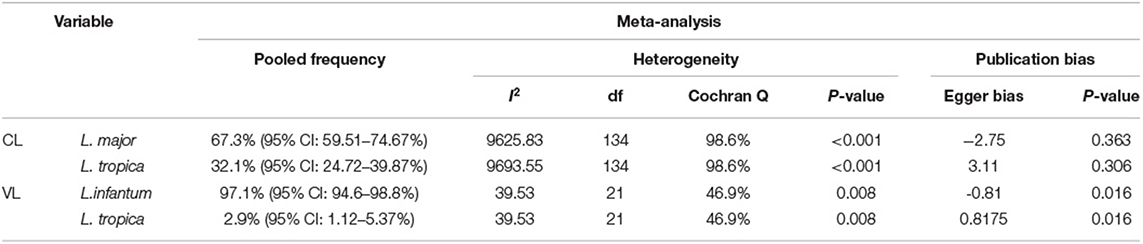
Table 3. The pooled frequency, heterogeneity, and publication bias of Leishmania species that causative VL and CL leishmaniasis.
In addition, the pooled frequency of causative agents of VL isolates was 97.1% (95% CI: 94.6–98.8%) for L. infantum and 2.9% (95% CI: 1.12–5.37%) for L. tropica (Table 3 and Supplementary Figures 3, 4). Also, other clinical forms of leishmaniasis were reported as follow: ML (n = 12), DCL (n = 5), MCL (n = 3), and PKDL (n = 2). ML cases caused by L. major (n = 7), L. tropica (n = 2), L. infantum (n = 2), and a mix of L. major/L. tropica (n = 1) whereas, all DCL and MCL cases caused by L. major, but two causative agents of PKDL were identified as L. infantum.
Leishmaniasis remains a major community health-based challenge with worldwide distribution, particularly in Iran (165). Identification of species is essential in diagnosis, treatment and epidemiological studies (165). We attempted to determine the etiological agents of human cutaneous and visceral leishmaniasis and their geographical distribution in Iran over two decades ago in the current systematic review and meta-analysis study.
According to the finding of the meta-analysis, most Leishmania isolates identified in Iran belonged to CL than VL cases. Every year, a large number of CL cases with a wide distribution are reported in 19 of 31 Iranian provinces, primarily in the central, southwest, east, and northeast regions. Some evidence suggests that the CL incidence rate overall has been decreasing in recent years, from 37/100,000 in 2007 to 22/100,000 in 2013. This decrease in incidence could be because appropriate public health measures such as education to residents, case finding and management, treatment, control of reservoir hosts, and distribution of repellents and nets treated with permethrin in the endemic focus of the disease have been accomplished (166).
However, at the same time, it seems like the distribution of CL has been extended to a new area (166, 167). In contrast, VL is mainly endemic in restricted regions of Iran, notably the northwest (Ardabil province) and southwest (Fars province) (7).
As illustrated by the finding of the subgroup analysis, the pool frequency of L. major and L. tropica, as causative agents of CL was 67.32% (95% CI: 59.51–74.67%) and 32.1% (95% CI: 24.72–39.87%), respectively. It can be concluded that the distribution of ZCL is higher than ACL form. According to a systematic review study conducted by Foroutan et al. rodents are the most important reservoirs of Leishmania species in many foci of ZCL throughout Iran (168). The most important of these rodent reservoirs are Rhombomys opimus, Meriones libycus, and Nesokia indica. The finding of this study showed that L. major has been reported as the predominant species of these rodents. The role of rodents in the spread of ZCL is evident (168).
The findings of this study demonstrated that the main causative agent of ZCL cases in the 14 provinces is L. major and the main causative agent of ACL cases in the five provinces is L. tropica (Figure 2). Although L. tropica was formerly common in many large urban areas, it has also been observed in rural areas and small cities in Iran (169). According to the findings of the two studies, four isolates of L. turanica were found in CL patients in the Gonbad-Kavous and Turkmen Sahara districts of Golestan province, which are the known oldest ZCL foci (90, 121). Nevertheless, it should be noted that L. major is the principal agent of ZCL in Iran. Besides, 14 isolates of L. infantum have been reported to cause CL cases (14, 22, 36, 47, 74, 122). A review of the literature showed that cases of L. infantum as the causative agent of CL have previously been identified in the Mediterranean (170), Southeast European countries, such as Portugal, Spain, Italy, and France (171) and the Americas (172), which is consistent with the findings.
On the other hand, the pool frequency of causative agents of VL isolates was 97.1% (95% CI: 94.6–98.8%) for L. infantum and 2.9% (95% CI: 1.12–5.37%) for L. tropica. The result of this study revealed that the main causative agent of VL in Iran is L. infantum. According to the results of a recent systematic review, the prevalence of HVL infection has been decreased in Iran throughout the last two decades. The maximum (3%, 95% CI: 1–5%) and minimum (0.5%, 95% CI, 0.2–0.7%) pooled prevalence of HVL was estimated in the northern and western Iranian provinces, respectively (173). It should be noted that the reason for reporting the relatively high number of cases of VL in Tehran, central Iran, as shown in Figure 3, is that these patients were referred to Tehran University Hospitals from other regions for diagnosis and treatment follow-up. Therefore, these reported cases did not belong to Tehran.
Nonetheless, despite the Iranian Center for Disease Control's (CDC) efforts to monitor and prevent HVL, new human cases of VL continue to emerge in old endemic foci. On the other hand, the disease has also emerged in new non-endemic areas of the country, such as Golestan province in north-eastern Iran (174). However, geo-climatic and environmental factors play the most important role in the emergence/reemergence of HVL in an area (174). Reasonable steps to monitor VL and prevent its spread to other areas should be taken in this respect.
Currently, molecular approaches are used for species identification, genotyping, and determine polymorphisms in Leishmania parasites (175). In most cases, these methods have replaced the isoenzyme method, which is the standard method for determining the species and strain of the Leishmania parasite (176). Molecular techniques have the potential to be more sensitive and rapid. In addition to high sensitivity and specificity, molecular methods can differentiate relapse from reinfection of disease (177).
Several DNA markers were used for DNA amplification of Leishmania spp. in the included articles, in which most kDNA and ITS1markers were used for the diagnosis of identification of species. Our finding showed that the kDNA-based PCR was the most sensitive diagnostic method for leishmaniasis and the ITS1-based PCR could be used as a sensitive/specific method to identify the Leishmania species. It is interesting to know that ITS1 is less sensitive compared to kDNA minicircles, because the copy number of rDNA (<200) is lower than the copy number of kDNA minicircles (tens of thousands). Therefore, it is more desirable to use specific primers for ITS regions and kDNA genes to diagnose the disease (103).
Phylogenetic analyses targeting the ITS1 gene are valuable and reliable tools in genetic analytical characterization of Leishmania parasite. This region is highly conserved among species (178). The ITS region as a target for differentiation of Leishmania at species and strain level has been used in different studies (102, 105, 132). As a whole, it should be noted that apply of two genetic markers simultaneously could provide more data regarding genetic map of the Leishmania parasite particularly in an endemic focus.
Notwithstanding that the DNA-based methods have proven to be very efficient in the identification and distinguish of Leishmania species, these methods also have limitations. One of these limitations is the exquisite sensitivity of these methods, and consequently false-positive PCR (179). For resolving this problem, it is necessary to use positive and negative control in each experiment simultaneously. Furthermore, preventing PCR contamination requires that this method be performed in reference laboratories. The specificity of PCR is generally controlled by several variables, including primer design, target genes, amount and purity of DNA, and type of enzyme (180).
In the end, the issue of the Leishmania RNA virus has become an interesting topic (181). Leishmania RNA virus (or LRV) is a genus of double-stranded RNA (dsRNA) virus in the family Totiviridae. LRVs exist within many species of the Leishmania isolates (181). Nowadays, Leishmania RNA virus is being extensively surveyed because it might be an important virulence factor of the infection (182). According to previous evidence, studies have been conducted to investigate the presence of Leishmania RNA virus in Iran. It is interesting to know that Leishmania RNA virus has been detected in many L. major species and one L. infantum isolated from a VL patient, and one L. tropica isolated from a CL patient in Iran (110, 183).
One of the limitations of this study was that some authors did not report isolates to belong to which province and isolates were introduced to as Iranian isolates. In addition, the limitations of the present study include: (a) use of different diagnostic techniques in the two included studies without similar results, (b) available studies with no sufficient information on identification of Leishmania species, and (c) variability of the sample size of the included studies. Also, people commuting between urban and rural areas has made it difficult to determine the main source of infection.
Our study reconfirms that CL and VL remain important infectious diseases in Iran. In this regard, the main causative agent of ZCL and ACL in Iran is L. major and L. tropica, respectively. In addition, the findings of this study demonstrated that the main causative agent of VL in Iran is L. infantum. The current study provides the geographical distribution of causative species in CL and VL forms in Iran and is a source of data to help researchers and public health workers in comprehensive investigations and developing prevention programs. Based on current findings, two markers kDNA and ITS1 can be used to accurately diagnose and determine Leishmania species using molecular methods. Our findings highlight the need for the implementation of control measures among the patients of both CL and VL. Further attention and monitoring will be needed to improve the surveillance and effective control to reduce the incidence of leishmaniasis in Iran.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
HH and RS conceived the presented idea and wrote the manuscript. MF and MM reviewed and commented on the findings of this work. HH, AB, and SG initially searched the literature studies and collected the data. SH analyzed and interpreted the data and methods. All authors provided critical feedback and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to express their thankfulness to all authors that their valuable publications were included in the current review.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.661674/full#supplementary-material
1. Tamiru HF, Mashalla YJ, Mohammed R, Tshweneagae GT. Cutaneous leishmaniasis a neglected tropical disease: community knowledge, attitude and practices in an endemic area, Northwest Ethiopia. BMC Infect Dis. (2019) 19:855. doi: 10.1186/s12879-019-4506-1
2. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microb Infect Dis. (2004) 27:305–18. doi: 10.1016/j.cimid.2004.03.004
3. McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermat. (2018) 154:1032–9. doi: 10.1001/jamadermatol.2018.2133
4. Ruiz-Postigo JA, Grout L, Saurabh J. Global leishmaniasis surveillance, 2017-2018, and first report on 5 additional indicators/Surveillance mondiale de la leishmaniose, 2017-2018, et premier rapport sur 5 indicateurs supplementaires. Weekly Epidemiol Record. (2020) 95:265–80. Available online at: https://reliefweb.int/report/world/weekly-epidemiological-record-wer-19-june-2020-vol-95-25-pp-265-280-enfr
5. Al-Salem W, Herricks JR, Hotez PJ. A review of visceral leishmaniasis during the conflict in South Sudan and the consequences for East African countries. Paras Vect. (2016) 9:460. doi: 10.1186/s13071-016-1743-7
6. Holakouie-Naieni K, Mostafavi E, Boloorani AD, Mohebali M, Pakzad R. Spatial modeling of cutaneous leishmaniasis in Iran from 1983 to (2013). Acta Tropica. (2017) 166:67–73. doi: 10.1016/j.actatropica.2016.11.004
7. Sharifi I, Aflatoonian MR, Parizi MHD, Hosseininasab A, Mostafavi M, Bamorovat M, et al. Visceral leishmaniasis in Southeastern Iran: a narrative review. Iran J Parasit. (2017) 12:1–11.
8. Saberi R, Moin-Vaziri V, Hajjaran H, Niyyati M, Taghipour N, Kheirandish F, et al. Identification of Leishmania species using N-acetylglucosamine-1-phosphate transferase gene in a zoonotic cutaneous leishmaniasis focus of Iran. J Vect Borne Dis. (2018) 55:14. doi: 10.4103/0972-9062.234621
9. Hajjaran H, Mohebali M, Teimouri A, Oshaghi MA, Mirjalali H, Kazemi-Rad E, et al. Identification and phylogenetic relationship of Iranian strains of various Leishmania species isolated from cutaneous and visceral cases of leishmaniasis based on N-acetylglucosamine-1-phosphate transferase gene. Infect Genet Evolu. (2014) 26:203–12. doi: 10.1016/j.meegid.2014.05.026
10. Akhoundi M, Downing T, Votýpka J, Kuhls K, Lukeš J, Cannet A, et al. Leishmania infections: molecular targets and diagnosis. Mol Aspects Med. (2017) 57:1–29. doi: 10.1016/j.mam.2016.11.012
11. Moher D, Liberati A, Tetzlaff J, Altman D. Academia and clinic annals of internal medicine preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annu Intern Med 151:264–269. (2009) doi: 10.7326/0003-4819-151-4-200908180-00135
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Europ J Epidem. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
13. Alimohammadian MH, Almasi H, Khabiri A, Hatam G, Mahboudi F, Tehrani SR, et al. Identification of species and characteristics of an outbreak of cutaneous leishmaniasis in a new focus of Iran. Iran Biomed J. (1999) 3:31–9. Available online at: http://ibj.pasteur.ac.ir/article-1-847-en.html
14. Motazedian H, Noamanpoor B, Ardehali S. Characterization of Leishmania parasites isolated from provinces of the Islamic Republic of Iran. EMHJ-Eastern Med Health J. (2002) 8:338–44.
15. Tashakori M, Azhdari S, Kariminia A, Ali MM, Mahboudi F. Characterization of Leishmania species and L. major strains in different endemic areas of cutaneous leishmaniasis in Iran. IBG. (2003) 7:43–50. Available online at: http://ibj.pasteur.ac.ir/article-1-537-en.html
16. Hajjaran H, Mohebali M, Razavi M, Mojtabavi J, Houshmand B. Identification of Leishmania species isolated from human cutaneous leishmaniasis, using random amplified polymorphic DNA (RAPD-PCR). Iran. J. Public Health. (2004) 33:8–15. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?ID=13302
17. Motazedian M, Karamian M, Ardehali S, Handjani F. Characterization of Leishmania parasites from archived geimsa-stained slides using nested polymerase chain reaction. JMR. (2004) 33:8–15. Available online at: https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=48462
18. Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS MED. (2006) 3:e162. doi: 10.1371/journal.pmed.0030162
19. Mahnaz T, Katrin K, Amer A-J, Isabel M, Gabriele S, Safar F, et al. Leishmania major: genetic heterogeneity of Iranian isolates by single-strand conformation polymorphism and sequence analysis of ribosomal DNA internal transcribed spacer. Acta Tropica. (2006) 98:52–8. doi: 10.1016/j.actatropica.2006.01.010
20. Akhavan A, Yaghoobi-Ershadi M, Hasibi F, Jafari R, Abdoli H, Arandian M, et al. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of southern Iran. J Arthrop Born Dis. (2007) 1–8. Available online at: https://jad.tums.ac.ir/index.php/jad/article/view/3
21. Maraghi S, Zadeh AS, Sarlak A, Ghasemian M, Vazirianzadeh B. Identification of cutaneous leishmaniasis agents by nested Po-Lymerase chain reaction (Nested-PCR) in Shush City, Khuzestan Province, Iran. Iran J Parasit. (2007) 13–5. Available online at: https://ijpa.tums.ac.ir/index.php/ijpa/article/view/29
22. Kazemi-Rad E, Mohebali M, Hajjaran H, Rezaei S, Mamishi S. Diagnosis and characterization of Leishmania species in Giemsa-stained slides by PCR-RFLP. Iran J Public Health. (2008) 54–60. Available online at: https://ijph.tums.ac.ir/index.php/ijph/article/view/2073
23. Shahbazi F, Shahabi S, Kazemi B, Mohebali M, Abadi AR, Zare Z. Evaluation of PCR assay in diagnosis and identification of cutaneous leishmaniasis: a comparison with the parasitological methods. Parasit Res. (2008) 103:1159–62. doi: 10.1007/s00436-008-1111-4
24. Fazaeli A, Fouladi B, Hashemi SS, Sharifi I. Clinical features of cutaneous leishmaniasis and direct pcrbased identification of parasite species in a new focus in Southeast of Iran. Iran J Public Health. (2008) 37:44–51. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?id=117574
25. Mohajery M, Hajjaran H, Shamsiyan AA, Tavakkol Afshari J, Saadabadi F. Identification of Leishmania Species Causing Cutaneous Leishmaniasis by RAPD-PCR. Med J Mashhad Univers Med Sci. (2008) 51:79–86. Available online at: http://eprints.mums.ac.ir/id/eprint/7716
26. Alimoradi S, Hajaran H, Mohebali M, Mansouri F. Molecular identification of Leishmania species isolated from human cutaneous leishmaniasis by RAPD-PCR. Iranian J Publ Health. (2009) 38:44–50. Available online at: https://ijph.tums.ac.ir/index.php/ijph/article/view/3188
27. Rahbarian N, Mesgarian A, Mahmoudirad M, Hajaran H, Shahbazi F, Mesgarian Z, et al. Identification of Leishmania species isolated from human cutaneous leishmaniasis using PCR method. J Res Health Sci. (2009) 9:48–51.
28. Razmjou S, Hejazy H, Motazedian MH, Baghaei M, Emamy M, Kalantary M. A new focus of zoonotic cutaneous leishmaniasis in Shiraz, Iran. Trans R Soc Trop Med Hygiene. (2009) 103:727–30. doi: 10.1016/j.trstmh.2008.12.013
29. Emami MM, Yazdi M, Nilforoushzadeh M. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of central Iran. Trans R Soc Trop Med Hygiene. (2009) 103:1257–62. doi: 10.1016/j.trstmh.2009.04.020
30. Fazaeli A, Fouladi B, Sharifi I. Emergence of cutaneous leishmaniasis in a border area at south-east of Iran: an epidemiological survey. J Vect Borne Dis. (2009) 46:36.
31. Khalili M, Nourollahi-Fard S. Detection and genotyping of cutaneous leishmaniasis species in the southeast of Iran: restriction enzyme analysis (RFLP). Tehran Univer Med J. (2009) 67:168–72. Available online at: https://tumj.tums.ac.ir/article-1-466-en.pdf
32. Pirstani M, Sadraei J, Dalimi A, Vaeznia H. Determination of Leishmania species causing cutaneous leishmaniasis in Mashhad by PCR-RFLP method. Arch Razi Instit. (2009) 64:39–44. doi: 10.22092/ARI.2009.103804
33. Sharifi I, Fekri AR, Aflatoonian MR, Khamesipour A, Mahboudi F, Dowlati Y, et al. Leishmaniasis recidivans among school children in Bam, South-east Iran, 1994–2006. Int J Dermat. (2010) 49:557–61. doi: 10.1111/j.1365-4632.2010.04419.x
34. Doudi M, Hejazi SH, Razavi MR, Narimani M, Khanjani S, Eslami G. Comparative molecular epidemiology of Leishmania major and Leishmania tropica by PCR-RFLP technique in hyper endemic cities of Isfahan and Bam, Iran. Med Sci Monitor. (2010) 16:CR530-CR5.
35. Saki J, Meamar A, Oormazdi H, Akhlaghi L, Maraghi S, Mohebali M, et al. Mini-exon genotyping of leishmania species in khuzestan province, southwest Iran. Iran J Parasit. (2010) 5:25.
36. Pourmohammadi B, Motazedian M, Hatam G, Kalantari M, Habibi P, Sarkari B. Comparison of three methods for diagnosis of cutaneous leishmaniasis. Iran J Parasi. (2010) 5:1–8.
37. Mahmoudi MR, Mohajeri M, Tavakol AJ, Shakeri MT, Yazdanpanah MJ, Berenji F, et al. Molecular identification of Leishmania species causing cutaneous leishmaniasis in Mashhad, Iran. JJM. (2010) 3:195–200. Available online at: https://sites.kowsarpub.com/jjm/articles/72551.html
38. Mesgarian F, Rahbarian N, Mahmoudi Rad M, Hajaran H, Shahbaz F, Mesgarian Z, et al. Identification of Leishmania species isolated from human cutaneous Leishmaniasis in Gonbad-e-Qabus city using a PCR method during 2006-2007. Tehran Univ Med J. (2010) 68:250–6. Available online at: http://tumj.tums.ac.ir/article-1-355-en.html
39. Mohajery M, Shamsian SA, Rezaee A, Hasan Poor K, Shakeri MT, Farnoosh G, et al. Evaluation of mulecular epidemiology of cutaneous leishmaniasis in Sabzevar. Med J Mashhad Univers Med Sci. (2010) 53:138–44. doi: 10.22038/mjms.2010.5376
40. Fakhar M, Mikaeili F, Hatam G, Habibi P, Karamian M, Motazedian M, et al. A molecular epidemiology survey of cutaneous Leishmaniasis in patients referring to Parasitology Lab at Shiraz School of Medicine and the importance of PCR assay. J Jahrom Univer Med Sci. (2010) 8:2–6. doi: 10.29252/jmj.8.1.2
41. Hamzavi Y, Nomanpour B, Karaji AG. Identification of species of Leishmania isolated from patients with cutaneous leishmaniasis in Kermanshah; using RAPD-PCR technique. J Kermanshah Univ Med Sci. (2010) 14:167–270. Available online at: https://sites.kowsarpub.com/jkums/articles/79482.html
42. Ghasemian M, Maraghi S, Samarbafzadeh A, Jelowdar A, Kalantari M. The PCR-based detection and identification of the parasites causing human cutaneous leishmaniasis in the Iranian city of Ahvaz. Ann Trop Med Parasit. (2011) 105:209–15. doi: 10.1179/136485911X12899838683520
43. Farahmand M, Nahrevanian H, Shirazi HA, Naeimi S, Farzanehnejad Z. An overview of a diagnostic and epidemiologic reappraisal of cutaneous leishmaniasis in Iran. Brazil J Infect Dis. (2011) 15:17–21. doi: 10.1590/S1413-86702011000100004
44. Hajjaran H, Vasigheh F, Mohebali M, Rezaei S, Mamishi S, Charedar S. Direct diagnosis of Leishmania species on serosity materials punctured from cutaneous leishmaniasis patients using PCR-RFLP. J Clin Lab Analysis. (2011) 25:20–4. doi: 10.1002/jcla.20377
45. Sharifi I, Poursmaelian S, Aflatoonian MR, Ardakani RF, Mirzaei M, Fekri AR, et al. Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropica in rural communities of Bam district after the earthquake, Iran. Trop Med Int Health. (2011) 16:510–3. doi: 10.1111/j.1365-3156.2011.02729.x
46. Sharifi I, Nakhaei N, Aflatoonian M, Parizi MH, Fekri A, Safizadeh H, et al. Cutaneous leishmaniasis in Bam: a comparative evaluation of pre-and post-earthquake years (1999–2008). Iran J Public Health. (2011) 40:49–56.
47. Khademvatan S, Neisi N, Maraghi S, Saki J. Diagnosis and identification of Leishmania spp. from Giemsa-stained slides, by real-time PCR and melting curve analysis in south-west of Iran. Ann Trop Med Parasit. (2011) 105:559–65. doi: 10.1179/2047773211Y.0000000014
48. Mohammadi Azni S, Rasi Y, Oshaghi MA, Yaghoubi Ershadi M, Mohebali M, Abaie M, et al. Diagnosis and characterization of leishmania species in patients and rodents Giemsa-stained slides by PCR-RFLP in Damghan district, Iran. Avicenna J Clin Med. (2011) 17:5–9. Available online at: http://sjh.umsha.ac.ir/article-1-250-en.html
49. Pour R, Sharifi I, Kazemi B, Zarean M. Identification of nonresponsive isolates to Glucantime in patients with cutaneous Leishmanaisis in Bam. J Kerman Univer Med Sci. (2011) 18:123–34. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?ID=191002
50. Mohammadi Azni S, Rassi Y, Oshaghi MA, Yaghoobi Ershdi MR, Mohebali M, Abai MR, et al. Determination of parasite species of cutaneous leishmaniasis using Nested PCR in Damghan – Iran, during 2008. J Gorgan Uni Med Sci. (2010) 1359–65. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?id=258744
51. Poresmaeliyan S, Mirzaei M, SHarifi E, Zarean M. The prevalence of cutaneous leishmaniasis in the city and suburb of Mohammadabad, Jiroft district and identification of parasite species by Nested-PCR, 2008. J Kerman Univer Med Sci. (2011) 18:218–27. Available online at: http://jkmu.kmu.ac.ir/article_16565.html
52. Azizi K, Soltani A, Alipour H. Molecular detection of Leishmania isolated from cutaneous leishmaniasis patients in Jask County, Hormozgan Province, Southern Iran, 2008. Asian Pacific J Trop Med. (2012) 5:514–7. doi: 10.1016/S1995-7645(12)60090-X
53. Khosravi S, Hejazi H, Hashemzadeh-Chaleshtori M, Eslami G, Yousofi Darani H. Molecular diagnosis of Old World leishmaniasis: real-time PCR based on tryparedoxin peroxidase gene for the detection and identification of Leishmania spp. J Vect Borne Dis. (2012) 49:15–8.
54. Sharifi F, Sharifi I, Zarean M, Parizi MH, Aflatoonian M, Harandi MF, et al. Spatial distribution and molecular identification of Leishmania species from endemic foci of south-eastern Iran. Iran J Parasit. (2012) 7:45–52.
55. Mahmoudzadeh-Niknam H, Ajdary S, Riazi-Rad F, Mirzadegan E, Rezaeian A, Khaze V, et al. Molecular epidemiology of cutaneous leishmaniasis and heterogeneity of Leishmania major strains in Iran. Trop Med Int Health. (2012) 17:1335–44. doi: 10.1111/j.1365-3156.2012.03078.x
56. Shiee MR, Mohebali M, Doroodgar A, Teimouri A, Afzali H, Shirzadi MR. A molecular and parasitological survey on cutaneous leishmaniasis patients from historical city of Kashan in Isfahan province, center of Iran. Asian Pacific Journal of Tropical Disease. (2012) 2:421–5. doi: 10.1016/S2222-1808(12)60093-0
57. Hashemi N, Hashemi M, Eslami G, Bidabadi LS, Hejazi SH. Detection of Leishmania parasites from cutaneous Leishmaniasis patients with negative direct microscopy using NNN and PCR-RFLP. J Isfahan Med School. (2012) 29:169. Available online at: http://jims.mui.ac.ir/index.php/jims
58. Mirzaei M, Sharifi I, Poursmaelian S. A new focus of anthroponotic cutaneous leishmaniasis and identification of parasite species by nested PCR in Jiroft, Iran. Comp Clin Pathol. (2012) 21:1071–5. doi: 10.1007/s00580-011-1231-6
59. Mohammadi F, Narimani M, Nekoian S, Bidabadi LS, Mohammadi F, Hosseini SM, et al. Identification and isolation of the cause of cutaneous Leishmaniasis in Isfahan Using ITS-PCR method. J Isfahan Med School. (2012) 30:175.
60. Saghafipour A, Rassi Y, Abai MR, Oshaghi Ma, Yaghoobi Arshadi Mr, Mohebali M, et al. Identification of Leishmania species in patients and reservoir rodents using PCR–RFLP in the central county of Qom province in 2010. J Arak Univer Med Sci. (2012) 15:1–10. Available online at: http://jams.arakmu.ac.ir/article-1-1249-en.html
61. Baghaei A, Jasbi E, Akhoundi M, Mirzaei H, Dehnam O. Microscopic and molecular detection of Leishmania species among suspected patients of cutaneous leishmaniasis using ITS-r DNA in Fars Province. SSU_Journals. (2012) 20:464–73. Available online at: http://jssu.ssu.ac.ir/article-1-2136-en.html
62. Baghaei A, Parvizi P, Amirkhani A, Honarvar M, Badiei F. Identification of Leishmania using microscopic and molecular methods in suspected patients of Cutaneous Leishmaniasis by targeting ITS-rDNA gene, Golestan province, Iran (2009-10). J Gorgan Uni Med Sci. (2012) 14:72–81. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?ID=274014
63. Kazemi-Rad E, Mohebali M, Khadem-Erfan MB, Saffari M, Raoofian R, Hajjaran H, et al. Identification of antimony resistance markers in Leishmania tropica field isolates through a cDNA-AFLP approach. Exp Parasitol. (2013) 135:344–9. doi: 10.1016/j.exppara.2013.07.018
64. Akhoundi M, Hajjaran H, Baghaei A, Mohebali M. Geographical distribution of Leishmania species of human cutaneous leishmaniasis in Fars province, southern Iran. Iran J Parasit. (2013) 8:85.
65. Maraghi S, Mardanshah O, Rafiei A, Samarbafzadeh A, Vazirianzadeh B. Identification of cutaneous leishmaniasis agents in four geographical regions of Khuzestan province using Nested PCR. Jundishapur J Microbiol. (2013) 6:1–4. doi: 10.5812/jjm.4866
66. Kheirandish F, Sharafi AC, Kazemi B, Mohebali M, Sarlak A, Tarahi MJ, et al. Identification of Leishmania species using PCR assay on giemsa-stained slides prepared from cutaneous leishmaniasis patients. Iran J Parasit. (2013) 8:382.
67. Yaghoobi-Ershadi MR, Shahbazi F, Darvishi M, Akhavan AA, Jafari R, Khajeian M, et al. Molecular epidemiological study of cutaneous leishmaniasis in the focus of Bushehr city, southwestern Iran. J Arthropod-Borne Dis. (2013) 7:113.
68. Kheirandish F, Sharafi AC, Kazemi B, Bandehpour M, javad Tarahi M, Khamesipour A. First molecular identification of Leishmania species in a new endemic area of cutaneous leishmaniasis in Lorestan, Iran. Asian Pacific J Trop Med. (2013) 6:713–7. doi: 10.1016/S1995-7645(13)60124-8
69. Saadabadi F, Mohajery M, Poostchi E, Shamsian SAA. Identification of Leishmania species causing cutaneous leishmaniasis using Random Amplified Polymorphic DNA (RAPD-PCR) in Kharve, Iran. Rep Biochem Mol Biol. (2013) 1:69.
70. Hajjaran H, Mohebali M, Mamishi S, Vasigheh F, Oshaghi MA, Naddaf SR, et al. Molecular identification and polymorphism determination of cutaneous and visceral leishmaniasis agents isolated from human and animal hosts in Iran. BioMed Res Int. (2013) 2013:326. doi: 10.1155/2013/789326
71. Oryan A, Shirian S, Tabandeh M-R, Hatam G-R, Randau G, Daneshbod Y. Genetic diversity of Leishmania major strains isolated from different clinical forms of cutaneous leishmaniasis in southern Iran based on minicircle kDNA. Infect Genet Evol. (2013) 19:226–31. doi: 10.1016/j.meegid.2013.07.021
72. Aflatoonian MR, Sharifi I, Poursmaelian S, Hakimi-Parizi M, Ziaali N. The emergence of anthroponotic cutaneous leishmaniasis following the earthquake in southern villages of Bam district, southeastern Iran, 2010. J Arthro Borne Dis. (2013) 7:8.
73. Taghizadeh N, Jafari M, Borjian Boroujeni A, Hejazi S, Azizi H. Detection and identification of Leishmania isolates from patients with cutaneous leishmaniasis (CL) in Isfahan (central region of Iran) by PCR method. Arch Razi Instit. (2013) 68:153–8. doi: 10.7508/ari.2013.02.010
74. Badirzadeh A, Mohebali M, Ghasemian M, Amini H, Zarei Z, Akhoundi B, et al. Cutaneous and post kala-azar dermal leishmaniasis caused by Leishmania infantum in endemic areas of visceral leishmaniasis, northwestern Iran 2002–2011: a case series. Pathog Global Health. (2013) 107:194–7. doi: 10.1179/2047773213Y.0000000097
75. Farash BRH, Mohajery M, Fata A, Shamsian SA, Rezaee A, Yazdanpanah MJ. Anthroponotic cutaneous leishmaniasis in torghabeh-shandiz, a region with rural texture (a molecular study). Jundishapur J Microbiol. (2013) 6:e8274. doi: 10.5812/jjm.8274
76. Mohammadi AMA, Khamesipour A, Khatami A, Javadi A, Nassiri-Kashani M, Firooz A, et al. Cutaneous leishmaniasis in suspected patients referred to the center for research and training in skin diseases and leprosy, Tehran, Iran from 2008 to 2011. Iran J Parasitol. (2013) 8:430.
77. Beiranvand E, Kalantari M, Rastgar HA, Amraee K. Molecular identification of Leishmania species isolated from human cutaneous leishmaniasis in Poledokhtar District, Lorestan Province, Iran. Jundishapur J Microbiol. (2013) 6:e8103. doi: 10.5812/jjm.8103
78. Karamian M, Faroghi Bojd MS, Hemmati M, Saadatjoo A, Barati DA. Molecular identification of cutaneous leishmaniasis agents in Birjand, Iran. J Birjand Univ Med Sci. (2013) 20:183–90. Available online at: http://journal.bums.ac.ir/article-1-1378-en.html
79. Pagheh AS, Fakhar M, Mesgarian F, Rahimi-Esboei B, Badiee F. Incidence trend of rural cutaneous leishmaniasis in Gonbad-e-Qabus city, (Golestan, Iran) during 2009-2012. J Mazandaran Univ Med Sci. (2013) 23:27–33. Available online at: http://jmums.mazums.ac.ir/article-1-2627-en.html
80. Mirzaie F, Eslami G, Yosefi MH, Pestehchian N. Molecular identification of Leishmania isolates obtained from patients suspected as having cutaneous leishmaniasis referred to reference laboratories from Yazd province in central Iran. Adv Biomed Res. (2013) 2:92. doi: 10.4103/2277-9175.122525
81. Spotin A, Rouhani S, Parvizi P. The associations of Leishmania major and Leishmania tropica aspects by focusing their morphological and molecular features on clinical appearances in Khuzestan Province, Iran. Biomed Res Int. (2014) 2014:913510. doi: 10.1155/2014/913510
82. Tolouei S, Hejazi SH, Ghaedi K, Hasheminia SJ. Identification of Leishmania isolates from healing and nonhealing cutaneous leishmaniasis patients using internal transcribed spacer region PCR. Jundishapur J Microbiol. (2014) 7:e9529. doi: 10.5812/jjm.9529
83. Shirian S, Oryan A, Hatam G-R, Panahi S, Daneshbod Y. Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch Pathol Lab Med. (2014) 138:235–40. doi: 10.5858/arpa.2013-0098-OA
84. Salehi Gh, Fata A, Mohaghegh MA, Mousavi SM, Rafatpanah H, Movahedi A. Molecular identification of Leishmania species in Taybad district, Iran. Asian Pacific J Trop Dis. (2014) 4 (2):S535–S539. doi: 10.1016/S2222-1808(14)60672-1
85. Eslami G, Hajimohammadi B, Jafari AA, Mirzaei F, Gholamrezai M, Anvari H, et al. Molecular identification of Leishmania tropica infections in patients with cutaneous leishmaniasis from an endemic central of Iran. Trop Biomed. (2014) 31:592–9.
86. Arjmand R, Saberi S, Tolouei S, Chizari Z, Nobari RF, Fard SS, et al. Identification of Leishmania isolates from Varzaneh city, Isfahan province, Iran using nested polymerase chain reaction method. Adv Biomed Res. (2014) 3:131. doi: 10.4103/2277-9175.139131
87. Hassanpour K, Aghamollaei H, Golpich M, Amani J, Taheri A, Farnoosh G. Molecular epidemiological study of cutaneous leishmaniasis in the east north of Iran. Asian Pacific J Trop Dis. (2014) 4:S540–S4. doi: 10.1016/S2222-1808(14)60673-3
88. Ghatee MA, Sharifi I, Kuhls K, Kanannejad Z, Harandi MF, de Almeida ME, et al. Heterogeneity of the internal transcribed spacer region in Leishmania tropica isolates from southern Iran. Exp Paras. (2014) 144:44–51. doi: 10.1016/j.exppara.2014.06.003
89. Bordbar A, Parvizi P. High density of Leishmania major and rarity of other mammals' L eishmania in zoonotic cutaneous leishmaniasis foci, I ran. Trop Med Int Health. (2014) 19:355–63. doi: 10.1111/tmi.12258
90. Moravvej H, Vesal P, Abolhasani E, Nahidi S, Mahboudi F. Comorbidity of Leishmania major with cutaneous sarcoidosis. Indian J Dermatol. (2014) 59:316. doi: 10.4103/0019-5154.131453
91. Karimian Shirazi M, Razmi G, Naghibi A. Molecular Identification of leishmania species causing cutaneous Leishmaniasis In Mashhad area, Iran. J Birjand Univers Med Sci. (2014) 21:237–45. Available online at: http://journal.bums.ac.ir/article-1-1473-en.html
92. Abdolmajid F, Ghodratollah SS, Hushang R, Mojtaba MB, Ali MM, Abdolghayoum M. Identification of Leishmania species by kinetoplast DNA-polymerase chain reaction for the first time in Khaf district, Khorasan-e-Razavi province, Iran. Trop Parasitol. (2015) 5:50–4. doi: 10.4103/2229-5070.145587
93. Shamsian S, Rezaei A, Akbarzadeh A, Hosseini Farash B. Molecular Identification of Leishmania tropica in an endemic border city for zoonotic cutaneous leishmaniasis (ZCL) in northeastern Iran. J Microb Exp. (2015) 2:53–8. doi: 10.15406/jmen.2015.02.00053
94. Spotin A, Rouhani S, Ghaemmaghami P, Haghighi A, Zolfaghari MR, Amirkhani A, et al. Different morphologies of Leishmania major amastigotes with no molecular diversity in a neglected endemic area of zoonotic cutaneous leishmaniasis in Iran. Iran Biomed J. (2015) 19:149–59. doi: 10.7508/ibj.2015.03.004
95. Mohebali M, Darabi H, Hajjaran H, Shirzadi MR, Fouladvand M, Charehdar S, et al. Molecular and parasitological study of cutaneous leishmaniasis in Bushehr province, southwest of the Islamic Republic of Iran: a cross-sectional study during 2009-2012. J Parasit Dis. (2015) 39:371–6. doi: 10.1007/s12639-013-0370-x
96. Doroodgar A, Sadr F, Razavi MR, Doroodgar M, Asmar M, Doroodgar M. A new focus of zoonotic cutaneous leishmaniasis in Isfahan Province, Central Iran. Asian Pacific J Trop Dis. (2015) 5(Suppl. 1):S54–S8. doi: 10.1016/S2222-1808(15)60857-X
97. Kolivand M, Fallah M, Salehzadeh A, Davari B, Poormohammadi A, Pazoki Ghohe H, et al. An epidemiological study of cutaneous leishmaniasis using active case finding among elementary school students in Pakdasht, Southeast of Tehran, Iran 2013-2014. J Res Health Sci. (2015) 15:104–8.
98. Gholami-Parizad E, Maleki Ravasan N, Gholami-Parizad E, Karimian F, Karimian B. Frequency and molecular identification of leishmania parasites in smears prepared from skin lesions of patients referred to health centers of ilam province by digestion of the rDNA-ITS1 gene. Pathob Res. (2015) 18:75–85. Available online at: http://mjms.modares.ac.ir/article-30-2489-en.html
99. Hezari F, Niyyati M, Tabaei SJS, Mohebali M, Vaziri VM, Behniafar H, et al. Frequency of cutaneous leishmaniasis and species identification in suspected individuals from Golestan province, Northern Iran in 2014. Iran J Public Health. (2016) 45:1348.
100. Haddad MHF, Ghasemi E, Maraghi S, Tavala M. Identification of Leishmania species isolated from human cutaneous leishmaniasis in Mehran, Western Iran using nested PCR. Iran J Parasit. (2016) 11:65.
101. Naseri A, Fata A, Rezai A, Hedayatimoghadam M, Berengi F, Akbarzadeh O, et al. Molecular identification of leishmania species in Torbat-e Heydarieh, Khorasan Razavi province, Iran. Int J Med Res Health Sci. (2016) 5:87–92.
102. Ghasemloo H, Rasti S, Delavari M, Doroodgar A. Molecular diagnosis of clinical isolates of Cutaneous leishmaniasis using ITS1 and KDNA genes and genetic polymorphism of Leishmania in Kashan, Iran. Pak J Biol Sci. (2016) 19:136. doi: 10.3923/pjbs.2016.136.142
103. Rasti S, Ghorbanzadeh B, Kheirandish F, Mousavi SG, Pirozmand A, Hooshyar H, et al. Comparison of molecular, microscopic, and culture methods for diagnosis of cutaneous leishmaniasis. J Clin Lab Analysis. (2016) 30:610–5. doi: 10.1002/jcla.21910
104. Mirahmadi H, Khorashad AS, Sohrabnahad A, Heydarian P, Bizhani N. Species identification and molecular typing of Leishmania spp. using targeting HSP70 gene in suspected patients of cutaneous leishmaniasis from Sistan and Baluchestan Province, Southeast Iran. Iran J Parasit. (2016) 11:489.
105. Dabirzadeh M, Hashemi M, Maroufi Y. Study of genetic variation of Leishmania major based on internal transcribed spacer 1 (ITS1) in chabahar, Iran. Jundish J Microb. (2016) 9:33498. doi: 10.5812/jjm.33498
106. Sharifi-Rad M, Dabirzadeh M, Sharifi I, Babaei Z. Leishmania major: genetic profiles of the parasites isolated from Chabahar, Southeastern Iran by PPIP-PCR. Iran J Parasit. (2016) 11:290.
107. Sarkari B, Ahmadpour NB, Motazedian MH, Mirjalali H, Akhoundi M, Mohebali M, et al. Inter-and intraspecific variations of leishmania strains isolated from patients with cutaneous and visceral leishmaniases in Fars Province, South of Iran. Iran J Med Sci. (2016) 41:209.
108. Abedi-Astaneh F, Hajjaran H, Yaghoobi-Ershadi MR, Hanafi-Bojd AA, Mohebali M, Shirzadi MR, et al. Risk mapping and situational analysis of cutaneous leishmaniasis in an endemic area of central Iran: A GIS-based survey. PLoS ONE. (2016) 11:e0161317. doi: 10.1371/journal.pone.0161317
109. Hajjaran H, Mahdi M, Mohebali M, Samimi-Rad K, Ataei-Pirkooh A, Kazemi-Rad E, et al. Detection and molecular identification of Leishmania RNA virus (LRV) in Iranian Leishmania species. Arch Virol. (2016) 161:3385–90. doi: 10.1007/s00705-016-3044-z
110. Izadi S, Mirhendi H, Jalalizand N, Khodadadi H, Mohebali M, Nekoeian S, et al. Molecular epidemiological survey of cutaneous leishmaniasis in two highly endemic metropolises of Iran, application of FTA cards for DNA extraction from Giemsa-stained slides. Jundish J Microb. (2016) 9:32885. doi: 10.5812/jjm.32885
111. Karamian M, Kuhls K, Hemmati M, Ghatee MA. Phylogenetic structure of Leishmania tropica in the new endemic focus Birjand in East Iran in comparison to other Iranian endemic regions. Acta Tropica. (2016) 158:68–76. doi: 10.1016/j.actatropica.2016.02.010
112. Mohammadpour I, Motazedian MH, Handjani F, Hatam GR. Cutaneous leishmaniasis of the eyelids: a case series with molecular identification and literature review. Kor J Parasit. (2016) 54:787. doi: 10.3347/kjp.2016.54.6.787
113. Soltan SS, Spotin A, Ebrahimi S, Alaeenovin E, Bordbar A, Parvizi P. Various morphologic shapes of Leishmania major amastigotes with low genetic structure among people afflicted with zoonotic cutaneous leishmaniasis in khuzestan province. IJIDTM. (2016) 21:61–74. Available online at: https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=524027
114. Pazoki Ghohe H, Pagheh AS, Fakhar M, Tavakoli G, Nazar E, Kiani M. Molecular identification of leishmania species isolated from patients with cutaneous leishmaniasis in pakdasht district, Iran, 2009-2014. J Mazandaran Univers Med Sci. (2016) 26:241–6. Available online at: http://jmums.mazums.ac.ir/article-1-9018-en.html
115. Rezai A, Moghaddas E, Bagherpor MR, Naseri A, Shamsian SA. Identification of leishmania species for cutaneous leishmaniasis in gonabad, bardaskan and kashmar, central khorasan, 2015. Jundishapur J Microbiol. (2017) 10:e44469. doi: 10.5812/jjm.44469
116. Kermanjani A, Akhlaghi L, Oormazdi H, Hadighi R. Isolation and identification of cutaneous leishmaniasis species by PCR–RFLP in Ilam province, the west of Iran. J Parasitic Dis. (2017) 41:175–9. doi: 10.1007/s12639-016-0772-7
117. Mohammadiha A, Dalimi A, Mahmoodi MR, Parian M, Pirestani M, Mohebali M. The PCR-RFLP-based detection and identification of the Leishmania species causing human cutaneous leishmaniasis in the Khorasan-Razavi Province, Northeast of Iran. J Arthropod Borne Dis. (2017) 11:383–92.
118. Nemati S, Fazaeli A, Hajjaran H, Khamesipour A, Anbaran MF, Bozorgomid A, et al. Genetic diversity and phylogenetic analysis of the Iranian Leishmania parasites based on HSP70 gene PCR-RFLP and sequence analysis. Kor J Parasit. (2017) 55:367. doi: 10.3347/kjp.2017.55.4.367
119. Motalleb G, Mirahmadi H, Ahmad Z-Z, Mehravaran A. Cytochrome b and Molecular Typing of Leishmania spp. in a Passive Sampling of Suspected Patients with Cutaneous Leishmaniasis in Sistan and Baluchestan Province, Eastern Iran. Iran J Parasit. (2017) 12:534.
120. Rastaghi ARE, Spotin A, Khataminezhad MR, Jafarpour M, Alaeenovin E, Najafzadeh N, et al. Evaluative assay of nuclear and mitochondrial genes to diagnose leishmania species in clinical specimens. Iran J Public Health. (2017) 46:1422.
121. Behravan M, Moin-Vaziri V, Haghighi A, Rahbarian N, Taghipour N, Abadi A, et al. Molecular identification of Leishmania species in a re-emerged focus of cutaneous leishmaniasis in Varamin district, Iran. J Arthropod-Borne Dis. (2017) 11:124.
122. Mohammadpour I, Motazedian MH, Handjani F, Hatam GR. Lip leishmaniasis: a case series with molecular identification and literature review. BMC Infect Dis. (2017) 17:96. doi: 10.1186/s12879-016-2178-7
123. Saghafipour A, Vatandoost H, Zahraei-Ramazani AR, Yaghoobi-Ershadi MR, Jooshin MK, Rassi Y, et al. Epidemiological study on cutaneous leishmaniasis in an endemic area, of Qom province, central Iran. J Arthropod-Borne Dis. (2017) 11:403–13.
124. Fata A, Rezai A, Moghaddas E, Mousavi VFS, Shamsian SA. Identification of Cutaneous Leishmaniasis Species in the Dargaz City, Iran. J Isfahan Med School. (2017) 34:1582–89. Available online at: https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=532733
125. Akia A, Hamzavi Y. Diagnosis and molecular typing of leishmania in patients with cutaneous leishmaniasis. J Mazandaran Univer Med Sci. (2017) 26:22–30. Available online at: http://jmums.mazums.ac.ir/article-1-9636-en.html
126. Mirahmadi H, Rezaee N, Mehravaran A, Heydarian P, Raeghi S. Detection of species and molecular typing of Leishmania in suspected patients by targeting cytochrome b gene in Zahedan, southeast of Iran. Vet World. (2018) 11:700. doi: 10.14202/vetworld.2018.700-705
127. Namazi MJ, Dehkordi AB, Haghighi F, Mohammadzadeh M, Zarean M, Hasanabad MH. Molecular detection of Leishmania species in northeast of Iran. Comp Clin Pathol. (2018) 27:729–33. doi: 10.1007/s00580-018-2658-9
128. Teimouri A, Mohebali M, Kazemirad E, Hajjaran H. Molecular identification of agents of human cutaneous leishmaniasis and canine visceral leishmaniasis in different areas of Iran using internal transcribed spacer 1 PCR-RFLP. J Arthropod-Borne Dis. (2018) 12:162. doi: 10.18502/jad.v12i2.42
129. Zahirnia AH, Bordbar A, Ebrahimi S, Spotin A, Mohammadi S, Ghafari SM, et al. Predominance of Leishmania major and rare occurrence of Leishmania tropica with haplotype variability at the center of Iran. Brazil J Infect Dis. (2018) 22:278–87. doi: 10.1016/j.bjid.2018.07.005
130. Ghatee MA, Mirhendi H, Marashifard M, Kanannejad Z, Taylor WR, Sharifi I. Population structure of Leishmania tropica causing anthroponotic cutaneous leishmaniasis in southern Iran by PCR-RFLP of kinetoplastid DNA. BioMed Res Int. (2018) 2018:198. doi: 10.1155/2018/6049198
131. Mousavi T, Shokohi S, Abdi J, Naserifar R, Ahmadi M, Mirzaei A. Determination of genetic diversity of Leishmania species using mini-circle kDNA, in Iran-Iraq countries border. Trop Parasit. (2018) 8:77. doi: 10.4103/tp.TP_3_18
132. Ramezany M, Sharifi I, Babaei Z, Ghasemi Nejad Almani P, Heshmatkhah A, Keyhani A, et al. Geographical distribution and molecular characterization for cutaneous leishmaniasis species by sequencing and phylogenetic analyses of kDNA and ITS1 loci markers in south-eastern Iran. Pathogens Global Health. (2018) 112:132–41. doi: 10.1080/20477724.2018.1447836
133. Askari A, Sharifi I, Aflatoonian M, Babaei Z, Almani PGN, Mohammadi M, et al. A newly emerged focus of zoonotic cutaneous leishmaniasis in South-Western Iran. Microbial Pathogen. (2018) 121:363–8. doi: 10.1016/j.micpath.2018.04.053
134. Mirzaei A, Ahmadipour F, Cannet A, Marty P, Delaunay P, Perrin P, et al. Immunodetection and molecular determination of visceral and cutaneous Leishmania infection using patients' urine. Infecti Genet Evolut. (2018) 63:257–68. doi: 10.1016/j.meegid.2018.05.021
135. Fata A, Moghaddas E, Rezee A, Abdali A, Jarahi L, Shamsian A. Epidemiological study of cutaneous leishmaniasis and identification of etiological species. J Mazandaran Univer Med Sci. (2018) 27:123–31. Available online at: http://jmums.mazums.ac.ir/article-1-10299-en.html
136. Gholamian-Shahabad MR, Azizi K, Asgari Q, Kalantari M, Moemenbellah-Fard MD. Sandflies species composition, activity, and natural infection with Leishmania, parasite identity in lesion isolates of cutaneous leishmaniasis, central Iran. J Paras Dis. (2018) 42:252–8. doi: 10.1007/s12639-018-0994-y
137. Mohammadiha A, Dalimi A, Mohebali M, Sharifi I, Mahmoudi M, Mirzaei A, et al. Molecular identification and phylogenetic classification of Leishmania spp. isolated from human cutaneous leishmaniasis in Iran: a cross-sectional study. Iran J Parasit. (2018) 13:351.
138. Mirzapour A, Spotin A, Behniafar H, Azizi H, Maleki B, Shakeraminia H, et al. Intra-Species Diversity of Leishmania major and L. tropica from Clinical Isolates of Cutaneous Leishmaniasis in Southwest Iran Inferred by ITS1-rDNA. Iran J Public Health. (2019) 48:893. doi: 10.18502/ijph.v48i5.1806
139. Razavinasab SZ, Sharifi I, Aflatoonian MR, Babaei Z, Mohammadi MA, Salarkia E, et al. Expansion of urban cutaneous leishmaniasis into rural areas of southeastern Iran: clinical, epidemiological and phylogenetic profiles explored using 7SL high resolution melting-PCR analysis. Transbound Emerg Dis. (2019) 66:1602–10. doi: 10.1111/tbed.13186
140. Mohammadpour I, Hatam GR, Handjani F, Bozorg-Ghalati F, PourKamal D, Motazedian MH. Leishmania cytochrome b gene sequence polymorphisms in southern Iran: relationships with different cutaneous clinical manifestations. BMC Infect Dis. (2019) 19:98. doi: 10.1186/s12879-018-3667-7
141. Barazesh A, Motazedian MH, Fouladvand M, Hatam G, Tajbakhsh S, Ebrahimi S, et al. Molecular identification of species caused cutaneous leishmaniasis in southern zone of Iran. J Arthropod-Borne Dis. (2019) 13:198. doi: 10.18502/jad.v13i2.1246
142. Ghobakhloo N, Motazedian MH, Naderi S, Ebrahimi S. Isolation of Crithidia spp. from lesions of immunocompetent patients with suspected cutaneous leishmaniasis in Iran. Trop Med Int Health. (2019) 24:116–26. doi: 10.1111/tmi.13042
143. Mirahmadi H, Gholizadeh S, Raeghi S, Sadat Roointan E, Rezaee N, Mehravaran A. KDNA and molecular typing of leishmania spp. Of cutaneous leishmaniasis patients in sistan and baluchestan province with low amount of parasite. J KermanUnivers Med Sci. (2019) 26:1–11. doi: 10.22062/jkmu.2019.87269
144. Ziaei Hezarjaribi H, Emadi N, Asfaram S, Geran Orimi T, Derakhshani-niya M, Fakhar M. Molecular Detection of Leishmania spp. in Negative Smears of Patients with Cutaneous Leishmaniasis in Bam, Southeast Iran. J Mazandaran Univer Med Sci. (2019) 29:123–7. Available online at: http://jmums.mazums.ac.ir/article-1-12648-en.html
145. Zare-Zadeh A, Motalleb G, Mirahmadi H, Salimi Khorashad A. ITS-rDNA and molecular typing of Leishmania spp. in suspected patients with cutaneous leishmaniasis in Sistan and Balochestan province, Iran. J Cell Mol Res. (2019) 32:125–35. Available online at: http://magiran.com/p1996206
146. Sarkari B, Fakhar M, Ebrahimi M, Hatam G, Kalantari M, Rezanejad H. Characterization of Leishmania parasites isolated from Kala-azar patients in Kohgiloyeh and BoyerAhmad, using semi-nested PCR. Armaghane Danesh. (2006) 11:27–34. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?id=62124
147. Alborzi A, Rasouli M, Shamsizadeh A. Leishmania tropica–isolated patient with visceral leishmaniasis in southern Iran. Am J Trop Med Hyg. (2006) 74:306–7. doi: 10.4269/ajtmh.2006.74.306
148. Motazedian M, Fakhar M, Motazedian MH, Hatam G, Mikaeili F. A urine-based polymerase chain reaction method for the diagnosis of visceral leishmaniasis in immunocompetent patients. Diagn Microb Infect Dis. (2008) 60:151–4. doi: 10.1016/j.diagmicrobio.2007.09.001
149. Alborzi A, Pouladfar GR, Fakhar M, Motazedian MH, Hatam GR, Kadivar MR. Isolation of Leishmania tropica from a patient with visceral leishmaniasis and disseminated cutaneous leishmaniasis, southern Iran. Am J Trop Med Hyg. (2008) 79:435–7. doi: 10.4269/ajtmh.2008.79.435
150. Fayzi M, Fanid L, Fayzi A, Pour M, Farajnia S, Nakhlband A. Detection of Leishmania infantum minicircle kinetoplast DNA in bone marrow and peripheral blood samples of paediatric patients from children's hospital of Tabriz Medical University. Biotechnology. (2008) 7:175–81. doi: 10.3923/biotech.2008.175.181
151. Fakhar M, Keyghobadi M, Akramipour R, Ghadiri K, Limouei M. Characterization of Leishmania isolated from Kala-azar infected patients in Kermanshah using PCR. J Kermanshah Univ Med Sci. (2011) 15:138–44.
152. Fakhar M, Motazedian M, Hashemi S, Golkar A. Detection of Leishmania parasites species isolated from patients suffering from kala-azar using one-step PCR. J Jahrom Univers Med Sci. (2011) 9:21–6. doi: 10.29252/jmj.9.2.21
153. Mohammadiha A, Mohebali M, Haghighi A, Mahdian R, Abadi A, Zarei Z, et al. Comparison of real-time PCR and conventional PCR with two DNA targets for detection of Leishmania (Leishmania) infantum infection in human and dog blood samples. Exp Parasit. (2013) 133:89–94. doi: 10.1016/j.exppara.2012.10.017
154. Fakhar M, Kia AA, Gohardehi S, Sharif M, Mohebali M, Akhoundi B, et al. Emergence of a new focus of visceral leishmaniasis due to Leishmania infantum in Golestan Province, north-eastern of Iran. J Paras Dis. (2014) 38:255–9. doi: 10.1007/s12639-013-0307-4
155. Hosseininasab A, Sharifi I, Mohammad Hossein D, Zarean M, Dadkhah M. Causes of pediatric visceral leishmaniasis in southeastern Iran. Iran J Parasit. (2014) 9:584.
156. Ghasemian M, Gharavi MJ, Akhlaghi L, Mohebali M, Meamar AR, Aryan E, et al. SYBR green-based detection of Leishmania infantum DNA using peripheral blood samples. J Paras Dis. (2016) 40:81–7. doi: 10.1007/s12639-014-0452-4
157. Sarkari B, Ahmadpour NB, Moshfe A, Hajjaran H. Molecular evaluation of a case of visceral leishmaniasis due to Leishmania tropica in Southwestern Iran. Iran J Parasit. (2016) 11:126.
158. Asfaram S, Fakhar M, Mohebali M, Mardani A, Banimostafavi ES, Hezarjaribi HZ, et al. Asymptomatic human blood donors carriers of Leishmania infantum: potential reservoirs for visceral leishmaniasis in northwestern Iran. Transf Apheresis Sci. (2017) 56:474–9. doi: 10.1016/j.transci.2017.06.001
159. Asfaram S, Pagheh A, Fakhar M, Gheraghali F, Rezai MS. Case series of visceral Leishmaniasis (kala-azar) in mazandaran and golestan provinces, North of Iran. J Mazandaran Univer Med Sci. (2017) 26:373–81. Available online at: http://jmums.mazums.ac.ir/article-1-9282-en.html
160. Dalimi A, Mohammadiha A, Mohebali M, Mirzaei A, Mahmoudi M. Molecular identification and intra-species variations among Leishmania infantum isolated from human and canine visceral leishmaniasis in Iran. Iran J Parasit. (2018) 13:567.
161. Masoori L, Kheirandish F, Haghighi A, Mohebali M, Akhoundi B, Taghipour N, et al. Molecular-based detection of Leishmania infantum in human blood samples in a new focus of visceral leishmaniasis in Lorestan Province, Iran. J Arthropod-Borne Dis. (2018) 12:67.
162. Layegh Gigloo A, Sarkari B, Rezaei Z, Hatam GR, Davami MH. Asymptomatic Leishmania infected children: a seroprevalence and molecular survey in a rural area of Fars Province, Southern Iran. J Trop Med. (2018) 2018:7247. doi: 10.1155/2018/8167247
163. Rezaei Z, Sarkari B, Dehghani M, Gigloo AL, Afrashteh M. High frequency of subclinical Leishmania infection among HIV-infected patients living in the endemic areas of visceral leishmaniasis in Fars province, southern Iran. Parasit Res. (2018) 117:2591–5. doi: 10.1007/s00436-018-5949-9
164. Ghatee MA, Mirhendi H, Karamian M, Taylor WR, Sharifi I, Hosseinzadeh M, et al. Population structures of Leishmania infantum and Leishmania tropica the causative agents of kala-azar in Southwest Iran. Parasitol Res. (2018) 117:3447–58. doi: 10.1007/s00436-018-6041-1
165. Kumar A, Pandey SC, Samant M. DNA-based microarray studies in visceral leishmaniasis: identification of biomarkers for diagnostic, prognostic and drug target for treatment. Acta Tropica. (2020) 2020:105512. doi: 10.1016/j.actatropica.2020.105512
166. Shirzadi M, Gouya M. National Guidelines for Cutaneous Leishmaniasis Surveillance in Iran. Tehran Iran: Ministry of Health and Medical Education (MOH) Zoonoses Control Department (2012). p. 1–78.
167. Norouzinezhad F, Ghaffari F, Norouzinejad A, Kaveh F, Gouya MM. Cutaneous leishmaniasis in Iran: results from an epidemiological study in urban and rural provinces. Asian Pacif J Trop Biomed. (2016) 6:614–9. doi: 10.1016/j.apjtb.2016.05.005
168. Foroutan M, Khademvatan S, Majidiani H, Khalkhali H, Hedayati-Rad F, Khashaveh S, et al. Prevalence of Leishmania species in rodents: a systematic review and meta-analysis in Iran. Acta Trop. (2017) 172:164–72. doi: 10.1016/j.actatropica.2017.04.022
169. Karimi T, Sharifi I, Aflatoonian MR, Aflatoonian B, Mohammadi MA, Salarkia E, et al. A long-lasting emerging epidemic of anthroponotic cutaneous leishmaniasis in southeastern Iran: population movement and peri-urban settlements as a major risk factor. Parasit Vect. (2021) 14:1–4. doi: 10.1186/s13071-021-04619-3
170. del Giudice P, Marty P, Lacour JP, Perrin C, Pratlong F, Haas H, et al. Cutaneous leishmaniasis due to Leishmania infantum: case reports and literature review. Arch Dermat. (1998) 134:193–8. doi: 10.1001/archderm.134.2.193
171. Lachaud L, Dedet JP, Marty P, Faraut F, Buffet P, Gangneux JP, et al. Surveillance of leishmaniases in France, 1999 to 2012. Eurosurveillance. (2013) 18:20534. doi: 10.2807/1560-7917.ES2013.18.29.20534
172. De Lima H, Rodríguez N, Feliciangeli MD, Barrios MA, Sosa A, Agrela I, et al. Cutaneous leishmaniasis due to Leishmania chagasi/Le. infantum in an endemic area of Guarico State, Venezuela. Trans R Soc Trop Med Hyg. (2009) 103:721–6. doi: 10.1016/j.trstmh.2008.11.019
173. Rostamian M, Bashiri H, Yousefinejad V, Bozorgomid A, Sohrabi N, Raeghi S, et al. Prevalence of human visceral leishmaniasis in Iran: a systematic review and meta-analysis. Comp Immunol Microb Infect Dis. (2020) 2020:101604. doi: 10.1016/j.cimid.2020.101604
174. Ghatee MA, Taylor WR, Karamian M. The geographical distribution of cutaneous leishmaniasis causative agents in Iran and its neighboring countries, a review. Front Public Health. (2020) 8:11. doi: 10.3389/fpubh.2020.00011
175. Reimão JQ, Coser EM, Lee MR, Coelho AC. Laboratory diagnosis of cutaneous and visceral leishmaniasis: current and future methods. Microorganisms. (2020) 8:1632. doi: 10.3390/microorganisms8111632
176. de Morais RCS, de Melo MGN, de Goes TC, e Silva RP, de Morais RF, de Oliveira Guerra JA, et al. Duplex qPCR for Leishmania species identification using lesion imprint on filter paper. Exp Parasit. (2020) 219:108019. doi: 10.1016/j.exppara.2020.108019
177. Sundar S, Singh OP. Molecular diagnosis of visceral leishmaniasis. Mol Diagn Therapy. (2018) 22:443–57. doi: 10.1007/s40291-018-0343-y
178. Dávila A, Momen H. Internal-transcribed-spacer (ITS) sequences used to explore phylogenetic relationships within Leishmania. Ann Trop Med Parasit. (2000) 94:651–4. doi: 10.1080/00034983.2000.11813588
179. Vega-López F. Diagnosis of cutaneous leishmaniasis. Curr Opin Infect Dis. (2003) 16:97–s101. doi: 10.1097/00001432-200304000-00006
180. Lorenz TC. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. JoVE. (2012) 2012:e3998 doi: 10.3791/3998
181. Saberi R, Fakhar M, Mohebali M, Anvari D, Gholami S. Global status of synchronizing Leishmania RNA virus in Leishmania parasites: a systematic review with meta-analysis. Transbound Emerg Dis. (2019) 66:2244–51. doi: 10.1111/tbed.13316
182. Ito MM, Catanhêde LM, Katsuragawa TH, Silva Junior CF, Camargo LM, Mattos Rde G, et al. Correlation between presence of Leishmania RNA virus 1 and clinical characteristics of nasal mucosal leishmaniosis. Braz J Otorhinolaryngol. (2015) 81:533–40. doi: 10.1016/j.bjorl.2015.07.014
183. Saberi R, Fakhar M, Hajjaran H, Ataei-Pirkooh A, Mohebali M, Taghipour N, et al. Presence and diversity of Leishmania RNA virus in an old zoonotic cutaneous leishmaniasis focus, northeastern Iran: haplotype and phylogenetic based approach. Int J Infect Dis. (2020) 101:6–13. doi: 10.1016/j.ijid.2020.08.033
Keywords: Leishmania major, Leishmania tropica, Leishmania infantum, DNA-based molecular method, human, Iran
Citation: Hajjaran H, Saberi R, Borjian A, Fakhar M, Hosseini SA, Ghodrati S and Mohebali M (2021) The Geographical Distribution of Human Cutaneous and Visceral Leishmania Species Identified by Molecular Methods in Iran: A Systematic Review With Meta-Analysis. Front. Public Health 9:661674. doi: 10.3389/fpubh.2021.661674
Received: 31 January 2021; Accepted: 31 May 2021;
Published: 25 June 2021.
Edited by:
Herbert Leonel de Matos Guedes, Federal University of Rio de Janeiro, BrazilReviewed by:
Muhammad Imran Khan, The University of Haripur, PakistanCopyright © 2021 Hajjaran, Saberi, Borjian, Fakhar, Hosseini, Ghodrati and Mohebali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Homa Hajjaran, aGFqYXJhbmhAdHVtcy5hYy5pcg==; Reza Saberi, ci5zYWJlcmlAbWF6dW1zLmFjLmly
†ORCID: Homa Hajjaran orcid.org/0000-0001-5877-2845
Reza Saberi orcid.org/0000-0002-7906-7034
Alireza Borjian orcid.org/0000-0003-1440-4922
Mahdi Fakhar orcid.org/0000-0002-4690-6938
Seyed Abdollah Hosseini orcid.org/0000-0002-2990-1123
Mehdi Mohebali orcid.org/0000-0002-4164-9514
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.