- 1Histopathology Department, The Royal Marsden Hospital, London, United Kingdom
- 2Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy
- 3Nursing Homes Villa Elisa and Arici-Sega, Brescia, Italy
- 4Nursing Home Casa Industria, Brescia, Italy
Background: COVID-19 outbursts have been registered worldwide within care homes with asymptomatic transmission combined with shortage/inaccuracy of diagnostic tests undermining the efforts at containment of the disease. Nursing facilities in Lombardy (Italy) were left with no, or limited, access to testing for 8 weeks after the outbreak of COVID-19.
Methods: This study includes 246 residents and 286 workers of three different nursing homes in Brescia-Lombardy. Clinical questionnaires and rapid serology tests were devised to integrate the data of the first available RT-PCR screening. Follow-up serology after 60-days was performed on 67 of 86 workers with positive serology or clinically suspicious.
Findings: Thirty-seven residents and 18 workers had previous positive RT-PCR. Thorough screening disclosed two additional RT-PCR-positive workers. Serology screening revealed antibodies in 59 residents and 48 workers, including 32/37 residents and all workers previously positive at RT-PCR. Follow up serology disclosed antibodies in two additional workers with recent symptoms at the time of screening. The professionals in close contact with residents had more infections (47/226–20.79% vs. 1/60–1.66%; p = 0.00013 Fisher exact-test). A suspicious clinical score was present in 44/64 residents and in 41/50 workers who tested positive with either method with totally asymptomatic disease more frequent among residents 28.1 vs. 10.0% (p = 0.019 Fisher exact-test).
Interpretation: Based on the available RT-PCR ± results at the time of symptoms/contacts, our integrated clinical and serological screening demonstrated sensitivity 89% and specificity 87%. This multimodal assessment proved extremely useful in understanding the viral spread in nursing homes, in defining its stage and in implementing protective measures. Rapid serology tests demonstrated efficient and particularly suited for older people less able to move/cooperate.
Introduction
Older adults with or without serious underlying medical conditions are at high risk for severe forms of COVID-19 (1, 2). Old people cared for in nursing homes frequently bear co-morbidities, are highly dependent on assistance and continuous close contact by multiple carers and are frequently hosted in shared rooms. In these circumstances the occurrence of severe outbreaks is easily predictable (3, 4).
Italy was the first country in Europe to be hit by SARS-Cov2, and Lombardy the first and most affected region, especially the provinces of Brescia and Bergamo (5).
Specific behavioral restrictions, including lockdown and social distancing, have been enacted in care homes in Brescia from February 28th, a week after the beginning of the COVID-19 outbreak in Lombardy and well in advance of European Community guidelines released in March. Nevertheless, outbursts of infection in many nursing homes were registered.
In Lombardy, the impact of COVID-19 epidemics was so overwhelming that no tests were available for several weeks except for those patients presenting with severe symptoms to Accident and Emergency (6).
In this chaotic emergency situation, care homes were laced at “the back of the queue.” Virology swabs were available for symptomatic residents and healthcare workers (HCW) only from April 4th and a staff-only RT-PCR screening was undertaken at the end of April.
Given the shortage of any other laboratory tests, rapid serology tests associated with recording of clinical data appeared a promising integration of virology screening in identifying false negative RT-PCR cases (7) and asymptomatic infections and in tailoring additional protective measures.
Materials and Methods
The cohort of this study included residents and staff of three differently sized care homes in Brescia (Italy) managed by the same medical direction during the COVID-19 outbreak: “CI,” a long-established geriatric care home for up to 130 residents in central historic buildings, “VE” and “AS,” hosting up to 62 and 120 residents in modern buildings on the outskirts of town. The number of single rooms was, respectively, 4 (CI), 30 (VE), and 48 (AS). Rooms occupied by three or more residents were present in the historic central building only (CI). The smallest nursing home (VE) had no registered COVID-related deaths amongst residents at the time of our screening, while several cases had been already registered in both other residences.
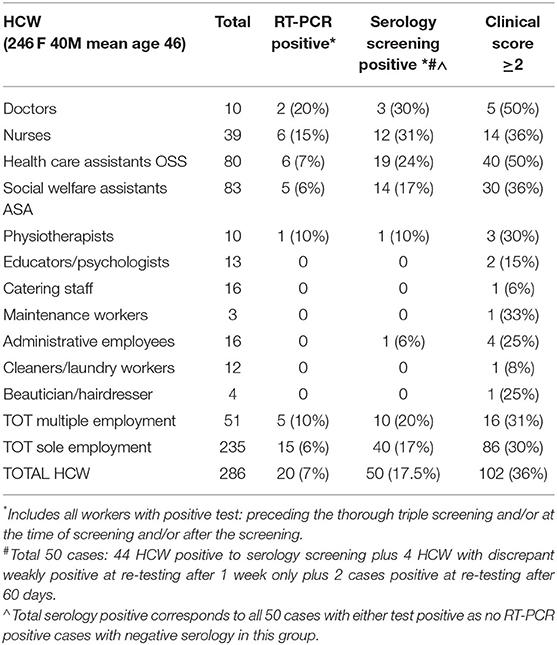
The research involved 532 persons including 246 residents (age range 27–100; mean 83; males 28%) and 286 regular and shift HCW (age range 23–70 years; mean 46; males 14%) (Tables 1, 2). The adherence to the study was voluntary: 13 residents and 7 HCW refused participation. A single HCW was excluded because she was quarantined at home. Testing was non-practicable for one resident.
The residents included 184 people with dementia, 58 of which had Alzheimer's or other dementia with wandering, and 20 in persistent vegetative state, including all six residents aged <65 (age range 23–61).
Regular HCW included professionals in close contact with residents (doctors, nurses, healthcare assistants, social health workers, physiotherapists, hairdressers, and beauticians) plus cleaners, catering staff, administrative workers. Shift workers were represented by night shift doctors and nurses and amounted to 51 workers.
Integrated triple screening of HCW was performed between April 23 and May 14, 2020.
Double clinico-serological screening of residents was performed May 19–June 30, 2020.
Data analysis was undertaken between May and July 2020.
Clinical Assessment
The clinical questionnaire devised for all HCW included a list of 33 symptoms linked to COVID-19, exposure to proven infected persons, precautional isolation measures, results of RT-PCR tests, data about underlying health conditions and routine or recent treatments. The list of symptoms (Supplementary Table 1) was drawn from the existing literature on March–April 2020 (1, 8–11) or inferred from the reports of the self-help Facebook “Coronavirus, CoV-2 and COVID-19 group for doctors only” created at the beginning of the European outburst and joined by more than 100.000 Italian and European doctors. A simplified list comprising the 18 more objectifiable symptoms (Supplementary Table 1) was completed by their doctors for all residents considered less able to report their symptoms. Symptoms referring to the period of SARS-Cov2 diffusion in Italy only were recorded, starting in February 2020. Exclusion of usual symptoms related to chronic conditions or to allergy was asked.
The degree of suspicion of COVID-19 was graded using a self-made scoring algorithm obtained by the combination of relevant symptoms, exposure to infected persons and, when available, chest computerized tomography-scan or ultrasound (Table 3). Scores ≥2 were considered suspicious and followed-up independently of test results.
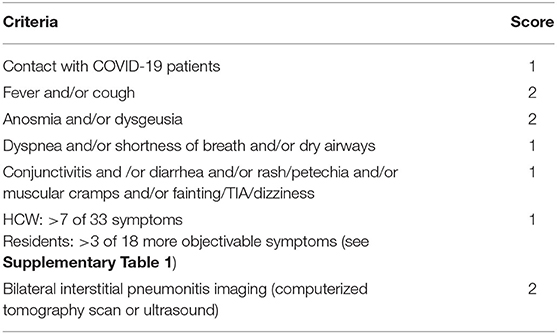
Table 3. Clinical pre-test scoring of COVID-19 probability: a total score ≥2 is considered suspicious of infection, independently of negative tests.
RT-PCR Testing
All the 286 HCW underwent RT-PCR at the time of our integrative screening. Previous RT-PCR tests results were available in 41 workers and 146 residents (Table 4). A single nursing home (AS) had all 88 residents screened for SARS-CoV-2, as a systematic screening was performed after a hotspot of COVID-19 was detected. All remaining HCV and residents had been tested because of evocative symptoms or contact with affected people.
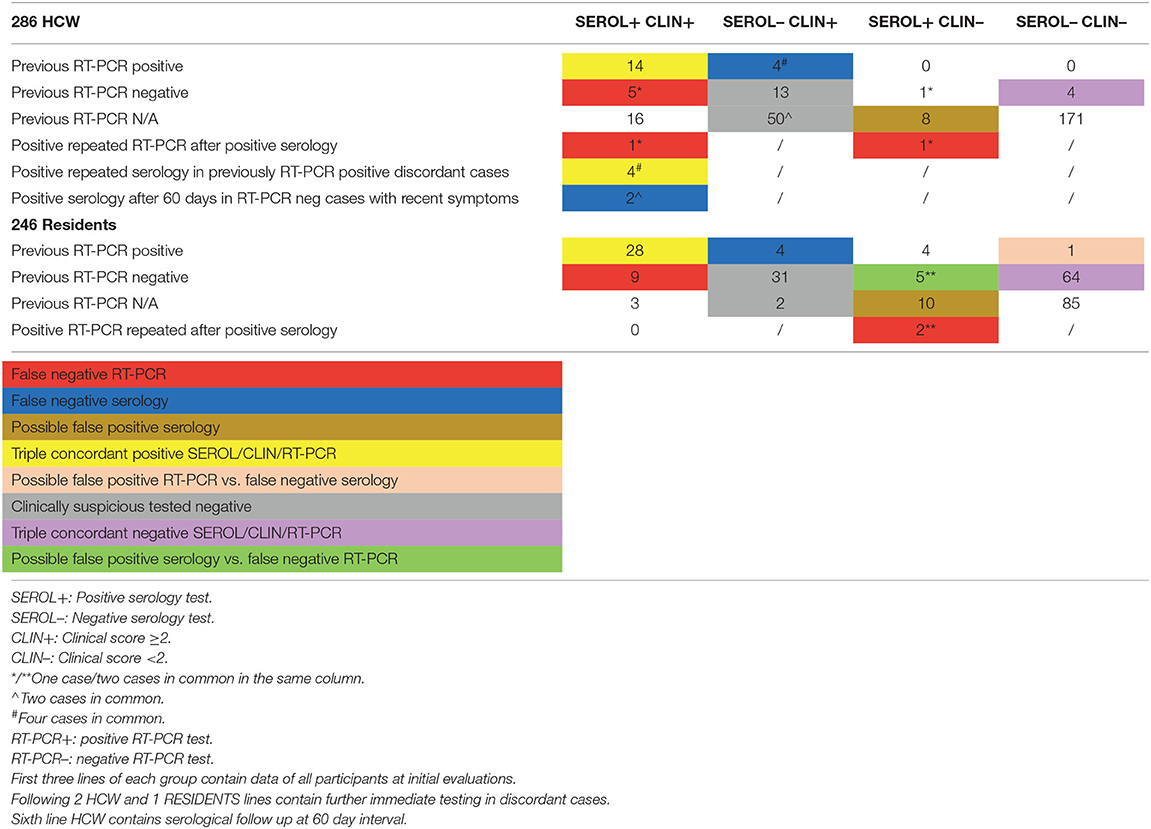
Table 4. Integrated screening interpretation: a case was considered positive for COVID-19 when 2 of 3 parameters where satisfied—a negative serology or RT-PCR test result was considered false negative only if associated with COVID-19 related symptoms or CT imaging and a positive test.
All RT-PCR tests were run in the same laboratory using RADI COVID-19 detection Kit (KHMedical), on ABI 7500 Bio-Rad CFX 96 platform. The lower limit of detection of this kit is 0.66 copies/microliter.
Serology Testing
Serology screening used the rapid point-of-care test® Core tests COVID-19 IgM/IgG Ab, whose instructions report IgM&IgG sensitivity 97.6% and specificity 100%. Tests reading and photographic recording were performed 15 min after deposing the finger blood sample just collected. Weak lines for IgM and IgG were considered positive, according to the report by Li et al. (12) and in consideration of the principle of maximum safety.
All those screened positive with immunoassay with no or negative RT-PCR were immediately isolated and a further swab test was taken.
Serology with the identical lot rapid Core test was repeated after 1 week in 4 HCW with discrepant negative serology screening and previous positive RT-PCR and at 60-day interval in 67 of 86 HCW with positive serology screening or with recent/suspicious clinical scores and negative tests.
Unlike HCW, residents with discrepant serology and positive virology tests did not repeat a serology test due to difficulty in getting an informed consent for further assessments. A 60-day serological follow up was also not performed.
Statistical Analysis
Data were reported as counts and percentages. Comparison between independent proportions (groups) were performed using Fisher Exacts-test. Trends in proportions were tested using Chi-square test for trends. All analysis assumed a 5% significance level and were performed using R software (version 4.0.5).
Informed Consent and Ethical Approval
Informed consent was obtained from all HCW and residents or their representatives.
Formal review and approval by the ethic committee of Spedali Civili of Brescia (NP 4378) was obtained for this study.
Funding
Voluntary donations and some residual funding from previous different research studies.
Results
A flowchart with all the results obtained is provided (Supplementary Figure 1).
Residents
A thorough virology screening was not available for the 246 residents.
Previous RT-PCR results were available for 146 residents (59.3%—Table 1).
32/59 residents positive for serology had also been previously identified with RT-PCR while 5 cases with previous positive RT-PCR turned out negative at serology (Table 4).
Paired positive IgM and IgG bands were present in 57 of 59 seropositive cases, five of which showing weak bands. Two asymptomatic cases showed isolated IgM. No isolated IgG positivity was found.
Overall, 18/64 residents (28.1%) with positivity to either test reported complete absence of symptoms. Mild symptoms and a clinical score 0–1 were present in further 2 cases. A clinical history suspicious of COVID-19 scoring ≥2 was associated with at least one positive test in 44 residents but was also present in 31 residents with both tests negative (Table 4).
In our study the virus was rapidly spreading between residents with Alzheimer's disease or other dementias associated with wandering (30/58), while the contagion amongst all other residents (28/188) was significantly less frequent (51.7 vs. 15.9%—p < 0.001 Fisher exact-test). Actually, the rate of infection in residents with dementia unassociated with wandering was comparable to the group with no cognitive impairment (7/42–16.9%—p-value NS).
The number of infections amongst the 20 residents in persistent vegetative state was instead inferior in comparison with all other residents (1/20–5% vs. 57/226–25.2%—p = 0.051 Fisher exact-test).
No correlation was found between the rate of infected residents and the different size, in terms of number of residents, of these three nursing homes: 23/107 (21.5%) vs. 28/88 (31.8%) vs. 7/51 (13.7%)—p = 0.58 (Test for trends in proportions—p value NS).
Staff
All 286 workers had SARS-CoV-2 RT-PCR, clinical and serological screening (Tables 2, 4).
Previous RT-PCR results were available for 41 HCW (14.3%).
14/44 HCW initially seropositive also had a previous positive RT-PCR result. Repeated RT-PCR in those identified with specific antibodies was positive in one additional HCW, who was thereafter isolated.
Paired positive IgM and IgG bands were present in all cases except for a single asymptomatic case showing a weak IgM band only.
Four symptomatic workers with previous positive RT-PCR and discrepant negative serology, were re-tested after 1 week with the same test-kit and all showed very weak bands for both IgM and IgG (Table 4). These four serology tests, although ultimately evaluated as positive in Table 2, have been considered false negative in Table 4, where all results are reported by chronological phases.
Follow up serology after 60 days, performed in 78% (67 of 86) of seropositive or clinically suspicious/recent HCW, yielded two additional serology-positive cases whose suspicious clinical history was recent at the time of the screening. These two additional cases have been considered serology-positive in Table 2, based on final information provided by this study.
HCW in close contact with the residents (including doctors, nurses, health care assistants, personal care-givers, physiotherapists, hairdressers, and beauticians) had significantly more infections (20.79 vs. 1.66% in remaining HCW; p = 0.00013 Fisher exact-test—Table 2). Familial contact with ill relatives was present in 11 HCW only, including the single administrative employee that tested positive.
The seroprevalence in the single nursing home with no COVID-related deaths amongst residents was markedly inferior (8%) to that found in the two nursing homes with known cases (19%) and appeared comparable to 7.5% seroprevalence of the general population in Lombardy at the time.1
The rate of positive tests was also greater amongst HCW with additional external activities in comparison to those with exclusive activity within their nursing home (20 vs. 16%—Table 2).
Among all 50 HCW with ultimately proven positivity for SARS-CoV2 (serology and/or RT-PCR) in our study, 40% only had fever and 50% reported cough (Supplementary Table 1).
At final evaluation of all test results, 9 seropositive HCW (18%) had been considered clinically non-suspicious (clinical score 0–1), 5 of which (10%) reported complete absence of symptoms. All 5 totally asymptomatic HCW had negative RT-PCR test while serology was frankly positive for both IgM and IgG in 2 cases, showed a weak IgM band only in a third case, and paired weak IgM and IgG bands in the last 2 cases. Close contact with COVID-19 patients was reported in two of them. A suspicious clinical score ≥2 was present in 41 HCW with at least one positive test but also in 61 workers who finally tested negative (Table 4).
Follow up serology after 60 days, performed in 78% (67 of 86) of seropositive or clinically suspicious/recent cases, provided identical either paired IgM and IgG or isolated weak IgM positivity patterns to those previously obtained, with no case showing disappearance of IgM or appearance of IgG. As mentioned, paired IgM and IgG bands were also demonstrated in two of the four workers with recent suspicious symptoms and negative initial serology screening.
Isolation and RT-PCR Testing of Seropositive Cases
Viral RNA was still present in two workers and two residents re-tested after being found seropositive, three of whom were completely asymptomatic.
Sensibility and Specificity of Clinico-Serological Screening
On the basis of the available RT-PCR ± data of participants at the time of symptoms or contacts with infected people, sensibility and specificity for on-site serology was 83 and 84%, sensibility and specificity for our clinical score was 91 and 56%, while integrated clinical scoring and on-site serology had sensibility 89% and specificity 87%.
Discussion
Most SARS-CoV-2 diagnostic tests so far available represent “blunt weapons,” whose approval for emergency use in response to the pandemics by FDA and EC has not implied a proper estimate of performance characteristics and limitations with extensive validation tests.
Symptoms
Symptoms of COVID-19 are poor indicators of the disease as people affected may experience a wide range of symptoms from mild to a myriad of different symptoms, and a proportion of cases between 6.3 and 96.0% (13) is asymptomatic.
Despite our scoring system intentionally devised to be highly sensitive, unexpected positive tests were still present in 9 workers and 20 residents with clinical scores below the threshold, and entirely asymptomatic disease was more frequent amongst residents (18/64–28.1% vs. 5/50–10.0%; p = 0.019). A similar difference has been observed elsewhere in long-term facilities (14, 15) and is most probably justified by a lesser degree of awareness and/or by difficulty in communicating with older people is particularly concerning when planning strategies for transmission containment in care homes.
In our study, the list of symptoms had been simplified since the vast majority of participants were not able to adequately report more subtle and subjective signs. This has allowed homogeneity and comparison within the same group, but it has made it impossible to assess atypical COVID-related symptoms described in older people elsewhere (16, 17). The significant paired difference in proven infection rates (64/246–26.0% vs. 50/286–17.5%; p = 0.020 Fisher exact-test) might be correlated with more common asymptomatic or atypical forms amongst residents.
Asymptomatic transmission is held responsible for failure of symptoms-based screenings and monitoring strategies in nursing homes (18–21). The awareness that a larger proportion of infected residents have no symptoms increases the urgency for their frequent regular testing (22).
RT-PCR
It is considered the gold standard to identify COVID-19: however, nasal and oropharyngeal swabs are charged with a considerable quote (30–40%) of false negative results (23, 24).
In our study 7–8% of residents and workers tested at the time of symptoms/possible contact showed single or multiple false negative virology tests on nasopharyngeal swabs. Repetition of tests proved worthwhile, allowing detection of viral RNA in four cases previously testing negative.
In our study, the residents had an easier access to the virological tests in comparison to HCW. This was due to specific dispositions of our Regional Health System at the beginning of the pandemics, when the availability of tests was still not meeting the demand. In fact, access to swab testing was guaranteed to all the residents with suspicious symptoms or possible contact with an infected person after 6 weeks of the pandemics. The same option was offered to those HCW that were by profession in direct contact with the residents and directly employed by the nursing home, not including the shift workers.
Serology
Antibody testing misses early infections but can be useful to understand the prevalence in a community, particularly when no tests have been previously available, and it may disclose false negative cases at antigen testing (25). The advent and the gradual expansion of the population vaccinated with SARS-CoV-2 vaccines, negatively impacts on the informative value provided by serology testing in the absence of associated clinical information and dynamic evaluation of antibody level changes.
Rapid Point-Of-Care Tests
Accurate point-of-care virology and immunology tests are particularly needed for effective monitoring of an infection in vulnerable and enclosed settings (12, 26–29).
A variety of serology tests has been internationally approved since the start of COVID-19 pandemics. The diagnostic performance of quantitative serology is generally considered superior to rapid qualitative tests, but its supply has been late and is still not meeting the demand.
Point-of-care antibody tests have demonstrated efficiency comparable to that of laboratory tests (30), have been successfully used in a series of studies so far (27, 28, 31–33), and represented a valid alternative to unavailable quantitative serology in our study.
Point-of care serology tests are cheap and easy to use in number of different clinical situations (12, 26) and are particularly useful in the case of poorly cooperative older residents. Despite controversy over their accuracy (31, 34), the authors decided to follow the encouragement of WHO to use these tests in research studies to establish their usefulness. Their results require a nuanced interpretation and correlation with RT-PCR and clinical information (27, 35).
In our study, rapid on-site serology has been able to confirm symptoms in 35 persons, 20 of whom (6 HCW and 14 residents) had previous negative RT-PCR results and to unveil a previous COVID in 24 further persons with no suspicious symptoms and negative or no previous RT-PCR.
Four HCW and 5 residents had a positive RT-PCR but a negative first serology test.
Interpretation of IgM results appears particularly challenging (36). Similar to other studies of the same period (34), no case with isolated IgG was found.
Although high levels of IgM have been reported 22–24 days after symptom onset at serial serology testing (37) and several studies have shown simultaneous production of IgM and IgG against SARS-CoV-2 (12, 24, 37), the unchanged paired IgM and IgG positivity in all tests repeated after 60 days with no case with IgM disappearance is disturbing. Similarly, the two asymptomatic cases with isolated IgM and with identical positivity pattern at re-testing after 60 days raise doubts about a possible false positive result.
The combination of qualitative and quantitative antibody testing could represent a safer strategy to obtain reliable data.
Comparative evaluations of rapid tests for COVID-19 infection in care homes are urgently needed too (28).
Identifying Weaknesses
Similar to other studies (7, 38), serology allowed a retrospective evaluation of the control strategies adopted. Besides continuous attention to maintain adherence to the measures put in place and regular testing, identifying weaknesses is crucial to safety in nursing homes.
Laboratory testing capacity was lagging for several weeks after the COVID-19 outbreak in Italy (6). While our regional government focused on hospitals and locating available ICU beds, Lombardy's nursing homes were in many ways left to fend for themselves with no access to RT-PCR testing for weeks. This has negatively interfered with the ability to contain the spread of disease.
The size of a care home is related to the probability of viral contamination, in relation to the number of residents and staff and the presence/ absence of shift workers. This seems confirmed by our results, but the small number of care homes studied does not allow significant conclusion.
The structure of the building itself and the proportion of available single rooms are determinant factors in containment of infection. Although the data of our three nursing homes do not provide confirmation of this hypothesis, a greater representative sample size would be needed to assess it.
The high rate of infected staff in close contact with residents is probably linked to lack of using PPE during the initial days of the outbreak, when no cases had yet been identified or no PPE was available.
Tracing contacts and clarifying the chain of viral transmission in the confined crowded environment of a nursing home might be difficult (35), especially in the absence of laboratory testing capacity. The quick viral spread observed amongst residents with wandering dementia, should be considered when planning strategies to contain the spread of the disease. Moreover, the scientific community has been questioning the ethical implications of implementing testing and restrictive measures such as isolation for residents with dementia. These people are unable to understand the significance of such measures that deeply modify their daily routine (39–41). It is well-documented that reduced social interactions have a greater impact on the residual capacities of these subjects compared to the general population. Therefore, future guidelines relating to restrictive measures must also take this into consideration (22).
Our study highlights the difficulty to gain access to RT-PCR for SARS-CoV-2 infection at the beginning of the pandemics in Italy, the potential contribution of clinical score associated with RT-PCR for SARS-CoV-2 infection diagnosis, and the usefulness of rapid serology tests to assess the level of the COVID-19 outbreak diffusion in nursing homes.
Our data show that a combined strategy with nasopharyngeal swabs, rapid on-site serology and accurate anamnestic monitoring has contributed to reveal the real diffusion of SARS-CoV-2. Our approach has increased the awareness of a previous infection in people that could not get a virology test or had a false negative in the past, clarifying the chain of transmission and identifying symptomatic and asymptomatic persons actually carrying the virus.
The contribution of rapid serology tests in unveiling previous infections is now less in the more developed countries where vaccines have been already administered to the people in nursing and care homes. However, our proposed triple screening strategy could still be a valuable option for daily practice in countries with reduced or no national health service. It could also guide the prioritization of the more vulnerable residents for access to vaccines or the decision to omit the second dose in case of a previous documented immunization (42).
However, continuous monitoring of symptoms and implementation of protective measures is mandatory given the frequent appearance of new variants of SARS-CoV-2 worldwide that might escape the protection provided by the vaccines currently available.
Conclusions
Although escaping infection so far, vulnerable residents and staff of nursing homes are still at risk for COVID-19, especially in countries where the availability of laboratory testing is not meeting the demand of a regular monitoring strategy and vaccines are not yet available.
Our data show that an integrated approach with continuous monitoring of symptoms, coupled with periodic rapid on-site antigen and antibody testing of residents and staff could be part of a strategy surveillance system to increase protection. This is particularly relevant where laboratory testing for SARS-CoV-2 is not available or not in keeping with the international recommendations for timely identification and control of outbreaks2.
Additional studies addressing the performance of point-of-care tests in long-term facilities are urgently needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was reviewed and approved by the Ethics Committee of Spedali Civili of Brescia (NP 4378). The patients/participants or their relatives provided their written informed consent to participate in this research.
Author Contributions
AS and EM prepared the manuscript, performed rapid serological tests, clinical assessment, and analyzed the results. SC performed the statistical analysis. GG and VR provided medical care, clinical information of residents, supplied RT-PCR test results of residents, and staff included in the study. All authors contributed feedback, revisions of the manuscript draft, read, and approved the final manuscript.
Funding
The cost for serology tests has been met mostly from voluntary donations. Some residual funding from previous different research studies was also used.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Maria Giuseppina Mazzocchi, who helped with identifying relevant research underpinning this article and Miss Susan Gunning, who helped to improve the English text, all staff and residents of the three care homes, their families and all those who have made this study possible.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.649524/full#supplementary-material
Supplementary Figure 1. Flowchart of triple screening study. RES, Residents; HCW, Health Care Workers; SEROL, Serology (+ positive; − negative); clin neg, Below the threshold of clinical assessment (score 0–1); PREV RT-PCR, Previous RT-PCR (+ positive; − negative; N/A Not Available).
Supplementary Table 1. List of symptoms and their prevalence in 50 HCW and 64 residents with proven positivity for SARS-CoV2 (PCR and/or serology positive).
Footnotes
1. ^https://www.istat.it/it/files//2020/08/ReportPrimiRisultatiIndagineSiero.pdf (accessed on August 4, 2020).
2. ^https://www.ecdc.europa.eu/sites/default/files/documents/Infection-prevention-and-control-in-healthcare-settings-COVID-19_5th_update.pdf (accessed on May 5, 2021).
References
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
2. Lloyd-Sherlock P, Ebrahim S, Geffen L, McKee M. Bearing the brunt of covid-19: older people in low and middle income countries. BMJ. (2020) 368:m1052. doi: 10.1136/bmj.m1052
3. Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet (London, England). (2020) 395:931–4. doi: 10.1016/S0140-6736(20)30567-5
4. Thompson DC, Barbu MG, Beiu C, Popa LG, Mihai MM, Berteanu, et al. The impact of COVID-19 pandemic on long-term care facilities worldwide: an overview on international issues. BioMed Res Int. (2020) 2020:8870249. doi: 10.1155/2020/8870249
5. Ciminelli G, Garcia-Mandicó S. COVID-19 in Italy: an analysis of death registry data. J Public Health (Oxford, England). (2020) 42:723–30. doi: 10.1093/pubmed/fdaa165
6. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet (London, England). (2020) 395:1225–8. doi: 10.1016/S0140-6736(20)30627-9
7. Stowell SR, Guarner J. Role of serology in the coronavirus disease 2019 pandemic. Clin Infect Dis. (2020) 71:1935–6. doi: 10.1093/cid/ciaa510
8. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. (2020) 34:101623. doi: 10.1016/j.tmaid.2020.101623
9. Dong X, Cao YY, Lu XX, Zhang JJ, Du H, Yan YQ, et al. Eleven faces of coronavirus disease 2019. Allergy. (2020) 75:1699–709. doi: 10.1111/all.14289
10. Siordia JA Jr. Epidemiology and clinical features of COVID-19: a review of current literature. J Clin Virol. (2020) 127:104357. doi: 10.1016/j.jcv.2020.104357
11. Wang HY, Li XL, Yan ZR, Sun XP, Han J, Zhang BW. Potential neurological symptoms of COVID-19. Ther Adv Neurol Disord. (2020) 13:1756286420917830. doi: 10.1177/1756286420917830
12. Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. (2020) 92:1518–1524. doi: 10.1002/jmv.25727
13. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. (2020) 173:362–7. doi: 10.7326/M20-3012
14. Heudorf U, Müller M, Schmehl C, Gasteyer S, Steul K. COVID-19 in long-term care facilities in Frankfurt am Main, Germany: incidence, case reports, lessons learned. GMS Hyg Infect Control. (2020) 15:Doc26. doi: 10.3205/dgkh000361
15. Graham N, Junghans C, Downes R, Sendall C, Lai H, McKirdy A, et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. (2020) 81:411–9. doi: 10.1016/j.jinf.2020.05.073
16. Blain H, Rolland Y, Benetos A, Giacosa N, Albrand M, Miot S, et al. Atypical clinical presentation of COVID-19 infection in residents of a long-term care facility. Eur Geriatr Med. (2020) 11:1085–8. doi: 10.1007/s41999-020-00352-9
17. Blain H, Gamon L, Tuaillon E, Pisoni A, Giacosa N, Albrand M, et al. Atypical symptoms, SARS-CoV-2 test results and immunisation rates in 456 residents from eight nursing homes facing a COVID-19 outbreak. Age Ageing. (2021) 50:641–8. doi: 10.1093/ageing/afab050
18. Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. (2020) 382:2081–90. doi: 10.1056/NEJMoa2008457
19. McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, et al. Epidemiology of covid-19 in a long-term care facility in King County, Washington. N Engl J Med. (2020) 382:2005–11. doi: 10.1056/NEJMoa2005412
20. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles' heel of current strategies to control covid-19. N Engl J Med. (2020) 382:2158–60. doi: 10.1056/NEJMe2009758
21. Roxby AC, Greninger AL, Hatfield KM, Lynch JB, Dellit TH, James A, et al. Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med. (2020) 180:1101–5. doi: 10.1001/jamainternmed.2020.2233
22. Blain H, Rolland Y, Schols J, Cherubini A, Miot S, O'Neill D, et al. August 2020 interim EuGMS guidance to prepare European long-term care facilities for COVID-19. Eur Geriatr Med. (2020) 11:899–913. doi: 10.1007/s41999-020-00405-z
23. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. (2020) 323:1843–4. doi: 10.1001/jama.2020.3786
24. Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. (2020) 71:2027–34. doi: 10.1093/cid/ciaa344
25. Abbasi J. The promise and peril of antibody testing for COVID-19. JAMA. (2020) 323:1881–3. doi: 10.1001/jama.2020.6170
26. Walensky RP, Del Rio C. From mitigation to containment of the COVID-19 pandemic: putting the SARS-CoV-2 genie back in the bottle. JAMA. (2020) 323:1889–90. doi: 10.1001/jama.2020.6572
27. Pallett S, Rayment M, Patel A, Fitzgerald-Smith S, Denny SJ, Charani, et al. Point-of-care serological assays for delayed SARS-CoV-2 case identification among health-care workers in the UK: a prospective multicentre cohort study. Lancet Respir Med. (2020) 8:885–94. doi: 10.1016/S2213-2600(20)30315-5
28. Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. (2020) 8:CD013705. doi: 10.1002/14651858.CD013705
29. Peeling RW, Wedderburn CJ, Garcia PJ, Boeras D, Fongwen N, Nkengasong, et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. (2020) 20:e245–9. doi: 10.1016/S1473-3099(20)30517-X
30. Tuaillon E, Bolloré K, Pisoni A, Debiesse S, Renault C, Marie, et al. Detection of SARS-CoV-2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J Infect. (2020) 81:e39–45. doi: 10.1016/j.jinf.2020.05.077
31. Demey B, Daher N, François C, Lanoix JP, Duverlie G, Castelain S, et al. Dynamic profile for the detection of anti-SARS-CoV-2 antibodies using four immunochromatographic assays. J Infect. (2020) 81:e6–10. doi: 10.1016/j.jinf.2020.04.033
32. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet (London, England). (2020) 396:535–44. doi: 10.1016/S0140-6736(20)31483-5
33. Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, Nielsen PB, Pries-Heje M, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. (2020) 20:1401–8. doi: 10.1016/S1473-3099(20)30589-2
34. Hoffman T, Nissen K, Krambrich J, Rönnberg B, Akaberi D, Esmaeilzadeh, et al. Evaluation of a COVID-19 IgM and IgG rapid test; an efficient tool for assessment of past exposure to SARS-CoV-2. Infect Ecol Epidemiol. (2020) 10:1754538. doi: 10.1080/20008686.2020.1754538
35. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. (2020) 383:e38. doi: 10.1056/NEJMp2015897
36. Van Elslande J, Houben E, Depypere M, Brackenier A, Desmet S, André, et al. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect. (2020) 26:1082–7. doi: 10.1016/j.cmi.2020.05.023
37. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. (2020) 26:845–8. doi: 10.1038/s41591-020-0897-1
38. Yong S, Anderson DE, Wei WE, Pang J, Chia WN, Tan CW, et al. Connecting clusters of COVID-19: an epidemiological and serological investigation. Lancet Infect Dis. (2020) 20:809–15. doi: 10.1016/S1473-3099(20)30273-5
39. Fahed M, Barron GC, Steffens DC. Ethical and logistical considerations of caring for older adults on inpatient psychiatry during the COVID-19 pandemic. Am J Geriatr Psychiatry. (2020) 28:829–34. doi: 10.1016/j.jagp.2020.04.027
40. Iaboni A, Cockburn A, Marcil M, Rodrigues K, Marshall C, Garcia MA, et al. Achieving safe, effective, and compassionate quarantine or isolation of older adults with dementia in nursing homes. Am J Geriatr Psychiatry. (2020) 28:835–8. doi: 10.1016/j.jagp.2020.04.025
41. Liddell K, Ruck Keene A, Holland A, Huppert J, Underwood BR, Clark O, et al. Isolating residents including wandering residents in care and group homes: Medical ethics and English law in the context of Covid-19. Int J Law Psychiatry. (2021) 74:101649. doi: 10.1016/j.ijlp.2020.101649
Keywords: COVID-19, SARS–CoV−2, asymptomatic transmission, nursing home, on-site testing, COVID-19 symptoms, RT-PCR screening, rapid serological assays
Citation: Savio A, Calza S, Guerrini G, Romano V and Marchina E (2021) Rapid Point-Of-Care Serology and Clinical History Assessment Increase Protection Provided by RT-PCR Screening: A Pilot Study Involving Three Nursing Homes in Brescia, a Hotspot of Lombardy. Front. Public Health 9:649524. doi: 10.3389/fpubh.2021.649524
Received: 04 January 2021; Accepted: 27 May 2021;
Published: 24 June 2021.
Edited by:
Chiara de Waure, University of Perugia, ItalyReviewed by:
Xiaojiong Jia, Harvard Medical School, United StatesStéphanie Miot, INSERM U1178 Santé Mentale et Santé Publique, France
Copyright © 2021 Savio, Calza, Guerrini, Romano and Marchina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonella Savio, c2F2aW9hbnRvbmVsbGFAZ21haWwuY29t
 Antonella Savio
Antonella Savio Stefano Calza
Stefano Calza Gianbattista Guerrini3
Gianbattista Guerrini3 Valentina Romano
Valentina Romano Eleonora Marchina
Eleonora Marchina