- 1Department of Dentistry, School of Medicine, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 2Department of Human Anatomy, School of Medicine, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Background: Dental caries affects mastication, growth and development, and school attendance and has a long-term psychological effect on affected individuals. In developing countries, the prevalence of dental caries is increasing due to the growing consumption of sugary foods, poor tooth brushing habits, and a low level of awareness about dental caries. Even if there was a high prevalence of dental caries in sub-Saharan Africa, there is a paucity of data on the prevalence of dental caries in East Africa. Hence, this study aimed to determine the prevalence of dental caries and associated factors in East Africa.
Methods: A systematic search of articles was conducted in MEDLINE, Scopus, and Google Scholar using all the synonyms of dental caries in published literature (until December 2020) in East Africa. Important data were extracted using a standardized data extraction form prepared in Excel. Stata software (version 14.0) was used to calculate the pooled prevalence of dental caries. Besides, subgroup analysis was done based on country and dentition type. Moreover, associated factors of dental caries were assessed and the overall effect was presented in the form of odds ratios. The quality of the included studies was evaluated using the Joanna Briggs Institute reviewers' manual.
Results: The overall pooled prevalence of dental caries was found to be 45.7% (95% CI = 38.0–53.4). The pooled prevalence was high in Eritrea (65.2%, 95% CI = 49.2–81.1), followed by Sudan (57.8%, 95% CI = 36.0–79.7), and a low prevalence was found in Tanzania (30.7%, 95% CI = 21.5–39.9). Moreover, the subgroup analysis revealed a prevalence of 50% (95% CI = 38.4–62.1) in permanent dentition and 41.3% (95% CI = 33.5–49.2) in mixed dentition. The overall mean decayed, missed, and filled permanent (DMFT) and primary (dmft) teeth were 1.941 (95% CI = 1.561–2.322) and 2.237 (95% CI = 1.293–3.181), respectively. High DMFT scores were reported in Sudan (3.146, 95% CI = 1.050–5.242) and Uganda (2.876, 95% CI = 2.186–3.565). Being female (OR = 1.34, 95% CI = 1.24–1.46) and having poor tooth brushing habit (OR = 1.967, 95% CI = 1.67–2.33) were independent risk factors of dental caries.
Conclusion: The overall prevalence of dental caries was comparatively high. Being female and poor oral health practice were independent risk factors of dental caries. The Ministry of Health of the member countries, along with dental associations of each country, ought to offer due attention to strengthen the oral health program in schools and primary health care centers and the implementation of school water fluoridation.
Background
Dental caries is a bacterial infectious disease that affects the calcified tissue of the tooth and causes dissolution of the organic component and demineralization of the inorganic portion (1). It is caused by bacterial plaque deposition on the surface of the tooth (2, 3), and frequent consumption of fermentable carbohydrates facilitates the progression of cavitation. Oral microbes such as Streptococcus mutans metabolize fermentable carbohydrates and produce lactic acid, which lowers the oral pH to a level where the minerals of dentin and enamel dissolve easily (4–7).
Dental caries is a global public health problem and affects all human race (8). The treatment cost in low-income countries alone exceeds the total child health care cost (9). This disease is found in all socioeconomic strata and affects the quality of life, school attendance, eating practices, growth, and the development of children and has psychological impacts on the performance of patients (3–5). In developing countries, dental caries remains untreated due to inappropriate, unaffordable, and unavailable dental services and to the scarcity of professionals (10). Moreover, dental caries costs US $298 billion in direct treatment costs to the global economy, 4.6% of the global health budget, and 144 billion losses due to loss of productivity (11).
In developed countries, the prevalence of dental caries is declining due to advanced dental facilities and the increased awareness of oral hygiene (10, 12–14). However, an unprecedented increase in prevalence is reported in developing countries due to the growing consumption of sugary foods, poor tooth brushing habits, and the absence of adequate dental services (10, 14–16). In developing countries, especially sub-Saharan Africa, the prevalence of dental caries varies according to the population group and socioeconomic status (12). The prevalence rates were 40.98% in Ethiopia (17), 52.4% in Sudan (18), 50.3% in Kenya (19), and 40.2% in Tanzania (20).
In East African countries, there is scarcity of data on the prevalence of dental caries. Hence, this study aimed to determine the pooled prevalence of dental caries and associated factors in East Africa.
Methods and Materials
The results of the present review are reported according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guideline (21).
Inclusion and Exclusion Criteria
Studies that meet the following inclusion criteria were included in the systematic review and meta-analysis.
• Observational studies were done on the prevalence of dental caries and associated factors in East African countries.
• Data regarding dental caries in terms of proportion or DMFT/dmft
• Full-text published articles
• Studies that did not report specific outcomes quantitatively
• Abstracts, case reports, review articles, comments, posters, editorial reviews, and letters to the editor were excluded.
Search Strategy
A systematic search of studies was carried out in MEDLINE, Scopus, and Google Scholar databases without language restriction and included studies published up to December 2020. Article search was done using key terms. Besides, the reference lists of all relevant studies were screened. The search strategy was built using a combination of keywords for the main axes of the research questions. The search strategy used key terms related to (a) dental caries, tooth decay, or DMFT and (b) East Africa countries (Ethiopia, Djibouti, Somalia, Eritrea, Kenya, Burundi, Tanzania, Sudan, South Sudan, Rwanda, and Uganda). The search terms were predefined to allow a comprehensive search that includes text fields within records and Medical Subject Headings (MeSH terms) were used to help expand the search. The MeSH terms used for Scopus were: {(Dental Caries OR caries [Mesh] OR Tooth decay OR DMFT index [Mesh] OR decayed teeth) AND (risk factors [Mesh])}.
Study Selection
Database search results were pooled and duplicate studies were removed using Endnote and manually. After the duplicates were removed, the titles and abstracts were screened and studies that were irrelevant to the research question and outcome of the study were excluded. Full-text studies that have outcomes of interest were evaluated further using the inclusion criteria. Two dental surgeons (AT and BG) independently screened information at each stage. Disagreement was resolved by the involvement of a third independent reviewer (AM).
Data Extraction and Data Items
Data such as the first author's name, country, age, study design, sample size, and the prevalence of dental caries or decayed, missed, and filled permanent (DMFT)/primary (dmft) teeth were extracted from the selected studies by two investigators (AT and BG) independently. Important data were extracted using a standardized data extraction form prepared in Excel. The pooled prevalence or DMFT/dmft was extracted. Any disagreement between the two reviewers was resolved by discussion and consensus.
Language translator was used to translate articles published in other languages, which were translated into English, and to afford conditions for data extraction. In the case of missing data, a one-time contact was attempted to obtain the missing information from the corresponding author via e-mail.
Quality Assessment
The quality of evidence was assessed using the Joanna Briggs Institute (JBI) reviewers' manual for a systematic review of prevalence and incidence studies. The reviewers critically appraised the quality of the studies based on sample representativeness, participant recruitment, sample size estimation, reliability of the measurement, and the analysis of the outcomes. Studies with a quality assessment score of 50% and above were included in the review.
Data Analysis
Stata software version 14.0 (22) was used to determine the pooled estimates. The prevalence of dental caries was determined for individual studies and then the prevalence ratio (PR) was calculated considering 95% confidence interval (CI). The analysis was performed using the random effect model (Mantel–Haenszel model) (23). The extent and the significance of variations between the selected studies were determined by calculating the heterogeneity using Higgins' I2 statistics (24). Substantial heterogeneity was considered when P < 0.10 for the Q test and I2 ≥ 50%. Publication bias was assessed using the funnel plot and Egger's test (25). Subgroup analyses were performed based on country and dentition status. Besides, a sensitivity analysis (26) was done to determine the influential studies on the overall prevalence ratio. Moreover, associated factors of dental caries were assessed and the overall effect was determined in the forms of the odds ratios.
Results
Study Selection
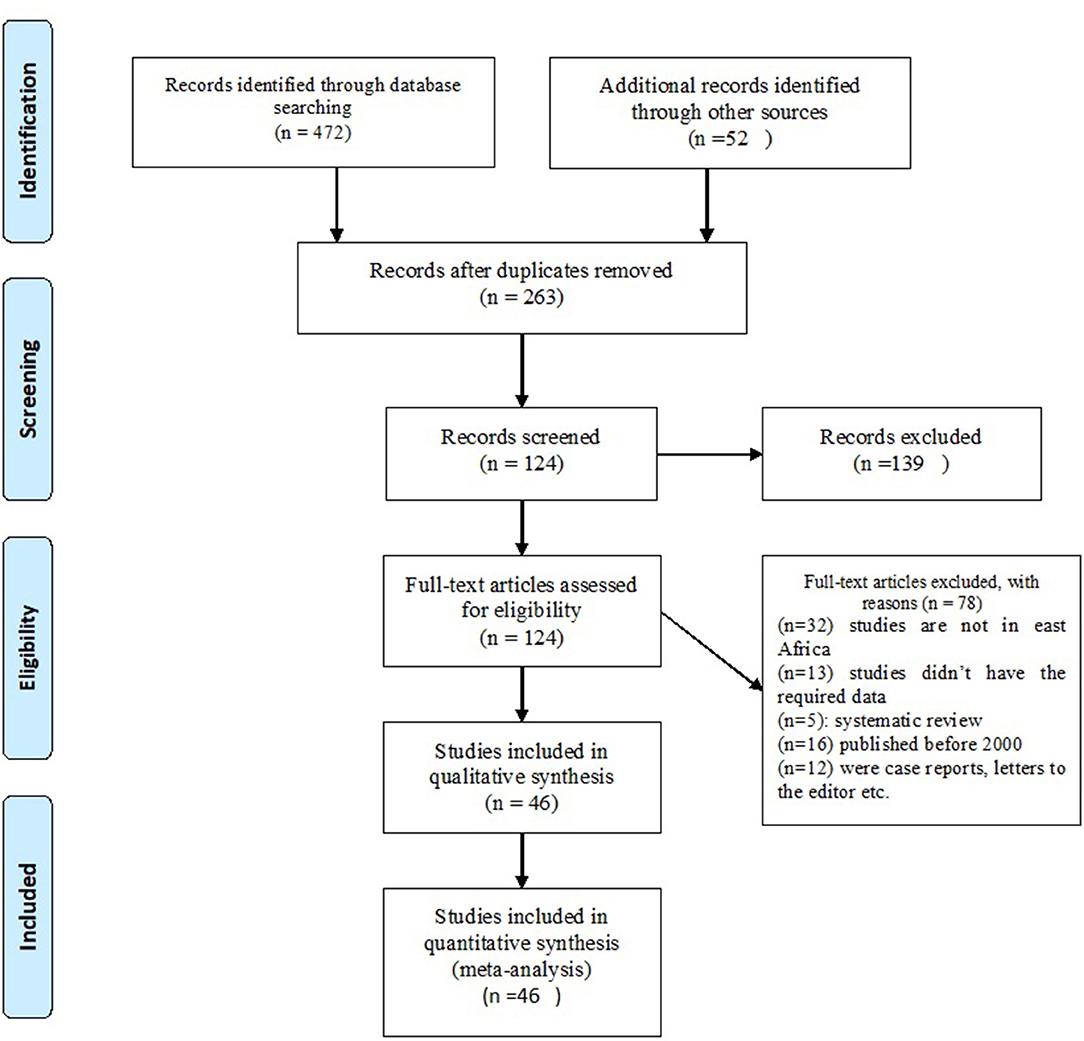
As shown in the flow diagram (Figure 1), 524 studies were searched from all databases. Of which, 261 were excluded as duplicates using Endnote 7 software and manually.
The remaining 263 studies were filtered according to the titles and abstracts; 139 studies were excluded due to unrelated themes. A full-text review was done for the remaining 124 studies and identified 44 studies that meet the inclusion criteria for this review.
Study Characteristics
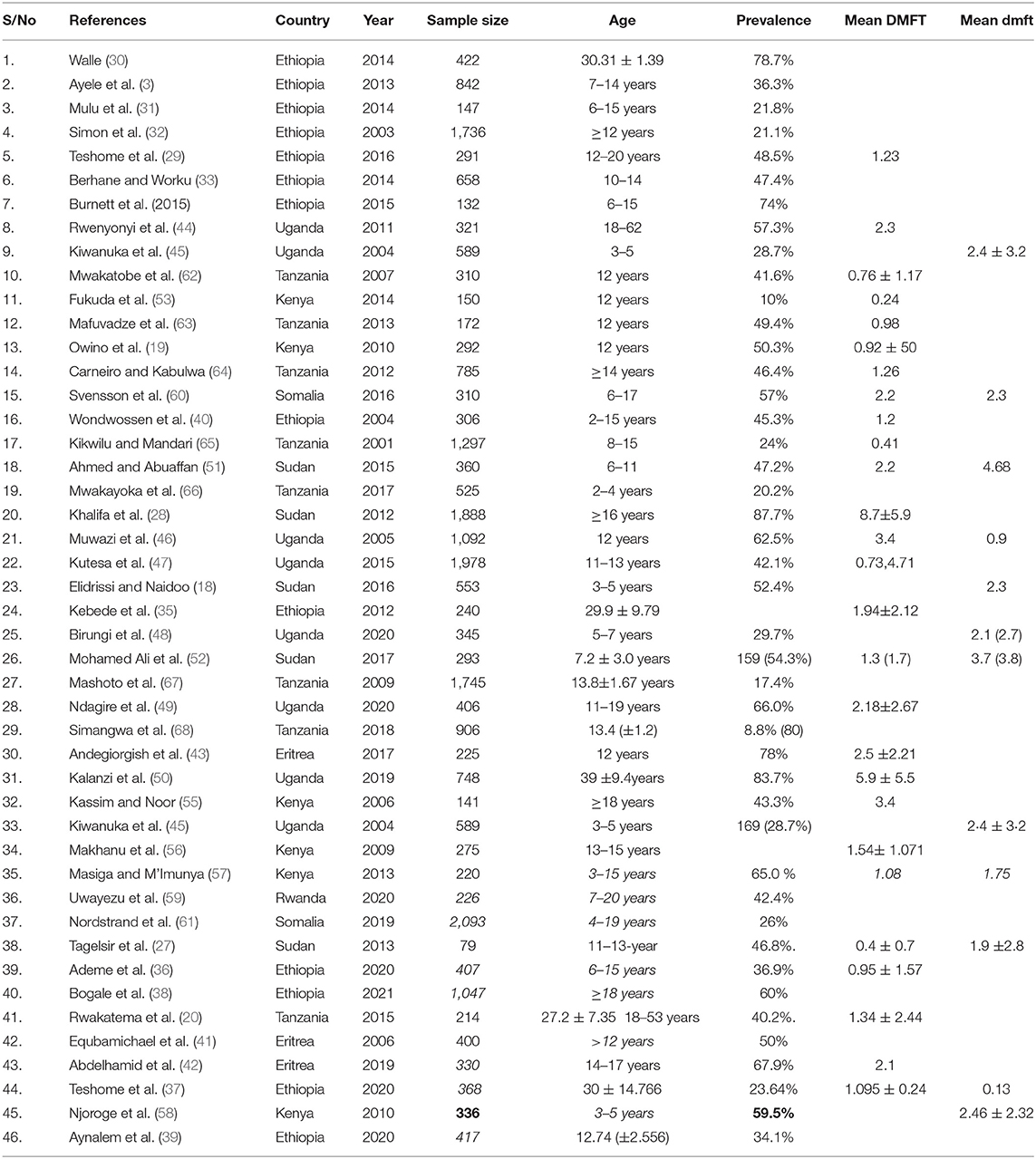
Forty-six studies meet the inclusion criteria, with 27,206 population. The sample sizes of the studies ranged from 79 (27) to 1,888 (28) study participants. The studies were conducted in Ethiopia (3, 29–40), Eritrea (41–43), Tanzania (26–33), Uganda (44–50), Sudan (18, 27, 28, 51, 52), Kenya (53–58), Rwanda (59), and Somalia (60, 61). All of the included studies were conducted between 2000 and 2020 (Table 1).
Prevalence of Dental Caries
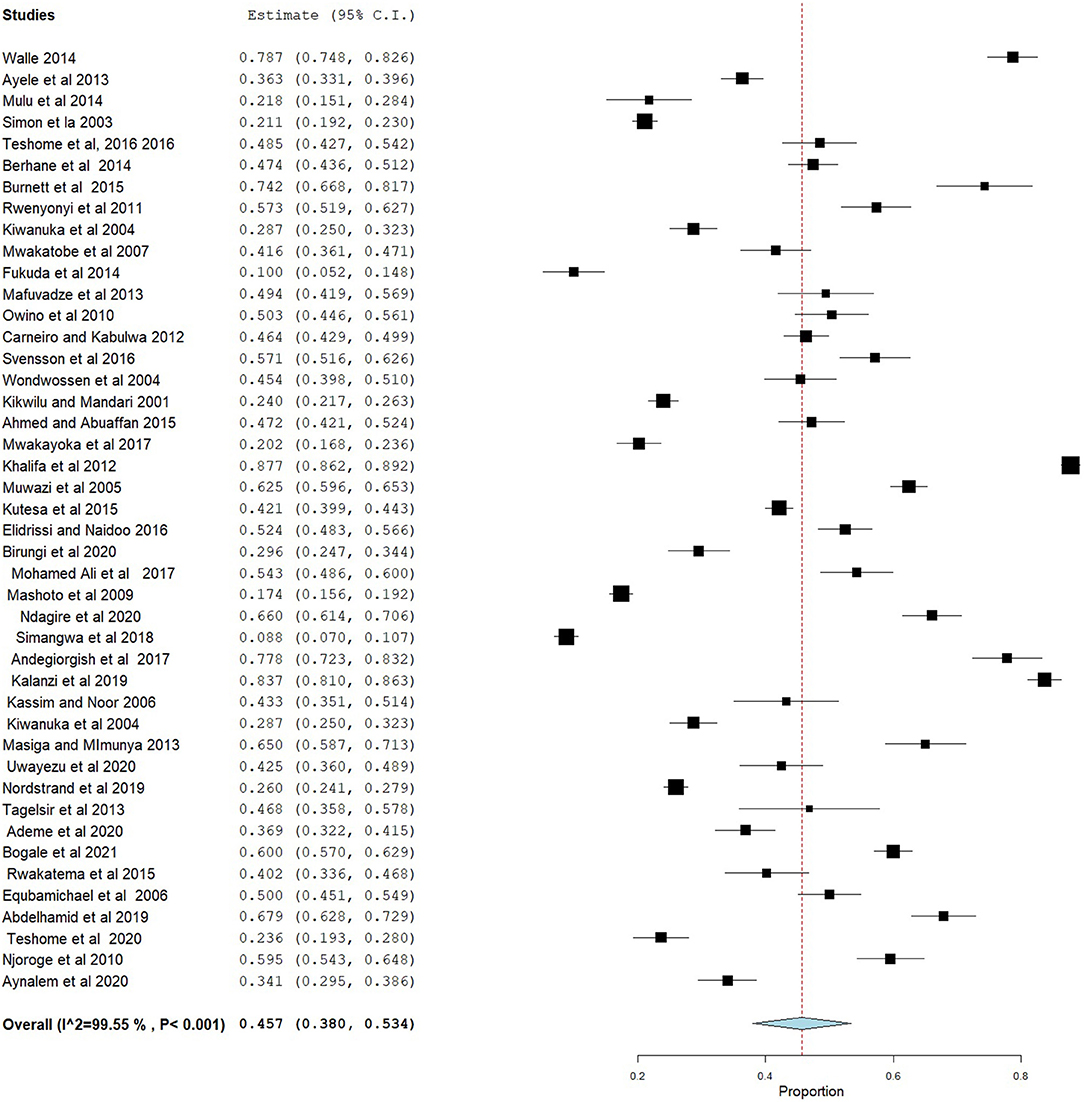
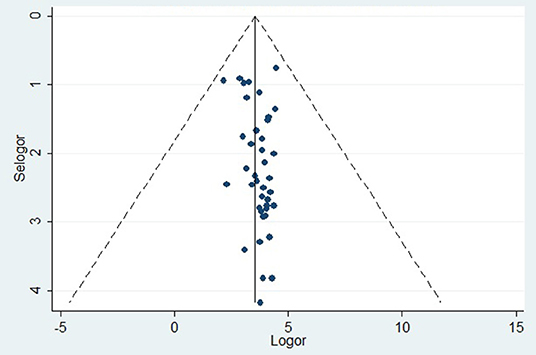
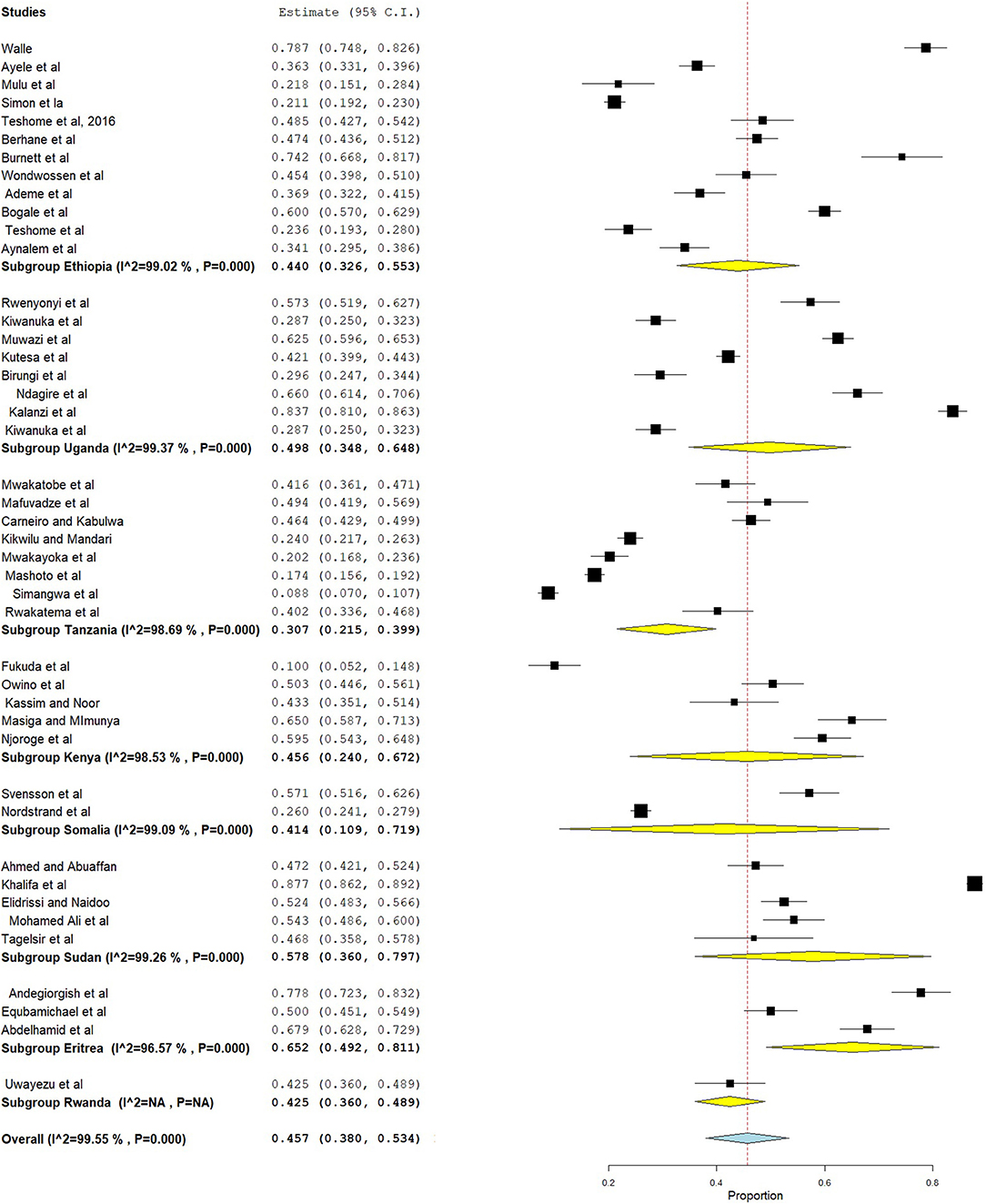
The prevalence of dental caries in this review ranged from 8.8% (68) to 87.7% (28). The DMFT of the region ranged from 0.24 (53) to 8.70 (28). Forty-four studies were included in the analysis of the pooled prevalence of dental caries. High heterogeneity was observed between the included studies, with an I2 value of 99.55%, and a random effect model was used. The overall pooled prevalence of dental caries in East Africa was found to be 45.7% (95% CI = 38.0–53.4), with heterogeneity (I2) of 99.55% (Figure 2). The funnel plot demonstrated a symmetrical distribution of the included studies (Figure 3). Moreover, the Begg's test and Egger's test showed the absence of publication bias (Pr > |z| = 0.398) and P = 0.155, respectively.
Subgroup Analysis
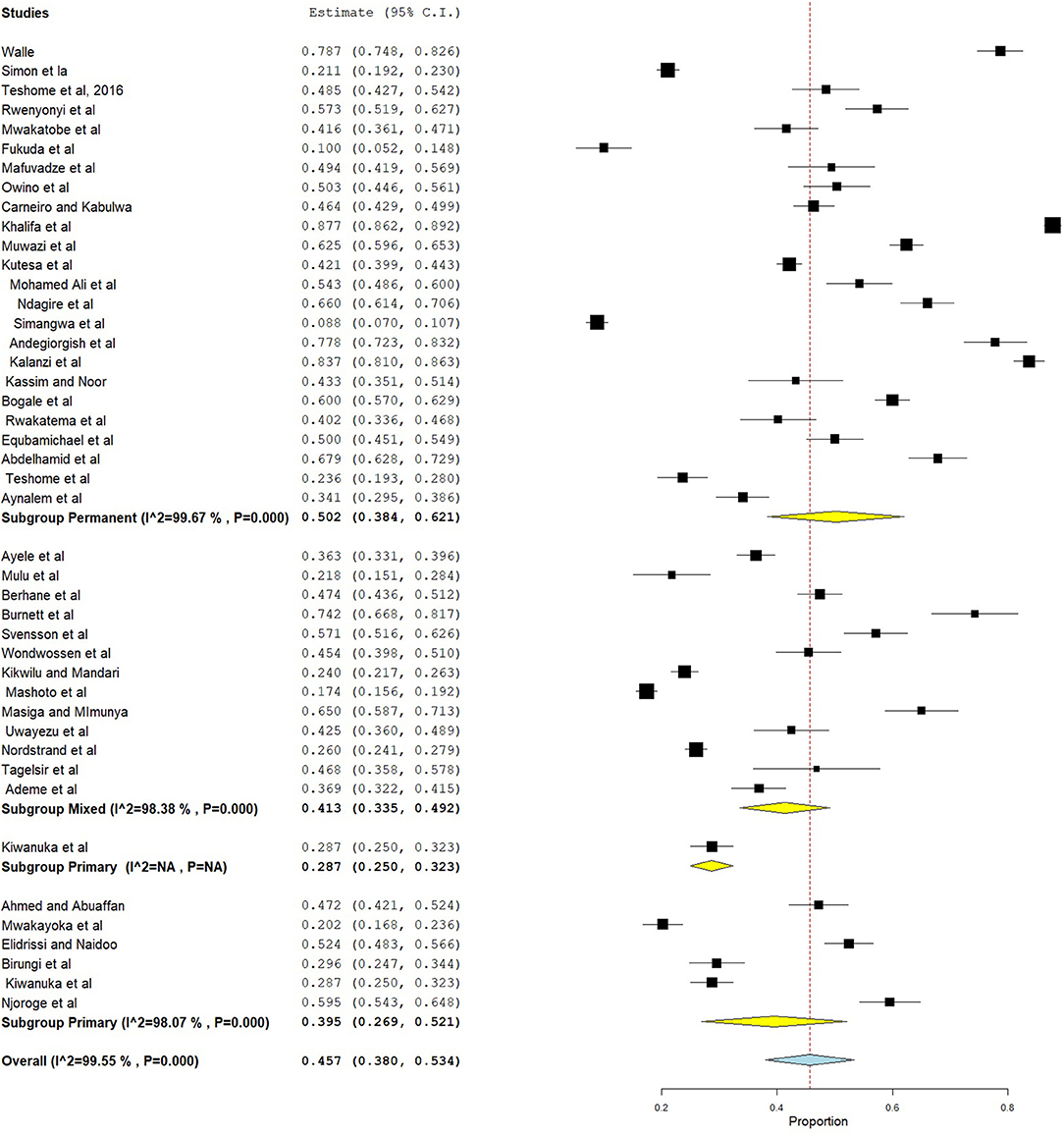
In this systematic review and meta-analysis, subgroup analysis was done based on country and dentition status (primary, mixed, or permanent dentition). Hence, the highest prevalence of dental caries was found in Eritrea, which was 65.2% (95% CI = 49.2–81.1), followed by Sudan (57.8%, 95% CI = 36.0–79.7) (Figure 4). Moreover, subgroup analysis revealed that the pooled prevalence of dental caries was high in those ≥12 years (50.2%, 95% CI = 38.4–62.1), followed by those 6–12 years (41.3%, 95% CI = 33.5–49.2) (Figure 5).
Sensitivity Analysis
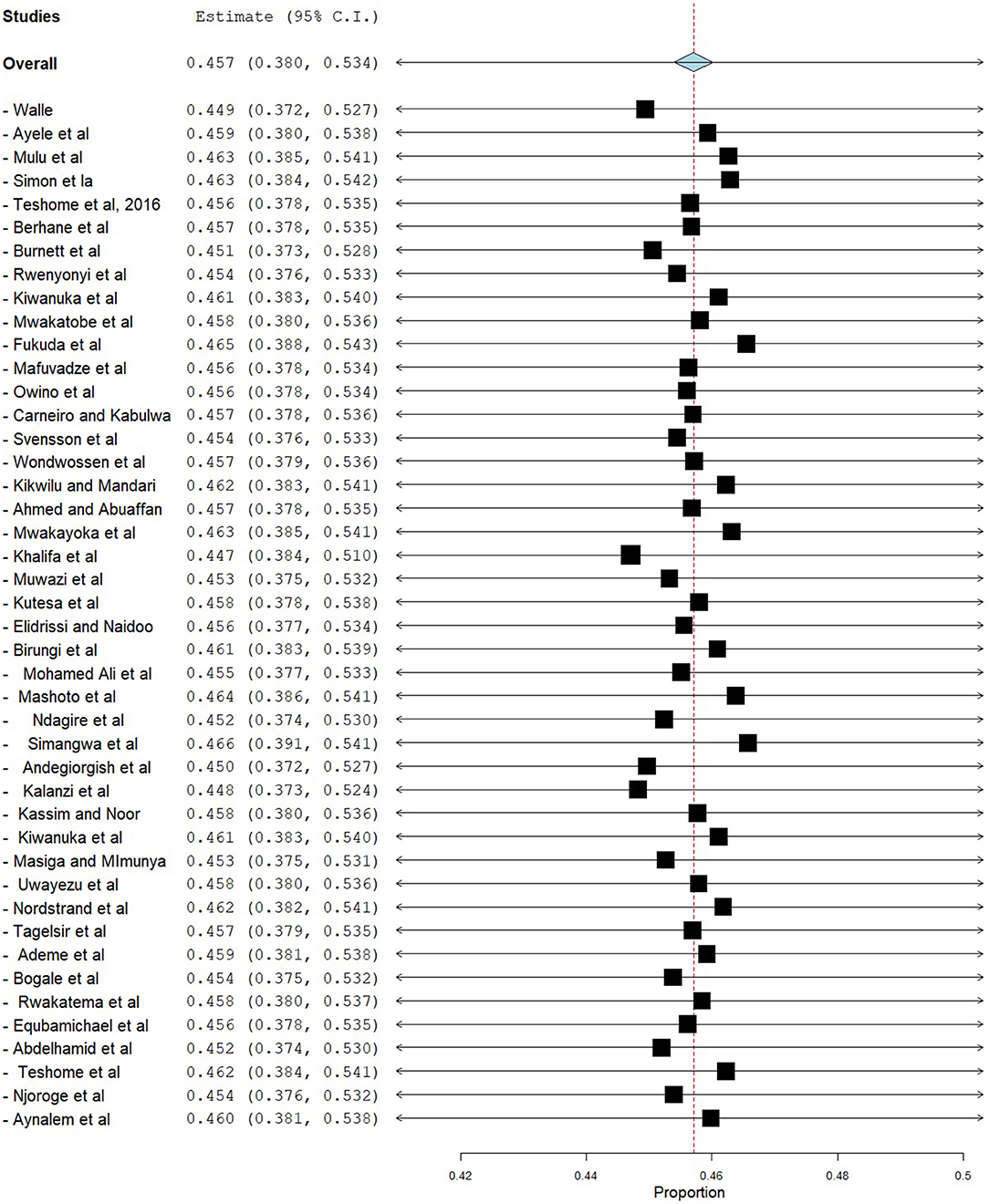
Sensitivity analysis was done using the leave-one-out method to identify the source of heterogeneity and found that the pooled prevalence did not depend on the outcome of a single study. After the removal of one study stepwise, the pooled prevalence ranged from 44.7% (95% CI = 38.4–51.0) to 46.6% (95% CI = 39.1–54.1) (Figure 6).
Decayed, Missed, and Filled Tooth and dmft
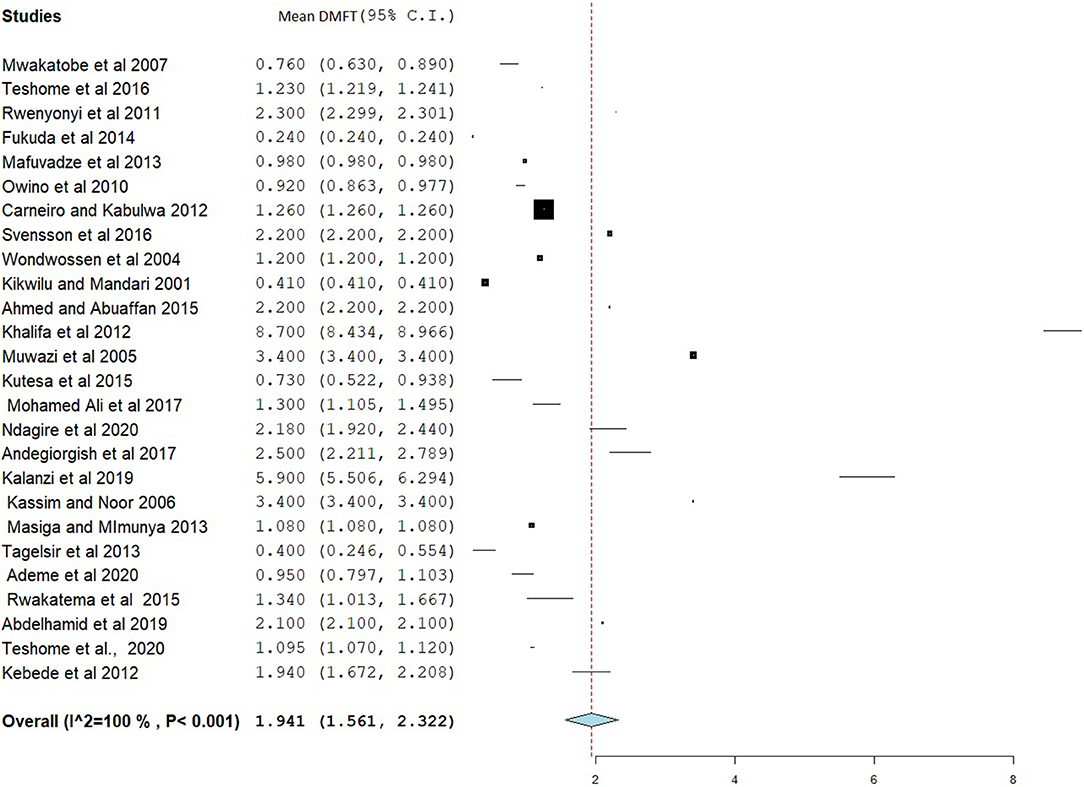
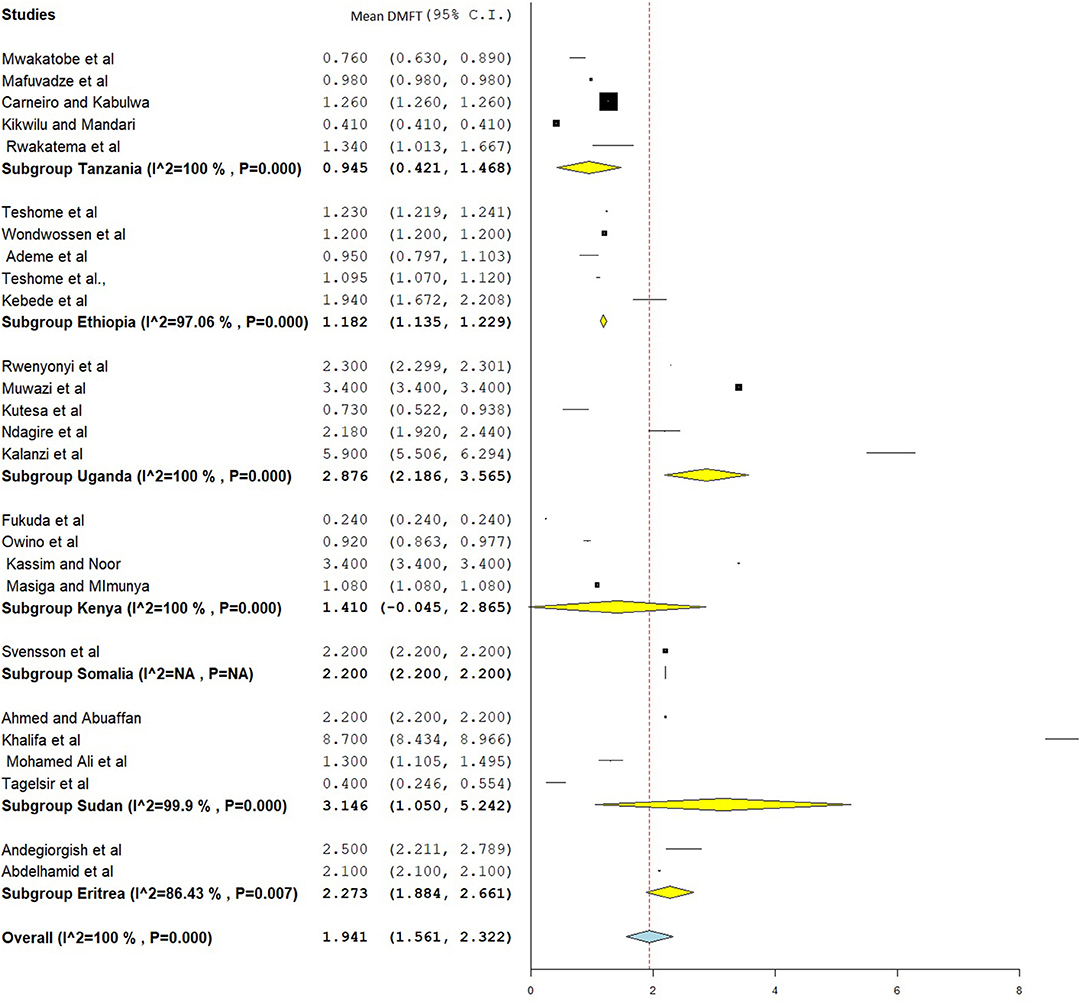
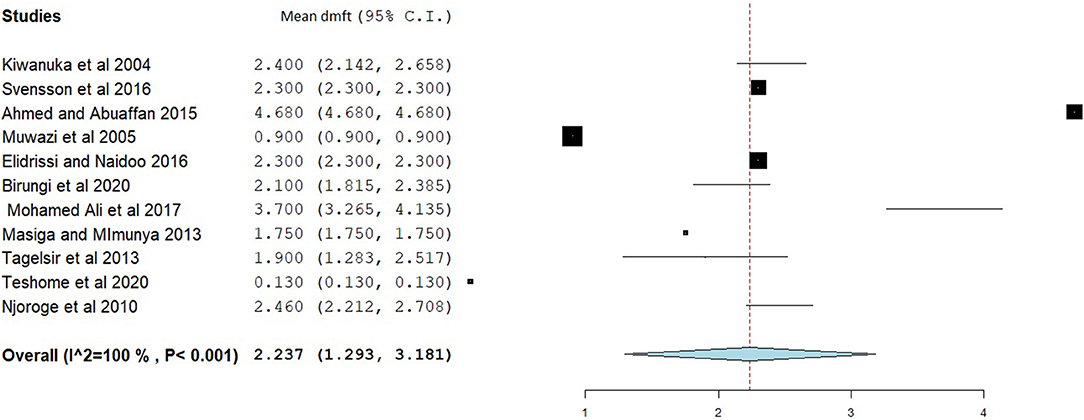
The overall mean DMFT score was 1.941 (95% CI = 1.561–2.322) (Figure 7). Subgroup analysis showed that the mean DMFT were 3.146 (95% CI = 1.050–5.242) in Sudan, 2.876 (95% CI = 2.186–3.565) in Uganda, 2.273 (95% CI = 1.884–2.661) in Eritrea, and 1.182 (95% CI = 1.135–1.229) in Ethiopia (Figure 8). Moreover, the mean decayed, missed, and filled teeth in primary dentition (dmft) were 2.237 (95% CI = 1.293–3.181) (Figure 9).
Factors Associated With Dental Caries
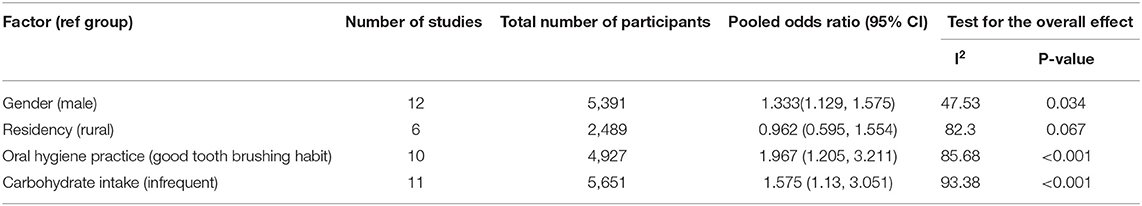
In this review, carbohydrate intake, oral hygiene practice, residency, and gender were tested for association with dental caries, and a separate meta-analysis was done for each variable. A total of 11 studies were included to estimate the association between sugary food intake and dental caries, and the pooled odds ratios showed that there was a statistically significant association between carbohydrate intake and dental caries (OR = 1.575, 95% CI = 1.13–3.051).
To determine the association between gender and dental caries, 12 studies were included. The pooled odds ratios indicated that females were 1.333 times more likely to develop dental caries as compared with males (OR = 1.333, 95% CI = 1.129–1.575). Besides, the pooled ORs did not show a statistically significant association between residency and dental caries (OR = 0.962, 95% CI = 0.595–1.554).
Finally, we assessed the association between tooth brushing practice and dental caries using the random effect model. A total of 10 studies were included in the analysis, and the results revealed that those who had no tooth brushing practice were 1.967 times at risk of developing dental caries (OR = 1.967, 95% CI = 1.205–3.211) (Table 2).
Risk of Bias Within Studies
The quality of the selected studies was assessed based on the JBI critical appraisal tool (69), and studies with a quality assessment score of 50% and above were included in the review.
Discussion
Dental caries is one of the neglected health problems in developing countries, including East African countries. Estimating the pooled prevalence and associated factors of dental caries in East Africa may contribute to informing policy makers in the region to design interventions and remedial actions. To date, there are no comprehensive data on the prevalence of dental caries and associated factors in East Africa.
The present meta-analysis revealed that 45.7% (95% CI = 38.0–53.4) of people had dental caries in East Africa, which is similar to a meta-analysis done in Ethiopia (40.98%, 95% CI = 31.62–50.34) (17) and a study done in China (41.15%) (70). However, the estimate of dental caries in the present study was lower than those in studies done in Gulf countries (64.7%) (71), Brazil (72.9%) (72), Kosovo (72.80%) (73), and China (67%, 95% CI = 56.0–77.0) (74). This difference might be due to differences in socioeconomic, dietary habits, oral hygiene practices, and knowledge and attitude of oral health prevention programs in these countries (75). In the subgroup analysis, a high prevalence of dental caries was found in Eritrea (65.2%, 95% CI = 49.2–81.1), followed by Sudan (57.8%, 95% CI = 36.0–79.7), and a low prevalence in Tanzania (30.7%, 95% CI = 21.5–39.9). This difference might be due to socioeconomic and dental facility differences between the countries in the region.
A subgroup analysis revealed that the prevalence of dental caries was age-dependent, with pooled prevalences of 39.5% (95% CI = 26.9, 52.1) in primary dentition, 41.3% (95% CI = 33.5–49.2) in mixed dentition, and 50.2% (95% CI = 38.4–62.1, P = 0.000) in permanent dentition. This finding is in line with a national oral health survey done in China (76) and Palestine (77), where 55.3% and 54.35% of children above 12 years had dental caries, respectively. Moreover, a meta-analysis done in Eastern Mediterranean countries found prevalences of 65% (45–85%) in primary dentition, 66% (59–73%) in mixed dentition, and 70% (64–75%) in permanent dentition (78).
The pooled estimates showed that the mean DMFT in East Africa was 1.941 (95% CI = 1.561–2.322), which is similar to studies done in India (DMFT = 1.95) (79) and Iran (DMFT = 2.33, 95% CI = 2.12–2.54) (80). However, this result is low compared to studies done in Arab League countries (DMFT = 2.469, 95% CI = 2.019–2.919) (81), Gulf countries (DMFT = 2.57) (71), and the Saudi population (DMFT = 3.34) (82). A meta-analysis in Southeast Asian countries found a mean DMFT of 0.51, which is low compared to the present study. Subgroup analysis found a high DMFT in Sudan (DMFT = 3.146) and a low DMFT in Tanzania (DMFT = 0.945). This might be due to differences in the way of life between the populations of the countries involved in the study.
This study found a mean dmft of 2.237 (95% CI = 1.293–3.181) in primary dentition, which is low compared to studies done in Saudi Arabia (dmft = 5.38, 95% CI = 4.314–6.436) (82), Gulf State countries (dmft = 5.136 ± 0.038) (71), and Arab League countries (dmft = 4.341, 95% CI = 3.714–4.969). This difference might be due to socioeconomic status differences and dietary practice differences between the countries.
The pooled analysis showed that females were 33.3% more likely to develop dental caries than males (OR = 1.333, 95% CI = 1.24–1.46), which is in line with a study done in China (42.88 vs. 39.77%) (70). The higher prevalence of caries among females might be due to the earlier eruption of teeth in girls, easier access to food supplies by women, and frequent snacking during food preparation and pregnancy (83). Moreover, consumption of carbohydrates increases the chance of developing dental caries by 1.575 times (OR = 1.575, 95% CI = 1.13–3.051), which corresponds with studies done in Ethiopia (17), Kenya (84), and Brazil (85). This might be due to the easy fermentation of carbohydrates by cariogenic bacteria into lactic acid, which facilitates the dissolution and destruction of the hard tissue of the teeth.
The present study revealed that there was a statistically significant association between poor tooth brushing habits and dental caries. Participants with poor oral hygiene practice were 1.967 times at risk of developing dental caries (OR = 1.967, 95% CI = 1.205–3.211). This is similar to a study done in Spain (OR = 1.83, 95% CI = 1.07–3.15) (86). However, the result is incomparable to a previous study done in Ethiopia (OR = 0.71, 95% CI = 0.17–2.96). This might be because few studies were included in the pooled estimates of the previous study (17). Nevertheless, the present study did not find a statistically significant association between residency and dental caries (OR = 0.962, 95% CI = 0.595–1.554), which is against the results found in Spain (OR = 1.3, 95% CI = 1.02–1.80) (86) and Ethiopia (adjusted OR = 1.6, 95% CI = 1.2–4.3) (87), where urban residents are at high risk of developing dental caries than rural residents.
Strengths and Limitations of the Study
This study used multiple databases to search all the relevant studies for systematic review and meta-analysis. Moreover, the two reviewers, to minimize error, independently did data extraction. This was also the first meta-analysis in the region and provided baseline data on the prevalence of dental caries in East Africa. There was no language restriction.
The authors faced certain limitations during this study. Although we used a comprehensive search of articles, there is a scarcity of studies in some countries of the region. Secondly, most of the studies present the status of dental caries in terms of percentage, and only a few studies used DMFT/dmft, which is one of the indicators of the severity of the disease.
Conclusion
The overall prevalence of dental caries was comparatively high. Being female and having poor oral health practice were independent risk factors of dental caries. The Ministry of Health of the member countries, along with dental associations of each country, ought to offer due attention to strengthen the oral health programs in schools and primary health care centers and the implementation of school water fluoridation.
Author Contributions
AT contributed to the conceptualization, methodology, analysis, validation, writing the original draft, writing the final version, and editing. AM and BG helped with the methodology, analysis, validation, writing the original draft, writing the final version, and editing. All authors have read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the University of Gondar and all the authors of the original articles.
Abbreviations
CI, confidence interval; CINAHL, cumulative index to nursing and allied health literature; DMFT, decayed, missed, and filled permanent tooth; dmft, decayed, missed, and filled primary tooth; EMBASE, Excerpta Medica database; JBI, Jonna Briggs Institute; PRISMA, preferred reporting items for systematic reviews and meta-analysis; SEAR, South-East Asian Region.
References
1. Pakshir HR. Dental education and dentistry system in Iran. Med Princ Pract. (2003) 12 (Suppl. 1):56–60. doi: 10.1159/000069844
2. Maher R, Khan A, Rahimtoola S, Bratthall D. Prevalence of mutans streptococci and dental caries in Pakistani children. JPMA J Pak Med Assoc. (1992) 42:213–5.
3. Ayele FA, Taye BW, Ayele TA, Gelaye KA. Predictors of dental caries among children 7–14 years old in Northwest Ethiopia: a community based cross-sectional study. BMC Oral Health. (2013) 13:7. doi: 10.1186/1472-6831-13-7
4. Franco e Franco TCC, Amoroso P, Marin JM, de Avila FA. Detection of Streptococcus mutans and Streptococcus sobrinus in dental plaque samples from Brazilian preschool children by polymerase chain reaction. Braz Dent J. (2007) 18:329–33. doi: 10.1590/S0103-64402007000400011
5. Tinanoff N, Kanellis MJ, Vargas CM. Current understanding of the epidemiology mechanisms, and prevention of dental caries in preschool children. Pediatr Dent. (2002) 24:543–51.
6. Amoroso P, de Ávila FA, Gagliardi CM. Prevalence of different Streptococci species in the oral cavity of children and adolescents. Braz J Oral Sci. (2016) 2:164–8.
7. Oda Y, Hayashi F, Okada M. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in patients with intellectual disabilities. BMC Oral Health. (2015) 15:1–5. doi: 10.1186/s12903-015-0087-6
8. Al Agili DE. A systematic review of population-based dental caries studies among children in Saudi Arabia. Saudi Dent J. (2013) 25:3–11. doi: 10.1016/j.sdentj.2012.10.002
9. Kathmandu RY. The burden of restorative dental treatment for children in Third World countries. Int Dent J. (2002) 52:1–9. doi: 10.1111/j.1875-595X.2002.tb00589.x
10. Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. (2005) 83:661–9.
11. Listl S, Galloway J, Mossey PA, Marcenes W. Global economic impact of dental diseases. J Dent Res. (2015) 94:1355–61. doi: 10.1177/0022034515602879
12. Cleaton-Jones P, Fatti P. Dental caries trends in Africa. Community Dent Oral Epidemiol. (1999) 27:316–20. doi: 10.1111/j.1600-0528.1999.tb02027.x
13. Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century–the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. (2003) 31:3–24. doi: 10.1046/j.2003.com122.x
14. da Silveira Moreira R. Epidemiology of dental caries in the world. Oral Health Care Pediatric Res Epidemiol Clin Pract. (2012) 8:149–68. doi: 10.5772/31951
15. Josefczyk ME. The State of Oral Health on the African Continent. Lynchburg, VI: Liberty University (2015).
17. Zewdu T, Abu D, Agajie M, Sahilu T. Dental caries and associated factors in Ethiopia: systematic review and meta-analysis. Environ Health Prev Med. (2021) 26:21. doi: 10.1186/s12199-021-00943-3
18. Elidrissi SM, Naidoo S. Prevalence of dental caries and toothbrushing habits among preschool children in Khartoum State, Sudan. Int Dent J. (2016) 66:215–20. doi: 10.1111/idj.12223
19. Owino RO, Masiga MA, Macigo FG. Dental caries, gingivitis and the treatment needs among 12-year-olds. East Afr Med J. (2010) 87:25–31. doi: 10.4314/eamj.v87i1.59950
20. Rwakatema DS, Ananduni KN, Katiti VW, Msuya M, Chugulu J, Kapanda G. Oral health in nursing students at Kilimanjaro Christian Medical Centre teaching hospital in Moshi, Tanzania. BMC Oral Health. (2015) 15:1–8. doi: 10.1186/s12903-015-0008-8
21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
22. StataCorp LP. Stata data analysis and statistical Software. Spec Ed Release. (2007) 10:733. doi: 10.1515/9783110617160
23. Hall DB, Woolson RF, Clarke WR, Jones MF. 16 Cochran-mantel-haenszel techniques: applications involving epidemiologic survey data. Bioenvironm Public Health Stat. (2000) 18:483–500. doi: 10.1016/S0169-7161(00)18018-6
24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
25. Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis. Publ Bias Meta Anal Prev Assess Adjust. (2005) 1:1–7. doi: 10.1002/0470870168
26. Saltelli A. Sensitivity analysis for importance assessment. Risk Anal. (2002) 22:579–90. doi: 10.1111/0272-4332.00040
27. Tagelsir A, Khogli AE, Nurelhuda NM. Oral health of visually impaired schoolchildren in Khartoum State, Sudan. BMC Oral Health. (2013) 13:33. doi: 10.1186/1472-6831-13-33
28. Khalifa N, Allen PF, Abu-bakr NH, Abdel-Rahman ME, Abdelghafar KO. A survey of oral health in a Sudanese population. BMC Oral Health. (2012) 12:5. doi: 10.1186/1472-6831-12-5
29. Teshome A, Yitayeh A, Gizachew M. Prevalence of dental caries and associated factors among Finote Selam primary school students aged 12–20 years, Finote Selam Town, Ethiopia. Age. (2016) 12:15–7.
30. Walle M. Khat chewing and self rated oral health out comes in Bahir Dar, North West Ethiopia. Am J Health Res. (2014) 2:344–9. doi: 10.11648/j.ajhr.20140206.14
31. Mulu W, Demilie T, Yimer M, Meshesha K, Abera B. Dental caries and associated factors among primary school children in Bahir Dar city: a cross-sectional study. BMC Res Notes. (2014) 7:949. doi: 10.1186/1756-0500-7-949
32. Simon C, Tesfaye F, Berhane Y. Assessment of the oral health status of school children in Addis Ababa. Ethiop Med J. (2003) 41:245–56.
33. Berhane HY, Worku A. Oral health of young adolescents in Addis Ababa—a community-based study. Open J Prev Med. (2014) 2014:640–8. doi: 10.4236/ojpm.2014.48073
34. Burnett D, Aronson J, Asgary R. Oral health status, knowledge, attitudes and behaviours among marginalized children in Addis Ababa, Ethiopia. J Child Health Care. (2016) 20:252–61. doi: 10.1177/1367493515569328
35. Kebede B, Kemal T, Abera S. Oral health status of patients with mental disorders in southwest Ethiopia. PLoS ONE. (2012) 7:e39142. doi: 10.1371/journal.pone.0039142
36. Ademe D, Admassu D, Balakrishnan S. Analysis of salivary level Lactobacillus spp. and associated factors as determinants of dental caries amongst primary school children in Harar town, eastern Ethiopia. BMC Pediatr. (2020) 20:1–9. doi: 10.1186/s12887-020-1921-9
37. Teshome A, Andualem G, Derese K. Dental caries and associated factors among patients attending the university of gondar comprehensive hospital dental clinic, North West Ethiopia: a hospital-based cross-sectional study. Clin Cosmet Investig Dent. (2020) 12:191. doi: 10.2147/CCIDE.S247179
38. Bogale B, Engida F, Hanlon C, Prince MJ, Gallagher JE. Dental caries experience and associated factors in adults: a cross-sectional community survey within Ethiopia. BMC Public Health. (2021) 21:1–12. doi: 10.1186/s12889-021-10199-9
39. Aynalem YA, Alamirew G, Shiferaw WS. Magnitude of dental caries and its associated factors among governmental primary school children in Debre Berhan Town, North-East Ethiopia. Pediatr Health Med Ther. (2020) 11:225. doi: 10.2147/PHMT.S259813
40. Wondwossen F, Åstrøm AN, Bjorvatn K, Bårdsen A. The relationship between dental caries and dental fluorosis in areas with moderate- and high-fluoride drinking water in Ethiopia. Community Dent Oral Epidemiol. (2004) 32:337–44. doi: 10.1111/j.1600-0528.2004.00172.x
41. Equbamichael M, Fissihaye T, Hagos A, Tsadu G, Kiflom A. Prevalence of dental caries in high school students in Asmara, Eritrea. J Eritrean Med Assoc. (2006) 1:36–9. doi: 10.4314/jema.v1i1.52680
42. Abdelhamid N, Bahta H, Mohammed S, Dhanni C, Elfatih AM. Prevalence of dental caries and evaluation of mean DMFT index among secondary school students in Asmara, Eritrea. Afr J Oral Health. (2019) 8:1–7.
43. Andegiorgish AK, Weldemariam BW, Kifle MM, Mebrahtu FG, Zewde HK, Tewelde MG, et al. Prevalence of dental caries and associated factors among 12 years old students in Eritrea. BMC Oral Health. (2017) 17:1–6. doi: 10.1186/s12903-017-0465-3
44. Rwenyonyi CM, Muwazi LM, Buwembo W. Assessment of factors associated with dental caries in rural communities in Rakai District, Uganda. Clin Oral Investig. (2011) 15:75–80. doi: 10.1007/s00784-009-0363-4
45. Kiwanuka SN, AAstrøm AN, Trovik TA. Dental caries experience and its relationship to social and behavioural factors among 3–5-year-old children in Uganda. Int J Paediatr Dent. (2004) 14:336–46. doi: 10.1111/j.1365-263X.2004.00570.x
46. Muwazi LM, Rwenyonyi CM, Tirwomwe FJ, Ssali C, Kasangaki A, Nkamba ME, et al. Prevalence of oral diseases/conditions in Uganda. Afr Health Sci. (2005) 5:227–33.
47. Kutesa A, Kasangaki A, Nkamba M, Muwazi L, Okullo I, Rwenyonyi CM. Prevalence and factors associated with dental caries among children and adults in selected districts in Uganda. Afr Health Sci. (2015) 15:1302–7. doi: 10.4314/ahs.v15i4.33
48. Birungi N, Fadnes LT, Engebretsen IM, Lie SA, Tumwine JK, AAstrøm AN, et al. Caries experience and oral health related quality of life in a cohort of Ugandan HIV-1 exposed uninfected children compared with a matched cohort of HIV unexposed uninfected children. BMC Public Health. (2020) 20:1–12. doi: 10.1186/s12889-020-08564-1
49. Ndagire B, Kutesa A, Ssenyonga R, Kiiza HM, Nakanjako D, Rwenyonyi CM. Prevalence, severity and factors associated with dental caries among school adolescents in Uganda: a cross-sectional study. Braz Dent J. (2020) 31:171–8. doi: 10.1590/0103-6440202002841
50. Kalanzi D, Mayanja-Kizza H, Nakanjako D, Mwesigwa CL, Ssenyonga R, Amaechi BT. Prevalence and factors associated with dental caries in patients attending an HIV care clinic in Uganda: a cross sectional study. BMC Oral Health. (2019) 19:1–8. doi: 10.1186/s12903-019-0847-9
51. Ahmed TES, Abuaffan A. Correlation between body mass index and dental caries among a sample of Sudanese children. Braz Dent Sci. (2015) 18:42–51. doi: 10.14295/bds.2015.v18i3.1149
52. Mohamed Ali H, Mustafa M, Hasabalrasol S, Elshazali OH, Nasir EF, Ali RW, et al. Presence of plaque, gingivitis and caries in Sudanese children with congenital heart defects. (2017) 21:1299–307. doi: 10.1007/s00784-016-1884-2
53. Fukuda H, Ogada CN, Kihara E, Wagaiyu EG, Hayashi Y. Oral health status among 12-year-old children in a rural Kenyan community. J Dent Oral Health. (2014) 1:1–5. doi: 10.17303/jdoh.2014.201
54. Owino RO, Macigo FG, Onyango FJ. Pattern and aetiology of mandibular fractures at Kenyatta national hospital. Afr J Oral Health Sci. (2003) 4:178–80.
55. Kassim BA, Noor MA. Oral health status among Kenyans in a rural arid setting: dental caries experience and knowledge on its causes. East Afr Med J. (2006) 83:100–5. doi: 10.4314/eamj.v83i2.9396
56. Makhanu M, Opinya G, Mutave RJ. Dental fluorosis, caries experience and snack intake of 13-15 year olds in Kenya. East Afr Med J. (2009) 86:120–4. doi: 10.4314/eamj.v86i3.54963
57. Masiga MA, M'Imunya JM. Prevalence of dental caries and its impact on quality of life (QoL) among HIV-infected children in Kenya. J Clin Pediatr Dent. (2013) 38:83–7. doi: 10.17796/jcpd.38.1.62l1q94650j5l815
58. Njoroge NW, Kemoli AM, Gatheche LW. Prevalence and pattern of early childhood caries among 3-5 year olds in Kiambaa, Kenya. East Afr Med J. (2010) 87:134–7. doi: 10.4314/eamj.v87i3.62199
59. Uwayezu D, Gatarayiha A, Nzayirambaho M. Prevalence of dental caries and associated risk factors in children living with disabilities in Rwanda: a cross-sectional study. Pan Afr Med J. (2020) 36:193.
60. Svensson I, Gustafsson J, Uleskog E, Mathisson C, Molla N, Kahlmeter A, et al. Oral condition and background factors in Somali immigrant children newly arrived in Sweden. Swed Dent J. (2016) 40:153–64.
61. Nordstrand MA, Saxe DS, Mohammed MA, Adam MB. Health and disease among Somali primary school children in Hargeisa. Glob Health Action. (2019) 12:1598648. doi: 10.1080/16549716.2019.1598648
62. Mwakatobe AJ, Mumghamba EG. Oral health behavior and prevalence of dental caries among 12-year-old school-children in Dar-es-Salaam, Tanzania. Tanzan Dent J. (2007) 14:1–7. doi: 10.4314/tdj.v14i1.37563
63. Mafuvadze BT, Mahachi L, Mafuvadze B. Dental caries and oral health practice among 12 year old school children from low socio-economic status background in Zimbabwe. Pan Afr Med J. (2013) 14:164. doi: 10.11604/pamj.2013.14.164.2399
64. Carneiro LC, Kabulwa MN. Dental caries, and supragingival plaque and calculus among students, Tanga, Tanzania. ISRN Dent. (2012) 2012:245296. doi: 10.5402/2012/245296
65. Kikwilu EN, Mandari GJ. Dental caries and periodontal conditions among primary school children in Morogoro municipality, Tanzania. East Afr Med J. (2001) 78:152–6. doi: 10.4314/eamj.v78i3.9083
66. Mwakayoka H, Masalu JR, Kikwilu EN. Dental caries and associated factors in children aged 2-4 years old in Mbeya City, Tanzania. J Dent. (2017) 18:104.
67. Mashoto KO, AAstrøm AN, David J, Masalu JR. Dental pain, oral impacts and perceived need for dental treatment in Tanzanian school students: a cross-sectional study. Health Qual Life Outcomes. (2009) 7:1–9. doi: 10.1186/1477-7525-7-73
68. Simangwa LD, AAstrøm AN, Johansson A, Minja IK, Johansson A-K. Oral diseases and oral health related behaviors in adolescents living in Maasai population areas of Tanzania: a cross-sectional study. BMC Pediatr. (2019) 19:1–14. doi: 10.1186/s12887-019-1655-8
69. The Joanna Briggs Institute. Joanna Briggs Institute Reviewers' Manual: 2014 Edition. Kronoberg County: The Joanna Briggs Institute (2014)
70. Cheng Y-H, Liao Y, Chen D-Y, Wang Y, Wu Y. Prevalence of dental caries and its association with body mass index among school-age children in Shenzhen, China. BMC Oral Health. (2019) 19:270. doi: 10.1186/s12903-019-0950-y
71. Alayyan W, Al Halabi M, Hussein I, Khamis A, Kowash M. A systematic review and meta-analysis of school children's caries studies in gulf cooperation council states. J Int Soc Prev Community Dent. (2017) 7:234.
72. Tambelini CA, Ramos DM, Poli-Frederico RC, Tomasetti CS de C, Barata T de JE, Maciel SM. Dental caries in adolescents and its association with excess weight and sociodemographic factors in Londrina, Paraná, Brazil. Rev Odonto Ciênc. (2010) 25:245–9.
73. Kamberi B, Koçani F, Begzati A, Kelmendi J, Ilijazi D, Berisha N, et al. Prevalence of dental caries in kosovar adult population. Int J Dent. (2016) 2016:4290291. doi: 10.1155/2016/4290291
74. Wang Y, Xing L, Yu H, Zhao L. Prevalence of dental caries in children and adolescents with type 1 diabetes: a systematic review and meta-analysis. BMC Oral Health. (2019) 19:213. doi: 10.1186/s12903-019-0903-5
75. Petersen PE, Kwan S. Evaluation of community-based oral health promotion and oral disease prevention–WHO recommendations for improved evidence in public health practice. Community Dent Health. (2004) 21 (4 Suppl.):319–29. doi: 10.6719/emhj.20.050
76. Wang H-Y, Petersen PE, Bian J-Y, Zhang B-X. The second national survey of oral health status of children and adults in China. Int Dent J. (2002) 52:283–90. doi: 10.1111/j.1875-595X.2002.tb00632.x
77. Mahfouz M, Abu Esaid A. Dental caries prevalence among 12-15 year old palestinian children. Int Sch Res Not. (2014) 2014:785404. doi: 10.1155/2014/785404
78. Kale S, Kakodkar P, Shetiya S, Abdulkader R. Prevalence of dental caries among children aged 5-15 years from 9 countries in the Eastern Mediterranean Region: a meta-analysis. East Mediterr Health. (2020) 26:726–35. doi: 10.26719/emhj.20.050
79. Janakiram C, Antony B, Joseph J, Ramanarayanan V. Prevalence of dental caries in India among the WHO index age groups: a meta-analysis. J Clin Diagn Res. (2018) 12:1–7. doi: 10.7860/JCDR/2018/32669.11956
80. Soltani MR. Dental caries status and its related factors in Iran: a meta-analysis. J Dent. (2020) 21:158. doi: 10.30476/DENTJODS.2020.82596.1024
81. Khan SQ. Dental caries in Arab League countries: a systematic review and meta-analysis. Int Dent J. (2014) 64:173–80. doi: 10.1111/idj.12092
83. Lukacs JR, Largaespada LL. Explaining sex differences in dental caries prevalence: saliva, hormones, and “life-history” etiologies. Am J Hum Biol. (2006) 18:540–55. doi: 10.1002/ajhb.20530
84. Punitha VC, Amudhan A, Sivaprakasam P, Rathanaprabu V. Role of dietary habits and diet in caries occurrence and severity among urban adolescent school children. J Pharm Bioallied Sci. (2015) 7 (Suppl. 1):S296–300. doi: 10.4103/0975-7406.155963
85. de Melo MMDC, de Souza WV, de Lima MLC, Braga C. Factors associated with dental caries in preschoolers in Recife, Pernambuco State, Brazil. Cad Saude Publica. (2011) 27:471–85. doi: 10.1590/S0102-311X2011000300008
86. Obregón-Rodríguez N, Fernández-Riveiro P, Piñeiro-Lamas M, Smyth-Chamosa E, Montes-Martínez A, Suárez-Cunqueiro MM. Prevalence and caries-related risk factors in schoolchildren of 12- and 15-year-old: a cross-sectional study. BMC Oral Health. (2019) 19:120. doi: 10.1186/s12903-019-0806-5
Keywords: dental caries, DMFT, prevalence, oral health, tooth decay
Citation: Teshome A, Muche A and Girma B (2021) Prevalence of Dental Caries and Associated Factors in East Africa, 2000–2020: Systematic Review and Meta-Analysis. Front. Public Health 9:645091. doi: 10.3389/fpubh.2021.645091
Received: 23 December 2020; Accepted: 15 March 2021;
Published: 29 April 2021.
Edited by:
Morenike Oluwatoyin Folayan, Obafemi Awolowo University, NigeriaReviewed by:
Duangporn Duangthip, The University of Hong Kong, Hong KongGuglielmo Campus, University of Bern, Switzerland
Wondimeneh Shiferaw, Debre Berhan University, Ethiopia
Copyright © 2021 Teshome, Muche and Girma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amare Teshome, dGVmZXJhZGVuQGdtYWlsLmNvbQ==
 Amare Teshome
Amare Teshome Abebe Muche2
Abebe Muche2