
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 09 February 2021
Sec. Health Economics
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.608375
Background: The aim of this study is to evaluate the pharmacoeconomic profile of adding enzalutamide to first-line treatment for metastatic, hormone-sensitive prostate cancer (mHSPC) from the US and Chinese payers' perspectives.
Materials and Methods: A Markov model with three health states: progression-free survival (PFS), progressive disease (PD), and death, was constructed. All patients were assumed to enter the model in the PFS state and transit according to the transition structure. Efficacy data were derived from the ENZAMET trial and Weibull distribution curves were modeled to fit the survival curves. Costs in the model included cost of drugs, best-supportive care (BSC), follow-up, tests, and adverse events (AEs)-related treatments. The primary endpoint of the study was incremental cost-effectiveness ratio (ICER). In addition, the impact of several key parameters on the results of the cost-effectiveness analysis was tested with one-way sensitivity analyses and probabilistic sensitivity analyses.
Results: Overall, ICERs were $430,933.95/QALY and $225,444.74/QALY of addition of enzalutamide to androgen deprivation therapy (ADT) vs. ADT from the US and Chinese payers' perspective, respectively. The most influential factors were the utility for the PFS state and the cost of enzalutamide. At the willingness-to-pay (WTP) thresholds of $100,000.00/QALY in the US and $28,988.40/QALY in China, the probability of adding enzalutamide to first-line treatment being a cost-effective option for mHSPC was 0%.
Conclusions: Based on the data from the ENZAMET trial and the current price of enzalutamide, adding enzalutamide to first-line treatment is not cost-effective for patients with mHSPC from the US and Chinse payers' perspectives.
Prostate cancer is the second most frequently diagnosed cancer and ranks the fifth in cancer-related death in men worldwide. It was estimated that almost 1.3 million new cases and 359,000 deaths occurred in 2018 (1). Most patients with prostate cancer are diagnosed with localized disease, however, 10–20% of the patients are expected to be diagnosed with locally advanced or metastatic disease, for whom the standard first-line treatment is androgen deprivation therapy (ADT) (2). Bilateral orchiectomy and luteinizing hormone-releasing hormone (LHRH) agonists/antagonists represent the two main methods for ADT (3, 4). Recent years, the efficacy and safety of adding docetaxel or abiraterone to ADT, have been investigated in the first-line treatment for advanced/metastatic prostate cancer. All these drugs were demonstrated to significantly prolong the survivals in patients with metastatic hormone-sensitive prostate cancer (mHSPC) (5–8).
Enzalutamide is an oral androgen receptor inhibitor, which is designed to solve acquired resistance to first-generation non-steroidal antiandrogens, including bicalutamide, nilutamide, and flutamide (9). In previous trials, enzalutamide has been demonstrated to prolong the survivals in metastatic castration-resistant prostate cancer (mCRPC) (10, 11). Encouraged by the significant benefit of enzalutamide in mCRPC, the effects of adding enzalutamide to first-line treatment that included testosterone suppression with or without early docetaxel was investigated in the ENZAMET trial based on the hypothesis that adding enzalutamide to first-line therapy would delay the emergence of castration resistance and thereby improve survivals (12). As expected, enzalutamide was associated with significantly longer progression-free survival (PFS) and overall survival (OS) than standard care in men with mHSPC receiving testosterone suppression, with or without early docetaxel.
Although enzalutamide has shown significant benefit in the first-line treatment for patients with mHSPC, addition of enzalutamide may also increase health-care expenditures, which results in difficult treatment decisions for patients, doctors and policy makers. Moreover, health-care expenditures are growing rapidly and have become a major public health concern worldwide recently (13, 14). Thus, evaluation of a novel treatment options from both efficacy and pharmacoeconomic profile is of great significance. The aim of this study is to evaluate the cost-effectiveness of adding enzalutamide to first-line treatment in men with mHSPC from the US and Chinese payers' perspectives.
To investigate the cost-effectiveness of adding enzalutamide to first-line treatment for patients with mHSPC compared to standard care, a Markov decision model, which included three health states [PFS, progressive disease (PD) and death], was constructed. In the model, all patients were assumed to enter in the PFS state and then transit from one state to another state according to the transition structure presented in Figure 1. Time horizon of the model was defined as 20 years, after which almost all patients were expected to be dead. Markov cycle length was set at 1 month, which was consistent with the length of treatment periods. Health utilities for the PFS state and the PD state were derived from previous literature (Table 1) (15, 16). Health utility values in the model were assumed to be invariable despite the impact of AEs. Incremental cost-effectiveness ratio (ICER) was regarded as the primary endpoint of the study. Willingness-to-pay (WTP) threshold in the analysis was set at $28,988.40/quality-adjusted life year (QALY) (3 × per capita GDP of China, 2018) for China and $100,000.00/QALY for US, respectively. Cost and effectiveness were discounted at an annual rate of 3% for China and 3.5% for US, in line with the guidelines. This study was approved by the Ethics Committee of West China Hospital, Sichuan University. The model was developed and performed using the Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and TreeAge software (TreeAge, Williamstown, MA, USA, 2011).
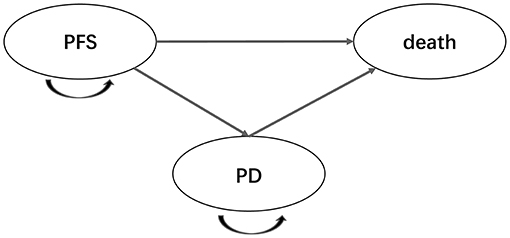
Figure 1. Markov model diagram for patients with metastatic, hormone-sensitive prostate cancer. PFS, progression-free survival; PD, progressive disease.
A cohort population, which reflected the participant population of the ENZAMET trial, were modeled. Enzalutamide was administered at a dose of 160 mg daily. Participants in the standard care group received a conventional non-steroidal antiandrogen (i.e., bicalutamide 50 mg daily, nilutamide 150 mg daily, or flutamide 250 mg three times a day). All participants in the ENZAMET trial received standard background therapy, including LHRHA (goserelin, leuprorelin, triptorelin, and degarelix) or surgical castration. For patients who received docetaxel, docetaxel should be administered at 75 mg/m2 every 21 days for a maximum of 6 cycles.
Efficacy data in the model were derived from the ENZAMET trial, and these data were used to estimate the transition probabilities between health stages (Table 1). Survival data were collected from the survival curves using the Web Plot Digitizer software (https://apps.automeris.io/wpd/). The estimated transition probabilities were modeled to fit the survival curves (Figure 2). To simplify the model, only AEs with a frequency ≥5% were included. The cost in the model were categorized into cost of drug, best-supportive care (BSC), follow-up (reflect the frequency of drug administration), tests and adverse events (AEs)-related treatments. The unit prices of drugs and tests in China were retrieved from the national drug prices or West China Hospital, Sichuan University, China. For analysis from the US payers' perspective, the unit prices of drugs were obtained from RED BOOK Online®, and unit cost of tests, drug administration, follow-up, tests, AEs-related treatments and BSC were retrieved from the CMS clinical laboratory fee schedule files and previously published literatures (Table 2) (17–23). To calculate the dose of docetaxel, participants with a body surface area of 2.1 or 1.72 m2 were assumed to reflect the patients in US and China (16, 24). All cost was converted into US dollars.
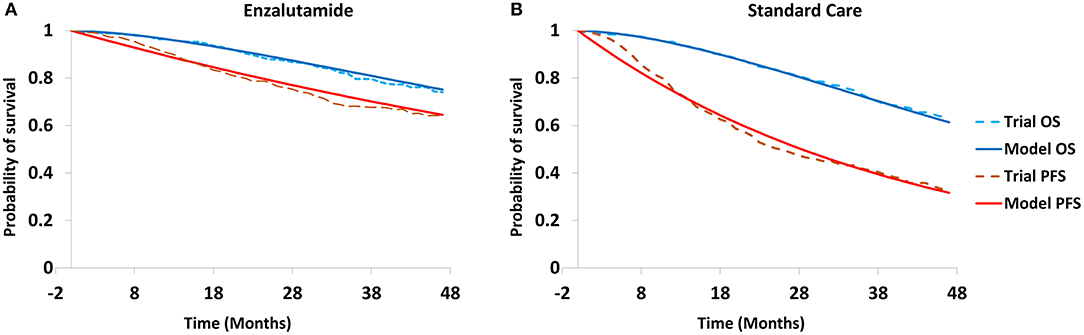
Figure 2. Modeled survival curves for enzalutamide group (A) and standard care group (B). PFS, progression-free survival; OS, overall survival.
The robustness of the analysis was tested with a series of one-way sensitivity analyses on several parameters, such as duration of PFS, duration of OS, cost of enzalutamide. In the one-way sensitivity analyses, parameters ranged between ±20%, and tornado diagrams were used to display the results of the one-way sensitivity analyses. Moreover, probabilistic sensitivity analyses were also performed based on the Monte Carlo simulations with 1,000 iterations.
The results of the base case analysis were shown in Table 3. Over a lifetime horizon of 20 years, adding enzalutamide to first-line treatment gained an effectiveness of 6.21 QALYs at a cost of $1,396,827.63 from the US payers' perspective, while from the Chinese payers' perspective, the effectiveness and cost were 5.70 QALYs and $516,510.76. On the other hand, the cost and effectiveness in the standard care group were $483,247.66, 4.09 QALYs and $83,656.86, 3.78 QALYs from the US payers' perspective and the Chinese payers' perspective, respectively. Overall, ICERs were $430,933.95/QALY and $225,444.74/QALY of adding enzalutamide to first-line treatment vs. standard care from the US payers' perspective and the Chinese payers' perspective, indicating adding enzalutamide to first-line treatment was not cost-effective vs. standard care at the WTP threshold of $28,988.40/QALY in China and $100,000.00/QALY in the US.
To investigate the impact of key variables on the robustness of our results, one-way sensitivity analyses were performed, and the results of the one-way sensitivity analyses were presented in a tornado diagram. As shown in Figure 3, the utility for the PFS state and the cost of enzalutamide were the most influential factors both from the US payers' perspective and the Chinese payers' perspective.
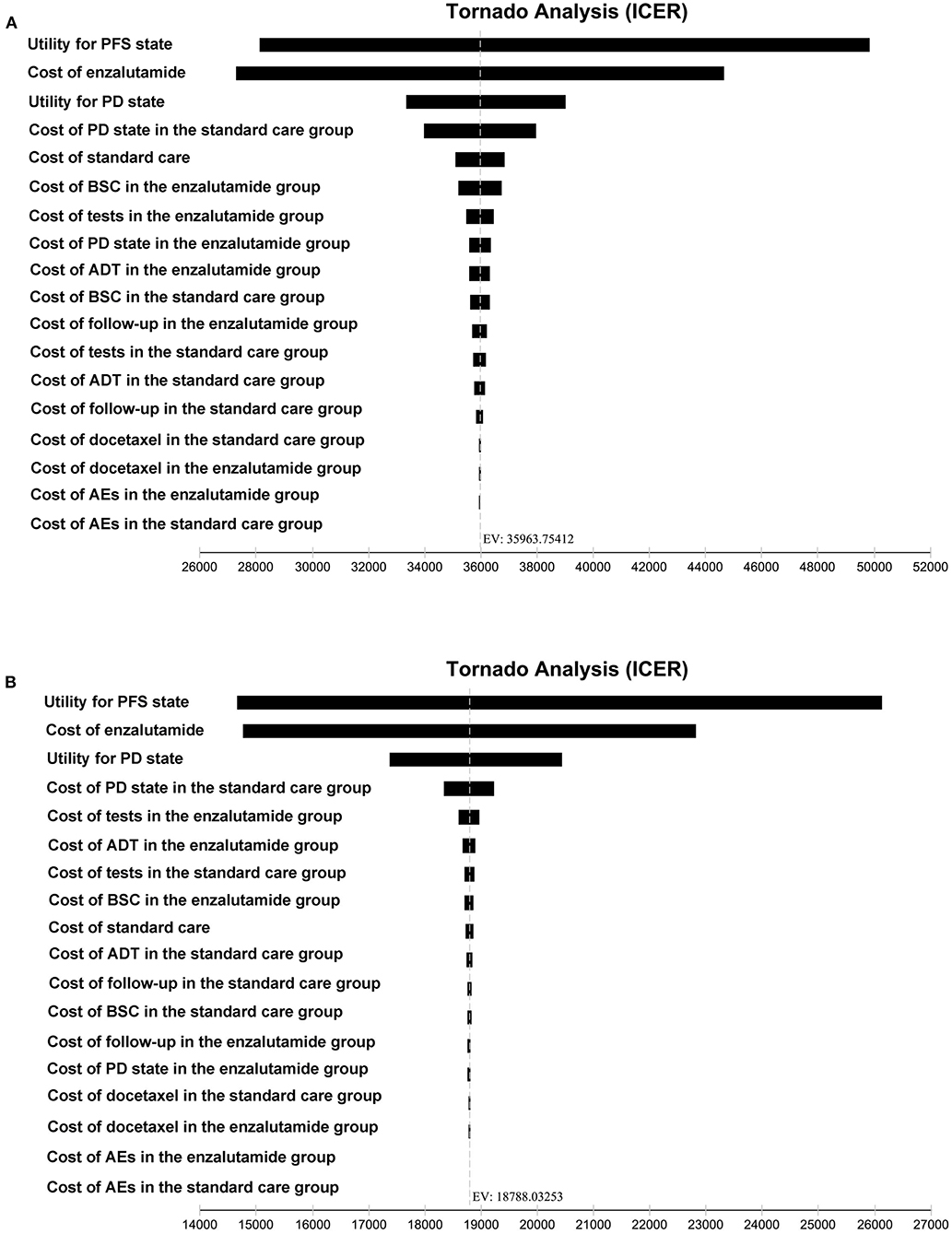
Figure 3. Tornado diagram for one-way sensitivity analyses. (A) The US payers' perspective. (B) The Chinese payers' perspective. PFS, progression-free survival; PD, progressed disease; BSC, best-supportive care; ADT, androgen-deprivation therapy; AEs, adverse events; ICER, incremental cost-effectiveness ratio.
We also performed a probabilistic sensitivity analysis to assess the uncertainty of the resultant ICER based on the Monte Carlo simulations with 1,000 iterations. The probabilities of adding enzalutamide to first-line treatment or standard care as the cost-effective option were 0 and 100% both from the Chinese and US payers' perspectives at the WTP threshold set in the model.
Prostate cancer represents one of the most commonly diagnosed cancer in men around the world. For locally advanced or metastatic diseases, ADT was previously considered as the standard first-line treatment. However, more and more novel drugs, such as docetaxel or abiraterone, were demonstrated to significantly improved the survivals in mHSPC. Recently, the results of the ENZAMET trial, which investigated the effect of adding enzalutamide to first-line treatment, was released. Consistent with the significant benefit achieved in mCRPC, adding enzalutamide to first-line treatment also significantly prolonged the survivals in patients with mHSPC. However, the price of enzalutamide was much higher than first-generation non-steroidal antiandrogens (bicalutamide, nilutamide, and flutamide). Thus, in this study, we evaluated the pharmacoeconomic profile of adding enzalutamide to first-line treatment in men with mHSPC from the US and Chinese payers' perspectives. Based on the analysis, the ICERs were $430,933.95/QALY and $225,444.74/QALY of adding enzalutamide to first-line treatment vs. standard care from the US payers' perspective and Chinese payers' perspective, respectively. From the pharmacoeconomic profile, adding enzalutamide to first-line treatment was not a cost-effective treatment option based on the data from the ENZAMET trial and the current price of enzalutamide.
Recently, a series of novel treatment options have been approved for the treatment of mHSPC. Despite the significant benefits achieved by these treatment options, novel treatment regimens may also cause heavy burden of health-care expenditures. To provide more evidences for selecting the optimal treatment regimen, more and more cost-effectiveness analyses were conducted to evaluate novel treatment regimens from the pharmacoeconomic profile. However, the conclusion of cost-effectiveness analysis in different regions were inconsistent. For example, two Chinese studies demonstrated that docetaxel combined with ADT is not a cost-effective treatment compared with ADT alone for mHSPC in the Chinese setting (25, 26). However, in the similar studies based on the US and Brazil, the addition of docetaxel with ADT in high-volume metastatic HSPC appears to be an economically attractive treatment approach (27, 28). Thus, in this study, we evaluated the cost-effectiveness of enzalutamide plus first-line treatment in men with mHSPC from both the US and Chinese payers' perspectives, which could extend the application of the results of the analysis. Our analysis, based on current costs and the results of ENZAMET trial after a median follow-up of 3 years, indicated that adding enzalutamide to first-line treatment is unlikely to be a cost-effective regimen from both the US and Chinese payers' perspectives.
Although adding enzalutamide to first-line treatment is not a cost-effective option treatment both in the US and China, we wonder whether the factors affecting the model are the same from the two perspectives. Thus, we performed a series of one-way sensitivity analyses to investigate the impact of key variables on the robustness of our results. The utility for the PFS state and the cost of enzalutamide were the most influential factors from the US payers' perspective. Meanwhile, the model was also sensitive to the utility for the PD state and the cost of PD state in the standard care group. The most influential factors from the Chinese payers' perspective were the same with that from the US payers' perspective. Moreover, the utility for the PD state and the cost of PD state in the standard care group were also another two factors mostly influencing the model.
This is the first study to assess the cost-effectiveness of enzalutamide in the first-line treatment of mHSPC, however, the pharmacoeconomic profile of enzalutamide has been evaluated in mCRPC. In a study, Pollard et al. performed a cost-effectiveness analysis to compare the contemporary treatment options for mCRPC, which shows that all currently available treatment options, including enzalutamide, exceed the commonly used WTP threshold of $100,000 per life year saved. In another study, Barqawi et al. evaluated the cost-effectiveness of abiraterone plus prednisone, cabazitaxel plus prednisone and enzalutamide for visceral mCRPC after docetaxel therapy resistance. Based on the cost-effectiveness analysis, enzalutamide was cost-effective in 92% of the time with a WTP threshold of $100,000/QALY for patients with visceral mCRPC after docetaxel therapy resistance (29, 30). The differences between the two study could be interpreted as the comparators were different. In Pollard's study, the standard care is docetaxel, which is relatively cheap. However, in Barqawi's study, abiraterone and cabazitaxel were also with high prices, which made enzalutamide as a cost-effective option.
The limitations of the study should also be addressed. First, the analysis was conducted based on the ENZAMET trial, and we merely investigated the cost-effectiveness of adding enzalutamide to first-line treatment vs. standard care for patients with mHSPC. We did not include other novel treatment options, such as abiraterone, as there are no head-to-head trials to compare the effect of these novel regimens. Second, the analysis included AEs with relatively frequent (>5%), expensive to treat or substantively affected quality of life. The cost of grade 1–2 AEs and AEs with low frequency were not included in the study. Fortunately, the results of the one-way sensitivity analyses demonstrated the economic results were not sensitive to AEs-related parameters. Third, several key parameters, such as the utility scores, cost of AE-related treatments, and cost of supportive care in the analysis, were derived from previously published literature, which may also undermine the correctness of our results. We believe that cost-effectiveness analysis based on real-world population may be more correct and representative.
In this study, we evaluated the pharmacoeconomic profile of adding enzalutamide to first-line treatment in men with mHSPC from the US and Chinese payers' perspectives. Based on the WTP threshold of $28,988.40/QALY for China and $100,000.00/QALY for US, enzalutamide, at its current price and based on 3-year follow-up from ENZAMET trial, is not cost-effective compared with standard care from the US and Chinse societal perspectives.
The data generated during this study are available from the corresponding author on reasonable request.
P-FZ and QL were responsible for the study conception, methodology, data analysis, draft writing, and editing. DX was responsible for the design, data collection and analysis, draft writing and editing. All authors contributed to the article and approved the submitted version.
This work was funded by the National Natural and Scientific Foundation of China (No. 81572988) and Science & Technology Department of Sichuan Province Funding Project (Nos. 2016FZ0108, 2018SZ0117, and 2018SZ0151).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bray F, Ferley J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr. Relat. Cancer. (2010) 17:R305–5. doi: 10.1677/ERC-10-0187
3. Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. (2008) 373:301–8. doi: 10.1016/S0140-6736(08)61815-2
4. Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. (2011) 378:2104–11. doi: 10.1016/S0140-6736(11)61095-7
5. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. (2015) 373:737–46. doi: 10.1056/NEJMoa1503747
6. Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. (2018) 36:1080–7. doi: 10.1200/JCO.2017.75.3657
7. Sternberg CN. RE: addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Eur. Urol. (2016) 69:1155–6. doi: 10.1016/j.eururo.2016.02.022
8. Fizazi K, Tran NP, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. (2017) 377:352–60. doi: 10.1056/NEJMoa1704174
9. Linder S, Der Poel HGV, Bergman AM, Zwart W, Prekovic S. Enzalutamide therapy for advanced prostate cancer: efficacy, resistance and beyond. Endocr. Relat. Cancer. (2018) 26:R31–R52. doi: 10.1530/ERC-18-0289
10. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. (2012) 367:1187–97. doi: 10.1056/NEJMoa1207506
11. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. (2014) 371:424–33. doi: 10.1056/NEJMoa1405095
12. Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. (2019) 381:121–31. doi: 10.1056/NEJMoa1903835
13. Dieleman JL, Templin T, Sadat N, Reidy P, Chapin A, Foreman K, et al. National spending on health by source for 184 countries between 2013 and 2040. Lancet. (2016) 387:2521–35. doi: 10.1016/S0140-6736(16)30167-2
14. Global Burden of Disease Health Financing Collaborator Network. Future and potential spending on health 2015-40: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet. (2017) 389:2005–30. doi: 10.1016/S0140-6736(17)30873-5
15. Liu M, Qu S, Liu Y, Yao X, Jiang W. Comparative clinical effects and cost–effectiveness of maximum androgen blockade, docetaxel with androgen deprivation therapy and ADT alone for the treatment of mHSPC in China. J. Comp. Eff. Res. (2019) 8:865–77. doi: 10.2217/cer-2018-0133
16. Ramamurthy C, Handorf EA, Correa AF, Beck JR, Geynisman DM. Cost-effectiveness of abiraterone versus docetaxel in the treatment of metastatic hormone naïve prostate cancer. Urol. Oncol. (2019) 37:688–95. doi: 10.1016/j.urolonc.2019.05.017
17. Chen Q, Ayer T, Nastoupil LJ, Rose AC, Flowers CR. Comparing the cost-effectiveness of rituximab maintenance and radioimmunotherapy consolidation versus observation following first-line therapy in patients with follicular lymphoma. Value Health. (2015) 18:189–97. doi: 10.1016/j.jval.2014.12.017
18. Gong CL, Hay JW. Cost-effectiveness analysis of abiraterone and sipuleucel-T in asymptomatic metastatic castration-resistant prostate cancer. J. Natl. Compr. Canc. Netw. (2014) 12:1417–25. doi: 10.6004/jnccn.2014.0139
19. Gu X, Zhang Q, Chu YB, Zhao YY, Zhang YJ, Kuo D, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer. (2019) 127:84–9. doi: 10.1016/j.lungcan.2018.11.029
20. Shi G, Park SH, Ren H, Xue M, Lu X, Dong P, et al. Cost analysis for different sequential treatment regimens for metastatic renal cell carcinoma in China. J. Med. Econ. (2018) 21:1150–8. doi: 10.1080/13696998.2018.1515769
21. Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J. Immunother. Cancer. (2018) 6:124. doi: 10.1186/s40425-018-0440-9
22. Bai Y, Xu Y, Wu B. Cost-effectiveness and budget impact analysis of apatinib for advanced metastatic gastric cancer from the perspective of health insurance system. Gastroenterol. Res. Pract. (2017) 2017:2816737. doi: 10.1155/2017/2816737
23. Wu B, Ye M, Chen H, Shen JF. Costs of trastuzumab in combination with chemotherapy for HER2-positive advanced gastric or gastroesophageal junction cancer: an economic evaluation in the chinese context. Clin. Ther. (2012) 34:468–79. doi: 10.1016/j.clinthera.2012.01.012
24. Wu B, Li T, Cai J, Xu Y, Zhao G. Cost-effectiveness analysis of adjuvant chemotherapies in patients presenting with gastric cancer after D2 gastrectomy. BMC Cancer. (2014) 14:984. doi: 10.1186/1471-2407-14-984
25. Zhang P, Wen F, Fu P, Yang Y, Li Q. Addition of docetaxel and/or zoledronic acid to standard of care for hormone-naive prostate cancer: a cost-effectiveness analysis. Tumori. (2017) 103:380–6. doi: 10.5301/tj.5000583
26. Zheng HR, Wen F, Wu YF, Wheeler JRC, Li Q. Cost-effectiveness analysis of additional docetaxel for metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy from a Chinese perspective. Eur. J. Cancer Care. (2017) 26:e12505. doi: 10.1111/ecc.12505
27. Beca J, Majeed H, Chan KKW, Hotte SJ, Loblaw A, Hoch JS. Cost-effectiveness of docetaxel in high-volume hormone-sensitive metastatic prostate cancer. Can. Urol. Assoc. J. (2019) 13:396–403. doi: 10.5489/cuaj.5889
28. Aguiar PN Jr, Barreto CMN, Gutierres BS, Tadokoro H, Lopes GL Jr. Cost effectiveness of chemohormonal therapy in patients with metastatic hormone-sensitive and non-metastatic high-risk prostate cancer. Einstein. (2017) 15:349–54. doi: 10.1590/S1679-45082017GS4017
29. Pollard ME, Moskowitz AJ, Diefenbach MA, Hall SJ. Cost-effectiveness analysis of treatments for metastatic castration resistant prostate cancer. Asian J. Urol. (2016) 4:37–43. doi: 10.1016/j.ajur.2016.11.005
30. Barqawi YK, Borrego ME, Roberts MH, Abraham I. Cost-effectiveness model of abiraterone plus prednisone, cabazitaxel plus prednisone and enzalutamide for visceral metastatic castration resistant prostate cancer therapy after docetaxel therapy resistance. J. Med. Econ. (2019) 22:1202–9. doi: 10.1080/13696998.2019.1661581
Keywords: prostate cancer, enzalutamide, cost-effectiveness, androgen deprivation therapy, first-line
Citation: Zhang P-F, Xie D and Li Q (2021) Adding Enzalutamide to First-Line Treatment for Metastatic Hormone-Sensitive Prostate Cancer: A Cost-Effectiveness Analysis. Front. Public Health 9:608375. doi: 10.3389/fpubh.2021.608375
Received: 10 October 2020; Accepted: 19 January 2021;
Published: 09 February 2021.
Edited by:
Nemanja Rancic, Military Medical Academy, SerbiaReviewed by:
Svetlana Ranko Radevic, University of Kragujevac, SerbiaCopyright © 2021 Zhang, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu Li, keythera@126.com
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.