- 1Department of Pulmonary and Critical Care Medicine, The Third Xiangya Hospital of Central South University, Changsha, China
- 2Department of Pulmonary and Critical Care Medicine, Central Hospital of Wuhan City, Wuhan, China
- 3Department of Nephrology, Xiangya Hospital of Central South University, Changsha, China
- 4Organ Fibrosis Key Laboratory of Hunan Province, Changsha, China
Background: A large number of people contracted moderate-type COVID-19 around the world. However, to our knowledge no studies have covered the clinical course of patients with moderate-type COVID-19. This study describes the clinical course of moderate-type patients with COVID-19 from Wuhan City and Yiyang City, and explores factors relevant to the length of hospitalization and symptoms relief.
Methods: The study analyzed the clinical course of 107 moderate-type patients with COVID-19 from the outbreak area (Wuhan) and the imported area (Yiyang), and used automatic linear modeling and multivariate linear regression analysis to explore the factors relevant to the length of hospitalization and symptoms relief. Furthermore, we created a scoring system to value the length of hospitalization and symptoms relief.
Results: Lymphopenia, elevated C-reactive protein, increased LDH, bilateral lung GGO (ground glass opacity), and lung consolidation were more likely to appear in ordinary inpatients with moderate-type COVID-19 from Wuhan (P < 0.05), compared to infected medical staff from Wuhan and ordinary inpatients with moderate-type COVID-19 from Yiyang. Meanwhile, the length of hospitalization and symptoms relief was longer in ordinary patients with moderate-type COVID-19 from Wuhan (P < 0.05). Onset of symptoms to admission, ESR, leucocytes count, and bilateral lung GGO were linearly related to the length of hospitalization (P < 0.05); onset of symptoms to admission, leucocytes count, bilateral lung GGO, and lung consolidation were linearly related to the length of symptoms relief (P < 0.05). By using the scoring system, we found that the time of hospitalization and symptoms relief lengthened as the scores increased.
Conclusions: This study described the clinical course of patients with moderate-type COVID-19, and found that ordinary patients with moderate-type COVID-19 in outbreak areas were more serious and needed stronger treatment and longer treatment time. Onset of symptoms to admission, ESR, leucocytes count, and bilateral lung GGO can be effective predictors of the length of hospitalization. And onset of symptoms to admission, leucocytes count, bilateral lung GGO, and lung consolidation can be effective predictors of the amount of time until symptoms relief. Most importantly, we have created a scoring system, which could contribute to the diagnosis and treatment of COVID-19.
Introduction
In December 2019, an emerging pneumonia caused by severe acute respiratory syndrome-novel coronavirus 2 (SARS-CoV-2) infection, named coronavirus disease-19 (COVID-19), appeared in Wuhan, Hubei Province (1). Most of the early patients were exposed to the Huanan seafood wholesale market (2). Like MERS-COV and SARS-COV, the coronavirus (SARS-CoV2) belonged to the beta coronavirus family (3). Current research suggested that it may have originated from bats and pangolins (4, 5). SARS-CoV-2 can be passed from person to person (6). The World Health Organization has identified COVID-19 as a global pandemic (7). So far, around 100,000 cases of COVID-19 have been reported in China, and around 119,000,000 cases have been reported outside of China, which posed an enormous threat to human health.
The clinical features of COVID-19 have been reported in severe and non-severe patients, as well as in surviving and deceased patients (8, 9). However, at present no studies have reported the clinical course of patients with moderate-type COVID-19. According to the 7th Chinese edition of the COVID-19 Diagnosis and Treatment Guidelines, patients are divided into four types based on their results of clinical examination, including mild-type, moderate-type, serious-type, and critical-type (10). There was a large number of patients with moderate-type COVID-19 around the world. A study reported that 262 patients were confirmed to have COVID-19 from 57 hospitals in Beijing, and 192 (73%) of them had moderate-type COVID-19 (11). In another study about COVID-19 from Renmin Hospital of Wuhan University, moderate patients also accounted for 56% (12). Our data showed that 65% (66/101) of patients in Central Hospital of Wuhan and 72% (43/60) of patients in Yiyang had moderate-type COVID-19. Studies from Egypt, USA, and Spain reported that 51.5, 36, and 44.6% of patients had moderate cases, respectively (13–15).
It is meaningful to pay attention to patients with moderate-type COVID-19. On the one hand, the large number of patients with moderate-type COVID-19 is a dangerous source of infection. On the other hand, the sooner the moderate-type patients are cured, the sooner they can be released from isolation and return to normal life, which is beneficial to reduce social panic and promote the restoration of social and economic life. For patients with moderate-type COVID-19, the relevant factors for disease progression have not been completely understood, which led to the vagueness of criteria for these patients to hospital admission in the US CDC (Centers for Disease Control and Prevention) COVID-19 guidelines (16). In China, almost all patients with COVID-19 received free treatment in the hospital, which is beneficial for us to describe and compare the clinical course of patients with moderate-type COVID-19 in the outbreak area (Wuhan) and in the imported area (Yiyang).
The aims of the study were to describe the clinical course of moderate-type patients with COVID-19 from Wuhan City and Yiyang City, and to explore factors relevant to the length of hospitalization and symptoms relief. The results of our research can be helpful for the appropriate treatment of moderate-type patients with COVID-19 from outbreak areas and imported areas. The study also found related factors to predict the length of hospitalization and symptoms relief, and offered an effective scoring system to value the severity and treatment of COVID-19.
Methods
Study Design and Participants
A total of 107 moderate-type patients discharged from December 16, 2019 to February 21, 2020 were enrolled in this study. This retrospective cohort study included three groups of moderate-type patients with COVID-19: ordinary inpatients from the Central Hospital of Wuhan, infected medical staff from the Central Hospital of Wuhan, and ordinary inpatients from Yiyang City. Due to death after pneumonia exacerbation, two cases of ordinary inpatients with moderate-type COVID-19 from Wuhan were excluded from the study. Among all the confirmed patients in Yiyang, 43 cases of inpatients with moderate-type COVID-19 were enrolled, and none of them were medical workers. At present, all the patients with COVID-19 in Yiyang have been completely cured and discharged. All data were collected through the hospital electrical records system. This study was approved by the Ethic Committee of COVID-19 designated hospitals (Ethic number: K-20040).
Data Collection
Demographic data, medical history, contact history, comorbidities, symptoms, signs, laboratory tests (such as leucocytes count, lymphocytes count, C-reactive protein, erythrocyte sedimentation rate (ESR), D-dimer, lactate dehydrogenase (LDH), creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), etc.), chest computed tomography (CT) scans, and hospitalization treatment (i.e., antiviral treatment, glucocorticoid therapy, respiratory support, intravenous immunoglobulin therapy, and antibiotic therapy) were collected from the electronic medical records system. Unilateral GGO (ground glass opacity), bilateral lung GGO, and lung consolidation were all based on the results from radiologists. All data were checked by two physicians (XLV and ML).
Definition
All the enrolled patients were diagnosed and discharged based on the 7th Chinese edition of the COVID-19 Diagnosis and Treatment Guidelines (10). The diagnostic criteria of moderate-type COVID-19 included: presenting clinical symptoms of the respiratory tract; having radiological evidence of viral pneumonia; respiratory rate <30 per min; oxygen saturation >93% when breathing ambient air, or ratio of arterial oxygen tension (PaO2) over inspiratory oxygen fraction (FIO2) >300 mmHg (1 mmHg equals to 0.133 kPa); lung imaging indicating multilocular lesions or progression of lesions <50% within 48 h. The criteria for discharge included: at least 3 days without fever, substantial improvement in both lungs in chest CT, clinical remission of respiratory symptoms, and two pharyngeal swab samples obtained at least 24 h apart that were negative for SARS-CoV-2 RNA (10). The date of onset was defined as the day when symptoms appeared. Onset of symptoms to admission was defined as the time from the day of symptoms onset to the day when patients were admitted to the hospital. The length of symptoms relief was defined as the time from the day of symptoms onset to the day when symptoms were improved to discharge.
Laboratory Procedures
To confirm SARS-CoV-2 infection, pharyngeal swab specimens provided by Wuhan Central Hospital or designated Hospitals of Yiyang were detected by RT-PCR in the local Center for Disease Control and Prevention. During hospitalization, the doctor re-examined the patients based on the alleviation of symptoms and chest CT scans. The frequency of laboratory tests and imaging tests was determined by the attending physicians.
Statistical Analysis
Continuous and categorical variables were presented as median (IQR) and n (%), respectively. We used the Whitney U test, χ2 test, or Fisher's exact test to compare the differences of inpatients with moderate-type COVID-19 from Wuhan City and Yiyang City. To explore factors relevant to the length of hospitalization and symptoms relief, we used automatic linear modeling. Then the predicted factors were subjected to multiple linear regression analysis. The correlation scores of these factors was decided by their regression coefficient (β). When the regression coefficient (β) was < 0.05, the correlation score was 1 point; when the regression coefficient (β) was < 0.01, the correlation score was 2 points. The total correlation score was calculated for each patient by summation of the score points. And comparisons between the total score groups were analyzed with one-way ANOVA, and comparisons between two groups were analyzed with a least significant difference (LSD) test. All statistics were analyzed using SPSS (the version 20.0 software). For unadjusted comparisons, a two-sided α of < 0.05 was considered statistically significant.
Results
Comparison of the Characteristics of Moderate-Type COVID-19 in Ordinary Patients and Infected Medical Staff From Wuhan
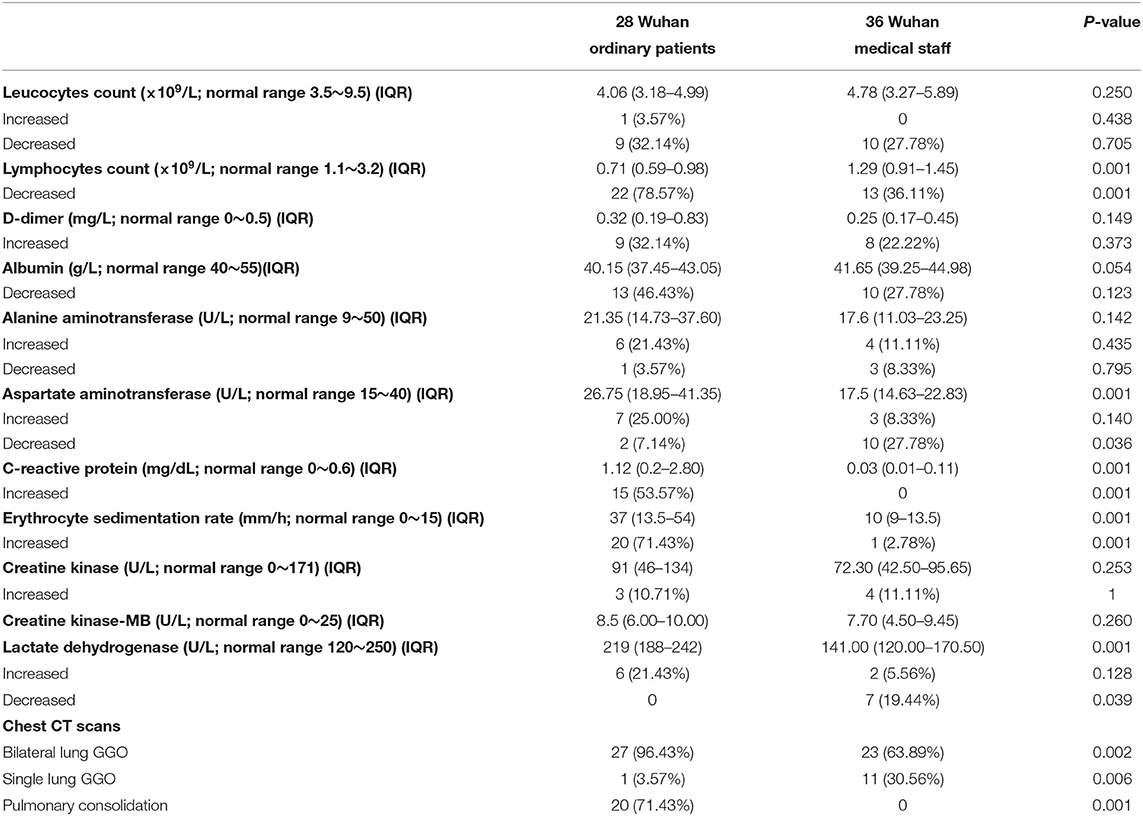
Ordinary hospitalized patients with moderate-type COVID-19 were older than infected medical staff [51 years (IQR, 41.5–59.75) vs. 34 years (IQR, 28–37)] (P < 0.001), and there was no significant difference in gender distribution (Table 1). Six ordinary patients had visited the Huanan seafood wholesale market, and none of infected medical staff had been to the seafood market. Ordinary patients had more comorbidities [5 (17.86%) vs. 0] (P = 0.030), as well as a longer time from the onset of symptoms to hospital admission [6 days (IQR, 3.25–8.75) vs. 3 days (IQR, 2-5.75)] (P = 0.027) (Table 1). Compared with ordinary patients, infected medical staff were more prone to suffer from fatigue [4 (14.29%) vs. 14 (38.89%)] (P = 0.030) and myalgia [2 (7.14%) vs. 16 (44.44%)] (P = 0.001). Other symptoms presented no significant differences between the two groups (Table 1). Compared with infected medical staff, the levels of AST, C-reactive protein, ESR, and LDH in ordinary patients were significantly increased (P < 0.01), and lymphocytes were significantly decreased (P < 0.001) (Table 2). Chest CT scans showed that ordinary patients had more bilateral lung GGO and lung consolidation (P < 0.01), while unilateral GGO was more likely to appear in infected medical staff (P < 0.01) (Table 2). There was no significant differences between treatment (i.e., antiviral treatment, glucocorticoid therapy, respiratory support, intravenous immunoglobulin therapy, and antibiotic therapy) (Table 1). However, compared with infected medical staff, the length of hospitalization of ordinary patients was longer [26 days (IQR, 17.75–30.25) vs. 21 days (IQR, 18–25)] (P = 0.041), as well as the length of symptoms relief [32 days (IQR, 24.5–36) vs. 26 days (IQR, 23–28)] (P = 0.002) (Table 1).
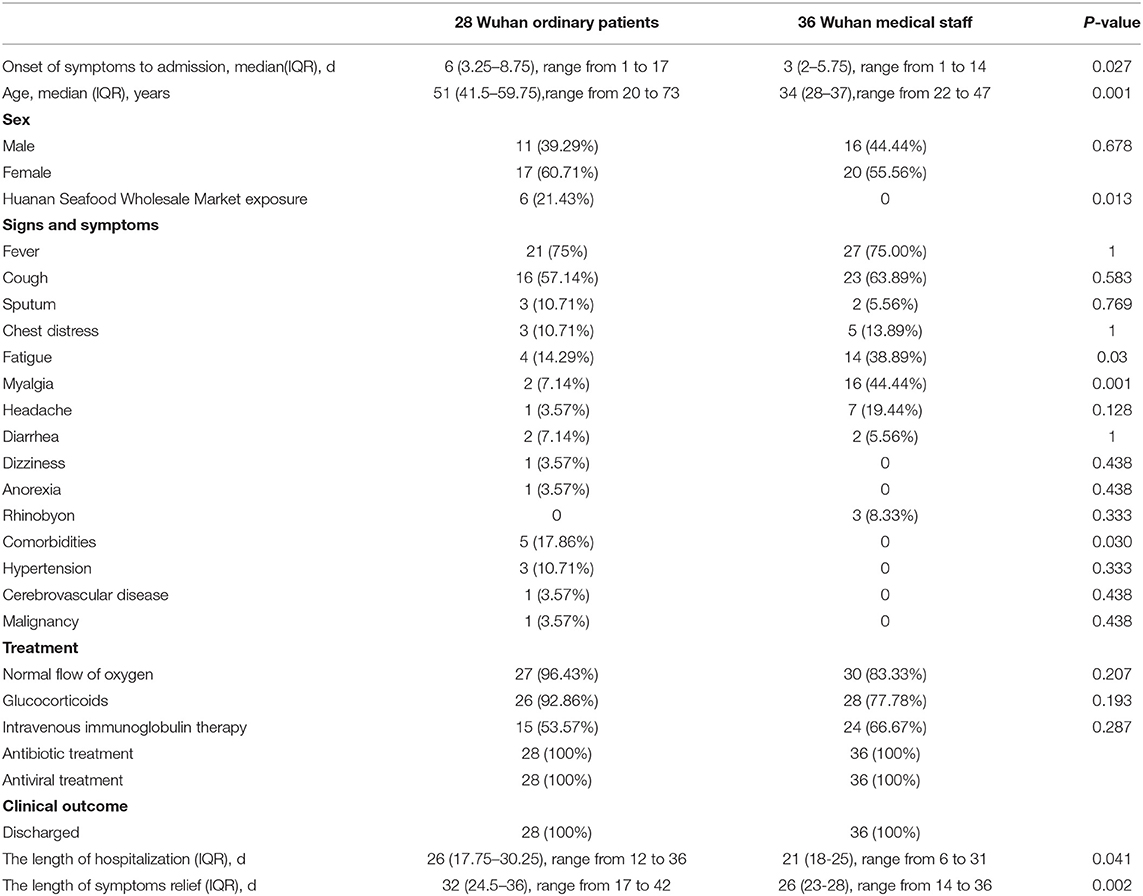
Table 1. Demographic, clinical characteristics, treatment, and outcome of inpatients with moderate-type COVID-19 from Wuhan.
Comparison of the Characteristics of Moderate-Type COVID-19 in Ordinary Patients From Wuhan and Yiyang
There was no significant difference in ordinary patients from Wuhan (28 cases) and Yiyang (43 cases) in age, gender, comorbidities, and the time from the onset of symptoms to hospital admission (Table 3). The ordinary patients from Wuhan were more prone to present with fever (P = 0.001), and other symptoms had no significant differences between the two groups (Table 3). Compared with ordinary patients from Yiyang, ordinary patients from Wuhan had fewer white blood cells and lymphocytes (P < 0.01), while C-reactive protein and LDH were increased significantly (P < 0.01) (Table 4). Chest CT scans showed that more bilateral lung GGO and lung consolidation were seen in Wuhan ordinary patients (P < 0.01), while unilateral GGO was more likely to appear in Yiyang patients (P < 0.01) (Table 4). All patients in both groups received antiviral treatment, and the number of patients receiving a normal flow of oxygen, antibiotics, systemic glucocorticoid, and intravenous immunoglobulin was more in Wuhan than that in Yiyang (P < 0.001) (Table 3). Compared with Yiyang patients, the length of hospitalization of Wuhan ordinary patients was longer {26 days (IQR, 17.75–30.25) vs. 11 days [IQR, (8–15)]} (P < 0.001), as well as the length of symptoms relief [32 days (IQR, 24.5–36) vs. 16 days (IQR, 14–20)] (P < 0.001) (Table 3).
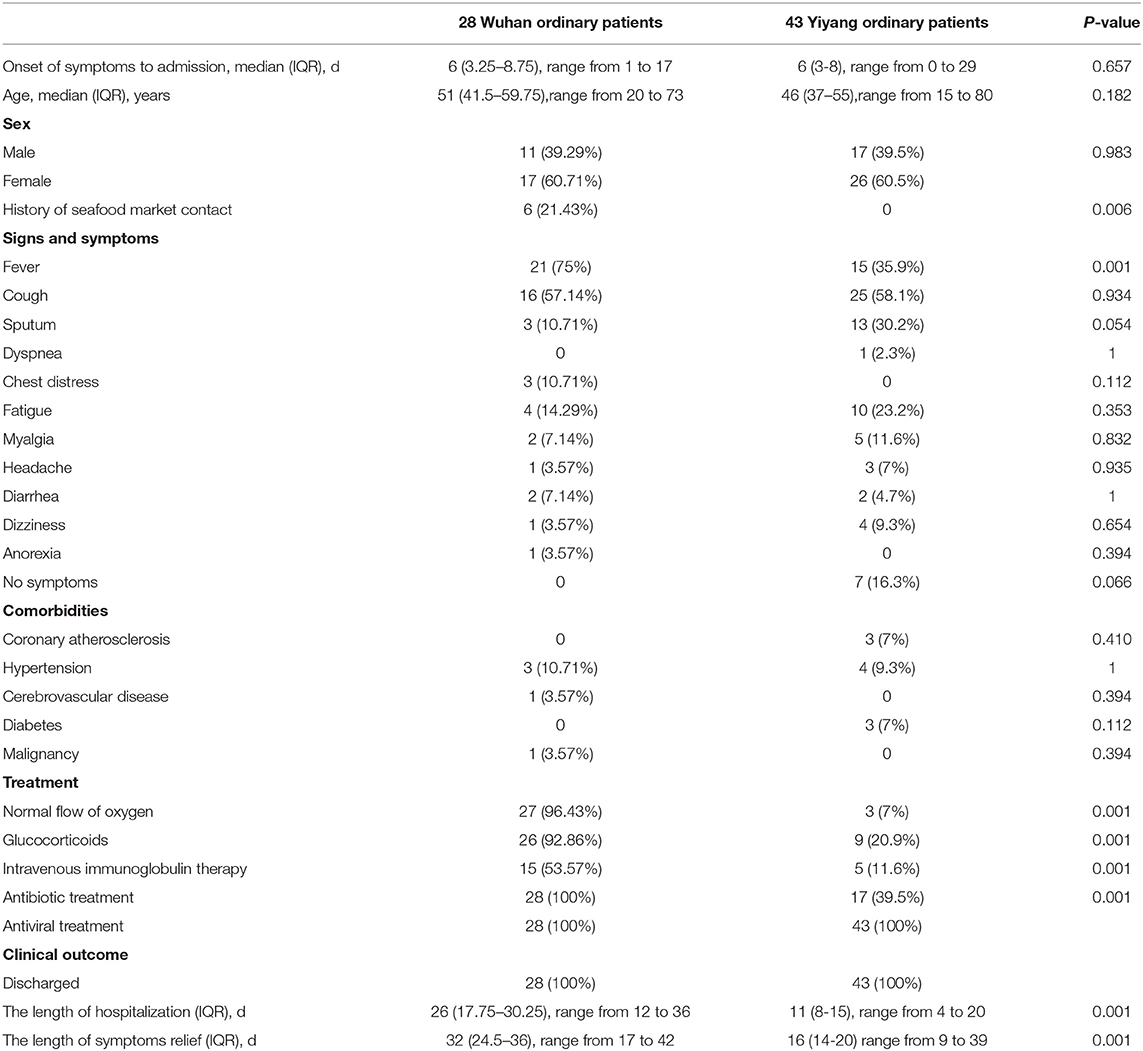
Table 3. Demographic, clinical characteristics, treatment, and outcome of ordinary patients with moderate-type COVID-19 from Wuhan and Yiyang.
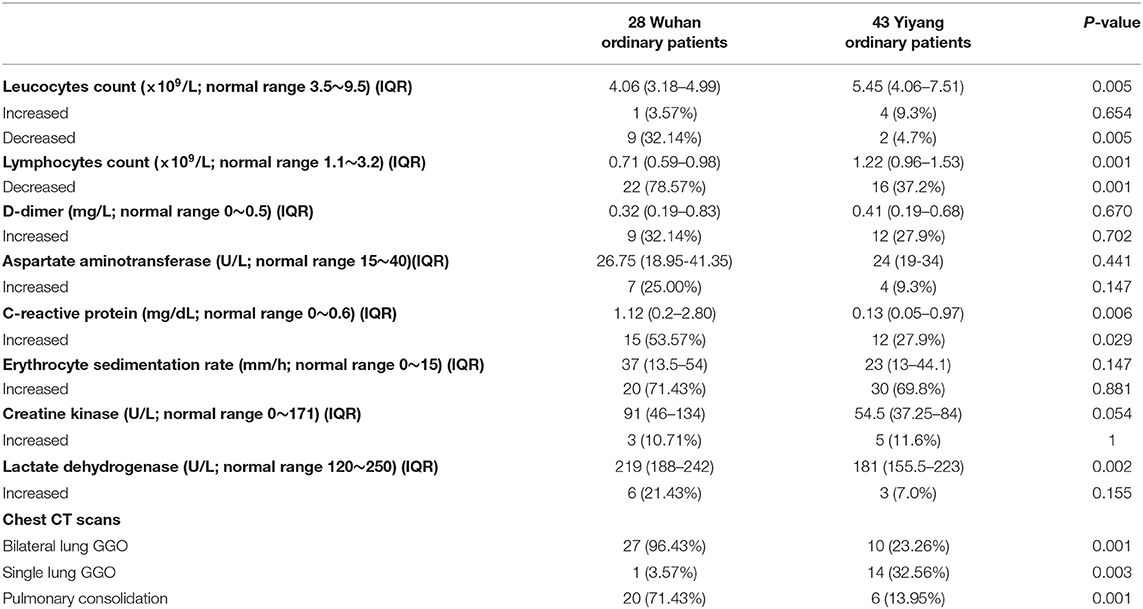
Table 4. Laboratory examination of ordinary patients with moderate-type COVID-19 from Wuhan and Yiyang.
Sixteen Yiyang patients had travel history in Wuhan. In order to verify whether the travel history affected the above results, we compared the characteristics of the patients. It was found that patients with travel history were more likely to present fatigue (P = 0.016). There were no obvious differences in other aspects (Supplementary Tables 1, 2).
Analyzing the Relevant Factors of the Length of Hospitalization and Symptoms Relief of Inpatients With Moderate-Type COVID-19
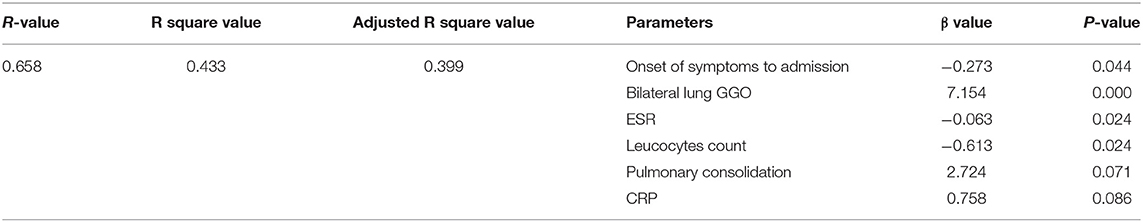
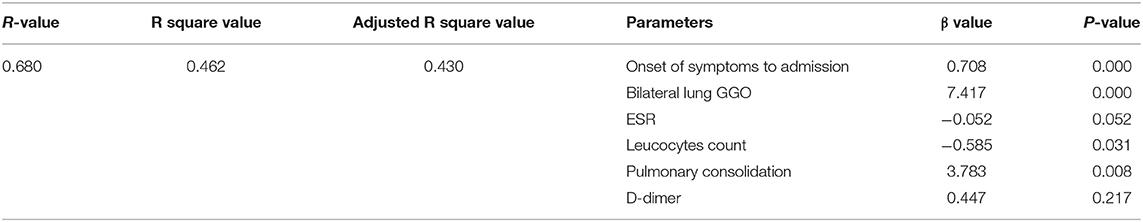
The tests of inflammation (leucocytes count, C-reactive protein, ESR), systemic damage (LDH, CK), lymphopenia (lymphocytes count), coagulopathy (D-dimer), and lung injuries (bilateral lung GGO and lung consolidation) were reported to be related to the severity of COVID-19 (17–20). In order to value the length of hospitalization and symptoms relief, we analyzed these above clinical indicators together with onset of symptoms to admission and demography (gender and age). The length of hospitalization and symptoms relief were similar to normal distributions (Figures 1, 2), so we used automatic linear modeling to predict the relevant factors. We found that factors related to the length of hospitalization included onset of symptoms to admission, ESR, CPR, leucocytes count, bilateral lung GGO, and lung consolidation (Figure 3); factors related to the length of symptoms relief included onset of symptoms to admission, ESR, D-dimer, leucocytes count, bilateral lung GGO, and lung consolidation (Figure 4). Then multiple linear regression analysis was used to confirm the results. As to the length of hospitalization, the R value and R square value were 0.658 and 0.433, respectively (Table 5). As to the length of symptoms relief, the R value and R square value were 0.680 and 0.462, respectively (Table 6). The results indicated that these relevant factors were linearly related to the length of hospitalization and symptoms relief, and can explain more than 40% of the variability of them. But the results of regression coefficient (β) showed that onset of symptoms to admission, ESR, leucocytes count, and bilateral lung GGO were linearly related to the length of hospitalization (P < 0.05); onset of symptoms to admission, leucocytes count, bilateral lung GGO, and lung consolidation were linearly related to the length of symptoms relief (P < 0.05) (Tables 5, 6). Except for bilateral lung GGO, onset of symptoms to admission, ESR, and leucocytes count were negatively correlated with the length of hospitalization (Tables 5, 6). And except for leucocytes count, onset of symptoms to admission, bilateral lung GGO, and lung consolidation were positively correlated with the length of symptoms relief (Tables 5, 6).
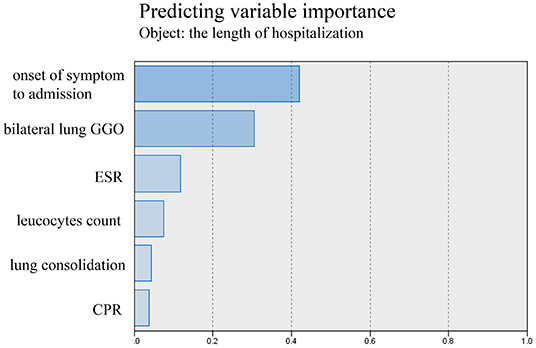
Figure 3. Using automatic linear modeling to predict relevant factors of the length of hospitalization.
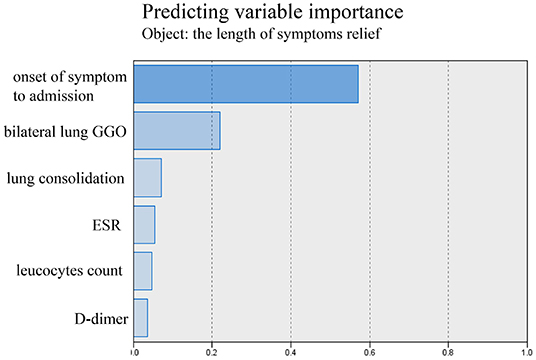
Figure 4. Using automatic linear modeling to predict relevant factors of the length of symptoms relief.
Analyzing the Difference of the Length of Hospitalization and Symptoms Relief of Inpatients With Moderate-Type COVID-19 in Scoring Groups
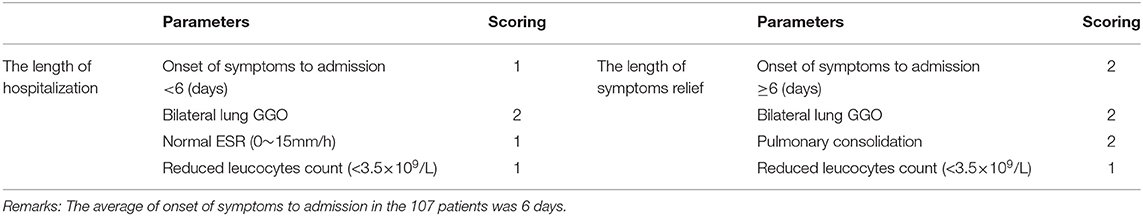
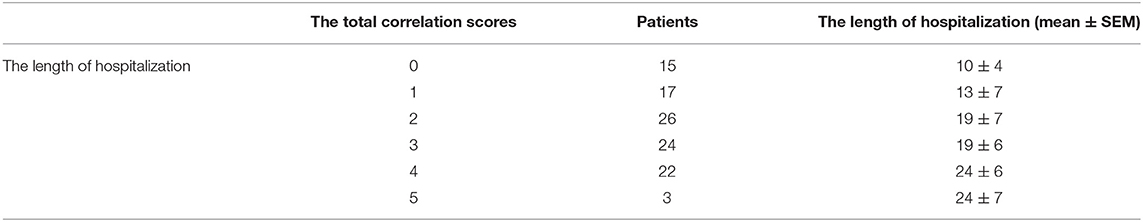
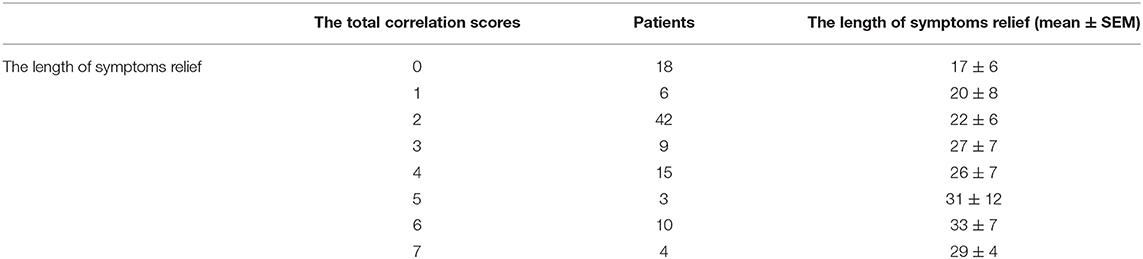
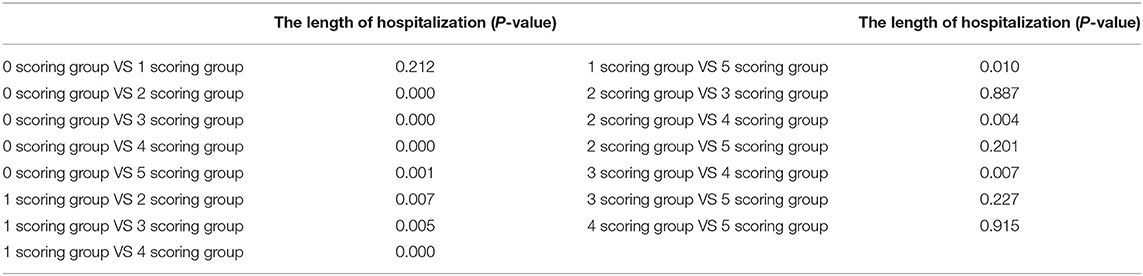
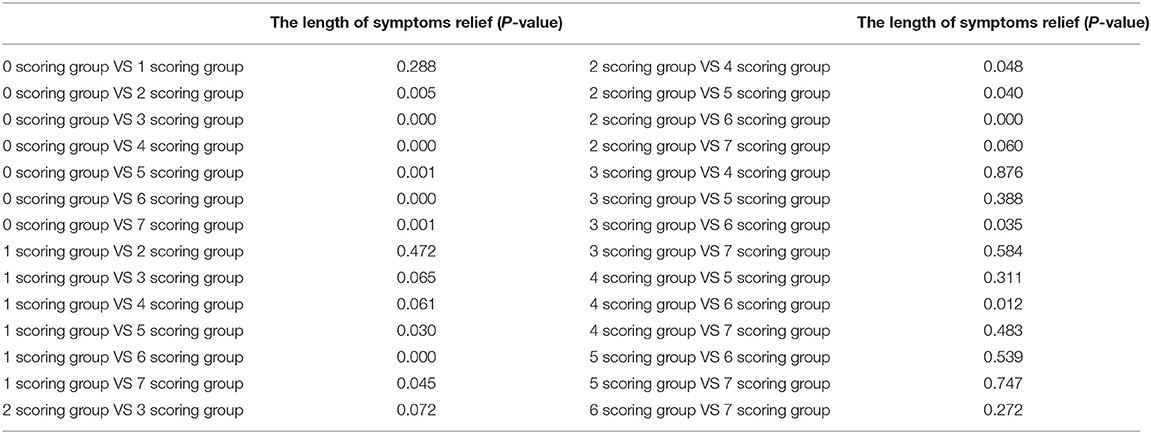
In order to conveniently use these related factors for clinical evaluation, we defined that when the regression coefficient (β) was <0.05, the related factor scored 1 point; when the regression coefficient (β) was <0.01, the related factor scored 2 points. Combining the clinical situation and the results of multiple linear regression analysis, the scoring criteria of relevant factors for the length of hospitalization and symptoms relief are described in Table 7, and all other conditions scored 0 points. The total correlation score was calculated for each patient by summation of the score points (Supplementary Materials). Then comparisons between the total score groups were analyzed with one-way ANOVA. With increasing scores, the time of hospitalization and symptoms relief of inpatients with moderate-type COVID-19 lengthened (Tables 8, 9). Compared with the 0 and 1 scoring groups, other scoring groups had a longer time of hospitalization (P < 0.01); except the 5 scoring group, the length of hospitalization in the 4 scoring group was longer than other scoring groups (P < 0.01) (Table 10). Compared with the 0 scoring group, other scoring groups had a longer time of symptoms relief, except the 1 scoring group (P < 0.01). Compared with the 1 and 2 scoring groups, the length of symptoms relief in above 4 scoring groups was longer (P < 0.1). Compared with the 3 and 4 scoring groups, the length of symptoms relief in the 6 scoring group was longer (P < 0.05) (Table 11).
Discussion
Pneumonia (COVID-19) caused by the infection of the new coronavirus (SARS-CoV-2) has become the first worldwide pandemic disease in the 21st century (21). The genomic characterization suggested that SARS-CoV-2 belonged to the beta coronavirus family (22). However, it is more contagious than SARS-COV (6), and has infected about 119,000,000 people around the world up to now, posting a serious burden on the world health system. In order to quickly cut off the spread of the virus, effectively treating infected patients is the key to control the outbreak of COVID-19. Our study and other studies showed that a large number of patients had moderate-type COVID-19 (11–15). However, the relevant factors for disease progression in patients with moderate-type COVID-19 were not completely clear. This study described the characteristics of patients with moderate-type COVID-19 in the outbreak area (Wuhan) and the imported area (Yiyang), and found that onset of symptoms to admission, ESR, leucocytes count, and bilateral lung GGO were linearly related to the length of hospitalization; onset of symptoms to admission, leucocytes count, bilateral lung GGO, and lung consolidation were linearly related to the length of symptoms relief. We also offered an effective scoring system to predict the length of hospitalization and symptoms relief, which could help to determine the severity and treatment of moderate-type patients with COVID-19.
Our study enrolled 71 ordinary patients (28 inpatients from Wuhan and 43 inpatients from Yiyang) with moderate-type COVID-19, and about 20% of them contracted or previously had comorbidities. However, a previous report from Wuhan showed a larger number of COVID-19 patients (30%) with comorbidities (23). The possible reason was that severe patients were excluded in this study. D-dimer [Wuhan 9 (32.14%) vs. Yiyang 12 (27.9%)], C-reactive protein [Wuhan 15 (53.57%) vs. Yiyang 12 (27.9%)], and ESR [Wuhan 20 (71.43%) vs. Yiyang 30 (69.8%)] were increased in more than 20% of the ordinary patients. Lymphopenia occurred in 22 ordinary patients [78.57%] from Wuhan and 16 [37.2%] from Yiyang. These results suggested that ordinary patients with moderate-type COVID-19 may develop abnormal coagulation, inflammation, and immunodeficiency. Results of chest CT scans indicated that bilateral lung GGO and lung consolidation were observed more in the ordinary patients with moderate-type COVID-19 from Wuhan than in those from Yiyang, suggesting that the ordinary patients in Wuhan had more severe lung injuries. From the results of serum tests and CT scans, we found that the ordinary patients with moderate-type COVID-19 in Wuhan (the outbreak area) had more serious disease than those patients in Yiyang (the imported area). The reasons may be as follows: Wuhan was the outbreak area; the virus loads invading the patient were greater; and the chance of repeated infection of patients was increased. The virus loads are related to the severity of COVID-19, which may contribute to the guidance on clinical diagnosis and treatment (24). However, there were no relevant studies to clearly explain this phenomenon. Except antiviral treatment, ordinary patients from Yiyang received fewer other treatments than those from Wuhan, suggesting that the treatment between the outbreak area and the imported area was different. Although the treatment of ordinary inpatients in Wuhan had been strengthened, the length of hospitalization and symptoms relief were still much longer, which implied systemic glucocorticoids and intravenous immunoglobulin cannot effectively clear the SARS-CoV-2 infection and shorten the course of disease.
In addition, we compared the clinical course of ordinary patients (28 cases) and infected medical staff (36 cases) from Wuhan. When displaying symptoms, medical staff would go to hospital more promptly than ordinary patients [6 (IQR 3.25–8.75] vs. 3 (IQR 2–5.75)], which may be due to occupational factors. Similar to ordinary patients, the most common symptoms of infected medical staff were fever and cough. However, the early symptoms of viral infection (fatigue and myalgia) were more likely to appear in infected medical staff, due to their earlier hospitalization. Compared with infected medical staff, the indicators of organ damage (AST, LDH) and inflammation (C-reactive protein, ESR) in ordinary patients were significantly increased, and lymphocytes were significantly reduced. Furthermore, chest CT scans showed that ordinary patients had more bilateral lung GGO and lung consolidation. These results of serum tests and imaging suggested that the ordinary patients in Wuhan were more serious ill than infected medical staff. Part of the reason may be that infected medical staff were younger and had no underlying diseases, which were reported to be related to the severity of COVID-19 (9). The occupational factors also allowed infected medical staff to protect themselves better and seek medical treatment quickly, which implied that protective measures and timely medical treatment might inhibit the progress of COVID-19 and reduce the severity of the disease. Consistent with the severity of COVID-19, the length of hospitalization and symptoms relief were longer in ordinary patients with moderate-type COVID-19.
According to the above results, compared to infected medical staff from Wuhan and ordinary inpatients with moderate-type COVID-19 from Yiyang, lymphopenia, elevated C-reactive protein, increased LDH, bilateral lung GGO, and lung consolidation were more likely to appear in ordinary inpatients with moderate-type COVID-19 from Wuhan. These indicators were reported to be related to the severity of the pneumonia (25–27). So the length of hospitalization and symptoms relief were longer in ordinary patients with moderate-type COVID-19 from Wuhan.
In this study, apart from describing the characteristics of patients with moderate-type COVID-19 in the outbreak area (Wuhan) and the imported area (Yiyang), we also found that onset of symptoms to admission, ESR, leucocytes count, and bilateral lung GGO were linearly related to the length of hospitalization; onset of symptoms to admission, leucocytes count, bilateral lung GGO, and lung consolidation were linearly related to the length of symptoms relief. Onset of symptoms to admission was negatively correlated with the length of hospitalization and positively correlated with the length of symptoms relief. The result suggested that some moderate-type patients may recover without treatment, which was consistent with the US CDC COVID-19 guidelines (16). But prompt medical treatment can shorten the process of this disease. Normal ESR and reduced leucocytes count seemed to accompany a longer length of hospitalization, and leucocytes count was also negatively correlated with the length of symptoms relief. Leucocytes count was an important indicator related to inflammation and immunity (28, 29), so we speculated that appropriate inflammation and immunity may help moderate patients clear the virus. Ali A Ghweil and his colleagues reported that reduced leucocytes count was more likely to appear in patients with severe COVID-19 (30). Functional exhaustion of both innate and adaptive immune response was positively related to the severity of COVID-19 (31). The World Health Organization (WHO) has published guidance recommending no corticosteroids for those with non-severe COVID-19 (32), which seemed to prove our speculation to some extent. Different from our results, ESR was reported to be positively related to the severity of this disease in some studies (33–35). The reason might be that severe-type patients with COVID-19 were also included in these studies. Lung injuries (bilateral lung GGO or lung consolidation) have been confirmed to be an important indicator of the severity of this disease (27), as they were accompanied with a longer period of hospitalization and symptoms relief. Moreover, the total correlation scores can work well to evaluate the length of hospitalization and symptoms relief in all 107 inpatients with moderate-type COVID-19. With increasing scores, the time of hospitalization and symptoms relief of inpatients lengthened. The results suggested that the scoring system was an effective instrument to determine the severity and the treatment of COVID-19.
Limitations
There were several limitations in this study. First, the study did not include patients with moderate-type COVID-19 who worsened or died. Second, since the patients in this study were not severe, the attending physicians reviewed the serum tests and imaging examinations less frequently and not in full during the hospitalization. So we could not describe the changes of the treatment process of these patients in detail. Third, due to the small number of cases in this study, multicenter or nationwide studies are warranted to validate these findings.
Conclusions
This study described the clinical course of patients with moderate-type COVID-19, and found that patients with moderate-type COVID-19 in outbreak areas had a more serious infection and needed stronger treatment and longer treatment time. Onset of symptoms to admission, ESR, leucocytes count, and bilateral lung GGO can be effective predictors of the length of hospitalization. And onset of symptoms to admission, leucocytes count, bilateral lung GGO, and lung consolidation can be effective predictors of the length of symptoms relief. Most importantly, we have created an effective scoring system to determine the length of hospitalization and symptoms relief in inpatients with moderate-type COVID-19, which could contribute to the diagnosis and treatment of COVID-19.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethic Committee of Central Hospital of Wuhan; the Ethic Committee of Fourth People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XLi, XLv, CS, and JM collected the epidemiological and clinical data. XLi, XLv, YZ, and YJ summarized all data. XLi, MJ, RH, and ML drafted the manuscript. JM and XLi revised the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Emergency Project of Prevention and Control for COVID-19 of Central South University (Grant 16026000 to JM).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.593109/full#supplementary-material
References
1. Li H, Liu L, Zhang DY, Xu JY, Dai HP, Tang N, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. (2020) 395:1517–20. doi: 10.1016/S0140-6736(20)30920-X
2. Chen NS, Zhou M, Dong X, Qu JM, Gong FY, Han Yang, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
3. Ceribelli A, Motta F, De SM, Ansari AA, Ridgway WM, Gershwin ME, et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J. Autoimmun. (2020) 109:102442. doi: 10.1016/j.jaut.2020.102442
4. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat. Med. (2020) 26:450–2. doi: 10.1038/s41591-020-0820-9
5. Lam TT, Jia N, Zhang YW, Shum MH, Jiang JF, Zhu HC, et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. (2020) 583:282–5. doi: 10.1038/s41586-020-2169-0
6. Liu T, Hu JX, Kang M, Li FL, Zhong HJ, Xiao JP, et al. Transmission dynamics of 2019 novel coronavirus (2019-nCoV). bioRxiv [Preprint]. (2020) 919787. doi: 10.1101/2020.01.25.919787
7. Benvenuto D, Giovanetti M, Salemi M, Prosperi M, De FC, Junior AL, et al. The global spread of 2019-nCoV: a molecular evolutionary analysis. Pathog Glob Health. (2020) 114:64–7. doi: 10.1080/20477724.2020.1725339
8. Gong J, Ou JY, Qiu XP, Jie YS, Chen YQ, Yuan LX, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk Nomogram in Wuhan and Guangdong, China. Clin. Infect. Dis. (2020) 71:833–40. doi: 10.1093/cid/ciaa443
9. Zhou F, Yu T, Du RH, Fan GH, Liu Y, Liu ZB, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
10. China National Health Commission. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. 7th ed. (2020). Available online at: http://kjfy.meetingchina.org/msite/news/lists/cn/1163.html
11. Tian SJ, Hu N, Lou J, Chen K, Kang XQ, Xiang ZJ, et al. Characteristics of COVID-19 infection in Beijing. J. Infect. (2020) 80:401–6. doi: 10.1016/j.jinf.2020.02.018
12. Liu R, Ma QF, Han HF, Su HW, Liu F, Wu KL, et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin. Chem. Lab. Med. (2020) 58:1121–4. doi: 10.1515/cclm-2020-0220
13. Ramadan HK, Mahmoud MA, Aburahma MZ, Elkhawaga AA, El-Mokhtar MA, Sayed IM, et al. Predictors of severity and co-infection resistance profile in COVID-19 patients: first report from upper egypt. Infect Drug Resist. (2020) 13:3409–22. doi: 10.2147/IDR.S272605
14. Miatech JL, Yaslik CP, Tarleton HE, West D, Kellum W, McKnight M, et al. Retrospective analysis of inflammatory markers and patient characteristics in hospitalized Covid-19 patients: an early experience in Louisiana. Cureus. (2020) 12:e10257. doi: 10.7759/cureus.10257
15. Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. (2020) 46:2200–11. doi: 10.1007/s00134-020-06192-2
16. National Center for Immunization and Respiratory Diseases (NCIRD). Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). (2020). Available online at: https://www.cdc.gov/ncird/index.html
17. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. (2020) 57:389–99. doi: 10.1080/10408363.2020.1770685
18. Zhu YJ, Du ZQ, Zhu YF, Li WF, Miao HJ, Li Z. Evaluation of organ function in patients with severe COVID-19 infections. Med Clin. (2020) 155:191–6. doi: 10.1016/j.medcle.2020.05.015
19. Yarza R, Bover M, Paredes D, López-López F, Jara-Casas D, Castelo-Loureiro A, et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur. J. Cancer. (2020) 135:242–50. doi: 10.1016/j.ejca.2020.06.001
20. Zhang YH, He LW, Chen HX, Lu SY, Xiong YF, Liu J, et al. Manifestations of blood coagulation and its relation to clinical outcomes in severe COVID-19 patients: retrospective analysis. Int J Lab Hematol. (2020) 42:766–72. doi: 10.1111/ijlh.13273
21. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report-75. Geneva: World Health Organization (2020).
22. Lu RJ, Zhao X, Li J, Niu PH, Yang B, Wu HL, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
23. Huang CL, Wang YM, Li XW, Ren LL, Zhao JP, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
24. Liu Y, Yan L, Wan LG, Xiang TX, Le AP, Liu JM, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. (2020) 20:656–7. doi: 10.1016/S1473-3099(20)30232-2
25. Wang DW, Hu B, Hu C, Zhu FF, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
26. Wan SX, Xiang Y, Fang W, Zheng Y, Li BQ, Hu YJ, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. (2020) 92:797–806. doi: 10.1002/jmv.25783
27. Liu KC, Xu P, Lv WF, Qiu XH, Yao JL, Gu JF, et al. CT manifestations of coronavirus disease-2019: a retrospective analysis of 73 cases by disease severity. Eur J Radiol. (2020) 126:108941. doi: 10.1016/j.ejrad.2020.108941
28. Venturini L, Bacchi S, Capelli E, Lorusso L, Ricevuti G, Cusa C. Modification of immunological parameters, oxidative stress markers, mood symptoms, and well-being status in CFS patients after probiotic intake: observations from a pilot study. Oxid Med Cell Longev. (2019). doi: 10.1155/2019/1684198
29. Preissner KT, Fischer S, Deindl E. Extracellular RNA as a versatile DAMP and alarm signal that influences leukocyte recruitment in inflammation and infection. Front Cell Dev Biol. (2020) 8:619221. doi: 10.3389/fcell.2020.619221
30. Ghweil AA, Hassan MH, Khodeary A, Mohamed AO, Mohammed HM, Abdelazez AA, et al. Characteristics, outcomes and indicators of severity for COVID-19 among sample of ESNA quarantine hospital's patients, egypt: a retrospective study. Infect Drug Resist. (2020) 13:2375–83. doi: 10.2147/IDR.S263489
31. Ruetsch C, Brglez V, Crémoni M, Zorzi K, Fernandez C, Boyer-Suavet S, et al. Functional exhaustion of Type I and II interferons production in severe COVID-19 patients. Front Med. (2020) 7:603961. doi: 10.3389/fmed.2020.603961
32. WHO. Corticosterids for COVID-19: Living guidance 2 September 2020. (2020). Available online at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Corticosteroids-2020.1 (assessed September 14, 2020).
33. Izcovich A, Ragusa MA, Tortosa F, Lavena MM, Agnoletti C, Bengolea A, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE. (2020) 15:e0241955. doi: 10.1371/journal.pone.0241955
34. Yao ZX, Zheng XY, Zheng Z, Wu K, Zheng JH. Construction and validation of a machine learning-based nomogram: a tool to predict the risk of getting severe coronavirus disease 2019 (COVID-19). Immun Inflamm Dis. (2021). doi: 10.1002/iid3.421. [Epub ahead of print].
Keywords: COVID-19, SARS-CoV-2, moderate type, treatment, clinical course
Citation: Liao X, Lv X, Song C, Jiang M, He R, Han Y, Li M, Zhang Y, Jiang Y and Meng J (2021) A Retrospective Cohort Study on the Clinical Course of Patients With Moderate-Type COVID-19. Front. Public Health 9:593109. doi: 10.3389/fpubh.2021.593109
Received: 09 August 2020; Accepted: 19 March 2021;
Published: 26 April 2021.
Edited by:
Mehdi Mirsaeidi, University of Miami, United StatesReviewed by:
Varadha Balaji Venkadakrishnan, Dana–Farber Cancer Institute, United StatesAbdolrazagh Hashemi Shahraki, University of Miami, United States
Copyright © 2021 Liao, Lv, Song, Jiang, He, Han, Li, Zhang, Jiang and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Meng, mengjie@csu.edu.cn
 Xiaohua Liao
Xiaohua Liao Xin Lv1
Xin Lv1 Mao Jiang
Mao Jiang Yupeng Jiang
Yupeng Jiang