
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 30 October 2020
Sec. Children and Health
Volume 8 - 2020 | https://doi.org/10.3389/fpubh.2020.595379
Background: It has been estimated that 27.8 million neonates will die worldwide between 2018 and 2030 if no improvements in neonatal and maternal care take place. The aim of this study was to determine the rate, risk factors, and causes of neonatal mortality in Jordan.
Methods: In August 2019, an electronic stillbirths and neonatal deaths surveillance system (JSANDS) was established in in three large cities through five hospitals. Data on all births, neonatal mortality and their causes, and other characteristics in the period between August 2019 and January 2020 were exported from the JSANDS and analyzed.
Results: A total of 10,328 births [10,226 live births (LB) and 102 stillbirths] were registered in the study period, with a rate of 14.1 deaths per 1,000 LBs; 76% were early neonatal deaths and 24% were late deaths. The odds of deaths in the Ministry of Health hospitals were almost 21 times (OR = 20.8, 95% CI: 2.8, 153.1) higher than that in private hospitals. Low birthweight and pre-term babies were significantly more likely to die during the neonatal period compared to full-term babies. The odds of neonatal mortality were significantly higher among babies born to housewives compared to those who were born to employed women (OR = 2.7; 95% CI: 1.2, 6.0). Main causes of neonatal deaths that occurred pre-discharge were respiratory and cardiovascular disorders (43%) and low birthweight and pre-term (33%). The main maternal conditions that attributed to these deaths were complications of the placenta and cord, complications of pregnancy, and medical and surgical conditions. The main cause of neonatal deaths that occurred post-discharge were low birthweight and pre-term (42%).
Conclusions: The rate of neonatal mortality have not decreased since 2012 and the majority of neonatal deaths occurred could have been prevented. Regular antenatal visits, in which any possible diseases or complications of pregnant women or fetal anomalies, need to be fully documented and monitored with appropriate and timely medical intervention to minimize such deaths.
Neonatal mortality is a public health problem worldwide primarily in low- and middle-income countries. Although extensive progress has been completed in reducing neonatal mortality over the last three decades, increased efforts to improve progress are still needed to achieve the 2030 SDG target (1). Even though there is a global decrease in neonatal mortality, the rate of decrement is considerably lower than that of the post-neonatal under five mortalities (2).
It has been estimated that 27.8 million neonates will die between 2018 and 2030 (1) if no improvements in neonatal mortality take place. A study conducted in 186 countries revealed that the risk of early neonatal death is very high across a range of countries and contexts (3). Of all neonatal deaths, about half occurred within 24 h of birth and around one third occurred in the first 6 h after birth (4). According to the Jordan Perinatal and Neonatal Mortality study (5), the neonatal mortality rates was 14.9 per 1,000 live births (LB).
In low- and middle-income countries, the majority of neonatal deaths occur without a clear cause of death (i.e., pre-maturity) (6, 7). It is difficult to confirm the cause because there are many factors that could be linked to the exact underlying cause of neonatal mortality, however, literature has categorized causes into those related to maternal or fetal conditions (8). Neonatal deaths often occur due to an illness presenting as an emergency, either soon after birth or later, due to infections such as tetanus or community-acquired infections (9). Data on causes of neonatal deaths and the timing around neonatal deaths are often sparse and less reliable than all-cause mortality data, and these data result in uncertain estimates, which poses substantial challenges to the generation of evidence-based interventions to prevent neonatal deaths. Improved data on where and when neonatal death occur and what causes delays is key to designing context-specific community and strategies (9).
The scarcity of data in Jordan on stillbirths and neonatal mortality, especially early mortality is generally linked to the fact that some births are not registered (10, 11). In addition, the existing sources of data on neonatal mortality are likely to be biased or incomprehensive. Given the fact that almost all (99.7%) births occur in Jordan are institutionalized (the birth occurs in hospital) (12), improving a reporting system of neonatal deaths in Jordanian hospitals is critical for tracking progress and taking appropriate actions.
As a result of this limitation, an electronic stillbirths and neonatal deaths surveillance system (JSANDS) was developed and established in five large hospitals in Jordan in August 2019. The JSANDS was developed by researchers at a leading university in Jordan with the collaboration of the Jordanian Ministry of Health as a secure on-line data entry system to collect, organize, analyze, and disseminate reliable data on neonatal deaths, and related causes. Additionally, the system registers births to use them as a denominator for mortality measures (13). The definition of the stillbirths and neonatal deaths used in the system were based on the international standards set by the World Health Organization and CDC. Given the scarcity, incomprehensiveness, and inconsistency of national data about causes and risk factors of stillbirths and neonatal mortality, the current study utilized the data from JSANDS aiming to determine the rate, risk factors, and causes of neonatal mortality in Jordan.
The information on all births and related outcomes that registered in the JSANDS surveillance system from August 2019 to January 2020 were retrieved and analyzed. We included all the births and deaths (stillbirths and neonates) within the indicated 6-months period of data collection. Any birth, stillbirth, or a neonatal death occurred within the five hospitals and entered into the JSANDS system were included in the study. If the birth or death is not registered on the JSANDS, they will be excluded.
The authors understand that the study period is limited and not long enough. However, within this 6 months' period, there were a total of over than 10,000 registered births on the JSANDS that give us a glimpse of the NMR and related causes in Jordan. It is worth mentioning that the total births in Jordan during 2019 are 197,278 in 2019 (14). Based on this number, the half yearly total births in 2019 is 98,650. Thus, the % of coverage by the five included hospitals out of the total births in Jordan during 2019 is 9.55%. We will utilize the data registered in the JSANDS to run periodic, perhaps annual, analysis of the rate, causes, and risk factors of Neonatal mortality rate in Jordan.
All births and neonatal deaths occurred in the five selected hospitals were completely registered. These five selected hospitals, from three major governorates, cover the vast majority of births and deaths occur in all regions in the north, east, and south of Jordan. In particular, we included one university teaching, referral hospital in Northern Jordan, which receives labor and delivery cases from all regions and suburbs in Northern Jordan. We also included another university teaching, governmental hospital affiliated with the Ministry of Health in Northern Jordan, which is specialized for maternal-related issues including births, and also receives maternal deliveries from all sectors in the region. A big private hospital in the North of Jordan was also included from several private and governmental sectors in the region. In the north east region, we included a large specialized hospital for maternal and child health and covers the whole Al-Mafraq governorate. Finally, we selected another specialized, referral hospital in the Southern region of Jordan which covers the majority of births within the region.
The extracted data included sociodemographic characteristics of the mother and father, birth data (i.e., gestational age, mode of delivery, and multiplicity), the newborn data (status, birth weight) and causes of neonatal deaths. In the current study, neonatal mortality was defined as any death that happened within the first 28 days of life. Neonatal mortality rate was calculated as the number of neonatal deaths per 1,000 live births (LB). The definition of the stillbirths and neonatal deaths used in the system were based on the international standards set by the World Health Organization and Centre for Disease Control.
Causes of neonatal deaths were identified according to the International Classification of Diseases-Perinatal Mortality (ICD-PM), which is part of the 10th version of the International Classification of Diseases (ICD-10) and report perinatal deaths (15). A training was held in the five hospitals for all healthcare providers on how to assign cause of death. The doctor (usually pediatrician) who is responsible for the follow up of the neonate has the primary responsibility to fill the form for the death, assign the cause of death, and write the ICD-10 code accordingly. The ICD-10 codes were used to provide a unified language for reporting and monitoring diseases allowing a standardized comparison and sharing of data among the five hospitals.
Causes of deaths related to fetal/neonatal condition or related to maternal condition were registered. First, the main disease or condition in the newborn is identified, in which the main disease or condition of the newborn who has died is entered. Other diseases or conditions in the newborn were also reported, if any. The main underlying maternal disease or condition affecting the newborn that contributed mostly to the neonatal death was then reported.
Last but not least, the research team verified all deaths registered through the JSANDS with those documented on paper and electronic medical records in the hospital to avoid any missing death. We found only 1% inconsistency between deaths registered through the JSANDS and those registered on paper mainly due to some delay in entering the death case to the JSANDS. However, all deaths tend to be registered on the JSANDS within 1 day of the occurrence of death.
The IBM SPSS version 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp) was used for data analysis. Data were described using rates and percentages for categorical data and means and standard deviations for continuous variables. The NMR was calculated as the number of neonatal deaths by 1,000 LB. The distribution of neonatal deaths according to studied characteristics were tested using Chi-square test. Multivariable analysis using binary logistic regression was used to determine factors associated with neonatal mortality. Backward stepwise selection method was used to select the variables to be included in the regression model. A p-value of <0.05 was considered statistically significant.
During the period from August 2019 to January 2020, a total of 10,328 births (10,226 LB and 102 stillbirths) were registered. The women's age ranged between 15 and 48 years with a mean (SD) of 29.1 (6.1) year. The majority of women (81%) were between 19 and 35 years of age, and 16.5% were older than 35 years. More than half of women (55%) had high school education or less. Almost three quarters (74%) of women had family income <714 US$. The majority of women were housewives (90%). The rate of cesarean section rate was 49% (27% planned CS and 22% emergency CS) (Table 1).
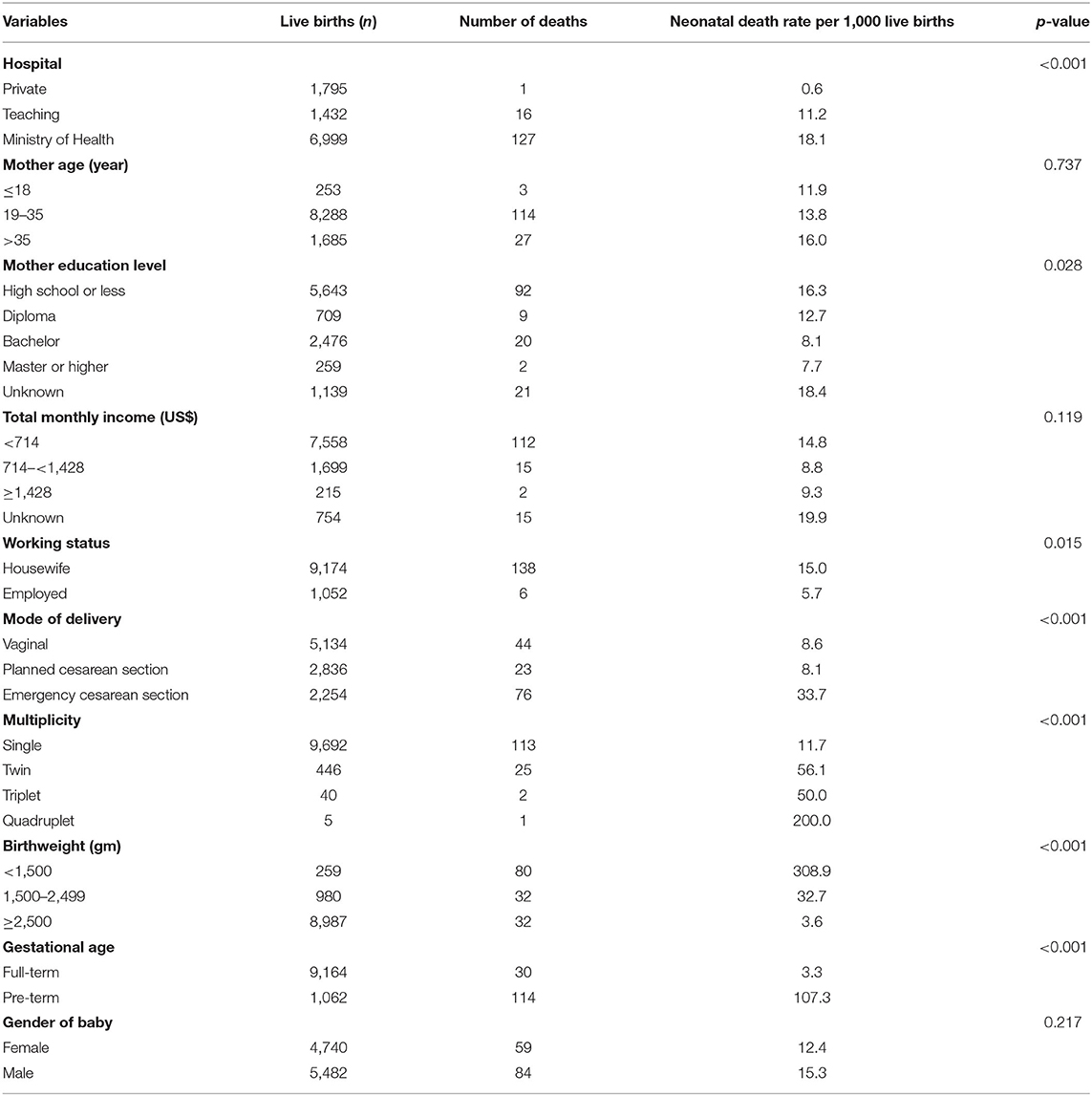
Table 1. Neonatal mortality rate according to the sociodemographic, maternal, clinical and relevant characteristics of women and births' characteristics.
A total of 10,328 (10,226 LB and 102 stillbirths) were registered in the five hospitals (68.4% from the three MOH hospitals, 14% from the teaching hospital, and 18% from the private hospital). Of all births, 46% were females. Almost 95% of total births were singleton, 87% weighed 2,500 grams or more, 10% weighed 1,500–2,499 grams, and 3% weighed <1,500 grams.
Of the total 10,226 LB, 144 were neonatal deaths. The overall NMR rate was 14.1 per 1,000 LB. Of neonatal deaths, 76% were early neonatal deaths and 24% were late neonatal deaths. Almost 25% of neonatal deaths occurred in the first day, 19% in the second day, 16% in the third day, 3% in the fourth day, 7% in the fifth day (Figure 1).
Table 1 shows the NMR according to the maternal, clinical and relevant characteristics. The NMR per 1,000 LB varied significantly according to mother's educational level, multiplicity, birthweight, mode of delivery, and gestational age. However, the neonatal deaths did not vary significantly according to health sector and mother's age, income, and working status.
Multivariable analysis (Table 2) showed that the odds of deaths in the Ministry of Health hospitals were almost 21 times (OR = 20.8, 95% CI: 2.8, 153.1) higher than that in private hospitals. The odds of death among very low birthweight (<1,500) were 31.8 (95% CI 18.8, 53.8) higher than those with birthweight ≥2,500 grams. Also, pre-term infants were significantly more likely (OR = 13.0; 95% CI: 7.8, 21.6) to die during the neonatal period compared to full-term babies. The odds of neonatal mortality was significantly higher among babies born to housewives compared to those who were born to employed women (OR = 2.7; 95% CI: 1.2, 6.0).
Table 3 shows the main causes of neonatal deaths in Jordan. The main leading cause of death was respiratory and cardiovascular disorders which contributed to 43% of pre-discharged deaths and 33% of post-discharged deaths. Of all neonatal deaths classified as respiratory and cardiovascular diseases, the majority (45/55; 81.8%) were due to respiratory distress of newborn, 8 (14.5%) were due to pulmonary hemorrhage originating in the perinatal period, and 2 (3.6%) were due to neonatal aspiration syndromes. The second leading cause of death was low birthweight and pre-term which contributed to 33% of pre-discharged deaths and 42% of post-discharged deaths, followed by congenital malformation deformations and chromosomal abnormalities which contributed to 19% of pre-discharged deaths and 8% of post-discharged deaths. Congenital malformations of heart were the most congenital malformations (7/24, 29.2%)
For the main maternal diseases or conditions affecting fetus/infant, the most common reported condition was complication of placenta, cord, and membrane which contributed to 55% of the 31 deaths that had maternal causes, followed by maternal complications of pregnancy (Fetus and newborn affected by maternal complications of pregnancy), and lastly maternal medical and surgical conditions (Table 3).
The neonatal death rate in the current study is almost similar (14.9 per 1,000 live births) to the 2016 Jordan Perinatal and Neonatal Mortality study using the same cut-off point of gestational weeks (≥20 weeks) (5) indicating that the rate has flatten since 2015 and has not shown a significant decline. Despite the tremendous efforts such as better quality medical care and availability of more advanced medical equipment, still more work is needed to accomplish the Sustainable Developmental Goal by 2030, particularly in regions with high NMR (1) including Jordan.
Our findings were similar to those in Batieha et al. (5) study in which congenital anomalies was a leading cause of death. Literature revealed that although several congenital anomalies could be avoided, they still are important causes of neonatal deaths (16). Congenital malformation was reported constantly across many classification systems (17), which could be preventable by pre-natal folic acid with multivitamin supplements that is proved to decrease the incidence of congenital abnormalities such as neural tube defects (18, 19).
One of the most powerful predictors of neonatal mortality is gestational age at birth.
There is a significant variation in mortality between babies born at 24 weeks and those born at full term (20), reflecting the great impact of immaturity on newborn survival. An exposure that increases pre-term births will therefore increase neonatal mortality. Other causes of neonatal death are congenital malformations, birth trauma, birth asphyxia, and hospital-acquired infection (21, 22). Some risk factors were identified in a recent national study including pre-maturity, low birth weight, maternal age <20 years, history of neonatal death or stillbirth, pre-eclampsia, scarce antenatal care, congenital anomalies, and gestational age before 37 weeks (5).
Assessing the magnitude and etiology of these important events and predicting their risk factors begin with accurately defining and reporting perinatal deaths (23, 24). A strategy for regionalized and cohesive perinatal network should be developed (10, 11) to reduce perinatal morbidity and mortality and improve survival for pre-term infants and other high-risk newborns. Mortality data should be available by geographical area, rural or urban, place of death, timing, underlying cause, and other data such as socio-economic status (25). This can help stakeholder to detect priorities and plan and monitor progress.
Similar to our findings, previous studies also showed that a strong predictor of neonatal death is immaturity as usually reflected by the age in gestational weeks at birth. Neonatal mortality can differ significantly between pre-mature babies and their counterparts full-term infants born at 39–40 weeks of gestation (20). Moreover, the findings of the national Jordan Perinatal and Neonatal Mortality study (5) are congruent with our findings where pre-maturity, gestational age before 37 weeks, low birth weight, multiple pregnancy were the most common risk factors associated with neonatal deaths. Low birth weight may result from both fetal growth restriction and pre-term birth, which are associated with placental dysfunction and subsequent poor fetal outcomes (25).
Previous literature reported similar findings. For instance, a study conducted in 60 low and middle income countries found that NMR was significantly higher among twins vs. singleton newborn babies even after adjusting for birth weight (26). Another study in Bangladesh also found that NMR was much higher among newborn babies born before 34 gestational weeks, twins or triplets, and first child in the family (27).
Congruent with the current study findings, previous studies showed that emergency CS was associated with higher NMR and that cesarean section rates higher than 10% are not associated with reduction in NMR, and hence should be avoided as much as possible (28). It is worth mentioning that the NMR has increased significantly in the last three decades including Jordan, surpassing the WHO recommendations of 10–15% CS as the maximum rates (29, 30).
Interestingly, the current study showed that NMR did not vary significantly according to mother' age, income, and working status but mother's high school or less of education was associated with higher rates of neonatal deaths probably due to less awareness about how and when to access medical care, especially in emergency situations as well as higher influence by family traditions and culture. Incongruent with our findings, maternal age of 30–35 years was associated with higher NMR (27, 31). The latest national study showed that maternal age <20 years was associated with higher rates of neonatal deaths (5). However, some research suggested that advanced maternal age is associated with placental dysfunction that may increase the risk of neonatal deaths and stillbirths (32) or to existing maternal medical condition (33). Also, newborn babies of richer families who also have a high educational level have higher chances to survive than those born to a poor family with lower educational level (34).
Despite the fact that the majority of neonatal deaths can be prevented with efficient interventions, such as access to emergency obstetric and neonatal care (35), some disadvantaged women and newborns who are most vulnerable to death and chronic morbidity have poor access to vital healthcare services (36, 37). Nonetheless, understanding the social and geographical pattern of NMR is crucial for stakeholders to increase access to effective interventions with focus on the poorest populations (36, 38). This will ensure that every pregnant woman and newborn baby have equal access to lifesaving interventions (39).
Complications of placenta was also found to be associated with higher NMR. Placental dysfunction is linked to intrauterine growth restriction, pre-term birth, and birth defects (40) resulting in inadequate oxygen supply to the fetus and thus increasing the probability of pre-term births and/or low birth weight.
Our findings are somehow congruent with the 2016 national study that revealed maternal diseases such as pre-eclampsia, mother's hospitalization during the current pregnancy, and poor antenatal care can all lead to neonatal deaths. It is surprising that in the national study, only a third of neonatal deaths had received optimum medical care (5). Other studies conducted in low-income countries like Pakistan have also specified several contributing factors to neonatal deaths such as inadequate training, insufficient medical care, low competence of healthcare providers and a lack of resources (41). Nonetheless, the national study showed also that a large proportion of neonatal deaths are preventable or possibly preventable thus providing optimal intrapartum, and direct post-partum care is likely to result in reduction of NMR (5).
However, not all births are registered in Jordan, especially if the birth results in stillbirth or early neonatal death before discharge from the hospital and the majority of neonatal deaths are not reported either (10, 11). About 30% of children <5 years do not have a birth certificate (42), and parents do not usually issue a death certificate for the majority of neonatal deaths (36). In the absence of reliable and standardized vital registration and administrative data in many countries, modeling of neonatal mortality rates remains necessary for public health policy and priority setting and monitoring.
Thus, there is a lack of credible data on causes of stillbirths and neonatal deaths making it challenging to develop appropriate interventions to avoid such deaths. The current study fills the gap in such data and hence, encourage stakeholders and policy makers to design and implement timely, evidence-based interventions to regions that register high number of stillbirths and neonatal deaths.
In the current study, having a neonatal mortality rate of 14.1 per 1,000 total births, which is somehow similar to the latest national study indicates that Jordan still falling behind in achieving the SDG of reducing NMR (5). The current study, therefore, highlights an immediate attention to accelerate appropriate efforts to prevent such deaths. This is vital as recent literature reported that with no improvements in neonatal mortality, 27·8 million neonates will die in the period from 2018 to 2030 (1). Yet, if policy makers initiate and implement interventions and improve quality of care to the point that NMR–in the countries that are still behind- would match the SDG target, then 5 million newborn babies could survive. A particular emphasis need to be toward births because a third of all neonatal deaths occur on the day of birth globally and about three-quarters of neonatal deaths occur during the first week of life (36, 43).
Despite the several strengths of the study mentioned earlier in the Methods section, the study has some limitations that need to be acknowledged. The JSANDS system did not include all hospitals in Jordan, and this could have limited the generalizability of the findings at a national level. However, the five selected hospitals were from three major governorates in Jordan and cover the vast majority of births and deaths occur in all regions in Jordan. Two of these hospitals are large university teaching, referral hospitals, and two of them are only specialized with maternal and child health.
The data exported from the JSANDS for this study were over a period of 6 months, which could have hindered estimating the accurate NMR in Jordan. However, even with this relatively short period, the JSANDS was able to provide an accurate estimates of the rate of neonatal mortality and related causes of deaths, as documented in previous national literature. We will utilize the data registered in the JSANDS in the future to run periodic, perhaps annual, analysis of the rate, causes, and risk factors of Neonatal mortality rate in Jordan. Analyzing the data over a longer period is expected to provide more reliable rates of the current situation in Jordan. Nonetheless, even with the current limitation of the study, our findings, using an innovative electronic surveillance system, are considered a baseline for stakeholders to start developing appropriate interventions and policies to decrease neonatal mortality in Jordan, especially preventable ones.
The main aim of the current study was to test the association of neonatal and maternal risk factors contributing to neonatal deaths, some of which are preventable, need to be incorporated in policies aimed at reducing NMR. Based on the current findings, the majority of neonatal deaths occurred in the current study could have been prevented with regular antenatal visits, in which all possible diseases or complications of pregnant women or fetal anomalies need to be fully documented and monitored with appropriate and timely medical intervention to minimize such deaths. Specialized care provided to low birthweight neonates and those with respiratory problems by experienced healthcare providers is vital. Additionally, the current study reported the findings of NMR extracted from a national neonatal and stillbirth surveillance system. As the majority of neonatal deaths are not reported in Jordan, investing in the health information systems to improve data registration will encourage appropriate use of interventions to reduce NMR. Finally, there is a need to design and implement evidence-based interventions to mothers and newborn babies who most need it.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The study was ethically approved by the Institutional Review Board (IRB) at Jordan University of Science and Technology and the Ministry of Health in Jordan. The data used in this manuscript was retrieved electronically from the JSANDS surveillance system (www.jsands.jo). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
NA-S: data collection, writing, original draft preparation, reviewing, and editing. YK: principal investigator, conceptualization, methodology, project administration, and funding acquisition. KS, MA, and AB: data collection, writing, reviewing, and editing. All authors have approved the final version of the manuscript.
This research was supported and funded by the International Development Research Centre/Canada (IDRC) and the United Nations International Children's Emergency Fund (UNICEF). The funding source had no role other than financial support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all nurses and doctors in the five participating hospitals for their support, especially those who have been members of the death review committees.
1. Hug L, Alexander M, You D, Alkema L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. (2019) 7:e710–20. doi: 10.1016/S2214-109X(19)30163-9
2. UNICEF, WHO, The World Bank, United Nations Population Division. Levels and Trends in Child Mortality: Report 2019 Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. UNICEF (2019).
3. Oza S, Lawn JE, Hogan DR, Mathers C, Cousens SN. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000–2013. Bull World Health Organ. (2014) 93:19–28. doi: 10.2471/BLT.14.139790
4. Baqui AH, Mitra DK, Begum N, Hurt L, Soremekun S, Edmond K, et al. Neonatal mortality within 24 hours of birth in six low- and lower-middle-income countries. Bull World Health Organ. (2016) 94:752–8B. doi: 10.2471/BLT.15.160945
5. Batieha A, Khader Y, Berdzuli N, Chua-Oon C, Badran E, Al-Sheyab N, et al. Level, causes and risk factors of neonatal mortality, in jordan: results of a national prospective study. Matern Child Health J. (2016) 20:1061–71. doi: 10.1007/s10995-015-1892-x
6. Goldenberg RL, Muhe L, Saleem S, Dhaded S, Goudar SS, Patterson J, et al. Criteria for assigning cause of death for stillbirths and neonatal deaths in research studies in low-middle income countries. J Matern Fetal Neonatal Med. (2019) 32:1915–23. doi: 10.1080/14767058.2017.1419177
7. Mengesha HG, Sahle BW. Cause of neonatal deaths in Northern Ethiopia: a prospective cohort study. BMC Public Health. (2017) 17:62. doi: 10.1186/s12889-016-3979-8
8. Lawn J, Cousens S, Zupan J, Team LNSS. 4 million neonatal deaths: when? where? why? Lancet. (2005) 365:891–900. doi: 10.1016/S0140-6736(05)71048-5
9. Gülmezoglu AM, Lawrie TA, Hezelgrave N, Oladapo OT, Souza JP, Gielen M, et al. Interventions to Reduce Maternal and Newborn Morbidity and Mortality. Disease Control Priorities. 3rd ed. Washington, DC: World Bank (2016). doi: 10.1596/978-1-4648-0348-2_ch7
10. Khader Y, Al-sheyab N, Alyahya M, Batieha A. Registration, documentation, and auditing of stillbirths and neonatal deaths in Jordan from healthcare professionals' perspectives: reality, challenges and suggestions. J Matern Fetal Neonatal Med. (2018) 33:3338–48. doi: 10.1080/14767058.2018.1531120
11. Khader YS, Alyahya M, Batieha A. Birth and neonatal death registrations in Jordan. In: Laher I, editor. Handbook of Healthcare in the Arab World. Cham: Springer International Publishing (2019). p. 1–12. doi: 10.1007/978-3-319-74365-3_116-1
12. Department of Statistics, ICF. Jordan Population and Family and Health Survey 2017-18. ICF (2019).
13. Khader YS, Alyahya M, Batieha A, Taweel A. JSANDS: a stillbirth and neonatal deaths surveillance system. In: 2019 IEEE/ACS 16th International Conference on Computer Systems and Applications (AICCSA). Abu Dhabi (2019). p. 1–5. doi: 10.1109/AICCSA47632.2019.9035335
15. World Health Organization. The WHO application of ICD-10 to deaths during the perinatal period: ICD-PM. WHO (2016).
16. Gatt M, England K, Grech V, Calleja N. Contribution of congenital anomalies to neonatal mortality rates in malta. Paediatric Perinatal Epidemiol. (2015) 29:401–6. doi: 10.1111/ppe.12206
17. Lawn J, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. (2016) 387:587–603. doi: 10.1016/S0140-6736(15)00837-5
18. Wilson RD, Wilson RD, Désilets V, Wyatt P, Langlois S, Gagnon A, et al. Pre-conceptional vitamin/folic acid supplementation 2007: the use of folic acid in combination with a multivitamin supplement for the prevention of neural tube defects and other congenital anomalies. J Obstet Gynaecol Can. (2007) 29:1003–13. doi: 10.1016/S1701-2163(16)32685-8
19. Wilson RD, Wilson RD, Audibert F, Brock J-A, Carroll J, Cartier L, et al. Pre-conception folic acid and multivitamin supplementation for the primary and secondary prevention of neural tube defects and other folic acid-sensitive congenital anomalies. J Obstet Gynaecol Can. (2015) 37:534–49. doi: 10.1016/S1701-2163(15)30230-9
20. Mathews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep. (2015) 64:1–30. Available online at: https://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_09.pdf
21. Heron M. Deaths: Leading Causes for 2013. National Vital Statistics Reports. National Center for Health Statistics (2016).
22. Khader YS, Alyahya M, Batieha A. Perinatal and neonatal mortality in Jordan. In Laher I, editor. Handbook of Healthcare in the Arab World. Cham: Springer International Publishing (2019). p. 1–22. doi: 10.1007/978-3-319-74365-3_161-1
23. Barfield W. Standard terminology for fetal, infant, and perinatal deaths. Pediatrics. (2016) 137:e20160551. doi: 10.1542/peds.2016-0551
24. Alyahya MS, Khader YS. Health care professionals' knowledge and awareness of the ICD-10 coding system for assigning the cause of perinatal deaths in Jordanian hospitals. J Multidiscip Healthc. (2019) 12:149–57. doi: 10.2147/JMDH.S189461
25. Blencowe H, Calvert PC, Lawn JE, Cousens S, Campbell OMR. Measuring maternal, foetal and neonatal mortality: challenges and solutions. Best Pract Res Clin Obstet Gynaecol. (2016) 36:14–29. doi: 10.1016/j.bpobgyn.2016.05.006
26. Bellizzi S, Sobel H, Betran AP, Temmerman M. Early neonatal mortality in twin pregnancy: findings from 60 low- and middle-income countries. J Glob Health. (2018) 8:010404. doi: 10.7189/jogh.08.010404
27. Al Kibria GM, Khanam R, Mitra DK, Mahmud A, Begum N, Moin SMI, et al. Rates and determinants of neonatal mortality in two rural sub-districts of Sylhet, Bangladesh. PLoS ONE. (2018) 13:e0206795. doi: 10.1371/journal.pone.0206795
28. Ye J, Zhang J, Mikolajczyk R, Torloni M, Gülmezoglu A, Betran A. Association between rates of caesarean section and maternal and neonatal mortality in the 21st century: a worldwide population-based ecological study with longitudinal data. BJOG. (2016) 123:745–53. doi: 10.1111/1471-0528.13592
29. Gibbons L, Belizan JM, Lauer JA, Betran AP, Merialdi M, Althabe F. Inequities in the use of cesarean section deliveries in the world. Am J Obstet Gynecol. (2012) 206:331.e1–19. doi: 10.1016/j.ajog.2012.02.026
30. Ye J, Betrán AP, Guerrero Vela M, Souza JP, Zhang J. Searching for the optimal rate of medically necessary cesarean delivery. Birth. (2014) 41:237–44. doi: 10.1111/birt.12104
31. Maniruzzaman M, Suri HS, Kumar N, Abedin MM, Rahman MJ, El-Baz A, et al. Risk factors of neonatal mortality and child mortality in Bangladesh. J Glob Health. (2018) 8:010417. doi: 10.7189/jogh.08.010421
32. Lean SC, Heazell AEP, Dilworth MR, Mills TA, Jones RL. Placental dysfunction underlies increased risk of fetal growth restriction and stillbirth in advanced maternal age women. Sci. Rep. (2017) 7:9677. doi: 10.1038/s41598-017-09814-w
33. Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Bommarito K, Madden T, Olsen MA, et al. Maternal age and risk of labor and delivery complications. Matern Child Health J. (2015) 19:1202–11. doi: 10.1007/s10995-014-1624-7
34. McKinnon B, Harper S, Kaufman JS, Bergevin Y. Socioeconomic inequality in neonatal mortality in countries of low and middle income: a multicountry analysis. Lancet Glob Health. (2014) 2:e165–73. doi: 10.1016/S2214-109X(14)70008-7
35. Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. (2005) 365:977–88. doi: 10.1016/S0140-6736(05)71088-6
36. Lawn J, Blencowe H, Oza S, You D, Lee A, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. (2014) 384:189–205. doi: 10.1016/S0140-6736(14)60496-7
37. Martines J, Paul VK, Bhutta ZA, Koblinsky M, Soucat A, Walker N, et al. Neonatal survival: a call for action. Lancet. (2005) 365:1189–97. doi: 10.1016/S0140-6736(05)71882-1
38. Chao F, You D, Pedersen J, Hug L, Alkema L. National and regional under-5 mortality rate by economic status for low-income and middle-income countries: a systematic assessment. Lancet Glob Health. (2018) 6:e535–47. doi: 10.1016/S2214-109X(18)30059-7
39. Lassi ZS, Bhutta ZA. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database Syst Rev. (2015) 3:CD007754. doi: 10.1002/14651858.CD007754.pub3
40. Malhotra A, Allison BJ, Castillo-Melendez M, Jenkin G, Polglase GR, Miller SL. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front Endocrinol. (2019) 10:55. doi: 10.3389/fendo.2019.00055
41. Ekirapa-Kiracho E, Waiswa P, Rahman MH, Makumbi F, Kiwanuka N, Okui O, et al. Increasing access to institutional deliveries using demand and supply side incentives: early results from a quasi-experimental study. BMC Int Health Hum Rights. (2011) 11:S11. doi: 10.1186/1472-698X-11-S1-S11
42. UNICEF. UNICEF Global Databases. Birth Registration Data. UNICEF: Division of Data Research and Policy (2017).
Keywords: neonate, mortality, risk factor, surveillance, Jordan
Citation: Al-Sheyab NA, Khader YS, Shattnawi KK, Alyahya MS and Batieha A (2020) Rate, Risk Factors, and Causes of Neonatal Deaths in Jordan: Analysis of Data From Jordan Stillbirth and Neonatal Surveillance System (JSANDS). Front. Public Health 8:595379. doi: 10.3389/fpubh.2020.595379
Received: 16 August 2020; Accepted: 07 October 2020;
Published: 30 October 2020.
Edited by:
Ziad Khatib, Austrian Agency for Health and Food Safety (AGES), AustriaReviewed by:
Manar AlAzzam, Al al-Bayt University, JordanCopyright © 2020 Al-Sheyab, Khader, Shattnawi, Alyahya and Batieha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nihaya A. Al-Sheyab, bmFzaGV5YWJAanVzdC5lZHUuam8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.