
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 05 November 2020
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 8 - 2020 | https://doi.org/10.3389/fpubh.2020.580637
 Laís Giuliani Felipetto1
Laís Giuliani Felipetto1 Pedro Irineu Teider-Junior1
Pedro Irineu Teider-Junior1 Felipe Fortino Verdan da Silva2
Felipe Fortino Verdan da Silva2 Ana Carolina Yamakawa3
Ana Carolina Yamakawa3 Louise Bach Kmetiuk4
Louise Bach Kmetiuk4 Anahi Chechia do Couto1
Anahi Chechia do Couto1 Camila Marinelli Martins5,6
Camila Marinelli Martins5,6 Eduarda Stankiwich Vaz1
Eduarda Stankiwich Vaz1 Leila Sabrina Ullmann7
Leila Sabrina Ullmann7 Helio Langoni3
Helio Langoni3 Jorge Timenetsky8
Jorge Timenetsky8 Andrea Pires dos Santos9
Andrea Pires dos Santos9 Alexander Welker Biondo9,10*
Alexander Welker Biondo9,10*Seroprevalence of Toxoplasma gondii has been extensively studied in a variety of different human populations. However, no study has focused on homeless populations. Accordingly, the present study aimed to assess the seroprevalence of anti-T. gondii antibodies and the risk factors associated in homeless persons from homeless shelter of São Paulo city, southeastern Brazil. In addition, anti-HIV antibodies and associated risk of T. gondii and HIV coinfection have been evaluated. Anti-T. gondii antibodies were detected by indirect fluorescent antibody test. In addition, anti-HIV levels were tested by chemiluminescence enzyme immunoassay, with positive samples confirmed by rapid immunoblot assay. Overall, IgG anti-T. gondii seropositivity was found in 43/120 (35.8%) homeless persons, with endpoint titers varying from 16 to 1,024. The only two pregnant women tested were negative for IgM by chemiluminescence enzyme immunoassay, with normal parturition and clinically healthy newborns in both cases. There were no statistical differences in the risk factors for anti-T. gondii serology (p > 0.05). Anti-HIV seropositivity was found in 2/120 (1.7%) homeless persons, confirmed as HIV-1. One HIV seropositive individual was also sero-reactive to IgG anti-T. gondii, and both were negative to IgM anti-T. gondii. This is the first study that reports the serosurvey of T. gondii in homeless persons worldwide. Despite the limited sample size available in the present study, our findings have shown that the prevalence of anti-T. gondii antibodies in homeless persons herein was lower than the general population, probably due to homeless diet habit of eating mainly processed food intake. No statistical differences were found regarding risk factors for anti-T. gondii exposure in homeless persons. Future studies should be conducted to fully establish risk factors for anti-T. gondii exposure in homeless persons.
Homeless persons have been described as one of the three most vulnerable populations, along with refugees and incarcerated persons (1). Morbidity and mortality of diseases have been reportedly higher in homeless than general population, probably due to social inequality associated with lack of settled home, job opportunity, and a series of family problems including drug addiction, mental health disorders, and social justice issues, mostly exacerbated by absence of health assistance (2). A population of 1.6 billion people without adequate housing has been estimated worldwide, of which 100 million are homeless (3, 4). In Brazil, the nationwide homeless population has been estimated in 101,854 individuals, with about 40.1% living in cities with more than 900,000 inhabitants and about 16,000 living on streets of São Paulo city (5, 6).
Toxoplasma gondii is a coccidian parasite relying on cats and other family Felidae as definitive hosts, which may shed fecal oocysts infecting a variety of homeothermic intermediate hosts (7). Human infection has been typically subclinical or asymptomatic, with the time of infection and transmission route not known in most cases (8, 9). Despite that, in immunodeficient people, such as in HIV-toxoplasmosis combination, the protozoan can cause severe clinical manifestations, with invasion into the central nervous system and encephalitis (10, 11).
The human T. gondii seroprevalence has been extensively reported, ranging from 0.8 to 77.5% worldwide (12), few reports are available for vulnerable populations including 123/597 (20.6%) aboriginal individuals of Thailand, 236/628 (37.6%) prisoners of Turkey, mostly (92.8%) males, and 63/199 (31.7%) pregnant refugee and borderline migrant women of Asia (13–15). In Brazil, seropositivity of T. gondii has been ranged from 14/65 (21.5%) urban students of northeastern region to 113/116 (97.4%) farmers of dairy cattle farm in central-western region (16, 17). In vulnerable populations of Brazil, the positivity reported was 131/231 (56.7%) inhabitants living in riverside communities of northern region and 119/148 (80.4%) indigenous of central-western region (18, 19).
Although T. gondii seroprevalence has been reported in others vulnerable populations, no study has focused on homeless populations. Accordingly, the present study aimed to assess the seroprevalence of anti-T. gondii and the associated risk factors for exposure in homeless persons from homeless shelter of São Paulo city, southeastern Brazil. In addition, anti-HIV antibodies and associated risk of T. gondii and HIV coinfection have been evaluated.
The present study represents a descriptive cross-sectional seroepidemiological approach of the homeless population from the western São Paulo city (23°33′1″S, 46°38′2″W) shelter (Social Center “Our Lady of Good Delivery”), responsible for daytime attendance of all the city region. The shelter is a Non-Governmental Organization, sponsored by a city partnership daily attending around 800–1,200 homeless persons, providing meals, medical assistance, job opportunities, and recreational activities.
São Paulo city, capital of São Paulo State, southeastern Brazil, has been ranked as the most populated city of Latin America with 11,253,500 people and the tenth-largest Gross Domestic Product (GDP) of the world, with a very high Human Development Index (HDI) (0.805). The city is located under a humid subtropical climate with average temperatures ranging from 19°C (winter) to 25°C (summer) (20).
The present study was conducted along with the city multi-task professionals team at the São Paulo City Secretary of Health, called “street outreach office,” which includes physicians, nurses, dentists, social assistants, and psychologists, based on the strategy of the Brazilian Unified Health System (21). This city official team offers permanent assistance and save clinical records of the homeless population, promoting health actions on a continuing care bond.
Epidemiological analyses were performed based on a questionnaire associated with homeless persons exposure to T. gondii and HIV, which included: (1) Demographic profile: sex, marital status, racial self-declaration, age, educational background, income, and city of origin; (2) Social profile: travel to other cities, communication with family, causes for becoming homeless, homelessness time, resting place, have children, have own children, pregnant woman, live alone, pet owner, use of licit and illicit drugs, alcohol consumption, tobacco use, marijuana use, cocaine use, crack use, assistance by the Psychosocial Care Centers (CAPS) as part of the free national Unified Health System; (3) Hygiene profile: bath frequency, change of clothes frequency, wash clothes, body lice (Pediculus humanus humanus) bites, and body lice presence (Supplementary Material). Refusal to fully or partially answer any question or incomplete answers were accepted and registered.
Blood samples of homeless persons were conveniently drawn from June to August 2018, which was the limited timeframe permission issued by the City Secretary of Health at the time. A minimal sampling of 71 individuals was calculated using commercially available software (Epi Info 7.7.7.6) based on an estimative of 16,000 homeless persons in São Paulo City and homeless HIV infection prevalence of 4.9% (22), the sampling was simple with 95% confidence and 5% accuracy. Homeless persons were recruited by government health officials and invited to participate voluntarily of research, and blood collection was performed by cephalic puncture. Samples were placed in tubes without anticoagulant and kept at 25°C until visible clot retraction. Serum was separated by centrifugation at 3,000 revolutions per minute for 10 min and stored at −20°C until processing.
In addition, the packed cell volume (PCV) by capillary tube centrifugation and total plasma protein (TPP) by refractometry were performed on the day of sampling and immediately given to the shelter administration. Due to the shelter demand, homeless persons were also examined for body lice (Pediculus humanus humanus) bites and presence, as previously described at the same shelter (23). The University made a clothing donation drive during the study and researchers offered clean clothes to all lice-infested homeless persons.
Detection of T. gondii antibodies was performed by indirect immunofluorescent antibody test (IFAT) (24), with serial serum dilutions of 1:16–1:4,096 performed in pH 7.2 phosphate-buffered saline solution (PBS) with the cut-off titer of ≥16 IU. Immunofluorescence slides were previously sensitized with 0.1% formaldehyde to inactivated tachyzoites of T. gondii (RH strain) obtained from an intraperitoneal lavage in Swiss mice after 3 days of inoculation. A commercial anti-human IgG antibody, conjugated with fluorescein isothiocyanate (Bethyl Laboratories, Montgomery, TX, USA) was used as secondary antibody. For positive samples, the highest titer was considered with at least 50% of fluorescence on the border of tachyzoites.
In addition, the detection of HIV was performed by chemiluminescent microparticle immunoassay (CMIA) (Alinity's HIV Ag/Ab Combo Reagent Kit, Abbott Laboratories, Chicago, IL, USA) used for the simultaneous qualitative detection of HIV p24 antigen and antibodies to HIV type 1 (HIV-1 group M and group O) and/or type 2 (HIV-2) in human serum. The resulting chemiluminescent reaction was measured as relative light units (RLU). Cases of reactive serology were confirmed by a commercially available rapid immunoblot assay (DPP HIV1/2®, Fiocruz, Rio de Janeiro, Brazil). Samples of the pregnant woman and the HIV-positive individuals were tested for T. gondii IgM presence by CMIA (Anility's Toxo IgM Reagent Kit, Abbott Laboratories, Chicago, IL, USA).
Statistical analysis was performed using SPSS 20.0 (25). Frequencies of T. gondii and HIV seropositivity (absolute and relative) were determined by the stratification of the observations according to demographic, social, and hygiene profiles. The Chi-Square test was used to determine the bivariate association between studied variables, and odds ratios (OR) were used for the association of T. gondii prevalence and potential risk factors.
Overall, anti-T. gondii antibodies were detected in 43/120 (35.8%, CI 95% 26.7–43.0%) homeless persons, with endpoint titers varying from 16 to 1,024. No statistical differences were found regarding risk factors for anti-T. gondii exposure (p > 0.05) in homeless persons (Table 1).
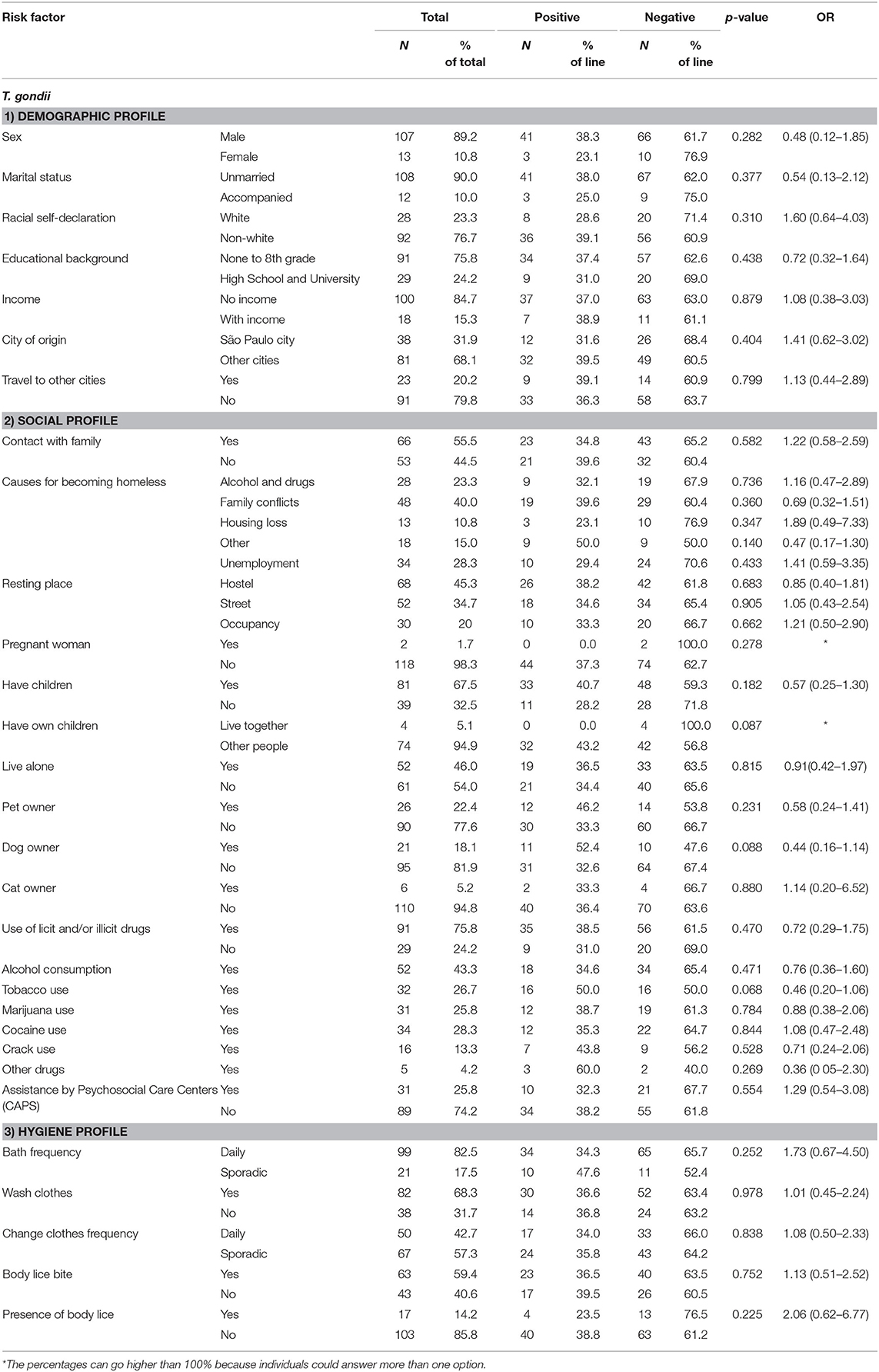
Table 1. Statistical results of univariate and multiple logistic regression models of associated risk factors for seropositivity of IgG anti-T. gondii antibodies in homeless persons.
Associated risk factors for the presence of anti-T. gondii were not statistically significant regarding educational background (p = 0.438), income (p = 0.805), resting place (hostels, street, occupancy) (p > 0.05), pregnancy (p = 0.567), pet owner (p = 0.399); cat owner (p = 0.916), bath frequency (p = 0.652), age (p = 0.223), and homelessness time (p = 0.827) (Table 2). The homeless persons sampled were mostly men counting 107/120 (89.2%) individuals, with 39/107 (36.8%) seropositive samples for T. gondii. On the other side, women accounted for 13/120 (10.8%) with 3/13 (23.1%) positive samples. Despite in lower number, eight women were within the reproductive age of 24–35 years old, and 7/8 (87.5%) presented negative serology for T. gondii, including the two pregnant homeless women exposed to infection. Fortunately, the two pregnant women tested negative for anti-T. gondii antibodies in both IgG by IFAT and IgM by CMIA, with normal parturition and clinically healthy newborns in both cases.
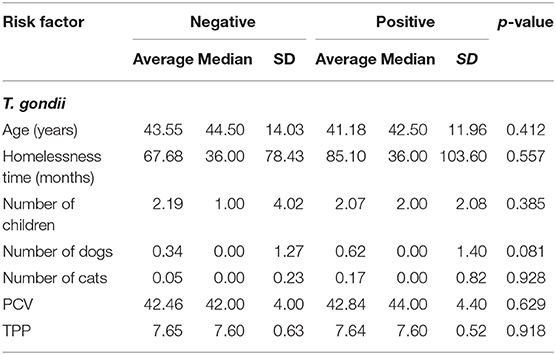
Table 2. Average, median, and standard deviation (SD) of T. gondii positive and negative homeless persons according to age (years), homelessness time (months), number of children, number of dogs, number of cats, packed cell volume (PCV), total plasma protein (TTP).
In addition, a total of 2/120 (1.7%, CI 95% 0.0–4.2%) anti-HIV seropositive homeless persons were detected by CMIA and confirmed by rapid immunoblot assay tests. No evaluation of HIV risk factors was made due to low seropositive rate.
To the authors' knowledge, this is the first study that reports the serosurvey of T. gondii in homeless persons and the associated risk factors.
The seroprevalence of anti- T. gondii antibodies herein (35.8%) was higher than other vulnerable populations, such as aborigines (20.6%), pregnant refugee, and borderline migrant women (31.7%), but similar to incarcerated populations (37.6%) (13–15). In the present study, a total of 91/114 (79.8%) homeless sampled herein declared not recently traveling to other cities, although 81/119 (68.1%) have been born or previously lived another city or region.
In Brazil, the anti-T. gondii seroprevalence herein was higher than the general population of the northeastern region, with 14/65 (21.5%) seropositive urban students, but lower than the central-western region, with 113/116 (97.4%) farmers from a single dairy cattle farm with domestic cats and potentially contaminated environment (16, 17). Interestingly, the seroprevalence of anti-T. gondii antibodies in the present study was lower than other Brazilian neglected populations, such as 131/231 (56.7%) persons of riverside communities in the northern and 119/148 (80.4%) indigenous persons in the central-western region (18, 19). In São Paulo, similar results were found, with 110/339 (32.4%) seropositive children from a low-socioeconomic community (26). Not surprisingly, a previous study has shown an association between high seropositivity for T. gondii and socioeconomic vulnerability in southern Brazil, with 526/715 (73.6%) seropositive individuals, particularly in low-income families (27).
Although low education and socioeconomic status have been associated with increased risk of T. gondii infection in different Brazilian studies (28–30), no statistical association with T. gondii infection was previously found regarding educational background and income, probably due to the broadly variable classification of and the low population homogeneity (31, 32). Similarly, no association was found in either education or income, likely associated to the impact of the vulnerable living style, with mostly drug addicts with poor eating habits.
Since the low socioeconomic status may be associated to malnutrition and might impair the host defense against protozoan infection, the relatively low seroprevalence of anti- T. gondii antibodies in homeless herein may be consequence of mainly consumption of ready-to-eat foods, as already indicated by previous studies on homelessness and food preparation facilities, which have reported dependence on charity meals, such as pre-prepared foods, processed foods or popular snacks (33–36). Not surprisingly, pre-processed ready-to-eat and meat-based foods have been shown to inactivate T. gondii cysts (37).
In addition, healthier and more expensive items, such as meat, fish, vegetables, and fruits have been less often consumed by homeless (33, 36, 38, 39), which may be a contributing factor to the low T. gondii seroprevalence found in this study. Hence, it is reasonable to speculate that the beneficial shelters, hostels, and meal services may have offered protection to the homeless population (36, 40) but not as nutritional good food habits when compared to the general population. Although no homeless person has been diagnosed with either anemia by packed cell volume (PCV) or hypoproteinemia by refractometry, such tests may not have enough sensitivity to detect chronic alimentary deficiencies, which should be further investigated.
A previous study with pregnant women has shown high seroprevalence of specific anti-T. gondii antibodies (68.4%; 333/487) and vertical transmission associated with social vulnerability in central Brazil (41). In the present study, despite negative for both IgG and IgM anti-T. gondii antibodies, the two pregnant women sampled, fortunately, gave birth to clinically healthy babies. T. gondii infection during pregnancy has been a significant problem, especially during the first months, and may result in spontaneous abortion, fetal and/or neonatal death or several congenital disabilities, such as hydrocephalus, central nervous system disorders, and chorioretinitis (42, 43). In the second and third trimester, newborns have usually been asymptomatic, with symptoms appearing late in childhood or early in adulthood, and may sporadically cause visual impairment (42, 44, 45). In addition, congenital toxoplasmosis may also be associated with reactivation of the chronical maternal infection, particularly in HIV-infected and immunosuppressed women (46). As 7/8 (87.5%) women herein were within reproductive age and presented negative serology for T. gondii, the homeless may be highly unprotected to infection during pregnancy.
Although the present study has shown no association between T. gondii infection and pet ownership, including stray cat owners, corroborating with previous studies in rural and other vulnerable populations of southern Brazil (27, 31), only 6/116 (5.2%) homeless persons owned a total of 11 cats. Outdoor lifestyle of stray cats may include hunting of birds and rodents, leading to raw meat dietary habits and increased risk of T. gondii ingestion (47). Nonetheless, human toxoplasmosis outbreaks may be attributed to exposure to infected cats, which may indicate an important role of cat oocyst excretion on infection spreading (48, 49), and homeless might be daily overexposed to environmental contamination. Another interestingly study has reported that 14/35 (40.0%) fresh vegetables and fruits collected from local producers and supermarket suppliers of Portugal and Spain were positive to T. gondii by PCR and microscopic autofluorescence (50). However, as mentioned above, homeless dietary habits of high intake of processed foods and low fresh meat, fish, vegetables and fruits may have led to lower T. gondii exposure. In fact, processing food methods, including high heating, curing, cooking, freezing, or chemical methods, have been shown to denature T. gondii proteins and remove or inactivated T. gondii oocysts, constituted by 90% cysteine and tyrosine (51).
Body lice (Pediculus humanus humanus) has been recently considered as a reemerging problem among homeless populations in France, Italy, USA, Colombia, and Brazil (23, 52–55). Even though body lice presence suggests social vulnerability and 17/120 (14.2%) of the homeless herein were infested with lice, no statistically risk of T. gondii exposure was found.
In addition to the T. gondii serosurvey, the HIV seroprevalence has been assessed herein. In the present study, 2/120 (1.7%) homeless persons was positive to anti-HIV antibodies, above the estimated prevalence of 0.4% for the general Brazilian population and lower than a recent study with 69/1,402 (4.9%) seropositive homeless (22, 56). Despite previous studies have shown a high prevalence of T. gondii and HIV co-infection, with 35.8% worldwide in a meta-analysis study and up to 88.4% of individuals coinfected in Ethiopia (57, 58), the analysis of associated risk factors herein has been impaired due to low HIV positive frequency.
One limitation of the present study was the relatively low number of samples, which was caused by the short timeframe sampling permission, associated to refusal of homeless persons in participating on the study. Difficulties in accessing such population may partially explain the lack of studies involving homeless and zoonoses, including toxoplasmosis. Although it may have generated insufficient data to provide strong basis for a statistical analysis, the results herein may still contribute on understanding homeless health and well-being. In addition, questionnaires to assess homeless information may be problematic, particularly regarding food intake and dietary habits, once such a population has often shown a chaotic lifestyle and a high prevalence of drug use and mental health disorders (36). Finally, further studies should be conducted in higher number and different homeless populations to fully establish the exactly impact of T. gondii in homeless persons worldwide.
In conclusion, this is the first study that reports the serosurvey of T. gondii in homeless persons worldwide. Despite the limited sample size available in the present study, our findings have shown that the frequency of anti-T. gondii antibodies in homeless persons was lower than the general population, probably due to homeless diet habit of eating mainly processed food intake. No statistical differences were found regarding risk factors for anti-T. gondii exposure in homeless persons. Future studies should be conducted to fully establish risk factors for anti-T. gondii exposure in homeless persons.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee in Human Research at the Federal University of Paraná (CAAE: 80099017.3.0000.0102, protocol number: 2.512.196), by the Ethics in Human Health Committee at the São Paulo City Secretary of Health (CAAE: 80099017.3.3004.0086, protocol number: 3.366.684) and by Ethics Committee in Human Research of the Clinics Hospital at the Federal University of Paraná (CAAE: 80099017.3.3005.0096, protocol number: 3.623.845), all subordinate to the National Human Ethics Research Committee of the Brazilian Ministry of Health. The Informed Consent Form was applied to all homeless persons, according to the ethical guidelines and principles of Federal University of Paraná. All participants research provided written informed consent. The patients/participants provided their written informed consent to participate in this study.
LF, PT-J, AY, LK, CM, LU, and AB: drafting and revision of the manuscript. LF, LU, HL, JT, AS, and AB: initiation, conception, design, and coordination of the research project. LF, FS, AC, CM, and EV: development of the intervention and evaluation materials. LF, PT-J, LK, CM, LU, AS, and AB: implementation of the intervention. All authors read and approved the final version of the manuscript.
AB research was funded through the Brazilian National Council for Scientific and Technological Development-CNPq (420999/2016-7). LF has been supported by graduate fellowships from the Coordination for the Improvement of Higher Education Personnel (CAPES).
CM is employed by the company AAC&T Research Consulting LTDA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are thankful to Dr. Mara Lúcia Gravinatti and the Social Center Our Lady of Good Delivery for helping with collection, sampling, and follow-up information. This manuscript has been released as a pre-print at Research Square (59).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.580637/full#supplementary-material
1. Aldridge RW, Story A, Hwang SW, Nordentoft M, Luchenski SA, Hartwell G, et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet. (2018) 391:241–50. doi: 10.1016/S0140-6736(17)31869-X
2. Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. (2014) 384:1529–40. doi: 10.1016/S0140-6736(14)61132-6
3. United Nations Organization. Commission on Human Rights. Economic, Social and Cultural Rights. Report of the Special Rapporteur on Adequate Housing as a Component of the Right. To an Adequate Standard of Living, Miloon Kothari. (2005). Available online at: https://documents-dds-ny.un.org/doc/UNDOC/GEN/G05/117/55/PDF/G0511755.pdf?OpenElement (accessed December 20, 2019).
4. United Nations Organization. Expert Group Meeting. Affordable Housing and Social Protection Systems for All to Address Homelessness. Department of Economic and Social Affairs. (2019). Available online at: https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2019/10/summary-egm-final-9sep.pdf (accessed December 20, 2019).
5. Institute of Economic Research Foundation. Pesquisa Censitária da População em Situação de Rua, Caracterização Socioeconômica da População Adulta em Situação de Rua e Relatório Temático de Identificação das Necessidades desta População na Cidade de São Paulo. (2015). Available online at: https://www.prefeitura.sp.gov.br/cidade/secretarias/upload/00-publicacao_de_editais/0005.pdf (accessed November 12, 2019).
6. Institute for Applied Economic Research. Atlas de Vulnerabilidade Social dos Municípios Brasileiros. (2015). Available online at: http://ivs.ipea.gov.br/images/publicacoes/Ivs/publicacao_atlas_ivs.pdf (accessed November 17, 2019).
7. Hill DE, Dubey JP. Toxoplasma gondii. Biology of Foodborne Parasites. Boca Raton: CRC Press (2015).
8. Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. (2002) 8:634–40. doi: 10.1046/j.1469-0691.2002.00485.x
9. Petersen E, Vesco G, Villari S, Buffolano W. What do we know about risk factors for infection in humans with Toxoplasma gondii and how can we prevent infections? Zoonoses Public Health. (2010) 57:8–17. doi: 10.1111/j.1863-2378.2009.01278.x
10. Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers. (2015) 1:15035. doi: 10.1038/nrdp.2015.35
11. Cong W, Dong XY, Meng QF, Zhou N, Wang XY, Huang SY, et al. Toxoplasma gondii infection in pregnant women: a seroprevalence and case–control study in Eastern China. Biomed Res Int. (2015) 2015:170278. doi: 10.1155/2015/170278
12. Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. (2009) 39:1385–94. doi: 10.1016/j.ijpara.2009.04.003
13. Fan CK, Liao CW, Wu MS, Su KE, Han BC. Seroepidemiology of Toxoplasma gondii infection among Chinese aboriginal and Han people residing in mountainous areas of northern Thailand. J Parasitol. (2003) 89:1239–42. doi: 10.1645/GE-3215RN
14. Yaman O, Yazar S, Çetinkaya Ü, Özcan Temel H, Balci E, Pehlivan I, et al. Kayseri Kapali.Cezaevi. Mahkumlarinda Toxoplasma gondii Seroprevalansi. Türkiye Parazitol Derg. (2009) 33:15–9.
15. van Enter BJD, Lau YL, Ling CL, Watthanaworawit W, Sukthana Y, Lee WC, et al. Seroprevalence of Toxoplasma gondii infection in refugee and migrant pregnant women along the Thailand-Myanmar border. Am J Trop Med Hyg. (2017) 97:232–5. doi: 10.4269/ajtmh.16-0999
16. de Amorim Garcia CA, Oréfice F, de Oliveira Lyra C, Gomes AB, França M, de Amorim Garcia Filho CA. Socioeconomic conditions as determining factors in the prevalence of systemic and ocular toxoplasmosis in Northeastern Brazil. Ophthalmic Epidemiol. (2004) 11:301–17. doi: 10.1080/09286580490515170
17. Santos GM, Silva SA, Barbosa AP, Campos DM. Investigação soroepidemiológica sobre a larva migrans visceral por Toxocara canis em usuários de serviços de saúde de Goiânia–GO. Ver Patol Trop. (2009) 38:197–206. doi: 10.5216/rpt.v38i3.7838
18. Amendoeira MRR, Sobral CAQ, Teva A, Lima JN, Klein CH. Inquérito sorológico para a infecção por Toxoplasma gondii em ameríndios isolados, Mato Grosso. Rev Soc Bras Med Trop. (2003) 36:671–6. doi: 10.1590/S0037-86822003000600005
19. Vitaliano SN, Mendonça GM, Sandres FAM, Camargo JSAA, Tarso P, Basano AS, et al. Epidemiological aspects of Toxoplasma gondii infection in riverside communities in the Southern Brazilian Amazon. Rev Soc Bras Med Trop. (2015) 48:301–6. doi: 10.1590/0037-8682-0040-2015
20. Brazilian Institute of Geography and Statistics. Censo Demográfico 2010: Panorama geral, São Paulo, Brasil. (2010). Available online at: https://cidades.ibge.gov.br/brasil/sp/sao-paulo/panorama (accessed December 12, 2019).
21. Paula HC, Daher DV, Koopmans FF, Faria MGA, Brandão PS, Scoralick GBF. Implementation of the street outreach office in the perspective of health care. Rev Brasil Enfermagem. (2018) 71:2843–7. doi: 10.1590/0034-7167-2017-0616
22. Grangeiro A, Holcman MM, Onaga ET, Alencar HDR, Placco ALN, Teixeira PR. Prevalência e vulnerabilidade à infecção pelo HIV de moradores de rua em São Paulo, SP. Rev Saúde Públ. (2012) 46:674–84. doi: 10.1590/S0034-89102012005000037
23. Gravinatti ML, Faccini-Martínez ÁA, Ruys SR, Timenetsky J, Biondo AW. Preliminary report of body lice infesting homeless people in Brazil. Rev Inst Med Trop São Paulo. (2018) 60:e9. doi: 10.1590/s1678-9946201860009
24. Camargo ME. Introdução às técnicas de imunofluorescência. Rev Bras Patol Clín. (1974) 10:143–69.
25. IBM Corp. Released in 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.
26. Francisco FM, Souza SLP, Gennari SM, Pinheiro SR, Muradian V, Soares RM. Seroprevalence of toxoplasmosis in a low-income community in the São Paulo municipality, SP, Brazil. Rev Inst Med Trop São Paulo. (2006) 48:167–70. doi: 10.1590/S0036-46652006000300009
27. Mareze M, Benitez ADN, Brandão APD, Pinto-Ferreira F, Miura AC, Martins FDC, et al. Socioeconomic vulnerability associated to Toxoplasma gondii exposure in southern Brazil. PLoS ONE. (2019) 14:e0212375. doi: 10.1371/journal.pone.0212375
28. Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Oréfice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis. (2003) 9:55–62. doi: 10.3201/eid0901.020160
29. Avelino MM, Campos D Jr, Parada JB, Castro AM. Risk factors for Toxoplasma gondii infection in women of childbearing age. Braz J Infect Dis. (2004) 8:164–74. doi: 10.1590/S1413-86702004000200007
30. Lopes-Mori FM, Mitsuka-Breganó R, Bittencourt LHFB, Dias RCF, Gonçalves DD, Capobiango JD, et al. Gestational toxoplasmosis in Paraná State, Brazil: prevalence of IgG antibodies and associated risk factors. Braz J Infect Dis. (2013) 17:405–9. doi: 10.1016/j.bjid.2012.12.003
31. Araújo AC, Villela MM, Sena-Lopes Â, Farias NAR, Faria LMJ, Avila LFC, et al. Seroprevalence of Toxoplasma gondii and Toxocara canis in a human rural population of Southern Rio Grande do Sul. Rev Inst Med Trop S Paulo. (2018) 60:e28. doi: 10.1590/s1678-9946201860028
32. Passos ADC, Bollela VR, Furtado JMF, Lucena MM, Bellissimo-Rodrigues F, Paula JS, et al. Prevalence and risk factors of toxoplasmosis among adults in a small Brazilian city. Rev Soc Bras Med Trop. (2018) 51:781–7. doi: 10.1590/0037-8682-0214-2017
33. Hickey C, Downey D. Hungry for Change: Social Exclusion, Food Poverty and Homelessness in Dublin. Dublin: Focus Ireland (2003).
34. Food Standards Agency. Research Into Food Poverty and Homelessness in Northern Ireland–Final Report. Belfast: Deloitte MCS Limited (2006). Available online at: http://webarchive.nationalarchives.gov.uk/20111206074236/http://www.food.gov.uk/multimedia/pdfs/homelessnifood.pdf (accessed December 14, 2019).
35. Alvarado-Esquivel C, Torres-Castorena A, Liesenfeld O, Estrada-Martínez S, Urbina-Álvarez JD. High seroprevalence of Toxoplasma gondii infection in a subset of Mexican patients with work accidents and low socioeconomic status. Parasit Vectors. (2012) 5:13. doi: 10.1186/1756-3305-5-13
36. Sprake EF, Russell JM, Barker ME. Food choice and nutrient intake amongst homeless people. J Hum Nutr Diet. (2014) 27:242–50. doi: 10.1111/jhn.12130
37. Mie T, Pointon AM, Hamilton DR, Kiermeier A. A qualitative assessment of Toxoplasma gondii risk in ready-to-eat smallgoods processing. J Food Prot. (2008) 71:1442–52. doi: 10.4315/0362-028X-71.7.1442
38. Rushton CM, Wheeler IE. The dietary intake of homeless males sleeping rough in Central London. J Hum Nutr Diet. (1993) 6:443–56. doi: 10.1111/j.1365-277X.1993.tb00389.x
39. Fallaize R, Seale JV, Mortin C, Armstrong L, Lovegrove JA. Dietary intake, nutritional status and mental wellbeing of homeless adults in Reading, UK. Br J Nutr. (2017) 118:707–14. doi: 10.1017/S0007114517002495
40. Li A, Dachner N, Tarasuk V. Food intake patterns of homeless youth in Toronto. Can J Public Health. (2009) 100:36–40. doi: 10.1007/BF03405490
41. Gontijo da Silva M, Clare Vinaud M, de Castro AM. Prevalence of toxoplasmosis in pregnant women and vertical transmission of Toxoplasma gondii in patients from basic units of health from Gurupi, Tocantins, Brazil, from 2012 to 2014. PLoS ONE. (2015) 10:e0141700. doi: 10.1371/journal.pone.0141700
42. Kieffer F, Wallon M. Congenital toxoplasmosis. Handb Clin Neurol. (2013) 112:1099–101. doi: 10.1016/B978-0-444-52910-7.00028-3
43. Abamecha F, Awel H. Seroprevalence and risk factors of Toxoplasma gondii infection in pregnant women following antenatal care at Mizan Aman General Hospital, Bench Maji Zone (BMZ), Ethiopia. BMC Infect Dis. (2016) 16:460. doi: 10.1186/s12879-016-1806-6
44. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. (2004) 363:1965–76. doi: 10.1016/S0140-6736(04)16412-X
45. Berrébi A, Assouline C, Bessières MH, Lathière M, Cassaing S, Minville V, et al. Long-term outcome of children with congenital toxoplasmosis. Am J Obstet Gynecol. (2010) 203:552.e1–552.e6. doi: 10.1016/j.ajog.2010.06.002
46. Azevedo KML, Setúbal S, Lopes VGS, Camacho LAB, Oliveira AR. Congenital toxoplasmosis transmitted by human immunodeficiency-virus infected women. Braz J Infect Dis. (2010) 14:186–9. doi: 10.1016/S1413-8670(10)70036-2
47. Ding H, Gao YM, Deng Y, Lamberton PHL, Lu DB. A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China. Parasit Vectors. (2017) 10:27. doi: 10.1186/s13071-017-1970-6
48. Teutsch SM, Juranek DD, Sulzer A, Dubey JP, Sikes RK. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. (1979) 300:695–9. doi: 10.1056/NEJM197903293001302
49. Torrey EF, Yolken RH. Toxoplasma oocysts as a public health problem. Trends Parasitol. (2013) 29:380–4. doi: 10.1016/j.pt.2013.06.001
50. Marques CS, Sousa S, Castro A, da Costa JMC. Detection of Toxoplasma gondii oocysts in fresh vegetables and berry fruits. Parasit Vectors. (2020) 13:180. doi: 10.1186/s13071-020-04040-2
51. Mirza Alizadeh A, Jazaeri S, Shemshadi B, Hashempour-Baltork F, Sarlak Z, Pilevar Z, et al. A review on inactivation methods of Toxoplasma gondii in foods. Pathog Glob Health. (2018) 112:306–19. doi: 10.1080/20477724.2018.1514137
52. Bonilla DL, Cole-Porse C, Kjemtrup A, Osikowicz L, Kosoy M. Risk factors for human lice and bartonellosis among the homeless, San Francisco, California, USA. Emerg Infect Dis. (2014) 20:1645–51. doi: 10.3201/eid2010.131655
53. Ly TDA, Touré Y, Calloix C, Badiaga S, Raoult D, Tissot-Dupont H, et al. Changing demographics and prevalence of body lice among homeless persons, Marseille, France. Emerg Infect Dis. (2017) 23:1894–7. doi: 10.3201/eid2311.170516
54. Faccini-Martínez ÁA, Márquez AC, Bravo-Estupiñan DM, Calixto OJ, López-Castillo CA, Botero-García CA, et al. Bartonella quintana and typhus group Rickettsiae exposure among homeless persons, Bogotá, Colombia. Emerg Infect Dis. (2017) 23:1876–9. doi: 10.3201/eid2311.170341
55. Liberato C, Magliano A, Romiti F, Menegon M, Mancini F, Ciervo A, et al. Report of the human body louse (Pediculus humanus) from clothes sold in a market in central Italy. Parasit Vectors. (2019) 12:201. doi: 10.1186/s13071-019-3458-z
56. Ministry of Health. Secretariat of Health Surveillance, 2018. Boletim Epidemiológico. HIV AIDS. (2018). Available online at: http://www.aids.gov.br/pt-br/pub/2018/boletim-epidemiologico-hivaids-2018 (accessed December 15, 2019).
57. Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. (2017) 4:e177–88. doi: 10.1016/S2352-3018(17)30005-X
58. Feleke DG, Gebreweld A, Zewde G. Toxoplasmosis in pregnant women and HIV/AIDS patients in Ethiopia: a systematic review and meta-analysis. J Parasitol Res. (2019) 2019:4670397. doi: 10.1155/2019/4670397
Keywords: homeless, Toxoplasma gondii, HIV, vulnerability, serology
Citation: Felipetto LG, Teider-Junior PI, da Silva FFV, Yamakawa AC, Kmetiuk LB, do Couto AC, Martins CM, Vaz ES, Ullmann LS, Langoni H, Timenetsky J, dos Santos AP and Biondo AW (2020) Serosurvey of Anti-Toxoplasma gondii Antibodies in Homeless Persons of São Paulo City, Southeastern Brazil. Front. Public Health 8:580637. doi: 10.3389/fpubh.2020.580637
Received: 15 July 2020; Accepted: 13 October 2020;
Published: 05 November 2020.
Edited by:
Alexandre Morrot, Federal University of Rio de Janeiro, BrazilReviewed by:
Phileno Pinge-Filho, State University of Londrina, BrazilCopyright © 2020 Felipetto, Teider-Junior, da Silva, Yamakawa, Kmetiuk, do Couto, Martins, Vaz, Ullmann, Langoni, Timenetsky, dos Santos and Biondo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Welker Biondo, YWJpb25kb0B1ZnByLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.