- 1Louisiana Health Sciences Center, School of Medicine, New Orleans, LA, United States
- 2Department of Public Health and Preventive Medicine, St. George's University, True Blue, Grenada
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer that lacks expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2). TNBC constitutes about 15–30 percent of all diagnosed invasive breast cancer cases in the United States. African-American (AA) women have high prevalence of TNBC with worse clinical outcomes than European-American (EA) women. The contributing factors underlying racial disparities have been divided into two major categories based on whether they are related to lifestyle (non-biologic) or unrelated to lifestyle (biologic). Our objective in the present review article was to understand the potential interactions by which these risk factors intersect to drive the initiation and development of the disparities resulting in the aggressive TNBC subtypes in AA women more likely than in EA women. To reach our goal, we conducted literature searches using MEDLINE/PubMed to identify relevant articles published from 2005 to 2019 addressing breast cancer disparities primarily among AA and EA women in the United States. We found that disparities in TNBC may be attributed to racial differences in biological factors, such as tumor heterogeneity, population genetics, somatic genomic mutations, and increased expression of genes in AA breast tumors which have direct link to breast cancer. In addition, a large number of non-biologic factors, including socioeconomic deprivation adversities associated with poverty, social stress, unsafe neighborhoods, lack of healthcare access and pattern of reproductive factors, can promote comorbid diseases such as obesity and diabetes which may adversely contribute to the aggression of TNBC biology in AA women. Further, the biological risk factors directly linked to TNBC in AA women may potentially interact with non-biologic factors to promote a higher prevalence of TNBC, more aggressive biology, and poor survival. The relative contributions of the biologic and non-biologic factors and their potential interactions is essential to our understanding of disproportionately high burden and poor survival rates of AA women with TNBC.
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer and the second leading cause of cancer death among women in the United States [American Cancer Society. Cancer Facts and Figures 2017]. Approximately 268,600 women will be diagnosed with BC, and nearly 41,760 will die with this malignancy in 2019 [American Cancer Society. Cancer Facts and Figures 2019].
Breast cancer is a heterogeneous disease consisting of distinct biological subtypes with a range of clinical, pathological, molecular, and genetic features and differing therapeutic responses and outcomes including the Black-White disparities in outcome (1). These differences have been demonstrated by molecular classification based on the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Using this approach, at least four intrinsic subtypes of breast cancer have been identified. These include luminal A [ER+ and/or PR+, HER2- and low Ki67 (<14%)], luminal B [ER+ and/or PR+, HER2-, and high Ki67 (>14%) or ER+ and/or PR+, HER2+], HER2 [ER-, PR-, HER2 amplification], triple negative [ER-, PR-, HER2-, basal markers, such as cytokeratin (CK) 5/6, CK 14, CK 17, and epidermal growth factor receptor]. While the terms basal-like subtype (characterized as ER−/PR−/HER2−, basal-markers+) and triple negative breast cancer (TNBC) have been used interchangeably in some studies, evidence has shown that although most TNBCs are basal-like, up to 20–30% of them are not; additionally, not all basal-like breast cancers are TNBCs (2–4).
A recent population study in the United States has described four molecular breast cancer subtypes as mentioned above, based on the expression of three tumor markers, namely estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2) (5, 6). Luminal A subtype constituted the majority (72.6%), TNBC 13%, luminal B 5% and HER2-enriched constituted 10% of all breast cancers diagnosed in 2011 (7). The presence of these four breast cancer subtypes varied notably with age and race. But we do not know whether it also varied with healthcare variables such as access to healthcare resources.
Prominent racial differences have been noted in the incidence of and mortality from breast cancer between African American (AA) and Non-Hispanic White (NHW) women. The 2017 CDC and NCI review of trends in population-based BC incidence and mortality rates in 1999–2014 by age and race in 2014 indicates that although the incidence rates are comparable for AA and NHW women for all ages and stages of diagnosis the mortality rates are very different (8). AA patients have an ~2-fold higher mortality incidence compared with the NHW women, resulting in a disproportionately higher (>65%) risk of death (United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report at: http://www.cdc.gov/USCS). In addition, a greater proportion of five-year breast cancer-specific survival rates are significantly lower in AAs (78.9%) compared with NHWs (88.6%) (9). The mortality disparity is especially noteworthy in light of the similar incidence rates of breast cancer among AA and NHW women.
The reasons underlying the health disparities in breast cancer outcome are multifactorial and complex. Many biological and non-biological factors are perceived to contribute to these disparities. Significant molecular differences have been identified in the biological properties between AA women and NH White women. The present review is undertaken to provide a comprehensive view of how these factors contribute to marked differences in age of onset, stage of presentation and survival between AA and NH White women and eventually the development and outcome of the TNBC disparity. An understanding of these factors and how do they contribute to disparities is critical in defining an in depth understanding of the marked differences in development, presentation, and outcome of breast cancer between Caucasian and AA women. To address inequities, we begin the article with a description of the pattern of TNBC disparities among AA and NH White women. Because obesity is a well-documented factor exerting a significant effect on the development of breast cancer, in the second section we addressed the potential link between obesity and TNBC in AA women. Several studies have suggested that tumor biology may contribute to the outcome disparities with TNBC in AA women. Therefore, in the third section of the article, we address the biological mechanisms of TNBC risk in AA women. There is increasing evidence that lower socioeconomic status disproportionately promotes aggressive biology in AA patients with TNBC. Thus, the fourth section encompasses the social determinants of TNBC risk in AA women. The article will provide comprehensive view of the relationship between biological and non-biological factors to facilitate our understanding of disparities in the risk of TNBC, and to guide future efforts to eliminate such disparities.
Methods
Search Criteria
Literature searches were conducted in MEDLINE/PubMed to identify relevant articles published from 2005 to 2019 addressing breast cancer disparities primarily among AA women compared to NHW women in the United States. The studies were selected based on the relevance of their full-text contents examining the nature and magnitude as well as the major risk factors associated with breast cancer disparities. When relevant to our review article, specific papers identified from the reference lists of published papers were also included. The combination of keywords- “biologic factors and breast cancer (BC) and African American (AA) women, non-biologic factors and BC and AA women, obesity and BC and AA women, triple negative breast cancer (TNBC) and AA women, Social determinants and BC and AA women, and socioeconomic status (SES) and BC and AA women" were used.
TNBC and AA Women
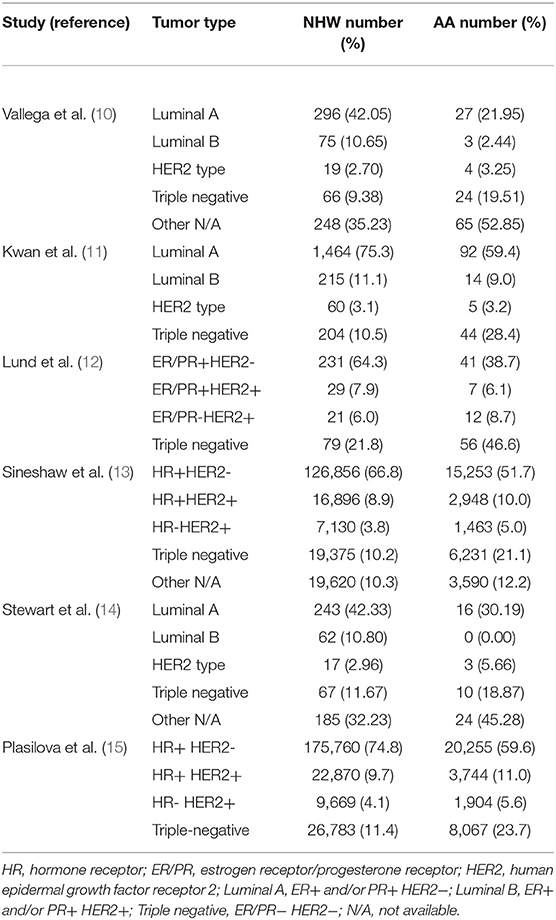
The distribution of breast cancer subtypes by race/ethnicity is illustrated in Table 1. As shown, the distribution of race/ethnicity within each subgroup compared with other subgroups varied significantly. The majority of the NHW and AA women had the luminal A (ER/PR+HER2-) subtype; NHW women had the highest prevalence compared with AA women.
Luminal B (ER/PR+HER2+) and HER2-overexpressing subtype was least common among both races/ethnicities. Compared with NHW women, AA women are twice as likely to be diagnosed with TNBC indicating a disproportionate burden of TNBC in this population (10–15). Highly aggressive features of the TNBCs, lack of viable therapeutic targets and earlier age at onset, may contribute to poor outcomes and explain in part the poorest survival observed among AA women (12). In most studies listed in Table 1, the most common subtype was luminal A (ER+/PR+/HER2-), followed by TNBC, with luminal B and HER2+ expressing subtypes being less common. Luminal A subtype generally has the most favorable prognosis, whereas TNBC subtype has the least favorable prognosis (12, 16–19).
Compared with the luminal A subtype, TNBC is disproportionately more common in younger or premenopausal women, especially young AA women (12, 20, 21). Many studies show that relative to women of European decent premenopausal AA and African women have a high prevalence of TNBC (22–25). The Carolina Breast Cancer Study showed that the prevalence of the basal-like subtype of TNBC (39%; 38/97 invasive cancers) was substantially higher in premenopausal AA women than the prevalence of TNBC observed in post-menopausal AA women (14%; 14/99 invasive cancers) or American women of European descent (16%; 48/300 invasive cancers) (p < 0.001 for both comparisons) (26). The high frequency of TNBC in premenopausal AA women has been also observed in several subsequent population-based studies (12, 27–29). TNBC is biologically more aggressive and has poor prognosis than the luminal A subtype (18, 30). In addition, the 5 year relative survival rates for AA women diagnosed with breast cancer (80%) is significantly lower than for NH White women (91%) across all ages and tumor stages and subtypes, and age-adjusted mortality rate for AA women (30.0/100,000) is the highest rate for any ethnic group studied [Cancer Statistics 2017 (31)].
The racial differences in TNBC strongly persisted in all age categories with steep increase in TNBC incidence with increasing age for AA women compared with NHW women (21). As shown, the TNBC incidence rates were ~2-fold higher for AA women compared to NHW women in all age categories with highest TNBC incidence rate in the 60–74 year-old category (21). TNBCs most frequently occur in women with germline BRCA1 mutations (24). However, <20–25% of AA women with TNBC have a BRCA1 germline mutation. These data suggest that the molecular events surrounding TNBC initiation in AA women may be distinct from those of non-African descent.
Obesity and TNBC in AA Women
Obesity is associated with increased risk of a variety of different cancer types. Of these obesity-associated cancer types, almost 13% of the cases worldwide, and nearly 20% of the cases in Europe and North America, are attributable to obesity (32, 33). In the United States, obesity rates have reached epidemic proportions. More than 60% of the adult US population falls in the overweight and obese categories as determined by BMI: 25–29.9 and >30 kg/m2, respectively (7, 34, 35). Results from the National Health and Nutrition Examination Survey (NHANES) have shown that obesity prevalence varied by sex, age, race, and socioeconomic status (25, 34–36). The recent 2-year NHANES survey showed that in the United States, 57.2% of AA women were obese vs. 38.2% NHW women (36). The overall prevalence of class 3 obesity (BMI > 40) in AA women was 16.8 vs. 9.7% in NHW women (36). Analyses of NHANES data from 2013 to 2014 showed that in addition to race, obesity and class 3 obesity prevalence also varied by age (36). As shown, the prevalence of obesity in AA women vs. NHW women was on an average 150% higher at all age groups, and the prevalence of class 3 obesity from ages 20–59 was 160% higher but at age ≥60 it was 240% higher (36).
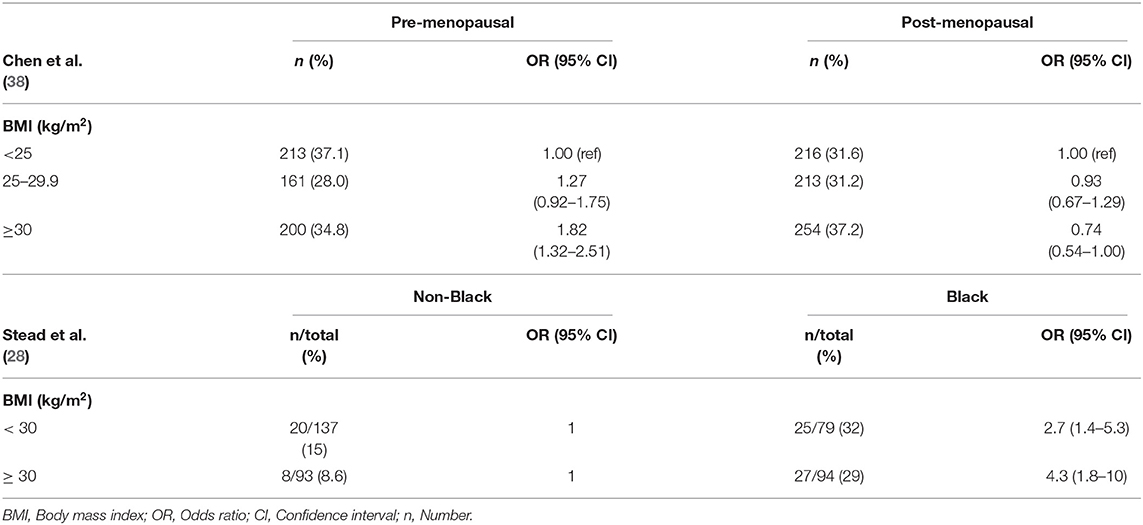
A number of studies have investigated the potential link between obesity and breast cancer. In premenopausal women at high risk for breast cancer as defined by the Gail score, risk of invasive breast cancer was significantly increased in overweight and obese women compared to women of BMI < 25 kg/m2 (37). A recent study by Chen et al., with a large cohort of predominantly NH White women suggests a heterogeneity in the relationship between BMI and breast cancer molecular subtype risk (38). Compared to women with BMI < 25 kg/m2, obese premenopausal women (BMI ≥ 30 kg/m2) had an 82% higher risk of TNBC, and those in the highest weight quartile (quartiles were classified according to the distribution among luminal A patients) had a 79% increased risk of TNBC compared to those in the lowest quartile. Among post-menopausal women, obesity was associated with reduced risk of TNBC (Table 2). While other studies have suggested an increased risk of TNBC in premenopausal women associated with higher BMI, no differences in risk were found among postmenopausal women (11, 39, 40). Another study performed in West Virginia, the only state in Appalachia with a 95% White population, the TNBC incidence increased with increasing BMI. In this study, TNBC tumors were found to be significantly more common in those patients who were classified as obese, 49.6 vs. 35.8%, respectively (P = 0.0098) (41).The majority of studies done in North America and Europe found that the prevalence of TNBC in NHW women with breast cancer ranged from ~10–13% (13–15) which contrasts with the 18.9% seen in the West Virginian patients (41). The study of Gershuni et al. (7), showed association between BMI and TNBC in Black women but no association in non-Black women. The prevalence of TNBC in overweight and obese Black women was 2-fold compared to that in normal weight Black women. On the other hand, the incidence of TNBC was not significantly different among normal weight, overweight, and obese non-Black women. Stead et al. (28) found that the prevalence of TNBC in AA women was 3-fold higher as compared with non-Black women (Table 2) (28). However, stratifying the dataset to Black vs. non-Black women, they showed that, 29% of obese Black women had triple-negative tumors compared with 8.6% of obese non-Black women [odds ratio (OR) = 4.3: 95 CI = 1.8–10; p = 0.0004]. (Applying White/Caucasian women as the reference category, the OR = 4.2 and 95% CI = 1.6–13). Similarly, 31% of non-obese Black women had triple-negative tumors compared with 15% of non-obese non-Black women (OR = 2.7, 95% CI = 1.4–5.3; p = 0.003). (Applying White/Caucasians women as the reference category, the OR = 2.5 and 95% CI = 1.2–5.4) (Table 2). These two ORs did not differ significantly from each other (p = 0.41), indicating that BMI does not seem to be correlated with triple-negative status among AA women (28). Pierobon and Frankenfeld (42) used meta-analysis to sum up the results of 11 epidemiologic studies published between May 2008 and February 2012 to evaluate the association between obesity (BMI) and risk of TNBC. There results, in a case-case comparison of TNBC, showed that obese premenopausal women had 43% greater risk of TNBC than non-obese premenopausal women, but that obesity did not correlate with risk of TNBC in postmenopausal women. The relationship between obesity and TNBC remains uncertain, except that chronic inflammatory conditions induced by obesity may activate molecular pathways that favor TNBC pathogenesis.
Racial Differences in Visceral Adipose Tissue and TNBC
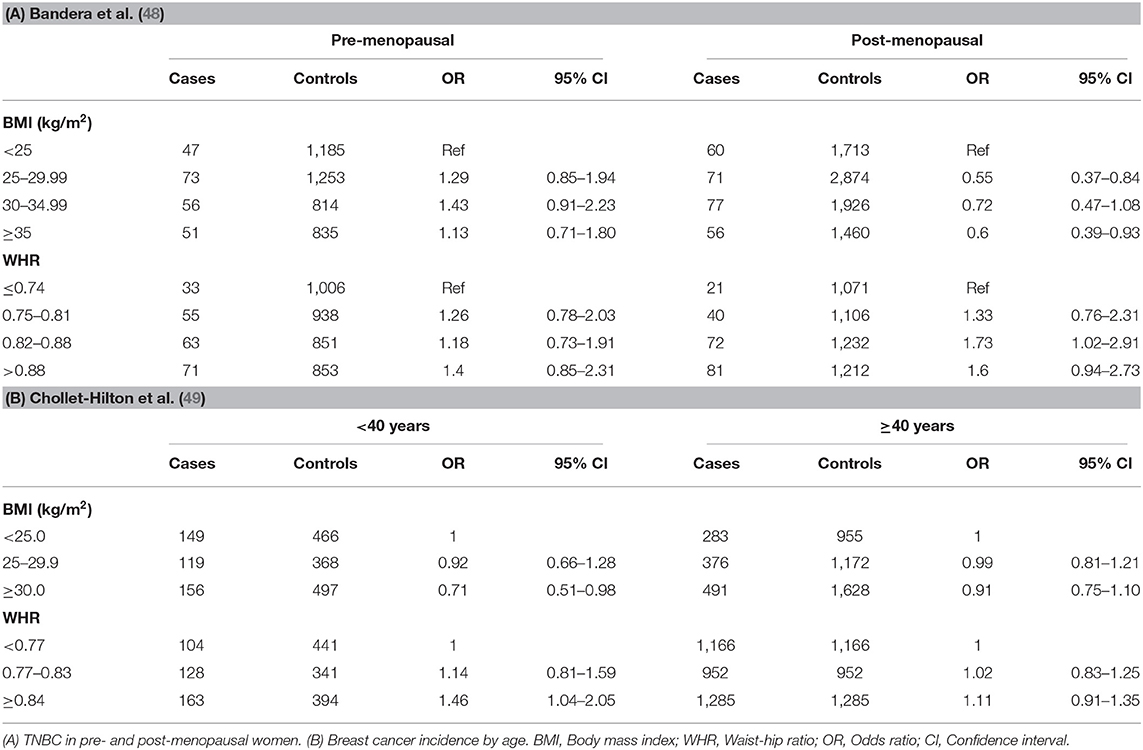
BMI is usually associated with general obesity and does not provide any information on body composition. Waist circumference (WC) and waist-hip ratio (WHR), on the other hand, have been used as measures of central or intra-abdominal obesity, defined as WHR above 0.90 for males and above 0.85 for females (43). A number of studies have shown measurement of WC or WHR to be the best anthropometric indicator of visceral adiposity which is a key component of the metabolic syndrome associated with hyperinsulinemia and insulin resistance (44–46). The correlations between excess adiposity and TNBC are stronger in AA women than in NHW women. In a population-based case controlled Carolina Breast Cancer epidemiological study of AA and NHW women, the WHR was compared between the highest (≥0.84) and lowest (<0.77) groups in relation to the TNBC. Across all women, premenopausal women and postmenopausal women with high WHR had a significantly increased risk of developing TNBC compared with the lowest WHR group (25). The prevalence TNBC and TNBC risk factors was the highest among premenopausal African American women (39). There was no significant association between increased BMI (BMI ≥30 kg/m2) and the risk of TNBC (25). The study suggested potential association between WHR and TNBC and a lack of association between WHR and increased BMI.
Several other studies have also reported WHR as a strong risk factor for TNBC in AA women (39, 47–49). As shown in Table 3, Bandera et al., reported that recent BMI was not significantly associated with premenopausal TNBC (9). For postmenopausal women, high recent BMI was associated with reduced risk of TNBC. On the other hand, higher WHR (Table 3) was associated with non-significant increases of TNBC among premenopausal women. Among postmenopausal women, higher WHR was a stronger risk for TNBC for third and fourth quartiles, compared to lowest (48). The studies by Chollet-Hinton et al. (49) and Harris et al. (47) also suggested that abdominal adiposity as represented by WHR is an important factor contributing to young-onset disease. Higher WHR was more strongly associated with young (<40 years) premenopausal women at breast cancer diagnosis compared with older age (≥40 years) at diagnosis (Table 3) (49). As shown, ORs for BMI were not significantly modified by age, though obese BMI (≥30 kg/m2) was more strongly associated with a reduced association among young women. The study by Harris et al. (47) indicated that WHR was more strongly associated with an increased risk of premenopausal ER-negative breast cancer than risk of ER-positive breast cancer suggesting that sex hormone-independent pathways are involved in the higher risk of ER-negative breast cancer.
Obesity and TNBC Development
Obesity may increase the incidence of TNBC in African and AA women through various biological mechanisms [reviewed in Chen et al. (38)]. In particular, centrally located adipose tissue creates a variety of physiological conditions that favor inflammation within the body. Dysregulation of certain biological functions such as cellular growth, angiogenesis stimulation, and extracellular matrix remodeling that favor tumor progression and relapse have been shown to result by adipokine secretion from adipose tissue. In addition, adipose tissue promotes an inflammatory response through the secretion of inflammatory mediators, such as tumor necrosis factor alpha (TNFα), interleukin 6 (IL-6), and retinol-binding protein-4 (RBP4). Studies have shown that TNFα promotes the growth of human breast cancer cells through the activation of several intracellular molecular pathways including NF-kappa B, MAP kinase and the PI3-K/AKT signaling pathways (50). Higher levels of circulating IL-6 is significantly associated with poor survival in patients with metastatic breast cancer (51, 52). IL-6 is also prognostic of early tumor size, tumor progression, and metastasis at various sites (53, 54).
Leptin is another cytokine predominantly produced by adipose tissue and is necessary for the development of normal breast gland and lactation. Serum leptin is significantly higher in obese than in normal weight individuals (55). This unusually high levels of leptin from excess adipose tissue may activate signaling that has been shown to induce relevant molecular pathways involved in proliferation, angiogenesis, and insulin-like growth factor 1 (IGF 1) expression resulting in tumor progression, invasion and metastasis (56, 57). On the contrary, adiponectin also secreted by adipose tissue, functions to block the tumorigenic effects of leptin. However, its expression is decreased in obese individuals and in those who are insulin resistant. This leads to an increased leptin to adiponectin ratio resulting in tumor proliferation in breast cancer cells (58). It has been shown that serum adiponectin is inversely related to breast cancer risk (59–61).
Several lines of evidence indicate a role for adiponectin in the pathways connecting insulin sensitivity to muscle morphology. Skeletal muscle is a heterogenous tissue comprising different fiber types which vary in their metabolic characteristic, that may play a role in the pathogenesis of obesity, hypertension, and diabetes. On the basis of these characteristics, muscle fiber are classified in to type I and type II fibers with type II further subdivided into type IIa and type IIb also known as type IIx (62, 63). Type I muscle fibers possess greater aerobic metabolic capacity because of higher myoglobin, capillary, and mitochondrial content. Type II muscle fibers, on the other hand, have lower aerobic ability, lower levels of myoglobin, less capillaries, and are better suited for anaerobic metabolism. Type IIa fibers have more aerobic potential than type IIx, but less aerobic potential than type I fibers (64). Higher proportion of type I muscle fibers has been significantly associated with higher serum adiponectin concentrations, whereas the proportion of type IIb muscle fibers was inversely associated (65).
The proportions of muscle fiber types vary by race, with more anaerobic (type II) fibers in NH Black people, and more of type I fibers in NH White people. Compared with type II muscle fibers and especially the type IIb (IIx), which are insulin resistant, type I muscle fibers are insulin sensitive (66, 67). Type II myofibers are also associated with obesity, hypertension, and diabetes, particularly in NH Black people (64, 68).
Obesity is a major risk factor for type 2 diabetes. Studies have shown that the proportion of type I muscle fibers correlate inversely and type IIx (IIb) muscle fibers correlate directly with the BMI and percent body fat (67, 69, 70). Obese diabetic individuals have a lower proportion of type I muscle fibers and a higher proportion of type IIx muscle fibers (67, 69, 70). In a study on the prevalence of muscle fiber type in African American Blacks, obese African American women showed a significantly more type IIx (IIb) muscle fibers than lean subjects (67). In addition, obese African American women showed significantly lower percentage of type I muscle fibers and a higher percentage of type IIx fibers than in obese Caucasians (67). These results are consistent with higher obesity prevalence, higher weight gain, and insulin resistance in African American Blacks.
Thus, because of an inherited lower amount of skeletal muscle fiber type I and higher amount of fiber type IIx (IIb), African American Blacks may be genetically predisposed to type 2 diabetes, decreasing oxidative ability and fat oxidation, resulting in increased accumulation of fat in muscular tissue.
Obesity is intimately linked to elevated circulating insulin levels, reduced insulin sensitivity, and insulin resistance (71). High blood glucose and insulin levels with corresponding insulin resistance have been correlated with poor outcomes in breast cancer patients (72–75). Human pulmonary tumor data have shown that cancer cells display increased glucose consumption which may lead to fueling of cancer cells (76). The increased glucose metabolism in cancer cells even in the presence of oxygen is known as the Warburg effect, which is defined by intense lipogenesis, increased aerobic glycolysis and low mitochondrial oxidative phosphorylation capability even in the presence of adequate oxygen (77). High blood glucose levels and hyperinsulinemia, which are frequent in obese individuals, may provide a selective growth advantage to the cancer cells (43).
Biologically, obese persons, and especially obese postmenopausal women, have increased serum levels of estrogens, estrone and estradiol, and decreased levels of sex hormone-binding globulin (SHBG), a glycoprotein which binds estradiol the major female steroid hormone and inhibits its function (78). As a result, this increased levels of the steroid hormones in obese persons may potentially enhance tumor progression and recurrence. Decreased levels of SHBG also increased the levels of circulating androgens which may contribute to tumor progression on their own and to tumor promoting effects by their further conversion to estrogens by adipose tissue (79). In addition, estrogens increase leptin production, which leads to breast cancer cell proliferation and cancer progression. It has been shown that excess adipose tissue contributes to increased circulating insulin and IGF-1 (80) resulting in poorer outcomes.
The impact of obesity on increased likelihood of premenopausal TNBC may partly explain the higher incidence of TNBC among young AA and Hispanic women compared to NH Whites. According to data from the 2013–2014 National Health and Nutrition Examination Survey, the prevalence of overweight or obesity (BMI ≥ 25 kg/m2) for women between 20 and 39 years of age was 56.7% for AA, and 33.2% for NH White women (36). If obesity alone is the determinant of ER/PR and HER2 status, then obese Black and non-Black women should have similar proportions of ER/PR and HER2-negative tumors. However, obese Black women have 4-fold more triple-negative tumors than obese non-Black women (28), suggesting other possible mechanisms that influence whole body obesity are crucial in determining ER/PR and HER2 expression.
Biological Mechanisms of TNBC Risk in AA Women
The 2017 CDC and NCI review of trends in population-based BC incidence and mortality among all racial groups in 2014 showed that although the incidence rates were comparable for AA and NHW American women (122.4/100,000 vs. 124.8/100,000), the mortality disparity was present (Table 4, ref. a). AA patients experience substantially higher breast cancer mortality than NHW women (28.1/100,000 vs. 20.0/100,000) (Table 4, ref. a). Compared to NHW women, 5-year survival was also worse for AA women (91 vs. 80%) (Table 4, ref. b). AA patients were most likely to be diagnosed at stage III (24.1%) with high tumor grade (64.6%) and TNBC (29.6%) (Table 4, ref. c). NHW women had the highest proportion of tumors diagnosed at stage I (45.6%) and ER-positive tumors (76.1%) (81). AA women had significantly fewer HR-positive tumors and significantly more TNBC than NHW women. There was no significant difference in HER2 positive tumors (Table 4, ref. d).
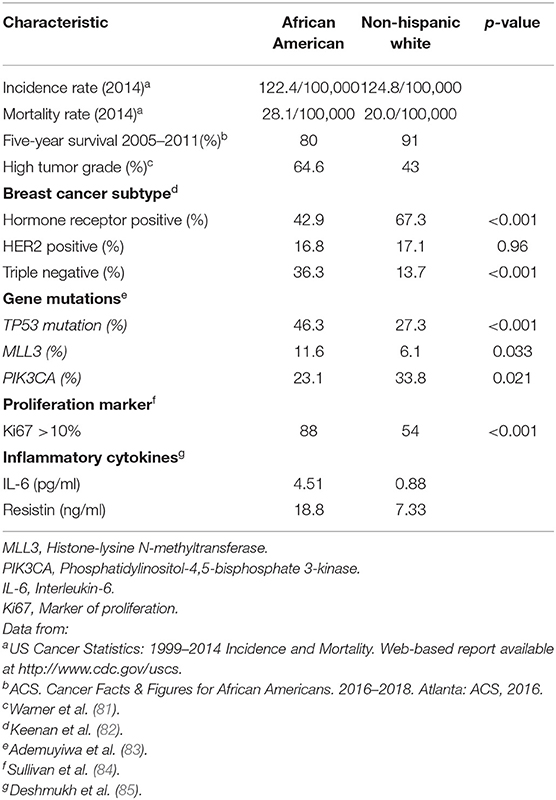
Table 4. Disparities in breast cancer risk and survival among African American and Non-Hispanic White women.
The reasons underlying the racial disparity in breast cancer outcome are multifactorial. Several socioeconomic issues, including income, access to care, and treatment delays, have been involved to play a critical role (86–90). However, many studies have found that the disparity remains even after adjustment for socioeconomic and treatment differences (87, 89, 90). Some studies have suggested that tumor biology may contribute to the inequity (91, 92). Although TNBCs are known to occur more frequently among AA women (26, 92–94), the influence of somatic genomic profiles on breast cancer disparity is still not clear. Somatic mutation analysis revealed racial differences in high prevalence (>5% in the TCGA dataset) genes (TP53, PIK3CA, MLL3) in all breast cancer patients, irrespective of clinical subtype (83). TP53 alterations were observed in 46% of all AA women vs. 27% of all Caucasians; p < 0.001, PIK3CA alterations: 23% in all AA women vs. 34% in all Caucasians; p 0.021, and MLL3 alterations: 12% in all AA women vs. 6% in all Caucasians; p value 0.034 (Table 4, ref. e). TP53 is a tumor suppressor gene and plays a key role in controlling cell proliferation, cell survival, and genomic integrity (95). Disrupting TP53 function promotes inappropriate survival leading to uncontrolled proliferation of damaged cells. Olivier et al. (95) have shown that TP53 mutations were more frequent in breast cancer tumors of ductal and medullar types, aggressive phenotype, and low hormone receptor content. The MLL3 (Histone-lysine N-methyltransferase) gene is tumor suppressor gene because it is often deleted in myeloid leukemia patients (96, 97). Recent studies have reported reduced MLL3 expression in many breast tumors (98). The phosphatidylinositol-3-kinase (PI3K) pathway is one of the most frequently enhanced oncogenic pathway in a variety of malignancies (99). In particular, breast cancer tumorigenesis is believed to depend on the PI3K pathway. Many studies have found mutations of the phosphatidylinositol-4,5-bisphosphatase-3-kinase (PIK3CA) gene coding for catalytic unit of PI3K mutations to be good prognostic markers in patients with early breast cancer (99).
Differential gene expression of primary breast cancer has discovered intriguing differences between races (14, 100–103). In the overall comparison between primary breast cancer tumors from AA and Caucasian American (CA) women, Steward et al. identified 342 differentially expressed genes and other transcripts (log2 fold-change > 1.0 or < −1.0 and P < 0.001) with few directly linked to breast cancer (14). Among 100 genes significantly overexpressed in AA breast tumors, resistin, an adipocytokine that induces insulin resistance and exerts proinflammatory effects (104, 105), showed 2.25 log2 fold-change (P = 3.05E-06). Breast cancer patients have significantly higher circulating levels of resistin compared to controls (106), and its higher level are associated with poor patient survival and more malignant clinical status (85, 107). Because of the strong link between obesity and cancer mortality, overexpression of resistin suggests important role of this gene in AA breast cancer (108–110). Similarly, a number of genes, including ADAM metallopeptidase with type 1 motif 15 (ADAMTS15: −1.29 log2 fold-change; P = 1.82E-04) have decreased expression in AA women. ADAMTS15 is a metalloprotease known to inhibit breast cancer cell migration. Thus, the decreased expression of this gene may have implications for the development and progression of breast cancer in AA women.
Social Determinants of TNBC Risk in AA Women
There is growing evidence that breast cancer patients with lower socioeconomic status (SES) are more likely to be diagnosed with advanced stages of breast cancer (111–114). The clinical outcomes in AA women with TNBC are worse when compared with European-American women who have the disease. AA patients were more likely to be diagnosed at a younger age, at a more advanced stage of the disease, to have larger tumors, to be unmarried, to live in lower SES neighborhoods, and to have public or no health insurance compared with European-American patients (all Ps < 0.05) (115). A number of other contributing factors may cause the deficiencies in treatment and care among AA breast cancer patients, such as less likely to receive the standard of care (116, 117), financial hardships caused by cancer care (118), need for time taken from work (119), and problems with travel (120, 121), which may also disproportionally affect the cancer treatment and care of AA women. Besides socioeconomic deprivation, survival may also be influenced by patient social context disproportionately affecting AA women, at the individual or neighborhood levels, and social inequality, leading to metabolic dysfunction associated with abdominal obesity (122–124).
In a large study of women diagnosed with invasive breast cancer in California, Tao et al. (115) found that disparities between AA and European-American women in breast cancer mortality varied according to breast cancer molecular subtype and the tumor stage. Within Stages II and III HR+/HER2- breast cancer, they found 31–39% higher rate of breast cancer specific deaths in AA than European-American patients after adjustment for tumor characteristics, first course of treatment, demographic factors, neighborhood SES, and insurance status (115). However, these factors, especially neighborhood SES, fully explained overall mortality differences in Stage I HR+/HER2-, Stage I and II HR+/HER2+, and Stage II TNBC, suggesting that early detection and early diagnosis plays a critical role in efforts to eliminate disparities. This finding is consistent with prior reports of a substantial impact of neighborhood SES on racial disparities in breast cancer mortality (13, 87, 114). Prior studies focusing on TNBC cancer reported that several molecular factors (see Biological mechanisms of breast cancer risk in AA women), as well as epidemiological factors, including reproductive and patient demographic factors (125), differed in prevalence among racial groups, indicating clear association between unequal living standards and increased levels of co-morbid disease.
Using the univariate multinomial logistic regression model, in their studies on associations between sociodemographic, tumor characteristics, and breast cancer subtypes, Llanos and collaborators (126) reported that compared to the luminal A subtype as the referent group, women with TNBCs were more likely to be younger at diagnosis, African American, and of lower SES (less than college educated, and income below the state median of $70,000). Additionally, women with TNBCs were more likely to have tumors that were self-detected, poorly differentiated, higher stage, larger, p53 positive and Ki67 positive and were less likely to have a history of benign breast disease. The findings of this study support associations between sociodemographic and clinicopathological characteristics of tumors, and biomarker status-based BC subtypes, specifically showing that the more aggressive tumor phenotypes were more likely to occur among women who were diagnosed at younger age, African American, and/or of lower SES.
Higher prevalence of the socioeconomic disadvantages experienced by AA women in their communities partially explains the breast cancer outcome disparities observed between AA women and White American women. Recent US Census Bureau reports show that poverty levels in AA women are more than twice as high as in White American populations (25.8 vs. 11.6%) (29). Despite overall drop in uninsured rates for all Americans from 16.0% in 2010 to 11.5% in 2014, disparities in this socioeconomic parameter persist, with 11.9% of the AA population being uninsured compared to 8.2% of the White American population (127). Barriers in healthcare access resulting in diagnostic and treatment delays explain partially variances in BC stage distribution and mortality disparities. Among BC cases regional disease is diagnosed in approximately one-third of the AA patients compared with one-quarter of White American patients, and localized disease is detected in approximately one-half vs. two-thirds, respectively (128).
There is a strong correlation between socio-demographic characteristics, lifestyle, diet, and obesity. Among AA women the prevalence of obesity (BMI≥30 kg/m2) is higher (58.6%) compared to non-Hispanic White women (33.4%) (34). Because AA women have higher incidence of obesity, and obesity predicts poor survival, it is speculated that obesity is a potential driver of aggressive TNBC biology in AA women. The mechanistic link between obesity, insulin signaling, and aggressive subtypes of TNBC is increasingly being supported. Tissue inflammation as a result of obesity promotes production of elevated levels of inflammatory cytokines (IL-6, IL-8, TNF-α, and leptin). IL-6 and IL-8 signaling leads to activation of STAT3, NF-κB, and EZH2 signaling pathways and predicts poor prognosis in women with TNBC (129).
Additionally, there is also a clear association between unequal standards of living and increased rates of co-morbid disease. Disparities in income, lack of access to fresh vegetables and nutritious food, lack of healthcare access, unsafe neighborhoods, and lack of physical activity can promote co-morbid diseases such as obesity and diabetes, which in turn may drive signaling pathways associated with aggressive biology in TNBC (Figure 1). Obesity and accompanying tissue inflammation increase tissue factors, such as inflammatory cytokines, increased activation of insulin-like growth factor 1 receptor (IGF1R), and increased expression of vascular endothelial growth factor-activated genes that contribute to aggressive breast cancer biology (38). Taken together, it is clear that disparities in physical activity, access to healthy food, and a lack of safe neighborhoods disproportionately promote obesity and poor metabolic health in AA women and aggressive TNBC biology.
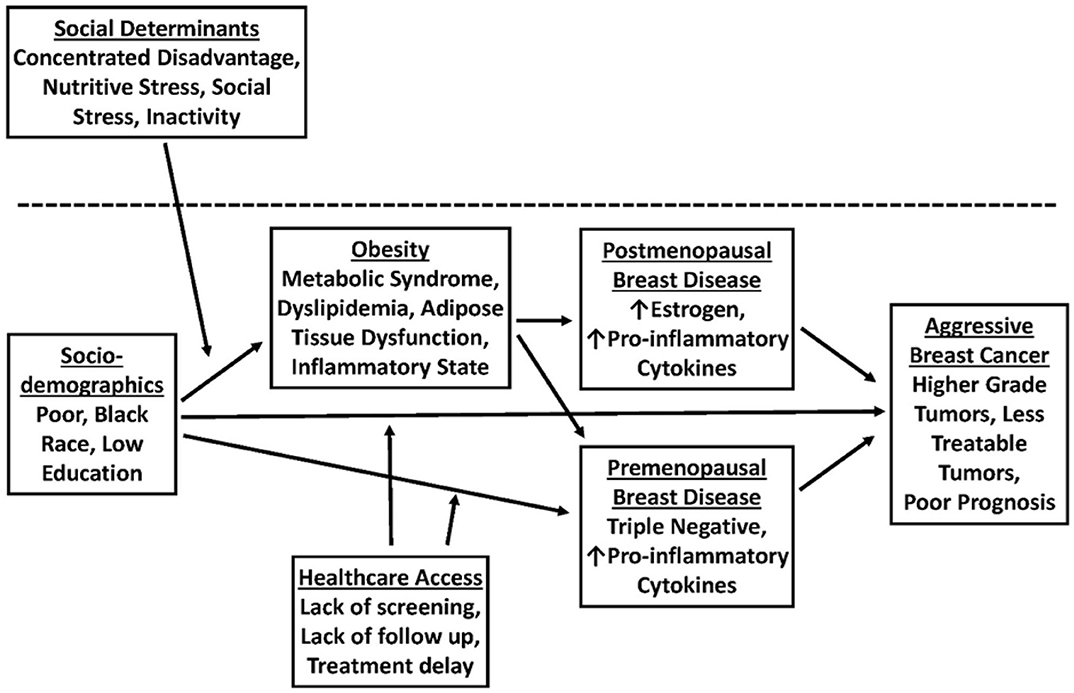
Figure 1. A model for the role of obesity in promoting breast cancer (BC) disparities in African American (AA) vs. Non-Hispanic White (NHW) women.
Conclusions and Future Direction
Significant disparities exist between AA and European-American women in the incidence and nature of breast cancer. AA women have twice the odds of being diagnosed with TNBCs than NHW women (Table 1). The reasons underlying the racial disparity in breast cancer outcome are multifactorial. Breast cancer risk factors have been divided into two major categories based on whether they are related to lifestyle (non-biologic) or related to factors unrelated to lifestyle (biologic). The poor prognosis of AA women with breast cancer has been attributed to both biologic and non-biologic factors. TNBC is more common among AA and western sub-Saharan African breast cancer (BC) patients compared with White/Caucasian Americans (WA). In a number of studies, striking similarities in disease epidemiology, risk factors, tumor biology, and genetics were observed between African and AA breast cancer patients, suggesting that West African ancestry is associated with inherited susceptibility for TNBC (24, 130). Further, altered expression levels of several genes associated with cellular growth and differentiation, invasion, and metastasis have been found in breast cancer tumors of AA women to a greater extent in NH White women and are considered to be important contributors to the disparities (14).
Central obesity measures, such as waist circumference (WC) or waist-to-hip ratio (WHR), are associated with an array of hormonal and metabolic changes and may be a better predictor of the risk of premenopausal breast cancer than overall adiposity. Waist circumference or WHR in premenopausal women has been found to be associated with higher levels of insulin-like growth factors or androgen levels, and thus central adiposity may be particularly relevant to premenopausal breast cancer risk (131, 132). Studies have shown that risk of TNBC tumors was reduced for women with a high BMI, but increased for those with central obesity, in particular, AA women (48). These and other findings support the notion that TNBC tumors may be more linked with the components of the metabolic syndrome (central obesity, insulin resistance, decreased tolerance to glucose, dyslipidemia, and hypertension) than by estrogens (133, 134).
A major drawback in performing studies that investigate the potential association between obesity and breast cancer is that the studies are simply driven by anthropomorphic measurements rather than by the metabolic health of the individual, although BMI and WHR likely play different roles in different breast cancer subtypes. The Edmonton Obesity Staging System (EOSS) is a five-stage system of obesity classification (stages 0–4) which is complemented by a clinical staging system that considers the metabolic, physical, and psychological parameters to provide meaningful framework for medical decision-making in optimal obesity treatment and pharmacologic interventions (135). A patient with obesity related risk factors (stage 1) but diagnosed with type 2 diabetes would be categorized as EOSS stage 2 (136). Patients with obesity-related end organ damage or severe disabilities from obesity-related chronic diseases would be classified in higher stages of EOSS (stages 3 and 4) (135). EOSS has been reported to be a better predictor of mortality than BMI or metabolic complications (137). Biological differences in body composition among NHW and AA women modulate risks resulting from obesity and obesity associated increased risk of breast cancer subtypes and comorbidities. EOSS may be useful for clinicians in the identification of breast cancer patients at higher mortality risk, and provide a framework to aid decision making to reduce mortality rates.
While biological differences contribute to breast cancer disparities, it is also generally recognized that social and behavioral factors play a major role in the racial differences observed in breast cancer mortality. Barriers to healthcare access because of low socio-economic status (SES) lead significantly to disparities in the outcome of breast cancer (115). Low SES is associated with higher risk of aggressive premenopausal breast cancer as well as late-stage diagnosis and poorer survival in AA women (13, 130, 138). Chu et al. explained the role of race/ethnicity in overall survival of TNBC patients using the data from a single hospital in Louisiana (139). After controlling for socioeconomic status (SES) and standard of care, they found that the overall survival of TNBC patients were not dependent on race/ethnicity. Chu et al., also reported that in indigent population, race or ethnicity had no impact on ER-negative breast cancer as well as other breast cancer outcomes (140, 141). These results support the idea that access to health care as a potential driver of unequal TNBC survival between AA and CA women. Our recent study on the TNBC cases from the Louisiana Tumor Registry suggested that neighborhood disadvantage (as measured by CDI, concentration disadvantage index) was associated with more advanced stages of TNBC at diagnosis and poorer stage-specific survival (142). Although TNBC incidence was higher in AA, the CDI did not fully explain the disparities, suggesting a role of biological differences.
Although many epidemiological studies rely on self-declared race, we do acknowledge the heterogeneity of individual genetic makeup (genetic admixture). Recent studies have described the limitations of using self-reported race and suggested to use ancestry informative markers to characterize individual's biological ancestry (143–145). Advancement of next generation sequencing should help us to perform genome wide association study to identify genetic factors responsible for health disparities among different racial and ethnic population. It is important to adjust for genetic race and ethnicity in the analyses of genetic susceptibility of diseases, however at present there is no single accepted standard method to characterize race and/or ethnicity (144, 146, 147).
Cancer outcomes are a function of a combination of factors including intrinsic biological factors, modifiable behavioral risk factors and decision-making, as well as characteristics of interactions between the medical system of patients and the health care system itself. Knowing how biological, social, and health-care variables work together to affect outcomes will benefit clinicians, researchers, and policy makers to pave the way to identify more innovative approaches to address breast cancer disparities and how do they combine to determine breast cancer risk.
Author Contributions
OP conceived the original idea and wrote the manuscript with inputs from FH, DD, AL, and RS. LM provided critical feedback and guidance in the preparation of the manuscript. All authors contributed to the final version of the manuscript and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Bolin Liu, M.D., for his critical reading and helpful comments on this manuscript. We are also thankful to the Department of Genetics, Louisiana State University, New Orleans, Louisiana, for providing financial support for the publication of this article.
References
1. Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. (2009) 20:628–35. doi: 10.1093/annonc/mdn675
2. Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, et al. How basal are triple-negative breast cancers? Int J Cancer. (2008) 123:236–40. doi: 10.1002/ijc.23518
3. Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. (2008) 14:1368–76. doi: 10.1158/1078-0432.CCR-07-1658
4. Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. (2009) 27:1160–7. doi: 10.1200/JCO.2008.18.1370
5. Bastien RR, Rodriguez-Lescure A, Ebbert MT, Prat A, Munarriz B, Rowe L, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. (2012) 5:44. doi: 10.1186/1755-8794-5-44
6. Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. (2014) 106:dju055. doi: 10.1093/jnci/dju055
7. Gershuni V, Li YR, Williams AD, So A, Steel L, Carrigan E, et al. Breast cancer subtype distribution is different in normal weight, overweight, and obese women. Breast Cancer Res Treat. (2017) 163:375–81. doi: 10.1007/s10549-017-4192-x
8. Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. (2017) 152:485–93. doi: 10.1001/jamasurg.2017.0005
9. Bandera EV, Maskarinec G, Romieu I, John EM. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv Nutr. (2015) 6:803–19. doi: 10.3945/an.115.009647
10. Vallega KA, Liu N, Myers JS, Yu K, Sang QX. Elevated resistin gene expression in African American estrogen and progesterone receptor negative breast cancer. PLoS ONE. (2016) 11:e0157741. doi: 10.1371/journal.pone.0157741
11. Kwan ML, Kushi LH, Weltzien E, Maring B, Kutner SE, Fulton RS, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. (2009) 11:R31. doi: 10.1186/bcr2261
12. Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. (2009) 113:357–70. doi: 10.1007/s10549-008-9926-3
13. Sineshaw HM, Gaudet M, Ward EM, Flanders WD, Desantis C, Lin CC, et al. Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National cancer data base. (2010-2011). Breast Cancer Res Treat. (2014) 145:753–63. doi: 10.1007/s10549-014-2976-9
14. Stewart PA, Luks J, Roycik MD, Sang QX, Zhang J. Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS ONE. (2013) 8:e82460. doi: 10.1371/journal.pone.0082460
15. Plasilova ML, Hayse B, Killelea BK, Horowitz NR, Chagpar AB, Lannin DR. Features of triple-negative breast cancer: analysis of 38,813 cases from the national cancer database. Medicine. (2016) 95:e4614. doi: 10.1097/MD.0000000000004614
16. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. (2001) 98:10869–74. doi: 10.1073/pnas.191367098
17. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor. (ER)-negative, progesterone receptor. (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. (2007) 109:1721–8. doi: 10.1002/cncr.22618
18. Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. (2009) 7:4–13. doi: 10.3121/cmr.2008.825
19. Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor. (ER), progesterone receptor. (PR), and the human epidermal growth factor receptor 2. (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. (2009) 15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x
20. Trivers KF, Lund MJ, Porter PL, Liff JM, Flagg EW, Coates RJ, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. (2009) 20:1071–82. doi: 10.1007/s10552-009-9331-1
21. Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women: Implications for breast cancer screening recommendations. Cancer. (2011) 117:2747–53. doi: 10.1002/cncr.25862
22. Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. (2014) 15:e625–34. doi: 10.1016/S1470-2045(14)70364-X
23. Sturtz LA, Melley J, Mamula K, Shriver CD, Ellsworth RE. Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer. (2014) 14:62. doi: 10.1186/1471-2407-14-62
24. Dietze EC, Sistrunk C, Miranda-Carboni G, O'Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. (2015) 15:248–54. doi: 10.1038/nrc3896
25. Dietze EC, Chavez TA, Seewaldt VL. Obesity and triple-negative breast cancer: disparities, controversies, and biology. Am J Pathol. (2018) 188:280–90. doi: 10.1016/j.ajpath.2017.09.018
26. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. (2006) 295:2492–502. doi: 10.1001/jama.295.21.2492
27. Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's surveillance, epidemiology, and end results database. Cancer. (2007) 110:876–84. doi: 10.1002/cncr.22836
28. Stead LA, Lash TL, Sobieraj JE, Chi DD, Westrup JL, Charlot M, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. (2009) 11:R18. doi: 10.1186/bcr2242
29. Stark A, Kleer CG, Martin I, Awuah B, Nsiah-Asare A, Takyi V, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer. (2010) 116:4926–32. doi: 10.1002/cncr.25276
30. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. (2009) 36:237–49. doi: 10.1053/j.seminoncol.2009.03.001
31. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. (2017) 67:7–30. doi: 10.3322/caac.21387
32. Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer. (2015) 22:R365–86. doi: 10.1530/ERC-15-0400
33. Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. (2015) 16:36–46. doi: 10.1016/S1470-2045(14)71123-4
34. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. (2012) 307:491–7. doi: 10.1001/jama.2012.39
35. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. (2014) 311:806–14. doi: 10.1001/jama.2014.732
36. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. (2016) 315:2284–91. doi: 10.1001/jama.2016.6458
37. Cecchini RS, Costantino JP, Cauley JA, Cronin WM, Wickerham DL, Land SR, et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res. (2012) 5:583–92. doi: 10.1158/1940-6207.CAPR-11-0482
38. Chen L, Cook LS, Tang MT, Porter PL, Hill DA, Wiggins CL, et al. Body mass index and risk of luminal, HER2-overexpressing, and triple negative breast cancer. Breast Cancer Res Treat. (2016) 157:545–54. doi: 10.1007/s10549-016-3825-9
39. Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. (2008) 109:123–39. doi: 10.1007/s10549-007-9632-6
40. Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. (2011) 130:587–97. doi: 10.1007/s10549-011-1616-x
41. Vona-Davis L, Rose DP, Hazard H, Howard-McNatt M, Adkins F, Partin J, et al. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev. (2008) 17:3319–24. doi: 10.1158/1055-9965.EPI-08-0544
42. Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. (2013) 137:307–14. doi: 10.1007/s10549-012-2339-3
43. Amadou A, Hainaut P, Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol. (2013) 2013:906495. doi: 10.1155/2013/906495
44. Guagnano MT, Manigrasso MR, Capani F, Davi G. [The “problem obesity”: viewpoint of the internist]. Ann Ital Chir. (2005) 76:407–11.
45. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. (2006) 444:881–7. doi: 10.1038/nature05488
46. Ness-Abramof R, Apovian CM. Waist circumference measurement in clinical practice. Nutr Clin Pract. (2008) 23:397–404. doi: 10.1177/0884533608321700
47. Harris HR, Willett WC, Terry KL, Michels KB. Body fat distribution and risk of premenopausal breast cancer in the Nurses' Health study II. J Natl Cancer Inst. (2011) 103:273–8. doi: 10.1093/jnci/djq500
48. Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER consortium. Breast Cancer Res Treat. (2015) 150:655–66. doi: 10.1007/s10549-015-3353-z
49. Chollet-Hinton L, Olshan AF, Nichols HB, Anders CK, Lund JL, Allott EH, et al. Biology and etiology of young-onset breast cancers among premenopausal African American women: results from the AMBER consortium. Cancer Epidemiol Biomarkers Prev. (2017) 26:1722–9. doi: 10.1158/1055-9965.EPI-17-0450
50. Rivas MA, Carnevale RP, Proietti CJ, Rosemblit C, Beguelin W, Salatino M, et al. TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp Cell Res. (2008) 314:509–29. doi: 10.1016/j.yexcr.2007.10.005
51. Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. (2003) 88:1721–6. doi: 10.1038/sj.bjc.6600956
52. Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. (2003) 103:642–6. doi: 10.1002/ijc.10833
53. Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. (1999) 19:1427–32.
54. Ahmed OI, Adel AM, Diab DR, Gobran NS. Prognostic value of serum level of interleukin-6 and interleukin-8 in metastatic breast cancer patients. Egypt J Immunol. (2006) 13:61–8.
55. Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. (2006) 207:12–22. doi: 10.1002/jcp.20472
56. Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin–a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. (2002) 94:1704–11. doi: 10.1093/jnci/94.22.1704
57. Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J Cell Biochem. (2008) 105:956–64. doi: 10.1002/jcb.21911
58. Grossmann ME, Ray A, Dogan S, Mizuno NK, Cleary MP. Balance of adiponectin and leptin modulates breast cancer cell growth. Cell Res. (2008) 18:1154–6. doi: 10.1038/cr.2008.293
59. Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. (2004) 89:1102–7. doi: 10.1210/jc.2003-031804
60. Sultana R, Kataki AC, Borthakur BB, Basumatary TK, Bose S. Imbalance in leptin-adiponectin levels and leptin receptor expression as chief contributors to triple negative breast cancer progression in Northeast India. Gene. (2017) 621:51–8. doi: 10.1016/j.gene.2017.04.021
61. Sun H, Zou J, Chen L, Zu X, Wen G, Zhong J. Triple-negative breast cancer and its association with obesity. Mol Clin Oncol. (2017) 7:935–42. doi: 10.3892/mco.2017.1429
62. Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve. (2001) 24:654–61. doi: 10.1002/mus.1051
63. Ceaser T, Hunter G. Black and White race differences in aerobic capacity, muscle fiber type, and their influence on metabolic processes. Sports Med. (2015) 45:615–23. doi: 10.1007/s40279-015-0318-7
64. Ama PF, Simoneau JA, Boulay MR, Serresse O, Theriault G, Bouchard C. Skeletal muscle characteristics in sedentary black and Caucasian males. J Appl Physiol. (1986) 61:1758–61. doi: 10.1152/jappl.1986.61.5.1758
65. Ingelsson E, Arnlov J, Zethelius B, Vasan RS, Flyvbjerg A, Frystyk J, et al. Associations of serum adiponectin with skeletal muscle morphology and insulin sensitivity. J Clin Endocrinol Metab. (2009) 94:953–7. doi: 10.1210/jc.2008-1772
66. Kern M, Wells JA, Stephens JM, Elton CW, Friedman JE, Tapscott EB, et al. Insulin responsiveness in skeletal muscle is determined by glucose transporter. (Glut4) protein level. Biochem J. (1990) 270:397–400. doi: 10.1042/bj2700397
67. Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. (2002) 282:E1191–6. doi: 10.1152/ajpendo.00416.2001
68. Nielson J, Christensen DL. Glucose intolerance in the West African Diaspora: a skeletal muscle fibre type distribution hypothesis. Acta Physiol (Oxf). (2011) 202:605–16. doi: 10.1111/j.1748-1716.2011.02272.x
69. Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, et al. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care. (2006) 29:895–900. doi: 10.2337/diacare.29.04.06.dc05-1854
70. Stuart CA, McCurry MP, Marino A, South MA, Howell ME, Layne AS, et al. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab. (2013) 98:2027–36. doi: 10.1210/jc.2012-3876
71. Ros Perez M, Medina-Gomez G. [Obesity, adipogenesis and insulin resistance]. Endocrinol Nutr. (2011) 58:360–9. doi: 10.1016/j.endoen.2011.05.004
72. Railo MJ, von Smitten K, Pekonen F. The prognostic value of insulin-like growth factor-I in breast cancer patients. results of a follow-up study on 126 patients. Eur J Cancer. (1994) 30A:307–11. doi: 10.1016/0959-8049(94)90247-X
73. Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, Gumbs AA, et al. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. (1997) 57:3079–83.
74. Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. (2002) 20:42–51. doi: 10.1200/JCO.2002.20.1.42
75. Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol. (2011) 29:54–60. doi: 10.1200/JCO.2010.29.3183
76. Nolop KB, Rhodes CG, Brudin LH, Beaney RP, Krausz T, Jones T, et al. Glucose utilization in vivo by human pulmonary neoplasms. Cancer. (1987) 60:2682–9. doi: 10.1002/1097-0142(19871201)60:11<2682::AIDCNCR2820601118>3.0.CO;2-H
77. Warburg O. On the origin of cancer cells. Science. (1956) 123:309–14. doi: 10.1126/science.123.3191.309
78. Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. (1989) 129:1120–31. doi: 10.1093/oxfordjournals.aje.a115234
79. Schapira DV, Kumar NB, Lyman GH. Obesity, body fat distribution, and sex hormones in breast cancer patients. Cancer. (1991) 67:2215–8. doi: 10.1002/1097-0142(19910415)67:8<2215::AIDCNCR2820670836>3.0.CO;2-Q
80. Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, et al. Effect of obesity on total and free insulin-like growth factor. (IGF)-1, and their relationship to IGF-binding protein. (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. (1997) 21:355–9. doi: 10.1038/sj.ijo.0800412
81. Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. (2015) 33:2254–61. doi: 10.1200/JCO.2014.57.1349
82. Keenan T, Moy B, Mroz EA, Ross K, Niemierko A, Rocco JW, et al. Comparison of the genomic landscape between primary breast cancer in african american versus white women and the association of racial differences with tumor recurrence. J Clin Oncol. (2015) 33:3621–7. doi: 10.1200/JCO.2015.62.2126
83. Ademuyiwa FO, Tao Y, Luo J, Weilbaecher K, Ma CX. Differences in the mutational landscape of triple-negative breast cancer in African Americans and Caucasians. Breast Cancer Res Treat. (2017) 161:491–9. doi: 10.1007/s10549-016-4062-y
84. Sullivan HC, Oprea-Ilies G, Adams AL, Page AJ, Kim S, Wang J, et al. Triple-negative breast carcinoma in African American and Caucasian women: clinicopathology, immunomarkers, and outcome. Appl Immunohistochem Mol Morphol. (2014) 22:17–23. doi: 10.1097/PAI.0b013e318281148e
85. Deshmukh SK, Srivastava SK, Bhardwaj A, Singh AP, Tyagi N, Marimuthu S, et al. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. (2015) 6:11231–41. doi: 10.18632/oncotarget.3591
86. Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Med Oncol. (2013) 30:419. doi: 10.1007/s12032-012-0419-1
87. Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California cancer registry, 2000-2010. BMC Cancer. (2013) 13:449. doi: 10.1186/1471-2407-13-449
88. Sheppard VB, Isaacs C, Luta G, Willey SC, Boisvert M, Harper FW, et al. Narrowing racial gaps in breast cancer chemotherapy initiation: the role of the patient-provider relationship. Breast Cancer Res Treat. (2013) 139:207–16. doi: 10.1007/s10549-013-2520-3
89. Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. (2013) 148:516–23. doi: 10.1001/jamasurg.2013.1680
90. Tannenbaum SL, Koru-Sengul T, Miao F, Byrne MM. Disparities in survival after female breast cancer diagnosis: a population-based study. Cancer Causes Control. (2013) 24:1705–15. doi: 10.1007/s10552-013-0246-5
91. Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. (2013) 310:389–97. doi: 10.1001/jama.2013.8272
92. Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. (2015) 313:165–73. doi: 10.1001/jama.2014.17322
93. Gleason MX, Mdzinarishvili T, Sherman S. Breast cancer incidence in black and white women stratified by estrogen and progesterone receptor statuses. PLoS ONE. (2012) 7:e49359. doi: 10.1371/journal.pone.0049359
94. Perez CA, Zumsteg ZS, Gupta G, Morrow M, Arnold B, Patil SM, et al. Black race as a prognostic factor in triple-negative breast cancer patients treated with breast-conserving therapy: a large, single-institution retrospective analysis. Breast Cancer Res Treat. (2013) 139:497–506. doi: 10.1007/s10549-013-2550-x
95. Olivier M, Langerod A, Carrieri P, Bergh J, Klaar S, Eyfjord J, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. (2006) 12:1157–67. doi: 10.1158/1078-0432.CCR-05-1029
96. Chen C, Liu Y, Rappaport AR, Kitzing T, Schultz N, Zhao Z, et al. MLL3 is a haploinsufficient 7q tumor suppressor in acute myeloid leukemia. Cancer Cell. (2014) 25:652–65. doi: 10.1016/j.ccr.2014.03.016
97. Weirich S, Kudithipudi S, Kycia I, Jeltsch A. Somatic cancer mutations in the MLL3-SET domain alter the catalytic properties of the enzyme. Clin Epigenetics. (2015) 7:36. doi: 10.1186/s13148-015-0075-3
98. Wang XX, Fu L, Li X, Wu X, Zhu Z, Dong JT. Somatic mutations of the mixed-lineage leukemia 3. (MLL3) gene in primary breast cancers. Pathol Oncol Res. (2011) 17:429–33. doi: 10.1007/s12253-010-9316-0
99. Mukohara T. PI3K mutations in breast cancer: prognostic and therapeutic implications. Breast Cancer.(2015) 7:111–23. doi: 10.2147/BCTT.S60696
100. Martin DN, Boersma BJ, Yi M, Reimers M, Howe TM, Yfantis HG, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS ONE. (2009) 4:e4531. doi: 10.1371/journal.pone.0004531
101. Field LA, Love B, Deyarmin B, Hooke JA, Shriver CD, Ellsworth RE. Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer. (2012) 118:1334–44. doi: 10.1002/cncr.26405
102. Grunda JM, Steg AD, He Q, Steciuk MR, Byan-Parker S, Johnson MR, et al. Differential expression of breast cancer-associated genes between stage- and age-matched tumor specimens from African- and Caucasian-American Women diagnosed with breast cancer. BMC Res Notes. (2012) 5:248. doi: 10.1186/1756-0500-5-248
103. Kroenke CH, Sweeney C, Kwan ML, Quesenberry CP, Weltzien EK, Habel LA, et al. Race and breast cancer survival by intrinsic subtype based on PAM50 gene expression. Breast Cancer Res Treat. (2014) 144:689–99. doi: 10.1007/s10549-014-2899-5
104. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. (2001) 409:307–12. doi: 10.1038/35053000
105. Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. (2003) 300:472–6. doi: 10.1016/S0006-291X(02)02841-3
106. Sun CA, Wu MH, Chu CH, Chou YC, Hsu GC, Yang T, et al. Adipocytokine resistin and breast cancer risk. Breast Cancer Res Treat. (2010) 123:869–76. doi: 10.1007/s10549-010-0792-4
107. Lee YC, Chen YJ, Wu CC, Lo S, Hou MF, Yuan SS. Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol Oncol. (2012) 125:742–50. doi: 10.1016/j.ygyno.2012.02.032
108. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
109. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. (2004) 4:579–91. doi: 10.1038/nrc1408
110. Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. (2008) 117:1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714
111. Gordon NH, Crowe JP, Brumberg DJ, Berger NA. Socioeconomic factors and race in breast cancer recurrence and survival. Am J Epidemiol. (1992) 135:609–18. doi: 10.1093/oxfordjournals.aje.a116340
112. Richardson JL, Langholz B, Bernstein L, Burciaga C, Danley K, Ross RK. Stage and delay in breast cancer diagnosis by race, socioeconomic status, age and year. Br J Cancer. (1992) 65:922–6. doi: 10.1038/bjc.1992.193
113. Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. (1998) 279:1801–7. doi: 10.1001/jama.279.22.1801
114. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. (2002) 94:490–6. doi: 10.1093/jnci/94.7.490
115. Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA. Breast cancer mortality in african-american and non-hispanic white women by molecular subtype and stage at diagnosis: a population-based study. Cancer Epidemiol Biomarkers Prev. (2015) 24:1039–45. doi: 10.1158/1055-9965.EPI-15-0243
116. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. (2002) 94:334–57. doi: 10.1093/jnci/94.5.334
117. Du Xianglin L, Gor BJ. Racial disparities and trends in radiation therapy after breast-conserving surgery for early-stage breast cancer in women, 1992 to 2002. Ethn Dis. (2007) 17:122–8.
118. Moore KA. Breast cancer patients' out-of-pocket expenses. Cancer Nurs. (1999) 22:389–96. doi: 10.1097/00002820-199910000-00007
119. Chirikos TN, Russell-Jacobs A, Jacobsen PB. Functional impairment and the economic consequences of female breast cancer. Women Health. (2002) 36:1–20. doi: 10.1300/J013v36n01_01
120. Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. (2000) 92:269–71. doi: 10.1093/jnci/92.3.269
121. Lamont EB, Hayreh D, Pickett KE, Dignam JJ, List MA, Stenson KM, et al. Is patient travel distance associated with survival on phase II clinical trials in oncology? J Natl Cancer Inst. (2003) 95:1370–5. doi: 10.1093/jnci/djg035
122. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. (2006) 24:1342–9. doi: 10.1200/JCO.2005.03.3472
123. Gerend MA, Pai M. Social determinants of black-white disparities in breast cancer mortality: a review. Cancer Epidemiol Biomarkers Prev. (2008) 17:2913–23. doi: 10.1158/1055-9965.EPI-07-0633
124. Keita AD, Judd SE, Howard VJ, Carson AP, Ard JD, Fernandez JR. Associations of neighborhood area level deprivation with the metabolic syndrome and inflammation among middle- and older- age adults. BMC Public Health. (2014) 14:1319. doi: 10.1186/1471-2458-14-1319
125. Araujo MA, Silveira CB, Melo SP. [Experiences of pregnant and post-partum women with HIV]. Rev Bras Enferm. (2008) 61:589–94. doi: 10.1590/S0034-71672008000500010
126. Llanos AA, Chandwani S, Bandera EV, Hirshfield KM, Lin Y, Ambrosone CB, et al. Associations between sociodemographic and clinicopathological factors and breast cancer subtypes in a population-based study. Cancer Causes Control. (2015) 26:1737–50. doi: 10.1007/s10552-015-0667-4
127. Fregene A, Newman LA. Breast cancer in sub-Saharan Africa: how does it relate to breast cancer in African-American women? Cancer. (2005) 103:1540–50. doi: 10.1002/cncr.20978
128. Woodward WA, Huang EH, McNeese MD, Perkins GH, Tucker SL, Strom EA, et al. African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer. (2006) 107:2662–8. doi: 10.1002/cncr.22281
129. Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. (2013) 73:3470–80. doi: 10.1158/0008-5472.CAN-12-4524-T
130. Williams DR, Mohammed SA, Shields AE. Understanding and effectively addressing breast cancer in African American women: Unpacking the social context. Cancer. (2016) 122:2138–49. doi: 10.1002/cncr.29935
131. Connolly BS, Barnett C, Vogt KN, Li T, Stone J, Boyd NF. A meta-analysis of published literature on waist-to-hip ratio and risk of breast cancer. Nutr Cancer. (2002) 44:127–38. doi: 10.1207/S15327914NC4402_02
132. Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev. (2003) 4:157–73. doi: 10.1046/j.1467-789X.2003.00108.x
133. Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. (2010) 121:479–83. doi: 10.1007/s10549-009-0591-y
134. Hauner D, Hauner H. Metabolic syndrome and breast cancer: is there a link? Breast Care. (2014) 9:277–81. doi: 10.1159/000365951
135. Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes. (2009) 33:289–95. doi: 10.1038/ijo.2009.2
136. Canning KL, Brown RE, Wharton S, Sharma AM, Kuk JL. Edmonton obesity staging system prevalence and association with weight loss in a publicly funded referral-based obesity clinic. J Obes. (2015) 2015:619734. doi: 10.1155/2015/619734
137. Kuk JL, Ardern CI, Church TS, Sharma AM, Padwal R, Sui X, et al. Edmonton obesity staging system: association with weight history and mortality risk. Appl Physiol Nutr Metab. (2011) 36:570–6. doi: 10.1139/h11-058
138. Dunn BK, Agurs-Collins T, Browne D, Lubet R, Johnson KA. Health disparities in breast cancer: biology meets socioeconomic status. Breast Cancer Res Treat. (2010) 121:281–92. doi: 10.1007/s10549-010-0827-x
139. Chu QD, Henderson AE, Ampil F, Li BD. Outcome for patients with triple-negative breast cancer is not dependent on race/ethnicity. Int J Breast Cancer. (2012) 2012:764570. doi: 10.1155/2012/764570
140. Chu QD, Smith MH, Williams M, Panu L, Johnson LW, Shi R, et al. Race/ethnicity has no effect on outcome for breast cancer patients treated at an academic center with a public hospital. Cancer Epidemiol Biomarkers Prev. (2009) 18:2157–61. doi: 10.1158/1055-9965.EPI-09-0232
141. Chu QD, Burton G, Glass J, Smith MH, Li BD. Impact of race and ethnicity on outcomes for estrogen receptor-negative breast cancers: experience of an academic center with a charity hospital. J Am Coll Surg. (2010) 210:585–92. doi: 10.1016/j.jamcollsurg.2010.01.025
142. Hossain F, Danos D, Prakash O, Gilliland A, Ferguson TF, Simonsen N, et al. Neighborhood social determinants of triple negative breast cancer. Front Public Health. (2019) 7:18. doi: 10.3389/fpubh.2019.00018
143. Baye TM, Wilke RA. Mapping genes that predict treatment outcome in admixed populations. Pharmacogenomics J. (2010) 10:465–77. doi: 10.1038/tpj.2010.71
144. Sucheston LE, Bensen JT, Xu Z, Singh PK, Preus L, Mohler JL, et al. Genetic ancestry, self-reported race and ethnicity in African Americans and European Americans in the PCaP cohort. PLoS ONE. (2012) 7:e30950. doi: 10.1371/journal.pone.0030950
145. Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics. (2015) 9:1. doi: 10.1186/s40246-014-0023-x
146. Barnholtz-Sloan JS, Chakraborty R, Sellers TA, Schwartz AG. Examining population stratification via individual ancestry estimates versus self-reported race. Cancer Epidemiol Biomarkers Prev. (2005) 14:1545–51. doi: 10.1158/1055-9965.EPI-04-0832
Keywords: triple negative breast cancer, racial disparities, African-American women, non-hispanic whites, socioeconomic status, obesity, body mass index, waist-hip ratio
Citation: Prakash O, Hossain F, Danos D, Lassak A, Scribner R and Miele L (2020) Racial Disparities in Triple Negative Breast Cancer: A Review of the Role of Biologic and Non-biologic Factors. Front. Public Health 8:576964. doi: 10.3389/fpubh.2020.576964
Received: 27 June 2020; Accepted: 20 October 2020;
Published: 22 December 2020.
Edited by:
Aldo Rosano, National Institute for the Analysis of Public Policy, ItalyReviewed by:
Victoria Seewaldt, Beckman Research Institute, City of Hope, United StatesIvana Kulhánová, Charles University, Czechia
Copyright © 2020 Prakash, Hossain, Danos, Lassak, Scribner and Miele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Om Prakash, b3ByYWthQGxzdWhzYy5lZHU=
 Om Prakash
Om Prakash Fokhrul Hossain
Fokhrul Hossain Denise Danos
Denise Danos Adam Lassak
Adam Lassak Richard Scribner2
Richard Scribner2 Lucio Miele
Lucio Miele