- 1Public Health Department, Henrietta Schmoll School of Health, Saint Catherine University, Saint Paul, MN, United States
- 2Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, United States
- 3Minneapolis VA Health Care System, Minneapolis, MN, United States
- 4Division of Infectious Disease and International Medicine, School of Medicine, University of Minnesota, Minneapolis, MN, United States
Background: Many antimicrobial-resistant infections are community-acquired, yet community carriage of microorganisms by healthy individuals is poorly characterized. We assessed microorganism carriage on the hands of Minnesota State Fair attendees and explored associated factors.
Methods: Minnesota State Fair attendees (in 2014) from households with ≥2 members (≥1 member being <19 years old [a child]) were eligible to participate. Participants provided biological samples via a hand plating technique and completed a questionnaire on factors potentially related to microorganism carriage. Using presumptive taxonomic identifications and disk-diffusion-determined resistance phenotypes, hand-culture isolates were classified by microbial type; types were grouped into four broad categories based on inferred pathogenicity and consistency with the skin microbiota. Descriptive statistics, X2 tests, and generalized linear mixed-effects models were used to explore associations between survey and culture data.
Results: We enrolled 206 participants from 82 households during 2 days; 50% of subjects were children. Overall, 99.5% (205/206) of hand samples yielded microorganisms. Most were non-pathogenic, whether skin microbiota (98.5% of participants) or non-skin microbiota (93.2% of participants). Only 2.4% (5/206) of samples yielded antibiotic-resistant bacteria. Children were more likely than adults to carry potentially pathogenic (OR = 3.63, 95% CI: 1.66–7.93) and presumably non-pathogenic (OR = 6.61, 95% CI: 1.67–26.15) non-skin microorganisms.
Conclusions: Large community gatherings can serve as efficient sites for estimating the prevalence of microorganism carriage. A small proportion of participants carried antimicrobial-resistant pathogens on their hands; most carried non-pathogenic microorganisms, and no exposures specific to the state fair were associated with microorganism carriage.
Introduction
In 1846, Ignaz Semmelweis hypothesized that infectious particles could be transferred from person to person via a clinician's hands (1). Hands have subsequently been implicated in innumerable infections, with transmission occurring both directly (person-to-person) and via contaminated surfaces (2–5). Today, hospital infection preventionists prioritize proper hand hygiene performance by healthcare workers (6, 7). Studies of pathogen spread within hospitals have documented every location a clinician's hand touches in a day and assessed whether “fist bump” greetings would be less transmission-prone than traditional handshakes (5, 8). Yet, each year in the United States an estimated 1.7 million hospital-acquired infections occur, resulting in 99,000 deaths—many of which are attributable to inadequate hand hygiene (9). These infections account for over $9.8 billion in healthcare expenses (10).
Most infections, however, are community-acquired (11–14). Several studies that sampled the built environment have detected pathogens—especially antimicrobial-resistant bacteria—in community locations, including computer labs, public transit systems, and on gym equipment (15–18). Although this identifies possible indirect transmission routes for community-acquired infections it does not address human carriage. Identifying which microorganisms are carried by individuals in the community is important to understand the circulation and potential transmission of pathogens in a community setting.
The annual Minnesota State Fair, which hosts approximately 2 million attendees of all ages over 12 days each August (19). Despite the Fair's location in the Minneapolis-St. Paul metropolitan area, it attracts urban and rural Minnesotans alike (20). In this study we attempted to assess the distribution of microorganisms, including potential pathogens and antimicrobial-resistant organisms, on the hands of State Fair attendees, who represent a subset of Minnesota residents. We also estimated associations between specific human behaviors and microorganisms, to identify possible ways to reduce pathogen carriage. Among the behaviors assessed were the frequency and methods of handwashing, known to reduce infection with many forms of pathogens (21, 22). Additionally, we asked participants about sites they visited at the fair, particularly animal buildings and whether they touched the animals, as it is well-established that contact with animals contribute to pathogen transmission (23). We also sought to understand potential exposures from participants' behaviors outside of the Fair that could expose them to pathogens including gardening (24), gyms and team sports (25–27), public transportation (28), and employment as a daycare (29) or healthcare provider (30–32) or with livestock (33).
Methods
We enrolled participants in this cross-sectional survey at the Minnesota State Fair. The primary outcome was the presence of specific microorganisms on participants' hands, as assessed by contact plating. We collected data on participants' demographic characteristics, handwashing behaviors in and outside of the home, and activities while at the fair. The consent procedures in this study were approved by the University of Minnesota Institutional Review Board (No. 1406M51101).
Participant Selection
Participants were enrolled on August 31 and September 1, 2014 in the University of Minnesota “Driven to Discover” research building at the Minnesota State Fair. The study was publicized prior to the fair on a University of Minnesota website, which listed the study objectives, eligibility criteria, and location and times of data collection.
At the fair, study staff greeted potential participants as they entered the research building. For individuals who indicated an interest in participating, study staff screened for eligibility and elicited oral informed consent or assent, as appropriate. Eligibility criteria included: having ≥2 family members at the fair who were willing to participate, ≥1 of whom was <19 years old; family members living together for ≥30 days prior to enrollment; and age ≥3 and ≤ 70 years. Participation was incentivized via a drawing for one of six prize packages, to be mailed to the winners after the fair's conclusion.
Specimen Collection and Processing
For hand sampling, participants placed their dominant hand directly on a tryptic soy agar plate containing 5% sheep blood (Becton Dickinson, Franklin Lakes, New Jersey) according to a standardized technique [S1]. Study staff used a sterile plastic rod to distribute the sample across the agar plate. Plates were refrigerated overnight at 2°C and transported to the laboratory the following morning.
In the laboratory, plates were incubated overnight at 37°C. Any colonies were assessed for morphology (color, shape, texture, and hemolysis), and one colony each for up to three predominant morphotypes was selected for gram-staining. Gram-positive cocci were assessed for coagulase and catalase production. For catalase-positive, coagulase-positive organisms (presumptive Staphylococcus aureus), CHROMagar plates (Becton Dickinson, Franklin Lakes, New Jersey) were used to differentiate methicillin-susceptible from methicillin-resistant S. aureus (MSSA and MRSA). Gram-negative bacilli (excluding coccobaccilli) were examined for lactose fermentation and oxidase production. Pseudomonas aeruginosa were identified with colonial morphology on T7 agar followed by an oxidase positive result. Identification of Lactobacillus after gram-staining was the presence of alpha hemolysis and a catalase negative result. Species identification was attempted for other gram-negative fermenting bacilli including Stenotrophomas and other Pseudonomas sp. using the API-20E system (34–36) (bioMerieux, Inc., Hazelwood, MO), and antibiotic susceptibility was determined using a standardized disk diffusion method (37), and breakpoints from the Clinical and Laboratory Standards Institute (CLSI).
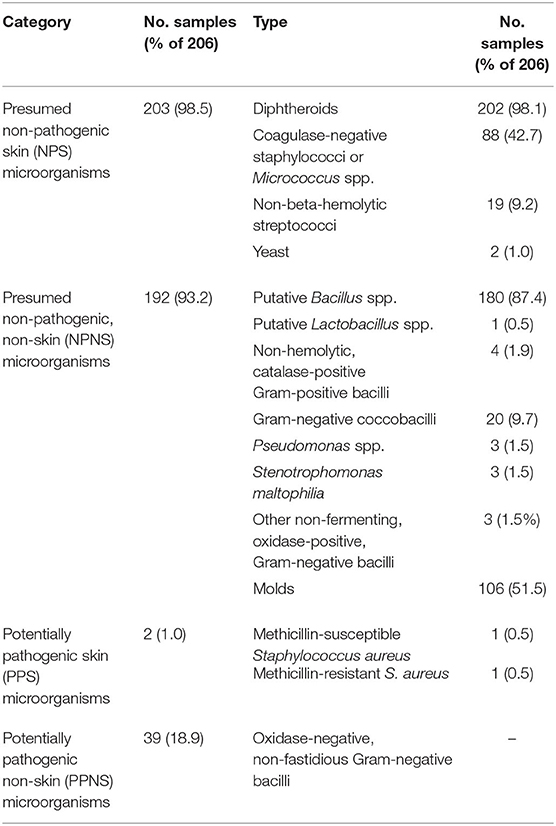
The resulting identifications were used to classify the isolates initially into 15 different microbial types based on presumptive identity and relevant resistance phenotypes (Table 1). These 15 types were further collapsed, arbitrarily, into four larger, quasi-homogenous categories for ease of presentation and quantitative analysis. Categories were based on a combination of the organism's (i) likelihood of occurring in the skin microbiota and (ii) potential pathogenicity in healthy hosts, vs. only in immunocompromised hosts. Presumed non-pathogenic skin (NPS) microorganisms included diphtheroids, coagulase-negative staphylococci, non-beta-hemolytic streptococci, Micrococcus spp., and yeast. Presumed non-pathogenic, non-skin (NPNS) microorganisms included putative Bacillus spp., putative Lactobacillus spp., non-hemolytic catalase-positive Gram-positive bacilli, Gram-negative coccobacilli, Pseudomonas spp., Stenotrophomonas maltophilia, other non-fermenting, oxidase-positive, Gram-negative bacilli, and molds. Potentially pathogenic skin (PPS) microorganisms included beta-hemolytic streptococci, MSSA, and MRSA. Potentially pathogenic non-skin (PPNS) microorganisms included oxidase-negative, non-fastidious Gram-negative bacilli.
Questionnaire
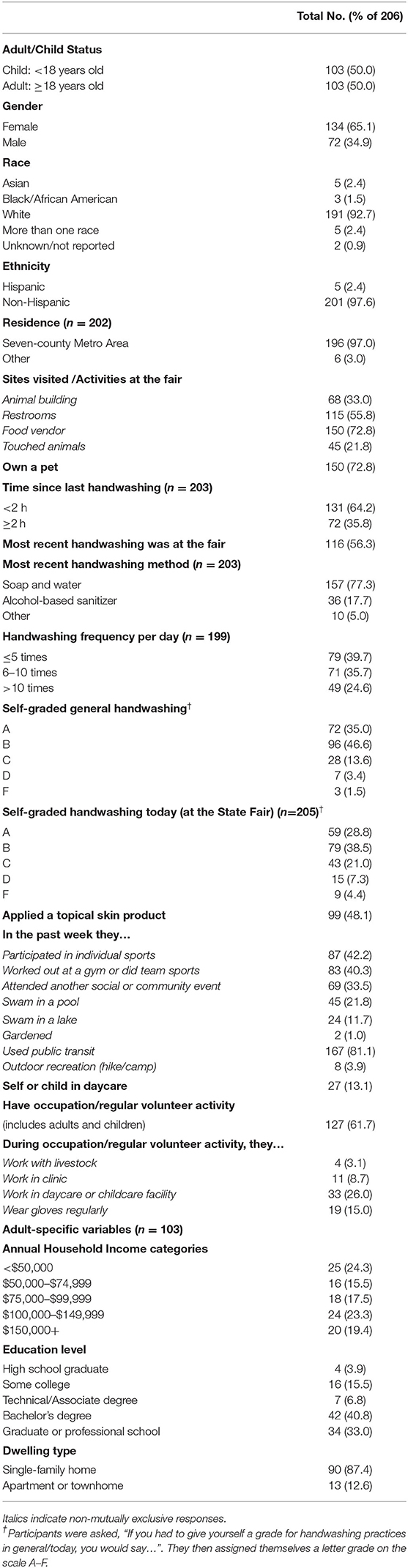
Study staff interviewed participants using REDCap electronic data capture tools (38). The questionnaire captured (i) demographic variables: self-identified gender, race, ethnicity, age (categorized as <19 years [child] vs. ≥19 years [adult]), educational level, family household income, residence in the seven-county metro area (as determined by ZIP code), and housing type; (ii) possible exposures and activities at home or at the State Fair that might influence hand carriage of specific microorganisms; and (iii) hygiene and health-related variables [S2]. Assessed home exposures included animal ownership, recreational activities, events attended in the past week, and regular employment type and volunteer activities. Assessed State Fair exposures included sites the participant had visited since arrival at the fair, which such site immediately preceded study enrollment, and whether the participant had touched any animals while at the fair. The health and hygiene-related questions addressed hand-washing behaviors (when, where, and how hand hygiene was performed most recently), and topical skin product use (Table 2).
Statistical Analyses
The prevalence of the 15 microbial types and corresponding four microorganism categories was used to describe the overall diversity of microorganisms present. We used cross-tabular analyses of X2 and Fisher's exact tests to identify statistical associations between these taxa and questionnaire responses. Given the exploratory nature of the study, tests were not adjusted for multiple comparisons. We excluded from type-specific analyses any microbial types identified in <3 samples (n = 4), leaving 10 type-specific analyses (Table 1). We also evaluated associations between each of the four microorganism categories in Table 1 and conducted an additional analysis of questionnaire responses vs. antibiotic-resistant organisms (regardless of type or category).
For the four microorganism categories and the combined antibiotic-resistant organisms, we further evaluated associations with questionnaire responses using univariable generalized linear mixed-effects models, with a binomial distribution and a logit link (lme4 package in R, version 3.4.0). A household-level random intercept was included to account for correlation among family members. For microorganisms associated with at least one questionnaire variable, we further analyzed thee associations in multivariable mixed-effects models. Multivariable models were constructed for each microorganism category using the statistically significant variables from the univariable models, and then examined in the presence of the each of the remaining questionnaire variables. For variables associated with a particular microbial category, odds ratios (OR) and 95% confidence intervals (CI) were calculated from regression models using standard methods (39).
Results
Participant Characteristics
During the 2-day enrollment period we enrolled 206 State Fair attendees (103 adults, 103 children), from 82 households; each participant contributed a hand culture. Of the participants, 65.1% (n = 134) were female, 92.7% (n = 191) were white, and 97.1% (n = 201) were non-Hispanic. Most lived in the seven-county metro area (97.0%, n = 196) and were pet owners (72.8%, n = 150). Of the participants, 81.1% (n = 167) used public transportation within the past week, 60.3% (n = 120), usually washed their hands ≥6 times per day, and 48.1% (n = 99) had applied a topical skin product (e.g., makeup, sunscreen, insect repellent) the day of study participation. All adults (100.0%, n =103) and some children (23.3%, n = 24) were employed or had regular volunteer activities. Most adults had a bachelor's degree or higher (73.8%, n = 76), an annual household income >$50,000 (75.7%, n = 78), and owned a single-family home (87.4%, n = 90). Participants were less likely to assign themselves an “A” or “B” grade (good) on handwashing at the State Fair (67.3%) than with their usual practice (81.6%) (Table 2).
Participants' activities at the State Fair included, eating from food vendors (78.2%; n = 150), using public restrooms (55.8%; n = 115), visiting animal buildings (33.0%; n = 68), and touching animals (21.8%; n = 45). Among all participants, the most recent hand washing involved soap and water (77.3%; n = 157) and had occurred at the fair (in a restroom or at a handwashing station; 56.3%; n = 116). Common behaviors outside of the fair in the past week included use of public transit (81.1%, n = 167), participation in individual sports (42.2%, n = 87), gym use or team sports (40.3%, n = 83) and attendance at other social or community events (33.5%, n = 69).
Microbiological Samples
Of the 206 hand samples, 205 (99.5%) were culture-positive. The most commonly identified microbial type was diphtheroids (98.1%, n = 202), followed by putative Bacillus spp. (87.4%, n = 180) and molds (51.5%, n = 106) (Table 1). Presumed non-pathogens, whether or not typically skin-associated (i.e., NPS and NPNS microorganisms), occurred in 98.5 and 93.2% of samples, respectively. By contrast, potential pathogens, whether or not typically skin-associated (i.e., PPS and PPNS microorganisms), occurred in only 1.0 and 18.9% of samples, respectively. Antibiotic-resistant bacteria, detected in 2.4% (n = 5) samples, included sulfamethoxazole-resistant Pseudomonas putida (n = 1), cephalosporin-resistant Pseudomonas putida (n = 2) and Stenotrophomonas maltophilia (n = 1), and methicillin-resistant S. aureus (n = 1).
Host Factors Associated With Presence of Microorganisms
Ten of the 15 individual microorganism types had sufficient prevalence for statistical analysis, of which, three were associated with demographic/behavioral factors. Non-beta-hemolytic streptococci were less likely among participants who took public transit within the past week (n = 167) (6.6 vs. 20.5%, p = 0.01) or with team sport participation or a gym workout in the past week (n = 84) (4.1 vs. 16.9%, p < 0.01). Putative Bacillus spp. carriage was more likely among participants who had a pet (n = 150) (75.6 vs. 53.8%, p = 0.04) or swam in a pool in the past week (n = 45) (97.8 vs. 84.5%, p = 0.03). Mold carriage was more common among children (n = 103) than adults (n = 103) (66.0 vs. 34.9%, p < 0.01).
Regarding the four broader microbial categories, NPS and PPS microorganisms were unassociated with demographic/behavioral factors. NPNS microorganisms were more common among children than adults (98.1 vs. 88.3%, p = 0.01) and among participants who swam in a pool within the past week (100.0 vs. 91.3%, p = 0.04). PPNS microorganism were more common among males (n = 72) than females (n = 134) (27.8 vs. 14.2%, p = 0.02) and were less likely among children than adults (9.7 vs. 28.2%, p < 0.01). Additionally, antibiotic-resistant microorganisms (irrespective of category membership) were more common among adults with a high school degree or some college (n = 20) than those with at least an associate or technical degree (n = 83) (15.0 vs. 0.0%, p < 0.01).
Generalized Linear Mixed-Effects Models
We used univariable and multivariable generalized linear mixed-effects models to assess associations between demographic/behavioral factors and microorganism categories with sufficient prevalence (NPS, NPNS, PPNS and antibiotic-resistant organisms) [S3]. In univariable analyses, NPNS microorganisms were significantly more likely among children (OR = 6.66, 95% CI: 1.45–30.55, p = 0.01), less likely among outdoor activity participants (OR = 0.10, 95% CI: 0.02–0.46, p < 0.01) and among those who used a topical skin product the day of the fair (OR = 0.03–0.91, p < 0.01). When combined in a multivariable model, adult/child status and participation in an outdoor activity remained significant predictors of NPNS microorganism carriage (Table 3).
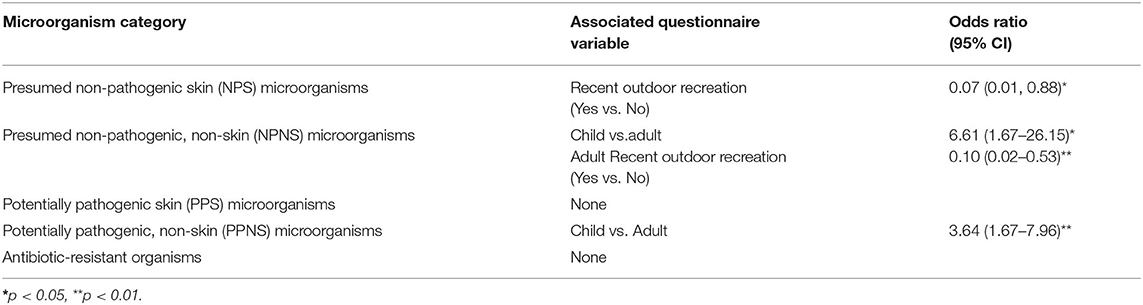
Table 3. Questionnaire variables statistically significantly associated with microorganism categories in univariable/multivariable generalized linear mixed models for 206 Minnesota State Fair attendees (2014).
PPNS microorganisms were more likely among children (OR = 3.64, 95% CI: 1.67–7.96, p < 0.01) and males (OR = 2.33, 95% CI: 1.15–4.73, p = 0.02) (Table 3). When these variables were combined in a multivariable model, sex was no longer predictive of PPNS microorganisms, and the coefficient for adult/child changed by 9.5%, suggesting slight confounding by sex of the association of adult/child with PPNS microorganisms. An exploratory X2 analysis showed that females accounted for a significantly higher proportion of adults (72.8%) than children (57.3%) (p = 0.03); however, in the multivariable model an interaction term between adult/child status and sex was not statistically significant.
Given the importance of adult/child status as a predictor of NPNS and PPNS microorganisms, we examined the relationship between adult/child status and other demographic/behavioral factors using X2 tests. Notably, handwashing frequency was greater for adults than children (p < 0.01). However, handwashing frequency was not a significant univariable or multivariable predictor of carriage of either NPNS or PPNS microorganisms.
In the univariable assessment for NPS microorganisms, those who participated in an outdoor activity in the past week had significantly lower odds of carriage than those who had not (OR: 0.07, 95% CI: 0.01–0.88, p = 0.04), which was inconsistent with the results of the tabular analyses. Antibiotic-resistant microorganisms were associated with work with livestock (OR: 13.22, 95% CI 0.19–19.56, p = 0.05), but this analysis used only the subset of participants in the study who were employed.
Discussion
This cross-sectional study sought to describe the types of microorganisms found on the hands of community-dwellers attending the Minnesota State Fair and to determine whether such carriage corresponds with demographic/behavioral factors. Among the 206 participants, the most common microorganisms were non-pathogenic, whether skin microbiota (NPS; 98.1% of participants) or not (NPNS; 93.2% of participants). The most prevalent NPNS microorganisms were Bacillus spp. (87.4% of participants), which occur in soil, a common exposure in the peri-domestic environment and on the State Fair grounds. Other common NPNS microorganisms were molds, which can be found in humid, dark, and damp areas, including soil with decomposing vegetation. Participants may have been exposed to molds at home through gardening or yard work or at any of several fairground sites, including animal barns (hay and straw) and landscaping, farming, and gardening exhibits.
Only two participants (1.0%) had detectable hand carriage of S. aureus, which in only one instance was MRSA (0.5%, 95% CI: 0.1–2.7%). The 2003–2004 National Health and Nutrition Examination Survey (NHANES) reported a MRSA prevalence of 1.5% (95% CI: 1.2–1.8%) from nasal swabs, and the National Institute for Occupational Safety and Health estimates a 1% general-population MRSA prevalence (40, 41). Our result aligns with these national estimates, despite some differences in sampling technique and population. Notably, we did not identify any pathogenic E. coli, which has been implicated in outbreaks at recent state and county fairs (42–44).
Although children were consistently more likely than adults to have detectable hand-source microorganisms, including various PPNS microorganisms, and although adults washed their hands more frequently than children, handwashing was unassociated with microorganism carriage. Unmeasured exposure differences between adults and children, as well as the variation in skin microbiota by age (45), may explain the observed differences in colonization by adult/child status.
Despite the differences in microorganisms carried by children and adults, most detected microorganisms were non-pathogenic, without associated concerns regarding community transmission either by behaviors participants engaged in at the State Fair, or activities in their daily lives. The Minnesota State Fair strongly encourages hand hygiene, in partnership with the Minnesota Department of Health. Handwashing stations are readily available throughout the fairgrounds, and promotional items, including signs and handheld fans that say, “I'm a fan of handwashing,” remind attendees to wash their hands. Conceivably, study participants' hand hygiene practices offered sufficient protection against carriage of pathogenic microorganisms. However, participants were less likely to assign themselves a “good” grade on handwashing at the State Fair than their usual practice. Perhaps their perception of poor hand hygiene practices at the State Fair actually made them more cautious of what they touched and when and how they washed their hands. As precedent for this phenomenon, nursing students who perceived higher risk of infection were more likely to exhibit appropriate hand hygiene practices than those who perceived a lower risk (46).
Strengths and Limitations
This cross-sectional study used the Minnesota State Fair as a setting for assessing hand carriage of microorganisms by healthy individuals. Participants were willing to incorporate study activities, including biological sample collection, into their time at the State Fair. This model conceivably could be applied to other large community gatherings for recruiting into a range of point-prevalence or longitudinal studies. Indeed, since 2014 more than 103,000 Minnesota State Fair attendees have participated in research during their fair visit, evidence of this enrollment strategy's usefulness for many investigators (47).
Nonetheless, despite the potential for recruiting a wide range of State Fair attendees, participants in this study may not have been representative of the average fairgoer. The affiliation of the enrollment site with a university likely contributed to a sample that was more educated and health-conscious than the Minnesota population as a whole. Notably, 97.0% of study participants were from the seven-county Twin Cities metro area, overrepresenting the predominantly urban metro area relative to the total Minnesota population (48). Likewise, compared with U.S. Census estimates, participants also were more likely to be white (92.7 vs. 86.3%), Non-Hispanic (97.1 vs. 94.8%), and to have at least a bachelor's degree (73.8 vs. 34.8%). Moreover, 75.7% earned at least $50,000 per year, and 58.2% at least $75,000, whereas the statewide median income is $65,699 (49).
Because this was an exploratory study, and of the small sample size, we did not adjust the analyses for multiple comparisons. Future investigations of prevalence, characteristics, and epidemiology of microbial hand contamination in the community should consider using a larger sample size or narrower range of exposure variables to describe associations more fully. Additionally, we studied only hand carriage. Therefore, we cannot exclude colonization at other sites, which also may contribute to community transmission of microorganisms (50).
Conclusions
Our study demonstrated the feasibility using a large community event to conduct a prevalence survey for microorganism carriage. The main findings were that (i) most participants carried non-pathogenic microorganisms, few carried pathogens, especially antimicrobial-resistant pathogens; (ii) adults and children differed with respect to both microorganism carriage and handwashing frequency; (iii) handwashing habits, behaviors at the fair and in daily life, were unassociated with microorganism carriage among community members.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study involving human participants was reviewed and approved by the University of Minnesota Institutional Review Board (No. 1406M1101). Written informed consent to participate in this study was provided by the participants or the participants' legal guardian/next of kin.
Author Contributions
MM and BM conceived of the study idea. JJ and CC supported MM and BM in designing the protocols and survey materials. SJ, FN, MM, and BM collected the data. CC performed all laboratory procedures and reported prevalence results to the rest of the team. SJ, FN, MM, BM, and RB conducted the statistical analyses. MM and BM wrote the manuscript with significant editorial contributions from JJ. All authors contributed to the article and approved the submitted version.
Funding
The Clinical and Translational Science Institute and the Division of Epidemiology and Community Health at the University of Minnesota provided initial funding for this project through the Driven to Discover initiative. The use of REDCap was supported by the National Institutes of Health's National Center for Advancing Translational Sciences, grant UL1TR002494. Additional support was provided by St. Catherine University's Academic and Professional Development Committee for an undergraduate research assistant. This work was also supported in part by Office of Research and Development, United States Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health's National Center for Advancing Translational Sciences, the Department of Veterans Affairs, or the authors' respective institutions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Logan Spector and Ellen Demerath for their initial encouragement for the project. Billie S. Slater assisted in the microbiology laboratory.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.574444/full#supplementary-material
References
1. Semmelweis IP. Die Aetiologie, Der Begriff Und Die Prophylaxis Des Kindbettfiebers. Budapest and Vienna: Hartleben (1861).
2. Edmonds-Wilson SL, Nurinova NI, Zapka CA, Fierer N, Wilson M. Review of human hand microbiome research. J Dermatol Sci. (2015) 80:3–12. doi: 10.1016/j.jdermsci.2015.07.006
3. Bellissimo-Rodrigues F, Pires D, Soule H, Gayet-Ageron A, Pittet D. Assessing the likelihood of hand-to-hand cross-transmission of bacteria: an experimental study. Infect Control Hosp Epidemiol. (2017) 38:553–8. doi: 10.1017/ice.2017.9
4. Jumaa PA. Hand hygiene: simple and complex. Int J Infect Dis. (2005) 9:3–14. doi: 10.1016/j.ijid.2004.05.005
5. Pittet D, Allegranzi B, Sax H, Dharan S, Lúcia Pessoa-Silva C, Donaldson L, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. (2006) 6:641–652. doi: 10.1016/S1473-3099(06)70600-4
6. Walker JL, Sistrunk WW, Higginbotham MA, Burks K, Halford L, Goddard L, et al. Hospital hand hygiene compliance improves with increased monitoring and immediate feedback. Am J Infect Control. (2014) 42:1074–8. doi: 10.1016/j.ajic.2014.06.018
7. Song X, Stockwell DC, Floyd T, Short BL, Singh N. Improving hand hygiene compliance in health care workers: strategies and impact on patient outcomes. Am J Infect Control. (2013) 41:101–5. doi: 10.1016/j.ajic.2013.01.031
8. Mela S, Whitworth DE. The fist bump: a more hygienic alternative to the handshake. Am J Infect Control. (2014) 42:916–7. doi: 10.1016/j.ajic.2014.04.011
9. Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep. (2007) 122:160–6. doi: 10.1177/003335490712200205
10. Septimus E, Yokoe DS, Weinstein RA, Perl TM, Maragakis LL, Berenholtz SM. Maintaining the momentum of change: the role of the 2014 updates to the Compendium in Preventing Healthcare-Associated Infections. Infect Control Hosp Epidemiol. (2014) 35:460–3. doi: 10.1086/675820
11. Siegman-Igra Y, Fourer B, Orni-Wasserlauf R, Golan Y, Noy A, Schwartz D, et al. Reappraisal of community-acquired bacteremia: a proposal of a new classification for the spectrum of acquisition of bacteremia. Clin Infect Dis. (2002) 34:1431–9. doi: 10.1086/339809
12. Corrado RE, Lee D, Lucero DE, Varma JK, Vora NM. Burden of adult community-acquired, health-care-associated, hospital-acquired, and ventilator-associated pneumonia: New York City, 2010 to 2014. Chest. (2017) 152:930–42. doi: 10.1016/j.chest.2017.04.162
13. Mork RL, Hogan PG, Muenks CE, Boyle MG, Thompson RM, Sullivan ML, et al. Longitudinal, strain-specific Staphylococcus aureus introduction and transmission events in households of children with community-associated meticillin-resistant S aureus skin and soft tissue infection: a prospective cohort study. Lancet Infect Dis. (2019) 20:188–98. doi: 10.1016/S1473-3099(19)30570-5
14. Henriksen DP, Nielsen SL, Laursen CB, Hallas J, Pedersen C, Lassen AT. How well do discharge diagnoses identify hospitalised patients with community-acquired infections? - A validation study. PLoS ONE. (2014) 9:e0092891. doi: 10.1371/journal.pone.0092891
15. Kassem II, Sigler V, Esseili MA. Public computer surfaces are reservoirs for methicillin-resistant staphylococci. ISME J. (2007) 1:265–8. doi: 10.1038/ismej.2007.36
16. Yeh PJ, Simon DM, Millar JA, Alexander HF, Franklin D. A diversity of antibiotic-resistant Staphylococcus spp. in a public transportation system. Osong Public Heal Res Perspect. (2011) 2:202–9. doi: 10.1016/j.phrp.2011.11.047
17. Garcia SA, McKenzie JF, Patterson T, Rohde RE. Snapshot prevalence and characterization of Staphylococcus species, including MRSA, in a student athletic facility: an undergraduate research project. Clin Lab Sci. (2012) 25:156–64. doi: 10.29074/ascls.25.3.156
18. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. (2003) 36:131–9. doi: 10.1086/345436
19. Minnesota State Fair. About the Fair | Minnesota State Fair (2019). Available online at: https://www.mnstatefair.org/about-the-fair/ (accessed October 27, 2020).
20. Minnesota State Fair. History | Minnesota State Fair. (2019). Available online at: https://www.mnstatefair.org/get-involved/media/history/ (accessed October 27, 2020).
21. Burton M, Cobb E, Donachie P, Judah G, Curtis V, Schmidt WP. The effect of handwashing with water or soap on bacterial contamination of hands. Int J Environ Res Public Health. (2011) 8:97–104. doi: 10.3390/ijerph8010097
22. Bloomfield SF, Aiello AE, Cookson B, O'Boyle C, Larson EL. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am J Infect Control. (2007) 35:27–64. doi: 10.1016/j.ajic.2007.07.001
23. Conrad CC, Stanford K, Narvaez-Bravo C, Callaway T, McAllister T. Farm fairs and petting zoos: a review of animal contact as a source of zoonotic enteric disease. Foodborne Pathog Dis. (2017) 14:59–73. doi: 10.1089/fpd.2016.2185
24. Baumgardner DJ. Soil-related bacterial and fungal infections. J Am Board Family Med. (2012) 25:734–44. doi: 10.3122/jabfm.2012.05.110226
25. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. (2007) 26:16–26. doi: 10.1016/j.clindermatol.2007.10.006
26. Haghverdian BA, Patel N, Wang L, Cotter JA. The sports ball as a fomite for transmission of Staphylococcus aureus. J Environ Health. (2018) 80:8–13. doi: 10.1016/B978-0-12-809671-0.00002-4
27. Markley JD, Edmond MB, Major Y, Bearman G, Stevens MP. Are gym surfaces reservoirs for Staphylococcus aureus? A point prevalence survey. Am J Infect Control. (2012) 40:1008–9. doi: 10.1016/j.ajic.2012.01.015
28. Hsu T, Joice R, Vallarino J, Abu-Ali G, Hartmann EM, Shafquat A, et al. Urban transit system microbial communities differ by surface type and interaction with humans and the environment. mSystems. (2016) 1:e00018-16. doi: 10.1128/mSystems.00018-16
29. Cosby CM, Costello CA, Morris WC, Haughton B, Devereauux MJ, Harte P, et al. Microbiological analysis of food contact surfaces in child care center. Appl Environ Microbiol. (2008) 74:6918–22. doi: 10.1128/AEM.00547-08
30. Montoya A, Schildhouse R, Goyal A, Mann JD, Snyder A, Chopra V, et al. How often are health care personnel hands colonized with multidrug- resistant organisms? A systematic review and meta-analysis. Am J Infect Control. (2019) 47:693–703. doi: 10.1016/j.ajic.2018.10.017
31. Brunetti L, De Caro F, Boccia G, Cavallo P, Capunzo M. Surveillance of nosocomial infections: a preliminary study on yeast carriage on hands of healthcare workers. J Prev Med Hyg. (2008) 49:63–8. doi: 10.15167/2421-4248/jpmh2008.49.2.118
32. Ferng Y, Clock SA, Wong-Mcloughlin J, DeLaMora PA, Perlman JM, Gray KS, et al. Multicenter study of hand carriage of potential pathogens by neonatal ICU healthcare personnel. J Pediat Infect Dis Soc. (2015) 4:276–9. doi: 10.1093/jpids/piu022
33. LeJeune J, Kersting A. Zoonoses: an occupational hazard for livestock workers and a public health concern for rural communities. J Agric Saf Health. (2010) 16:161–79. doi: 10.13031/2013.32041
34. Devenish JA, Barnum DA. Evaluation of the API 20E system for the identification of gram-negative nonfermenters from animal origin. Can J Comp Med. (1982) 46:80–4.
35. Shayegani M, Maupin PS, McGlynn DM. Evaluation of the API 20E system for identification of nonfermentative Gram-negative bacteria. J Clin Microbiol. (1978) 7:539–45.
36. Denton M, Kerr KG. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev. (1998) 11:57–80. doi: 10.1128/CMR.11.1.57
37. Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. (2012) 1–13. Available online at: https://www.asm.org/Protocols/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Pro (accessed October 27, 2020).
38. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
39. Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. (2010) 19:227–9. Available online at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2938757&tool=pmcentrez&rendertype=abstract (accessed October 27, 2020).
40. Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. (2008) 197:1226–34. doi: 10.1086/533494
41. U.S. Department of Health & Human Services. CDC - MRSA and the Workplace - NIOSH Workplace Safety and Health Topic. (2015). Available online at: https://www.cdc.gov/niosh/topics/mrsa/default.html (accessed December 2, 2020).
42. Taylor DB. Toddler dies from E. Coli linked to contact with animals at San Diego County Fair (June 30, 2019). New York Times. Available online at: https://www.nytimes.com/2019/06/30/health/e-coli-san-diego.html (accessed October 27, 2020).
43. Olson J. E.coli outbreak linked to State Fair birth exhibit (September 17, 2019). Star Tribune. Available online at: http://www.startribune.com/e-coli-outbreak-linked-to-state-fair-birth-exhibit/560593792/ (accessed October 27, 2020).
44. Centers for Disease Control and Prevention (CDC). Notes from the field: Escherichia coli O157:H7 gastroenteritis associated with a state fair - North Carolina, 2011. MMWR Morb Mortal Wkly Rep. (2012) 60:1745–6.
45. Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiool. (2011) 9:244–53. doi: 10.1038/nrmicro2537
46. Oyapero A, Oyapero O. An assessment of hand hygiene perception and practices among undergraduate nursing students in Lagos State: a pilot study. J Educ Heal Promot. (2018) 7:150. doi: 10.4103/jehp.jehp_56_17
47. University of Minnesota. History & Funding: 2013 - Present - D2D: The Driven to Discover Research Facility. (2017). Available online at: http://d2d.umn.edu/history-funding/ (accessed December 2, 2020).
48. Egbert A, Brower S. Greater Minnesota: Refined & Revisited. St. Paul, MN: Minnesota State Demographic Center (2017).
49. U.S. Department of Commerce. American FactFinder - Community Facts. Available online at: https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml?src=bkmk (accessed December 2, 2020).
Keywords: microbial diversity, prevalence, hand hygiene, epidemiology–descriptive, community gatherings
Citation: Mason MR, Morawski BM, Bayliss RL, Noor FM, Jama SH, Clabots CL and Johnson JR (2020) Prevalence, Characteristics, and Epidemiology of Microbial Hand Contamination Among Minnesota State Fair Attendees (2014). Front. Public Health 8:574444. doi: 10.3389/fpubh.2020.574444
Received: 19 June 2020; Accepted: 20 November 2020;
Published: 16 December 2020.
Edited by:
Marco Cassone, University of Michigan, United StatesReviewed by:
Maya Nadimpalli, Institut Pasteur, FranceNa Hong, Digital China Health Technologies Co. Ltd, China
Copyright © 2020 Mason, Morawski, Bayliss, Noor, Jama, Clabots and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meghan R. Mason, bXJtYXNvbkBzdGthdGUuZWR1
 Meghan R. Mason
Meghan R. Mason Bozena M. Morawski2
Bozena M. Morawski2 Connie L. Clabots
Connie L. Clabots James R. Johnson
James R. Johnson