- 1Department of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Clinical Laboratory, Tongji Medical College, Union Hospital, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Intensive Care Unit, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
Background: The clinical characteristics of coronavirus disease 2019 (COVID-19) have been well-studied, while effective predictors for clinical outcome and research on underlying mechanisms are scarce.
Methods: Hospitalized COVID-19 pneumonia patients with definitive clinical outcome (cured or died) were retrospectively studied. The diagnostic performance of the leucocyte subsets and other parameters were compared using the area under the receiver operating characteristic curve (AUC). Further, the correlations between leucocyte subsets and inflammation-related factors associated with clinical outcome were subsequently investigated.
Results: Among 95 subjects included, 56 patients were cured, and 39 died. Older age, elevated aspartate aminotransferase, total bilirubin, serum lactate dehydrogenase, blood urea nitrogen, prothrombin time, D-dimer, Procalcitonin, and C-reactive protein levels, decreased albumin, elevated serum cytokines (IL2R, IL6, IL8, IL10, and TNF-α) levels, and a decreased lymphocyte count indicated poor outcome in patients with COVID-19 pneumonia. Lymphocyte subset (lymphocytes, T cells, helper T cells, suppressor T cells, natural killer cells, T cells+B cells+NK cells) counts were positively associated with clinical outcome (AUC: 0.777; AUC: 0.925; AUC: 0.900; AUC: 0.902; AUC: 0.877; AUC: 0.918, resp.). The neutrophil-to-lymphocyte ratio (NLR), neutrophil to T lymphocyte count ratio (NTR), neutrophil percentage to T lymphocyte ratio (NpTR) effectively predicted mortality (AUC: 0.900; AUC: 0.905; AUC: 0.932, resp.). Binary logistic regression showed that NpTR was an independent prognostic factor for mortality. Serum IL6 levels were positively correlated with leucocyte count, neutrophil count, and eosinophil count and negatively correlated with lymphocyte count.
Conclusion: These results indicate that leucocyte subsets predict the clinical outcome of patients with COVID-19 pneumonia with high efficiency. Non-self-limiting inflammatory response is involved in the development of fatal pneumonia.
Introduction
In December 2019, a new type of β-coronavirus named the 2019 novel coronavirus (SARS-CoV-2) emerged in Wuhan, China, and spread rapidly throughout the world (1). As of May 31, 2020, 5.9 million cases of coronavirus disease 2019 (COVID-19) have been confirmed, including 367 thousand deaths (2). It was most likely initially a zoonotic infectious disease, but effective transmission between people was soon discovered (3). The clinical symptoms of SARS-CoV-2 infection are variable, including being asymptomatic, upper respiratory tract disease, viral pneumonia, fatigue, gastrointestinal symptoms, respiratory failure, and even death (4, 5). Its clinical characteristics have been well-evaluated, but effective predictors for clinical outcome and research on the underlying mechanisms are scarce (6). Identification of effective predictors could help to judge the prognosis and optimal intervention measures for COVID-19 patients at an early stage.
Therefore, 95 hospitalized COVID-19 pneumonia patients with definitive clinical outcome (cured or died) were retrospectively selected between February 5, 2020, and March 11, 2020. The clinical characteristics of the 95 hospitalized patients are described. Moreover, factors predicting the prognosis of patients with COVID-19 pneumonia were investigated in this study.
Materials and Methods
Study Design and Participants
This retrospective case-control study enrolled a total of 95 cases admitted to Tongji hospital between February 5, 2020, and March 8, 2020, with confirmed COVID-19 pneumonia. In brief, the patients were all hospitalized probable subjects in four wards of Tongji hospital with (1) positive throat swab nucleic acid test by real-time RT-PCR methods or positive for SARS-CoV-2-specific antibodies, (2) chest radiographic evidence of pneumonia, (3) rehabilitation discharge or died in hospital between February 20, 2020, and March 11, 2020. Patients who were discharged from hospital in 24 h or died within 24 h after hospitalization were excluded from this study. This study was reviewed and approved by the ethics committee of Tongji Hospital, Huazhong University of Science and Technology (IRB ID:TJ- IRB20200343).
Data Collection
Clinical, laboratory, and radiological results were collected from electronic medical records. Data were obtained with standardized forms for all subjects involved. Two researchers independently collected and reviewed the data.
Outcomes
Two outcomes were evaluated: “cured” or “died.” The criteria for rehabilitation discharge or being cured were (1) no fever for at least 3 days; (2) substantial improvement in chest CT scan or X-ray images; (3) negative nucleic acid test two consecutive times with at least a 24-h interval between them.
Statistical Analysis
Descriptive analyses of the continuous variables were expressed as mean or median with interquartile range (IQR). Categorical variables were described as frequency rates and percentages. Differences in continuous variables were analyzed using t-tests when normally distributed or otherwise with the Mann-Whitney test. Categorical variables were compared using the χ2 test. The ROC curve was used to analyze the predictive factors, and the area under the ROC curve (AUC) was calculated. Correlation analysis was evaluated by the Pearson test. The appropriate sample size for inferences was determined based on the Wilcoxon statistics, where the statistical power was 0.8 (1–β) and α = 0.05. Forward stepwise, binary logistic regression was performed on the covariates. All tests were two-sided, and P < 0.05 was considered statistically significant. The statistical software SPSS 23.0 was used in this study.
Results
The Characterization of Patients
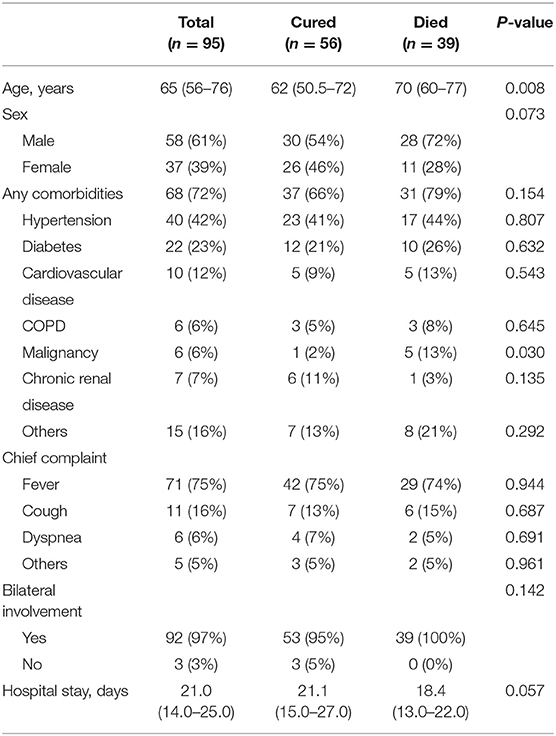
A total of 95 COVID-19 patients (58 men and 37 women) were included in the study (Table 1). The median age was 65 years old. As of March 11, 56 patients (58.9%) had been discharged, and 39 (41.1%) had died. The all-cause mortality rate in these COVID-19 patients was 22.4%. Of the 95 patients, 68 (71.6%) had one or more pre-existing diseases. Hypertension (40 [42.1%]), diabetes (22 [23.2.1%]), cardiovascular disease (10 [10.5%]), and malignancies (6 [6.3%]) were the most common. The chief complaints were fever (71 [74.7%]), cough (13 [13.7%]), dyspnea (6 [6.3%]), and other uncommon symptoms. Of the 95 patients, bilateral involvement was detected in chest CT or X-ray images of 92 (97%) patients. The mean hospitalization duration was 21 (14.0–25.0) days. People who died were significantly older (70 years [IQR 60–77] vs. 62 years [IQR 50.5–72]; p = 0.008), more likely to have malignancies (5 [13%] vs. 1 [2%]; p = 0.030), while other variables (i.e., gender, hypertension, diabetes, cardiovascular disease, COPD, hospital stay) were similar between the two groups.
Laboratory Parameters in the Cured and Died Groups
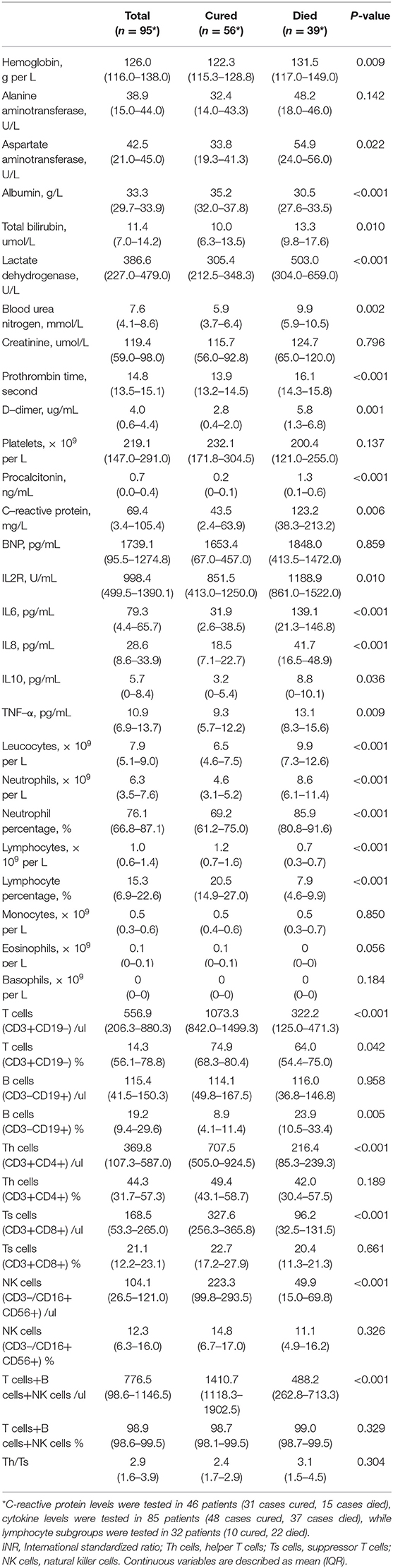
The blood count of patients on admission showed a decrease in lymphocytes, especially in the died group (Table 2). Consistent with this, most lymphocyte subsets (T cells, Th cells, Ts cells, and NK cells) detected by flow cytometry were significantly higher in survivors than in non-survivors. Meanwhile, leucocyte count and neutrophil count were higher in deceased patients (leucocyte 9.9 × 109 per L [7.3–12.6]; neutrophil 8.6 × 109 per L [6.1–11.4]) than in cured patients (leucocyte 8.6 × 109 per L [6.1–11.4], p < 0.001; neutrophil 4.6 × 109 per L [3.1–5.2], p = 0.001). Prothrombin time and D-dimer levels were higher in deceased patients (PT 16.1 s [14.3–15.8]; D-dimer 5.8 mg/L [1.4–6.8]) than in cured patients (PT 13.9 s [13.2–14.5], p < 0.001; D-dimer 2.8 mg/L [0.4–2.0], p = 0·001). Blood urea nitrogen levels, C-reactive protein (CRP) levels and Procalcitonin levels were higher in deceased patients (BUN 9.9 mmol/L [5.9–10.5]; CRP 123.2 mg/L [38.3–213.2]; Procalcitonin 1.3 ng/mL [0.1–0.6]) than in cured patients (BUN 5.9 mmol/L [3.7–6.4]), p < 0.001; CRP 43.5 mg/L [2.4–63.9], p = 0.006; Procalcitonin 0.2 ng/mL [0–0.1], p < 0.001). Serum albumin levels were lower in deceased patients (albumin 30.5 g/L [27.6–33.5]) than in cured patients (albumin 35.2 g/L [32.0–37.8], p = 0.022), while serum total bilirubin and lactate dehydrogenase levels were higher in deceased patients (T-Bil 13.3 μmol/L [9.8–17.6]; LDH 503.0 U/L [304.0–659.0]) than in cured patients (T-Bil 10.0 μmol/L [6.3–13.5], p = 0.010; LDH 305.4 U/L [212.5–348.3], p < 0.001), indicating hepatic dysfunction in more patients in the died group. The levels of most cytokines (IL2R, IL6, IL8, IL10, and TNF-α) were significantly higher in non-survivors than in survivors. No significant differences in serum creatine and BNP levels existed between deceased patients and cured patients (p > 0.05). As determined based on the Wilcoxon statistics (7), the sample size needed for evaluating CRP, Procalcitonin, LDH, IL6, neutrophil percentage, lymphocyte percentage, and T cells were 31 patients (18 cases cured, 13 cases died), 244 patients (144 cases cured, 100 cases died), 41 patients (24 cases cured, 17 cases died), 56 patients (33 cases cured, 23 cases died), 29 patients (17 cases cured, 12 cases died), 27 patients (16 cases cured, 11 cases died), and 103 patients (61 cases cured, 42 cases died), respectively.
Prognostic Factors of Patients With COVID-19 Pneumonia
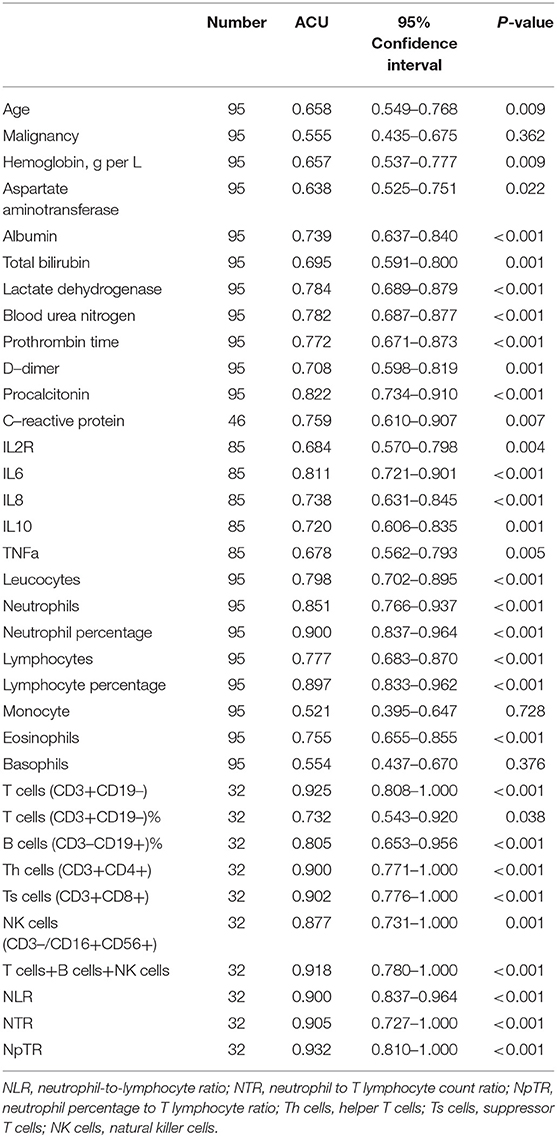
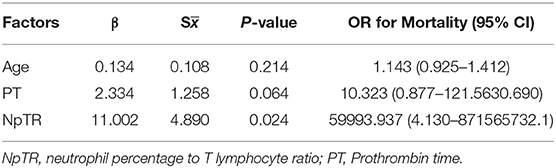
ROC curve analysis was performed to evaluate the prognostic factors for COVID-19. The neutrophil-to-lymphocyte ratio (NLR) is a well-known marker of systemic inflammation (8). Therefore, we evaluated the efficiency of NLR and other potential predictors (neutrophil to T lymphocyte count ratio, NTR; neutrophil percentage to T lymphocyte ratio, NpTR) in predicting mortality. As shown in Table 3 and Figures 1A–C, factors associated with peripheral blood cell count (neutrophils, neutrophil percentage, lymphocyte percentage, T cells, B cells, Th cells, Ts cells, NK cells, T cells+B cells+NK cells, NLR, NTR, and NpTR) and inflammation-associated factors (Procalcitonin, IL6) showed good prognostic values, among which NpTR was the most predominant predictive factor for the clinical outcome (0.932; 95% CI: 0.810–1.000, p < 0.001; Figure 1C). Furthermore, binary logistic regression models showed that NpTR was the independent prognostic factor for death (OR =59993.937, 95% CI: 4.130–871565732.1; p = 0.024; Table 4). The Nagelkerke R value was 0.811. The condition indexes for age, LDH, and NpTR were 2.6, 5.4, and 18.6, respectively. To minimize potential confounding effects of age, a matched case-control study was performed (each deceased patient was matched with one cured patient with an age difference of 4 years or less). Both the matched and unmatched analyses yielded similar results (Supplementary Table 1).
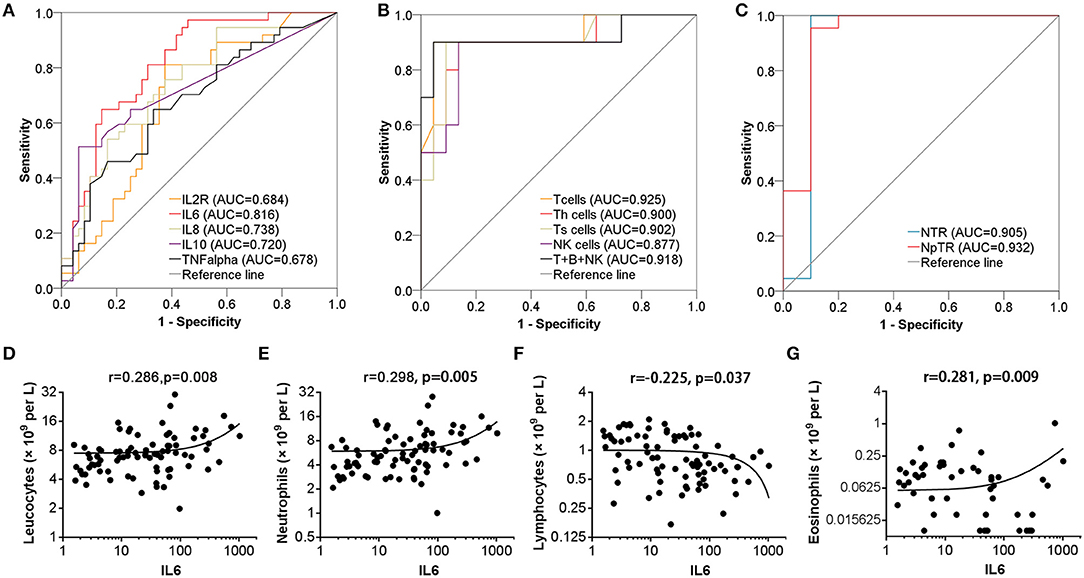
Figure 1. Efficiency of cytokine and leucocyte subsets in predicting the clinical outcome of patients with COVID-19 pneumonia. (A–C) Efficiency of serum cytokine and leucocyte subsets in predicting the mortality of patients. (D–G) Serum IL6 levels were positively correlated with lymphocyte count, neutrophil count, and eosinophil count and negatively correlated with lymphocyte count.
Correlations Between Leucocyte Subsets and Inflammation-Related Factors
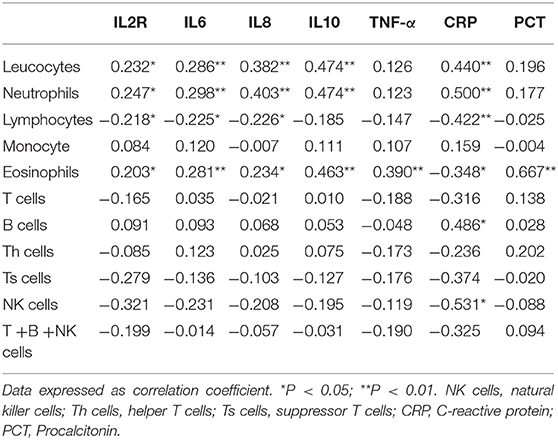
We subsequently explored the correlations between leucocyte subsets and inflammation-related factors (IL2R, IL6, IL8, TNF-α, CRP, and Procalcitonin) associated with clinical outcome. Interestingly, serum IL6 levels were positively correlated with leucocyte count, neutrophil count, and eosinophil count (r = 0.286, p = 0.008; r = 0.298, p = 0.005; r = 0.281, p = 0.009, resp.) and negatively correlated with lymphocyte count (r = −0.226, p = 0.037) but not monocyte count, as shown in Table 5 and Figures 1D–G. Meanwhile, leucocytes, neutrophils, and eosinophils were positively correlated with most inflammatory factors (IL2R, IL6, IL8, TNF-α, CRP, and Procalcitonin), as shown in Table 5. No significant correlations were found between lymphocyte subsets and inflammatory factors (IL2R, IL6, IL8, TNF-α, and Procalcitonin) (p > 0.05).
Discussion
We retrospectively analyzed the clinical characteristics and laboratory parameters in 95 COVID-19 patients with definitive clinical outcome and found that leucocyte subsets and inflammatory factors showed good prognostic values and that non-self-limiting inflammatory response may be involved in the development of fatal pneumonia induced by SARS-CoV-2 infection.
As previously reported, older age, a coagulation disorder, bacterial infection, malfunctions in the liver, heart, or kidney, and changes in blood cell count are associated with the prognosis of COVID-19 patients (4, 6). Similarly, we found that older age, lower albumin, and higher serum LDH levels, BUN levels, and PT indicated poor outcome. Patients with increased levels of organ damage-associated biomarkers were more likely to develop complications such as fetal acute lung injury (ALI) and multiple organ dysfunction syndrome. Moreover, the leucocyte count or neutrophil count was higher in the died group and predicted the mortality in COVID-19 patients, verified by ROC curves, while lymphocyte subset (lymphocytes, T cells, Th cells, Ts cells, NK cells, T cells+B cells+NK cells) counts were positively associated with cure rate, among which the AUC for T cells was the highest (AUC: 0.925, P < 0.001). NLR is a well-known marker of systemic inflammation (8), so we evaluated NLR and other potential predictors (NTR, NpTR) originating from the existing parameters. ROC curves showed that NLR, NTR, and NpTR could effectively predict the mortality in patients with COVID-19 pneumonia (AUC: 0.900, P < 0.001; AUC: 0.905, P < 0.001; AUC: 0.932, P < 0.001, resp.).
Despite a series of studies, specific factors causing the high mortality of COVID-19 are incompletely understood. Respiratory failure is the main cause of mortality in COVID-19 patients (4, 9). Severe pneumonia caused by pathogenic human coronavirus is often associated with high viral load, massive inflammatory cell infiltration, and elevated cytokine responses resulting in ALI (10, 11). Consistent with the above findings, histological examination of lungs from patients dead from COVID-19 revealed extensive leucocyte infiltration, overactivated T lymphocytes being the predominant cell type (9). Hyper-inflammatory cytokines can amplify inflammatory responses by promoting unrestrained virus replication (12). Furthermore, coronavirus-infected patients may die of ALI despite successful viral elimination (13). Consistent with previous studies (4, 14), we found that higher levels of inflammation-associated factors (IL2R, IL6, IL8, TNF-α, CRP, and Procalcitonin) indicated poor outcome, indicating that powerful positive feedback between virus infection and hyperinflammation might be critical in lung destruction and disease morbidity. IL6 was a powerful predictor of mortality and closely correlated with leucocyte, neutrophil, lymphocyte, and eosinophil counts. Thus, IL6 receptor blockade (tocilizumab), which has been approved for a clinical COVID-19 treatment trial in China (ChiCTR2000029765), is a new and promising treatment for COVID-19. IL-10 was highly expressed in non-survivors, indicating the failure to limit and ultimately terminate hyperinflammation in severe patients (15). Meanwhile, lymphocytes play an important role in SARS-CoV-2 elimination, as indicated by the production of larger amounts of granzymes and perforin in a mild-moderate patient (16). There was no significant elevation of cytokines in this mild-moderate patient, further indicating the key role of cytokines in disease progression. Middle East respiratory syndrome coronavirus (MERS) can efficiently infect human T cells and induced apoptosis in human T lymphocytes (17), so it is speculated that both redistribution to the target organ and depletion contribute to lymphocyte decline, both of which indicate poor outcome. Studies have demonstrated that T-cell responses can inhibit the overactivation of innate immunity (18). As described in our study, leucocytes, neutrophils, and eosinophils were positively correlated with most inflammatory factors (IL2R, IL6, IL8, TNF-α, CRP, and Procalcitonin), indicating that neutrophils and eosinophils might contribute to cytokine release. Meanwhile, lymphocyte count significantly decreased in fatal cases and was negatively correlated with IL6 levels, indicating negative feedback between lymphocytes and IL6 during coronavirus infection. Dysfunction of lymphocytes in both virus clearance and inhibition of cytokine overactivation may contribute to excessive inflammatory response. Thus, non-self-limiting inflammatory response and lymphocyte dysfunction may be the key mechanisms in fatal pneumonia induced by SARS-CoV-2 infection. Probably because of the limited number of cases detected, no correlations were found between lymphocyte subsets and inflammatory factors.
This study does have some limitations. Firstly, owing to the retrospective nature of this study, results for viral load not available. Secondly, it is a single-center study with a limited number of cases. In addition, the majority of patients admitted to our hospital were critically ill, so population bias exists, though a confounding effect of age has been ruled out.
Conclusion
In summary, our results demonstrate that leucocyte subsets and inflammation-related factors predict the clinical outcome of patients with COVID-19 pneumonia with high efficiency, among which T cells and NpTR are most predominant. Non-self-limiting inflammatory response and lymphocyte dysfunction may be the key mechanisms in fatal pneumonia induced by SARS-CoV-2 infection. This provides evidence for laboratory diagnostics and clinical interventions.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Tongji Hospital of Huazhong University of Science and Technology. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SL and CY made substantial contributions to the study design. JG and CY were in charge of the draft manuscript. JL and SL took responsibility for data acquisition. JL made the main contributions to data analysis and interpretation. CY participated in the diagnosis and treatment of patients. CY made substantial revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (No. 81702989).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a pre-print at SSRN (Hu Zhiquan, Gan Jiahua, Li Jingjing, et al.) (16).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00299/full#supplementary-material
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. WHO. WHO Coronavirus Disease (COVID-19) Dashboard. (2020). Available from: https://covid19.who.int/ (accessed May 31, 2020).
3. Li Q, Med M, Guan X, Wu P, Wang X, Zhou L, et al. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
5. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
6. Wu C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 58:713–4. doi: 10.1016/j.jemermed.2020.04.007
7. Al-Sunduqchi MS. Determining the appropriate sample size for inferences based on the Wilcoxon statistics. 1990 (Ph.D. dissertation) under the direction of William C. Guenther. Dept. Of Statistics, University of Wyoming, Laramie, WY.
8. Liu X, Shen Y, Wang H, Ge H, Fei A, Pan S. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with sepsis: a prospective observational study. Mediators Inflamm. (2016) 2016:8191254. doi: 10.1155/2016/8191254
9. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–22. doi: 10.1016/S2213-2600(20)30076-X
10. Guery B, Poissy J, Mansouf L, Séjourné C, Ettahar N, Lemaire X, et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. (2013) 381:2265–72. doi: 10.1016/S0140-6736(13)60982-4
11. Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah M, Al Ajlan A, et al. Histopathology of middle east respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. (2018) 72:516–24. doi: 10.1111/his.13379
12. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. (2017) 39:529–39. doi: 10.1007/s00281-017-0629-x
13. Mayer K, Nellessen C, Hahn-Ast C, Schumacher H, Pietzonka S, Eis-Hübinger AM, et al. Fatal outcome of human coronavirus NL63 infection despite successful viral elimination by IFN-alpha in a patient with newly diagnosed ALL. Eur J Haematol. (2016) 97:208–10. doi: 10.1111/ejh.12744
14. Liu Y, Yang Y, Zhang C, Huang F, Wang Y, Yuan Y, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. (2020) 63:364–74. doi: 10.1007/s11427-020-1643-8
15. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. (2001) 19:683–765. doi: 10.1146/annurev.immunol.19.1.683
16. Thevarajan I, Nguyen NHO, Koutsakos M, Druce J, Caly L, van de Sandt CE, Jia X, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. (2020) 26:453–5. doi: 10.1038/s41591-020-0819-2
17. Chu H, Zhou j, Ho-Yin Wong B, Li C, Fuk-Woo ChanK, Cheng Z-S, et al. Middle east respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. (2016) 213:904–14. doi: 10.1093/infdis/jiv380
Keywords: SARS-CoV-2, COVID-19, prognosis, leucocyte, lymphocyte, cytokine
Citation: Gan J, Li J, Li S and Yang C (2020) Leucocyte Subsets Effectively Predict the Clinical Outcome of Patients With COVID-19 Pneumonia: A Retrospective Case-Control Study. Front. Public Health 8:299. doi: 10.3389/fpubh.2020.00299
Received: 04 May 2020; Accepted: 04 June 2020;
Published: 18 June 2020.
Edited by:
Zisis Kozlakidis, International Agency For Research On Cancer (IARC), FranceReviewed by:
Catherine Ropert, Federal University of Minas Gerais, BrazilXiaojiong Jia, Harvard Medical School, United States
Copyright © 2020 Gan, Li, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunguang Yang, Y2d5YW5nLWh1c3RAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Jiahua Gan1†
Jiahua Gan1† Chunguang Yang
Chunguang Yang