- 1CICS-UBI–Health Sciences Research Centre, University of Beira Interior, Covilhã, Portugal
- 2FCS–Faculty of Health Sciences, University of Beira Interior, Covilhã, Portugal
- 3Escola Superior da Saúde, IPG–Instituto Politécnico da Guarda, Guarda, Portugal
- 4DiMeC–Department of Medicine & Surgery, University of Parma, Parma, Italy
- 5FoRST–Fondazione per la Ricerca Scientifica Termale, Rome, Italy
- 6NuESA–Health & Environment Study Group, Faculty of Health Sciences, University of Beira Interior, Covilhã, Portugal
- 7Department of Immunoallergology, CHUCB–Cova da Beira University Hospital Centre, Covilhã, Portugal
Natural mineral (thermal) waters have been used for centuries as treatment for various diseases. However, the scientific background of such therapeutic action is mostly empiric and based on knowledge acquired over time. Among the various types of natural mineral waters, sulfurous thermal waters (STWs) are the most common type in the center of Portugal. STWs are characterized by high pH, poor mineralization, and the presence of several ions and salts, such as bicarbonate, sodium, fluoride, silica, and carbonate. Furthermore, these waters are indicated as a good option for the treatment of various illnesses, namely respiratory diseases (e.g., allergic rhinitis, asthma, and chronic obstructive pulmonary disease). From the sulfide species present in these waters, hydrogen sulfide (H2S) stands out due to its abundance. In healthy conditions, H2S-related enzymes (e.g., cystathionine β-synthase and cystathionine γ-lyase) are expressed in human lungs, where they have mucolytic, antioxidant, anti-inflammatory, and antibacterial roles, thus contributing to airway epithelium homeostasis. These roles occur mainly through S-sulfhydration, a post-translational modification through which H2S is able to change the activity of several targets, such as ion channels, second messengers, proteins, among others. However, in respiratory diseases the metabolism of H2S is altered, which seems to contribute somehow to the respiratory deterioration. Moreover, H2S has been regarded as a good biomarker of airway dysfunction and severity, and can be measured in serum, sputum, and exhaled air. Hence, in this review we will recapitulate the effects of STWs on lung epithelial-immune crosstalk through the action of its main component, H2S.
Introduction
Natural mineral waters from thermal springs (thermal waters) are used in Europe since ancient Greece for hygiene and later for the treatment of several diseases (e.g., respiratory, skin, and musculoskeletal diseases). Nowadays these waters are also used beyond their conventional purposes, namely with preventive, anti-stress, and aesthetic functions. The classification of thermal waters (35–40°C) is based upon their physicochemical features, which allows their subdivision into sulfurous, salso-bromo-iodic, bicarbonate, and bicarbonate-sulfate waters (1, 2). In fact, a beneficial link between sulfurous thermal water (STWs) and clinical improvement of several illnesses has been suggested (3–6). Such beneficial effects may be due to analgesic, antioxidant (7), antibacterial (8), and anti-inflammatory (9) properties of STWs. Thus, the main advantages of the therapeutic use of STWs lies in the fact that these provide a non-aggressive treatment, without considerable side effects, and which also has preventive properties (6, 8, 10, 11). Nevertheless, knowledge associated with the clinical properties of STWs in the context of respiratory diseases is mainly empiric, acquired over a period of centuries, since few well-designed clinical studies exist. Furthermore, although there are some very interesting in vitro studies on the effects of STWs on cells of the immune system, such studies are scant. Hence, the question of how exactly these waters modulate the observed clinical amelioration is poorly understood.
In this non-systematic review, we will analyze recent and past data obtained from a number of studies that have contributed toward the elucidation of the mechanisms of action of STWs on the lung epithelia-immune interface. To do that, we have performed a compilation of PubMed publications combining the following search terms “sulfurous thermal waters,” and “hydrogen sulfide” with the terms “allergic rhinitis,” “asthma,” “chronic obstructive pulmonary disease” (COPD) “lung,” and ”lung endothelial cells” with Boolean operator “AND” and “OR.” Various combinations were used, in order to focus on specific search questions of the various analyzed topics. No limitations were made in duration of the study or the demographic data of subjects. Literature published in the last 30 years was included. The inclusion criteria in this review were studies conducted with STW, H2S-enriched waters, or H2S for airway diseases, allergic, chronic, rhinosinusitis, COPD, or biological targets and effects of H2S.
The following outcome parameters examined were included in this review: mucocilliary clearance time, nasal respiratory flow and resistance, adverse effects, and immunoglobulin values.
Overall, in the different searches performed, 7,345 studies were obtained. Of these, in total, 7,114 studies were excluded by reading the title or the abstract since they were not relevant or focused on cellular features or disease-related aspects that were not related to the topic of this review. Of the remaining 231 studies, 59 were excluded from analysis because of redundant information of lower quality than that in included studies or because the work focused on issues that were not fully relevant.
Thermal Waters: Composition and Biochemical Properties
Depending on their geographical localization, STWs present different physicochemical characteristics. These differences are due to their diverse chemical composition and to the presence of varying amounts of ions and salts, resulting in different therapeutic indications (2, 12).
Portugal is a country with abundant natural mineral (thermal) waters from north to south, as well as in the Portuguese islands, and the frequent visits to bath spas are quite common by the Portuguese population. Among the different types of thermal waters, sulfurous ones are the most common in the north and center of Portugal, with Termas das Caldas da Felgueira (13), Termas de São Pedro do Sul (14), Ferreira et al. (15), and Termas de Unhais da Serra (16) being some of the more representative ones in terms of sulfur-predominant waters. These thermal waters are alkaline (pH = 8.4–8.9), poorly mineralized, and are indicated for the treatment of respiratory, circulatory, digestive, rheumatic and musculoskeletal, as well as metabolic-endocrine diseases (12).
Concerning respiratory diseases, the therapeutic exposure to STWs is performed mostly through inhalation (6, 11, 17), and recently, significant clinical efficacy (e.g., nasal resistance and nasal flow improvement, and reduction of mucocilliary clearance time) was demonstrated when adult and elderly patients underwent hydrogen sulfide (H2S)-enriched nasal water inhalations (18). Inhalator exposure efficacy depends upon various aspects which may affect particle deposition in the airways. These include adopted nebulizer, particle size, airway caliber, and patient's breathing pattern. For instance, the nebulizer must be able to produce particles with a diameter <3 μm in order to reach the bronchioli (11). Side effects are another aspect that must always be taken into consideration, whatever the implemented therapy. Even though STWs are generally well-accepted as a safe therapeutic tool due to their low number of side effects (10), these can still occur. In a systematic review and meta-analysis, Keller et al. (19), analyzed all side effects occurring in the pooled total patient population that took part in 13 clinical studies. Focusing on sulfurous waters, after 90 days of STWs treatment, only 19 out of 370 patients presented some adverse events. From those, 13 experienced mild nasal irritations and a sensation of burning, 5 suffered from very limited epistaxis, and one from dermatological hypersensitivity. Moreover, it is of note that even when subjects presented those effects, most of them were local and reversible (19). However, despite common use STWs to achieve a state of well-being and disease amelioration, the cellular and molecular bases underlying these beneficial effects remain unclear.
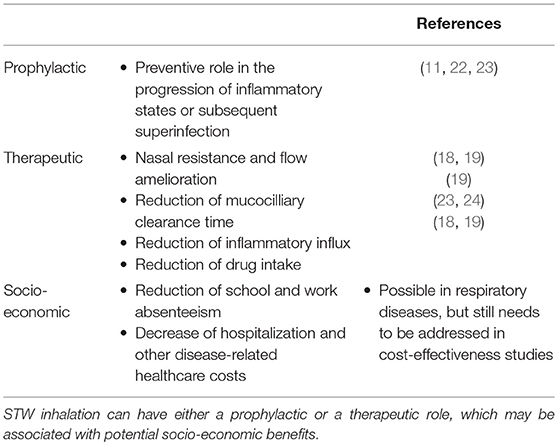
It was recently found that STWs can induce the production of moderate amounts of neutrophil-attracting chemokines, and low levels of tumor necrosis factor α and interleukin (IL)-6 (9). Even though pro-inflammatory mediators are frequently linked to detrimental situations, moderate inflammatory stress may be regarded as positive, according to hormesis theory (20). Thus, mild stress can stimulate body systems repair, in order to prevent further and more severe damage, provided that this state does not involve the accumulation of irreversible changes (20). In addition, with time, inflammation may change the composition of nasal, sinusal, and lung bacterial flora, which may be associated with the development of resistant strains of bacteria (21). This means that inadequately controlled inflammation may lead to bacterial superinfection. Thus, for subjects with inflammatory respiratory disease, which is stable or in its initial phases, STWs may be a good complement to drug therapy since they may contribute toward prevention of recurrent infections caused by various bacteria (11) and viruses (22), and subsequently avoid the progression to a chronic state. Additionally, thermal waters might be also a useful tool in post-operative recovery in cases of chronic rhinosinusitis with or without polyposis, as observed by Staffieri et al. (23). These authors detected a significant reduction of the numbers of inflammatory cells (particularly eosinophils and mast cells) in the nasal mucosa of patients who were treated with sulfurous-arsenical-ferruginous thermal water inhalation after 6 months of having undergone endoscopic sinus surgery (23). Hence, by playing a preventive role in the progression of inflammatory states, STWs may have a prophylactic effect against further inflammation or subsequent superinfection. Furthermore, as a prophylactic agent, STWs may also reduce or even avoid additional hospital costs and degradation of patients' quality of life which are associated with events, such as frequent infection-induced exacerbations or prolonged hospitalizations. Thus, either as prophylactic or therapeutic tools, STWs may not only improve patients' general health and disease-related clinical parameters, but may also show other benefits from a social and financial point of view (e.g., reduction of drug-related costs, decreased hospitalization and disease-specific healthcare costs, national health care decongestion, and a decrease in school and work absenteeism), although some of these benefits were shown in rheumatological diseases and still need to be addressed in cost-effectiveness studies of STWs treatments for respiratory diseases (see Table 1).
In thermal waters H2S, hydrosulfide ion, and sulfide anion are the most common sulfide species present, with H2S being the most abundant (7).
Biological Properties of Hydrogen Sulfide
Due to its inflammable and corrosive nature, H2S was thought to be a poisonous gas. However, in the last decades, it has been reported, along with nitric oxide and carbon monoxide, as a gaseous signaling molecule (25). Indeed, in contrast to nitric oxide, H2S is relatively stable in body fluids, appearing as a promising therapeutic agent in several diseases. Nevertheless, it is important to take into account that H2S can easily pass from water to air (26) and it is a gas which is soluble in water as well as in physiological liquids, and which is volatilized and broken down in vivo (namely in the lungs due to the abundant presence of oxygen) (27). Thus, H2S frequently mentioned in studies may indicate a mix of H2S and hydrosulfide and sulfide species, alongside with their effects (25, 28, 29).
H2S is a colorless gas with potent reducing properties resulting from geothermal activities (sulfurous mineral water and volcanoes) and found in vegetable proteins (broccoli, garlic), and synthetic compounds (NaHS, GYY4137) (28, 30–34) (Figure 1A). In the human body, H2S is produced by a variety of cells (e.g., epithelial, vascular, and smooth muscle cells), and is mainly synthesized from L-cysteine via cytoplasmic and mitochondrial cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) enzymes (30, 35). Endogenous H2S is also generated by the combined action of cysteine aminotransferase, localized in the cytosol and 3-mercaptopyruvate sulfurtransferase, present in the mitochondria (28, 30, 34, 36). Apart from these enzymatic pathways, H2S can also derive from indirect or secondary endogenous sources, particularly from glucose (via glycolysis), glutathione (GSH), inorganic and organic polysulfides, elemental sulfur, and even from bacterial sources present within the gastrointestinal and pulmonary tracts (29, 31, 33) (Figure 1B). After H2S synthesis, it can either act on its biological targets or be stored as a bound sulfane sulfur pool, through oxidative formation of hydrodisulfides/persulfides, hydropolysulfides, and polysulfides) (33, 37) as well as an acid-labile sulfur pool, through metal-sulfur clusters, free hydrosulfide ion, and persulfides (29, 34) (Figures 1C,D). In the presence of oxygen, H2S undergoes oxidation in the mitochondria by sulfide quinone reductase and also via methylation in the cytoplasm, through thiol-S-methyltransferase (30, 38) (Figure 1E). In addition, free H2S can be scavenged by methemoglobin and molecules with metal or disulfide bonds (Figure 1E), and excreted together with biological fluids (urine and flatus), as well as exhaled in breath (Figure 1F) (30, 35).
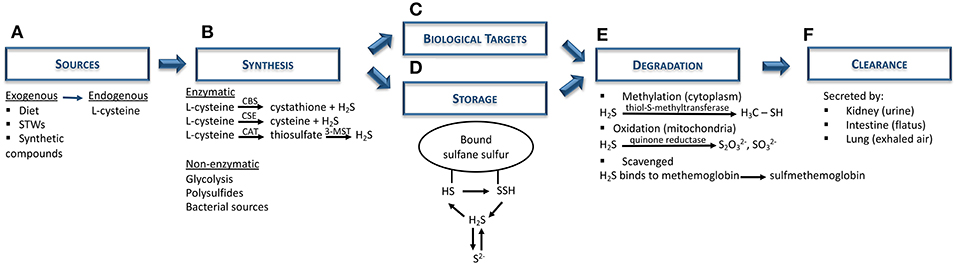
Figure 1. Hydrogen sulfide metabolism: synthesis, storage, degradation, and clearance. (A) Both exogenous (diet; sulfurous thermal waters; synthetic compounds, e.g. NaHS) and endogenous (L-cysteine) are the main sources of H2S biosynthesis. (B) H2S is synthesized mainly via CBS and CSE enzymes, although it can also be produced through the combination of CAT and 3-MST. Nevertheless, non-enzymatic sources (e.g., polysulfides, bacterial sources) can also be H2S sources. (C) Subsequently, H2S can either act on its biological targets or (D) be stored as bound sulfane sulfur and acid-labile sulfur pools. (E) In order to maintain H2S levels balanced, this gas undergoes further degradation via oxidation in mitochondria (quinone reductase) or, methylation in the cytoplasm (thiol-S-methyltransferase), or it can be scavenged by binding to hemoglobin. (F) Finally, H2S is excreted by the kidney (urine), intestine (flatus) or lung (exhaled air). CAT, cysteine aminotransferase; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; H2S, hydrogen sulfide; HS, hydrosulfide; 3-MST, 3-mercaptopyruvate sulfurtransferase; NaHS, sodium hydrosulfide; S2−, acid-labile sulfur; SSH, hydrodisulfide; STWs, sulfurous thermal waters.
Due to its high ability to diffuse across lipid membranes without the need of a transporter, H2S can easily reach its molecular targets in a variety of cells, including those in respiratory, cardiovascular, and neuronal systems (25, 28, 35, 39, 40). Its stability, storage, and release depend upon pH, temperature, and oxygen concentration of the environment. Thus, at physiological pH only a third of total sulfur amount is in the H2S form. At acidic pH, however, H2S is the only form present. In contrast, at an alkaline pH only the bisulfide form exists (28). Oxygen also influences H2S stability since the presence of oxygen induces its conversion to sulfur, which can be further oxidized to hyposulfite, sulfites, and sulfates. In other words, under aerobic conditions H2S is consumed and consequently its effective concentration decreases (29, 41). These findings corroborate the hypothesis that H2S may act as a cellular oxygen sensor (42). Indeed, the decrease in H2S oxidation under hypoxic conditions has been associated with the production of significant amounts of H2S by airway epithelial cells (AECs) (43), akin to production via reduction of preexisting sulfites in mitochondria (44). Fu et al. suggested that this occurs as a result of CSE enzyme translocation to mitochondria, thereby allowing H2S synthesis even after a stress-inducing stimulus, such as hypoxia (45). However, a significant increment of mitochondrial H2S levels and a decrease of its clearance can, therefore, lead to harmful effects, including vasoconstriction and pro-apoptotic effects (46).
The measurement of H2S has turned out to be of major importance to ascertain its putative role in a number of diseases. Although various methods have been used for measuring H2S levels in blood and plasma/serum, such as the methylene blue method, sulfite-sensitive ion selective electrodes, and others, these methods have provided discrepant results, with H2S levels in plasma ranging between 1 and 100 μM. This high variability has been attributed to the limitation and lack of sensitivity of the techniques (29, 47), to its capacity to diffuse through cellular membranes, and to the extremely short H2S half-time in vivo (48). Hence, to overcome this obstacle it is mandatory to perform precise biological measurements of H2S.
Regarding the airways, different approaches are used to quantify H2S levels. On the one hand, Saito et al. have suggested that sputum H2S may be a better biomarker than serum H2S in lung-related diseases, since its serum levels may reflect other diseases of peripheral organs (49). Moreover, induced sputum was shown to be an effective sample to assess and identify asthma subtypes (50). However, Saito et al. found a negative correlation between H2S levels in sputum and serum in COPD patients with acute exacerbations (51). This led the authors to propose that an increase in H2S levels in sputum may reflect sequestration of H2S from the circulation into the lungs, with the sputum-to-serum H2S ratio being a good predictor of exacerbations (51). On the other hand, the measurement of exhaled H2S is a non-invasive technique that is not affected by oral conditions (52) and which has been shown to positively correlate with the lung function, particularly forced expiratory volume in 1s (53). Nevertheless, unlike exhaled nitric oxide, H2S levels in exhaled air are not used as an H2S metabolism detector (54). Therefore, either exhaled or sputum H2S could become promising and accurate indicators of airway diseases and airway disease phenotypes.
Biological Targets of Hydrogen Sulfide
Most of the biological activities of H2S are exerted through protein S-sulfhydration, a post-translational modification process whereas an H2S-derived sulfur group (sulfhydryl) is added to the thiol groups of reactive cysteine residues, originating hydropersulfide (35, 55) (Figure 2).
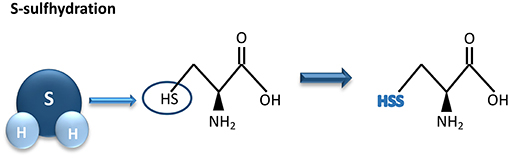
Figure 2. Hydrogen sulfide-mediated S-sulfhydration reaction. H2S adds a sulfhydril group to thiol groups of reactive cysteine residues, resulting in sulfhydration of its biological targets. H2S, hydrogen sulfide.
This formed group enhances reactivity of the protein and may be associated with an increment of its biological activity (55). The degree of protein S-sulfhydration can be influenced by cell pH as well as by the distance between the target amino acids and the active core of the protein (35). Unlike S-sulfhydration, S-nitrosylation caused by nitric oxide results in a reduction of cysteine reactivity. As an example, through S-sulfhydration the endothelial nitric oxide synthase (eNOS) activity increases, as a consequence of eNOS dimerization. In contrast, S-nitrosylation of eNOS induces the formation of eNOS monomers, leading to a reduction of its activity (56).
Thus, H2S is regarded as a gaseous signaling molecule that targets a number of important biological processes and pathways, and in this sense contributes to the maintenance of body homeostasis (35, 57, 58). The biological targets include ion channels, second messengers, signaling molecules, and transcriptions factors. Nevertheless, some of the observed H2S effects are contradictory and probably result from different cell types and concentrations used.
Ion Channels
H2S is able to interact with many ion channels and, as a consequence, alter their activity by inducing or inactivating their action. It has been reported that by sulfhydrating ATP-activated potassium channels () in smooth muscle cells (SMCs) (35, 59, 60) and small to medium calcium-dependent potassium channels in vascular endothelial cells (61), H2S can induce the opening of these channels, thereby allowing K+ ion influx, which leads to vasorelaxation.
In contrast, this molecule has the ability to inhibit big conductance calcium-activated potassium channels (35, 57, 61). Moreover, H2S also exerts a regulatory effect on intracellular calcium levels by stimulating or inhibiting the T/L-type calcium channels and in turn may up- or down-regulate several calcium-dependent signaling pathways and enzymes, depending upon cell type (35, 61, 62). Furthermore, through the reduction of inositol-1,4,5-triphosphate receptor present in airway SMCs, H2S can also affect calcium efflux in cells (62) (Figure 3A).
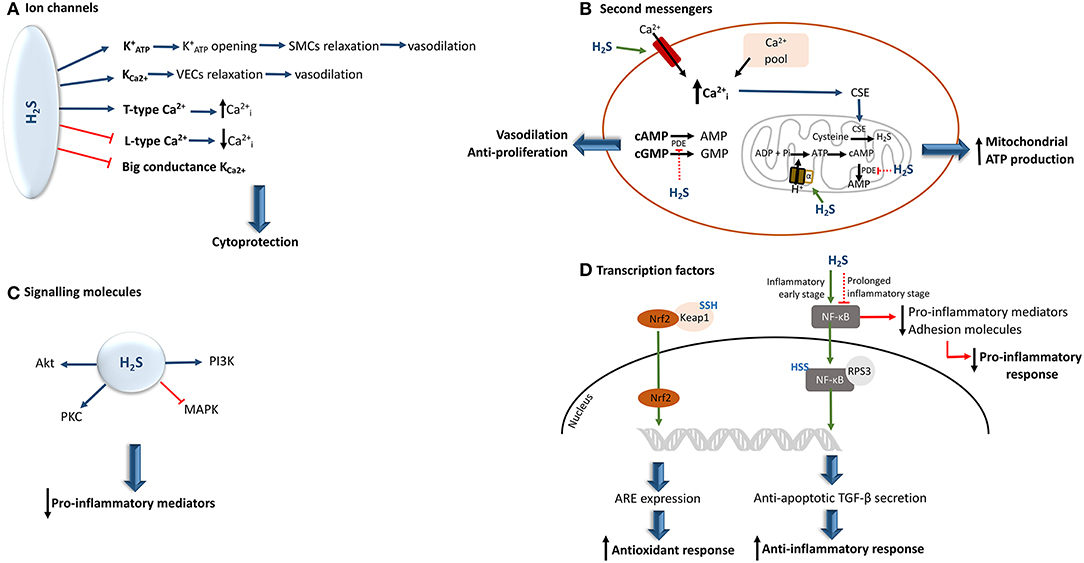
Figure 3. Hydrogen sulfide interaction and effects upon the activity of its biological targets. (A) In a physiological concentrations, H2S can either activate or inhibit different ion channels, providing a cytoprotective effect to the cell. (B) By inducing an increment of intracellular calcium, H2S induces the translocation of CSE to the mitochondria, resulting in the generation of H2S. In parallel, the H2S-mediated S-sulfhydration of the mitochondrial alpha subunit of ATP synthase, allows the augmentation of cAMP levels. This results in the increase of mitochondrial ATP production. Moreover, H2S can also inhibit PDE activity, allowing the increase of cAMP and cGMP net levels, thereby conferring vasodilatory and anti-proliferative properties (C) If, on the one hand, H2S seems to be able to prevent MAPK activation, on the other hand it increases the levels of other kinases, including PI3K/Akt, and protein kinase C, some of which can inhibit the production of pro-inflammatory cytokines. (D) The H2S-induced S-sulfhydration of Keap 1 induces the translocation of Nrf2 to the nucleus leading to the expression of ARE, which results in the augmentation of antioxidant responses. Moreover, H2S can induce NF-κB activation or inhibition. This depends upon the stage of underlying inflammation. ARE, antioxidant response elements; , intracellular calcium; CSE, cystathionine γ-lyase; , ATP-activated potassium channels; Keap 1, Kelch-like ECH-associated protein 1; MAPK, mitogen-activated protein kinase; NF-kB, nuclear factor-kappa B; Nrf2, nuclear factor erythroid-related factor 2; PI3K, phosphoinositide 3-kinase; PDE, phosphodiesterase; PKB, protein kinase B; PKC, protein kinase C; SMCs, smooth muscle cells; TGF-β, transforming growth factor-β; SSH, S-sulfhydration; VECs, vascular endothelial cells; the green arrow indicates that the pathway is stimulated and the red arrow indicates that the pathway is inhibited.
Second Messengers
Second messengers are important intracellular signaling molecules involved in several cellular pathways and their availability can be affected by H2S in a direct or indirect way.
Bucci et al. have observed that H2S has the ability to suppress the degradation of cyclic nucleotides by inhibiting the activity of phosphodiesterase, an enzyme that is responsible for their conversion into AMP and GMP (63). Thus, cAMP and cGMP availability increases, making these molecules available for intracellular signal transduction pathways that they are involved in.
The interaction of H2S with calcium ion channels directly affects calcium availability in cells. Therefore, the interaction of this gaseous signaling molecule with calcium ion channels and intracellular calcium pools, can lead to a rise in intracellular calcium by inducing its influx and release, respectively (35). This subsequently induces endothelial proliferation. In parallel, the enhancement of calcium levels can also prompt the translocation of CSE from cytosol to mitochondria, thereby stimulating H2S synthesis inside this organelle, and subsequent mitochondrial ATP production (45). On the other hand, the decrease in intracellular calcium can also be induced by H2S via inhibition of calcium channels, and in turn this suppression seems to be involved in the induction of airway smooth muscle relaxation (64). Moreover, L-type calcium channel inhibition by H2S induces membrane permeability and, subsequently, decreases elastase release (65) (Figure 3B).
Recently, it was reported that, under pathological conditions, the S-sulfhydration of the alpha subunit of ATP synthase supports its activation. In this manner, mitochondrial bioenergetics is maintained (66).
Signaling Molecules
Signaling molecules act as signal transmitters allowing the cross-talk between cells. H2S has also been reported as capable of modulating the activity of several signaling molecules involved in biological processes, including phosphorylation, oxidation and degradation of proteins.
Thus, H2S has been shown to regulate the activity of the mitogen-activated protein kinase (MAPK). In primary human lung endothelial cells, exogenous H2S was able to prevent MAPK activation (67). H2S has also been reported to increase the levels of other kinases, including PI3K/Akt, and protein kinase C, some of which can inhibit the production of pro-inflammatory cytokines (48, 57, 67–69) (Figure 3C).
Transcription Factors
Transcription factors are proteins responsible for regulating genetic information transcription and can also be a target for H2S action. Thus, by undergoing S-sulfhydration transcription factors can induce up- or downregulation of gene expression.
The inactivation of nuclear factor-kappa B (NF-κB) through S-sulfhydration, blocks its translocation to the nucleus, which leads to the suppression of pro-inflammatory mediators and production of adhesion molecules (40). Nonetheless, depending upon the specific inflammatory stage, an opposite effect can be observed (70). Thus, in an initial phase of the inflammatory response, pro-inflammatory cytokine tumor necrosis factor α (TNF-α) can induce CSE transcription, thereby enhancing H2S synthesis. Hence, the newly generated H2S sulfhydrates the p65 subunit of NF-kB, promoting its binding to the coactivator ribosomal protein S3, which results in an increment of anti-inflammatory cytokine transforming growth factor-β secretion as well as the stimulation of anti-apoptotic transcriptional activity (57, 70). However, it should be highlighted that in both situations the inflammatory response is diminished, and consequently, oxidative stress decreases.
Moreover, as a consequence of S-sulfhydration of Kelch-like ECH-associated protein 1 (a negative regulator of factor erythroid-related factor 2 activity), the nuclear factor erythroid-related factor 2 is then translocated to the nucleus. This results in the expression of antioxidant response elements (68, 71) (Figure 3D).
Consequently, S-sulfhydration appears to play an important role in protecting cellular damage, since it has vital implications in anti-inflammatory as well as in antioxidant defenses.
Biological Effects of Hydrogen Sulfide in the Lung and Associated Immune System Cells
The respiratory mucosal epithelium is the first internal line of defense by acting as a major physical barrier between internal and external environments. The airway surface liquid (comprising mucus and periciliary liquid layers) covers this epithelial surface made up of goblet cells, ciliated cells, basal cells, and club–Clara cells (72–74). It is produced by the epithelial cells, which are also in contact with respiratory gases, thereby constituting the air-liquid interface (75). A connective tissue fibroblast-rich layer is located underneath the epithelial surface, and is involved in the maintenance of tissue homeostasis and wound healing (76). This cell layer also contains a variety of cell types, including cells of the innate and adaptive immune system (76). In this layer is located another lung barrier formed by endothelial cells, which separate the bloodstream and vessel walls. The endothelium also has other functions (e.g., blood and oxygen supply, nutrient delivery, and immune cell trafficking) (75, 76).
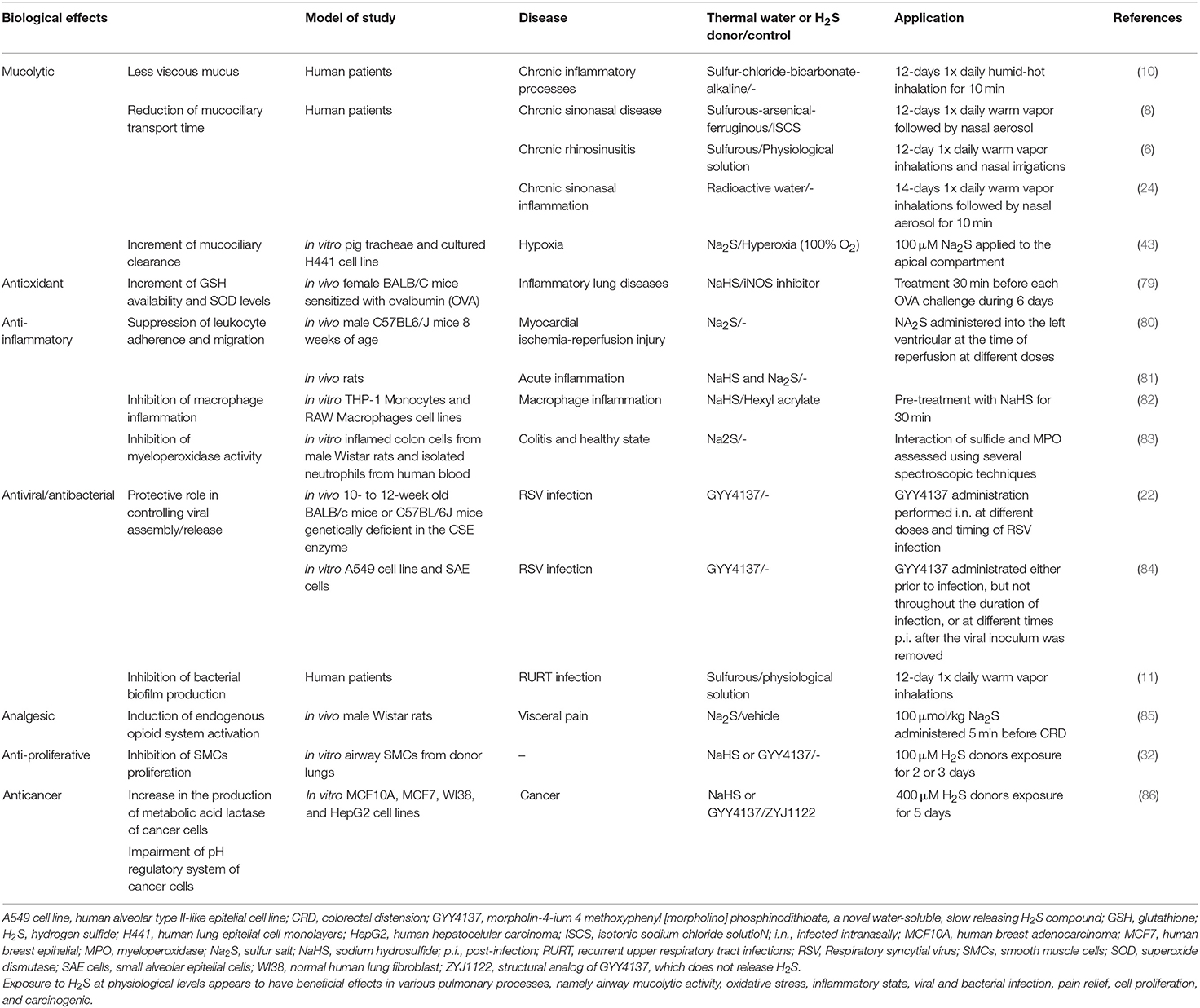
In physiological conditions, H2S-related enzymes are expressed in the human lungs, namely in SMCs and primary fibroblasts (60, 77). It is now acknowledged that H2S is required for the development of lung vasculature and alveolarisation (78), and in other lung functions, including airway tone and pulmonary circulation (30). Furthermore, H2S appears to be involved in various processes namely airway mucolytic activity, oxidative stress, inflammatory state, cell proliferation, and apoptosis (10, 30). These will be briefly reviewed below (see also Table 2).
Mucolytic Effect
Mucins are secreted by goblet cells, submucosal glands in upper airways (75, 87), and alveolar cells in lower airways (74). By secreting mucins, the mucus traps and absorbs potential harmful pathogens and irritants which are subsequently removed from the respiratory tract by the process of ciliary beating which mediates appropriate mucociliary clearance (73). An effective removal of mucus protects the respiratory epithelium, acting as a vital role in airways homeostasis (88). Both endogenous and exogenous H2S show positive effects upon the respiratory tract by modulating the mucolytic activity. This appears to result from the interactions between H2S and the disulfide bonds of mucins, resulting in breakage of the latter, which allows the mucus to become less viscous (10). The production of endogenous H2S induces the opening of and the activation of the cAMP pathway (30). Additionally, exogenous H2S inhibits Na+/K+-ATPase and calcium-sensitive potassium channels in human bronchiolar epithelia, thereby triggering electrolyte absorption (33, 89).
This leads to an increase in mucociliary clearance, and therefore the elimination of foreign microorganisms can be more effective (43). Krause et al. also proposed that inhibition of transepithelial sodium absorption (via inhibition of Na+/K+-ATPase) is favored under acute hypoxia, in order to avoid H2S degradation, as well as exogenous H2S exposure (43). Additionally, an amelioration of mucociliary function was observed with inhalation of exogenous H2S, as confirmed by a substantial reduction of mean mucociliary transport time in patients with chronic rhinosinusitis (8, 24).
Antioxidant Effect
In a well-controlled environment, reactive oxygen species (ROS) are responsible for cell signaling activation, enhancement of pro-inflammatory cytokines acting as mediators of immunity, and in addition, ROS are also able to protect the cells/tissues. Simultaneously, a balance is achieved with a tight regulation performed by antioxidants (e.g., reduced glutathione, GSH) which directly scavenge those species that are formed, thereby inhibiting any excessive production, by removing or repairing cellular damage or modifications induced by these reactive species.
In the lungs, exposure to H2S promotes a boost of antioxidant effects, such as (i) increase in GSH availability, and superoxide dismutase levels (28, 79, 90), and (ii) generation of ATP, replacing oxygen in mitochondrial respiration (91). The reported H2S-induced mitochondrial protection related to this antioxidant effect is known to result from mitochondrial cytochrome c oxidase inhibition and also from its capacity to modulate cellular respiration, thereby preventing the generation of ROS (48). Consequently, there is an increase in scavenging capacity (30, 34, 92), as well as an induction of endogenous antioxidant defenses (39). Hence, H2S antioxidant features seem to involve both an indirect action and an induction of endogenous antioxidant defenses. For instance, by stimulating cysteine and cysteine transporter activity, H2S induces an augmentation of substrate levels that are necessary for GSH production (29). Unlike large size antioxidants, H2S can easily cross both plasma and mitochondrial membranes. This allows H2S to more promptly reach its biological targets, and therefore it is considered to be more effective at diminishing cellular oxidative stresses, and at increasing antioxidant defenses (80). Braga et al. demonstrated that antioxidant effects of STWs, an exogenous H2S source, provide protection against oxidative DNA damage (93).
Anti-inflammatory Effect
As the first line in contact with foreign species, AECs can act either as a modulator (during homeostasis) or as a sensor (post-injury lung homeostasis) of innate and adaptive immune systems (74). In healthy states, macrophages are the main immune cell type present in the airways, accounting for about 60–70% of all cells. Neutrophils represent 30–40% of total cells, while eosinophils are rare, not exceeding 2% (94). Beneath the epithelial layer, dendritic cells, macrophages, and mast cells can be found in the lamina propria. Dendritic cells and macrophages, in particular, can detect pathogens that have crossed the lung epithelium, phagocytose and destroy by microbicidal mechanisms, thereby contributing toward avoidance of significant infections.
Under a normal state the recognition of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) occurs through specific membrane or cytosolic receptors present on epithelial cells, and results in the activation of several signaling pathways (e.g., via MAPK, NF-κB, among others) (95–97). Therefore, pro-inflammatory cytokines, chemokines, antimicrobial proteins, and antiviral substances are produced and released by AECs, which allows them to have a crucial role in the recruitment and activation of innate (e.g., macrophages, eosinophils, dendritic cells) and adaptive (e.g., T and B cells) immune cells (96, 97). The presence of these cells allows several responses (phagocytosis, dendritic cell maturation, chemotaxis, inflammasome activation, and others) to be triggered (97). Subsequently, cells of the adaptive immune system (e.g., T and B cells) are activated. Thus, when homeostasis is affected, the usual function of AECs is reduced and pro-inflammatory activities increase significantly.
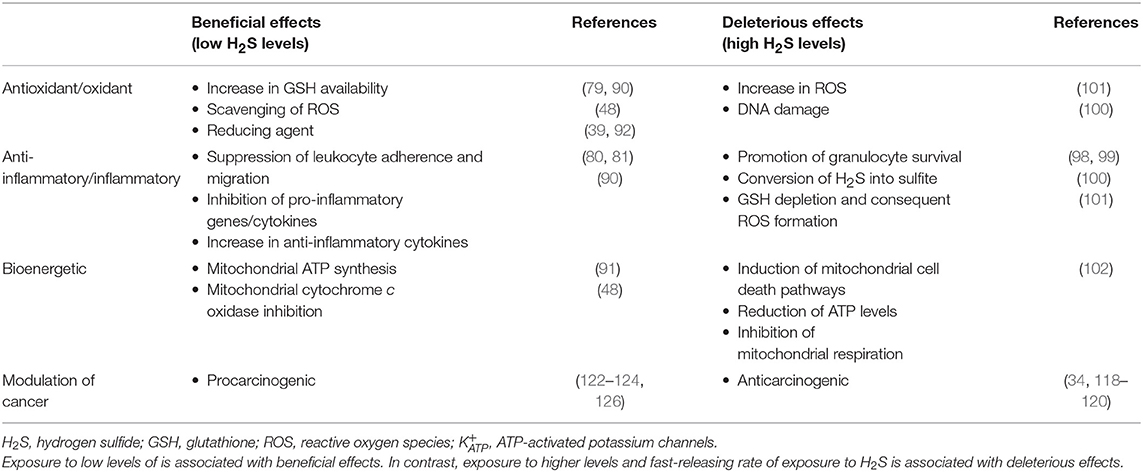
The anti-inflammatory properties of H2S can in part be explained by its potent reducing, antioxidant, and scavenging features. Nevertheless, there are controversial results concerning H2S properties, since it appears to exert both pro- and anti-inflammatory effects. Some authors have shown an in vitro promotion of granulocyte survival via H2S-induced inhibition of caspase-3-cleavage and p38 phosphorylation (98, 99) and in an oxidative stress environment, activated neutrophils seem to be able to convert H2S into sulfite, which is associated with inflammation (100). Moreover, there are studies suggesting that this gaseous transmitter is involved in GSH depletion and ROS formation (101), and consequently induction of mitochondrial cell death pathways (102). Meanwhile, others have suggested the participation of H2S in some key anti-inflammatory pathways, such as: (i) suppression of leukocyte adherence and migration, mediated by activation in endothelial cells and leukocytes (81), (ii) inhibition of oxidized low-density lipoprotein-induced macrophage inflammation via NF-κB suppression (82), leading to a reduction of several pro-inflammatory cytokines (e.g., IL-1β, IL-6, and IL-8) (103–105), and (iii) reduction of neutrophil toxic effects by inhibiting myeloperoxidase activity (83). However, these discrepancies may be related to differences in H2S concentration. In fact, beneficial effects of H2S generally prevail at lower concentrations, whereas deleterious effects are observed at higher levels and at fast-releasing rates, which is comparable to effects seen with carbon monoxide and cyanide (28, 34, 48). In addition, inhalation of H2S at high acute levels or chronic lower-level exposure can also induce lung inflammation and toxicity (48).
Nonetheless, it has been known for some time that STWs can exert a direct anti-inflammatory effect in the lung. Exposure to these natural mineral waters may induce increased release of IL-10 levels by in vitro exposed primary human monocytes (9). Furthermore, an increase in the levels of IL-10 in saliva were also described in patients who had been treated with STWs (9). Moreover, STWs can simultaneously up-regulate immunoglobulin (Ig) A (an anti-inflammatory immunoglobulin) levels, as well as down-regulate the levels of IgE (a pro-inflammatory immunoglobulin) and cytokines secreted by eosinophils (90). This suggests that by stimulating anti-inflammatory defenses, STWs decrease the formation of ROS, and therefore modulate the pro-inflammatory state.
Antiviral and Antibacterial Effects
In several pulmonary diseases (asthma, COPD, among others) recurrent respiratory tract infections may occur due to a fragile protective mechanism system, which may enhance a pro-inflammatory state (106). As these diseases progress, viral and bacterial infections may become more frequent, and increasingly more difficult to treat. Consequently, the patients' health state worsens and their quality of life decreases.
Using AECs, Chen et al. shown a link between CSE inhibition and a significant increase in viral replication and chemokine secretion, which are reduced when these cells are exposed to a slow-releasing H2S donor (107). Serum H2S levels are significantly decreased in COPD subjects with very symptomatic exacerbations induced by bacteria and viruses (Type I; Anthonisen criteria), compared to control subjects. However, when COPD exacerbations are less symptomatic (Type II or III; Anthonisen criteria), serum H2S levels are higher than those in control subjects (107). Moreover, when COPD patients who exacerbated are treated with antibiotics, serum H2S levels are lower than in those patients who did not require antibiotic treatment. Taken together, these results indicate that endogenous H2S synthesis is induced in order to counter the infections-mediated exacerbations (107). Currently, the most frequent therapies used to treat lung viral and bacterial infections are antiviral and antibiotics. However, over time, antibiotic-resistant bacteria may be increasingly observed.
To overcome these problems, some studies focused on the clinical efficacy of therapeutic S-based compounds (e.g., H2S donors, STWs) (22, 84). Previous data showed that these compounds exhibit a protective role as an antiviral agent since they were able to control viral assembly/release (84). In turn, an improvement in viral infection-induced airway hyperresponsiveness can be prompt and may be associated with a decrease in the expression of pro-inflammatory mediators as well as in inflammatory cell influx into the lung (22). Furthermore, a study where patients with chronic sinonasal disease inhaled STWs showed a significant nasal flow improvement in these patients, which was associated with lowering the numbers of nasal bacteria (8). Also, a study in children with frequent upper respiratory infections showed an important reduction in frequency, duration, severity, and social impact of the infectious episodes with STWs treatment (11). This antibacterial effect may be due to H2S toxicity since only sulfur-bacteria and some microorganisms survive in the presence of S-based compounds, such as those present in STWs (108). This anti-pathogenic role also seems to be linked to the inhibition of bacterial biofilm production, as a result of blockage of the synthesis of microbial adhesins, thereby interfering with bacterial adherence to epithelia and reducing pro-inflammatory potential (6). Also, Varrichio et al. observed a significant reduction of bacteria in children with respiratory infections treated with salso-sulfide thermal water (17). In this context, Benedetti et al. proposed NF-kB pathway as a target of H2S donors to reduce bacteria-induced inflammation (40).
Thus, S-based compounds can be a good complementary approach to conventional drug therapy, thereby increasing clinical efficacy of these treatments.
Analgesic Effect
Whether and how exactly H2S can modulate an anti-nociception effect remains to be clarified. In patients with perennial allergic rhinitis (AR), a previous study involving inhalation of heated water showed that heat therapy may contribute to relief of perinasal pain and can somehow prevent nasal congestion (109). However, in this study, the heated water was not described in terms of H2S content. Nevertheless, hyperthermia acts as a stimulus of neuroendocrine responses, with an increase in opioid (β-endorphin and Met-encephalin) and adrenocorticotropic hormone levels (110, 111). As mentioned previously, the main compound present in STWs is H2S, and since this therapy is usually performed through inhalation at a temperature between 36 and 38°C (13, 14, 16), STWs therapy can somehow mediate an analgesic effect in respiratory diseases, mainly in the upper airways, during treatment. This can be explained in part, by the high temperatures of STWs but also by the role of H2S in the activation of . In fact, some studies have suggested that the H2S-mediated analgesic effect is based upon the opening of neuronal , which in turn is related with endogenous opioid system activation (85, 112).
Anti-proliferative Role
In the lung, cAMP and cGMP are responsible for mediating endothelium-dependent dilatation, since they are involved in lung vascular homeostasis. In fact, a reduction in their levels may lead to pulmonary hypertension, a disease characterized by high blood pressure that affects the lungs' arteries which become narrowed, blocked or destroyed (113). Since H2S inhibits the activity of phosphodiesterase, cGMP net levels increase (63). Therefore, H2S might eventually act as a good auxiliary agent in the treatment of pulmonary hypertension by preventing the proliferation of vascular SMCs and consequently promoting vasodilation of lung blood vessels.
Perry et al. proposed that both endogenous and exogenous H2S might be able to control airway SMCs proliferation and cytokine release, namely IL-8, by suppressing these cell types. This occurs through inhibition of extracellular signal-regulated kinase 1/2 phosphorylation and extracellular signal-regulated kinase-1/2 p38 MAPK, with CBS, and not CSE, the main enzyme involved in the endogenous H2S production (32). Although H2S inhibits the proliferation of SMCs, it promotes the growth of endothelial cells (60, 114).
The inhibitory effect upon cell growth induced by exogenous H2S was also observed by a reduction in lymphocyte subset proliferation, with a subsequent decrease in T cell-derived IL-2 production (115). Similar results were obtained in an in vitro study using STWs (116). This anti-proliferative effect was also shown by Baskar et al. when exogenous H2S exposure caused human lung fibroblast cell death by inducing an increase in DNA lesions in these cells (102). In parallel, cell cycle regulators (p53, p21, and Ku proteins) were activated, being responsible for cell death via apoptosis (102). Therefore, the anti-proliferative effect prevents further degradation of DNA and allows the removal of injured cells by a controlled cell death process. In accordance with this, H2S has been shown to exert a direct suppression effect on human lung fibroblast migration, proliferation, and transdifferentiation into myofibroblasts (117).
Anticancer Effect
During the last decade, some data have suggested various anticancer effects related to H2S action (118–121). It was proposed that H2S-mediated anticancer role is based upon the combined capacity of H2S to increase the production of metabolic acid lactase and to impair pH regulatory system of cancer cells (86).
However, in contrast, and despite the anticancer efficacy in various types of cancer cells (e.g., gastric carcinoma, renal tumor), other studies have shown that H2S may induce tumor cell proliferation, and migration, as well as angiogenesis, via inhibiting apoptosis (122–124). Jia et al. observed hepatoma cell suppression when CBS/H2S pathway was inhibited (125). Moreover, Szczesny et al. have also found that besides normal lung epithelial cells, epithelial lung adenocarcinoma cells also have the ability to synthesize H2S, in order to maintain their bioenergetics function and to increase its mitochondrial DNA repair processes. In this sense, cancer cell viability was preserved (126).
Hence, in a cancer situation the type of treatment must be re-assessed since H2S seems to be advantageous for cancer cell proliferation. Nevertheless, the H2S-induced inhibitory effects on cancer cell proliferation depend not only on H2S dose and length of exposure, but also on tumor types. In this context, a continuous and prolonged exposure to low H2S concentrations significantly affects cancer cell survival activity (127). In turn, with continuous, high-level exposure to H2S, the cell survival rate is severely affected in both cancer and non-cancer cells (128). Oláh et al. have also indicated varying H2S levels as an explanation for these inconsistent findings regarding anticancer effect of both H2S inhibitors and donors (129). Nevertheless, these authors suggested that if on the one hand optimal endogenous H2S concentration may induce proliferation of cancer cells, on the other hand both endogenous H2S inhibition (decrease of its levels to below optimal concentration) and exogenous delivery (increment of its levels to above optimal concentration) may suppress cancer cell proliferation (129). Clearly, H2S-mediated effects have to be further studied, namely with various cancer cells lines in vitro as well as in animal models of various cancers, before any firm conclusions can be drawn.
It should also be taken into account that H2S may have a biphasic biological effect, since at low-to-moderate concentrations H2S has a physiological role, whereas at higher concentrations, it exerts a more pathological role, as summarized in Table 3.
Effects of Hydrogen Sulfide in Respiratory Diseases
The human nose and lung are continually exposed to indoor and outdoor agents (e.g., allergens, tobacco smoke, and pathogens). As a result, the airway mucosal epithelium, as an internal line of defense needs to play numerous roles in order to eliminate these foreign agents, thereby allowing normal airway function. On the other hand, after chronic injury and inflammation, the dysregulation of airway epithelial cell function may lead to the pathogenesis of lung diseases, such as AR, asthma, and COPD. These airways diseases are characterized by frequent or persistent respiratory symptoms and intermittent or persistent airflow limitation (130, 131). Moreover, besides the involvement of inflammatory cells and oxidative stress in the pathogenesis of AR, asthma, and COPD, there are various studies suggesting that these patients present alterations in H2S metabolism (132–137).
Allergic Rhinitis
AR is a chronic inflammatory disease that affects the nasal airways, which become inflamed and engorged after exposure to an allergen to which patients are sensitized. In the nasal mucosa of AR patients, the relevant allergens bind to membrane-bound IgE on mast cells. Cross-linking of various IgE molecules by the allergen induces mast cell activation with rapid release of various pro-inflammatory mediators which are involved in development of acute symptoms. Eosinophils and lymphocytes are also involved, being crucial to the development of chronic symptoms (138, 139). In fact, this inflammatory process disturbs some features of the nasal mucosa, since inflammation is associated with swelling of sinusoidal capacitance vessels, reduction of nasal airway passages size, and an increase in mucus production (140). In addition, nasal airway remodeling may also take place in AR, although whether this really occurs remains controversial. In fact, some studies claim that remodeling is involved, since structural changes (e.g., nasal tissue glandular hypertrophy, collagen or extracellular matrix deposition) were observed, as compared with healthy controls (141, 142). However, in contrast other studies have not found such an association (143, 144).
Park et al. have shown that H2S can be found in human nasal mucosa as well as in the plasma of healthy subjects. This may be partially explained by the presence of CBS and CSE in human nasal epithelium (134). While CBS is mainly distributed in the superficial epithelium and submucosal glands, CSE is exclusively localized in vascular endothelium and surrounding smooth muscles (133, 134). In the nasal and sinus mucosa, the amount of H2S was shown to be increased in mild and moderate/severe persistent AR (134). In this context, there is an enhancement of human H2S-synthesized enzymes, mRNA and protein levels, which consequently leads to a significant increment of H2S levels in human airway (134). This may indicate a compensatory mechanism to attempt to revert the pro-inflammatory state.
Although there are few data specifically regarding STWs-treatment of AR, an amelioration in AR patients suffering from allergen-specific non-seasonal rhinitis when treated with a S-based compound water was observed, and this was associated with a reduction in total IgE and an increase in IgA serum levels (145). In accordance with this, it was suggested that STWs may exert an immunomodulatory activity by inducing an increase in IgA levels in nasal mucus (90). Another study also showed significant amelioration when AR patients were treated with STWs, namely in terms of a significant decrease in nasal flow resistance and nasal mucociliary transport time in 84% of subjects (146). Likewise, a significant reduction of IgE and an increase in IgA levels as well as an improvement of subjective symptomatology assessment scale were also observed (146). Data from these studies suggest a compensatory mechanism in order to reduce the presence of pro-inflammatory mediators and an improvement of the inflammatory state. However, clearly more thorough studies on the immunomodulatory and anti-inflammatory effects of STWs treatments in AR patients (as well as in patients with chronic rhinosinusitis) are needed.
Due to common immunopathophysiology, there is a close relationship between AR and asthma (“single airways concept”). In fact, AR is regarded as a risk factor for the development of asthma (147). Thus, although few studies have been carried out with STWs treatment in patients with asthma, we shall now analyse this context.
Asthma
Asthma is a heterogeneous disease, usually characterized by chronic airway inflammation. Asthma is thus regarded as a long-term inflammatory disorder that results in excessive smooth muscle contraction, hyper-responsiveness, and variable airflow obstruction and bronchospasm. The development and progression of asthma are associated with airway inflammation (inflammatory cells and cytokines), mitochondrial dysfunction, and oxidative stress (148). Excessive airway mucus production is a feature of asthma, particularly in more severe stages, and mucus may even block the airways, which may lead to death by suffocation (149). The difficulty in controlling asthma is partially explained by its clinical and cellular phenotype heterogeneity. Thus, asthma patients may be grouped into different clusters which are associated with preferential features. In this context, one partial but possible subdivision of patients may be into those who preferentially present an eosinophilic bronchial infiltrate and those that have a preferentially neutrophilic profile (150, 151). In addition, it is fundamental to remember that AECs and SMCs are also some of the major cell types involved in asthma pathology, since they are sources of excessive mucus production and the mediators of cell contraction, respectively. This is supported by the underlying episodes of bronchial smooth muscle contraction in asthma patients (152). Likewise, both AECs and SMCs are responsible for facilitating the amplification of lung inflammation, by recruiting T cells, which contributes to an uncontrolled pro-inflammatory environment. All of these actions are associated with histological changes in the airways (e.g., increased bronchial wall thickness, mucous metaplasia, and smooth muscle hyperplasia and hypertrophy) which may lead to the impairment of pulmonary function (153).
In human studies, a significant decrease in H2S serum levels was shown in patients with stable asthma, in comparison with healthy subjects. Such a decrease was even more pronounced in asthmatic patients with severe acute exacerbations. It should be pointed out that a similar type of reduction in H2S serum levels was also observed in smokers compared to non-smokers. Moreover, such a decrease was negatively correlated with the percentage of sputum neutrophils in patients with acute asthma (137). Furthermore, both serum H2S levels and lung function parameters were found to be decreased in asthmatic children, suggesting a positive correlation between these two parameters (136). In contrast, Saito et al. found elevated H2S levels in the sputum of asthmatic subjects, but without significant differences between mild and severe stages (49). These authors also observed a positive correlation between sputum H2S levels and sputum neutrophil counts. Nevertheless, serum H2S levels presented a negative correlation with sputum macrophage (49) and eosinophil counts (53).
Previous data have also shown that H2S donors induce vascular smooth muscle relaxation, while they suppress the proliferation of airway SMCs and IL-8 release in humans (32). Since airway SMCs express high amounts of H2S-related enzymes, it appears that the lack of H2S may be involved in the progression and worsening of asthmatic airway obstruction.
However, exposure to environmental H2S levels in excessive concentrations may have deleterious effects in asthma patients. In an epidemiological study carried out in northeast Nebraska (USA), a positive correlation was observed between the frequency of hospital visits by pediatric and adult asthma patients, due to exacerbations, and high (>30 ppb) outdoor total reduced sulfur and/or general H2S levels on the previous day (154). However, it should be noted that actual individual levels of exposure to these sulfur species were not recorded but only inferred. In contrast, Bates et al. did not observe a significant increase in the risk of development of symptoms in asthmatic subjects when exposed to H2S from geothermal activity in New Zealand (155). This suggests that, in certain settings, or in prolonged ambient exposure inhalation of high levels of environmental sulfur species may trigger asthma exacerbations, although this must be studied with more robust study designs and compared with intermittent and controlled exposure which is the situation observed in Spas.
Chronic Obstructive Pulmonary Disease
COPD is an airways disease caused by significant exposure to noxious particles or gases. The chronic, persistent, airflow limitation associated with COPD is caused by a mixture of small airways disease (e.g., obstructive bronchiolitis) and parenchymal destruction (emphysema) (131). Thus, COPD is characterized by an irreversible persistent decline of airflow associated with an enhanced chronic inflammatory response and emphysematous changes (156). It is well-known that COPD non-smokers and smokers share epithelial susceptibilities features, with direct or indirect cigarette smoking exposure being the main trigger for the development of this disease. In addition, most COPD patients have other chronic co-morbidities, with a proportion of them also having features of asthma–potential Asthma-COPD overlap/ACO (157, 158).
COPD is well-characterized by severe lung structural and functional changes, namely basal, goblet and mucous cell hyperplasia, as well as airway fibrosis (159, 160). Furthermore, a chronic influx of inflammatory cells (T lymphocytes, neutrophils, and alveolar macrophages) in bronchial wall and lumen is also involved in the pathophysiology of the disease (161). As a result of the pro-inflammatory actions, particularly those induced by neutrophil-derived enzymes, the lung parenchyma is destroyed in many cases, leading to emphysema (106, 161, 162).
In a small study performed by Sun et al. using human peripheral lung tissue samples from six patients with COPD, as well as from eleven healthy non-smokers and seven smokers with normal lung function, despite H2S levels being quite similar among the groups, a significant decrease in CSE protein levels and an increase in its mRNA levels was observed in COPD patients as well as in smokers (135). However, in contrast, CBS mRNA levels were reduced in COPD patients as well as in smokers, suggesting that H2S metabolism may be altered in the lung tissue of COPD patients, just as it is in smokers (135). Moreover, the same group showed a negative correlation between exhaled levels of H2S and sputum eosinophilia, in a larger group of 77 COPD patients, suggesting that increased levels of exhaled H2S may predict a non-eosinophilic phenotype in these patients (163). Nevertheless, temporal stability of such phenotypes must be confirmed, since it may vary over time. In fact, Chen et al. also showed that serum H2S levels in COPD subjects vary longitudinally, with higher levels being more frequently seen in a stable state than in acute exacerbations of COPD (132). Both in healthy subjects and in COPD patients with acute exacerbations, H2S levels were more significantly reduced in smokers than in non-smokers. Also, in this study, regarding serum H2S levels, a negative correlation with sputum neutrophils, and a positive association with sputum lymphocytes and macrophages was observed in all patients with COPD. Finally, it seems that H2S availability is also related to the stage of airway obstruction in COPD since its levels are significantly decreased in the more advanced stage III than in the milder stage I (132). Thus, the authors justify the enhancement of serum H2S levels in stable COPD patients as a compensatory mechanism. Nevertheless, increased levels of H2S were observed in COPD subjects with an exacerbation, in comparison with patients with stable COPD, healthy smokers, and non-smokers (51). Despite certain contradictions founded in the previous studies, all indicated significant changes of H2S and H2S-synthesized enzymes levels in COPD subjects. These H2S metabolism alterations seems to contribute, at least in part, to exacerbations and worsening of this respiratory disease state, affecting general lung function.
A significant reduction of oxidative stress and an amelioration of symptoms in subjects suffering from moderate to severe COPD were observed after a 12-day inhalation with STWs and 1 month after the end of the treatment (92). Similar beneficial effects have been observed in patients with chronic rhinosinusitis (6). Further studies focusing on mechanisms underlying such STWs-driven improvement in COPD patients are warranted.
In summary, in spite of generally effective drug-based treatments for most cases of AR, bronchial asthma, and COPD, some patients still show a sub-optimal response to such treatments. Furthermore, over time, long-term high-dose therapy may be associated with the development of some adverse effects. Thus, additional thermal spa complementary therapeutic tool mainly for subjects whose symptoms are not adequately controlled with the usual drug-based therapeutic approach seems to be a good option. With supplementary STWs treatment it may be possible to regain symptom control and eventually reduce baseline drug therapy. Another aspect which must be borne in mind is that with STWs treatment at Spas, additional psychological components may also contribute toward clinical improvement. These components include leisure time, opportunity for relaxation, being aware of regular clinical monitoring, and various cultural aspects, all of which may play a part in Spa treatment-associated final results (164).
Conclusions and Future Perspectives
Mainly due the presence of H2S, STWs might be an advantageous and promising option as an add-on non-pharmacological complementary therapy for respiratory diseases, such as AR, chronic rhinitis/rhinosinusitis, bronchial asthma and COPD, since these natural mineral waters have been associated with significant, quick onset, and relatively long-lasting improvement of clinical parameters in patients with these diseases. Furthermore, H2S-rich STWs have various effects upon inflammatory and immunological parameters that may contribute toward their clinical efficacy. It may thus be possible to use STWs in a preventive way in terms of disease progression and exacerbations, although this needs to be better ascertained. Moreover, if correctly applied, no significant side effects have been reported with STWs. Nevertheless, in spite of the potential benefits of STWs in respiratory diseases, further elucidation of the mechanisms underlying such benefits warrants further studies. To that end, in vitro models appear to be a promising choice for studying the effects of STWs on human respiratory mucosa. Recently, Epithelix Sàrl (Switzerland) and MatTek Corporation (USA) have developed an in vitro 3D human lung models with a morphologically and functionally differentiated structure as well as an ability to be maintained in a homeostatic state for a long period of time and to be a ready-to-use model (165, 166). In addition, this 3D cell model can also be used to perform safety testing of occupational and environmental chemicals, pharmaceutical development, drug delivery, and inflammatory responses (167–172). Overall, since these in vitro 3D models seem to reproduce human biological responses, they are an interesting choice in the study of various respiratory diseases, such as AR, asthma, and COPD, namely in terms of their response to STWs exposure, and this is one of the current interests of our research, which is in accord with current ethical concerns regarding the use of animals in research investigations, i.e., the 3R (Replace, Reduce, Refine) and animal welfare.
Contribution of this Review to the Field
This review is novel andignificantly contributes toward having a clearer and more organized view of the role of sulfurous thermal/natural mineral waters (STWs), in inhalational treatment of respiratory diseases, such as rhinitis, asthma or chronic obstructive pulmonary disease. Furthermore, this review also recapitulates the main effects of STWs on lung epithelial-immune crosstalk through the action of its main component, H2S, thereby establishing a relationship between clinical efficacy of these treatments and the underlying immunopathological and immunotherapeutic mechanisms.
Author Contributions
JV and AE carried out literature searches and wrote the manuscript. EC checked the biochemical aspects. FA checked the immunological aspects. MV and LT-B checked the clinical aspects. All authors made contributions to the review. EC, FA, MV, and LT-B checked the final version of the manuscript.
Funding
This work was supported by CENTRO 04-3928-FEDER-000010, financed by PROVERE, CENTRO 2020, Portugal 2020, and FEDER and National Funds by FCT–Foundation for Science and Technology (Project UID/Mul]/00709).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
STWs, Sulfurous thermal waters; H2S, Hydrogen sulfide; IL, Interleukin; COPD, Chronic obstructive pulmonary disease; MAPK, Mitogen-activated protein kinase; NF-κb, Nuclear factor-kappa B; AR, Allergic rhinitis.
References
1. Ciprandi G, Cristofolini M, Mira E. Comano thermal water inhalations in the treatment of allergic rhinitis: preliminary results. Eur Ann Allergy Clin Immunol. (2016) 48:220–3.
2. Portugal TSO. Classification of Waters. (2018). Retrieved from http://www.termasdeportugal.pt/classificacao/ (accessed October, 2017).
3. Costantino M, Filippelli A, Quenau P, Nicolas J-P, Coiro V. Sulphur mineral water and SPA therapy in osteoarthritis. Therapie. (2012) 67:43–8. doi: 10.2515/therapie/2012002
4. Kovács C, Pecze M, Tihanyi Á, Kovács L, Balogh S, Bender T. The effect of sulphurous water in patients with osteoarthritis of hand. Double-blind, randomized, controlled follow-up study. Clin Rheumatol. (2012) 31:1437–42. doi: 10.1007/s10067-012-2026-0
5. Mirandola P, Gobbi G, Malinverno C, Carubbi C, Ferné F, Artico M, et al. Impact of sulphurous water politzer inhalation on audiometric parameters in children with otitis media with effusion. Clin Exp Otorhinolaryngol. (2013) 6:7–11. doi: 10.3342/ceo.2013.6.1.7
6. Salami A, Dellepiane M, Strinati F, Guastini L, Mora R. Sulphurous thermal water inhalations in the treatment of chronic rhinosinusitis. Rhinology. (2010) 48:71–6. doi: 10.4193/Rhin09.065
7. Braga P, Sambataro M, Dal Sasso M, Culici M, Alfieri M, Nappi G. Antioxidant effect of sulphurous thermal water on human neutrophil bursts: chemiluminescence evaluation. Respiration. (2008) 75:193–201. doi: 10.1159/000107976
8. Staffieri A, Abramo A. Sulphurous-arsenical-ferruginous (thermal) water inhalations reduce nasal respiratory resistance and improve mucociliary clearance in patients with chronic sinonasal disease: preliminary outcomes. Acta Otolaryngol. (2007) 127:613–7. doi: 10.1080/00016480600951525
9. Prandelli C, Parola C, Buizza L, Delbarba A, Marziano M, Salvi V, et al. Sulphurous thermal water increases the release of the anti-inflammatory cytokine IL-10 and modulates antioxidant enzyme activity. Int J Immunopathol Pharmacol. (2013) 26:633–46. doi: 10.1177/039463201302600307
10. Costantino M, Lampa E, Nappi G. Effectiveness of sulphur spa therapy with politzer in the treatment of rhinogenic deafness. Acta Otorhinolaryngol Ital. (2006) 26:7–13.
11. Salami A, Dellepiane M, Crippa B, Mora F, Guastini L, Jankowska B, et al. Sulphurous water inhalations in the prophylaxis of recurrent upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. (2008) 72:1717–22. doi: 10.1016/j.ijporl.2008.08.014
12. DGS. Despacho Conjunto de 1989. (1989). Retrieved from https://www.dgs.pt/saude-ambiental/areas-de-intervencao/estabelecimentos-termais.aspx (accessed October, 2017).
13. Termas das Caldas-da-Felgueira. Characteristics of the Water. (2018). Retrieved from http://www.termasdeportugal.pt/estanciastermais/Caldas-da-Felgueira (accessed October, 2017). (accessed October, 2017).
14. Termas de São-Pedro-do-Sul. Characteristics of the Water. (2018). Retrieved from http://www.termasdeportugal.pt/estanciastermais/Termas-de-Sao-Pedro-do-Sul (accessed October, 2017).
15. Ferreira M, Costa P, Bahia M. Effect of São Pedro do Sul thermal water on skin irritation. Int J Cosmet Sci. (2010) 32:205–10. doi: 10.1111/j.1468-2494.2010.00527.x
16. Termas de Unhais-da-Serra. Characteristics of the Water. (2018). Retrieved from http://www.termasdeportugal.pt/estanciastermais/Termas-de-Unhais-da-Serra (accessed October, 2017).
17. Varricchio A, Giuliano M, Capasso M, Del Gaizo D, Ascione E, De Lucia A, et al. Salso-sulphide thermal water in the prevention of recurrent respiratory infections in children. Int J Immunopathol Pharmacol. (2013) 26:941–52. doi: 10.1177/039463201302600412
18. Neri M, Sansone L, Pietrasanta L, Kisialiou A, Cabano E, Martini M, et al. Gene and protein expression of CXCR4 in adult and elderly patients with chronic rhinitis, pharyngitis or sinusitis undergoing thermal water nasal inhalations. Immun Ageing. (2018) 15:10. doi: 10.1186/s12979-018-0114-y
19. Keller S, König V, Mösges R. Thermal water applications in the treatment of upper respiratory tract diseases: a systematic review and meta-analysis. J Allergy. (2014) 2014:17. doi: 10.1155/2014/943824
20. Toussaint O, Remacle J, Dierick J-F, Pascal T, Frippiat C, Royer, et al. Approach of evolutionary theories of ageing, stress, senescence-like phenotypes, calorie restriction and hormesis from the view point of far-from-equilibrium thermodynamics. Mech Ageing Dev. (2002) 123:937–46. doi: 10.1016/S0047-6374(02)00031-3
21. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. (2010) 5:e8578. doi: 10.1371/journal.pone.0008578
22. Ivanciuc T, Sbrana E, Ansar M, Bazhanov N, Szabo C, Casola A, et al. Hydrogen sulfide is an antiviral and antiinflammatory endogenous gasotransmitter in the airways. Role in respiratory syncytial virus infection. Am J Respir Cell Mol Biol. (2016) 55:684–96. doi: 10.1165/rcmb.2015-0385OC
23. Staffieri A, Marino F, Staffieri C, Giacomelli L, D'Alessandro E, Maria Ferraro S, et al. The effects of sulfurous-arsenical-ferruginous thermal water nasal irrigation in wound healing after functional endoscopic sinus surgery for chronic rhinosinusitis: a prospective randomized study. Am J Otolaryngol. (2008) 29:223–9. doi: 10.1016/j.amjoto.2007.07.002
24. Passali D, De Corso E, Platzgummer S, Streitberger C, Lo Cunsolo S, Nappi G, et al. SPA therapy of upper respiratory tract inflammations. Eur Arch Otorhinolaryngol. (2013) 270:565–70. doi: 10.1007/s00405-012-2024-5
25. Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. (2002) 16:1792–8. doi: 10.1096/fj.02-0211hyp
26. Spedding J, Vujcich M. Exchange of H2S between air and water. J Geoph Res Oceans. (1982) 87:8853–6. doi: 10.1029/JC087iC11p08853
27. Braga PC, Sasso MD, Culici M, Falchi M, Spallino A, Nappi G. Free radical–scavenging activity of sulfurous water investigated by electron paramagnetic resonance (EPR) spectroscopy. Exp Lung Res. (2012) 38:67–74. doi: 10.3109/01902148.2011.641668
28. Carbajo J, Maraver F. Sulphurous mineral waters: new applications for health. Evid Based Complement Alternat Med. (2017) 2017:11. doi: 10.1155/2017/8034084
29. Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. (2013) 35:5–20. doi: 10.1016/j.niox.2013.07.002
30. Bazhanov N, Ansar M, Ivanciuc T, Garofalo R, Casola A. Hydrogen sulfide: a novel player in airway development, pathophysiology of respiratory diseases, and antiviral defenses. Am J Respir Cell Mol Biol. (2017) 57:403–10. doi: 10.1165/rcmb.2017-0114TR
31. Benavides G, Squadrito G, Mills R, Patel H, Isbell T, Patel R, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. (2007) 104:17977–82. doi: 10.1073/pnas.0705710104
32. Perry M, Hui C, Whiteman M, Wood M, Adcock I, Kirkham P, et al. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am J Respir Cell Mol Biol. (2011) 45:746–52. doi: 10.1165/rcmb.2010-0304OC
33. Pouokam E, Althaus M. Epithelial electrolyte transport physiology and the gasotransmitter hydrogen sulfide. Oxid Med Cell Longev. (2016) 2016:13. doi: 10.1155/2016/4723416
34. Predmore B, Lefer D, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. (2012) 17:119–40. doi: 10.1089/ars.2012.4612
35. Wallace J, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. (2015) 14:329. doi: 10.1038/nrd4433
36. Yuan S, Patel R, Kevil C. Working with nitric oxide and hydrogen sulfide in biological systems. Am J Physiol Lung Cell Mol Physiol. (2015) 308:L403–15. doi: 10.1152/ajplung.00327.2014
37. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. (2012) 92:791–896. doi: 10.1152/physrev.00017.2011
38. Mishanina T, Libiad M, Banerjee R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nature Chem Biol. (2015) 11:457–64. doi: 10.1038/nchembio.1834
39. Benedetti F, Curreli S, Krishnan S, Davinelli S, Cocchi F, Scapagnini G, et al. Anti-inflammatory effects of H2S during acute bacterial infection: a review. J Transl Med. (2017) 15:100. doi: 10.1186/s12967-017-1206-8
40. Benedetti F, Davinelli S, Krishnan S, Gallo R, Scapagnini G, Zella D, et al. Sulfur compounds block MCP-1 production by Mycoplasma fermentans-infected macrophages through NF-κB inhibition. J Transl Med. (2014) 12:145. doi: 10.1186/1479-5876-12-145
41. Kimura H. Metabolic turnover of hydrogen sulfide. Front Physiol. (2012) 3:101. doi: 10.3389/fphys.2012.00101
42. Olson K, Dombkowski R, Russell M, Doellman M, Head S, Whitfield N, et al. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol. (2006) 209:4011–23. doi: 10.1242/jeb.02480
43. Krause N, Kutsche H, Santangelo F, DeLeon E, Dittrich N, Olson K, et al. Hydrogen sulfide contributes to hypoxic inhibition of airway transepithelial sodium absorption. Am J Physiol. (2016) 311:R607–17. doi: 10.1152/ajpregu.00177.2016
44. Olson K. Hydrogen sulfide as an oxygen sensor. Antioxid Redox Signal. (2015) 22:377–97. doi: 10.1089/ars.2014.5930
45. Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H(2)S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci. (2012) 109:2943–8. doi: 10.1073/pnas.1115634109
46. Stein A, Bailey S. Redox biology of hydrogen sulfide: implications for physiology, pathophysiology, and pharmacology. Redox Biol. (2013) 1:32–9. doi: 10.1016/j.redox.2012.11.006
47. Olson K, DeLeon E, Liu F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide. (2014) 41:11–26. doi: 10.1016/j.niox.2014.05.012
48. Calvert J, Coetzee W, Lefer D. Novel insights into hydrogen sulfide–mediated cytoprotection. Antioxid Redox Signal. (2010) 12:1203–17. doi: 10.1089/ars.2009.2882
49. Saito J, Zhang Q, Hui C, Macedo P, Gibeon D, Menzies-Gow A, et al. Sputum hydrogen sulfide as a novel biomarker of obstructive neutrophilic asthma. J Allergy Clin Immunol. (2013) 131:232–4 e233. doi: 10.1016/j.jaci.2012.10.005
50. Simpson J, Scott R, Boyle M, Gibson P. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. (2006) 11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x
51. Saito J, Mackay A, Rossios C, Gibeon D, Macedo P, Sinharay R, et al. Sputum-to-serum hydrogen sulfide ratio in COPD. Thorax. (2014) 69:903–9. doi: 10.1136/thoraxjnl-2013-204868
52. Wang P, Zhang G, Wondimu T, Ross B, Wang R. Hydrogen sulfide and asthma. Exp Physiol. (2011) 96:847–52. doi: 10.1113/expphysiol.2011.057448
53. Zhang J, Wang X, Chen Y, Yao W. Correlation between levels of exhaled hydrogen sulfide and airway inflammatory phenotype in patients with chronic persistent asthma. Respirology. (2014) 19:1165–9. doi: 10.1111/resp.12372
54. Saito J, Sato S, Hasunuma H, Ishimaru Y, Kanegae H, Kudo S, et al. Off-line fractional exhaled nitric oxide measurement is useful to screen allergic airway inflammation in an adult population. J Asthma. (2007) 44:805–10. doi: 10.1080/02770900701645595
55. Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. (2012) 13:499–507. doi: 10.1038/nrm3391
56. Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal. (2014) 7:ra87. doi: 10.1126/scisignal.2005478
57. Li L, Rose P, Moore P. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. (2011) 51:169–87. doi: 10.1146/annurev-pharmtox-010510-100505
58. Wu C. The role of hydrogen sulphide in lung diseases. Biosci Horizons. (2013) 6:hzt009. doi: 10.1093/biohorizons/hzt009
59. Sen N. Functional and molecular insights of hydrogen sulfide signaling and protein sulfhydration. J Mol Biol. (2017) 429:543–61. doi: 10.1016/j.jmb.2016.12.015
60. Wang R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens. (2011) 20:107–12. doi: 10.1097/MNH.0b013e3283430651
61. Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol. (2010) 37:753–63. doi: 10.1111/j.1440-1681.2010.05351.x
62. Ryu A, Thompson M, Venkatachalem S, Pabelick C, Prakash Y. Effect of hydrogen sulfide on [Ca2+]i regulation in airway smooth muscle. FASEB J. (2009) 23:622–5.
63. Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. (2010) 30:1998–2004. doi: 10.1161/ATVBAHA.110.209783
64. Castro-Piedras I, Perez-Zoghbi J. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J Physiol. (2013) 591:5999–6015. doi: 10.1113/jphysiol.2013.257790
65. Braga P, Dal Sasso M, Culici M, Spallino A, Marabini L, Bianchi, et al. Effects of sulphurous water on human neutrophil elastase release. Ther Adv Respir Dis. (2010) 4:333–40. doi: 10.1177/1753465810376783
66. Módis K, Ju Y, Ahmad A, Untereiner A, Altaany Z, Wu L, et al. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol Res. (2016) 113:116–24. doi: 10.1016/j.phrs.2016.08.023
67. Wang T, Wang L, Zaidi S, Sammani S, Siegler J, Moreno-Vinasco L, et al. Hydrogen sulfide attenuates particulate matter–induced human lung endothelial barrier disruption via combined reactive oxygen species scavenging and Akt activation. Am J Respir Cell Mol Biol. (2012) 47:491–6. doi: 10.1165/rcmb.2011-0248OC
68. Polhemus D, Lefer D. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. (2014) 114:730–7. doi: 10.1161/CIRCRESAHA.114.300505
69. Spassov S, Donus R, Ihle P, Engelstaedter H, Hoetzel A, Faller S. Hydrogen sulfide prevents formation of reactive oxygen species through PI3K/Akt signaling and limits ventilator-induced lung injury. Oxid Med Cel Long. (2017) 2017:14. doi: 10.1155/2017/3715037
70. Sen N, Paul B, Gadalla M, Mustafa A, Sen T, Xu R, et al. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell. (2012) 45:13–24. doi: 10.1016/j.molcel.2011.10.021
71. Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal. (2013) 18:1906–19. doi: 10.1089/ars.2012.4645
72. Rokicki W, Rokicki M, Wojtacha J, Dzeljijli A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir Torakochirurgia Pol. (2016) 13:26–30. doi: 10.5114/kitp.2016.58961
73. Soleas J, Paz A, Marcus P, McGuigan A, Waddell T. Engineering airway epithelium. J Biomed Biotechnol. (2012) 2012:10. doi: 10.1155/2012/982971
74. Weitnauer M, Mijošek V, Dalpke A. Control of local immunity by airway epithelial cells. Mucosal Immunol. (2015) 9:287–98. doi: 10.1038/mi.2015.126
75. Todoroff J, Vanbever R. Fate of nanomedicines in the lungs. Curr Opin Col Interf Sci. (2011) 16:246–54. doi: 10.1016/j.cocis.2011.03.001
76. Shoda T, Futamura K, Orihara K, Emi-Sugie M, Saito H, Matsumoto K, et al. Recent advances in understanding the roles of vascular endothelial cells in allergic inflammation. Allergol Int. (2016) 65:21–9. doi: 10.1016/j.alit.2015.08.001
77. Chen Y, Wang R. The message in the air: hydrogen sulfide metabolism in chronic respiratory diseases. Respir Physiol Neurobiol. (2012) 184:130–8. doi: 10.1016/j.resp.2012.03.009
78. Madurga A, Golec A, Pozarska A, Ishii I, MiŽíková I, Nardiello C, et al. The H2S-generating enzymes cystathionine β-synthase and cystathionine γ-lyase play a role in vascular development during normal lung alveolarization. Am J Physiol Lung Cell Mol Physiol. (2015) 309:L710–24. doi: 10.1152/ajplung.00134.2015
79. Campos D, Ravagnani G, Gurgueira S, Vercesi A, Teixeira S, Costa S, et al. Increased glutathione levels contribute to the beneficial effects of hydrogen sulfide and inducible nitric oxide inhibition in allergic lung inflammation. Int Immunopharmacol. (2016) 39:57–62. doi: 10.1016/j.intimp.2016.07.009
80. Elrod J, Calvert J, Morrison J, Doeller J, Kraus D, Tao L, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. (2007) 104:15560–5. doi: 10.1073/pnas.0705891104
81. Zanardo R, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace J. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. (2006) 20:2118–20. doi: 10.1096/fj.06-6270fje
82. Du J, Huang Y, Yan H, Zhang Q, Zhao M, Zhu M, et al. Hydrogen sulfide suppresses oxidized low-density lipoprotein (Ox-LDL)-stimulated monocyte chemoattractant protein 1 generation from macrophages via the nuclear factor κB (NF-κB) pathway. J Biol Chem. (2014) 289:9741–53. doi: 10.1074/jbc.M113.517995
83. Pálinkás Z, Furtmüller Paul G, Nagy A, Jakopitsch C, Pirker Katharina F, Magierowski M, et al. Interactions of hydrogen sulfide with myeloperoxidase. Br J Pharmacol. (2015) 172:1516–32. doi: 10.1111/bph.12769
84. Li HMY, Escaffre O, Ivanciuc T, Komaravelli N, Kelley JP, Coletta C, et al. Role of hydrogen sulfide in paramyxovirus infections. J Virol. (2015) 89:5557–68. doi: 10.1128/JVI.00264-15
85. Distrutti E, Cipriani S, Renga B, Mencarelli A, Migliorati M, Cianetti S, et al. Hydrogen sulphide induces μ opioid receptor-dependent analgesia in a rodent model of visceral pain. Mol Pain. (2010) 6:36. doi: 10.1186/1744-8069-6-36
86. Lee Z, Teo X, Tay E, Tan C, Hagen T, Moore P, et al. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. Br J Pharmacol. (2014) 171:4322–36. doi: 10.1111/bph.12773
87. Zhu Y, Abdullah L, Doyle S, Nguyen K, Ribeiro C, Vasquez P, et al. Baseline goblet cell mucin secretion in the airways exceeds stimulated secretion over extended time periods, and is sensitive to shear stress and intracellular mucin stores. PLoS ONE. (2015) 10:e0127267. doi: 10.1371/journal.pone.0127267
88. Bustamante-Marin X, Ostrowski L. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol. (2017) 9:a028241. doi: 10.1101/cshperspect.a028241
89. Althaus M, Urness K, Clauss W, Baines D, Fronius M. The gasotransmitter hydrogen sulphide decreases Na(+) transport across pulmonary epithelial cells. Br J Pharmacol. (2012) 166:1946–63. doi: 10.1111/j.1476-5381.2012.01909.x
90. Pane G, Gaggero G, Mora F. Recent results about antioxidant and immunomodulatory effects of sulphurous water. J Biol Res. (2011) 1:148–9. doi: 10.4081/4657
91. Wallace J, Ferraz J, Muscara M. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxid Redox Signal. (2012) 17:58–67. doi: 10.1089/ars.2011.4351
92. Contoli M, Gnesini G, Forini G, Marku B, Pauletti A, Padovani A, et al. Reducing agents decrease the oxidative burst and improve clinical outcomes in COPD patients: a randomised controlled trial on the effects of sulphurous thermal water inhalation. Sci World J. (2013) 2013:7. doi: 10.1155/2013/927835
93. Braga P, Ceci L, Marabini L, Nappi G. The antioxidant activity of sulphurous thermal water protects against oxidative DNA damage: a comet assay investigation. Drug Res (Stuttg). (2013) 3:198–202. doi: 10.1055/s-0033-1334894
94. Gorska K, Krenke R, Korczynski P, Kosciuch J, Domagala-Kulawik JRC. Eosinophilic airway inflammation in chronic obstructive pulmonary disease and asthma. J Physiol Pharmacol. (2008) 59:261–70.
95. Hiemstra PS, McCray PB, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. (2015) 45:1150–62. doi: 10.1183/09031936.00141514
96. Hoving J, Wilson Gillian J, Brown Gordon D. Signalling C-Type lectin receptors, microbial recognition and immunity. Cell Microbiol. (2014) 16:185–94. doi: 10.1111/cmi.12249
97. Whitsett J, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. (2015) 16:27–35. doi: 10.1038/ni.3045
98. Hu L-F, Wong Peter T, Moore Philip K, Bian J-S. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. (2007) 100:1121–8. doi: 10.1111/j.1471-4159.2006.04283.x
99. Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase. Lab Invest. (2006) 86:391–7. doi: 10.1038/labinvest.3700391
100. Mitsuhashi H, Yamashita S, Ikeuchi H, Kuroiwa T, Kaneko Y, Hiromura K, et al. Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock. (2005) 24:529–34. doi: 10.1097/01.shk.0000183393.83272.de
101. Truong D, Eghbal MA, Hindmarsh W, Roth S, O'Brien P. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev. (2006) 38:733–44. doi: 10.1080/03602530600959607
102. Baskar R, Li L, Fau-Moore P, Moore P. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J. (2007) 21:247–55. doi: 10.1096/fj.06-6255com
103. Kloesch B, Liszt M, Steiner G, Bröll J. Inhibitors of p38 and ERK1/2 MAPkinase and hydrogen sulphide block constitutive and IL-1β-induced IL-6 and IL-8 expression in the human chondrocyte cell line C-28/I2. Rheumatol Int. (2012) 32:729–36. doi: 10.1007/s00296-010-1682-0
104. Pan L-L, Liu X-H, Gong Q-H, Wu D, Zhu Y-Z. Hydrogen sulfide attenuated tumor necrosis factor-α-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS ONE. (2011) 6:e19766. doi: 10.1371/journal.pone.0019766
105. Sieghart D, Liszt M, Wanivenhaus A, Bröll H, Kiener H, Klösch B, et al. Hydrogen sulphide decreases IL-1β-induced activation of fibroblast-like synoviocytes from patients with osteoarthritis. J Cell Mol Med. (2014) 19:187–97. doi: 10.1111/jcmm.12405
106. Pandey R, Singh M, Singhal U, Gupta K, Aggarwal S. Oxidative/nitrosative stress and the pathobiology of chronic obstructive pulmonary disease. J Clin Diagn Res. (2013) 7:580–8. doi: 10.7860/JCDR/2013/4360.2832
107. Chen Y-H, Yao W-Z, Gao J-Z, Geng B, Wang PP, Tang CS. Serum hydrogen sulfide as a novel marker predicting bacterial involvement in patients with community-acquired lower respiratory tract infections. Respirology. (2009) 14:746–52. doi: 10.1111/j.1440-1843.2009.01550.x
108. Albertini M, Dachà M, Teodori L, Conti M. Drinking mineral waters: biochemical effects and health implications–the state-of-the-art. Int J Environ Health. (2007) 1:153–69. doi: 10.1504/IJENVH.2007.012230
109. Georgitis J. Nasal hyperthermia and simple irrigation for perennial rhinitis. Changes in inflammatory mediators. Chest. (1994) 106:1487–92. doi: 10.1378/chest.106.5.1487
110. Britschka Z, Teodoro W, Velosa A, de Mello S. The efficacy of Brazilian black mud treatment in chronic experimental arthritis. Rheumatol Int. (2007) 28:39–45. doi: 10.1007/s00296-007-0371-0
111. Vescovi P, Gerra G, Pioli G, Pedrazzoni M, Maninetti L, Passeri M. Circulating opioid peptides during thermal stress. Horm Metab Res. (1990) 22:44–6. doi: 10.1055/s-2007-1004846
112. Ocaña M, Cendán C, Cobos E, Entrena J, Baeyens J. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol. (2004) 500:203–19. doi: 10.1016/j.ejphar.2004.07.026
113. Bubb K, Trinder S, Baliga R, Patel J, Clapp L, MacAllister R, et al. Inhibition of phosphodiesterase 2 augments cGMP and cAMP signaling to ameliorate pulmonary hypertension. Circulation. (2014) 130:496–507. doi: 10.1161/CIRCULATIONAHA.114.009751
114. Wang R, Szabo C, Ichinose F, Ahmed A, Whiteman M, Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol Sci. (2015) 36:568–78. doi: 10.1016/j.tips.2015.05.007
115. Mirandola P, Gobbi G, Sponzilli I, Pambianco M, Malinverno C, Cacchioli A, et al. Exogenous hydrogen sulfide induces functional inhibition and cell death of cytotoxic lymphocytes subsets. J Cell Physiol. (2007) 213:826–33. doi: 10.1002/jcp.21151
116. Valitutti S, Castellino F, Musiani P. Effect of sulfurous (thermal) water on T lymphocyte proliferative response. Ann Allergy. (1990) 65:463–8.
117. Fang L-P, Lin Q, Tang C-S, Liu X-M. Hydrogen sulfide suppresses migration, proliferation and myofibroblast transdifferentiation of human lung fibroblasts. Pulm Pharmacol Ther. (2009) 22:554–61. doi: 10.1016/j.pupt.2009.07.003
118. Breza J, Soltysova A, Hudecova S, Penesova A, Szadvari I, Babula P, et al. Endogenous H2S producing enzymes are involved in apoptosis induction in clear cell renal cell carcinoma. BMC Cancer. (2018) 18:591. doi: 10.1186/s12885-018-4508-1
119. Du S, Huang Y, Jin H, Wang T. Protective mechanism of hydrogen sulfide against chemotherapy-induced cardiotoxicity. Front Pharmacol. (2018) 9:32. doi: 10.3389/fphar.2018.00032
120. Murata K, Fujimoto K, Kitaguchi Y, Horiuchi T, Kubo K, Honda T. Hydrogen peroxide content and ph of expired breath condensate from patients with asthma and COPD. J COPD. (2014) 11:81–7. doi: 10.3109/15412555.2013.830094
121. Ma Y, Yan Z, Deng X, Guo J, Hu J, Yu Y, et al. Anticancer effect of exogenous hydrogen sulfide in cisplatin resistant A549/DDP cells. Oncol Rep. (2018) 39:2969–77. doi: 10.3892/or.2018.6362
122. Liu H, Chang J, Zhao Z, Li Y, Hou J. Effects of exogenous hydrogen sulfide on the proliferation and invasion of human Bladder cancer cells. J Cancer Res Ther. (2017) 13:829–32. doi: 10.4103/jcrt.JCRT_423_17
123. Ma Z, Bi Q, Wang Y. Hydrogen sulfide accelerates cell cycle progression in oral squamous cell carcinoma cell lines. Oral Dis. (2015) 21:156–62. doi: 10.1111/odi.12223
124. Zhen Y, Wu Q, Ding Y, Zhang W, Zhai Y, Lin X, et al. Exogenous hydrogen sulfide promotes hepatocellular carcinoma cell growth by activating the STAT3-COX-2 signaling pathway. Oncol Lett. (2018) 15:6562–70. doi: 10.3892/ol.2018.8154
125. Jia H, Ye J, You J, Shi X, Kang W, Wang T. Role of the cystathionine β-synthase/H2S system in liver cancer cells and the inhibitory effect of quinolone-indolone conjugate QIC2 on the system. Oncol Rep. (2017) 37:3001–9. doi: 10.3892/or.2017.5513
126. Szczesny B, Marcatti M, Zatarain J, Druzhyna N, Wiktorowicz J, Nagy P, et al. Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Sci Rep. (2016) 6:36125. doi: 10.1038/srep36125
127. Lee Z, Zhou J, Chen C-S, Zhao Y, Tan C-H, Li L, et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE. (2011) 6:e21077. doi: 10.1371/journal.pone.0021077
128. Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. (2007) 21:1699–706. doi: 10.1096/fj.06-7407com
129. Oláh G, Módis K, Törö G, Hellmich M, Szczesny B, Szabo C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem Pharmacol. (2018) 149:186–204. doi: 10.1016/j.bcp.2017.10.011
130. GINA. GINA Report Strategy for Asthma Management and Prevention. (2018). Available online at: https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf (accessed January 2019).
131. GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD. (2019). Available online at: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-POCKET-GUIDE-FINAL_WMS.pdf (accessed January 2019).
132. Chen Y-H, Yao W-Z, Geng B, Ding Y-L, Lu M, Zhao M-W, et al. Endogenous hydrogen sulfide in patients with COPD. Chest. (2005) 128:3205–11. doi: 10.1378/chest.128.5.3205
133. Hwang J, Jun Y, Park S, Kim T, Lee K, Hwang S, et al. Endogenous production of hydrogen sulfide in human sinus mucosa and its expression levels are altered in patients with chronic rhinosinusitis with and without nasal polyps. Am J Rhinol Allergy. (2014) 28:12–9. doi: 10.2500/ajra.2014.28.3972
134. Park Se J, Kim Tae H, Lee Seung H, Ryu Hyei Y, Hong Ki H, Jung Jong Y, et al. Expression levels of endogenous hydrogen sulfide are altered in patients with allergic rhinitis. Laryngoscope. (2013) 123:557–63. doi: 10.1002/lary.23466
135. Sun Y, Wang K, Li M-X, He W, Chang J-R, Liao C-C, et al. Metabolic changes of H2S in smokers and patients of COPD which might involve in inflammation, oxidative stress and steroid sensitivity. Sci Rep. (2015) 5:14971. doi: 10.1038/srep14971
136. Tian M, Wang Y, Lu Y, Yan M, Jiang Y, Zhao D. Correlation between serum H2S and pulmonary function in children with bronchial asthma. Mol Med Rep. (2012) 6:335–8. doi: 10.3892/mmr.2012.904
137. Wu R, Yao W, Chen Y, Geng B, Tang C. Plasma level of endogenous hydrogen sulfide in patients with acute asthma. Beijing Da Xue Xue Bao. (2008) 40:505–8.
138. Liu Y, Yao Y, Wang Z, Ning Q, Liu Z. Novel innate and adaptive lymphocytes: the new players in the pathogenesis of inflammatory upper airway diseases. Clin Exp Allergy. (2018) 48:620–31. doi: 10.1111/cea.13128
139. Togias A. Unique mechanistic features of allergic rhinitis. J Allergy Clin Immunol. (2000) 105:S599–604. doi: 10.1067/mai.2000.106885
140. Horak F, Stübner U, Zieglmayer R, Harris AG. Effect of desloratadine versus placebo on nasal airflow and subjective measures of nasal obstruction in subjects with grass pollen-induced allergic rhinitis in an allergen-exposure unit. J Allergy Clin Immunol. (2002) 109:956–61. doi: 10.1067/mai.2002.124657
141. Kim T, Lee J, Lee H, Lee S, Cho W, Ju Y, et al. Remodelling of nasal mucosa in mild and severe persistent allergic rhinitis with special reference to the distribution of collagen, proteoglycans, and lymphatic vessels. Clin Exp Allergy. (2010) 40:1742–54. doi: 10.1111/j.1365-2222.2010.03612.x
142. Mori S, Fujieda S, Sunaga H, Fox SB, Saito H. Expression of platelet-derived endothelial cell growth factor and vascularity in the nasal mucosa from allergic rhinitis. Clin Exp Allergy. (2000) 30:1637–44. doi: 10.1046/j.1365-2222.2000.00903.x
143. Braunstahl G, Fokkens W, Overbeek S, KleinJan A, Hoogsteden H, Prins J. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy. (2003) 33:579–87. doi: 10.1046/j.1365-2222.2003.01652.x
144. Eifan A, Orban N, Jacobson M, Durham S. Severe persistent allergic rhinitis. Inflammation but no histologic features of structural upper airway remodeling. Am J Respir Crit Care Med. (2015) 192:1431–9. doi: 10.1164/rccm.201502-0339OC
145. Barbieri M, Salami A, Mora F, Casazza A, Sovatzis A, Teglia R, et al. Behavior of serum IgE and IgA in patients with allergic rhinitis treated with iodine bromide thermal water. Acta Otorhinolaryngol Ital. (2002) 22:215–9.
146. Mora R, Chiarlone M, Crippa B, Barbieri M. Nasal irrigations with sulphurea water in the treatment of the oral allergy syndrome. Medicina Clinica Termale. (2002) 14:287–90.
147. Bousquet J, Khaltaev N, Cruz A, Denburg J, Fokkens W, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA). Allergy. (2008) 63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x
148. Reddy P. Mitochondrial dysfunction and oxidative stress in asthma: implications for mitochondria-targeted antioxidant therapeutics. Pharmaceuticals. (2011) 4:429–56. doi: 10.3390/ph4030429
149. WHO. Asthma–Fact Sheet (2018). Retrieved from http://www.who.int/en/news-room/fact-sheets/detail/asthma (accessed December 2018).
150. Gibeon D, Chung K. The investigation of severe asthma to define phenotypes. Clin Exp Allergy. (2012) 42:678–92. doi: 10.1111/j.1365-2222.2012.03959.x
151. McGrath K, Icitovic N, Boushey H, Lazarus S, Sutherland E, Chinchilli V, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. (2012) 185:612–9. doi: 10.1164/rccm.201109-1640OC
152. Bara I, Ozier A, De Lara JT, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J. (2010) 36:1174–84. doi: 10.1183/09031936.00019810
153. Erle D, Sheppard D. The cell biology of asthma. J Cell Biol. (2014) 205:621–31. doi: 10.1083/jcb.201401050
154. Campagna D, Kathman S, Pierson R, Inserra S, Phifer B, Middleton D, et al. Ambient hydrogen sulfide, total reduced sulfur, and hospital visits for respiratory diseases in northeast Nebraska, 1998–2000. J Expo Anal Environ Epidemiol. (2004) 14:180–7. doi: 10.1038/sj.jea.7500313
155. Bates M, Garrett N, Crane J, Balmes J. Associations of ambient hydrogen sulfide exposure with self-reported asthma and asthma symptoms. Environ Res. (2013) 122:81–7. doi: 10.1016/j.envres.2013.02.002
156. WHO. COPD–Fact Sheets. (2018). Retrieved from http://www.who.int/mediacentre/factsheets/fs315/en/ (accessed December, 2018).
157. Negewo N, McDonald V, Gibson P. Comorbidity in chronic obstructive pulmonary disease. Respir Investig. (2015) 53:249–58. doi: 10.1016/j.resinv.2015.02.004
158. Westerik J, Metting E, van Boven J, Tiersma W, Kocks J, Schermer T. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. (2017) 18:31. doi: 10.1186/s12931-017-0512-2
159. Crystal RG. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2014) 190:1355–62. doi: 10.1164/rccm.201408-1492PP
160. Shaykhiev R, Crystal R. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc. (2014) 11:S252–8. doi: 10.1513/AnnalsATS.201402-049AW
161. MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. (2001) 429:195–207. doi: 10.1016/S0014-2999(01)01320-6
162. Vogelmeier C, Criner G, Martinez F, Anzueto A, Barnes P, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. (2017) 195:557–82. doi: 10.1164/rccm.201701-0218PP
163. Zhang J, Wang X, Chen Y, Yao W. Exhaled hydrogen sulfide predicts airway inflammation phenotype in COPD. Respir Care. (2015) 60:251–8. doi: 10.4187/respcare.03519
164. Gutenbrunner C, Bender T, Cantista P, Karagülle Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int J Biometeorol. (2010) 54:495–507. doi: 10.1007/s00484-010-0321-5
165. Epithelix. Epithelix Sàrl. (2018). Retrieved from www.epithelix.com (accessed October, 2017).
166. MatTek. (2018). Retrieved from https://www.mattek.com/ (accessed October, 2017).
167. Frieke Kuper C, Gröllers-Mulderij M, Maarschalkerweerd T, Meulendijks N, Reus A, van Acker F, et al. Toxicity assessment of aggregated/agglomerated cerium oxide nanoparticles in an in vitro 3D airway model: the influence of mucociliary clearance. Toxicol In Vitro. (2015) 29:389–97. doi: 10.1016/j.tiv.2014.10.017
168. Huang S, Wiszniewski L, Constant S, Roggen E. Potential of in vitro reconstituted 3D human airway epithelia (MucilAir™) to assess respiratory sensitizers. Toxicol In Vitro. (2013) 27:1151–6. doi: 10.1016/j.tiv.2012.10.010
169. Reus A, Maas W, Jansen H, Constant S, Staal Y, van Triel J, et al. Feasibility of a 3D human airway epithelial model to study respiratory absorption. Toxicol In Vitro. (2014) 28:258–64. doi: 10.1016/j.tiv.2013.10.025
170. Sexton K, Balharry D, BéruBé K. Genomic biomarkers of pulmonary exposure to tobacco smoke components. Pharmacogenet Genomics. (2008) 18:853–60. doi: 10.1097/FPC.0b013e328307bddf
171. Sharma M, Schoop R, Hudson J. The efficacy of Echinacea in a 3-D tissue model of human airway epithelium. Phytother Res. (2010) 24:900–4. doi: 10.1002/ptr.3051
Keywords: sulfurous thermal waters, hydrogen sulfide, S-sulfhydration, allergic rhinitis, asthma, chronic obstructive pulmonary disease
Citation: Viegas J, Esteves AF, Cardoso EM, Arosa FA, Vitale M and Taborda-Barata L (2019) Biological Effects of Thermal Water-Associated Hydrogen Sulfide on Human Airways and Associated Immune Cells: Implications for Respiratory Diseases. Front. Public Health 7:128. doi: 10.3389/fpubh.2019.00128
Received: 20 February 2019; Accepted: 08 May 2019;
Published: 05 June 2019.
Edited by:
Fabrizio Bianchi, Italian National Research Council, ItalyReviewed by:
Levent Dalar, Istanbul Bilim University, TurkeyYue-Wern Huang, Missouri University of Science and Technology, United States
Copyright © 2019 Viegas, Esteves, Cardoso, Arosa, Vitale and Taborda-Barata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luís Taborda-Barata, dGFib3JkYWJhcmF0YUBmY3NhdWRlLnViaS5wdA==
 Joana Viegas
Joana Viegas Ana Filipa Esteves
Ana Filipa Esteves Elsa M. Cardoso
Elsa M. Cardoso Fernando A. Arosa
Fernando A. Arosa Marco Vitale4,5
Marco Vitale4,5 Luís Taborda-Barata
Luís Taborda-Barata