- 1Faculty of Pharmacy, Medical University of Sofia, Sofia, Bulgaria
- 2National Council on Prices and Reimbursement, Sofia, Bulgaria
Background: Policy makers face a lot of challenges in the process of drug reimbursement decision-making, especially in the context of entering the market of more and more innovative medicinal products (MPs). The aim of the current study is to make an overview of the reimbursement system development and to evaluate the access of innovative medicines, which have entered the EU-market in the period 2015–2017, in Bulgaria as reference example for middle-income European country.
Methods: A literature and a legislative systematic review regarding the Bulgarian reimbursement system as well as a defining the number of available innovative reimbursed MPs in 2017 in Bulgaria was made.
Results: The reimbursement legislation in Bulgaria is quite unstable due to constant changes, which have been made, especially in the recent years. Despite this fact, the reimbursement process in Bulgaria is in accordance with the Transparency Directive. Bulgarian patients have a relatively delayed access to innovative medicines as only 5% of centrally authorized MPs in 2017 are available in the positive drug list (PDL), 16% of all in 2016 and 18%—in 2015. This could be explained by the long procedure for their appraisal in Bulgaria: the first step is issuing an opinion by the HTA Committee, followed by negotiation of discounts between the marketing authorization holder and the National Health Insurance Fund and making a final decision by the National Council on Prices and Reimbursement (NCPR) for the inclusion into the PDL.
Conclusion: Optimization of the procedure for issuing reimbursement status for innovative MPs is needed, such as improvements in the process of conducting HTA reports and their appraisal, incorporation of adequate systems for following the effectiveness and safety of MPs in the real-world conditions, value-based pricing implementation, and increasing the financial control over the health insurance system.
Introduction
The policy makers are constantly facing the challenge to find the balance between the increased patients’ needs of innovative, high costly medicines and limited financial resources (1). The scarce resources and the increasing patients’ needs define the need for implementation of strict pharmacoeconomic evaluations for the purposes of making the right decision.
A lot of issues still exist, notably in the middle and upper-middle-income European countries (2). The economic situation in these countries is critical and there is an emergency need of more efficient reallocation of the resources especially in the pharmaceutical sector. Their health-care systems are not as stable as they should be due to a lot of reforms which have been made in the recent years (2). Rancic et al. concluded that the total health expenditures showed significant growth in the period 1995–2012 probably due to population aging (3). Pharmaceutical expenditures are a significant part of total health-care expenditures. For example, in Bulgaria the pharmaceutical expenditures increase every year, which leads to the annual budget deficit for National Health Insurance Fund (NHIF) (4). Therefore, more precise cost-containment measures should be applied as well as optimization of HTA usage in order to get better value for money (2, 5). Implementation of effective working generic policy and entering the market of biosimilar products are also possible measures (2). As Jakovljevic et al. highlighted there are some factors such as demographic crisis which could not be overcome and which is a main pharmaceutical expenditures driver in the next years (2, 6–8). Moreover, Bulgaria as the EU Member State with the lowest income per capita [only 47% of the EU average (9)] faces many challenges in ensuring the most innovative medicines for its citizens.
The aim of the current study is to make an overview of the reimbursement system development and to evaluate the access of innovative medicines, which have entered the EU-market in the period 2015–2017, in Bulgaria as reference example for middle-income European country.
Materials and Methods
The first part of the study was a literature and a legislative systematic review regarding the implemented reimbursement system in Bulgaria for the period 2000–2017. A search was made in the official websites of Bulgarian institutions such as Ministry of Health, NHIF, National Council on Prices and Reimbursement of Medicinal Products (MPs), National Centre for Public Health and Analyses, and Bulgarian Drug Agency in order to identify the latest legislative documents and guidelines for conducting of administrative pricing and reimbursement procedures.
The second part of the study included a search of all MPs (MPs) which received marketing authorization through the centralized procedure for the period 2015–2017. A comparison of the generated list of these MPs by the website of the European Medicines Agency (EMA) and the current Bulgarian Positive Drug List (PDL) was made. Therefore, the availability of the newest medicines in Bulgaria was analyzed.
The third part of the study presents a systematic and analytical review of the identified issues in the reimbursement process in Bulgaria on the basis of the authors’ point of view and officially published scientific studies.
Results
Reimbursement Legislation in Bulgaria
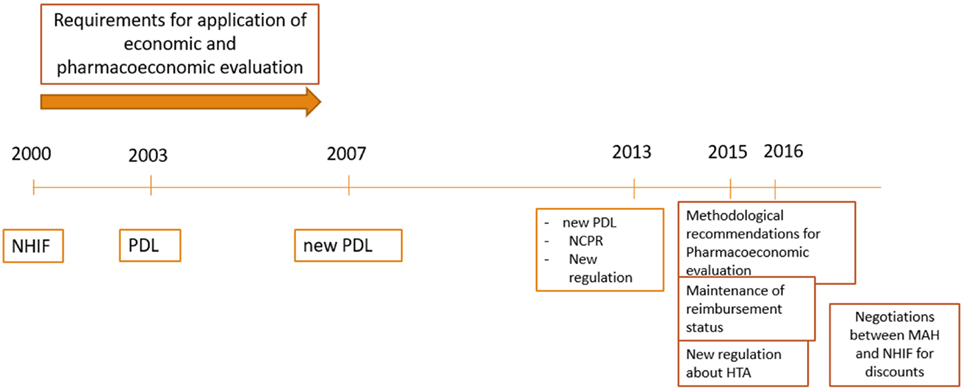
The Health Insurance Act (1998) introduced the mandatory health insurance in Bulgaria (Figure 1) (10). According to this law NHIF was founded in 1999 as an independent public institution (11). The NHIF reimburse MPs, medical devices, dietetic foods, foods for special purposes for treatment of obligatory health insured Bulgarian citizens, as well as for hospitalized patients. For the inclusion of the medicines in the reimbursement lists a methodological approach has been developed and published in 2000, in which several crucial points were stated:
1. economic analysis should precede the pharmacoeconomic analysis;
2. economic analysis includes directs costs, due to product application; market share, prices; additional costs etc.;
3. pharmacoeconomic analysis is a comparison of the costs and consequences of the product application and its competitors (12).
The Council Decree 81 in 2003 stipulates the criteria, conditions and procedures for including MPs in the Bulgarian PDL. Three groups of MPs in PDL were defined:
A. new MPs without a medicinal alternative in the clinical practice (new mechanism of action, new ATC code);
B. new medicines for which there is a therapeutic alternative with pharmacotherapeutic advantages (group A and B are innovative products);
C. MPs with a medicinal alternative in the clinical practice (generics).
A fixed percent of the reimbursement for each MP is defined (100, 75, 50, and 25%) on the basis of its importance for disease therapy and severity of the disease.
In 2007 after the Bulgarian accession to the EU new Regulation was issued and the structure of PDL was changed: ANNEX 1: for fully or partly reimbursed medicines paid by the NHIF; ANNEX 2: medicines paid by the hospital budgets; ANNEX 3: medicines paid by the Ministry of Health budget according to Health Insurance Law; ANNEX 4: medicines for the therapy of rare diseases, HIV, and prophylactics of infections. There were no particular recommendations or guidelines for the development and presentation of the pharmacoeconomic analysis.
The pricing and reimbursement decision process were merged and delegated to one institution in 2013. The National Council on Prices and Reimbursement (NCPR) was established as responsible body for inclusion and exclusion of MPs in the PDL (PDL) and for maintenance of their reimbursement status (13). The PDL was changed and there are now three main annexes and the time for decision was shortened (60 days). All innovative medicines should receive a positive opinion by the Health Technology Assessment Committee since 2015 before issuing the final decision by the Council (14, 15).
Pharmacoeconomic and HTA dossiers are prepared following the officially published methodological guidelines. Science-based efficacy, safety, and pharmacoeconomic evidence should be presented in the dossier. Schematic explanation of the reimbursement procedure is shown on Figure 2.
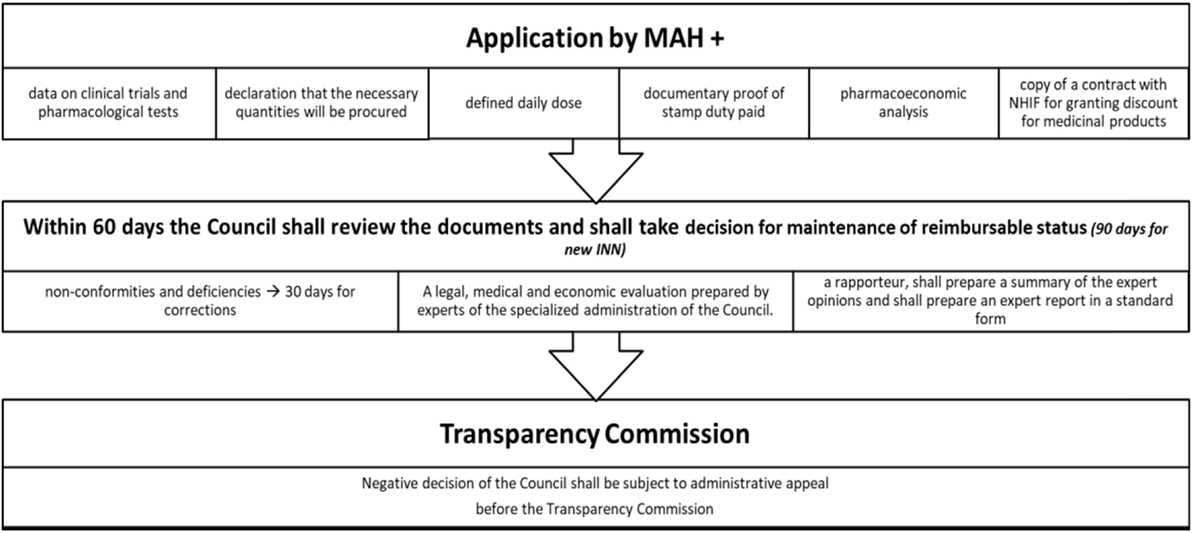
Figure 2. Procedure for inclusion of MPs in PDL. Abbreviations: MPs, medicinal products; PDL, positive drug list.
A number of discounts are possible and their level should be negotiated between the Marketing authorization holder (MAH) and the NHIF (16):
1. mandatory discount for reimbursement of Single Source Products (new INNs) (>10%);
2. mandatory discount for new INN and combinations—there is no particular percentage;
3. managed entry agreement—MAH should provide additional discount when the agreed annual expenditures of the MP for each relevant year is exceeded (if the forecast values exceeded to 10% then the discount is not lower than 25%; if the forecast values exceeded to 10–15% the discount is not lower than 50%; if the forecast values exceeded to 15–25 per cent then the discount is not lower than 75%; if the forecast values exceeded 25% then the discount is not lower than 90%);
4. growth discount—MAH should pay back 20% of the relevant rate of growth, when the total growth is higher than 3% from the negotiated (for e.g., the expected expenditures are 100 million BGN, but the real expenditures are 110 mill BGN then the MAH should pay back 20% of 10 million BGN). Exchange rate is 1 BGN = 0.51 Euro;
5. voluntary discounts—for multiple source products; every MAH could provide voluntary additional discounts.
Access and Affordability to Innovative MPs in Bulgaria
Bulgarian patients have a relatively delayed access to innovative medicines. The percentage of innovative MPs included in the Bulgarian PDL is far below 20%. The number of the newest medicines authorized through the centralized procedure by the EMA in 2017, is 83. Only three of them are reimbursed in Bulgaria and one has received a positive opinion by the HTA Committee. Logically, the number of reimbursed innovative MPs in Bulgaria, which entered the EU-market in 2015 and 2016, is higher than the following year: 18 and 16%, respectively (Figure 3). Some innovative products even do not apply for reimbursement and only register prices for non-reimbursable marketing.
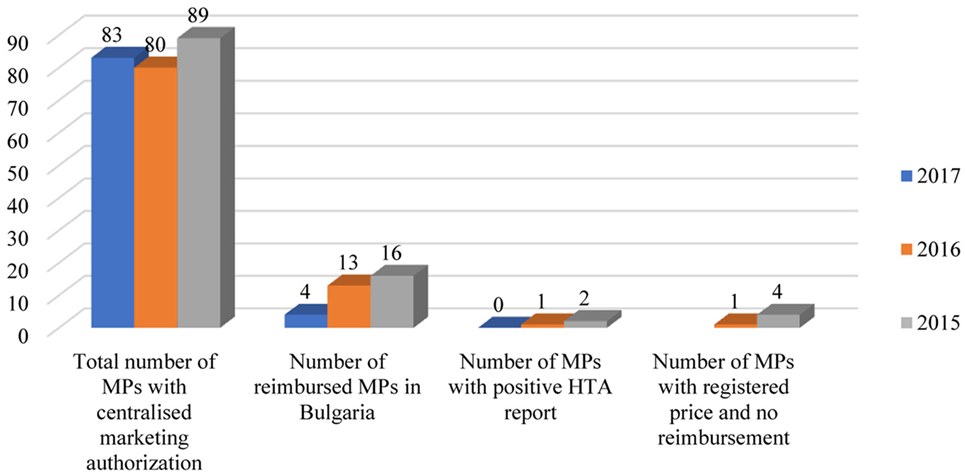
Figure 3. Reimbursement status of MPs in Bulgaria authorized through centralized procedure in the EU. Abbreviations: MA, marketing authorization; MPs, medicinal products; HTA, health technology assessment.
Despite the limited number of reimbursed innovative medicines, very important and promising therapies such as those for Hepatitis C, HIV, multiple myeloma, oncological conditions, etc. are ensured for all Bulgarian patients for whom there is no other option (Table 1).
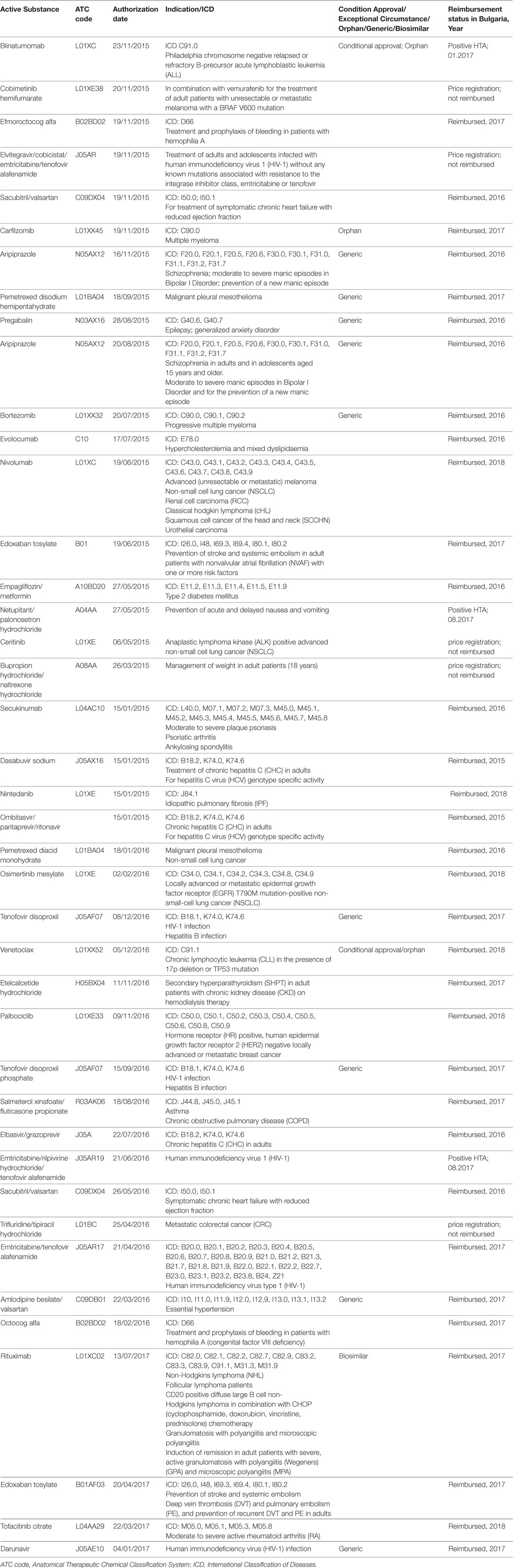
Table 1. Medicinal products with centralized marketing authorization, which are available in Bulgaria.
MPs Reimbursement Issues in Bulgaria As an Example for Middle-Income EU Country
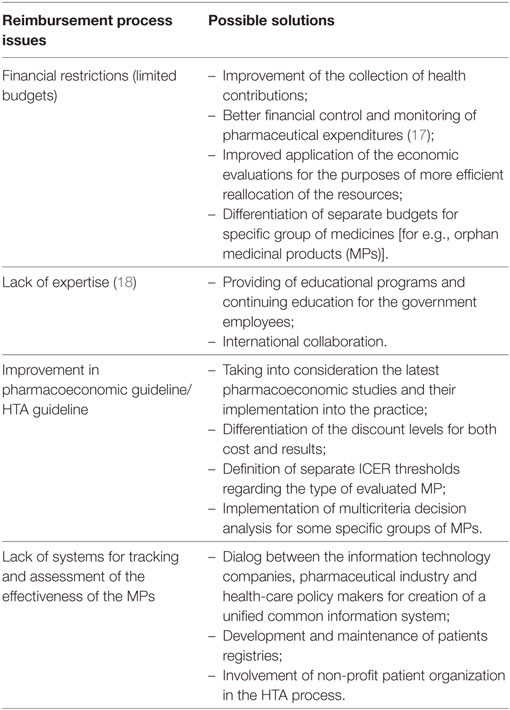
The financial limitations of low and middle-income countries are the main drivers for cost-containment measures introduction. In the context of medical and pharmaceutical development, the requirements to the NHIF are increasing. Therefore, more precise and regular financial control mechanisms should be implemented. Another serious problem in these countries is the lack of expertise and the limited local epidemiological data for the purposes of preparing a valuable pharmacoeconomic/HTA dossier. Some of the issues regarding the reimbursement process in Bulgaria and the possible solutions are highlighted in Table 2.
Discussion
The reimbursement policy in Bulgaria could be characterized by implementation of lots of rules for the inclusion of medicines into the PDL and a clear process of reimbursement performed by the National Council on Prices and Reimbursement (19). Despite the necessity of their further improvement, the available pharmacoeconomic and HTA guidelines give the possibility to the policy decision maker to step on a scientific basis in order to make the best possible reimbursement decision. Some problems such as lack of mechanisms for gathering effectiveness data from real-world studies, the periodic legislative changes and the lack of enough experts in the area could be highlighted. Further improvement in the legislative framework is needed in order to cope with the increasing reimbursement expenditures. Collaboration with other European countries could be useful in order to find the best solutions for the reimbursement practice in Bulgaria (20, 21). The process of development and improvement of reimbursement policy is slower, but it could ensure more options for providing innovative medicines to the population (22, 23) as it is the case in other Balkan countries such as Greece (2, 24), Croatia (25), Bosna and Herzegovina, and Republic of Serbia (26). Several crucial changes are proposed in Polish reimbursement system. One of these changes aims to create an innovative reimbursement budget, which will provide funding for reimbursement of innovative products developed by manufacturers with research and development activities with considerable impact on the Polish economy (27). Therefore, the patient access in Poland to innovative therapies could be significantly improved.
Our study confirms that the patient access to innovative medicines from the moment of their marketing authorization is delayed. The number of reimbursed innovative medicines as a percent of the centrally authorized by EMA is far below 20% which confirms some extent of limitations in the patient access. Similar results are presented by Inotai et al. for the patient access to original biologics and biosimilar in Central and Eastern European countries (CEE countries). The authors explain the results with the current implemented biosimilar policies in these countries (28), which means that some improvement in the local legislation is needed. Significant variations exist in uptake of biosimilars in Europe, which could be overcome with implementation of specific procedures and measures (29). While Western Balkan countries has proved through the years that are capable to ensure reimbursed medicines for patients with non-communicable diseases with some exceptions (30), there is still gaps in the knowledge about the patients access to innovative medicines in these countries. Study published in 2017 highlighted the large disparities in access to innovative therapy for metastatic melanoma among the European countries mostly in the Eastern European region (31). The Romanian HTA system implements criteria focused more on the costs and, therefore, it raises a barrier for the innovative medicines in the country (32).
The regulatory bodies especially in CEE countries are pressured in order to ensure new medicines (orphan MPs, innovative biological products, etc.) for severe life-threatening conditions with no available alternative (33). The budget constraints are inevitable, especially in the low- and middle-income countries. The policy makers are trying to balance in the context of deficit resources adopting various approaches. Performance based managed entry agreements for pharmaceuticals is a possible option which is partly applied in Bulgaria. Reassessment of treatments after their inclusion in the reimbursement lists gives a guarantee for collecting of more valuable evidence for effectiveness and cost-effectiveness of the new medicine (34). So, the public fund will be able to stop financing technologies with no proven value in the post reimbursement period. The crucial evidence, which should be taken into account when a reimbursement decision is made, is whether the new medicine brings additional benefits for those patients with no available alternative (23).
Strength of the current study is that it represents the development of Bulgarian reimbursement legislation since its formation in 2000 to these days. This review could be used for the purposes of making more valuable and evidence based decisions for further reforms in the system. As an example of a middle-income Balkan country, the case with Bulgarian reimbursement system could be used as a model for other Balkan countries, which are economically similar to Bulgaria and which are characterized with similar pricing and reimbursement requirements (35). To the best of our knowledge, this is the first study, which makes an attempt to present the access of Bulgarian patients to reimbursed innovative therapies, which received marketing authorization through the centralized procedure in the EU, and to give some recommendations for improvement of the reimbursement decision about these medicines. Further studies could focus more on the real financial burden of the innovative therapies.
Conclusion
Optimization of the procedure for issuing reimbursement status for innovative MPs is needed especially in the Balkan countries, where lots of issues exist. Improvements in the process of conducting HTA reports and their appraisal, incorporation of adequate systems for following the effectiveness and safety of MPs in the real-world conditions, value-based pricing implementation and increasing the financial control over the health insurance system could be some of the possible solutions. It is crucial the level of expertise in these countries to be enhanced through accreditation of shared master Health Technology Assessment programs. Shared experience among Balkan countries could provide additional valuable information regarding economic evaluation and appropriate reimbursement mechanisms for innovative medicines.
Author Contributions
All the authors have provided valuable contributions to the manuscript.
Conflict of Interest Statement
The authors certify that they do not have any conflict of interest to declare regarding the current study.
References
1. Pharmaceutical regulation in 15 European countries. Review. Health Systems in Transition (2016) 18(5). Available from: http://apps.who.int/medicinedocs/documents/s23163en/s23163en.pdf
2. Pejcic A, Jakovljevic M. Pharmaceutical expenditure dynamics in the Balkan countries. J Med Econ (2017) 20(10):1013–7. doi:10.1080/13696998.2017.1333514
3. Rancic N, Kovacevic A, Dragojevic-Simic V. Long-term health expenditure changes in selected Balkan countries. Front Public Health (2015) 3:152. doi:10.3389/fpubh.2015.00152
4. Iskrov G, Stefanov R. Prospects of risk-sharing agreements for innovative therapies in a context of deficit spending in Bulgaria. Front Public Health (2015) 3:64. doi:10.3389/fpubh.2015.00064
5. Vlãdescu C, Scîntee SG, Olsavszky V, Hernández-Quevedo C, Sagan A. Romania: health system review. Health Syst Transit (2016) 18(4):1–170.
6. Jakovljevic MB, Milovanovic O. Growing burden of non-communicable diseases in the emerging health markets: the case of BRICS. Front Public Health (2015) 3:65. doi:10.3389/fpubh.2015.00065
7. Jakovljevic MB, Vukovic M, Fontanesi J. Life expectancy and health expenditure evolution in Eastern Europe-DiD and DEA analysis. Expert Rev Pharmacoecon Outcomes Res (2016) 16:537–46. doi:10.1586/14737167.2016.1125293
8. Jakovljevic M, Laaser U. Population aging from 1950 to 2010 in seventeen transitional countries in the wider region of South Eastern Europe. SEEJPH (2015) 3:1–12. doi:10.4119/UNIBI/SEEJPH-2015-49
9. The World Bank in Bulgaria, Overview (2017). Available from: http://www.worldbank.org/en/country/bulgaria/overview
10. National Peoples’ Assembly. The Health Insurance Act. Governmental Newspaper. (1998). 70; last amended Gov, Newspaper 2009; 101.
11. National Health Insurance Fund (2009–2018). Available from: www.nhif.bg
12. Petrova G, Getov I. Methodological approach for economic and/or pharmacoeconomic assessment of the drugs (with H2-blokers practical example). Soc Med (2000) 1:37–40.
13. National Peoples’ Assembly. Law on the Medicinal Products in Human Medicine. (2007). 31, last amendment 2015; 48.
14. Ministry Council. Regulation on Conditions and Rules for Regulating and Registering the Prices of Medicinal Products – Amend. (2013). Govern. Newspaper 40/ 30 April.
15. Ministry of Health. Regulation for the Conditions and Order for Performing a Health Technology Assessment. (2015). Gov.
16. Ministry of Health. Regulation 10/2009 on the Terms, Rules for Reimbursement of Medicines, Medical Devices etc. by NHIF. (2009). 24 p. Gov. Newsp.
17. Benisheva-Dimitrova T, Sidjimova D, Cherneva D, Kralimarkov N. Pricing, reimbursement, and health technology assessment of medicinal products in Bulgaria. Int J Technol Assess Health Care (2017) 33(3):365–70. doi:10.1017/S0266462317000551
18. Danko D, Petrova G. Health technology assessment in the Balkans: opportunities for a balanced drug assessment system. Biotechnol Biotechnol Equip (2014) 28(6):1181–9. doi:10.1080/13102818.2014.978636
19. Petrova G, Benisheva T, Ivanova A. Pharmacoeconomics and the new Bulgarian positive list. Regul Aff J (2005) 16(3):175–9.
20. Seiter A. Pharmaceutical policy challenges in Central and Eastern Europe. Eurohealth (2010) 14(1):30–2.
21. García-Mochón L, Espín Balbino J, Olry de Labry Lima A, Caro Martinez A, Martin Ruiz E, Pérez Velasco R. HTA and decision-making processes in Central, Eastern and South Eastern Europe: results from a survey. Health Policy (2017). doi:10.1016/j.healthpol.2017.03.010
22. Tesar T, Hloska A, Wawruch M, Lehocka L, Snopkova M, Masarykova L. Introduction of health technology assessment for medicines in Slovakia. Int J Technol Assess Health Care (2017) 33:1. doi:10.1017/S026646231700006X
23. Zlatareva A. Analysis of the reimbursement policy in Central and Eastern European Countries – the policy of Poland, Romania, Hungary and the Czech Republic, PART II. World J Pharm Pharm Sci (2015) 4(9):64–76.
24. European Commission. State of Health in the EU Greece Country Health Profile. Paris/Brussels: OECD Publishing/European Observatory on Health Systems and Policies (2017).
25. Huic M, Tandara Hacek R, Svajger I. Health technology assessment in Central, Eastern, and South European countries: Croatia. Int J Technol Assess Health Care (2017) 33(3):376–83. doi:10.1017/S026646231700054X
26. Guzvic V, Catic T, Kostic M. Health technology assessment in Central-Eastern and South Europe countries: Bosnia and Herzegovina. Int J Technol Assess Health Care (2017) 33(3):390–5. doi:10.1017/S0266462317000058
27. Badora K, Caban A, Rémuzat C, Dussart C, Toumi M. Proposed changes to the reimbursement of pharmaceuticals and medical devices in Poland and their impact on market access and the pharmaceutical industry. J Mark Access Health Policy (2017) 5:1–11. doi:10.1080/20016689.2017.1381544
28. Inotai A, Csanadi M, Petrova G, Dimitrova M, Bochenek T, Tesar T, et al. Patient access, unmet medical need, expected benefits, and concerns related to the utilisation of biosimilars in Eastern European countries: a survey of experts. Biomed Res Int (2018). Article ID 9597362, 9 pages, doi:10.1155/2018/9597362
29. Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PLoS One (2017) 12(12):e0190147. doi:10.1371/journal.pone.0190147
30. Pekez-Pavlisko T, Racic M, Kusmuk S. Medicine availability and prescribing policy for non-communicable diseases in the Western Balkan countries. Front Public Health (2017) 5:295. doi:10.3389/fpubh.2017.00295
31. Kandolf Sekulovic L, Peris K, Hauschild A, Stratigos A, Grob JJ, Nathan P, et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first-line innovative treatments. Eur J Cancer (2017) 75:313–22. doi:10.1016/j.ejca.2017.01.012
32. Chiriac ND, Radu PC, Mazilu MA. Access gap to innovative treatments in Romania. Value Health (2017) 20:A690. doi:10.1016/j.jval.2017.08.1755
33. Kamusheva M, Tachkov K, Petrova G, Savova A, Manova M. Orphan medicinal products’ access to the Bulgarian pharmaceutical market – challenges and obstacles. Expert Opin Orphan Drugs (2018) 6(2):95–104. doi:10.1080/21678707.2018.1421063
34. Paris V, Slawomirski L, Colbert A, Delaunay N, Oderkirk J. New Health Technologies Managing Access, Value and Sustainability. Paris: OECD Publishing (2017). Available from: http://www.keepeek.com/Digital-Asset-Management/oecd/social-issues-migration-health/managing-new-technologies-in-health-care_9789264266438-en#page17
Keywords: reimbursement, Bulgaria, low and middle-income Balkan countries, innovative medicines, access, affordability, positive drug list
Citation: Kamusheva M, Vassileva M, Savova A, Manova M and Petrova G (2018) An Overview of the Reimbursement Decision-Making Processes in Bulgaria As a Reference Country for the Middle-Income European Countries. Front. Public Health 6:61. doi: 10.3389/fpubh.2018.00061
Received: 29 December 2017; Accepted: 16 February 2018;
Published: 05 March 2018
Edited by:
Nick Verhaeghe, Ghent University, BelgiumReviewed by:
Aleksandra Barac, Faculty of Medicine, University of Belgrade, SerbiaTomasz Holecki, Medical University of Silesia, Poland
Copyright: © 2018 Kamusheva, Vassileva, Savova, Manova and Petrova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guenka Petrova, Z3VlbmthLnBldHJvdmFAZ21haWwuY29t
 Maria Kamusheva
Maria Kamusheva Mariya Vassileva2
Mariya Vassileva2 Alexandra Savova
Alexandra Savova Manoela Manova
Manoela Manova Guenka Petrova
Guenka Petrova