
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 11 December 2017
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 5 - 2017 | https://doi.org/10.3389/fpubh.2017.00332
In 2011, Germany experienced one of the largest outbreaks of entero-hemorrhagic Escherichia coli (EHEC) ever reported. Four years thereafter, we systematically searched for scientific publications in PubMed and MEDPILOT relating to this outbreak in order to assess the pattern of respective research activities and to assess the main findings and recommendations in the field of public health. Following PRISMA guidelines, we selected 133 publications, half of which were published within 17 months after outbreak onset. Clinical medicine was covered by 71, microbiology by 60, epidemiology by 46, outbreak reporting by 11, and food safety by 9 papers. Those on the last three topics drew conclusions on methods in surveillance, diagnosis, and outbreak investigation, on resources in public health, as well as on inter-agency collaboration, and public communication. Although the outbreak primarily affected Germany, most publications were conducted by multinational cooperations. Our findings document how soon and in which fields research was conducted with respect to this outbreak.
In spring 2011, Germany experienced one of the largest outbreaks of entero-hemorrhagic Escherichia coli (EHEC) ever reported, almost 3,000 people fell ill with acute gastroenteritis, 855 of them developed hemolytic uremic syndrome (HUS). In total, 55 people died due to the infection (1). The outbreak affected primarily five states in Northern Germany but also visitors from 15 other countries and was linked to a smaller subsequent outbreak in France (2). In contrast to what would have been expected from previous outbreak reports and surveillance data, this outbreak was characterized by high case fatality, a higher proportion of HUS resulting from the EHEC infection (1.1 cases per 100,000 inhabitants in 2011 versus an average of 0.1 cases per 100,000 inhabitants yearly, in 2001–2010), a predominance of adult patients (3.10 HUS resulting from EHEC infection and 15.5 EHEC cases without HUS per 100,000 inhabitants in 2011 versus less than 0.0 HUS resulting from EHEC infection and EHEC cases without HUS per 100,000 inhabitants yearly, in 2001–2010) and a stronger predominance for female patients (HUS resulting from EHEC infection: 68% female patients, EHEC without HUS: 58% female patients) (1). The outbreak lasted 58 days, from May 8 to July 5, 2011. On May 25, 2011, seven days after the first notification of the outbreak, the causative pathogen was identified to be Shiga toxin (Verocytotoxin)-producing Escherichia coli O104:H4, a bacterium with a novel virulence profile in comparison to strains usually prevalent in Europe. From May 20 to July 8, 2011, the Robert Koch Institute (RKI), the German federal public health institute, together with local and state health and food safety agencies, conducted a total of 13 epidemiological field investigations, using different study designs. Initial investigations pointed at lettuce, raw tomatoes, and cucumbers as potential sources of the infection. On June 10—3 weeks after the first notification of the outbreak—epidemiological evidence supported that fenugreek sprouts, produced in Germany from seeds imported from Egypt, were the vehicle causing the outbreak (3). The outbreak resulted in massive challenges for hospitals, public health and food safety agencies, and intense international media coverage. There were significant economic repercussions on the agriculture industry of various European countries, particularly Spain, after the Hamburg secretary of health prematurely presented an unconfirmed laboratory result erroneously implicating cucumber imported from Spain as the potential source of infection (3). This, in turn, had international political and economic consequences, including a temporary embargo of food products exported from the European Union. Although epidemiological studies and investigations supported that fenugreek sprouts were the vehicle, various attempts to detect the pathogen in sprouts consumed by known patients had failed. In early evaluations of the outbreak, Krause et al. (3), Stark et al. (4), and Beutin and Martin (5) highlighted the uniqueness and size of the outbreak. They strongly recommended evaluating the experiences with the outbreak as best as possible in order to better prevent and control comparable situations in the future. In this work, we systematically reviewed the scientific literature related to this outbreak 4 years after its onset. The aim of the study was to trace the scientific process in order to assess to which extent the different disciplines were involved, to identify the collaborations established, and to find out which public health-related topics were researched. The results of this work can help public health authorities to better understand how the scientific community works under the conditions of a disease outbreak. With this knowledge, coordination and collaboration between public health authorities and scientists can be facilitated. This paper also encourages scientists from different disciplines to take a broader view during outbreak investigations, and to contemplate the challenges and potentials of interdisciplinary collaboration.
Following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (6), we searched for literature in PubMed and MEDPILOT using the search terms “EHEC” OR “O104:H4” OR “HUS” and their related Medical Subject heading (MESH) “Shigatoxigenic Escherichia coli” OR “hemolytic-uremic syndrome” in combination with “Germany” and its MESH term “Germany” appearing in title, abstract or key words for articles published between May 1, 2011 and April 30, 2015. We did not apply any restriction regarding the language of the publications. Since MEDPILOT does not allow retrospectively specifying exact search periods, we initially included publications from January 2011 to April 2015 and later manually excluded those published between January 1 and April 30, 2011. We also included any additional publications that were referenced in retrieved articles and met our inclusion criteria but were not listed in the two databases (Figure 1). We conducted a two-stage screening of the retrieved literature. First, titles and abstracts were screened. Second, eligibility for inclusion in the review was assessed by full-text screening of articles retained after the first screening. Two reviewers (EK and LK) independently screened the literature in duplicate. In the case of discrepant or uncertain results where the two reviewers could not reach consensus, a third reviewer (GK) was consulted. Besides the formal criterion of time of publication, studies had to meet the following criteria for inclusion: they had to be scientific articles published in a publicly accessible journal, but excluding work that was either non-original or not peer reviewed, such as government reports, editorials, commentaries, replies, meeting abstracts, and diaries (e.g., articles reflecting the course of the outbreak in daily intervals from a personal point of view). We also excluded all publications reporting on other outbreaks or strains, as well as publications on the outbreak in question but not constituting original research, such as treatment recommendations not based on research relating to this outbreak. We included articles on cases of O104:H4 infection outside Germany related to this outbreak. We then assigned the articles to one or more of the following pre-defined topics: Epidemiology, Food Safety, and Outbreak Reporting as Public Health topics and Medicine (including disease progression, non-microbiological diagnosis and treatment), and Microbiology (including microbiological diagnosis) as further topics. Contents of publications belonging to the topics medicine or microbiology though were not analyzed, as this would be beyond the public health scope of this review. We also recorded the countries of origin of the authors’ institutions in the categories, “Germany,” “non-German countries,” and international collaborations in the category “Germany and non-German countries.” We assigned each article to one or more of the subtopics listed in Table 1. Additionally, we assigned every article to one of the following three types of publication: review, situation report, and original research. We differentiated between reviews conducted for, or motivated by, the outbreak but not processing data on this outbreak (opportunity reviews); and reviews systematically processing publications on this outbreak (evaluation reviews). Situation reports comprised outbreak reports that were primarily descriptive in nature, while original research only included articles that reported analytical study designs. For the summarized analysis, situation reports and original research were comprised in the category “other publications.” The patterns of respective research activities, namely the publication latency differentiated by topic and the topics differentiated by countries of origin of the authors and by publication type, were assessed for all included publications. For all publications that belonged to the Public Health topics Epidemiology, Food Safety, and Outbreak Reporting, we extracted the main findings and recommendations using a data extraction form developed for this review.
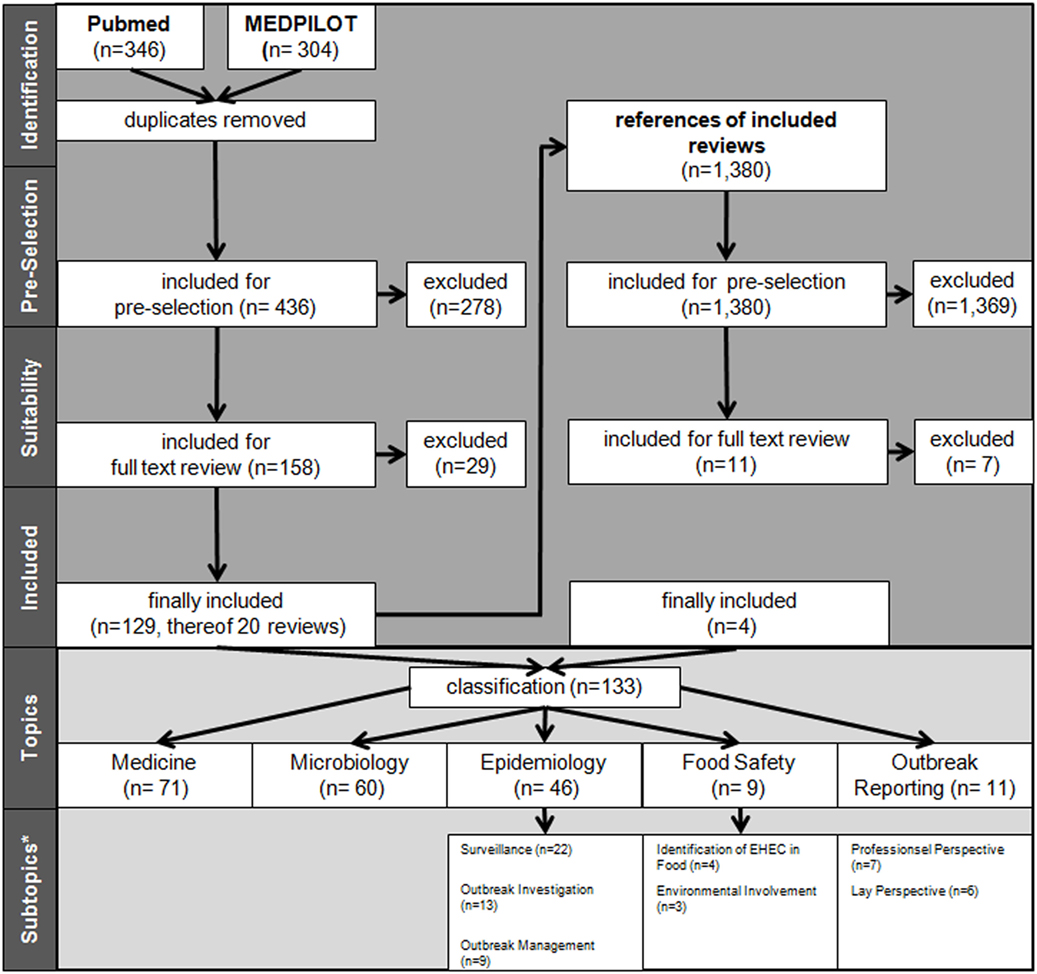
Figure 1. Flow process chart of literature search and selection. *Allocation for subtopics only for other types of articles and not for reviews. More than one topic/subtopic per paper (sums higher than totals).
By applying the search criteria, we found 346 publications in PubMed and 304 in MEDPILOT; 214 articles (32.9%) were duplicates, resulting in a total of 436 unique publications written in Chinese, Dutch, English, French, German, Polish, Russian, Spanish, or Swedish. Of these, 255 publications were excluded based on content criteria, 47 for being editorials, commentaries, replies, diaries, or meeting abstracts, and five because of being submitted or published before May 1, 2011, leaving 129 (29.6%) after full-text screening. We identified an additional 11 publications on scrutinizing reference lists of eligible reviews, of which four articles fulfilled our eligibility criteria. In total, 133 eligible publications (published in Chinese, Dutch, English, German, and Swedish language) were included in the systematic review (Figure 1; Table 2). Fifty-one articles were published within the first year after onset of the outbreak, 46 in the second year, 19 in the third, and 17 in the fourth year, with a peak of 19 publications in the last quarter of 2012 (Figure 2). In the first year after onset of the outbreak, 20 (24%) publications belonged to the category Epidemiology, ranging between 17 (26%) and 4 (14%) in the following years. Most publications in the first year after the outbreak belonged to the topic Microbiology (30 publications, 37%). The number of publications of this topic dropped to 16 (25%) in the second year and to 8 (29%) in the third year. In the first as well as in the second year after the outbreak, 24 publications appeared within the topic Medicine resulting in 29 and 37%, respectively, followed by 14 publications (50%) in the third year and 9 publications (41%) in the fourth year. As displayed in Figure 2, the topics Food Safety and Outbreak Reporting were the least frequently covered topics throughout the 4-year time period ranging from 6 to 0% for Food Safety and 4 to 8% for Outbreak Reporting. Half of the 133 articles were published within 17 months after the onset of the outbreak (reviews: median 16 months; other publication types: median 17 months). As Figure 2 reveals, we did not find any further literature related to the analyzed topics Outbreak Reporting, Food Safety, or Epidemiology after the third quarter of 2014. Publication latency for all other types of publications was lowest for the topic of Food Safety (median of 9 months) and highest for the topic of Outbreak Reporting (median 20 months). For reviews, publication latency was 11 months in the topic of Microbiology and 18 months in the topic of Medicine (Table 3). The extent of collaboration was determined within the different publication types and topics: International collaboration could be found in 3/20 reviews and in 28/113 other publication types. Most review articles were by institutions from non-German countries only (14/20), while other publication types were mostly by German institutions only (57/113). In total, international collaborations were seen in 31/133 articles. Most articles in the topic Medicine (42/71 = 59%) were exclusively authored by German institutions, whereas articles on Microbiology were mostly authored by non-German authors (32/60 = 53%). In the topic of Epidemiology the predominant authorship setup was German and international collaboration (19/46 = 41%) (Table 4). A total of 39 publications belonged to one or more of the topics Epidemiology, Food Safety, or Outbreak Reporting (Table 4). Their main findings and recommendations are displayed in Table 5. Within the topic of Epidemiology, conclusions and recommendations mainly addressed the following issues: (1) automatization of disease notification and changes in notification laws; (2) methodological improvements in surveillance; (3) personnel resources in public health; (4) factors related to secondary cases; (5) novel outbreak investigation methods; and (6) capacity and collaboration between different agencies and institutions. Within the topic of Food Safety, conclusions and recommendations addressed the issues of (1) sanitation of wastewater; (2) safety of drinking water; (3) methods to detect the pathogens in food/from food samples; and (4) hygienic handling of livestock. Regarding Outbreak Reporting, conclusions and recommendations mainly addressed the question of (1) timing of public information; (2) detail as well as coherence of communication; and (3) coordination of risk communication between different agencies and individuals.
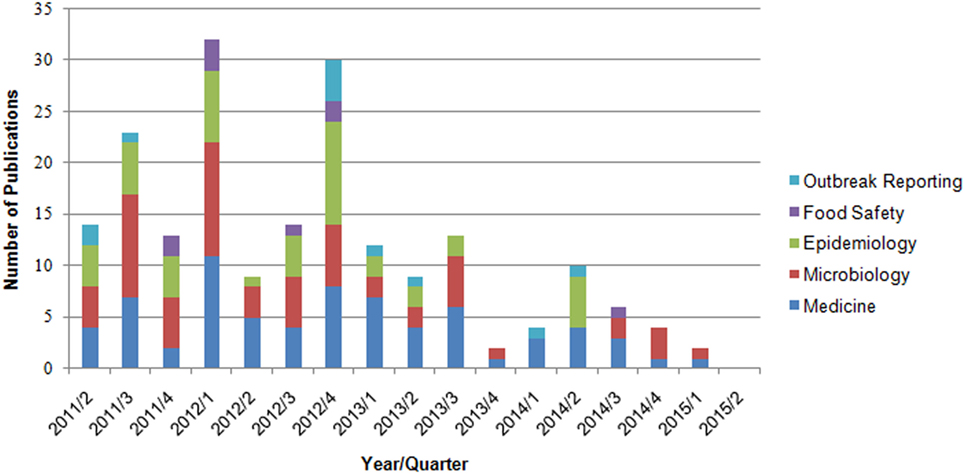
Figure 2. Number of publications by year/quarter and topic (n = 133 publications, one paper can be assigned to more than one topic).
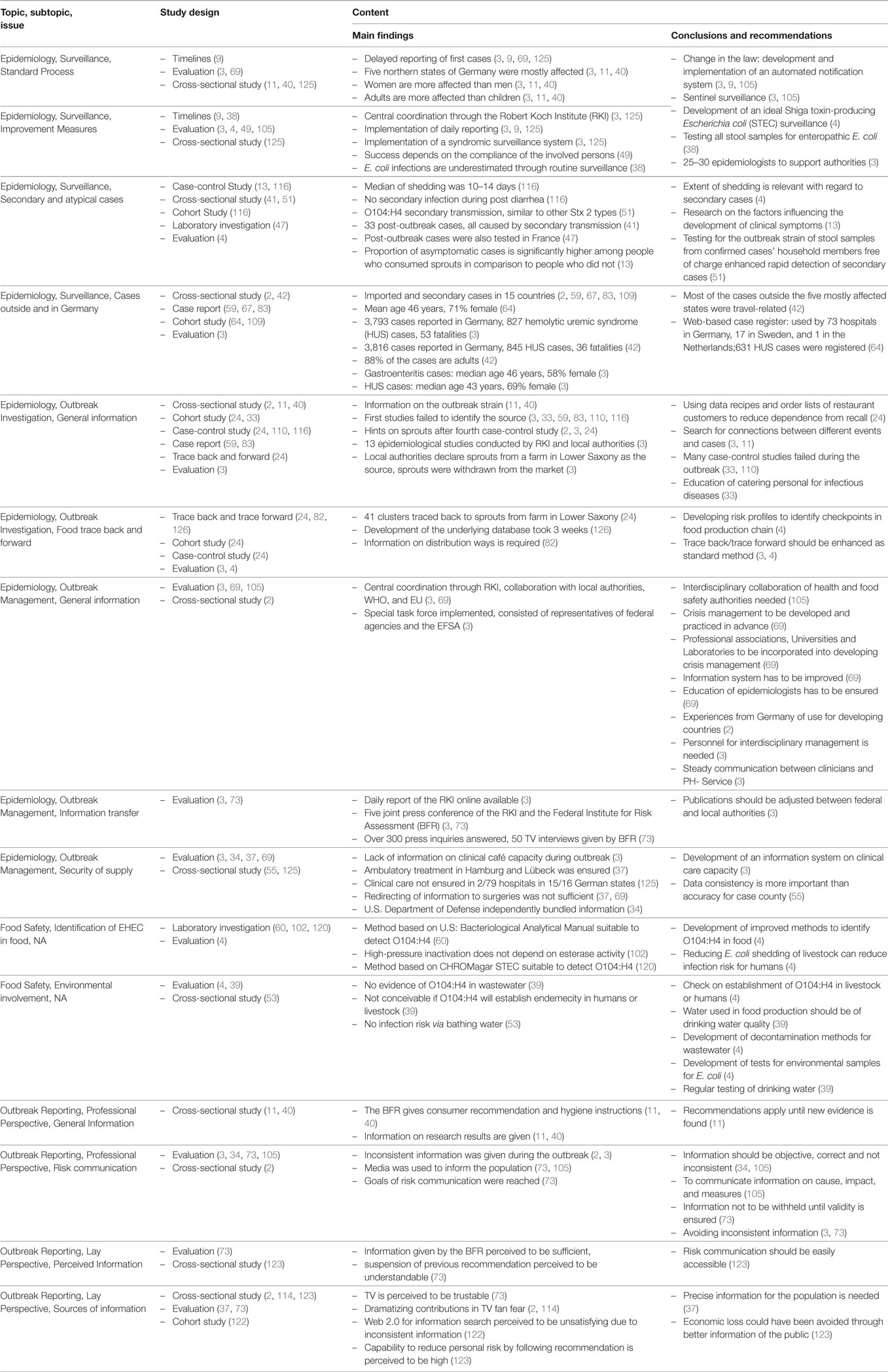
Table 5. Structural overview of main findings and recommendations of the extracted publications within the topics epidemiology, food safety, and outbreak reporting (n = 39).
We found a total number of 133 original publications directly related to one geographically and temporally rather confined outbreak. Although major publications appeared within days and weeks of the onset of the outbreak (11), the median latency of publications was 17 months with 17 papers (13% of the total) appearing as late as in the fourth year. This indicates that much of the research conducted with respect to this outbreak went beyond immediate outbreak investigations. It addressed research questions that are of relevance for food safety, surveillance, and outbreak investigation methods in general, but also relate to clinical and microbiological aspects of EHEC. This is further supported by the broad spectrum of topics and subtopics covered by these publications. We identified some characteristic differences among topics not only with respect to publication latency but also with respect to the composition of institutions contributing to the publications (Table 3). As expected, the majority of the articles were published exclusively by, or led by, German institutions. However, given that the outbreak primarily occurred in Germany, the proportion of non-German collaborators or lead authors appears to be high. Non-German participation or lead authorship was most common in Microbiological research on the causative E. coli strain and in reviews based on prior publications. This is easily explained by the fact that these kinds of publications did not require active involvement in the epidemiological investigation or clinical patient management (19, 118). This may also explain the somewhat unexpected observation that reviews tended to be published earlier than other types of publications (Table 3): while clinicians and public health agents were primarily occupied with managing the outbreak and later generating and analyzing the data derived from it, non-involved (and thus more likely non-German) specialists in the field could without any delay focus on analyzing the existing literature. Such division of tasks appears rational and useful, since early review on specific management questions could help those primarily involved in the management to adapt their interventions. Similarly, laboratories outside the primarily affected country can complement the microbiologic research related to the outbreak once an isolated strain is made available, thus supporting local laboratories to rapidly characterize the implicated pathogen. The EHEC outbreak was exceptional in many aspects and provided a unique opportunity for better understanding the disease (5). Some of the findings published on the outbreak may appear redundant (Table 5). This does not necessarily signify duplicate publication, since arriving at similar conclusions using different study populations or diverse analytic methods applied to the same study population is a desired process in science. It is also justified in an acute outbreak situation to quickly publish results of early analyses and later complement them with more complete or methodologically refined studies (73). We found many case reports, particularly in the field of Medicine. Due to their specific methodological limitations, individual case reports have a limited potential to contribute significantly to pressing research questions, unless a pooled analysis of cases is performed. Unfortunately, no such analysis seems to have been done so far. Our search strategy including two independent data bases and all languages is likely to have led to a rather comprehensive collection of publications. We believe our decision to exclude editorials, commentaries, replies and diaries, is unlikely to result in relevant original research findings to be missed. Furthermore, this approach ensured that only scientifically reviewed publications were included and it inhibited dissemination of politically motivated communications by stakeholders or interested parties. Nevertheless, larger and more comprehensive studies on the outbreak may still be published in the future, especially in the field of Medicine. The intention to address the patterns of respective research activities from diverse disciplines resulting from this outbreak, instead of addressing one specific research question, precluded us from conducting a meta-analysis. We limited data extraction to Public Health-related topics as we wanted to focus on the scientific output generated in this field. The observed latency of publications on the German EHEC outbreak 2011 highlights the fact that even several years after such an event, original research work continues to be published. We also found a considerable level of cooperation between large number of institutions from within and outside Germany. Our review was not designed to judge the content and scientific merit of these publications. We do not expect that the limitation to 4 years after onset of the outbreak influenced the results of this review as we observed that the number of publications decreased toward the end of the inclusion period. Moreover, novel findings and conclusions were less common in the later years of the observation period. This work reveals a unique map of how the EHEC outbreak was scientifically processed. Nevertheless, the large number of original publications found in our search, and the breadth of topics covered, suggests that the scientific community made appropriate use of this outbreak for research. Compared with the scientific output of the O157:H7 outbreak in the USA in 2009 and in Japan 1996, the EHEC outbreak in Germany has resulted in a higher number of publications. A preliminary search in PubMed indicates that the EHEC outbreak in Germany in 2011 has resulted in approximately four times as many publications as the E. coli O157:H7 outbreak in the USA and three times as many as the outbreak in Japan. The toll of the outbreak in terms of morbidity, mortality, and economic losses was undoubtedly high, which may explain the large body of scientific publications in diverse disciplines. The extent to which recommendations resulting from it have actually contributed to improvements in policies and practice merits follow-up research.
[PubMed {SCR:004846}].
GK conceived this study. EK led the literature review and conducted the descriptive and content analysis. EK and LK performed the systematic review. All authors (EK, LK, AS, OR, GK) contributed to the study design, interpretation of the data, and to the writing and revision of this paper. All authors agree to be accountable for the content of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Phillip Tarr, Washington University School of Medicine and Robert Tauxe, Centers for Disease Control and Prevention, Atlanta, for their encouragement and valuable advice. We also thank Helga Brink, Helmholtz Centre for Infection Research, for her administrative and editorial support, the librarians of Bielefeld University, and various colleagues who helped translating articles.
1. Robert Koch-Institut (RKI). AbschließendeDarstellung und Bewertung der epidemiologischenErkenntnisseim EHEC O104:H4 Ausbruch, Deutschland 2011. (2011). Available from: http://edoc.rki.de/documents/rki_ab/reeFNxULvsdZo/PDF/262b4Pk2TGGs.pdf
2. Rubino S, Cappuccinelli P, Kelvin DJ. Escherichia coli (STEC) serotype O104 outbreak causing haemolytic syndrome (HUS) in Germany and France. J Infect Dev Ctries (2011) 5(6):437–40. doi:10.3855/jidc.2172
3. Krause G, Frank C, Gilsdorf A, Mielke M, Schaade L, Stark K, et al. The 2011 HUS epidemic in Germany. Challenges for disease control: what should be improved? Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz (2013) 56(1):56–66. doi:10.1007/s00103-012-1585-1
4. Stark K, Bauerfeind R, Bernard H, Eckmanns T, Ethelberg S, Flieger A, et al. Experiences from the Shiga toxin-producing Escherichia coli O104:H4 outbreak in Germany and research needs in the field, Berlin, 28–29 November 2011. Euro Surveill (2012) 17(7):1–4. doi:10.2807/ese.17.07.20091-en
5. Beutin L, Martin A. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. J Food Prot (2012) 75(2):408–18. doi:10.4315/0362-028X.JFP-11-452
6. Ziegler A, Antes G, König I. Bevorzugte report items für systematischeÜbersichten und meta-analysen: das PRISMA-statement. Dtsch Med Wochenschr (2011) 136(08):e9–15. doi:10.1055/s-0031-1272978
7. Ahmed SA, Awosika J, Baldwin C, Bishop-Lilly KA, Biswas B, Broomall S, et al. Genomic comparison of Escherichia coli O104:H4 isolates from 2009 and 2011 reveals plasmid, and prophage heterogeneity, including Shiga toxin encoding phage stx2. PLoS One (2012) 7(11):e48228. doi:10.1371/journal.pone.0048228
8. Albers JM, Pavenstaedt H, Dlugos C, Lebiedz P, Ringelstein EB, Dittrich R, et al. An increased pulsatility index in transcranial Doppler sonography is associated with Shiga-toxin-related encephalopathy in hemolytic uremic syndrome. Cerebrovasc Dis (2012) 33(4):403–4. doi:10.1159/000335826
9. Altmann M, Wadl M, Altmann D, Benzler J, Eckmanns T, Krause G, et al. Timeliness of surveillance during outbreak of Shiga toxin-producing Escherichia coli infection, Germany, 2011. Emerg Infect Dis (2011) 17(10):1906–9. doi:10.3201/eid1710.111027
10. Artunc F, Amann K, Haap M. Hemolytic uremic syndrome following EHEC infection. Dtsch Med Wochenschr (2011) 136(38):1917. doi:10.1055/s-0031-1289123
11. Askar M, Faber MS, Frank C, Bernard H, Gilsdorf A, Fruth A, et al. Update on the ongoing outbreak of haemolytic uraemic syndrome due to Shiga toxin-producing Escherichia coli (STEC) serotype O104, Germany, May 2011. Euro Surveill (2011) 16(22):19883. doi:10.2807/ese.16.22.19883-en
12. Aurass P, Prager R, Flieger A. EHEC/EAEC O104:H4 strain linked with the 2011 German outbreak of haemolytic uremic syndrome enters into the viable but non-culturable state in response to various stresses and resuscitates upon stress relief. Environ Microbiol (2011) 13(12):3139–48. doi:10.1111/j.1462-2920.2011.02604.x
13. Balabanova Y, Klar S, Delere Y, Wilking H, Faber MS, Lassen SG, et al. Serological evidence of asymptomatic infections during Escherichia coli O104:H4 outbreak in Germany in 2011. PLoS One (2013) 8(9):e73052. doi:10.1371/journal.pone.0073052
14. Bannas P, Fraedrich K, Treszl A, Bley TA, Herrmann J, Habermann CR, et al. Shiga toxin-producing E. coli O104:H4 outbreak 2011 in Germany: radiological features of enterohemorrhagic colitis. Rofo (2013) 185(5):434–9. doi:10.1055/s-0032-1330520
15. Bauer A, Loos S, Wehrmann C, Horstmann D, Donnerstag F, Lemke J, et al. Neurological involvement in children with E. coli O104:H4-induced hemolytic uremic syndrome. Pediatr Nephrol (2014) 29(9):1607–15. doi:10.1007/s00467-014-2803-x
16. Beutin L, Hammerl JA, Reetz J, Strauch E. Shiga toxin-producing Escherichia coli strains from cattle as a source of the Stx2a bacteriophages present in enteroaggregative Escherichia coli O104:H4 strains. Int J Med Microbiol (2013) 303(8):595–602. doi:10.1016/j.ijmm.2013.08.001
17. Bielaszewska M, Idelevich EA, Zhang W, Bauwens A, Schaumburg F, Mellmann A, et al. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob Agents Chemother (2012) 56(6):3277–82. doi:10.1128/AAC.06315-11
18. Bielaszewska M, Mellmann A, Zhang W, Kock R, Fruth A, Bauwens A, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis (2011) 11(9):671–6. doi:10.1016/S1473-3099(11)70165-7
19. Bilinski P, Kapka-Skrzypczak L, Posobkiewicz M, Bondaryk M, Holownia P, Wojtyla A. Public health hazards in Poland posed by foodstuffs contaminated with E. coli O104:H4 bacterium from the recent European outbreak. Ann Agric Environ Med (2012) 19(1):3–10.
20. Bloch SK, Felczykowska A, Nejman-Falenczyk B. Escherichia coli O104:H4 outbreak-have we learnt a lesson from it? Acta Biochim Pol (2012) 59(4):483–8.
21. Borgatta B, Kmet-Lunacek N, Rello J. E. coli O104:H4 outbreak and haemolytic-uraemic syndrome. Med Intensiva (2012) 36(8):576–83. doi:10.1016/j.medin.2011.11.022
22. Braune SA, Wichmann D, von Heinz MC, Nierhaus A, Becker H, Meyer TN, et al. Clinical features of critically ill patients with Shiga toxin-induced hemolytic uremic syndrome. Crit Care Med (2013) 41(7):1702–10. doi:10.1097/CCM.0b013e31828a24a8
23. Brzuszkiewicz E, Thurmer A, Schuldes J, Leimbach A, Liesegang H, Meyer F, et al. Genome sequence analyses of two isolates from the recent Escherichia coli outbreak in Germany reveal the emergence of a new pathotype: entero-aggregative-haemorrhagic Escherichia coli (EAHEC). Arch Microbiol (2011) 193(12):883–91. doi:10.1007/s00203-011-0725-6
24. Buchholz U, Bernard H, Werber D, Bohmer MM, Remschmidt C, Wilking H, et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med (2011) 365(19):1763–70. doi:10.1056/NEJMoa1106482
25. Chattaway MA, Dallman T, Okeke IN, Wain J. Enteroaggregative E. coli O104 from an outbreak of HUS in Germany 2011, could it happen again? J Infect Dev Ctries (2011) 5(6):425–36. doi:10.3855/jidc.2166
26. Cheung MK, Li L, Nong W, Kwan HS. 2011 German Escherichia coli O104:H4 outbreak: whole-genome phylogeny without alignment. BMC Res Notes (2011) 4:533. doi:10.1186/1756-0500-4-533
27. Christner M, Trusch M, Rohde H, Kwiatkowski M, Schluter H, Wolters M, et al. Rapid MALDI-TOF mass spectrometry strain typing during a large outbreak of Shiga-toxigenic Escherichia coli. PLoS One (2014) 9(7):e101924. doi:10.1371/journal.pone.0101924
28. Cordesmeyer S, Peitz U, Godde N, Kasper HU, Hoffmann MW, Allemeyer E. Colonic ischaemia as a severe Shiga toxin/verotoxin producing Escherichia coli O104:H4 complication in a patient without haemolytic uraemic syndrome, Germany, June 2011. Euro Surveill (2011) 16:19895. doi:10.2807/ese.16.25.19895-en
29. Corogeanu D, Willmes R, Wolke M, Plum G, Utermohlen O, Kronke M. Therapeutic concentrations of antibiotics inhibit Shiga toxin release from enterohemorrhagic E. coli O104:H4 from the 2011 German outbreak. BMC Microbiol (2012) 12:160. doi:10.1186/1471-2180-12-160
30. Dammermann W, Schipper P, Ullrich S, Fraedrich K, Zur Schulze Wiesch J, Frundt T, et al. Increased expression of complement regulators CD55 and CD59 on peripheral blood cells in patients with EAHEC O104:H4 infection. PLoS One (2013) 8(9):e74880. doi:10.1371/journal.pone.0074880
31. Delannoy S, Beutin L, Burgos Y, Fach P. Specific detection of enteroaggregative hemorrhagic Escherichia coli O104:H4 strains by use of the CRISPR locus as a target for a diagnostic real-time PCR. J Clin Microbiol (2012) 50(11):3485–92. doi:10.1128/JCM.01656-12
32. Denamur E. The 2011 Shiga toxin-producing Escherichia coli O104:H4 German outbreak: a lesson in genomic plasticity. Clin Microbiol Infect (2011) 17(8):1124–5. doi:10.1111/j.1469-0691.2011.03620.x
33. Diercke M, Kirchner M, Claussen K, Mayr E, Strotmann I, Frangenberg J, et al. Transmission of shiga toxin-producing Escherichia coli O104:H4 at a family party possibly due to contamination by a food handler, Germany 2011. Epidemiol Infect (2014) 142(1):99–106. doi:10.1017/S0950268813000769
34. Dodd CC, Cooper MJ. Multidisciplinary response to the Escherichia coli 0104 outbreak in Europe. Mil Med (2012) 177(11):1406–10. doi:10.7205/MILMED-D-12-00097
35. Doulgere J, Otto B, Nassour M, Wolters-Eisfeld G, Rohde H, Magnus T, et al. Soluble plasma VE-cadherin concentrations are elevated in patients with STEC infection and haemolytic uraemic syndrome: a case-control study. BMJ Open (2015) 5(3):e005659. doi:10.1136/bmjopen-2014-005659
36. Dücker C, Dautel P, Wagner K, Przewozna J, Oehlerking S, Repenthin J, et al. Clinical symptoms, treatment and outcome of EHEC and EHEC-HUS patients treated as in-patients. Dtsch Med Wochenschr (2011) 136(36):1770–6. doi:10.1055/s-0031-1286099
37. Eisele M, Hansen H, Wagner H, von Leitner E, Pohontsch N, Scherer M. Epidemics and pandemics in general practice. What can we learn from the swine flu (H1N1) and EHEC outbreak? Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz (2014) 57(6):687–93. doi:10.1007/s00103-014-1970-z
38. Englund H, Hautmann W. Using an outbreak to study the sensitivity of the surveillance of enterohemorrhagic Escherichia coli and other enteropathic Escherichia coli in Bavaria, Germany, January to October 2011. Euro Surveill (2012) 17(34):20251. doi:10.2807/ese.17.34.20251-en
39. Exner M, Hartemann P, Hunter PR, Levi Y, Loret J, Mathys W, et al. Consensus report: E. coli O104:H4 (HUSEC041) and the potential threat to European water supplies. Int J Hyg Environ Health (2011) 214(6):500–1. doi:10.1016/j.ijheh.2011.08.001
40. Frank C, Faber MS, Askar M, Bernard H, Fruth A, Gilsdorf A, et al. Large and ongoing outbreak of haemolytic uraemic syndrome, Germany, May 2011. Euro Surveill (2011) 16(21):19878. doi:10.2807/ese.16.21.19878-en
41. Frank C, Milde-Busch A, Werber D. Results of surveillance for infections with Shiga toxin-producing Escherichia coli (STEC) of serotype O104:H4 after the large outbreak in Germany, July to December 2011. Euro Surveill (2014) 19(14):20760. doi:10.2807/1560-7917.ES2014.19.14.20760
42. Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med (2011) 365(19):1771–80. doi:10.1056/NEJMoa1106483
43. Ge S, Hertel B, Emden SH, Beneke J, Menne J, Haller H, et al. Microparticle generation and leucocyte death in Shiga toxin-mediated HUS. Nephrol Dial Transplant (2012) 27(7):2768–75. doi:10.1093/ndt/gfr748
44. Geerdes-Fenge HF, Lobermann M, Nurnberg M, Fritzsche C, Koball S, Henschel J, et al. Ciprofloxacin reduces the risk of hemolytic uremic syndrome in patients with Escherichia coli O104:H4-associated diarrhea. Infection (2013) 41(3):669–73. doi:10.1007/s15010-012-0387-6
45. Gerritzen A, Wittke J, Wolff D. Rapid and sensitive detection of Shiga toxin-producing Escherichia coli directly from stool samples by real-time PCR in comparison to culture, enzyme immunoassay and Vero cell cytotoxicity assay. Clin Lab (2011) 57(11–12):993–8.
46. Grad YH, Godfrey P, Cerquiera GC, Mariani-Kurkdjian P, Gouali M, Bingen E, et al. Comparative genomics of recent Shiga toxin-producing Escherichia coli O104:H4: short-term evolution of an emerging pathogen. MBio (2013) 4(1):e00452–412. doi:10.1128/mBio.00452-12
47. Grad YH, Lipsitch M, Feldgarden M, Arachchi HM, Cerqueira GC, FitzGerald M, et al. Genomic epidemiology of the Escherichia coli O104:H4 outbreaks in Europe, 2011. Proc Natl Acad Sci U S A (2012) 109(8):3065–70. doi:10.1073/pnas.1121491109
48. Greinacher A, Friesecke S, Abel P, Dressel A, Stracke S, Fiene M, et al. Treatment of severe neurological deficits with IgG depletion through immunoadsorption in patients with Escherichia coli O104:H4-associated haemolytic uraemic syndrome: a prospective trial. Lancet (2011) 378(9797):1166–673. doi:10.1016/S0140-6736(11)61253-1
49. Greutélaers B, Wadl M, Nachtnebel M, Rieck T, Hogan B, Adlhoch C, et al. Hospital surveillance during major outbreaks of community-acquired diseases. Pandemic Influenza Hospital Surveillance (PIKS) 2009/2010 and Surveillance of Bloody Diarrhea (SBD) 2011. Dtsch Med Wochenschr (2013) 138(13):632–7. doi:10.1055/s-0032-1332962
50. Hagel C, Krasemann S, Loffler J, Puschel K, Magnus T, Glatzel M. Up-regulation of Shiga toxin receptor CD77/Gb3 and interleukin-1beta expression in brain of EHEC patients with hemolytic uremic syndrome and neurologic symptoms. Brain Pathol (2014) 25(2):146–56. doi:10.1111/bpa.12166
51. Hauri AM, Gotsch U, Strotmann I, Krahn J, Bettge-Weller G, Westbrock H, et al. Secondary transmissions during the outbreak of Shiga toxin-producing Escherichia coli O104 in Hesse, Germany, 2011. Euro Surveill (2011) 16(31):19937. doi:10.2807/ese.16.31.19937-en
52. Hauswaldt S, Nitschke M, Sayk F, Solbach W, Knobloch JK. Lessons learned from outbreaks of Shiga toxin producing Escherichia coli. Curr Infect Dis Rep (2013) 15(1):4–9. doi:10.1007/s11908-012-0302-4
53. Heinemeyer E, Luden K, Monazahian M. On the risk of spread of E. coli/EHEC O104:H4 stx 2 positive bacteria via sewerage treatment plants during the 2011 EHEC outbreak in north Germany. Gesundheitswesen (2013) 75(8–9):512–4. doi:10.1055/s-0032-1327760
54. Ho C, Yuen K, Lau SKP, Woo PCY. Rapid identification and validation of specific molecular targets for detection of Escherichia coli O104:H4 outbreak strain by use of high-throughput sequencing data from nine genomes. J Clin Microbiol (2011) 49(10):3714–6. doi:10.1128/JCM.05062-11
55. Höhle M, an der Heiden M. Bayesian now casting during the STEC O104:H4 outbreak in Germany, 2011. Biometrics (2014) 70(4):993–1002. doi:10.1111/biom.12194
56. Huang X, Lu L, Deng X, Huang Q, Liang J, Zhang Y, et al. A notice from epidemiological investigation of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany, 2011. Zhonghua Liu Xing Bing Xue Za Zhi (2012) 33(1):111–4.
57. Ibarra C, Amaral MM, Palermo MS. Advances in pathogenesis and therapy of hemolytic uremic syndrome caused by Shiga toxin-2. IUBMB Life (2013) 65(10):827–35. doi:10.1002/iub.1206
58. Jansen A. Highlights from the clinical symposium on Shiga toxin-producing Escherichia coli/ haemolytic uremic syndrome, Berlin, September 2011. Euro Surveill (2011) 16:39. doi:10.2807/ese.16.39.19977-en
59. Januszkiewicz A, Szych J, Rastawicki W, Wolkowicz T, Chrost A, Leszczynska B, et al. Molecular epidemiology of a household outbreak of Shiga-toxin-producing Escherichia coli in Poland due to secondary transmission of STEC O104:H4 from Germany. J Med Microbiol (2012) 61(Pt 4):552–8. doi:10.1099/jmm.0.039289-0
60. Jinneman KC, Waite-Cusic JG, Yoshitomi KJ. Evaluation of Shiga toxin-producing Escherichia coli (STEC) method for the detection and identification of STEC O104 strains from sprouts. Food Microbiol (2012) 30(1):321–8. doi:10.1016/j.fm.2011.12.007
61. Kaper JB, O’Brien AD. Overview and historical perspectives. Microbiol Spectr (2014) 2:2. doi:10.1128/microbiolspec.EHEC-0028-2014
62. Karch H, Denamur E, Dobrindt U, Finlay BB, Hengge R, Johannes L, et al. The enemy within us: lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO Mol Med (2012) 4(9):841–8. doi:10.1002/emmm.201201662
63. Kern WV. Management of patients with EHEC/HUS. Lessons and perspectives from clinical infectious disease specialists. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz (2013) 56(1):87–94. doi:10.1007/s00103-012-1577-1
64. Kielstein JT, Beutel G, Fleig S, Steinhoff J, Meyer TN, Hafer C, et al. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome: an analysis of the German STECHUS registry. Nephrol Dial Transplant (2012) 27(10):3807–15. doi:10.1093/ndt/gfs394
65. Kleimann A, Toto S, Eberlein CK, Kielstein JT, Bleich S, Frieling H, et al. Psychiatric symptoms in patients with Shiga toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome. PLoS One (2014) 9(7):e101839. doi:10.1371/journal.pone.0101839
66. Knobloch JK, Hauswaldt S, Solbach W, Nitschke M, Sayk F. Antibiotic therapy in Shiga toxin producing Escherichia coli infection and colonization. GMS Infect Dis (2013). doi:10.3205/id000004
67. Kuijper EJ, Soonawala D, Vermont C, van Dissel JT. Household transmission of haemolytic uraemic syndrome associated with Escherichia coli O104:H4 in the Netherlands, May 2011. Euro Surveill (2011) 16(25):309–15. doi:10.2807/ese.16.25.19897-en
68. Laing CR, Zhang Y, Gilmour MW, Allen V, Johnson R, Thomas JE, et al. A comparison of Shiga-toxin 2 bacteriophage from classical enterohemorrhagic Escherichia coli serotypes and the German E. coli O104:H4 outbreak strain. PLoS One (2012) 7(5):e37362. doi:10.1371/journal.pone.0037362
69. Leidel J, Feil F. Structures and concepts for nationwide outbreak management in a federal state. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz (2013) 56(1):95–101. doi:10.1007/s00103-012-1578-0
70. Lipp MJ, Schirmer J, Feyerabend B, Stavrou GA, Cordruwisch W, Faiss S, et al. EHEC-associated colon stenosis after ulcerous-chronic haemorrhagic colitis and consecutive resulting ileus. Z Gastroenterol (2012) 50(5):453–6. doi:10.1055/s-0031-1299059
71. Löbel U, Eckert B, Simova O, Meier-Cillien M, Kluge S, Gerloff C, et al. Cerebral magnetic resonance imaging findings in adults with haemolytic uraemic syndrome following an infection with Escherichia coli, subtype O104:H4. Clin Neuroradiol (2014) 24(2):111–9. doi:10.1007/s00062-013-0231-0
72. Löwe B, Andresen V, Fraedrich K, Gappmayer K, Wegscheider K, Treszl A, et al. Psychological outcome, fatigue, and quality of life after infection with Shiga toxin-producing Escherichia coli O104. Clin Gastroenterol Hepatol (2014) 12(11):1848–55. doi:10.1016/j.cgh.2014.02.035
73. Lohmann M, Epp A, Roder B, Bol G. Risk communication of the Federal Institute for Risk Assessment during a food-related outbreak. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz (2013) 56(1):102–9. doi:10.1007/s00103-012-1589-x
74. Loirat C, Saland J, Bitzan M. Management of hemolytic uremic syndrome. Presse Med (2012) 41(3 Pt 2):e115–35. doi:10.1007/s00103-012-1589-x
75. Loman NJ, Constantinidou C, Christner M, Rohde H, Chan JZ, Quick J, et al. A culture-independent sequence-based metagenomics approach to the investigation of an outbreak of Shiga-toxigenic Escherichia coli O104:H4. JAMA (2013) 309(14):1502–10. doi:10.1001/jama.2013.3231
76. Loos S, Ahlenstiel T, Kranz B, Staude H, Pape L, Hartel C, et al. An outbreak of Shiga toxin-producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: presentation and short-term outcome in children. Clin Infect Dis (2012) 55(6):753–9. doi:10.1093/cid/cis531
77. Lorenzen JM, Menne J, Schmidt BMW, Schmidt M, Martino F, Dietrich R, et al. Circulating microRNAs in patients with Shiga-toxin-producing E. coli O104:H4 induced hemolytic uremic syndrome. PLoS One (2012) 7(10):e47215. doi:10.1371/journal.pone.0047215
78. Lukasz A, Beneke J, Menne J, Vetter F, Schmidt BMW, Schiffer M, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) in patients with Shiga toxin mediated haemolytic uraemic syndrome (STEC-HUS). Thromb Haemost (2014) 111(2):365–72. doi:10.1160/TH13-05-0387
79. Lukinmaa-Aberg S, Horsma J, Pasanen T, Mero S, Aulu L, Vaara M, et al. Applicability of DiversiLab repetitive sequence-based PCR method in epidemiological typing of enterohemorrhagic Escherichia coli (EHEC). Foodborne Pathog Dis (2013) 10(7):632–8. doi:10.1089/fpd.2012.1411
80. Lüth S, Frundt TW, Rosch T, Schlee C, Lohse AW. Prevention of hemolytic uremic syndrome with daily bowel lavage in patients with Shiga toxin-producing enterohemorrhagic Escherichia coli O104:H4 infection. JAMA Intern Med (2014) 174(6):1003–5. doi:10.1001/jamainternmed.2014.1175
81. Magnus T, Rother J, Simova O, Meier-Cillien M, Repenthin J, Moller F, et al. The neurological syndrome in adults during the 2011 northern German E. coli serotype O104:H4 outbreak. Brain (2012) 135(Pt 6):1850–9. doi:10.1093/brain/aws090
82. Manitz J, Kneib T, Schlather M, Helbing D, Brockmann D. Origin detection during food-borne disease outbreaks–a case study of the 2011 EHEC/HUS outbreak in Germany. PLoS Curr (2014) 6. doi:10.1371/currents.outbreaks.f3fdeb08c5b9de7c09ed9cbcef5f01f2
83. Marejkova M, Rohacova H, Reisingerova M, Petras P. An imported case of bloody diarrhea in the Czech Republic caused by a hybrid enteroaggregative hemorrhagic Escherichia coli (EAHEC) O104:H4 strain associated with the large outbreak in Germany, May 2011. Folia Microbiol (Praha) (2012) 57(2):85–9. doi:10.1007/s12223-011-0095-0
84. Maurer-Stroh S, Gunalan V, Wong W, Eisenhaber F. A simple shortcut to unsupervised alignment-free phylogenetic genome groupings, even from unassembled sequencing reads. J Bioinform Comput Biol (2013) 11(6):1343005. doi:10.1142/S0219720013430051
85. Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One (2011) 6(7):e22751. doi:10.1371/journal.pone.0022751
86. Menne J, Kielstein JT, Wenzel U, Stahl RAK. Treatment of typical hemolytic-uremic syndrome. Knowledge gained from analyses of the 2011 E. coli outbreak. Internist (Berl) (2012) 53(12):1420–30. doi:10.1007/s00108-012-3107-5
87. Menne J, Nitschke M, Stingele R, Abu-Tair M, Beneke J, Bramstedt J, et al. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ (2012) 345:e4565. doi:10.1136/bmj.e4565
88. Merabishvili M, de Vos D, Verbeken G, Kropinski AM, Vandenheuvel D, Lavigne R, et al. Selection and characterization of a candidate therapeutic bacteriophage that lyses the Escherichia coli O104:H4 strain from the 2011 outbreak in Germany. PLoS One (2012) 7(12):e52709. doi:10.1371/journal.pone.0052709
89. Monecke S, Mariani-Kurkdjian P, Bingen E, Weill F, Baliere C, Slickers P, et al. Presence of enterohemorrhagic Escherichia coli ST678/O104:H4 in France prior to 2011. Appl Environ Microbiol (2011) 77(24):8784–6. doi:10.1128/AEM.06524-11
90. Mora A, Herrrera A, Lopez C, Dahbi G, Mamani R, Pita JM, et al. Characteristics of the Shiga-toxin-producing enteroaggregative Escherichia coli O104:H4 German outbreak strain and of STEC strains isolated in Spain. Int Microbiol (2011) 14(3):121–41. doi:10.2436/20.1501.01.142
91. Moriel DG, Rosini R, Seib KL, Serino L, Pizza M, Rappuoli R. Escherichia coli: great diversity around a common core. MBio (2012) 3:3. doi:10.1128/mBio.00118-12
92. Neumann H, Hunstiger M, Langner C, Neurath MF, Vieth M. Bloody diarrhea caused by enterohemorrhagic Escherichia coli (EHEC). Endoscopy (2011) 43:E229–30. doi:10.1055/s-0030-1256596
93. Nitschke M, Sayk F, Hartel C, Roseland RT, Hauswaldt S, Steinhoff J, et al. Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA (2012) 307(10):1046–452. doi:10.1001/jama.2012.264
94. Page AV, Liles WC. Enterohemorrhagic Escherichia coli infections and the hemolytic-uremic syndrome. Med Clin North Am (2013) 97(4):681–95, xi. doi:10.1016/j.mcna.2013.04.001
95. Pareja-Tobes P, Manrique M, Pareja-Tobes E, Pareja E, Tobes R. BG7: a new approach for bacterial genome annotation designed for next generation sequencing data. PLoS One (2012) 7(11):e49239. doi:10.1371/journal.pone.0049239
96. Petruzziello-Pellegrini TN, Moslemi-Naeini M, Marsden PA. New insights into Shiga toxin-mediated endothelial dysfunction in hemolytic uremic syndrome. Virulence (2013) 4(6):556–63. doi:10.4161/viru.26143
97. Piérard D, de Greve H, Haesebrouck F, Mainil J. O157:H7 and O104:H4 Vero/Shiga toxin-producing Escherichia coli outbreaks: respective role of cattle and humans. Vet Res (2012) 43:13. doi:10.1186/1297-9716-43-13
98. Pritchard L, Holden NJ, Bielaszewska M, Karch H, Toth IK. Alignment-free design of highly discriminatory diagnostic primer sets for Escherichia coli O104:H4 outbreak strains. PLoS One (2012) 7(4):e34498. doi:10.1371/journal.pone.0034498
99. Qin J, Cui Y, Zhao X, Rohde H, Liang T, Wolters M, et al. Identification of the Shiga toxin-producing Escherichia coli O104:H4 strain responsible for a food poisoning outbreak in Germany by PCR. J Clin Microbiol (2011) 49(9):3439–40. doi:10.1128/JCM.01312-11
100. Radosavljevic V, Finke E, Belojevic G. Escherichia coli O104:H4 outbreak in Germany––clarification of the origin of the epidemic. Eur J Public Health (2014) 25(1):125–9. doi:10.1093/eurpub/cku048
101. Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med (2011) 365(8):709–17. doi:10.1056/NEJMoa1106920
102. Reineke K, Sevenich R, Hertwig C, Janssen T, Frohling A, Knorr D, et al. Comparative study on the high pressure inactivation behavior of the Shiga toxin-producing Escherichia coli O104:H4 and O157:H7 outbreak strains and a non-pathogenic surrogate. Food Microbiol (2015) 46:184–94. doi:10.1016/j.fm.2014.07.017
103. Richter AM, Povolotsky TL, Wieler LH, Hengge R. Cyclic-di-GMP signalling and biofilm-related properties of the Shiga toxin-producing 2011 German outbreak Escherichia coli O104:H4. EMBO Mol Med (2014) 6(12):1622–37. doi:10.15252/emmm.201404309
104. Riegel B, Broicher W, Wegscheider K, Andresen V, Brahler E, Lohse AW, et al. Quality of life one year post-Shiga toxin-producing Escherichia coli O104 infection––a prospective cohort study. Neurogastroenterol Motil (2015) 27(3):370–8. doi:10.1111/nmo.12503
105. Rissland J, Kielstein JT, Stark K, Wichmann-Schauer H, Stumpel F, Pulz M. The EHEC O104:H4 outbreak in Germany 2011––lessons learned? Gesundheitswesen (2013) 75(4):184–9. doi:10.1055/s-0033-1341444
106. Rohde H, Qin J, Cui Y, Li D, Loman NJ, Hentschke M, et al. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med (2011) 365(8):718–24. doi:10.1056/NEJMoa1107643
107. Ross B, Witzke O, Kribben A, Heintschel von Heinegg E, Buer J, Gerken G, et al. Managing EHEC in hospital routine. Dtsch Med Wochenschr (2012) 137(18):933–6. doi:10.1055/s-0032-1304923
108. Rund SA, Rohde H, Sonnenborn U, Oelschlaeger TA. Antagonistic effects of probiotic Escherichia coli Nissle 1917 on EHEC strains of serotype O104:H4 and O157:H7. Int J Med Microbiol (2013) 303(1):1–8. doi:10.1016/j.ijmm.2012.11.006
109. Samuelsson O, Follin P, Rundgren M, Rylander C, Selga D, Ståhl A. HUS-epidem in sommaren 2011 varallvarlig. Tyska och svenska erfarenheter av EHEC-utbrottet. Läkartidningen (2012) 109(25):1230–4.
110. Scharlach M, Diercke M, Dreesman J, Jahn N, Krieck M, Beyrer K, et al. Epidemiological analysis of a cluster within the outbreak of Shiga toxin-producing Escherichia coli serotype O104:H4 in Northern Germany, 2011. Int J Hyg Environ Health (2013) 216(3):341–5. doi:10.1016/j.ijheh.2012.10.001
111. Scheppach W, Reissmann N, Breunig E, Konwisorz A, Schwarz TF, Muller JG, et al. Segmental necrotizing colitis in a patient with E. coli O104:H4 infection. Z Gastroenterol (2012) 50(2):209–12. doi:10.1055/s-0031-1299289
112. Scheutz F, Nielsen EM, Frimodt-Moller J, Boisen N, Morabito S, Tozzoli R, et al. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro Surveill (2011) 16:24. doi:10.2807/ese.16.24.19889-en
113. Scholl D, Gebhart D, Williams SR, Bates A, Mandrell R. Genome sequence of E. coli O104:H4 leads to rapid development of a targeted antimicrobial agent against this emerging pathogen. PLoS One (2012) 7(3):e33637. doi:10.1371/journal.pone.0033637
114. Schulz C, Schutte K, Jacobi CA, Hulsemann JL, Malfertheiner P. TV news and concerns about––the EHEC-outbreak 2011 in Germany. Z Gastroenterol (2014) 52(3):277–80. doi:10.1055/s-0033-1350189
115. Simova O, Weineck G, Schuetze T, Wegscheider K, Panzer U, Stahl RAK, et al. Neuropsychological outcome after complicated Shiga toxin-producing Escherichia coli infection. PLoS One (2014) 9(7):e103029. doi:10.1371/journal.pone.0103029
116. Sin MA, Takla A, Flieger A, Prager R, Fruth A, Tietze E, et al. Carrier prevalence, secondary household transmission, and long-term shedding in 2 districts during the Escherichia coli O104:H4 outbreak in Germany, 2011. J Infect Dis (2013) 207(3):432–8. doi:10.1093/infdis/jis702
117. Soolsma J, Yo M, Bakker L, Kingma P, Gamadia L, Lobatto S. Een patiënt met hemolytisch-uremisch syndroom en infectie met enterohemorragische Escherichia coli (EHEC). Ned Tijdschr Geneeskd (2011) 155:A3809.
118. Soon JM, Seaman P, Baines RN. Escherichia coli O104:H4 outbreak from sprouted seeds. Int J Hyg Environ Health (2013) 216(3):346–54. doi:10.1016/j.ijheh.2012.07.005
119. Trachtman H, Austin C, Lewinski M, Stahl RAK. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol (2012) 8(11):658–69. doi:10.1038/nrneph.2012.196
120. Tzschoppe M, Martin A, Beutin L. A rapid procedure for the detection and isolation of enterohemorrhagic Escherichia coli (EHEC) serogroup O26, O103, O111, O118, O121, O145 and O157 strains and the aggregative EHEC O104:H4 strain from ready-to-eat vegetables. Int J Food Microbiol (2012) 152(1–2):19–30. doi:10.1016/j.ijfoodmicro.2011.10.009
121. Ullrich S, Bremer P, Neumann-Grutzeck C, Otto H, Ruther C, von Seydewitz CU, et al. Symptoms and clinical course of EHEC O104 infection in hospitalized patients: a prospective single center study. PLoS One (2013) 8(2):e55278. doi:10.1371/journal.pone.0055278
122. van Velsen L, van Gemert-Pijnen JEWC, Beaujean DJMA, Wentzel J, van Steenbergen JE. Should health organizations use web 2.0 media in times of an infectious disease crisis? An in-depth qualitative study of citizens’ information behavior during an EHEC outbreak. J Med Internet Res (2012) 14(6):e181. doi:10.2196/jmir.2123
123. de Vocht M, Cauberghe V, Sas B, Uyttendaele M. Analyzing consumers’ reactions to news coverage of the 2011 Escherichia coli O104:H4 outbreak, using the Extended Parallel Processing Model. J Food Prot (2013) 76(3):473–81. doi:10.4315/0362-028X.JFP-12-339
124. Vonberg RP, Höhle M, Aepfelbacher M, Bange FC, Belmar Campos C, Claussen K, et al. Duration of fecal shedding of Shiga toxin-producing Escherichia coli O104:H4 in patients infected during the 2011 outbreak in Germany: a multicenter study. Clin Infect Dis (2013) 56(8):1132–40. doi:10.1093/cid/cis1218
125. Wadl M, Rieck T, Nachtnebel M, Greutelaers B, an der Heiden M, Altmann D, et al. Enhanced surveillance during a large outbreak of bloody diarrhoea and haemolytic uraemic syndrome caused by Shiga toxin/verotoxin-producing Escherichia coli in Germany, May to June 2011. Euro Surveill (2011) 16(24):19893. doi:10.2807/ese.16.24.19893-en
126. Weiser AA, Gross S, Schielke A, Wigger J, Ernert A, Adolphs J, et al. Trace-back and trace-forward tools developed ad hoc and used during the STEC O104:H4 outbreak 2011 in Germany and generic concepts for future outbreak situations. Foodborne Pathog Dis (2013) 10(3):263–9. doi:10.1089/fpd.2012.1296
127. Weissenborn K, Donnerstag F, Kielstein JT, Heeren M, Worthmann H, Hecker H, et al. Neurologic manifestations of E coli infection-induced hemolytic-uremic syndrome in adults. Neurology (2012) 79(14):1466–73. doi:10.1212/WNL.0b013e31826d5f26
128. Wengenroth M, Hoeltje J, Repenthin J, Meyer TN, Bonk F, Becker H, et al. Central nervous system involvement in adults with epidemic hemolytic uremic syndrome. AJNR Am J Neuroradiol (2013) 34(5):1016–21, S1. doi:10.3174/ajnr.A3336
129. Werber D, King LA, Muller L, Follin P, Buchholz U, Bernard H, et al. Associations of age and sex with the clinical outcome and incubation period of Shiga toxin-producing Escherichia coli O104:H4 infections, 2011. Am J Epidemiol (2013) 178(6):984–92. doi:10.1093/aje/kwt069
130. Wu C, Hsueh P, Ko W. A new health threat in Europe: Shiga toxin-producing Escherichia coli O104:H4 infections. J Microbiol Immunol Infect (2011) 44(5):390–3. doi:10.1016/j.jmii.2011.07.001
131. Würzner R, Riedl M, Rosales A, Orth-Holler D. Treatment of enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome (eHUS). Semin Thromb Hemost (2014) 40(4):508–16. doi:10.1055/s-0034-1375298
132. Zhang W, Bielaszewska M, Bauwens A, Fruth A, Mellmann A, Karch H. Real-time multiplex PCR for detecting Shiga toxin 2-producing Escherichia coli O104:H4 in human stools. J Clin Microbiol (2012) 50(5):1752–4. doi:10.1128/JCM.06817-11
133. Zhang W, Bielaszewska M, Kunsmann L, Mellmann A, Bauwens A, Kock R, et al. Lability of the pAA virulence plasmid in O104:H4: implications for virulence in humans. PLoS One (2013) 8(6):e66717. doi:10.1371/journal.pone.0066717
134. Zhou K, Ferdous M, de Boer RF, Kooistra-Smid AMD, Grundmann H, Friedrich AW, et al. The mosaic genome structure and phylogeny of Shiga toxin-producing Escherichia coli O104:H4 is driven by short-term adaptation. Clin Microbiol Infect (2014) 21(5):468.e7–18. doi:10.1016/j.cmi.2014.12.009
Keywords: disease outbreaks, Shiga-toxigenic/entero-hemorrhagic Escherichia coli, hemolytic uremic syndrome, Germany, research
Citation: Köckerling E, Karrasch L, Schweitzer A, Razum O and Krause G (2017) Public Health Research Resulting from One of the World’s Largest Outbreaks Caused by Entero-Hemorrhagic Escherichia coli in Germany 2011: A Review. Front. Public Health 5:332. doi: 10.3389/fpubh.2017.00332
Received: 13 March 2017; Accepted: 23 November 2017;
Published: 11 December 2017
Edited by:
Alex W. Friedrich, University Medical Center Groningen, NetherlandsReviewed by:
Zisis Kozlakidis, University College London, United KingdomCopyright: © 2017 Köckerling, Karrasch, Schweitzer, Razum and Krause. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gérard Krause, Z2VyYXJkLmtyYXVzZUBoZWxtaG9sdHotaHppLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.