- Anesthesiology, Pain and Palliative Care Section, Kongsberg Hospital, Vestre Viken Hospital Trust, Kongsberg, Norway
The understanding of heart rate variability (HRV) has increased parallel with the development of modern physiology. Discovered probably first in 1847 by Ludwig, clinical applications evolved in the second part of the twentieth century. Today HRV is mostly used in cardiology and research settings. In general, HRV can be measured over shorter (e.g., 5–10 min) or longer (12 or 24 h) periods. Since 1996, most measurements and calculations are made according to the standard of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. As the first step, the series of times between successive R-peaks in the ECG are in milliseconds. It is crucial, however, to identify and remove extrasystoles and artifacts according to standard protocols. The series of QRS distances between successive heartbeats can be analyzed with simple or more sophisticated algorithms, beginning with standard deviation (SDNN) or by the square root of the mean of the sum of squares of differences between adjacent normal RR (rMSSD). Short-term HRV is frequently analyzed with the help of a non-parametric fast Fourier transformation quantifying the different frequency bands during the measurement period. In the last decades, various non-linear algorithms have been presented, such as different entropy and fractal measures or wavelet analysis. Although most of them have a strong theoretical foundation, their clinical relevance is still debated.
Introduction
Heartbeat varies over time. This has been observed early in medicine. Variations and patterns of heart beat have been associated with pathological conditions already 2,000 years ago (1). However, first in the last 100 years, conceptual ideas evolved, and understanding of involved mechanisms increased, in particular since 1996 when a standard was established and parameters defined (2).
The increasing interest in heart rate variability (HRV) can partially be explained by the feasibility of the method. Data can in principle be obtained by a simple one-channel ECG or even a pulse watch; data are processed by user-friendly programs. In reality, the issue is more complicated {(3) #2021} {(4) #2317} {(5) #2320}. Whether pulse watch-generated HRV calculations can be used is still a matter of debate {(6) #2326} {(7, 8) #2022} {(9) #2319}. Automated recognition of R-peaks is prone to errors {(10) #2323} and manual editing is still the gold standard, which impairs clinical use. No overall accepted normal values exist. In the beginning, HRV was first usually calculated based on 24-h recordings. Eventually, new algorithms were introduced (explained below) and clinical studies supported the use of short-term measurements. HRV has thus changed to an apparently simple point-of-care method obtained within 2–10 min with potential clinical value for the patients regarding risk stratification, individual therapeutic strategies, and even therapeutically in the form of HRV-biofeedback.
This review intends to give an overview of the developments of HRV in the last decades. It is basically descriptive. In earlier work (11), an extensive literature search was conducted, based mainly on the simple keyword “Heart Rate Variability” in the US National Library of Science (PubMed) and consecutive search in the reference lists of the identified articles. This review extends and updates this work although only the most central publications, chosen by the author will be discussed, for the sake of clarity.
Therefore, in this review, only a brief history of HRV will be presented. In the second part, the methods of signal measurement will be introduced. Most important algorithms for HRV analysis will be explained, but algorithms (still) not being used in clinical research or practice will not be mentioned. In addition, some possible confounding mechanisms of importance will be reported. Finally, a brief perspective of HRV for the future will be offered.
History
Pulse diagnosis has been early a part of ancient medicine and descriptions include its variation over timeWestern medical historians usually quote Galen as one of the first analyzing pulse patterns in human patients. Pulse diagnosis was, however, an important part of ancient Chinese and Indian medicine, too. In China, pulse diagnosis was investigated as early as between 800 and 200 BCE. For instance, in Chinese Medicine, Bian Que (扁鹊, about 500 BCE, also known as Qin Yueren, 秦 越人), living about one generation before Hippocrates described the “four diagnostic methods” of Traditional Chinese Medicine, in particular tongue and pulse diagnostics. All these forms of historical pulse analysis described patterns qualitatively. Quantitative measurements were first possible after the introduction of exact time measuring devices.
Variations of arterial blood pressure during the respiratory cycle was observed again in the eighteenth century, probably first of Stephen Hales. His observation of HRV was based on conducting measurements of blood pressure in some animal species (mostly dogs) by inserting fine cannulas into arteries and measuring the height to which the column of blood rose (12). Carl Ludwig (1816–1895) described a link between heartbeat fluctuations and respiration [respiratory sinus arrhythmia (RSA)] when investigating the frequency and pulse wave in dogs using a special instrument (“kymograph”) (13). One of the founders of experimental psychology, Wilhelm Wundt (1832–1920), made similar observations and introduced the notion of using physiological measures to investigated psychological mechanisms.
The French physiologist, Claude Bernard (1813–1878), introduced the term “milieu intérieur,” a basic principle to homeostasis. This internal environment is “constituted, in particular, by the fluids circulating in the body.” The American physiologist, Walter Bradford Cannon (1871–1945), expanded Bernards concept of homeostasis, beyond others by the two claims that the regulating system determining the state of the homeostasis consists of several connected subsystems. According to Cannon homeostasis is a consequence of self-organizing systems (termed self-government by Cannon). An important paradigm in HRV is based on Bernard’s and Cannon’s notion. Stable homeostasis is according to this concept connected to increased variability of HRV (14).
The classical model of autonomic control describes a balance between parasympathetic and sympathetic activation. It was also proposed by Cannon (15) and later expanded by Langley (16) who divided the autonomic outflows between sympathetic and parasympathetic elements, a division used until today. Cannon associated also increased activity in the sympathetic system with the evolutionary notion of “fight and flight.” In his seminal book, Langley erroneously defined the ANS as a purely visceral motor system, mediating the consequences of central nervous states to the periphery [today we know that 80% of vagal fibers are in reality afferent, providing important information to the brain regarding the state of the visceral organs (17)]. Hering described the functional relation between the amplitude of RSA and the vagal tone in 1910 (18). His son provided experimental data describing the baroreceptor reflex more exactly in 1927 (19).
Some years later, Adrian et al. published for the first time the behavior of the sympathetic nervous system in anesthetized rabbits and cats (20). At the same time, Maltzberg observed the association between cardiac disease and major depression, at this time termed “involution melancholia” (21), an association leading to important research in the last decades.
In 1965 investigations of the HRV of fetal ECGs revealed diminished variability after contractions when the fetus was distressed (22). This principle is still a cornerstone in monitoring fetus under labor. In cardiology, the relationship between the nervous system status and HRV was described by Wolf (23), 2 years after Valbona et al. described HRV changes in patients with serious brain damage (24).
Katona and Jih (25) introduced a non-invasive approach to measuring cardiac parasympathetic control in anesthetized dogs where they were able to control respiration rate. They introduced the notion that the magnitude of sinus arrhythmia is associated with changes in the vagal tone; assuming a linear association between vagal efferent activity and the change of heart period, and that during inspiration the cardiac vagal input is inhibited. Akselrod et al. applied power spectrum analysis of short-term HRV in an animal model, showing the association between different frequency ranges and the sympathetic and parasympathetic activity (26).
At this time, portable ECG-measurements became more frequent. Until then, HRV was mainly determined by measuring RR-distances with a ruler. Eventually, electrical circuits identifying the peak of R-waves and the time of the intervals with an accuracy of milliseconds were developed. First investigations of HRV were based on 24-h recordings by Holter monitoring. This changed when Axelrod started to analyze the frequency domain of HRV also in humans by using short-term HRV of 10 min or less (27). Earlier, spectral analysis methods were utilized in some study, investigating driver fatigue {(28) #2332}, the effect of aging on HRV {(29) #2333}, or in hypertension {(30) #2334}. Of particular importance at this time was also the increasing interest in non-linear phenomena. In particular Goldberger, the founder of the prominent website “Physionet,” began to focus more on non-linear algorithms (31–33). Looking closer on his articles shows relevant influences: he quotes May’s important article about evolutionary models (34) and Haken’s (35), and Shaw’s articles about chaos theory and strange attractors (36). Hermann Haken, physicist conducted research on self-organizing systems and founded at the end of 1960s synergetics, an interdisciplinary science investigating the formation and self-organization of patterns and structures in open systems far from equilibrium—characteristic for most physiological systems {(37) #2327}. Robert May introduced the use of models to test stability and fragility of systems {(38) #2328}. Robert Shaw was one of the pioneers of chaos theory at this time.
The breakthrough of HRV in cardiology occurred when the association between SDNN (explanation, see below) and the mortality after acute myocardial infarction was discovered. Probably, the first observation was made public by Australian group 1978, describing an association between sinus arrhythmia and survival after acute myocardial infarction {(39) #2329}. A landmark study of Kleiger et al. {(40) #1561} and several important cardiologic HRV studies followed, e.g., Ref. (41–44), frequently combining traditional cardiological measures with HRV. Bigger’s introduction of short-term measures (45) and Kleiger’s study were significant reasons to form the joint Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (2). The Task Force proposed minimal technical requirements, definitions, standardized the areas of Power bands in frequency domain and offered recommendations for clinical research and patient examinations. This article is still the most frequently cited HRV paper. Nearly every study after 1996 is based on this standard, and no major revision appeared until recently—the presentation of currently accepted linear measures is comprehensive, and the clinical signification of the non-linear parameters is still unclear. A recent joint position statement of the European Society of Cardiology and the European Heart Rhythm Association stated a lack of communication between mathematicians and engineers developing new algorithms and clinicians. It recommends, however, the combined use of linear and non-linear measures (46). A recent study provided reference values obtained by healthy individuals (7, 8), with limited relevance because they were recorded with Holter monitoring 24 h and are, therefore, not applicable for short-term measurements. The study was also criticized because of inconsistencies and unrealistic values, beyond others (3).
Already more than one century ago scientists observed and proposed associations between imbalances of the ANS and (pathological) mental states. Notions included that dysfunctional mental states might be associated with excessive vagal outflow (47), with imbalances between the sympathetic and parasympathetic system (48), or with excessive sympathetic outflow (49). Already Lacey and Lacey reported personality traits associated with greater HRV (50). Early work of Porges and Raskin showed mental state associations with HRV (51). This notion was later extended and elaborated by Porges (Polyvagal Theory) and Thayer (Neurovisceral Integration Model) (52–55).
Today, HRV has been used in more than 2,000 clinical trials and has been mentioned in more than 14,000 articles (46). It is used as an algorithm in sports watches and frequently appears in new Apps in electronic devices, mostly for health or training purposes (7, 8). The clinical use, however, is still invariant.
Probably the most relevant use of HRV in clinical practice is risk stratification. Several studies have shown clear associations between decreased HRV and the risk of sudden cardiac death (56–58) and the value of using HRV has been recognized (58–60). In some centers, HRV, together with other variables is used to identify patients at high risk for sudden cardiac death (61). This has consequences for treatment because the identified individuals received Automated Implantable Cardioverter-Defibrillators, an expensive, but a highly effective method. HRV is also established in the identification of cardiac autonomic neuropathy caused by diabetes and part of standardized examination protocols (62, 63). An emerging field is the use of HRV to predict systemic infections in critical care medicine. However, HRV is only utilized in some hospitals, and more often still not implemented in clinical practice (64, 65).
Based on the mentioned models and concepts above, HRV is also increasingly used in psychological research. The general hypothesis there is that higher levels of HRV parameters associated with activity in the parasympathetic system are also associated with better adaptivity to perturbations and better stress response. A recent meta-analysis confirmed this hypothesis, showing significant associations, although the absolute differences were small. Interestingly not only parasympathetic but also higher general HRV parameters were related to greater adaptivity (66). As an example, HRV has been used as a method in anxiety research. According to the neurovisceral model, anxiety disorders can be characterized by a breakdown of the inhibitory processes of the central autonomic network (67). This disinhibition is permanently linked to the continual state of excessive worry and mirrored by the decreased activity of the parasympathetic system. Several studies have investigated individuals with different kinds of anxiety disorders and have supported this notion in general anxiety (68), various forms of panic disorder (69), social anxiety (70), stress-associated anxiety (71), and trait anxiety (72). A closer look at these studies also shows the problems—e.g., in an experimental study looking on correlations between electric skin conductance, startle blink reflex and resting HRV (rMSSD) during conditioned fear inhibition and extinction. Higher rMSSD was associated with pronounced fear inhibition and extinction (indexed with startle blink potentiation), but the effect is most pronounced at the group level, and the scatter plot shows rather a point cloud instead of a clear regression line (73).
The newer history of HRV research is closely associated with the history of complexity research. As already mentioned, Ary Goldberger was inspired by publications of beyond others von Haken. He is one of the European representatives of a research tradition trying to understand systems. A system is regarded as a set of different parts (or subsystems) connected through positive and negative feedback circles. The fundamental notion of complexity science is that the whole system has more properties as the sum of properties of its parts. In other words, if you analyze the parts of the system separately, and add all results, there will be properties which cannot be explained out of this. Another term for this approach is non-linear science. Linear systems can be described by an addition of the equations describing its parts. If the subsystems interact, the system behaves non-linear and its behavior cannot be predicted by analyzing its parts. A set of equations characterizing a non-linear system can usually neither be solved with analytical mathematical methods {(74) #2335}.
Non-linear systems behave different compared to linear systems {(75) #2352}. Key notions are robustness and fragility. System robustness is often defined as the quality of a biological system or network to maintain its components, structure, and function despite both external changes and endogenous fluctuations {(76) #2337} {(77) #2338}. Fragility is connected to robustness. A property of complex systems is a conservation of sensitivity. When robustness is improved in one area, it leads to increased fragility in another {(78) #2339}. Complex systems are, therefore, robust, yet fragile by cascading failures initiated by tiny perturbations which may lead to a complete breakdown, or to a fundamental system change, termed emergence {(79) #2340}. Essential tools to study complexity are mathematical models and time-series analysis. HRV is the most used time-series analysis in medicine. The complexity paradigm has been explicitly used of Thayer and Lane in their neurovisceral model (55). The study of HRV has been influenced by dynamical systems theory, the study of fractal systems and chaos theory. It was also influenced by notions of self-organizing systems, network theories, and by modeling methods {(11) #1498}.
Methods
Investigating HRV needs a three-step approach. First, a condition should be defined where the measuring of heart rate signals and its variability gives relevant information. For the second, it is important to detect the signal as adequate as possible, to identify potential artifacts and manage them and at the end to obtain a time-series in milliseconds between the heartbeats which can be analyzed. The third step consists of different forms of analysis which again return various parameters to be used to analyze the state of the system.
Preparing a measurement of HRV should involve answers to several questions. The length of measurement is relevant for the kind of parameters of interest. When the focus is on basic parameters, a measurement period of 5 min or even less might be enough. When long-term fluctuations are relevant, a longer measurement period is necessary. Several non-linear parameters do also need a longer measurement period than 5 min. Details are given in Table 1. Recently, ultra-short-term analysis has been proposed for some parameters (80). According to these reports, the time domain measure root Mean Sum of Squared Distances (rMSSD) and the frequency domain measure High Frequency Power (HF, both explained in the next sections) can be reliably measured in time-series of 10–30 s.
Most algorithms for the analysis of short-term HRV require stationarity of the heart rhythm. The heart rhythm should not increase or decrease during the measurement period. An exact rule of stationarity would demand that the distribution of a time-series is invariant over time. A weaker rule demands only that mean and covariance are stable. When in time-series trends are occurring, they can probably distort the parameter calculation (45). Stationarity in measurement protocols is usually obtained by demanding a resting period for the individuals at least 5, but usually 10 min. In the case of measurements during tests (e.g., physical movement, stress tests) algorithms not needing stationarity should be considered.
Another precondition is of course that the heart rhythm is feasible for HRV analysis. Although some research groups have used HRV analysis in atrial fibrillation (AF) {(83) #1868} {(84) #1719} {(85) #2341}, in most cases individuals with AF have to be excluded. The same applies for participants with a high number of ectopic beats, with exception when heart rate turbulence will be analyzed, where ectopic beats are needed. Individuals with more than 20–30% ectopic beats are usually not feasible for HRV analysis {(86) #2343}. Before HRV parameters can be calculated, preprocessing of the raw data is necessary. Artifacts have to be removed, and ectopic beats have to be identified and handled. Several computer-based algorithms provide automatic identification and managing of ectopic beats, but most protocols include a manual review of the ECG signal {(86) #2343}. A typical way of management is to replace the distances between the QRS complex before and after the ectopic beat by the distance between these two QRS-complexes divided by two {(2) #1505}.
The sampling rate is an important issue. If the sampling rate of the signal is under a certain threshold, the calculated parameters might be distorted. Wittling showed that a sample rate below 256 Hz can already cause significant distortion with the example of a patient investigated after myocardial infarction (87) p 151. The Task Force recommends a sampling rate between at least 250 and 500 Hz. A lower sampling rate is only acceptable if appropriate interpolation algorithms are used, but not lower than 100 Hz (2). A recent exploration described stable measures at sampling rates of 125 Hz or lower (88).
In the last years, heart rate has been increasingly measured by photoplethysmography (PPG), as implemented in newer smart watches {(9) #2319}. For instance, the pulse watch Polar RS800cx, using an electrode belt and PPG measured with a finger cuff, compared with ECG showed moderate to excellent agreement levels. However, some values (LF and HF) had a lower correlation {(89) #2321}. Mobile phone technology showed excellent similarity between ECG signals and finger color changes taped with the camera lens, and the flash turned on {(90) #2324} {(91) #2322}. For instance, SDNN measured by ECG was 92.2 ± 5.3 and 92.3 ± 5.9 by the mobile phone in one study {(91) #2322}. These results seem promising, and this method has been used in some studies, e.g., {(92) #2325}. A recent review concluded that Pulse Rate Variability with PPG seems to work acceptable in healthy younger persons at rest, but not in movement or under stress conditions {(6) #2326}. Application of pulse watches with breast belts or PPG can only be recommended, if the particular equipment is first validated with a traditional ECG approach.
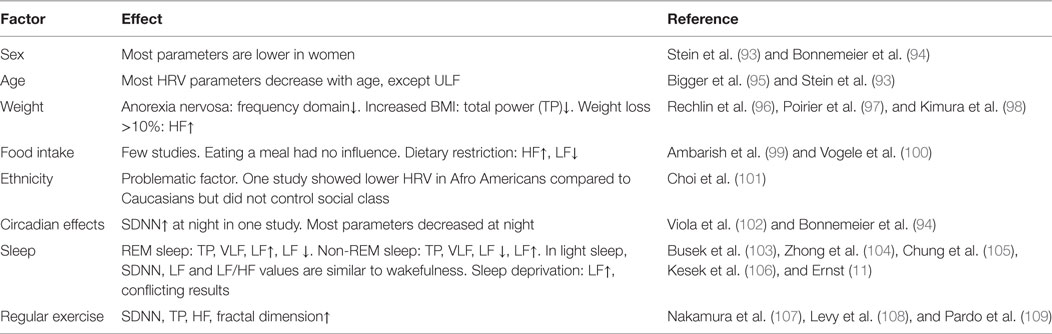
Several factors influence the measurement results. HRV results are clearly sex and age dependent. Also sleep, physical exercise, fasting, and position might distort HRV parameters. An overview is given in Table 2. A comprehensive overview is given in Ref. (11), chapter 4. One major problem regards reference values. Some reference values have been provided by the Task Force {(2) #1505}. In a review, 44 studies with together 21,438 participants were pooled and the results were considerably different {(4) #932}. A recent study provided reference values for 24-h recordings {(7, 8) #2020}, but received massive critic for inconsistencies and the methodological approach {(3) #2021}. Beyond others heterogeneity of study populations, measurement conditions (e.g., stressed or relaxed participants), or time of the measurement can have profound effect. Studies have, therefore, usually control groups instead of relating to reference values. On the other hand, some parameter values, such as SDNN < 50 ms are generally accepted as pathological {(3) #2021}.
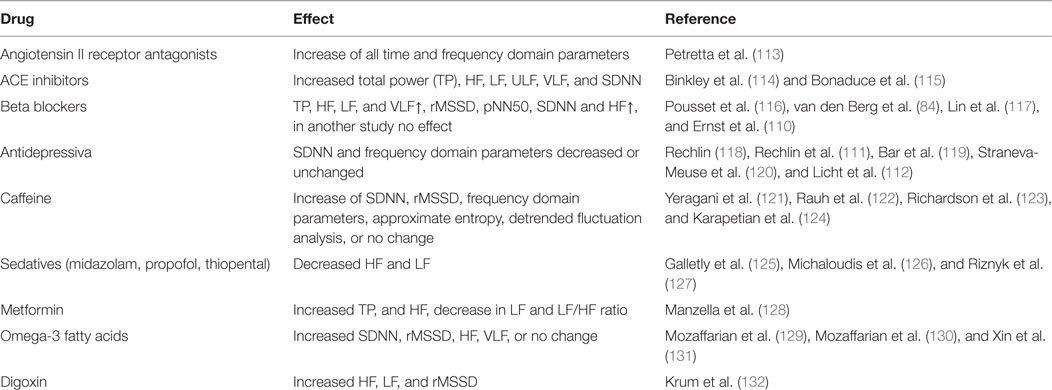
Different drugs might influence HRV parameters, but often the evidence is conflicting. Beta blockers are mentioned most frequently (2), but recently a negative study was published (110). In most studies, individuals taking beta blockers are excluded, analyzed separately or included into the statistical model. An overview of some drugs and its effects on HRV is given in Table 3. Different antidepressive drugs frequently showed effects on various HRV parameters. Amitryptilin and Doxepin, taken in a period of 2 weeks was associated with general decreased frequency domain parameters (111), the effect of tricyclic antidepressants, selective serotonin reuptake inhibitors and other antidepressants was confirmed in a larger study (112) Also here, a comprehensive overview can be found in Ref. (11), chapter 4.
Algorithms
Linear Algorithms
Time Domain
Time domain analysis measures the variation of the intervals between consecutive normal cardiac cycles. The SD of NN intervals (SDNN) is the most frequently used HRV parameter, formally the SD of all normal (“NN”) QRS distances. It correlates with total power (TP), often r > 0.9 (87) {(133) #2347}. Since TP is adjusted to the variance of the analyzed time-series within the particular time frame, this correlation is not surprising. The SD of the average NN intervals (SDANN), usually calculated over 5-min periods, needs longer measuring periods and cannot be applied in short-term HRV measures. pNN50 and rMSSD can be used both in short-term and long-term measurements. NN50 is the number of pairs of successive NNs that differ by more than 50 ms, pNN50, the proportion of NN50 divided by total number of NNs over (normally) a 24h-recording {(2) #1505} and is often interpreted as a proxy for cardiac parasympathetic activity {(134) #2345}. rMSSD stands for the square root of the mean squared differences of successive NN intervals (2, 135). Some reference values for time domain parameters are presented in Table 4. Importantly, time domain parameters depend on the length of the recording time. Longer periods generate more variability. Studies can, therefore, only be compared when they use the same measurement period {(136) #2350}.
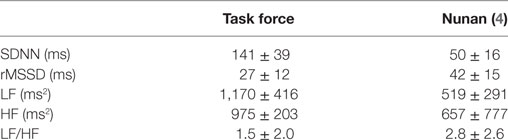
Table 4. Reference values for some heart rate variability values provided by the Task Force and Nunan {(4) #2317} {(2) #1505}.
Geometric Methods
Geometric methods are obtained from sequences of NN intervals. Several algorithms are described as geometric methods, such as the 24-h histogram, the HRV triangular index, the triangular interpolation of NN interval histograms, and the Poincaré-plot.
The triangular index (TI) constructs a triangle with the major peak of the histogram, its baseline width corresponding to the amount of RR interval variability, and its height corresponding to the total number of all RR intervals (137). It is based on the density distribution (the number of all NN intervals) divided by the maximum of the density distribution. TI uses time-series of NN intervals on a discrete scale, and the parameter is calculated by the total number of NN intervals divided through the number of NN intervals in the modal bin and dependent on the length of the bin, with other words on the precision of the discrete scale of measurement [Task Force 1996]. It has been used more frequently in the last years, e.g., in Ref. (138, 139).
The Poincaré-plot is constructed with pairs of following R–R intervals assumed implicitly that the current one significantly determines the next R–R interval. Under physiological conditions, the difference between the first and following QRS-intervals increases, but less under pathological conditions (135). Poincaré plots can be approached qualitatively by describing their different shapes (140) but they can also be measured by the SD12 index which is based on the length of the axis of a circle having its center at the average RR interval and being related to the plot itself (141). Its additional value to other linear domain parameters is limited since SD1 correlates closely to rMSSD and SD2 to SDNN (142).
Frequency Domain
The frequency domain (power spectral density) analysis in humans was introduced by Axelrod et al. (27). It describes the periodic oscillations in different frequencies of the heart rate signal, and quantifies the amount of different frequency bands (137). During preprocessing, the RR intervals have to be resampled to transform it to a real time-series, usually at 4 Hz to capture oscillations up to 2 Hz according to the Nyquist theorem {(143) #2349}. Most frequently, frequency domain is calculated non-parametrically with the fast Fourier transformation (FFT). Parametric methods in the discrete Fourier transformation are more complex and dependent on the used model. The investigated time-series has to be stationary; therefore, it cannot be applicated in patients with fast changing heart rates under the measurement period. Under certain circumstances FFT fails to find structures which can be found with, e.g., wavelet analysis (144).
Usual parameters include TP, VLF (very low frequency, <0.003–0.04 Hz), LF (low-frequency power, 0.04–0.15 Hz), HF (high frequency power, 0.15–0.4 Hz). A frequently used ratio is LF/HF. Frequency domain parameters can be applicated both in short- and long-term measurements, but not ULF (ultra low frequency, <0.003 Hz), which only can be used in Holter monitoring.
HF is frequently interpreted as a marker of the PNS and is influenced by the respiratory rate (135). It is to a certain degree the same as the RSA (45) and correlates with it (145). Parasympathetic regulation of the heart has a fast response after about 0.5 s and returns to baseline within 1 s (67).
LF is modulated both by the activity of the sympathetic and parasympathetic system. A high LF power is often explained as result of high sympathetic activity (mental, physical stress, sympathomimetic pharmacologic agents). Sympathetic input leads to changes in heart rate, however, more slowly as after parasympathetic input, with a peak after about 4 s and return to baseline after about 20 s (146). The LF/HF ratio mirrors the general sympathetic/parasympathetic balance and returns usually in rest a value between 1 and 2. VLF is a general proxy for physical activity and might mirror also sympathetic activity, but the causality is debated (135). Increased inflammatory parameters like CRP, Il-6, and WBC are correlated with low VLF (147). Some reference values for frequency domain parameters are presented in Table 4.
Non-Linear Algorithms
The difference between “linear” and “non-linear” methods in HRV is not as straightforward as in the general definition mentioned above. Principally, frequency domain analysis is based on already established patterns. In Fourier transformations, the presumed frame is a sinusoidal wave and in wavelet analysis predefined wavelet function. Both patterns are in principle non-linear, but the methods remain linear because in Fourier transformations the sine waves are added, same as in wavelet analysis the different wavelets. By contrast, non-linear methods are not based on prespecified structures but analyze temporal similarities in the signals. Entropy is frequently, but not entirely, described as a measure for regularity of the signals, whereas fractal methods investigate self-similarities within signals.
Entropy
An influential algorithm in HRV at the beginning of the 1990s was approximate entropy (ApEN) (148). It was first introduced in 1991 (149) and evaluates data sets for repeating structures and for the probability that other time periods in the data set with the same length of runs (m), tolerance (r), and length of RR intervals (n) have the same structures. ApEN returns a number between 0 and around 1. In normal adults, ApEN is around 1. Lower numbers of ApEn indicate higher regularity, higher values less patterns and low uniformity in the data set. ApEN can be used reliably down to 1,000 data points making it feasible for short-term-HRV of 20 min (148). ApEN has been used successfully in such different fields in endocrinology (secretion of ACTH and cortisol in patients with major depressive disorders) (150), HRV behavior in patients with a combination of unstable angina pectoris and depression (151), respiration patterns in panic disorders (152), or HRV of adolescents treated with anti-depressant drugs (153). ApEN and other similar tools are superior to detect unknown relations between seemingly unconnected systems. In one study investigating patients with cachexia due to COPD, they had in contrast to non-cachectic patients with similar disease and healthy controls an absent circadian rhythm of circulating leptin (154). A major problem of ApEN is probably a lack of internal consistency. Therefore, as alternative a different algorithm, termed “sample entropy” (SampEn) has been introduced (155). Similarly, it calculates the probability of identifying specific patterns in a short time-series and is defined as the negative natural logarithm of an estimate for predictability in finding specific matches in a short time-series {(155) #1566}. To set the exactness of pattern recognition, the length (m) of the subseries and the tolerance (r) for the patterns has to be predefined. It returns results between 0 and around 2, 0 represents, e.g., a sinus curve and a result near 2 complete chaos. SampEn needs far fewer data points compared to ApEN, and it can be applicated in time-series between 200 to 250 data points (156, 157). Several other entropy algorithms have been proposed, like Lempel Ziv entropy (158), Multiscale entropy (159), fuzzy entropy (160), or Renyi entropy (161). All have been used in clinical studies, but their significance is still unclear.
Fractal Analysis
The notion of fractality has been originally introduced and applied by Benoit Mandelbrot on spatial self-similarities in graphical plots of non-linear deterministic iterations (162). Used for heart rate time-series, it refers not to spatial, but to temporal self-similarities over a range of scales {(163) #2351}. A normal series of RR intervals is fractal-like and shows a scale-free 1/f fluctuation typical for self-organizing systems behaving between uncorrelated randomness and highly predictable behavior {(164) #1569} {(165) #1747}.
Detrended fluctuation analysis (DFA) determines the statistical self-affinity of a signal. When used to analyze heart rates, it yields to separate scaling regions, a short-term scaling exponent and a long-term scaling exponent. Peng et al. presented the short-term scaling exponent (also termed α1) calculated by DFA first in genetical data (166) and in the following year also in HRV (167). Its great advantage is that it can be used for non-stationary data from time-series and correlates with the randomness in the heart rate time-series, the lowest values (~0.5) ressembles an entirely random series; high values (1.5) signify a time-series being completely correlated (141). It has been used to predict cardiac mortality in different patient populations (165, 168). Unfortunately, it needs at least 1,000 beats and has, therefore, been used more frequently in Holter monitoring studies.
Other proposed algorithms include coarse grained spectral analysis (169), the Fano factor (170), dispersional analysis (170, 171), fractal dimension (172), correlation dimension (173), or the Largest Lyapunov Exponent (174). Their clinical value is still unclear.
Other Algorithms
Heart Rate Turbulence is normally not considered as a HRV parameter, but it is based, however, on a similar physiological background and can be applicated in comparable ways. Patients need to have ventricular extrasystoles (VES) because HRT is based on the reaction of the system afterward. Healthy individuals without any arrhytmias can not be investigated with HRT. The heart rate directly following a VES increases normally, to decrease a moment later. This pattern is changed or non-existent in patients after myocardial infarction (175). The algorithm returns the parameters turbulence onset and turbulence slope (176).
Outlook
Since the guidelines of the Task Force were published in 1996, the measurement and calculation of linear parameters have been highly standardized, making investigations comparable and meta-analysis possible. After the introduction of different non-linear parameters, expectations were high, but the role of non-linear algorithms is still unclear after more than two decades. As more than 70 different algorithms have been used (177). Today, HRV beyond its use in pulse watches is still not established in the clinical area although the methods are mature and have been tested extensively. The understanding, however, has increased substantially. Several models have extended its use into psychological and mental health research (55, 178, 179). Finally, 20 years after publishing the first globally used standard of measurement (2), a highly recommendable comprehensive methodological hands-on guideline summarizes state of the art and should be used a new standard (180). In addition, the role of non-linear methods has been recently evaluated and recapitulated (46). Also, a useful guideline to present HRV data has been published (181). HRV as a research and clinical tool is still underrecognized. It should be implemented in several clinical areas within a Bayesian paradigm to improve prediction, diagnosis, and therapy (182).
Author Contributions
GE has elaborated and written the whole review.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Billman GE. Heart rate variability – a historical perspective. Front Physiol (2011) 2:86. doi:10.3389/fphys.2011.00086
2. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J (1996) 17(3):354–81. doi:10.1093/oxfordjournals.eurheartj.a014868
3. Bauer A, Camm AJ, Cerutti S, Guzik P, Huikuri H, Lombardi F, et al. Reference values of heart rate variability. Heart Rhythm (2017) 14(2):302–3. doi:10.1016/j.hrthm.2016.12.015
4. Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol (2010) 33(11):1407–17. doi:10.1111/j.1540-8159.2010.02841.x
5. Selvaraj N, Jaryal A, Santhosh J, Deepak KK, Anand S. Assessment of heart rate variability derived from finger-tip photoplethysmography as compared to electrocardiography. J Med Eng Technol (2008) 32(6):479–84. doi:10.1080/03091900701781317
6. Schafer A, Vagedes J. How accurate is pulse rate variability as an estimate of heart rate variability? A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int J Cardiol (2013) 166(1):15–29. doi:10.1016/j.ijcard.2012.03.119
7. Sammito S, Bockelmann I. [Options and limitations of heart rate measurement and analysis of heart rate variability by mobile devices: a systematic review]. Herzschrittmacherther Elektrophysiol (2016) 27(1):38–45. doi:10.1007/s00399-016-0419-5
8. Sammito S, Bockelmann I. Reference values for time- and frequency-domain heart rate variability measures. Heart Rhythm (2016) 13(6):1309–16. doi:10.1016/j.hrthm.2016.02.006
9. Carpenter A, Frontera A. Smart-watches: a potential challenger to the implantable loop recorder? Europace (2016) 18(6):791–3. doi:10.1093/europace/euv427
10. Chen CL, Chuang CT. A QRS detection and R point recognition method for wearable single-lead ECG devices. Sensors (Basel) (2017) 17(9):1969. doi:10.3390/s17091969
12. Anonymous. Stephen Hales, parson and physiologist. JAMA (1962) 182(1):70–1. doi:10.1001/jama.1962.03050400072016
13. Ludwig C. Beiträge zur Kenntniss des Einflusses der Respirationsbewegungen auf den Blutlauf im Aortensystem. Arch Anat Physiol Leipzig (1847) 13:242–302.
14. Goldberger AL. Fractal variability versus pathologic periodicity: complexity loss and stereotypy in disease. Perspect Biol Med (1997) 40(4):543–61. doi:10.1353/pbm.1997.0063
17. Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiol Behav (2003) 79(3):503–13. doi:10.1016/S0031-9384(03)00156-2
18. Porges SW. The Polyvagal perspective. Biol Psychol (2007) 74(2):116–43. doi:10.1016/j.biopsycho.2006.06.009
19. Zimmer HG. Heinrich Ewald Hering and the carotid sinus reflex. Clin Cardiol (2004) 27(8):485–6. doi:10.1002/clc.4960270813
20. Adrian ED, Bronk DW, Phillips G. Discharges in mammalian sympathetic nerves. J Physiol (1932) 74(2):115–33. doi:10.1113/jphysiol.1932.sp002832
21. Maltzberg B. Mortality among patients with involution melancholia. Am J Psychiatry (1937) 93:1231–8. doi:10.1176/ajp.93.5.1231
22. Hon EH, Lee ST. The fetal electrocardiogram. 3. Display techniques. Am J Obstet Gynecol (1965) 91:56–60. doi:10.1016/0002-9378(65)90586-7
23. Wolf S. The end of the rope: the role of the brain in cardiac death. Can Med Assoc J (1967) 97(17):1022–5.
24. Vallbona C, Cardus D, Spencer WA, Hoff HE. Patterns of sinus arrhythmia in patients with lesions of the central nervous system. Am J Cardiol (1965) 16(3):379–89. doi:10.1016/0002-9149(65)90729-0
25. Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol (1975) 39(5):801–5.
26. Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science (1981) 213(4504):220–2. doi:10.1126/science.6166045
27. Axelrod S, Lishner M, Oz O, Bernheim J, Ravid M. Spectral analysis of fluctuations in heart rate: an objective evaluation of autonomic nervous control in chronic renal failure. Nephron (1987) 45(3):202–6. doi:10.1159/000184117
28. Egelund N. Spectral analysis of heart rate variability as an indicator of driver fatigue. Ergonomics (1982) 25(7):663–72. doi:10.1080/00140138208925026
29. Jennings JR, Mack ME. Does aging differentially reduce heart rate variability related to respiration? Exp Aging Res (1984) 10(1):19–23. doi:10.1080/03610738408258536
30. Pagani M, Lombardi F, Guzzetti S, Sandrone G, Rimoldi O, Malfatto G, et al. Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. J Hypertens Suppl (1984) 2(3):S383–5.
31. Goldberger AL, Findley LJ, Blackburn MR, Mandell AJ. Nonlinear dynamics in heart failure: implications of long-wavelength cardiopulmonary oscillations. Am Heart J (1984) 107(3):612–5. doi:10.1016/0002-8703(84)90120-0
32. Goldberger AL, Kobalter K, Bhargava V. 1/f-like scaling in normal neutrophil dynamics: implications for hematologic monitoring. IEEE Trans Biomed Eng (1986) 33(9):874–6. doi:10.1109/TBME.1986.325781
33. Goldberger AL, West BJ. Applications of nonlinear dynamics to clinical cardiology. Ann N Y Acad Sci (1987) 504:195–213. doi:10.1111/j.1749-6632.1987.tb48733.x
34. May RM. Stability in randomly fluctuating versus deterministic environments. Am Nat (1973) 107(957):621–50. doi:10.1086/282863
36. Shaw RS. Strange attractors, chaotic behaviour and information flow. Z Naturforsch A (1981) 36:80–112.
37. Haken H. Synergetics: an introduction: nonequilibrium phase transitions and self-organization in physics, chemistry and biology. 2nd ed. Springer Series in Synergetics. (Vol. 1), Berlin: Springer-Verlag (1978).
38. May RM. Stability and complexity in model ecosystems. 2nd ed. Monographs in Population Biology. (Vol. 6), Princeton, NJ: Princeton University Press (1974).
39. Wolf MM, Varigos GA, Hunt D, Sloman JG. Sinus arrhythmia in acute myocardial infarction. Med J Aust (1978) 2(2):52–3.
40. Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol (1987) 59(4):256–62. doi:10.1016/0002-9149(87)90795-8
41. La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation (1988) 78(4):816–24. doi:10.1161/01.CIR.78.4.816
42. Forslund L, Bjorkander I, Ericson M, Held C, Kahan T, Rehnqvist N, et al. Prognostic implications of autonomic function assessed by analyses of catecholamines and heart rate variability in stable angina pectoris. Heart (2002) 87(5):415–22. doi:10.1136/heart.87.5.415
43. Balanescu S, Corlan AD, Dorobantu M, Gherasim L. Prognostic value of heart rate variability after acute myocardial infarction. Med Sci Monit (2004) 10(7):CR307–15.
44. Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE, Cast Investigators. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol (2005) 16(1):13–20. doi:10.1046/j.1540-8167.2005.04358.x
45. Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology (1997) 34(6):623–48. doi:10.1111/j.1469-8986.1997.tb02140.x
46. Sassi R, Cerutti S, Lombardi F, Malik M, Huikuri HV, Peng CK, et al. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace (2015) 17(9):1341–53. doi:10.1093/europace/euv015
47. Eppinger H, Hess L. Vagotonie. Klinische Studie. Sammlung klinischer Abhandlungen über Pathologie und Therapie der Stoffwechsel- und Ernährungsstörungen. Berlin: Hirschwald (1910). 9/10 p.
48. Wenger MA. The measurement of individual differences in autonomic balance. Psychosom Med (1941) 3(4):427–34. doi:10.1097/00006842-194110000-00006
50. Lacey JI, Lacey BC. Verification and extension of the principle of autonomic response-stereotypy. Am J Psychol (1958) 71(1):50–73. doi:10.2307/1419197
51. Porges SW, Raskin DC. Respiratory and heart rate components of attention. J Exp Psychol (1969) 81(3):497–503. doi:10.1037/h0027921
52. Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology (1995) 32(4):301–18. doi:10.1111/j.1469-8986.1995.tb01213.x
53. Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord (2000) 61(3):201–16. doi:10.1016/S0165-0327(00)00338-4
54. Porges SW. The Polyvagal Theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med (2009) 76(Suppl 2):S86–90. doi:10.3949/ccjm.76.s2.17
55. Smith R, Thayer JF, Khalsa SS, Lane RD. The hierarchical basis of neurovisceral integration. Neurosci Biobehav Rev (2017) 75:274–96. doi:10.1016/j.neubiorev.2017.02.003
56. Huikuri HV, Makikallio TH, Peng CK, Goldberger AL, Hintze U, Moller M. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation (2000) 101(1):47–53. doi:10.1161/01.CIR.101.1.47
57. La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation (2003) 107(4):565–70. doi:10.1161/01.CIR.0000047275.25795.17
58. Maheshwari A, Norby FL, Soliman EZ, Adabag S, Whitsel EA, Alonso A, et al. Low heart rate variability in a 2-minute electrocardiogram recording is associated with an increased risk of sudden cardiac death in the general population: the atherosclerosis risk in communities study. PLoS One (2016) 11(8):e0161648. doi:10.1371/journal.pone.0161648
59. Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol (2010) 7(4):216–25. doi:10.1038/nrcardio.2010.3
60. Adabag S, Smith LG, Anand IS, Berger AK, Luepker RV. Sudden cardiac death in heart failure patients with preserved ejection fraction. J Card Fail (2012) 18(10):749–54. doi:10.1016/j.cardfail.2012.08.357
61. Gimeno-Blanes FJ, Blanco-Velasco M, Barquero-Perez O, Garcia-Alberola A, Rojo-Alvarez JL. Sudden cardiac risk stratification with electrocardiographic indices – a review on computational processing, technology transfer, and scientific evidence. Front Physiol (2016) 7:82. doi:10.3389/fphys.2016.00082
62. Schonauer M, Thomas A, Morbach S, Niebauer J, Schonauer U, Thiele H. Cardiac autonomic diabetic neuropathy. Diab Vasc Dis Res (2008) 5(4):336–44. doi:10.3132/dvdr.2008.047
63. Cygankiewicz I, Zareba W. Heart rate variability. Handb Clin Neurol (2013) 117:379–93. doi:10.1016/B978-0-444-53491-0.00031-6
64. Seely AJ, Macklem PT. Complex systems and the technology of variability analysis. Crit Care (2004) 8(6):R367–84. doi:10.1186/cc2948
65. Mazzeo AT, La Monaca E, Di Leo R, Vita G, Santamaria LB. Heart rate variability: a diagnostic and prognostic tool in anesthesia and intensive care. Acta Anaesthesiol Scand (2011) 55(7):797–811. doi:10.1111/j.1399-6576.2011.02466.x
66. Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev (2017) 74(Pt A):233–55. doi:10.1016/j.neubiorev.2016.12.032
67. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol (2006) 10(3):229–40. doi:10.1037/1089-2680.10.3.229
68. Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry (1996) 39(4):255–66. doi:10.1016/0006-3223(95)00136-0
69. Friedman BH, Thayer JF. Autonomic balance revisited: panic anxiety and heart rate variability. J Psychosom Res (1998) 44(1):133–51. doi:10.1016/S0022-3999(97)00202-X
70. Mezzacappa E, Tremblay RE, Kindlon D, Saul JP, Arseneault L, Seguin J, et al. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. J Child Psychol Psychiatry (1997) 38(4):457–69. doi:10.1111/j.1469-7610.1997.tb01531.x
71. Sgoifo A, Braglia F, Costoli T, Musso E, Meerlo P, Ceresini G, et al. Cardiac autonomic reactivity and salivary cortisol in men and women exposed to social stressors: relationship with individual ethological profile. Neurosci Biobehav Rev (2003) 27(1–2):179–88. doi:10.1016/S0149-7634(03)00019-8
72. Fuller BF. The effects of stress-anxiety and coping styles on heart rate variability. Int J Psychophysiol (1992) 12(1):81–6. doi:10.1016/0167-8760(92)90045-D
73. Wendt J, Neubert J, Koenig J, Thayer JF, Hamm AO. Resting heart rate variability is associated with inhibition of conditioned fear. Psychophysiology (2015) 52(9):1161–6. doi:10.1111/psyp.12456
74. Strogatz SH. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering. Studies in Nonlinearity. Reading, MA: Addison-Wesley (1994).
75. Goldberger A. Non-linear dynamics for clinicians: chaos theory, fractals, and complexity at the bedside. Lancet (1996) 347(9011):1312–4. doi:10.1016/S0140-6736(96)90948-4
76. Silva-Rocha R, de Lorenzo V. Noise and robustness in prokaryotic regulatory networks. Annu Rev Microbiol (2010) 64:257–75. doi:10.1146/annurev.micro.091208.073229
77. Masel J, Siegal ML. Robustness: mechanisms and consequences. Trends Genet (2009) 25(9):395–403. doi:10.1016/j.tig.2009.07.005
78. Kriete A. Robustness and aging–a systems-level perspective. Biosystems (2013) 112(1):37–48. doi:10.1016/j.biosystems.2013.03.014
79. Carlson JM, Doyle J. Complexity and robustness. Proc Natl Acad Sci U S A (2002) 99(Suppl 1):2538–45. doi:10.1073/pnas.012582499
80. Baek HJ, Cho CH, Cho J, Woo JM. Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemed J E Health (2015) 21(5):404–14. doi:10.1089/tmj.2014.0104
81. Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol (1997) 145(10):899–908. doi:10.1093/oxfordjournals.aje.a009049
82. Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME, et al. The association among autonomic nervous system function, incident diabetes, and intervention arm in the diabetes prevention program. Diabetes Care (2006) 29(4):914–9. doi:10.2337/diacare.29.04.06.dc05-1729
83. Stein KM, Borer JS, Hochreiter C, Devereux RB, Kligfield P. Variability of the ventricular response in atrial fibrillation and prognosis in chronic nonischemic mitral regurgitation. Am J Cardiol (1994) 74(9):906–11. doi:10.1016/0002-9149(94)90584-3
84. van den Berg MP, Haaksma J, Brouwer J, Tieleman RG, Mulder G, Crijns HJ. Heart rate variability in patients with atrial fibrillation is related to vagal tone. Circulation (1997) 96(4):1209–16. doi:10.1161/01.CIR.96.4.1209
85. Yamada A, Hayano J, Sakata S, Okada A, Mukai S, Ohte N, et al. Reduced ventricular response irregularity is associated with increased mortality in patients with chronic atrial fibrillation. Circulation (2000) 102(3):300–6. doi:10.1161/01.CIR.102.3.300
86. Peltola MA. Role of editing of R-R intervals in the analysis of heart rate variability. Front Physiol (2012) 3:148. doi:10.3389/fphys.2012.00148
87. Wittling WW, Ralf Arne W. Herzschlagvariabilität: Frühwarnsystem, Stress- und Fitnessindikator. Heiligenstadt: Eichsfeld-Verlag (2012).
88. Ellis RJ, Zhu B, Koenig J, Thayer JF, Wang Y. A careful look at ECG sampling frequency and R-peak interpolation on short-term measures of heart rate variability. Physiol Meas (2015) 36(9):1827–52. doi:10.1088/0967-3334/36/9/1827
89. Vasconcellos FV, Seabra A, Cunha FA, Montenegro RA, Bouskela E, Farinatti P. Heart rate variability assessment with fingertip photoplethysmography and polar RS800cx as compared with electrocardiography in obese adolescents. Blood Press Monit (2015) 20(6):351–60. doi:10.1097/MBP.0000000000000143
90. Peng RC, Zhou XL, Lin WH, Zhang YT. Extraction of heart rate variability from smartphone photoplethysmograms. Comput Math Methods Med (2015) 2015:516826. doi:10.1155/2015/516826
91. Scully CG, Lee J, Meyer J, Gorbach AM, Granquist-Fraser D, Mendelson Y, et al. Physiological parameter monitoring from optical recordings with a mobile phone. IEEE Trans Biomed Eng (2012) 59(2):303–6. doi:10.1109/TBME.2011.2163157
92. Roy RA, Boucher JP, Comtois AS. Heart rate variability modulation after manipulation in pain-free patients vs patients in pain. J Manipulative Physiol Ther (2009) 32(4):277–86. doi:10.1016/j.jmpt.2009.03.003
93. Stein PK, Kleiger RE, Rottman JN. Differing effects of age on heart rate variability in men and women. Am J Cardiol (1997) 80(3):302–5. doi:10.1016/S0002-9149(97)00350-0
94. Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol (2003) 14(8):791–9. doi:10.1046/j.1540-8167.2003.03078.x
95. Bigger JT Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Schneider WJ, Stein PK. RR variability in healthy, middle-aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation (1995) 91(7):1936–43. doi:10.1161/01.CIR.91.7.1936
96. Rechlin T, Weis M, Ott C, Bleichner F, Joraschky P. Alterations of autonomic cardiac control in anorexia nervosa. Biol Psychiatry (1998) 43(5):358–63. doi:10.1016/S0006-3223(97)00026-7
97. Poirier P, Hernandez TL, Weil KM, Shepard TJ, Eckel RH. Impact of diet-induced weight loss on the cardiac autonomic nervous system in severe obesity. Obes Res (2003) 11(9):1040–7. doi:10.1038/oby.2003.143
98. Kimura T, Matsumoto T, Akiyoshi M, Owa Y, Miyasaka N, Aso T, et al. Body fat and blood lipids in postmenopausal women are related to resting autonomic nervous system activity. Eur J Appl Physiol (2006) 97(5):542–7. doi:10.1007/s00421-006-0207-8
99. Ambarish V, Barde P, Vyas A, Deepak KK. Comparison between pre-prandial and post-prandial heart rate variability (HRV). Indian J Physiol Pharmacol (2005) 49(4):436–42.
100. Vogele C, Hilbert A, Tuschen-Caffier B. Dietary restriction, cardiac autonomic regulation and stress reactivity in bulimic women. Physiol Behav (2009) 98(1–2):229–34. doi:10.1016/j.physbeh.2009.05.018
101. Choi JB, Hong S, Nelesen R, Bardwell WA, Natarajan L, Schubert C, et al. Age and ethnicity differences in short-term heart-rate variability. Psychosom Med (2006) 68(3):421–6. doi:10.1097/01.psy.0000221378.09239.6a
102. Viola AU, Simon C, Ehrhart J, Geny B, Piquard F, Muzet A, et al. Sleep processes exert a predominant influence on the 24-h profile of heart rate variability. J Biol Rhythms (2002) 17(6):539–47. doi:10.1177/0748730402238236
103. Busek P, Vankova J, Opavsky J, Salinger J, Nevsimalova S. Spectral analysis of the heart rate variability in sleep. Physiol Res (2005) 54(4):369–76.
104. Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol (1985) (2005) 98(6):2024–32. doi:10.1152/japplphysiol.00620.2004
105. Chung MH, Kuo TB, Hsu N, Chu H, Chou KR, Yang CC. Sleep and autonomic nervous system changes – enhanced cardiac sympathetic modulations during sleep in permanent night shift nurses. Scand J Work Environ Health (2009) 35(3):180–7. doi:10.5271/sjweh.1324
106. Kesek M, Franklin KA, Sahlin C, Lindberg E. Heart rate variability during sleep and sleep apnoea in a population based study of 387 women. Clin Physiol Funct Imaging (2009) 29(4):309–15. doi:10.1111/j.1475-097X.2009.00873.x
107. Nakamura Y, Yamamoto Y, Muraoka I. Autonomic control of heart rate during physical exercise and fractal dimension of heart rate variability. J Appl Physiol (1985) (1993) 74(2):875–81.
108. Levy WC, Cerqueira MD, Harp GD, Johannessen KA, Abrass IB, Schwartz RS, et al. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol (1998) 82(10):1236–41. doi:10.1016/S0002-9149(98)00611-0
109. Pardo Y, Merz CN, Velasquez I, Paul-Labrador M, Agarwala A, Peter CT. Exercise conditioning and heart rate variability: evidence of a threshold effect. Clin Cardiol (2000) 23(8):615–20. doi:10.1002/clc.4960230813
110. Ernst G, Watne LO, Frihagen F, Wyller TB, Dominik A, Rostrup M. Decreases in heart rate variability are associated with postoperative complications in hip fracture patients. PLoS One (2017) 12(7):e0180423. doi:10.1371/journal.pone.0180423
111. Rechlin T, Claus D, Weis M. Heart rate analysis in 24 patients treated with 150 mg amitriptyline per day. Psychopharmacology (Berl) (1994) 116(1):110–4. doi:10.1007/BF02244880
112. Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch Gen Psychiatry (2008) 65(12):1358–67. doi:10.1001/archpsyc.65.12.1358
113. Petretta M, Spinelli L, Marciano F, Apicella C, Vicario ML, Testa G, et al. Effects of losartan treatment on cardiac autonomic control during volume loading in patients with DCM. Am J Physiol Heart Circ Physiol (2000) 279(1):H86–92.
114. Binkley PF, Haas GJ, Starling RC, Nunziata E, Hatton PA, Leier CV, et al. Sustained augmentation of parasympathetic tone with angiotensin-converting enzyme inhibition in patients with congestive heart failure. J Am Coll Cardiol (1993) 21(3):655–61. doi:10.1016/0735-1097(93)90098-L
115. Bonaduce D, Marciano F, Petretta M, Migaux ML, Morgano G, Bianchi V, et al. Effects of converting enzyme inhibition on heart period variability in patients with acute myocardial infarction. Circulation (1994) 90(1):108–13. doi:10.1161/01.CIR.90.1.108
116. Pousset F, Copie X, Lechat P, Jaillon P, Boissel JP, Hetzel M, et al. Effects of bisoprolol on heart rate variability in heart failure. Am J Cardiol (1996) 77(8):612–7. doi:10.1016/S0002-9149(97)89316-2
117. Lin JL, Chan HL, Du CC, Lin IN, Lai CW, Lin KT, et al. Long-term beta-blocker therapy improves autonomic nervous regulation in advanced congestive heart failure: a longitudinal heart rate variability study. Am Heart J (1999) 137(4 Pt 1):658–65. doi:10.1016/S0002-8703(99)70219-X
118. Rechlin T. The effect of amitriptyline, doxepin, fluvoxamine, and paroxetine treatment on heart rate variability. J Clin Psychopharmacol (1994) 14(6):392–5. doi:10.1097/00004714-199412000-00004
119. Bar KJ, Greiner W, Jochum T, Friedrich M, Wagner G, Sauer H. The influence of major depression and its treatment on heart rate variability and pupillary light reflex parameters. J Affect Disord (2004) 82(2):245–52. doi:10.1016/j.jad.2003.12.016
120. Straneva-Meuse PA, Light KC, Allen MT, Golding M, Girdler SS. Bupropion and paroxetine differentially influence cardiovascular and neuroendocrine responses to stress in depressed patients. J Affect Disord (2004) 79(1–3):51–61. doi:10.1016/S0165-0327(02)00352-X
121. Yeragani VK, Krishnan S, Engels HJ, Gretebeck R. Effects of caffeine on linear and nonlinear measures of heart rate variability before and after exercise. Depress Anxiety (2005) 21(3):130–4. doi:10.1002/da.20061
122. Rauh R, Burkert M, Siepmann M, Mueck-Weymann M. Acute effects of caffeine on heart rate variability in habitual caffeine consumers. Clin Physiol Funct Imaging (2006) 26(3):163–6. doi:10.1111/j.1475-097X.2006.00663.x
123. Richardson T, Baker J, Thomas PW, Meckes C, Rozkovec A, Kerr D. Randomized control trial investigating the influence of coffee on heart rate variability in patients with ST-segment elevation myocardial infarction. QJM (2009) 102(8):555–61. doi:10.1093/qjmed/hcp072
124. Karapetian GK, Engels HJ, Gretebeck KA, Gretebeck RJ. Effect of caffeine on LT, VT and HRVT. Int J Sports Med (2012) 33(7):507–13. doi:10.1055/s-0032-1301904
125. Galletly DC, Buckley DH, Robinson BJ, Corfiatis T. Heart rate variability during propofol anaesthesia. Br J Anaesth (1994) 72(2):219–20. doi:10.1093/bja/72.2.219
126. Michaloudis D, Kochiadakis G, Georgopoulou G, Fraidakis O, Chlouverakis G, Petrou A, et al. The influence of premedication on heart rate variability. Anaesthesia (1998) 53(5):446–53. doi:10.1046/j.1365-2044.1998.00323.x
127. Riznyk L, Fijalkowska M, Przesmycki K. Effects of thiopental and propofol on heart rate variability during fentanyl-based induction of general anesthesia. Pharmacol Rep (2005) 57(1):128–34.
128. Manzella D, Grella R, Esposito K, Giugliano D, Barbagallo M, Paolisso G. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: effect of metformin administration. Am J Hypertens (2004) 17(3):223–7. doi:10.1016/j.amjhyper.2003.11.006
129. Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation (2005) 112(13):1945–52. doi:10.1161/CIRCULATIONAHA.105.556886
130. Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation (2008) 117(9):1130–7. doi:10.1161/CIRCULATIONAHA.107.732826
131. Xin W, Wei W, Li XY. Short-term effects of fish-oil supplementation on heart rate variability in humans: a meta-analysis of randomized controlled trials. Am J Clin Nutr (2013) 97(5):926–35. doi:10.3945/ajcn.112.049833
132. Krum H, Bigger JT Jr, Goldsmith RL, Packer M. Effect of long-term digoxin therapy on autonomic function in patients with chronic heart failure. J Am Coll Cardiol (1995) 25(2):289–94. doi:10.1016/0735-1097(94)00417-O
133. Carrasco S, Gaitán MJ, González R, Yánez O. Correlation among poincareplot indexes and time and frequency domain measures of heart rate variability. J Med Eng Technol (2001) 25(6):240–8. doi:10.1080/03091900110086651
134. Penttila J, Kuusela T, Scheinin H. Analysis of rapid heart rate variability in the assessment of anticholinergic drug effects in humans. Eur J Clin Pharmacol (2005) 61(8):559–65. doi:10.1007/s00228-005-0953-2
135. Frenneaux MP. Autonomic changes in patients with heart failure and in post-myocardial infarction patients. Heart (2004) 90(11):1248–55. doi:10.1136/hrt.2003.026146
136. Campos LA, Pereira VL Jr, Muralikrishna A, Albarwani S, Brás S, Gouveia S. Mathematical biomarkers for the autonomic regulation of cardiovascular system. Front Physiol (2013) 4:279. doi:10.3389/fphys.2013.00279
137. Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly (2004) 134(35–36):514–22. doi:2004/35/smw-10321
138. da Silva DM, Macedo MC, Lemos LB, Vieira FC, Piropo US, Andrade HB, et al. Reliability analysis of the heart autonomic control parameters during hemodialysis sessions. Biomed Tech (Berl) (2016) 61(6):623–30. doi:10.1515/bmt-2015-0239
139. Goulart CD, Cabiddu R, Schneiders PB, Antunes San Martin E, Trimer R, Borghi-Silva A, et al. Is cardiac autonomic modulation during upper limb isometric contraction and Valsalva maneuver impaired in COPD patients? Int J Chron Obstruct Pulmon Dis (2017) 12:849–57. doi:10.2147/COPD.S130428
140. Woo MA, Stevenson WG, Moser DK, Trelease RB, Harper RM. Patterns of beat-to-beat heart rate variability in advanced heart failure. Am Heart J (1992) 123(3):704–10. doi:10.1016/0002-8703(92)90510-3
141. Stein PK, Reddy A. Non-linear heart rate variability and risk stratification in cardiovascular disease. Indian Pacing Electrophysiol J (2005) 5(3):210–20.
142. Guzik P, Piskorski J, Krauze T, Schneider R, Wesseling KH, Wykretowicz A, et al. Correlations between the Poincare plot and conventional heart rate variability parameters assessed during paced breathing. J Physiol Sci (2007) 57(1):63–71. doi:10.2170/physiolsci.RP005506
143. Mobli M, Hoch JC. Nonuniform sampling and non-Fourier signal processing methods in multidimensional NMR. Prog Nucl Magn Reson Spectrosc (2014) 83:21–41. doi:10.1016/j.pnmrs.2014.09.002
144. Togo F, Kiyono K, Struzik ZR, Yamamoto Y. Unique very low-frequency heart rate variability during deep sleep in humans. IEEE Trans Biomed Eng (2006) 53(1):28–34. doi:10.1109/TBME.2005.859783
145. Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, et al. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol (2010) 109(2):201–11. doi:10.1007/s00421-009-1341-x
146. Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology (1993) 30(2):183–96. doi:10.1111/j.1469-8986.1993.tb01731.x
147. Kop WJ, Stein PK, Tracy RP, Barzilay JI, Schulz R, Gottdiener JS. Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom Med (2010) 72(7):626–35. doi:10.1097/PSY.0b013e3181eadd2b
148. Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol (1994) 266(4 Pt 2):H1643–56.
149. Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A (1991) 88(6):2297–301. doi:10.1073/pnas.88.6.2297
150. Posener JA, Charles D, Veldhuis JD, Province MA, Williams GH, Schatzberg AF. Process irregularity of cortisol and adrenocorticotropin secretion in men with major depressive disorder. Psychoneuroendocrinology (2004) 29(9):1129–37. doi:10.1016/j.psyneuen.2004.01.004
151. Vigo DE, Nicola Siri L, Ladron De Guevara MS, Martinez-Martinez JA, Fahrer RD, Cardinali DP, et al. Relation of depression to heart rate nonlinear dynamics in patients > or =60 years of age with recent unstable angina pectoris or acute myocardial infarction. Am J Cardiol (2004) 93(6):756–60. doi:10.1016/j.amjcard.2003.11.056
152. Yeragani VK, Rao R, Tancer M, Uhde T. Paroxetine decreases respiratory irregularity of linear and nonlinear measures of respiration in patients with panic disorder. A preliminary report. Neuropsychobiology (2004) 49(2):53–7. doi:10.1159/000076410
153. Srinivasan K, Ashok MV, Vaz M, Yeragani VK. Effect of imipramine on linear and nonlinear measures of heart rate variability in children. Pediatr Cardiol (2004) 25(1):20–5. doi:10.1007/s00246-003-0468-5
154. Takabatake N, Nakamura H, Minamihaba O, Inage M, Inoue S, Kagaya S, et al. A novel pathophysiologic phenomenon in cachexic patients with chronic obstructive pulmonary disease: the relationship between the circadian rhythm of circulating leptin and the very low-frequency component of heart rate variability. Am J Respir Crit Care Med (2001) 163(6):1314–9. doi:10.1164/ajrccm.163.6.2004175
155. Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol (2000) 278(6):H2039–49.
156. Kuusela TA, Jartti TT, Tahvanainen KU, Kaila TJ. Nonlinear methods of biosignal analysis in assessing terbutaline-induced heart rate and blood pressure changes. Am J Physiol Heart Circ Physiol (2002) 282(2):H773–83. doi:10.1152/ajpheart.00559.2001
157. Heffernan KS, Fahs CA, Shinsako KK, Jae SY, Fernhall B. Heart rate recovery and heart rate complexity following resistance exercise training and detraining in young men. Am J Physiol Heart Circ Physiol (2007) 293(5):H3180–6. doi:10.1152/ajpheart.00648.2007
158. Cabiddu R, Trimer R, Borghi-Silva A, Migliorini M, Mendes RG, Oliveira AD Jr, et al. Are complexity metrics reliable in assessing HRV control in obese patients during sleep? PLoS One (2015) 10(4):e0124458. doi:10.1371/journal.pone.0124458
159. Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett (2002) 89(6):068102. doi:10.1103/PhysRevLett.89.068102
160. Ji L, Li P, Li K, Wang X, Liu C. Analysis of short-term heart rate and diastolic period variability using a refined fuzzy entropy method. Biomed Eng Online (2015) 14:64. doi:10.1186/s12938-015-0063-z
161. Cornforth DJ, Tarvainen MP, Jelinek HF. How to calculate Renyi entropy from heart rate variability, and why it matters for detecting cardiac autonomic neuropathy. Front Bioeng Biotechnol (2014) 2:34. doi:10.3389/fbioe.2014.00034
163. Mäkikallio T, Tapanainen JM, Tulppo MP, Huikuri HV. Clinical applicability of heart rate variability analysis by methods based on nonlinear dynamics. Card Electrophysiol Rev (2002) 6(3):250–5. doi:10.1023/A:1016381025759
164. Huikuri HV, Mäkikallio TH, Airaksinen KE, Seppänen T, Puukka P, Räihä IJ, et al. Power-law relationship of heart rate variability as a predictor of mortality in the elderly. Circulation (1998) 97(20):2031–6. doi:10.1161/01.CIR.97.20.2031
165. Makikallio TH, Huikuri HV, Makikallio A, Sourander LB, Mitrani RD, Castellanos A, et al. Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J Am Coll Cardiol (2001) 37(5):1395–402. doi:10.1016/S0735-1097(01)01171-8
166. Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic organization of DNA nucleotides. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics (1994) 49(2):1685–9.
167. Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos (1995) 5(1):82–7. doi:10.1063/1.166141
168. Perkiomaki JS, Makikallio TH, Huikuri HV. Fractal and complexity measures of heart rate variability. Clin Exp Hypertens (2005) 27(2–3):149–58. doi:10.1081/CEH-200048742
169. Yamamoto Y, Hughson RL. Coarse-graining spectral analysis: new method for studying heart rate variability. J Appl Physiol (1985) (1991) 71(3):1143–50.
170. Das M, Gebber GL, Barman SM, Lewis CD. Fractal properties of sympathetic nerve discharge. J Neurophysiol (2003) 89(2):833–40. doi:10.1152/jn.00757.2002
171. Bassingthwaighte JB, Raymond GM. Evaluation of the dispersional analysis method for fractal time series. Ann Biomed Eng (1995) 23(4):491–505. doi:10.1007/BF02584449
172. Martinis M, Knezevic A, Krstacic G, Vargovic E. Changes in the Hurst exponent of heartbeat intervals during physical activity. Phys Rev E Stat Nonlin Soft Matter Phys (2004) 70(1 Pt 1):012903. doi:10.1103/PhysRevE.70.012903
173. Osaka M, Saitoh H, Atarashi H, Hayakawa H. Correlation dimension of heart rate variability: a new index of human autonomic function. Front Med Biol Eng (1993) 5(4):289–300.
174. Ganz RE, Weibels G, Stacker KH, Faustmann PM, Zimmermann CW. The Lyapunov exponent of heart rate dynamics as a sensitive marker of central autonomic organization: an exemplary study of early multiple sclerosis. Int J Neurosci (1993) 71(1–4):29–36. doi:10.3109/00207459309000589
175. Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, et al. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet (1999) 353(9162):1390–6. doi:10.1016/S0140-6736(98)08428-1
176. Bauer A, Malik M, Schmidt G, Barthel P, Bonnemeier H, Cygankiewicz I, et al. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol (2008) 52(17):1353–65. doi:10.1016/j.jacc.2008.07.041
177. Smith AL, Owen H, Reynolds KJ. Heart rate variability indices for very short-term (30 beat) analysis. Part 1: survey and toolbox. J Clin Monit Comput (2013) 27(5):569–76. doi:10.1007/s10877-013-9471-4
178. Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behavior: a Polyvagal perspective. Infant Child Dev (2011) 20(1):106–18. doi:10.1002/icd.688
179. Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol (2014) 5:1040. doi:10.3389/fpsyg.2014.01040
180. Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Front Psychol (2017) 8:213. doi:10.3389/fpsyg.2017.00213
181. Quintana DS, Alvares GA, Heathers JA. Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry (2016) 6:e803. doi:10.1038/tp.2016.73
Keywords: heart rate variability, Holter monitoring, time domain, frequency domain, systems science, complexity theory
Citation: Ernst G (2017) Hidden Signals—The History and Methods of Heart Rate Variability. Front. Public Health 5:265. doi: 10.3389/fpubh.2017.00265
Received: 16 June 2017; Accepted: 14 September 2017;
Published: 16 October 2017
Edited by:
Robert Drury, ReThink Health/U Wisconsin Institute for Discovery, United StatesReviewed by:
Dirk Cysarz, Witten/Herdecke University, GermanyHarm Van Marwijk, University of Manchester, United Kingdom
Copyright: © 2017 Ernst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gernot Ernst, YnNlcm5nQHZlc3RyZXZpa2VuLm5v
 Gernot Ernst
Gernot Ernst