
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PROTOCOLS article
Front. Public Health , 03 October 2017
Sec. Aging and Public Health
Volume 5 - 2017 | https://doi.org/10.3389/fpubh.2017.00256
This article is part of the Research Topic Theory- and Evidence-Based Health Promotion Program Planning; Intervention Mapping View all 13 articles
Introduction: Epilepsy is a neurological disorder involving recurrent seizures. It affects approximately 5 million people in the U.S. To optimize their quality of life people with epilepsy are encouraged to engage in self-management (S-M) behaviors. These include managing their treatment (e.g., adhering to anti-seizure medication and clinical visit schedules), managing their seizures (e.g., responding to seizure episodes), managing their safety (e.g., monitoring and avoiding environmental seizure triggers), and managing their co-morbid conditions (e.g., anxiety, depression). The clinic-based Management Information Decision Support Epilepsy Tool (MINDSET) is a decision-support system founded on theory and empirical evidence. It is designed to increase awareness by adult patients (≥18 years) and their health-care provider regarding the patient’s epilepsy S-M behaviors, facilitate communication during the clinic visit to prioritize S-M goals and strategies commensurate with the patient’s needs, and increase the patient’s self-efficacy to achieve those goals.
Methods: The purpose of this paper is to describe the application of intervention mapping (IM) to develop, implement, and formatively evaluate the clinic-based MINDSET prototype and in developing implementation and evaluation plans. Deliverables comprised a logic model of the problem (IM Step 1); matrices of program objectives (IM Step 2); a program planning document comprising scope, sequence, theory-based methods, and practical strategies (IM Step 3); a functional MINDSET program prototype (IM Step 4); plans for implementation (IM Step 5); and evaluation (IM Step 6). IM provided a logical and systematic approach to developing and evaluating clinic-based decision support toward epilepsy S-M.
Epilepsy is a neurological disorder involving recurrent seizures (1). It affects approximately 5 million people in the US (1). Epilepsy onset is not age dependent but incidence rates peak before 5 and after 60 years of age (2). Greater psychosocial impact is likely when seizure onset is in adolescence compared to younger ages (2). Epilepsy can have adverse social, physical, and psychological consequences, compromising a person’s economic and social future. The direct costs of epilepsy care were estimated to range from $8,412 to $9,287 in 2013 and were markedly higher for sub-populations with uncontrolled or refractory epilepsy, or co-morbidity (3).
People with epilepsy (PWE) have varied disease severity. Regardless, PWE are encouraged to manage their treatment and lifestyle to provide optimal quality of life. The Managing Epilepsy Well (MEW) Network defines epilepsy S-M as the “processes a person uses to optimize seizure control, to minimize the effects of having a seizure disorder, and to maximize quality of life in partnership with their health-care provider” (4, 5). This includes both S-M behaviors that are specific to epilepsy as well as S-M behaviors for chronic care that are applicable to most chronic conditions (2). Epilepsy specific S-M encompasses managing treatment (e.g., adhering to anti-seizure medication and clinical visit schedules), managing seizures (e.g., planning for, and responding to, seizure episodes), managing safety (e.g., monitoring and avoiding environmental seizure triggers), and managing co-morbid conditions (e.g., anxiety, depression). Chronic care S-M encompasses management of lifestyle issues (e.g., adjusting typical behaviors to avoid seizures and/or to mitigate their adverse consequences), partnering actively with the health-care team (e.g., information sharing), and pursuing independence (e.g., invoking support, resources, and services when needed) (2, 6). Knowledge and self-efficacy to perform S-M behavior are associated with epilepsy S-M (5, 7–10). S-M practice can be compromised by co-morbidities including depression, anxiety, and cognitive dysfunction. These can also act directly as internal precipitants of seizures (2, 11, 12). The emergence of S-M research in epilepsy has co-occurred with the development of the MEW Research Network. The development described in this paper occurred as a MEW Network collaborative project, supporting the Network’s long-term objective to increase the number of adequately tested epilepsy S-M programs available to health-care providers (HCPs) and members of the epilepsy community. The aim of the Network is to contribute to applied research targeting the priority recommendations from the CDC Epilepsy Program and Living Well with Epilepsy 2003 to promoting S-M (5, 13). The Network’s objectives are to: (1) “develop and implement a coordinated, applied-research agenda”; (2) conduct rigorous research that promote S-M and quality of life suitable for application in diverse settings including homes, communities, and clinics; and (3) to identify and collaborate with stakeholders outside of the network to implement these activities (5). The importance of S-M for PWE and programs available to assist S-M are discussed in the needs assessment section below.
The Institute of Medicine (IOM) report, Epilepsy Across the Spectrum, promotes patient-centered care for epilepsy and related co-morbidities, including collaborative approaches (2). Partnership between the HCP, including clinicians, nurse educators, and community health workers and the patient (including the patient’s family or significant others), is important in facilitating S-M adherence. Consistent with the patient-centered model of caring for people with a chronic disease HCPs are well positioned to help their patients in meet S-M goals (2).
Shared decision involves HCPs and patients making decisions together based on the best evidence available. This promotes a two-way communication that incorporates clinician expertise (i.e., disease, options, probabilities, and prognosis) and patient expertise (i.e., “preferences, values, attitudes to risk, and social circumstances”) (14). Dual participation enables the best solution when varied options are available. Prompting patients before the clinical encounter can result in better shared decision-making and enable the transfer of more salient information from the HCP (14). Patient care plans or action plans can be useful, allowing patients to consider individual preferences on options and treatment goals prior to discussion. Systematic review indicates that shared decision-making can lead to better patient treatment adherence (15).
Health-care providers need to be able to clearly communicate the risks associated with epilepsy, the importance of S-M, potential side effects to treatment options, and resources and services that are available (2). Patients need to determine if the type and frequency of their S-M behavior adherence is appropriate; decide on S-M goals that they perceive as important and doable; and determine how to accomplish these behaviors in everyday life. Adoption of S-M behaviors can be undermined due to poor patient-HCP communication and/or a discrepancy in the perceptions about the patient’s attitudes to, and S-M abilities, regarding their epilepsy. Conversely, by reinforcing patient S-M, HCPs can instill greater commitment to monitoring and improving behaviors (16, 17). There are challenges to effectively incorporating S-M assistance within a brief clinical visit that limits the time to assess a patient’s S-M needs and adequately address them (18, 19).
The IOM report also cited the need for new tools to enhance S-M decision-making (2). A decision-support system (DSS), broadly defined, is a tool to support the decision-making process. Typically, such tools are used in the context of less well-structured problems, enable the incorporation of varied models and analytic techniques, provide easy use by non-experts, and are flexible in accommodating changes in circumstances. Text- and video-based materials exist to assist patients and their HCPs in complex decision-making toward outcomes reflective of patient values and preferences (2, 13, 20–24). Electronic health (eHealth) applications are emerging that support daily S-M monitoring and decision-making for epilepsy (25). mHealth is a subset of eHealth that pertains to the “practice of medicine and public health supported by mobile devices such as mobile phones, tablet computers, and PDAs” (26). Clinic-based DSSs have focused on the technical aspects of diagnostic and pharmacologic decisions (27–33) and less on the personal or social aspects of patient care (34). Facilitating patient and HCP epilepsy S-M decision-making, therefore, represents a novel application of decision support.
The clinic-based Management Information Decision Support Epilepsy Tool (MINDSET) was developed to (1) engage adult patients with epilepsy (≥18 years) and their HCPs in managing therapy and lifestyle to prevent seizures and maximize quality of life (2), (2) provide easily followed goal-based action plans for patient decision support between clinic visits (35), and (3) document patient-centric quality indicators for epilepsy care (36, 37).
Management Information Decision Support Epilepsy Tool is a DSS founded on theory and empirical evidence. It is designed to increase awareness by adult patients and their HCP regarding the patient’s epilepsy S-M behaviors, facilitate communication during the clinic visit to prioritize S-M goals and strategies commensurate with the patient’s needs, and to increase the patient’s self-efficacy to achieve those goals.
Intervention mapping is a stepped framework to guide the development of behavioral change interventions that enable developers to systematically apply social and behavioral science theories (38). The 6 steps of IM are to (1) assess needs and develop a logic model of the problem, (2) develop matrices of behavioral change objectives for the program, (3) identify theory-based methods and practical applications to be applied in the program, (4) produce program components and materials, (5) plan for program adoption, implementation, and sustainability, and (6) plan for evaluation (38). IM is widely used to develop behavioral change interventions worldwide. A recent systematic review has demonstrated significant increase in the uptake of disease prevention behaviors associated with IM-based interventions when compared to placebo control groups (39). IM has been successfully applied in the domain of chronic disease S-M (39). However, few applications of IM have been reported in the context of managing epilepsy and, to our knowledge, none in the context of support for patient and provider epilepsy S-M decision-making.
The purpose of this paper is to describe the application of IM to develop and formatively evaluate MINDSET to be a clinic-based tool for adult patient (≥18 years) and provider decision-making regarding the patient’s S-M. Plans for subsequent efficacy evaluation are briefly described.
IM steps 1 through 4 are the focus of this paper. Completion of these steps approximated 2 years of development time. The first 6 months of Year 1 involved completion of the logic model of the problem (IM Step 1) and defining program outcomes and objectives and the logic model of change (IM step 2). The remaining 6 months of year 1 involved program planning, developing the MINDSET design document (IM Step 3). The first 6 months of Year 2 involved producing a program prototype and the remaining 6 months of year 2 involved formative evaluation, including alpha- and usability-testing (IM step 4). Plans for implementation and evaluation (IM Steps 5 and 6) were commenced during the period of MINDSET formative testing.
Step 1 comprised establishing a planning group; conducting a needs assessment informed by the PRECEDE planning model that outlines the factors associated with the problem; defining the context of the intervention in terms of population, setting, and community; and stating program goals.
Task 1.1 Establish and Work with a Planning Group
Management Information Decision Support Epilepsy Tool development took place in collaboration with three neurology clinics varying in patient population, payer-base, epilepsy cases, and provider experience: Kelsey–Seybold Neurology Clinic (KS clinic) and their associated Education and Research Program, the Smith Clinic at Harris Health, and the University of Texas Physicians-Neurology Clinic (UT clinic). The clinics enabled access to patients and neurologists for a Patient-Provider Advisory Group (PPAG; described below) that provided ongoing input on MINDSET development through each step of the IM process and also provided a test-bed for formative assessment of MINDSET. These clinics were the test sites for the planned efficacy trial of MINDSET.
Collaborating Clinic Sites. The KS clinic operates within a large urban multispecialty medical organization comprising 21 clinics and over 325,000 diverse patients comprising primarily white (55%), African-American (23%), and Hispanic (19%) ethnicities who are mainly middle-class, employed, and with private insurance coverage primarily through HMO- or PPO-type plans. Patients with epilepsy are referred to the centralized KS neurology clinic. HCPs include general neurologists (n = 5), an epileptologist, and a nurse epilepsy specialist. The neurology department has an annual epilepsy case load of approximately 400 patients. The Epilepsy Education and Research Program was established at KS Clinic in 1987 with the goal to demystify epilepsy through patient and family education and training about epilepsy, its treatment, and management, and to develop and conduct research to improve the clinical management of epilepsy through participation in multicenter clinical drug trials and academic collaborations (3, 40, 41).
The Smith Clinic at Harris Health provides care to patients who are primarily Hispanic (40%), low-income, uninsured, and covered by Medicaid, and are referred from community health centers (n = 12) operated by a large public hospital system. Medical residents and students rotating through the clinic see up to 40 patients per clinic day under the supervision of attending faculty.
The UT clinic is a large urban multispecialty neurology clinic. Patients with epilepsy comprise white (56%), black (14%), Hispanic (4%), Asian (0.2%), and other/unknown (27%). Economic status and financial coverage for health care is diverse, predominantly commercially managed care (58%), and Medicare (31%). The clinic is a tertiary care referral center for neurological disorders, including the diagnosis of epilepsy and the management of difficult epilepsy.
Patient Provider Advisory Group. A PPAG was formed with representation from patients and HCPs from the three clinics and incorporated into the MINDSET research and planning team. The PPAG was consulted to review content (e.g., constructs, scales, and threshold scores for identifying “at-risk” patients); assess functionality, flow, and “look and feel;” test usability; and review evaluation plans. Patients for the advisory group were invited to join the PPAG by co-investigator clinicians and nurses on the basis of their being representative of the patient population, over 18 years of age, English speaking, engaged in epilepsy management issues, and interested in contributing to the field. The PPAG included three neurologists, one nurse educator, and eight patients with epilepsy. The PPAG met in a conference room at the KS Clinic. Patient members received an incentive payment of $30 per meeting.
Task 1.2 Conduct a Needs Assessment to Create a Logic Model of the Problem
Information gathered to inform the development of MINDSET was obtained through literature review, quantitative enquiry with the PPAG, empirical investigation of the association of S-M antecedents with PWE in Houston clinics, and clinic-based system task analysis (described in Step 4). The needs assessment was designed to inform a logic model of the problem, to provide background information on challenges experienced by PWE in epilepsy S-M, and the potential for technology to assist patient and HCP decision-making regarding epilepsy S-M.
Literature Review. A decision support tool for identifying patient S-M needs based on clinical, behavioral, and psychosocial variables requires identifying S-M behaviors and the clinical, behavioral, and psychosocial antecedents related to poor S-M as well as to identifying what other instruments/tools might be available in the field. To develop a logic model of the problem, the literature review addressed the medical management of epilepsy, epilepsy S-M behaviors, determinants of S-M behavior, and environmental factors associated with S-M. Data on the S-M interventions, DSSs in support of epilepsy management, and perceptions of PWE toward technology-based applications were also reviewed to understand the empirical and clinical context. Theories and models applicable to chronic disease management amenable to, or applied to, epilepsy were also reviewed, as were practice guidelines for epilepsy management. The research team developed problem statements, identified relevant electronic publication databases of Medline, PubMed, and PsychINFO, formulated database search strategies, and recommended an approach to synthesizing the literature. Data abstraction forms were developed and pilot-tested before they were used to abstract data from the identified relevant studies. Abstracted data were used to create evidence and information tables for expert review. We considered articles published in peer-reviewed journals, including review articles and surveys as well as practice guidelines. Abstracts, poster presentations, and editorial publications were excluded.
Medical Management and the Pathophysiology of Epilepsy. As with many chronic diseases, patients with epilepsy may undergo benign or malignant courses, but all will be affected significantly in some way (21, 42). Most patients with epilepsy undergo basic serological tests, EEG, and imaging studies, and have treatment initiated with a single anti-seizure medication (referred to, henceforth, by the common term anti-epilepsy drugs or AEDs) appropriate for the type of seizure, and age and gender of the patient. If the first agent does not control the seizures or has unacceptable toxicity, switching to a second or third appropriate agent occasionally provides better results. Some patients have seizures incompletely controlled with a single agent, but the addition of a second medication only allows a further 15% seizure control. The choice of a specific AEDs for a given patient is a fairly complex process, which needs to consider the individual’s tolerance for medication in general, seizure type, etiology of seizures, co-morbid conditions, concurrent medications, as well as non-medical factors such as employment and medication costs. Despite optimal pharmaceutical treatment, approximately 30% of patients will have recurrent seizures, and as many as 50% of patients with partial seizures will not attain complete seizure control with medication regimens. Patients who do not respond adequately to AEDs may be candidates for surgical treatment or other alternative regimens, including the ketogenic diet, vagal nerve stimulator, and control of precipitating factors (43). The pathophysiology of epilepsy varies between individual patients who may experience a number of different seizure types (e.g., generalized tonic-clinic seizures characterized by convulsions; absence seizures characterized by abrupt beginning and end, blank stares, and only a few second in duration; and complex partial seizures that are characterized by altered consciousness where there is no memory of the misplaced behavior demonstrated during the seizure) and varied stimulus onsets (43).
Epilepsy S-M Behaviors. For PWE, S-M comprises a number of adaptive behaviors that may assist in lowering seizures (44). S-M for PWE refers to a number of adaptive behaviors that may assist in lowering seizures. In recent years patient S-M has received more attention (2, 20). Behavioral risk factors contributing to seizures and co-morbidities include lack of adherence to AEDs, failure to monitor and protect against seizure triggers, lack of safety management to minimize the adverse consequences of seizures, failure to adhere to clinical visit regimens, and failure to adjust lifestyle behaviors to minimize risk of injury (2, 6, 45) (Figure 1). Epilepsy co-morbidities are associated with poor S-M and include cognitive dysfunction, depression, suicidal ideation, death resulting from a seizure or status epilepticus, and sudden unexpected death in epilepsy (SUDEP). Medication management (adherence), safety behaviors (e.g., cessation of driving), and daily activities (e.g., maintaining sleep and reducing stress and exposure to triggers of seizure triggers) may lower seizure frequency. The focus of seizure control is management of AEDs. AEDs require strict adherence and, even with this, may not completely control seizure activity in 30% of epilepsy patients (46). Compounding this is low AED adherence. Review data for claims indicates 39% of patients do not take their prescribed regimen (47). Failure to adhere to prescription is associated with increased likelihood of hospitalizations and ER visits (47). Poor adherence is related to significant adverse health effects and increased mortality (48, 49). Uncontrolled seizures place “challenging demands” on PWE and their family and strongly predict low quality of life (50), being related to injury, limits on driving, and restrictions on sporting and recreational activities. Other S-M activities, apart from AED adherence, can increase mood and quality of life. For example, self-monitoring can increase awareness of prodromal (early) features of seizures (e.g., “mood and premonitory triggers of blurred vision, hunger, thirst, tiredness”) (44). Such self-prediction is associated with favorable mood and increased confidence in one’s ability to accurately predict seizures (51).
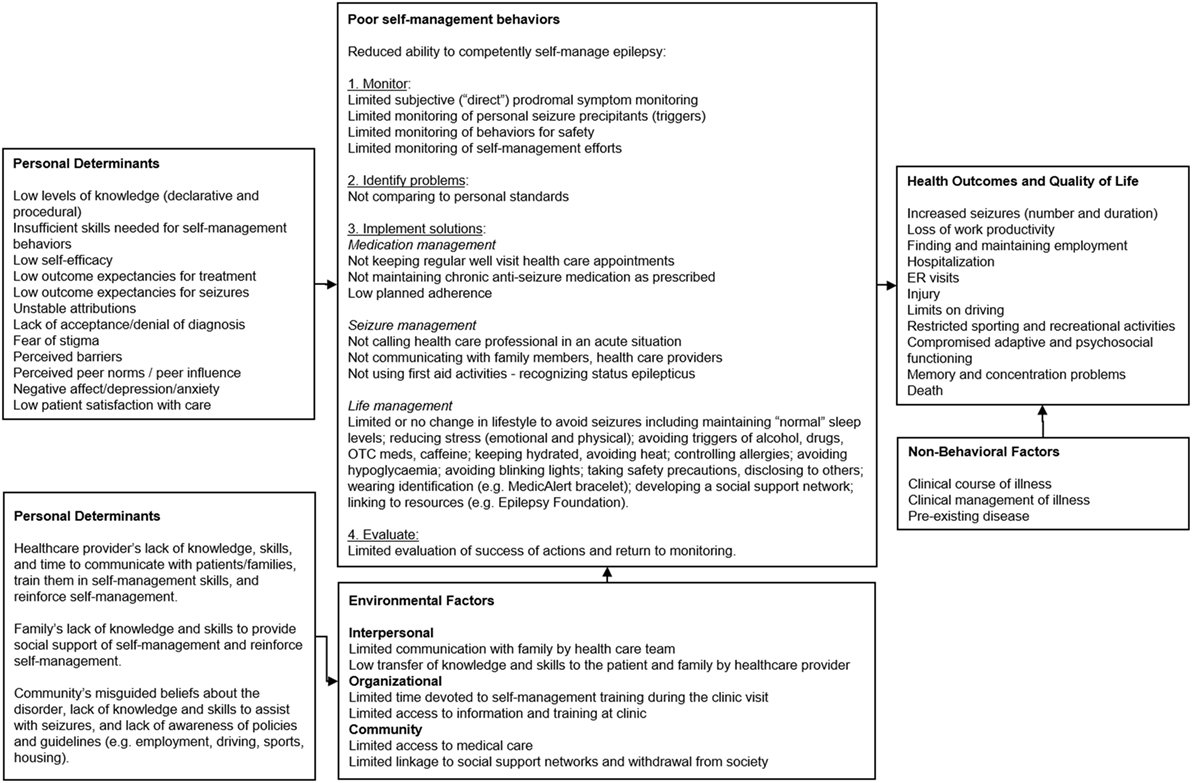
Figure 1. PRECEDE logic model of the problem for Management information Decision Support Epielpsy Tool (MINDSET) (101).
Personal Determinants of S-M Behavior. A range of factors provide antecedents for S-M behavior (Figure 1). Poor S-M could be due to the patient’s low levels of knowledge (declarative and procedural) and skill regarding epilepsy S-M behavior and goal setting, low self-efficacy or confidence to perform S-M behaviors, low outcome expectations (both in terms of causality of seizure onset as well as causality of treatment), and lack of attribution of S-M success to self-effort (particularly as this relates to patient control); lack of acceptance or denial of the diagnosis of epilepsy; fear of stigma related to epilepsy; perceived barriers to managing epilepsy, as well as unrealistic perceptions of how other PWE self-manage (2, 7–9, 11, 16, 52–62). Epilepsy is often associated with cognitive dysfunction, behavior problems, depression, and anxiety (12). Furthermore, seizures in epilepsy may be precipitated by psychological triggers such as stress and emotions such as anxiety and anger (12). Patient’s perceptions of, and satisfaction with, health services and clinical care are associated with health care utilization, an important aspect of S-M (2). Many PWE lack the behavioral capability to monitor and self-regulate behaviors that affect seizure susceptibility indicating a need for effective S-M programs (2). Such behaviors include AED adherence, exposure to environmental stimuli, overuse of drugs and alcohol, stress reduction, and ensuring adequate sleep (2).
Personal Determinants of the Environmental Factors. Interpersonal, organizational, and community factors impact PWE. Personal determinants of environmental factors involve HCPs, families, and the community (Figure 1) (2). Epilepsy management is compromised when families lack knowledge and skills for providing support for S-M, and HCPs lack the skills to effectively communicate with patients and families to train them on, and reinforce them for epilepsy management behaviors (2, 12). This is compounded by the general community’s misguided beliefs about epilepsy, lack of knowledge and skills to assist with seizures and support management, and lack of awareness of policies and guidelines regarding supporting PWE in important life functions, including employment, driving, sports, and housing (2).
S-M Interventions. Until recently, there were few evidence-based epilepsy educational programs (63). Reported results were encouraging. In a study among 100 adults with epilepsy with a two-day psycho-educational program (Sepulveda Epilepsy Education) significant effects were demonstrated that included greater serum AED levels (indicating better drug adherence), decreased use of hazardous medical S-M practices, greater understanding of epilepsy, and decreased fear of seizures in treatment group compared to the comparison group (64). A study among adult Nigerian patients, a two-day modular didactic psycho-educational program focused on adjusting to epilepsy and related psychoneurotic traits, depression, and stigma demonstrated significant improvement in knowledge of epilepsy, neurotic disorders, and depression, in the treatment group compared to the comparison group (65). A modular didactic educational program (MOSES, Modular Service Package Epilepsy) evaluated on a sample of 242 participants, demonstrated greater tolerance of AEDs, fewer side effects, improved knowledge and coping, and greater satisfaction with therapy in the treatment group compared to the comparison group (56). Interventions were mainly psycho-educational with minimal focus on S-M as previously defined. Recent evidence-based interventions that target S-M behaviors and/or co-morbidities for adults (≥18 years) include WebEase (Web Epilepsy Awareness, Support, and Education), UPLIFT (Using Practice and Learning to Increase Favorable Thoughts), PEARLS (Program to Encourage Active Rewarding Lives), HOBSCOTCH (HOme Based S-M and COgnitive Training CHanges lives), and PACES in Epilepsy (Program of Active Consumer Engagement in S-M) (66). WebEase is a self-paced online website where PWE can choose from among medication, stress, sleep, and personal tracking diary modules that provide tailored activities for learning, self-assessment, and goal setting (assessed at 1-week intervals). A national RCT (n = 148) demonstrated significant improvement in self-efficacy and medication adherence for those using WebEase (67). Other interventions have greater focus on co-morbidities of depression [UPLIFT (68), PEARLS (69)] and subjective memory complaints (HOBSCOTCH) (70) or a niche priority population consumers with active epilepsy (with seizures occurring within the last year) (PACES) (71). Tools to optimize decision-making for S-M for patients and providers within the clinic visit had not been reported.
Decision Support and eHealth Applications in Epilepsy Management and Patient Perceptions. The Centers for Disease Control and Prevention (CDC) Epilepsy Program supported the development of e-Tools as one of several approaches to address the gap in available epilepsy S-M tools (1). This vehicle has the potential to overcome barriers to care that PWE face such as lack of transportation and stigma (1). Despite this, a review of the literature on informatics applications for epilepsy management revealed an only recently emerging research effort (25). Of the 68 studies reviewed in the domains of patient monitoring and prevention, education, and therapy or guideline application most were descriptive (describing models, system development, or system installation) with only eight studies testing effectiveness (the impact on patient or provider behavior) using prospective design (25). PWE are well positioned to use emerging eHealth applications in epilepsy S-M. Over 50% of PWE have access to the Internet in a variety of settings (i.e., home, work, school, library) (72–74) and a recent cross-sectional study (19) with adult PWE (n = 183) indicated that most participants had access to computers and the Internet (95 and 60%, respectively) and used them to find health information (99 and 57%, respectively). Participants reported “searching for general information on epilepsy (43%), medication (30%), specific types of epilepsy (23%), and treatment (20%)” and most reported that they “likely would use an Internet-based S-M program to help control their epilepsy” (19). Counter-balancing this is needs assessment survey data of adults with epilepsy in the Pacific Northwest (n = 165) (75, 76) indicating that a majority of patients prefer in-person (individual or group) program delivery, reinforcing the importance of this over purely distance delivery (phone or Internet).
While the patients in the PPAG were veteran self-managers and mostly exhibiting good seizure control they expressed frustration regarding their relationship with their HCP. This applied particularly to patients from a large inner city community health clinic where brief “face time” with clinicians and frequent and consistent turnover of fellows hampered the development of an ongoing therapeutic relationship. They considered a tablet-based DSS within the context of a clinic visit as a positive addition. Many were using the internet to acquire information on epilepsy and most wanted greater communication with their HCP. There was general agreement for the potential of the epilepsy DSS as a clinical tool in facilitating patient-provider communication.
Review of Selected Theories, Models, and Practice Guidelines for Chronic Disease Management. Social cognitive theory (SCT) and self-regulation models (77–79) were consistently reported in the literature in the context of S-M of epilepsy and other chronic diseases (80, 81) and associated with key psychosocial determinants, including knowledge, outcome expectations, and self-efficacy and skills previously described. A tenet of SCT is that behavior is determined by the interaction of personal, environmental, and behavioral influences (77). Personal influencers include cognitions, such as personal values, beliefs, skills, outcome expectations, and self-efficacy. Environmental influencers include social or physical factors (e.g., influential role models, social or normative support). Self-regulation is a potent SCT concept for organizing health education in the management of chronic health disorders (82, 83). It comprises primary sub-functions of behavior self-monitoring (including antecedents and consequences); judgment of one’s behavior in comparison to optimal personal standards and environmental circumstances; and self-reaction (behavior to rectify drifts from optimal S-M) (84). The categorization of S-M behaviors in Figure 1 are informed by this self-regulation framework (Figure 1). The term self-regulation refers to both the patient’s management of his/her own care and the transfer of S-M tasks to the patient by the HCP as appropriate. Self-regulation has the potential of improving the patient’s autonomy and increasing adherence to medical regimens, which can improve medical outcome. Self-regulation necessitates a more prominent role of the patient in first determining, and then monitoring, behaviors and environment, and then modifying therapeutic regimens accordingly in collaboration with the HCP. Self-efficacy and outcome expectations have been described as determinants of epilepsy S-M behavior (10, 16).
The 5-A’s model of behavior change (81), quality-of-care criteria, and clinical guidelines for epilepsy (13, 31, 32, 85); informed MINDSET’s scope, components, and relevance within a clinical context (described in Step 3). Motivational enhancement therapy protocols (86) provided a means of eliciting decision-making within an mHealth program. Both motivational interviewing and shared decision-making supports the ethical principle of self-determination (87) Motivational enhancement protocols used to elicit movement toward behavioral change had been used in previous decision-support studies (88, 89).
Empirical Study of S-M Determinants in the Target Population. To collect additional data on determinants of poor epilepsy S-M in their priority population, the planning team conducted surveys with PWE receiving care at two clinics in the Houston area (n = 238) (10). The objective was to examine variation in S-M across diverse patient populations and explore the association between personal psychosocial factors (knowledge, self-efficacy, depression, and stigma) with S-M. A cohort of 437 patients previously enrolled in the CDC-funded Epilepsy Care and Outcomes Study (41) completed a 45-minute S-M survey within the context of their regular clinic visit. The survey comprised scales previously reported in epilepsy-related research, including the Epilepsy S-M Scale, Epilepsy Knowledge Scale, Epilepsy Self-efficacy Scale, Outcome expectations, Shared Control portion of the Multidimensional Desire for Control Scale, Personal Resource Questionnaire 85 Part 2, Center for Epidemiologic Studies Depression Scale, and Modified Parent Stigma Scale, and Patient Satisfaction Questionnaire-III. The justification for these scales was recent research that had focused on assessing the association of these factors and epilepsy-related S-M behaviors. DiIorio et al. (90) determined the association of the assistance aspect of social support with regimen-specific support (41). Self-efficacy was significantly associated with outcome expectancy and anxiety in the predicted directions and anxiety was significantly negatively associated with S-M (90). DiIorio et al. determined that self-efficacy and patient satisfaction explained the most variance in medication management (16). Self-efficacy was associated with social support, stigma, outcome expectations, and depressive symptoms. Stigma was associated with depressive symptoms (16). The overall fit of the model was improved by adding the direct association between stigma and outcome expectations for seizures to S-M (90). DiIorio et al. (91) identified depressive symptoms and seizure severity as significant antecedents of self-efficacy for epilepsy S-M. Also significant were predictors of social support and stigma (91). Self-efficacy, social support, depression, and perceived stigma were significantly related to S-M regardless of demographics, seizure frequency, or socio-economic status (p < 0.05). These findings suggested that the difficulties with S-M faced by many patients with epilepsy are similar irrespective of a patient’s background or characteristics and that the types of strategies to improve S-M appear similar regardless of population heterogeneity.
Task 1.3 Describe the Context for the Intervention, Including the Population, Setting, and Community
Management Information Decision Support Epilepsy Tool development was modestly focused on application for patients in the collaborating clinics (previously described). The heterogeneity offered in the clinic type (HMO, community clinic, and teaching hospital) and the patient population (demographics and epilepsy type) provided an excellent test-bed for development.
The family, significant others, and community sentiment regarding epilepsy were important environmental influences (Figure 1). Given the clinical setting, the priority environmental focus was the HCP. A caregiver component was considered, and though valid, represented an extension of project scope without universal relevance to PWE who may lack this social network, who are not accompanied to clinic visits, and who are not ready to involve others in their S-M. Broader community influencers, while important, were also outside the scope of the project.
Task 1.4 State Program Goals
Goals for MINDSET were to influence patient S-M behavior and to influence the mediating patient-provider communication regarding S-M. Respective patient and provider goals for MINDSET included:
1. Patients with epilepsy who use the MINDSET S-M DSS in the context of their usual clinic visit for three consecutive clinic visits over a 9-month period will report at least three fewer “at-risk” S-M behaviors (assessed by the Epilepsy S-M scale) compared to patients who do not use MINDSET.
2. HCPs who use the MINDSET S-M DSS in the context of their usual clinic visit will focus discussion on at least 3 “at-risk” S-M behaviors (assessed by the Epilepsy S-M scale) at every visit with every patient using MINDSET.
Step 2 comprised: identification of expected outcomes, performance objectives (POs), and determinants for the behavior and environment; the development of matrices of change objectives; and the construction of a logic model of change for the program. This step enabled the triangulation of data obtained in Step 1 (from theory, empirical findings, and participant involvement) to inform a logic model of change.
Task 2.1 State Expected Outcomes for Behavior and Environment
Expected Behavioral Outcomes. Management Information Decision Support Epilepsy Tool was designed to positively impact S-M behavior for epilepsy that encompassed three domains: Medication management, seizure management, and lifestyle management.
The expected behavioral outcomes for PWE related to each domain were as follows:
• Take AEDs as prescribed by the physician (medication management).
• Prepare for, and respond to, seizure episodes (seizure management).
• Alter behaviors to avoid seizure onset and seizure-related injury (lifestyle management).
Targeted health and quality of life outcomes included decreased seizures (number and duration) and AED side effects and improved daily functioning resulting in improved work productivity, less injury, and reduced ER visits, hospitalization, or death attributable to epilepsy (Figure 1).
Expected Environmental Outcomes. Management Information Decision Support Epilepsy Tool was designed for use in the clinic visit so S-M assessment and intervention needed to become a minimally invasive component of the clinic flow. Rather than manipulate varied clinic environments (which would be prohibitive when considering future dissemination), the environmental outcome focused on the interpersonal level of the HCP (neurologist and nurse educator). Therefore, the environmental outcome was focused at a personal level:
• HCP and/or nurse educator will support PWE to self-manage their condition.
Task 2.2 Specify POs for Health-Promoting Behavior and Environmental Outcomes
POs for Epilepsy S-M. Performance objectives were described for each S-M outcome: medication management, seizure management, and lifestyle management. These are listed in Figure 2 and were drawn mainly from review of existing literature and S-M measurement instrument domains (7). MINDSET and the patient action plan alert both the patient and HCP when change in S-M behavior is needed and cues them to decide on S-M priorities or goals based on available evidence and to agree on how best to achieve these S-M changes. The action plan provides the patient with an ongoing resource outside of the clinic visit on priority S-M performance objectives and strategies to achieve them (described further in Task 4.3 below).
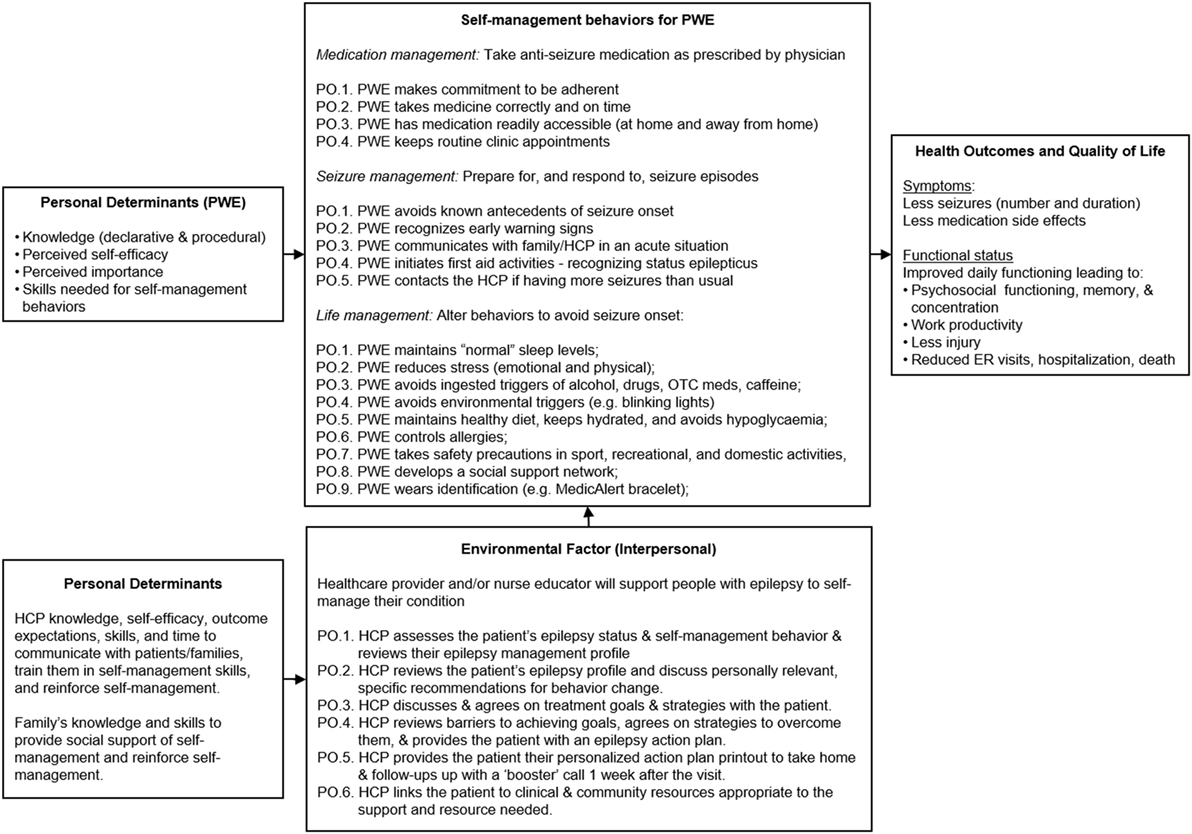
Figure 2. PRECEDE logic model of change for MINDSET (101).
POs for HCPs. The environmental focus for MINDSET was for the HCP to support S-M awareness raising and skills training (Figure 2). This included identification and review of S-M problems, and discussion with the patient to develop agreed upon S-M behavioral goals. The HCP’s behaviors were structured in accordance with the 5 A’s model and included requesting the patient complete data input into MINDSET, reviewing their epilepsy management profile, and acknowledging status (ASSESS); reviewing the patient’s epilepsy profile, reinforcing past management successes, and discussing personally relevant, specific recommendations for behavior change (ADVISE); reaching agreement on treatment goals with the patient (AGREE); reviewing barriers to achieving goals and agreeing on strategies to overcome them, and providing the patient with an action plan (ASSIST); reviewing epilepsy S-M change at each visit by comparing MINDSET epilepsy profile with that of the previous visit, arranging referrals appropriate to existing co-morbidities in the patient profile, and linking patients to appropriate community resources to provide the support needed (ARRANGE). The briefness of clinic encounters made it challenging to adequately review S-M and such assessments had lacked formality. There was opportunity for relative improvement in the HCP modus operandi as it pertained to S-M intervention.
Task 2.3 Select Determinants for Behavioral and Environmental Outcomes
Information obtained from the needs assessment phase (Step 1) and Step 2 literature review informed the specification of determinants for the POs. After reviewing findings from the empirical literature, SCT and self-regulation models, motivational enhancement therapy, and our own formative research (Task 1.2 above), we identified knowledge, self-efficacy, perceived importance, and skills as important and changeable determinants of epilepsy S-M for PWE (Table 1). Similarly, we identified knowledge, self-efficacy and skills, and outcome expectations as important and changeable determinants of the HCP’s behavior (Table 2).
Task 2.4 Construct Matrices of Change Objectives
Matrices were developed that cross-referenced behavioral POs with determinants to produce change objectives. The resulting cells of each matrix contained change objectives that stated what needed to change about a specific determinant (e.g., self-efficacy) for the patient to achieve a specific PO. Change objectives were produced for each relevant cell of the matrix. Example cells from the matrix for adherence to the prescription plan for AEDs are provided in Table 1. Similarly, a matrix was developed to describe the behaviors to be engaged in by the HCP that incorporated the MINDSET action plan into the clinic encounter (Table 2).
Task 2.5 Create a Logic Model of Change
A logic model provided an understanding of the types of functional components MINDSET would need to provide to impact both the patient’s S-M behaviors as well as the HCP-patient discussion of S-M in the clinic visit (Figure 2).
Step 3 comprised the generation of MINDSET’s scope and sequence, the choice of theory and evidence-based methods, and the design of practical applications to deliver change methods.
Task 3.1 Generate Program Themes, Components, Scope, and Sequence
The theoretical framework for MINDSET is based in SCT (77), self-regulation models (77, 78) the 5-A’s model of behavioral change (92), motivational enhancement therapy (86), quality-of-care criteria and clinical guidelines for epilepsy (13, 33, 34, 83), and formative studies (10, 93) drawn from the review of literature. The literature reviewed in Step 1 on decision support and S-M in epilepsy was particularly helpful in informing methods (10, 25, 94, 95).
The challenge was to develop a program to be able to fulfill five functional objectives involving both the patient and provider, without disrupting the flow of a typical clinic visit:
1. Increase patient awareness about their S-M behaviors.
2. Provide immediate feedback on S-M behaviors.
3. Provide a profile of the patient’s S-M behavior for the HCP.
4. Provide tailored S-M behavioral goals for the patient and HCP, including a printable S-M Action Plan.
5. Increase the potential for patient-provider communication of S-M problems and goal setting.
Management Information Decision Support Epilepsy Tool’s scope was contained to only relevant data necessary for the visit so as to not unduly intrude on the timing of events in the clinic flow and to not over-burden the patient. These objectives and our observation of the natural clinic flow suggested the scope and sequence of MINDSET. It was possible for the patient to enter and review their data in MINDSET in the waiting room prior to their visit, and then to provide this profile and the tailored action plan to the HCP for review and discussion in the clinic visit. MINDSET’s scope and sequence are more fully detailed in Step 4.2 below. The original working title for the program was “Brainstorm.” The PPAG advised against this title. While the notions of epilepsy as a brain-related disorder and thinking about management are apparent in this title the term “brainstorm” also has connotations with the erratic neural activity of a seizure and was considered too provocative by patients and providers. The MINDSET acronym, Management Information and Decision Support Epilepsy Tool, offered two contextually related meanings, that of the cognitive profile of the patient explored in the retrospective data input phase, and of “setting” one’s mind which relates to the prospective action plan phase.
Task 3.2 Choose Theory and Evidence-Based Change Methods
Individual Behaviors. Theoretical and empirically based methods for S-M education, included chunking of information into a meaningful framework of S-M domains, self-assessment of S-M behaviors, feedback of a S-M profile to the patient to give an assessment of their S-M status, reinforcement for behavioral successes, goal setting to address those behaviors that were a problem for S-M, tailoring of goals based on the patient’s individual profile, advance organizers and cue altering for S-M using behavioral strategies, self-monitoring of behaviors and environment, and facilitation and linkage to care/support as needed (Table 3). The research team selected methods based on (1) our previous work in decision support of chronic disease (96) and technology-based behavioral change interventions founded in self-regulation frameworks within varied health domains (97–99), (2) empirical evidence for use to impact the target determinants (exemplified in Table 3), and (3) the pragmatics of use in a tablet-based program. These methods and their related practical applications (Task 3.3) could all be delivered through repeated exposure to the MINDSET intervention in clinic visits over time. Their operationalization within MINDSET is described in Task 4.1 below.
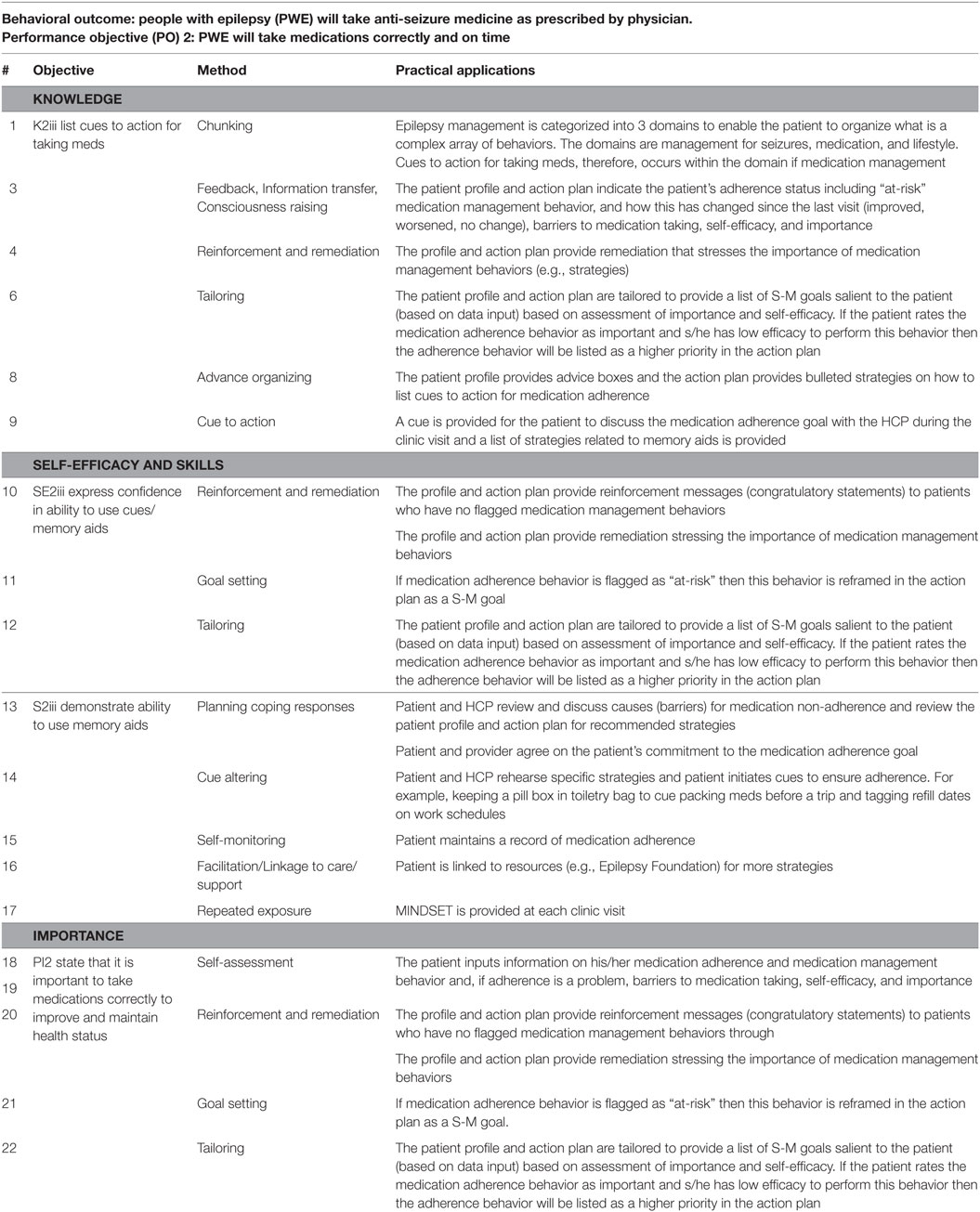
Table 3. Example of methods and practical applications used in MINDSET to impact the determinants (knowledge, self-efficacy, perceived importance, and skills) for adhering to prescribed medications.
Clinic Environment. Guidance on how MINDSET could align to existing guidelines, recommendations, and clinic flow was informed by the 5 A’s model, quality assurance guidelines, and clinic task analysis. The 5 A’s Behavior Change Model. The 5 A’s Behavior Change Model (used with the Improving Chronic Illness Care Chronic Care Model) provided a framework for developing the scope, contextual fit, and application of MINDSET at the interpersonal (patient-provider interaction) level (81). A tenet of the model is that chronic illness patients have a S-M Action Plan covering the 5 A’s elements (Assess, Advise, Agree, Assist, and Arrange). Quality-of-Care Measures. Quality-of-care measures for epilepsy management include an array of assessment, treatment, and counseling protocols representing the best practice recommendations (33, 34, 100). Published quality care measures for the clinical management of epilepsy were consulted to determine the context of use for the practice of medicine. Aligning MINDSET function within these protocols positioned it for ready acceptance for clinic use. Clinic Task Analysis. Task analysis was conducted to examine the clinic flow in each of the participating clinics to understand the on-site operation and to determine logical opportunities for intervention without compromising that clinic flow (101) (Figures 3 and 4). This involved shadowing patients through their clinic visit in each of the participating neurology clinics, examining data flow within the clinic for each patient, decision-making by HCP, interaction points between the patient and provider, and duration in each location.
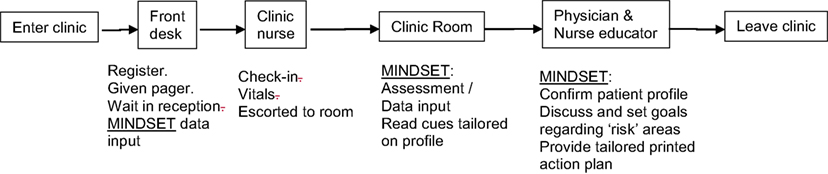
Figure 3. Management Information Decision Support Epilepsy Tool (MINDSET) use within the clinic visit and top-level flow (101).
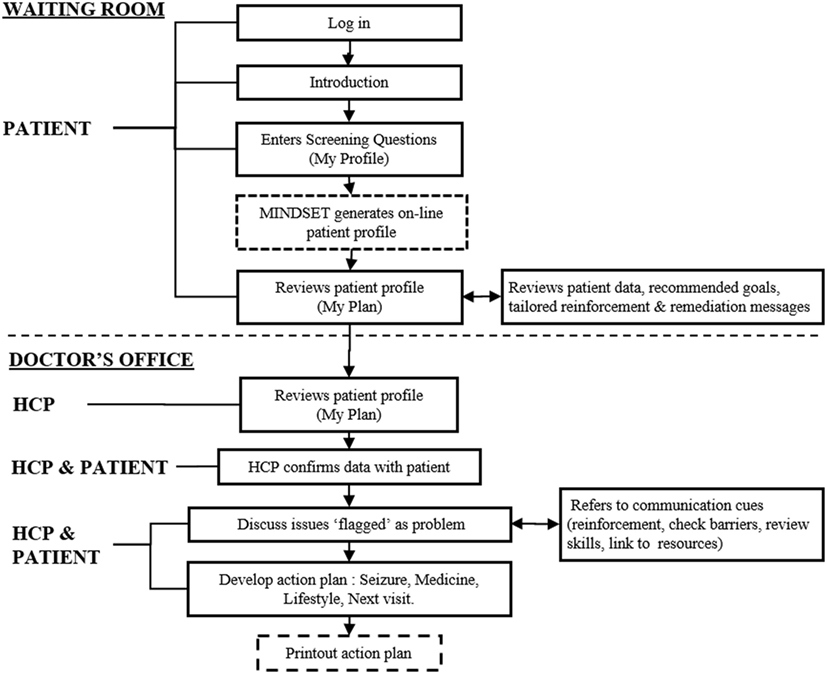
Figure 4. Management Information Decision Support Epilepsy Tool (MINDSET) upper-level flow (101).
Task 3.3 Select or Design Practical Applications to Deliver Change Methods
The planning team selected specific practical applications to operationalize the theory-based change methods in ways that fit the population and setting for the intervention. We designed MINDSET to be easy to use by physician and patient and portable to be able to accompany the patient through the clinic visit. A PC tablet-based tailored self-assessment approach appeared feasible for intervention delivery. Inclusion of data familiar and important to HCPs (e.g., seizure frequency and history, medication missed doses, and side effects) were included with the less familiar data on S-M behaviors for seizure, medication, and lifestyle management to provide added salience for use in the clinic setting. Clinic visit time constraints further suggested the advantages of tailoring data input such that patients would only enter their perceived self-efficacy and importance for “flagged” S-M problem behaviors. An action plan that could be printed in the clinic provided a vehicle for use by both patient and provider during the clinic visit as well as an ongoing reference by the patient between clinic visits.
Step 4 comprised refinement of the program’s structure and organization, planning for program materials, drafting of messages and materials, and pretesting, refinement, and production of materials.
Task 4.1 Refine Program Structure and Organization
Management Information Decision Support Epilepsy Tool is provided on a tablet-based platform to provide S-M decision support to patients (≥18 years) and HCPs during their clinic visit and a printable action plan to provide decision support to patients outside the clinic (102). Originally mounted on an Archos 101 Android tablet platform (and subsequently on a Windows-based Dell platform), the use of MINDSET in the clinic comprises: (1) data entry by the patient; (2) data review by the patient and HCP; and (3) discussion by the patient and HCP of issues, goals, and strategies in conjunction with a tailored action plan (102). MINDSET was designed for the patient to enter data in the waiting room, prior to seeing their HCP. Data represented three epilepsy S-M domains: medication; seizures; and lifestyle. The method of chunking (Table 4, #1) informed us in distilling the complexity of epilepsy S-M into questions assessing 3 management domains and 13 S-M sub-domains including medication S-M (“current AED prescriptions, medication adherence, adherence barriers, side effects, and medication, S-M behaviors”), seizure S-M (“the patient’s recent seizure history, including frequency and type, and seizure S-M behaviors”), and lifestyle S-M (“including mood, social life including sexual relationships, child care, employment, and driving, physical activity, safety, record keeping, social support, and clinic visits”) (102).
Patient Data Entry for Assessment. Scales were embedded in MINDSET to provide assessment of the critical behaviors and determinants previously identified (Tables 1 and 2). A design specification was that MINDSET be minimally intrusive of clinic flow and patient burden. Therefore, an assessment battery was designed that collected information based on theory and empirical relevance, availability of a comprehensive and psychometrically valid scale, and clinical practice needs. For this reason, the determinant of knowledge was not assessed in MINDSET, though addressed in tailored messaging and action plan feedback. Furthermore, in response to the need for utility for use, assessment was tailored such that data were collected only when necessary for a given patient. For example, data on perceived self-efficacy and importance were only collected on a behavior if that behavior was flagged as “at-risk” (less than optimal adherence frequency), described in Task 4.2 and Figure 5 below.
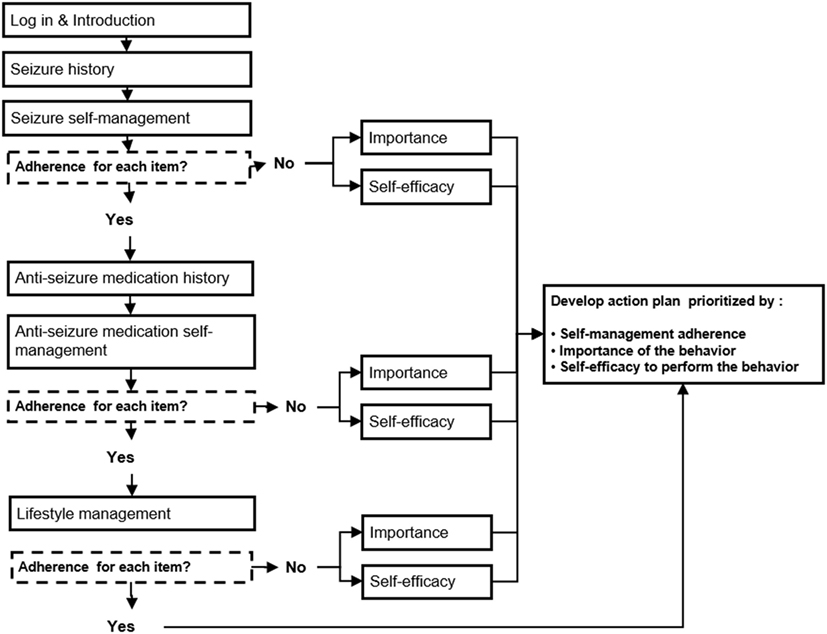
Figure 5. Management Information Decision Support Epilepsy Tool (MINDSET) decision flow to produce a tailored action plan (101).
Assessment of S-M Behaviors. Self-assessment was an important method applied within MINDSET (Table 4). Assessment of S-M behavior was collected using the 38-item Epilepsy S-M Scale (7, 8, 16, 55) that delineates behaviors regarding medication adherence, seizures, information, safety, and lifestyle. Responses were entered using a button selection on a 5-point Likert scale ranging from “never” to “always.” Perceived self-efficacy to perform S-M behaviors was assessed using a 33-item Epilepsy Self-Efficacy Scale (7). Responses were entered on a sliding scale (slider bar) adapted from motivational enhancement protocols (86) with a response set ranging from 0 to 10 with 0 being not at all confident (I cannot do at all) and 10 being extremely confident (Sure I can do) (8, 56). Self-efficacy items were completed for those behaviors flagged as “at-risk.” Also adapted from the use of decision rulers from motivational enhancement protocols was the assessment of importance. Responses were based on a sliding scale from 1 to 10 with 1 indicating not important and 10 indicating extremely important (86).
Assessment of Medication Side Effects and Barriers to Adherence. Medication side effects represent an important clinical parameter to inform AED prescription as well as motivation for medication adherence. Side effects were assessed using a 19-item Epilepsy Adverse Events profile assessing reported problems during the previous four weeks from a list of 19 adverse effects (Table 5) (34, 103–105). The scale assessed reported problems during the previous 4 weeks from a list of 19 adverse drug effects (Table 5). The original instrument used a 4-point Likert scale response set: 1. Never a problem; 2. Rarely a problem; 3. Sometimes a problem; 4. Always a problem (103). Barriers to AEDs were assessed using a list of 18 barriers to medication adherence (adapted from previous studies) and provided to patients reporting missed doses (Table 5) (88).
Assessment of Depression. Depression is a common co-morbidity of epilepsy that can compromise S-M practice. MINDSET was not initially designed to intervene on depression directly and S-M matrices were developed for patients who were physically and cognitively capable of S-M practice. However, the MINDSET planning team saw the potential of MINDSET providing neurologists with the benefit of rapid assessment. Depression was assessed using the 6-item Neurological Disorders Depression Inventory for Epilepsy (NDDI-E) Screening Tool that assesses the degree of depressive symptoms in the last week (106–108). Patients were “prompted to provide the answer that best described them over the last 2 weeks for ‘everything is a struggle’, ‘nothing I do is right’, ‘I feel guilty’, ‘I’d be better off dead’, ‘I feel frustrated’, and ‘I had difficulty finding pleasure’. The response set was a 4-point Likert scale ranging from never to always or often (102).” “NIDDI-E scores of above 15 were considered positive for depression, with specificity of 90%, sensitivity of 81%, and positive predictive value of 0.62” based on the mini international neuropsychiatric interview (MINI) (102, 107, 108).
Patient Review of the S-M Profile. Immediate feedback is an important method applied in MINDSET (Table 4, #3). A profile is produced by MINDSET. The patient can review this in the waiting area and then share it with the HCP (Figure 3). The profile summarizes responses on medication, seizures, and lifestyle, and flags “at-risk” behaviors based on a comparison of the frequency of the behavior to benchmarks. As previously described, the patient rates his/her self-efficacy (confidence) and perceived importance to perform any S-M behavior that is flagged (Table 4, #4). Based on programmed benchmarks for behavior (frequency), self-efficacy (degree of confidence), and perceived importance, the profile provides a prioritized list of behavioral issues for discussion, goal setting, and action. The profile has accompanying tailored advice boxes to increase awareness about strategies to improve S-M behaviors. If the patient reports no problems with S-M behaviors (i.e., he/she has no flagged behaviors), reinforcement is provided in a text-based congratulatory message (Table 4, #5). The advice boxes are also available to provide anticipatory guidance (or advance organizers) in the form of specific behavioral strategies to consider in the future (Table 4, #6). When sharing MINDSET, both the patient and HCP can tab to a list of recommended action items and discuss the items and set goals (Table 4, #7). “The process of using MINDSET is designed to promote shared decision-making where a patient and HCP can assess the need for improvements (both medical and psychosocial) and make subsequent informed treatment and behavioral change decisions (102).” The applications, messages, and cues for discussion (Table 4, #8) are designed to impact determinants of knowledge, self-efficacy, perceived importance, and skills (Table 1).
HCP-Patient Review and Discussion of the S-M Profile and Action Plan. Providing patients with a decision aid to document S-M behaviors and guide future S-M goals is consistent with other approaches to chronic disease management (e.g., asthma) (109). For epilepsy S-M, such tools have focused on acute seizure management and not broader S-M domains inclusive of medication or lifestyle behaviors (110). MINDSET flow and function provides the HCP with an intuitive scaffold to progress through the management steps of assess, advise, agree, assist, and arrange (Table 2), allowing a rapid review of a patient’s status, reviewing strategies to plan coping responses (Table 4, #9), to alter behavioral cues (Table 4, #10), to institute self-monitoring (Table 4, #11), and to link to family and community support as needed (Table 4, #12). The process of using MINDSET is reiterated at each clinic visit (Table 4, #13).
Task 4.2 Prepare Plans for Program Materials
A program design document provided the blueprint for MINDSET, informed by our understanding of patient characteristics, including knowledge, education, cognitive capacity, and time available for learning and discussion in the clinic setting (10). The team developed flowcharts to establish the function of MINDSET for the programmer, depicting the steps in the development of a tailored S-M action plan focused on AED adherence, seizure management, and lifestyle management (Figure 5). Flow charts and screen map mock-ups were developed as Powerpoint slides to depict MINDSET content, function, position of menu options, data entry components, patient profile display screens, and tailored feedback (bullets and cues). These “proof-of-concept” layouts illustrated what the patient and provider would see (the look and feel of the program).
Initial mock-ups depicted the following: (1) screening assessment and (2) decision support for intervention on S-M (Figure 6). The screening assessment consisted of computer-based prompts for the patient to input data (based on the data acquired from the screening tool, see Section “Task 4.1 Refine Program Structure and Organization” above) (10). The decision support was designed to provide feedback to both the patient and the provider in the form of confirmation of the patient’s profile on clinical and psychosocial variables; cues on discussion points during the clinic visit; and S-M goals and an action plan for after the clinic visit. The algorithm for prioritizing the S-M goals on the basis of patient self-report is illustrated in Figure 6. The development of flowcharts and screen maps was an iterative process and an essential one that helped guard against serious error or logical flows in the finished product. The design of an intuitive user interface was essential so that someone unfamiliar with the program could easily use it. A dedicated formative PPAG meeting, held at the KS Clinic conference room, provided a review and feedback on the design documents including content, design (interface) features, navigation, functionality, language, logistics of use and implementation in the clinic, orientation needs, and evaluation specifications. The aim was to uncover any concerns with these program elements as well as recommendations for improvement of MINDSET for PWE prior to programming. The PPAG was provided a simulated “walk through” of MINDSET from log-in through action plan review using projected screen “mock-ups” on Powerpoint slides. Flowcharts were used to illustrate MINDSET use in the context of the clinic visit. The PPAG had few concerns about the use of MINDSET within the clinics and the top-level flow of the program. Their concerns were mainly focused on clarity and completeness. Suggestions for improved clarify included defining medical terms (e.g., in describing seizure types) and specifying general terms (e.g., “wellness”). Suggestions for completeness included adding “choose all that apply” and “I don’t know” options to data collection items; adding dosage amounts for assessment of medication adherence; and addition of items focused on negotiating independence and privacy. Design document revisions were made in response to PPAG consensus.
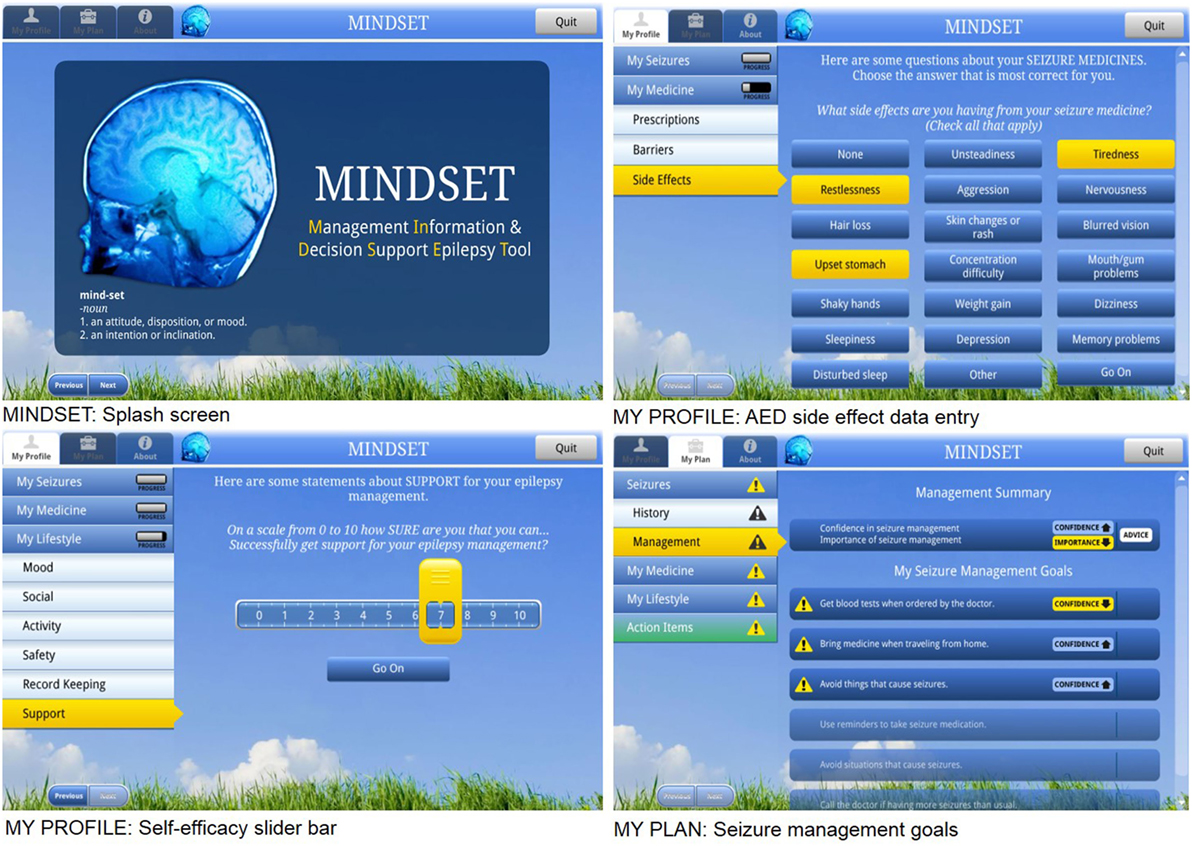
Figure 6. Management Information Decision Support Epilepsy Tool (MINDSET) user interface (101).
Task 4.3 Draft Messages, Materials, and Protocols
Programming followed a stepped sequence. At each developmental step all components of the program were taken one draft further toward completion, building upon the review of previous developmental steps. This process ensured that all elements of the program had been developed with the benefit of multiple reviews by the research team. Structured programming techniques were used to develop the program and reduce needed refinements. The Archos 101 Android tablet platform provided the first MINDSET hardware platform, later superseded by the Dell Latitude. Patients and providers interacted with MINDSET using a stylus or touch screen. The program was button and menu driven and designed for intuitive, easy navigation for both patient and provider with a limited depth of screens, ensuring providers could review the patient’s entire profile in two stylus button presses and not need to “drill down” for data deeper than two screens.
Data input was in the form of pre-existing items from the previously validated surveys embedded in MINDSET (see Task 4.1 Refine Program Structure and Organization above). Tailored messages were created from permutations of these data. Segue messages confirmed the patient’s self-efficacy (low/high) and perceived importance (low/high) regarding a particular flagged behavior and provided a cue to the need for further discussion and reference to the action plan (Table 6). Tailored messages in the action plan were guided by the 5-A’s model: Confirming the patient’s S-M profile (including citing the flagged behavior) and reinforcing S-M success (Assess), stating the behavior goal to mitigate the behavioral problem (Agree), providing strategies and recommendations specific to flagged behaviors (Assist) and cued discussion with the HCP (Advise) (Table 7).

Table 6. Tailored Segue Messages Based on Confidence and Importance Feedback Exemplified for Medication Management.
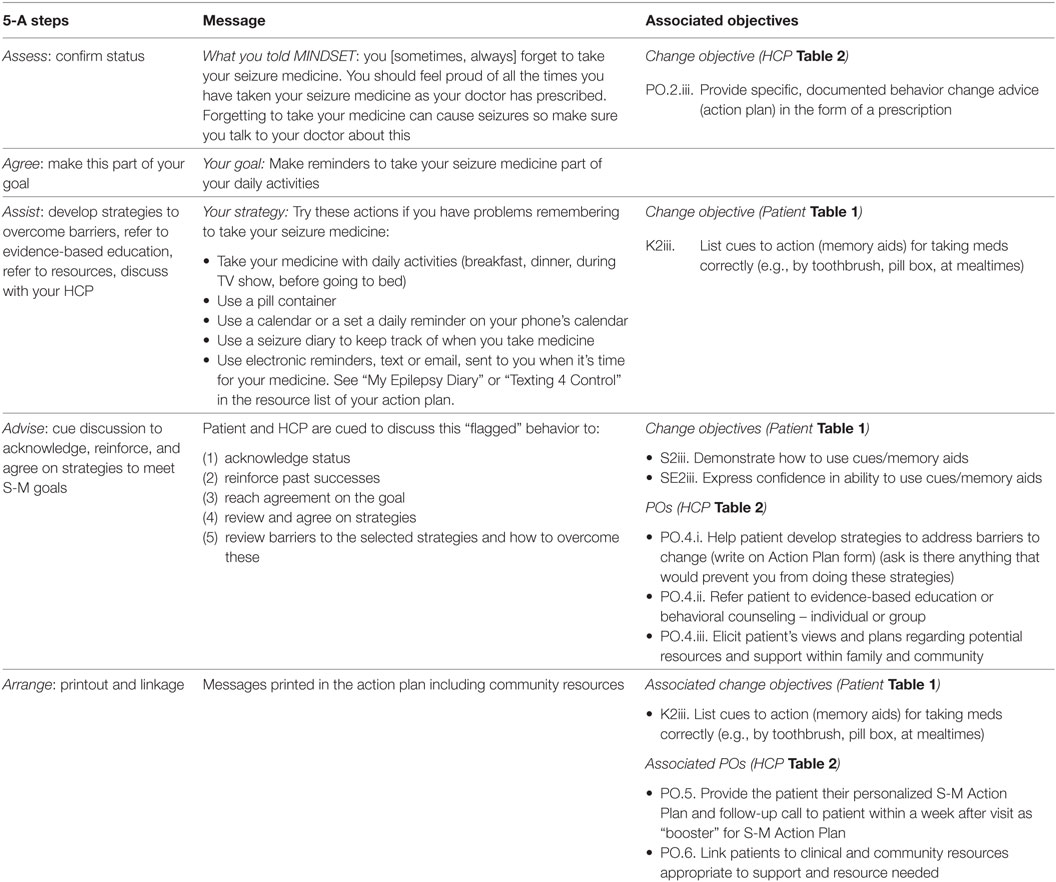
Table 7. Example Management Information Decision Support Epilepsy Tool (MINDSET) messaging and associated objectives for a patient who reports forgetting to take seizure medicine.
Task 4.4 Pretest, Refine, and Produce Materials
Upon completion MINDSET was pretested and refined through an in-house alpha test and a usability test.
MINDSET Alpha Test for Functionality. An in-house alpha test was conducted by the MINDSET research team to ensure all program components and functions conformed to the intentions of the designers, functioned appropriately, and presented no anomalies (“bugs”). Research team members each completed MINDSET, simulating a patient with particular epilepsy S-M profiles. They completed logs recording any problems encountered that included incorrect logic, program bugs, syntax errors, or interface design problems. They completed the problem log by recording their location in the program, the user initiated events that preceded the problem, and a description of the problem (including screen captures where appropriate). Problems were collated and sent to the programming team for further troubleshooting and revision prior to usability testing.
MINDSET Usability Testing with PPAG. Patient Provider Advisory Group patients from three clinic sites (36–53 years of age, prescribed at least one AED, mainly female and ethnic minority) and 4 HCP from the planning team were asked to use the MINDSET prototype in “laboratory” conditions (a dedicated conference room at Kelsey–Seybold clinic) not associated with their regular clinic visit. Hypotheses for usability testing were that patient ratings on usability parameters (measured on a usability survey) would exceed an a priori benchmark of 70% agreement and that HCPs would rate MINDSET features (measured on a features checklist) as providing improvement to their current practice. After an orientation, patients were asked to access all elements of MINDSET (the screening tool, patient profile, recommendations, and action plan) and to verbally describe and interpret what they were seeing and doing. Any problems (as previously described for alpha testing) were recorded and collated. Patients then completed a usability survey assessing the functions of MINDSET and were interviewed on how MINDSET could be improved in terms of content, function, and interface design. Data were gathered on the patient’s satisfaction with the user interface, ease of use (usability), acceptability, credibility, and applicability of the system to their needs using previously validated usability measures. HCPs were provided with a MINDSET tablet that had pre-loaded data on a patient whose profile indicated clinical and psychosocial S-M needs. Patients rated MINDSET favorably on usability parameters, providing 80 to 100% agreement that it was easy to use, likable, credible, understandable, and appealing. This exceeded a priori success criteria of at least 70% agreement (94, 95). Patients appreciated the opportunity to thoroughly review their epilepsy management: “It makes me look @ problems in my lifestyle/mood,” and to receive advice: “I love the advice sections”; “the advice sections were really useful for me”; and to organize their thoughts prior to the clinic encounter “:… opportunity to remember everything to discuss with doctor”; “the information and seizure history for the doctor is great”; and “Helped condense my thought and organized any questions I might have.” HCPs rated MINDSET as increasing the ease, thoroughness, accuracy, and communication in each of the S-M domains (“seizure history and management, medication management, lifestyle management, and providing an epilepsy action plan”) (102).
Reported barriers to use of MINDSET included that the questions (behavior and self-efficacy) seemed repetitive; that patients required assistance due to technical difficulties with the tablet that delayed system responsiveness (distinct from a need to clarify data input questions); and that, while patients advocated the use of MINDSET, they suggested the need for patience for data entry due to the extensive data input in the My Profile section. Modifications were made in response to these issues. These focused on technical/functional fixes, on adjusting clinic expectations on the time commitment for data entry, and alerting patients to the apparent repetition of data input items. The usability data indicated that MINDSET showed initial promise in facilitating the operationalization of S-M constructs for screening, management, and education; the application of clinical guidelines; and was feasible for clinic use. The HCPs rated MINDSET favorably on thoroughness; but also rated it as requiring more time for the clinic encounter.
Step 5 comprised describing potential program implementers, stating the outcomes and POs for implementation, constructing matrices of change objectives for implementation, and designing implementation interventions. An implementation intervention for wide scale adoption, implementation, and maintenance of MINDSET can be developed pending the intervention’s demonstrated efficacy to enhance epilepsy S-M behaviors.
Task 5.1 Identify Potential Program Implementers
Management Information Decision Support Epilepsy Tool was designed for use by HCPs in specialty neurology clinics managing outpatients with epilepsy. Thus, potential adopters included specialty clinic directors or upper-level administrators; potential implementers included HCPs such as neurologists, epileptologists, and nurse educators.
Task 5.2 State Outcomes and POs for Implementation
Performance objectives for adoption were brainstormed by the research team with consideration of the decision-makers in neurology clinics, and informed by the IM framework (38), and characteristics for diffusion of innovation (111). These included that implementers recognize a need for MINDSET and its relative advantage, and make a formal commitment to use. Steps drafted to date include that the clinic director will: Assess the need for an epilepsy S-M program among clinic patients; review MINDSET and note objectives and relative advantages for program adoption; obtain feedback from clinic staff on potential barriers to/advantages of adopting MINDSET; solicit experiences from other clinics that have used MINDSET; agree to adopt MINDSET by signing a memorandum of understanding for its use.
Task 5.3 Construct Matrices of Change Objectives for Implementation and Task 5.4 Design Implementation Interventions
Critical opportunities for MINDSET implementation within the clinic flow were identified from clinic task analysis of collaborating clinics. This enabled us to understand environmental constraints. MINDSET was designed to accommodate regular clinic visits in varied clinic settings previously described (Figures 3 and 4). Matrices of change objectives for clinic directors, HCPs, and clinic nurses to adopt and implement MINDSET and the development of an implementation intervention are pending determination of its effectiveness.
Management Information Decision Support Epilepsy Tool will be more likely adopted if it is efficacious with minimal disruption to clinic activities or clinic overhead. The thoroughness of the S-M assessment may be associated with greater time commitments but this may, in turn, be offset by its provision of a detailed record of (potentially) billable behavioral counseling activities in the clinic. Integration of the MINDSET data base with existing medical record systems would also enhance its appeal to HCPs. Emerging potential uses for MINDSET exist beyond its original design including as a tool for clinic-based community health workers and as an electronic behavioral assessment with the National Epilepsy Education and Awareness Collaborative (NEEAC).
Step 6 comprised effect and process evaluation questions, developing indicators and measures of assessment, and specifying an evaluation design.
Task 6.1 Write Effect and Process Evaluation Questions
The primary question to be addressed in planning the evaluation of MINDSET was: Does the use of MINDSET by a PWE and their HCP during multiple clinic visits over a 9 month period, including the use of a printed action plan between visits, improve the S-M behaviors and confidence of patients? Stated as an alternative testable empirical hypothesis: PWE who use MINDSET in the context of their usual clinic visit for three consecutive clinic visits over a 9-month period, and a printed action plan between visits, will report at least three fewer “at-risk” S-M behaviors (assessed by the Epilepsy S-M scale) compared to patients who do not use MINDSET.
Planned process evaluation questions included assessment of factors that mediate the success of MINDSET as well as facilitating its implementation (Table 8). These include intervention exposure, impact on patient-provider communication, and information seeking other than MINDSET. Sufficient exposure to MINDSET relates to implementation fidelity, that the patient was exposed to all components and completed them through to action plan printout. Incomplete exposure compromises the quality of the efficacy trial. Time-on-task data (both patient and patient and provider use) informs expectations for future implementation (e.g., time commitments) for adopting clinics. Assessment of the quality of the patient and provider clinic encounter when MINDSET is used allows a determination of correspondence between MINDSET cues and topics subsequently discussed in the clinic encounter. Exit interviews allow for a protracted discussion of the HCP’s experience in using MINDSET and recommended adjustments to facilitate its use and future adoption. Assessment of the degree to which the patients accessed other sources for information on S-M enables an accurate assessment of the degree to which MINDSET and the action plan (distinct from other sources) influenced S-M. Knowledge gained from clinic testing will inform implementation plans and program user manuals for those adopting MINDSET in the future.
Task 6.2 Develop Indicators and Measures for Assessment
From the outset the development of MINDSET focused on instruments and scales to assess patient’s S-M status and to provide indicators of S-M success over time. For this reason, measures for evaluation can closely correspond to those embedded in MINDSET. Planned impact measures include the epilepsy S-M scale, epilepsy self-efficacy scale, NDDI-E, and adverse effects scale previous described (refer to Step 4: Program Production and Table 8). Planned process measures were developed to assess the process evaluation constructs previously described (Table 8).
Task 6.3 Specify Evaluation Design
The planned evaluation design for MINDSET involves an RCT with a sample of patients randomly assigned to treatment (MINDSET and usual care) and comparison (usual care only) groups (n = 30 per group) at three clinic sites over three visits to evaluate its efficacy.
Planned Patient Recruitment. A total of 60 patients from the KS clinic (n = 20), BT clinic (n = 20), and UT clinic (n = 20) (previously described) would be invited to participate. Participants would include patients with a diagnosis of epilepsy who are 18 years of age and older, who can speak English, who are willing and able to complete MINDSET, and who have no other medical disorders that could inhibit their ability to use MINDSET or practice S-M activities. Participation would be based on clinician and nurse educator referral and ideally reflect the diversity of gender and race-ethnicity, average age and SES for the respective clinic populations.
Planned Pilot Efficacy Trial of MINDSET. Each clinical site would recruit 20 patients to participate in the randomized pre–post treatment and comparison group study. Once enrolled the patients would participate during three regular clinic visits that would be scheduled three months apart. They would be randomly assigned to one of two groups (30 in each group, 10 from each site) for receipt of the treatment (MINDSET plus usual care) or comparison (usual care only) condition.
At the first visit, a MINDSET research staff member would meet the patient at the clinic to confirm participation, answer questions, and, if they agree to participate, obtain signed consents. Consent and study protocols are subject to approval by human subjects internal review boards at the contributing university and clinical organizations. All patients would then complete a contact sheet, and a demographic survey. They would then input data into the assessment section of MINDSET (My Profile) prompted by screening questions. This would include data on seizures, AEDs, and lifestyle, as well as S-M behaviors (Epilepsy S-M Scale) and self-efficacy (Epilepsy Self-efficacy Scale) related to these domains. Data input would take place in the waiting room and clinic room while waiting for the clinic appointment.
Group 1 patients would use MINDSET to review their epilepsy S-M patient profile (My Plan) that indicates S-M challenges (“risk”), provides behavioral goals and associated advice about content, provides recommendations for discussion with the HCP, and also provides information on associated S-M resources (e.g., available through the American Epilepsy Society, Epilepsy Foundation, and MEW Network). During the clinic encounter, the provider and patient would refer to MINDSET. The HCP would be provided suggested action items based on the patient’s profile, could access the patient profile (My Plan) data and could confirm or modify these data after interviewing the patient. The patient and provider would have the opportunity to review recommended discussion points, goals for management, and the action plan. The provider will have the opportunity to provide the patient with a tailored printed action plan that reiterates the priority management goals discussed in the clinic encounter.
After completing initial assessment items in MINDSET, Group 2 patients would provide MINDSET back to the research staff member and continue their regular clinic visit in which they would meet with their providers as usual without the benefit of MINDSET information and prompting on discussion points and the action plan, and without the receipt of a printout of the action plan. Following the clinic visit both group 1 and group 2 patients would complete process measures of the clinic visit interaction checklist and clinic visit communication scale. All patients will then be provided $15 for their participation.
During the second visit, patients in Group 1 and Group 2 would again complete the assessment (My Profile) component of MINDSET. Group 1 patients would again use MINDSET to review their epilepsy S-M patient profile and recommended management goals (My Plan) and both HCP and the patient can use MINDSET to review and confirm data and develop the action plan. The HCP would also have access to any change in the patient data since the last clinic visit. Group 2 patients would again only complete initial assessment items in MINDSET, and then will provide MINDSET back to the research staff member and continue to the regular clinic visit. Again, following the clinic visit, both Group 1 and Group 2 patients would complete process measures (described below). During the third visit, both Group 1 and Group 2 patients would input their profile data using the assessment screening tool in MINDSET (My Profile). They will then return MINDSET to the research staff, complete a short exit interview.
Analysis. Comparisons of changes in scale scores on S-M and self-efficacy from O1 to O3 will be made and t-tests and one-way analysis of covariance of the changes will be used to address the evaluation hypothesis. The independent variable of interest in all cases will be group assignment: treatment or comparison. Measures of mediating factors including depression and pre-test scores will be used as covariates.
Limitations. This study represents a modest trial designed to be accomplished with available resources. While loss to follow-up is often an issue in such trials the small sample and previous success of the clinics in tacking and maintaining these patients indicates that a 20% attrition is realistic. A per-protocol analysis is planned in this efficacy trial to determine impact of the MINDSET intervention if received. Furthermore, because a limited number of providers (neurologists) are involved in the trial there is expected to be limited turnover and the capability of targeted training by the research staff. Despite this, any findings need to be interpreted in the light of acknowledged study limitations. We expect minimal between-patient contamination because the patients are not typically interacting with each other in these clinics. However, the study is subject to within-provider contamination because a limited number of providers will encounter both MINDSET (Group 1) and usual care (Group 2) patients. It is not possible to blind the provider in this type of trial because they are using MINDSET and the action plan with their patients. It is, therefore, possible that providers will be more attentive to lifestyle issues with all their patients, above what they might originally have been. The contamination will work against a Type I error, making any findings more robust. Further, the time constraints of a busy neurology clinic will likely limit providers to patient-specific cues (from the Action Plan) and not unduly influence general discussion. This remains to be determined. The trial has not been registered in a public trials registry.
Management Information Decision Support Epilepsy Tool is designed to address the need, identified in the 2012 IOM report, “Epilepsy Across The Spectrum,” for substantial engagement by patients and their HCPs to manage therapy and lifestyle issues so as to prevent seizures and maximize quality of life (3, 22, 24). It also provides a called for “structured approach to addressing and documenting patient-centric quality indicators for epilepsy patient care” (3, 22, 24). In epilepsy care, DSSs have focused on diagnostic and pharmacologic support, consistent with historic applications of such systems in medicine (26–31). This reflects the focus and enquiry into the “technical aspects of care, in contrast to personal or social concerns” (32). The development and testing of MINDSET may lead to a new patient-centered decision-making tool to assist in identifying initial steps toward epilepsy S-M (4) to identify patients needing more assistance, and to provide ongoing decision support through action plans. It is also responsive to the Healthy People 2020 objective (HC/HIT-1.1) to increase the proportion of persons receiving easy-to-understand instructions about what to do to take care of their illness or health condition (109). To date, there is a lack of attendance to S-M needs in clinical settings despite the availability of evidence-based interventions to promote epilepsy S-M outside clinic settings.
The IM framework has facility in developing DSSs that promote patient and provider decision-making regarding S-M. Advantages of the framework include the imposition of a systematic approach, thoroughness in detailing needs and solutions informed by theoretical- and empirical literature, encouraging “downstream” thinking regarding implementation, evaluation, and dissemination, and ensuring that priority populations are consulted throughout. Challenges for the use of IM are the time resource required to complete the process with maximum “textbook” fidelity due to the tension with resource constraints in research projects. The MINDSET development presented here represents one case study application for decision support for chronic disease S-M in clinic contexts. In this capacity it is contributive as a guide for future development in analogous domains and populations and application. However, there are necessary limitations in this work that are a basis for future recommendations. Developers are encouraged to apply a systematic application of core processes with each development task. These include posing questions, brainstorming answers, review findings from published research, accessing and using theory, identifying and addressing the need for new research, and formulating the working list of answers. This will ensure a more complete and continuous data feedback loop throughout. Also advised is a timeline that indexes dialog with the priority population with each development task rather than, for example, a calendar meeting schedule which may fail to involve the priority population fully and at the time when input is most helpful.
By providing tools and procedures for identifying and assisting patients with S-M needs, this study will make a significant contribution to the CDC-MEW goal of promoting S-M and self-determination principles in the care of PWE. IM was conducive to providing an innovative technological application to facilitate the dissemination of knowledge from social and behavioral research on epilepsy S-M into clinical practice.
This study was carried out in accordance with the recommendations of local human subject research internal review boards at the University of Texas and the Harris County Hospital District (Harris Health) with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the local human subject research internal review boards at the University of Texas and the Harris County Hospital District (Harris Health).
The authors (RS and CB) have both been involved in the development of the MINDSET epilepsy decision support system from early needs assessment and descriptive studies through the structure and function of the program.
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors and their academic institution have received no payment or services from a third party for any aspect of this research. There are no financial relationships with organizations or entities that could be perceived to influence, or that give the appearance of potentially influencing, what has been written in this work. No commercial patents or copyrights are or have been pending, issued, licensed, and/or received, and no royalties have been received from this research.
The reviewer FS and handling editor declared their shared affiliation.
We extend our deepest appreciation to the patients and clinicians who participated in the development of MINDSET and to our colleagues in the MEW Research Network for their consultation. This research received human research approval from the local human subject research internal review boards at the University of Texas and the Harris County Hospital District (Harris Health). Permission has been granted to publish this work from Jossey-Bass, San Francisco, CA, USA. This paper was originally published as a case study in Planning Health Promotion Programs: An Intervention Mapping Approach, Bartholomew Eldredge, L. K., Markham, C. M., Ruiter, R. A. C., Fernández, M. E., Kok, G., Parcel, G. S. (Eds.), San Francisco, CA, USA: Jossey-Bass.
This research was funded by Special Interest Project Grants (SIP07-006, SIP09-012, and SIP12-057) from the Centers for Disease Control and Prevention (CDC) Managing Epilepsy Well (MEW) Collaborating Center.
1. Kobau R, Price P, Hawkins N. News from the CDC: translating epilepsy self-management research to practice. Transl Behav Med (2012) 2(2):124–5. doi:10.1007/s13142-012-0122-y
2. Institute of Medicine. Epilepsy across the Spectrum: Promoting Health and Understanding. Washington, DC: National Academies Press (2012).
3. Begley CE, Durgin T. The direct cost of epilepsy in the United States: a systematic review of estimates. Epilepsia (2015) 56(9):1376–87. doi:10.1111/epi.13084
4. DiIorio C. Epilepsy Self-Management. Handbook of Health Behavior Research II: Provider Determinants. New York: Plenum Press (1997). p. 213–30.
5. Dilorio C, Bamps Y, Edwards AL, Escoffery C, Thompson NJ, Begley CE, et al. The prevention research centers’ managing epilepsy well network. Epilepsy Behav (2010) 19(3):218–24. doi:10.1016/j.yebeh.2010.07.027
6. Buelow JM. Epilepsy management issues and techniques. J Neurosci Nurs (2001) 33:260–9. doi:10.1097/01376517-200110000-00006
7. DiIorio C, Faherty B, Manteuffel B. Epilepsy self-management: partial replication and extension. Res Nurs Health (1994) 17:167–74. doi:10.1002/nur.4770170304
8. DiIorio C, Faherty B, Manteuffel B. The development and testing of an instrument to measure self-efficacy in individuals with epilepsy. J Neurosci Nurs (1992) 24:9–13. doi:10.1097/01376517-199202000-00004
9. Tedman S, Thornton E, Baker G. Development of a scale to measure core beliefs and perceived self-efficacy in adults with epilepsy. Seizure (1995) 4(3):221–31. doi:10.1016/S1059-1311(05)80065-2
10. Begley C, Shegog R, Iyagba B, Chen V, Talluri K, Dubinsky S, et al. Socioeconomic status and self-management in epilepsy: comparison of diverse clinical populations in Houston, TX. Epilepsy Behav (2010) 19(3):232–8. doi:10.1016/j.yebeh.2010.08.020
11. Jones JE, Hermann BP, Woodward JL, Barry JJ, Gilliam F, Kanner AM, et al. Screening for major depression in epilepsy with common self-report depression inventories. Epilepsia (2005) 46(5):731–5. doi:10.1111/j.1528-1167.2005.49704.x
12. Ramaratnam S, Baker GA, Goldstein LH. Psychological treatments for epilepsy. Cochrane Database Syst Rev (2008) 3:CD002029. doi:10.1002/14651858.CD002029.pub3
13. American Epilepsy Society. Centers for Disease Control and Prevention (U.S.). Epilepsy Foundation of America, National Association of Epilepsy Centers. Living Well with Epilepsy II Report of the 2003 National Conference on Public Health and Epilepsy: Priorities for a Public Health Agenda on Epilepsy. Landover, MD: Epilepsy Foundation of America (2004). Available from: http://www.cdc.gov/Epilepsy/pdfs/living_well_2003.pdf
14. Pickell WO, Elwyn G, Smith PEM. Shared decision-making in epilepsy management. Epilepsy Behav (2015) 47:78–82. doi:10.1016/j.yebeh.2015.01.033
15. Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, Van der Staak CP, deJong CA. Systematic review of effects of shared decision-making on patient satisfaction, treatment adherence, and health status. Psychother Psychosom (2008) 77:219–26. doi:10.1159/000126073
16. DiIorio C, Shafer PO, Letz R, Henry TR, Schomer DL, Yeager K. Project EASE: a study to test a psychosocial model of epilepsy medication management. Epilepsy Behav (2004) 5:926–36. doi:10.1016/j.yebeh.2004.08.011
17. Rothert M. Perspectives and Issues in Studying Patient’s Decisionmaking. Proceedings of the AHCPR Conference on Primary Care Research: Theory and Methods. Washington, DC: U.S. Department of Health (1991). p. 175–9.
18. DiIorio C, Reisinger E, Yeager K, Schomer DL, Henry TR, Shafer PO. A descriptive analysis of seizure events among adults who participated in a computer-based assessment. J Neurosci Nurs (2008) 40:124–41. doi:10.1097/01376517-200806000-00003
19. Escoffery C, DiIorio C, Yeager KA, McCarty F, Robinson E, Reisinger E, et al. Use of computers and the Internet for health information by patients with epilepsy. Epilepsy Behav (2008) 12(1):109–14. doi:10.1016/j.yebeh.2007.07.013
20. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med (2013) 368(1):6–8. doi:10.1056/NEJMp1209500
21. AHRQ. Management of Newly Diagnosed Patients with Epilepsy: A Systematic Review of the Literature. Summary, Evidence Report/Technology Assessment: Number 39. AHRQ Publication (2009). p. 01–E037. Available from: http://www.ahrq.gov/clinic/epcsums/epilepsum.htm
22. National Institute of Neurological Disorders and Stroke. Epilepsy Information Page: National Institute of Neurological Disorders and Stroke (NINDS). (2009). Available from: http://www.ninds.nih.gov/disorders/epilepsy/epilepsy.htm
23. National Institute of Neurological Disorders and Stroke. Seizures and Epilepsy: Hope Through Research: National Institute of Neurological Disorders and Stroke (NINDS). (2009). Available from: http://www.ninds.nih.gov/disorders/epilepsy/detail_epilepsy.htm
24. CDC. Center for Disease Control Framework for Epilepsy Prevention and Control. (2009). Available from: http://www.healingwithnutrition.com/edisease/epilepsy/cdcplan.html
25. Shegog R, Bamps YA, Patel A, Kakacek J, Escoffery C, Johnson EK, et al. Managing epilepsy well: emerging eTools for epilepsy self-management. Epilepsy Behav (2013) 29:133–40. doi:10.1016/j.yebeh.2013.07.002
27. Harding A. Biomedical Informatics Applications for Epilepsy Management: A Systematic Review [Master’s Thesis]. Houston, TX: University of Texas School of Public Health (2011).
28. Holden EW, Grossman E, Nguyen HT, Gunter MJ, Grebosky B, Von Worley A, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag (2005) 8:1–14. doi:10.1089/dis.2005.8.1
29. Vassilakis KM, Vorgia L, Micheloyannis S. Decision support system for classificationof epilepsies in childhood. J Child Neurol (2002) 17:357–63. doi:10.1177/088307380201700509
30. Smeets R, Talmon J, Meinardi H, Hasman A. Validating a decision support system for anti-epileptic drug treatment. Part I: initiating anti-epileptic drug treatment. Int J Med Inform (1999) 55:189–98. doi:10.1016/S1386-5056(99)00051-9
31. Smeets R, Talmon J, Meinardi H, Hasman A. Validating a decision support system for anti-epileptic drug treatment. Part II: adjusting anti-epileptic drug treatment. Int J Med Inform (1999) 55:199–209.
32. Korpinen L, Pietilä T, Peltola J, Peltola J, Nissilä M, Keränen T, et al. Evaluation of epilepsy expert – a decision support system. Comput Methods Programs Biomed (1994) 45:223–31. doi:10.1016/0169-2607(94)90206-2
33. Mishra RB. A decision table and rule based interpretation system for epileptic discharges. Int J Clin Monit Comput (1992) 9:165–78. doi:10.1007/BF01145170
34. Bernabeo E, Holmboe ES. Patients, providers, and systems need to acquire a specific set of competencies to achieve truly patient-centered care. Health Aff (2013) 32(2):250–8. doi:10.1377/hlthaff.2012.1120
35. U.S. Department of Health and Human Services. Healthy People 20/20. Washington, DC: U.S. Department of Health and Human Services (2013). Available from: http://www.healthypeople.gov/2020/default.aspx
36. Pugh MJ, Berlowitz DR, Montouris G, Bokhour B, Cramer JA, Bohm V, et al. What constitutes high quality of care for adults with epilepsy? Neurology (2007) 69(20):2020–7. doi:10.1212/01.WNL.0000291947.29643.9f
37. Pugh MJ, Berlowitz DR, Rao JK, Shapiro G, Avetisyan R, Hanchate A, et al. The quality of care for adults with epilepsy: an initial glimpse using the QUIET measure. BMC Health Serv Res (2011) 11:1. doi:10.1186/1472-6963-11-1
38. Bartholomew Eldredge LK, Markham CM, Ruiter RAC, Fernandez ME, Kok G, Parcel GS. Planning Health Promotion Programs: An Intervention Mapping Approach. 4th ed. San Francisco, CA: Jossey-Bass (2016).
39. Garba RM, Gadanya MA. The role of intervention mapping in designing disease prevention intervnetions: a systematic review of the literature. PLoS One (2017) 12(3):e0174438. doi:10.1371/journal.pone.0174438
40. Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia (1999) 40:502–6. doi:10.1111/j.1528-1157.1999.tb00748.x
41. Begley CE, Basu R, Reynolds T, Lairson DR, Dubinsky S, Newmark M, et al. Sociodemographic disparities in epilepsy care: results from the Houston/New York City health care use and outcomes study. Epilepsia (2009) 50(5):1040–50. doi:10.1111/j.1528-1167.2008.01898.x
42. Prinjha S, Chapple A, Herxheimer A, McPherson A. Many people with epilepsy want to know more: a qualitative study. Fam Pract (2005) 22(4):435–41. doi:10.1093/fampra/cmi024
43. Epilepsy Foundation of America, Inc. Epilepsy: You and Your Child, Information for Parents from the Epilepsy Foundation. Pamplet #365 YCP. Landover, MD: Epilepsy Foundation of America (2000).
44. Edward K, Cook M, Giandinoto J. An integrative review of the benefits of self-management interventions for adults with epilepsy. Epilepsy Behav (2015) 45:195–204. doi:10.1016/j.yebeh.2015.01.026
45. Shope JT. Compliance in children and adults: review of studies. Epilepsy Res Suppl (1988) 1:23–47.
46. Robinson E, DiIorio C, Depadilla L, McCarty F, Yeager K, Henry T, et al. Psycholosocial predictors of lifestyle management in adults with epilepsy. Epilepsy Behav (2008) 13:223–8. doi:10.1016/j.yebeh.2008.05.015
47. Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia (2008) 49(3):446–54. doi:10.1111/j.1528-1167.2007.01414.x
48. Faught E, Duh MS, Weiner JR, Guerin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM study. Neurology (2008) 71(20):1572–8. doi:10.1212/01.wnl.0000319693.10338.b9
49. Faught RE, Weiner JR, Guerin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia (2009) 50(3):501–9. doi:10.1111/j.1528-1167.2008.01794.x
50. Chung K, Liu Y, Ivey SL, Huang D, Chung C, Guo W, et al. Quality of life in epilepsy (QOLIE): insights about epilepsy and support groups from people with epilepsy (San Francisco bay Area, USA). Epilepsy Behav (2012) 24:256–63. doi:10.1016/j.yebeh.2012.02.003
51. Haut SR, Hall CB, Borkowski T, Tennen H, Lipton RB. Modeling seizure self-prediction: an e-diary study. Epilepsia (2013) 54:1960–7. doi:10.1111/epi.12355
52. Green LW, Kreuter MW. Health Promotion Planning: An Educational and Environmental Approach. 2nd ed. CA: Mayfield Publishing Co (1991).
53. Levine J, Rudy T, Kerns R. A two factor model of denial of illness: a confirmatory factor analysis. J Psychosom Res (1994) 38(2):99–110. doi:10.1016/0022-3999(94)90083-3
54. Sabaz M, Lawson JA, Cairns DR, Duchowny MS, Resnick TJ, Dean PM, et al. Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav (2003) 4:680–91. doi:10.1016/j.yebeh.2003.08.012
55. DiIorio C, Faherty B, Manteuffel B. Self-efficacy and social support in self-management of epilepsy. West J Nurs Res (1992) 14(3):292–307. doi:10.1177/019394599201400303
56. May TW, Pfafflin M. The efficacy of an educational treatment program for patients with epilepsy (MOSES): results of a controlled, randomized study. Modular service package epilepsy. Epilepsia (2002) 43(5):539–49. doi:10.1046/j.1528-1157.2002.23801.x
57. Anderson LA, DeVillis RF, Boyles B, Freussner JR. Patient’s perceptions of their clinical interactions: development of the multidimensional desire for control scales. Health Educ Res (1989) 4:383–97. doi:10.1093/her/4.3.383
58. Cramer JA, Westbrook LE, Devinsky O, Perrine K, Glassman MB, Camfield C. Development of the quality of life in epilepsy inventory for adolescents: the QOLIE-AD-48. Epilepsia (1999) 40(8):1114. doi:10.1111/j.1528-1157.1999.tb00828.x
59. Westbrook LE, Bauman LJ, Shinner S. Applying stigma theory to epilepsy: a test of a conceptual model. J Pediatr Psychol (1992) 17:633–49. doi:10.1093/jpepsy/17.5.633
60. Brandt PA, Weinhert C. The PRQ-A social support measure. Nurs Res (1981) 30:227–30. doi:10.1097/00006199-198109000-00007
61. Marshall GN, Hays RD, Sherbourne CD, Wells KB. The structure of patient satisfaction with outpatient medical care. Psychol Assess (1993) 5:477–83. doi:10.1037/1040-3590.5.4.477
62. McLeod JS, Austin JK. Stigma in the lives of adolescents with epilepsy: a review of the literature. Epilepsy Behav (2003) 4:112–7. doi:10.1016/S1525-5050(03)00007-6
63. Ramaratnam S, Baker GA, Goldstein LH. Psychological treatments for epilepsy. Cochrane Database Syst Rev (2003) 4:CD002029. doi:10.1002/14651858.CD002029
64. Helgeson DC, Mittan R, Tan SY, Chayasirisobhon S. Sepulveda epilepsy education: the efficacy of a psychoeducational treatment program in treating medical and psychosocial aspects of epilepsy. Epilepsia (1990) 31:75–82. doi:10.1111/j.1528-1157.1990.tb05363.x
65. Olley BO, Osinowo HO, Brieger WR. Psycho-educational therapy among Nigerian adult patients with epilepsy: a controlled outcome study. Patient Educ Couns (2001) 42:25–33. doi:10.1016/S0738-3991(00)00087-2
66. Martha Sajatovic M, Jobst BC, Shegog R, Bamps YA, Begley CE, Fraser RT, et al. The managing epilepsy well network advancing epilepsy self-management. Am J Prev Med (2017) 52(3S3):S241–5. doi:10.1016/j.amepre.2016.07.026
67. DiIorio C, Bamps Y, Walker ER, Escoffery C. Results of a research study evaluating WebEase, an online epilepsy self-management program. Epilepsy Behav (2011) 22(3):469–74. doi:10.1016/j.yebeh.2011.07.030
68. Thompson NJ, Patel AH, Selwa LM, Stoll SC, Begley CE, Johnson EK, et al. Expanding the efficacy of project UPLIFT: distance delivery of mindfulness based depression prevention to people with epilepsy. J Consult Clin Psychol (2015) 83(2):304–13. doi:10.1037/a0038404
69. Chaytor N, Ciechanowski P, Miller JW, Fraser R, Russo J, Unutzer J, et al. Long-term outcomes from the PEARLS randomized trial for the treatment of depression in patients with epilepsy. Epilepsy Behav (2011) 20(3):545–9. doi:10.1016/j.yebeh.2011.01.017
70. Caller TA, Ferguson RJ, Roth RM, Secore KL, Alexandre FP, Zhao W, et al. A cognitive behavioral intervention (HOBSCOTCH) improves quality of life and attention in epilepsy. Epilepsy Behav (2016) 57(Pt A):111–7. doi:10.1016/j.yebeh.2016.01.024
71. Fraser RT, Johnson EK, Lashley S, Barber J, Chaytor N, Miller JW, et al. PACES in epilepsy: results of a self-management randomized controlled trial. Epilepsia (2015) 56(8):1264–74. doi:10.1111/epi.13052
72. Elliott JO, Charyton C, Lu B, Moore JL. Serious psychological distress and health outcomes for persons with epilepsy in poverty. Seizure (2009) 18(5):332–8. doi:10.1016/j.seizure.2008.11.003
73. Epilepsy Foundation of America. About Epilepsy. Landover, MD (2013). Available from: http://www.epilepsyfoundation.org/aboutepilepsy/index.cfm/statistics.cfm
74. Zvarova J, Preiss J, Sochorova A. Analysis of data about epileptic patients using the GUHA method. Int J Med Inform (1997) 45(1–2):59–64. doi:10.1016/S1386-5056(97)00035-X
75. Fraser RT, Johnson EK, Miller JW, Temkin N, Barber J, Caylor L, et al. Managing epilepsy well: self-management needs assessment. Epilepsy Behav (2011) 20(2):291–8. doi:10.1016/j.yebeh.2010.10.010
76. Johnson EK, Fraser RT, Miller JW, Temkin N, Barber J, Caylor L, et al. A comparison of epilepsy self-management needs: provider and patient perspectives. Epilepsy Behav (2012) 25(2):150–5. doi:10.1016/j.yebeh.2012.07.020
77. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice-Hall Series in Social Learning Theory, xiii. Englewood Cliffs, NJ: Prentice-Hall, Inc. (1986). 617 p.
78. Clark NM. Management of chronic disease by patients. Annu Rev Public Health (2003) 24:289–313. doi:10.1146/annurev.publhealth.24.100901.141021
79. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol (2000) 55(1):68–78. doi:10.1037/0003-066X.55.1.68
80. Clark NM, Stoll S, Youatt EJ, Sweetman M, Derry R, Gorelick A. Adults with learning disabilities and epilepsy: knowledge about epilepsy before and after an educational package. Seizure (2010) 10(7):492–9. doi:10.1053/seiz.2001.0537
81. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med (2003) 26(1):1–7. doi:10.1207/S15324796ABM2601_01
82. Clark NM. Asthma self-management education. Research and implications for clinical practice. Chest (1989) 95:1110–3. doi:10.1378/chest.95.5.1110
83. Clark NM, Zimmerman BJ. A social cognitive view of self-regulated learning about health. Health Educ Res Theory Pract (1990) 5(3):371–9. doi:10.1093/her/5.3.371
84. Bandura A. Social cognitive theory of self-regulation. Special issue: theories of cognitive self-regulation. Organ Behav Hum Decis Process (1991) 50:248–87. doi:10.1016/0749-5978(91)90022-L
85. Fountain NB, Van Ness PC, Swain-Eng R, Tonn S, Bever CT. Quality improvement in neurology: AAN epilepsy quality measures. Report of the quality measurement and reporting subcommittee of the American Academy of Neurology. Neurology (2011) 76(4):94–9. doi:10.1212/WNL.0b013e318203e9d1
86. Velasquez MM, Gaddy-Maurer G, Crouch C, DiClemente CC. Group Treatment for Substance Abuse: A Stages-of-Change Therapy Manual. New York, NY: The Guilford Press (2001).
87. Pickrell WO, Elwyn G, Smith PEM. Shared decision-making in epilepsy management. Epilepsy Behav (2015) 47:78–82. doi:10.1016/j.yebeh.2015.01.033
88. Markham CM, Shegog R, Leonard AD, Bui TC, Paul ME. +CLICK: harnessing web-based training to reduce secondary transmission among HIV-positive youth. AIDS Care (2009) 21(5):622–31. doi:10.1080/09540120802385637
89. Shegog R, Markham CM, Leonard AD, Bui TC, Paul ME. “+CLICK”: pilot of a web-based training program to enhance ART adherence among HIV-positive youth. AIDS Care (2012) 24(3):310–8. doi:10.1080/09540121.2011.608788
90. DiIorio C, Hennessy M, Manteuffel B. Epilepsy self-management: a test of a theoretical model. Nurs Res (1996) 45:211–7. doi:10.1097/00006199-199607000-00004
91. Dilorio C, Shafer PO, Letz R, Henry TR, Schomer DL, Yeager K, et al. Behavioral, social, and affective factors associated with self-efficacy for self-management among people with epilepsy. Epilepsy Behav (2006) 9:158–63. doi:10.1016/j.yebeh.2006.05.001
92. Glasgow RE, Bull SS, Piette JD, Steiner JF. Interactive behavior change technology: a partial solution to the competing demands of primary care. Am J Prev Med (2004) 27(2):80–7. doi:10.1016/j.amepre.2004.04.026
93. Center for Managing Chronic Disease (University of Michigan). Contributing to Managing Epilepsy Well. Key Informants’ Perspectives on Managing Epilepsy Report. (2010). Available from: http://www.sph.emory.edu/ManagingEpilepsyWell/documents/reports/Key_Informants_Perspectives_on_Managing_Epilepsy_v4.pdf
94. Shegog R, Begley C, Iyagba B, Harding M, Dubinsky S, Newmark M, et al. MINDSET: The Development and Testing of a Clinic-Based Self-Management Decision Support System. Baltimore: American Epilepsy Society Special Interest Group Session on PEC. AES mtg (2011).
95. Shegog R, Begley C, Harding A, Dubinsky S, Newmark M, Ojukwu N, et al. MINDSET: Management Information & Decision Support Epilepsy Tool. Washington, DC: mHEALTH (2011).
96. Shegog R, Bartholomew LK, Sockrider MM, Czyzewski DI, Pilney S, Mullen PD, et al. Computer-based decision support for pediatric asthma management: description and feasibility of the stop asthma clinical system. Health Informatics J (2006) 12(4):259–73. doi:10.1177/1460458206069761
97. Prokhorov AV, Kelder SH, Shegog R, Murray N, Peters R Jr, Agurcia-Parker C, et al. Impact of a smoking prevention interactive experience (ASPIRE), an interactive, multimedia smoking prevention and cessation curriculum for culturally diverse high-school students. Nicotine Tob Res (2008) 10(9):1477–85. doi:10.1080/14622200802323183
98. Shegog R, Bartholomew LK, Parcel GS, Sockrider MM, Mâsse L, Abramson SL. Impact of a computer-assisted education program on factors related to asthma self-management behavior. J Am Med Inform Assoc (2001) 8(1):49–61. doi:10.1136/jamia.2001.0080049
99. Tortolero SR, Markham CM, Peskin MF, Shegog R, Addy RC, Escobar-Chaves SL, et al. It’s your game: keep it real: delaying sexual behavior with an effective middle school program. J Adolesc Health (2010) 46(2):169–79. doi:10.1016/j.jadohealth.2009.06.008
100. Hoch DB, Norris D, Lester JE, Marcus AD. Information exchange in an epilepsy forum on the world wide web. Seizure (1999) 8:30–4. doi:10.1053/seiz.1998.0217
101. Shegog R, Begley C. MINDSET: management information decision-support epilepsy tool intervention mapping case study. In: Bartholomew Eldredge LK, Markham CM, Ruiter RAC, Fernández ME, Kok G, Parcel GS, editors. Planning Health Promotion Programs: An Intervention Mapping Approach. San Francisco, CA: Jossey-Bass (2016). Available from: http://bcs.wiley.com/he-bcs/Books?action=resource&bcsId=10196&itemId=111903549X&resourceId=40707&chapterId=117731 (accessed September 15, 2017).
102. Shegog R, Begley C, Dubinsky S, Harding A, Goldsmith C, Hope O, et al. Description and feasibility of MINDSET: a clinic decision aid for epilepsy self-management. Epilepsy Behav (2013) 29:527–36. doi:10.1016/j.yebeh.2013.09.023
103. Panelli RJ, Kilpatrick R, Moore SM, Matkovic Z, D’Souza WJ, O’Brien TJ. The Liverpool adverse events profile: relation to AED use and mood. Epilepsia (2007) 48(3):456–63. doi:10.1111/j.1528-1167.2006.00956.x
104. Perucca P, Carter J, Vahle V, Gilliam FG. Adverse antiepileptic drug effects: toward a clinically and neurobiologically relevant taxonomy. Neurology (2009) 72:1223–9. doi:10.1212/01.wnl.0000345667.45642.61
105. Gillman FG, Fessler AJ, Baker G, Vahle V, Carter J, Attarian H. Systematic screening allows reduction of adverse antiepileptic drug effects: a randomized trial. Neurology (2004) 62:23–7. doi:10.1212/WNL.62.1.23
106. Friedman DE, Kung DH, Laowattana S, Kass JS, Hrachovy RA, Levin HS. Identifying depression in epilepsy in a busy clinical setting is enhanced with systematic screening. Seizure (2009) 18(6):429–33. doi:10.1016/j.seizure.2009.03.001
107. Gilliam FG, Barry JJ, Hermann BP, Meadow KJ, Vahle V, Manner AM. Rapid detection of major depression in epilepsy: a multicenter study. Lancet Neurol (2006) 5(5):399–405. doi:10.1016/S1474-4422(06)70415-X
108. Epilepsy Foundation. Neurological Disorder Depression Inventory for Epilpesy (NDDI-E) Screening Tool. (2013). Available from: http://www.epilepsyfoundation.org/aboutepilepsy/relatedconditions/Mood/NDDIE_Tool.cfm
109. US Department of Health and Human Services. National Asthma Education Program Expert Panel Report 3. Executive Summary: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Asthma Education Program, Office of Prevention, Education and Control, National Heart, Lung and Blood Institute, US Department of Health and Human Services (2007). p. 08–5846. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/summary-report-2007 (accessed September 15, 2017).
110. Le S, Shafer PO, Bartfeld E, Fisher RS. An online diary for tracking epilepsy. Epilepsy Behav (2011) 22:705–9. doi:10.1016/j.yebeh.2011.08.035
Keywords: epilepsy, self-management, decision support, intervention mapping, intervention, mobile health, electronic health, treatment
Citation: Shegog R and Begley CE (2017) Clinic-Based Mobile Health Decision Support to Enhance Adult Epilepsy Self-Management: An Intervention Mapping Approach. Front. Public Health 5:256. doi: 10.3389/fpubh.2017.00256
Received: 06 March 2017; Accepted: 08 September 2017;
Published: 03 October 2017
Edited by:
Robert A. C. Ruiter, Maastricht University, NetherlandsReviewed by:
Francine Schneider, Maastricht University, NetherlandsCopyright: © 2017 Shegog and Begley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ross Shegog, cm9zcy5zaGVnb2dAdXRoLnRtYy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.