
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 30 January 2025
Sec. Mood Disorders
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1547230
This article is part of the Research Topic Bipolar Disorder and Cognition: Cognitive Decline and Dementia View all 7 articles
 Douglas W. Barrett1
Douglas W. Barrett1 Roger E. Davis1
Roger E. Davis1 Jennifer E. Siegel-Ramsay2
Jennifer E. Siegel-Ramsay2 Amy Bichlmeier2
Amy Bichlmeier2 Jorge R. C. Almeida2
Jorge R. C. Almeida2 F. Gonzalez-Lima1*
F. Gonzalez-Lima1*Background: Converging evidence suggests that bipolar disorder (BD) involves mitochondrial dysfunction and prefrontal cortex (PFC) hypometabolism associated with cognitive impairment, which persists in remitted BD individuals. Transcranial infrared laser stimulation (TILS) provides safe, non-invasive brain stimulation that enhances PFC metabolism via photobiomodulation of mitochondrial respiration and tissue oxygenation. We tested the hypothesis that the neurocognitive deficits found in BD may be ameliorated by TILS treatments.
Methods: This is the first study to explore neurocognitive effects of repeated TILS administration in BD. Using an open-label design, 29 individuals with remitted BD received six weekly TILS treatments. Working memory and attention were assessed with trail-making and 2-back tasks sensitive to TILS cognitive effects in individuals with BD. Changes in PFC network interactions were measured with functional near-infrared spectroscopy (fNIRS) because this method can measure TILS effects on oxygen metabolism in the PFC of individuals with BD.
Results: Participants reported no adverse effects from treatment, confirming the safety of this intervention in individuals with BD. Cognitive test results showed that in people with remitted BD, TILS was effective at improving cognition, i.e., enhanced speed and accuracy in tasks reflecting cognitive flexibility, working memory, and attentional control. Antipsychotic medication improved TILS cognitive effects. The fNIRS results showed a significant reduction in PFC network correlations of oxygenated hemoglobin changes driven by cognitive task performance. The right-hemisphere frontopolar cortex showed greater TILS effects than its left-hemisphere counterpart.
Conclusions: Repeated TILS is a safe intervention to improve cognition in people with remitted BD. Continued antipsychotic medication may have contributed to the cognitive improvement. To confirm TILS efficacy, a sham-controlled, double-blinded randomized trial is needed.
Clinical trial registration: https://clinicaltrials.gov/, identifier NCT05354895.
Converging evidence suggests, at least in part, that bipolar disorder (BD) involves mitochondrial dysfunction (1) and reduced energy metabolism in prefrontal cortex (PFC) (2). Disruptions in brain networks mediating cognition and emotion (3) may underlie the deficits found in BD. These deficits are found even in individuals with remitted, non-cycling (euthymic) BD (4). Current clinical interventions for BD are mainly targeted for mood stabilization instead of treatment of mitochondrial deficits, and a small number of studies have focused on cognitive impairment (5).
Different neuromodulation strategies have been recently introduced in BD research (6). Transcranial infrared laser stimulation (TILS) is a safe, non-invasive low-level laser therapy that enhances brain metabolic energy production by delivering photons at 1064 nm wavelength through the forehead to the PFC [for review, see Gonzalez-Lima (7)]. The way TILS works differs from magnetic and electrical brain stimulation, such as transcranial magnetic stimulation or electroconvulsive therapy. While magnetic and electrical stimulation alter the electrical excitability of cell membranes, TILS works by having photons directly absorbed by mitochondria, which increases oxygen consumption and improves blood flow to enhance cognitive brain function. We chose TILS for BD patients because of its mitochondrial mechanism of action, which could help reduce certain cognitive impairments associated with BD by enhancing mitochondrial energy production in the PFC.
A form of transcranial photobiomodulation, TILS targets the mitochondrial enzyme cytochrome-c-oxidase (CCO), causing photo-oxidation of CCO and increasing tissue oxygenation in the PFC (8). TILS has been estimated to penetrate the PFC gray matter via a tissue optical window to infrared light at 1064 nm (9). We have previously demonstrated beneficial cognitive and emotional effects resulting from TILS (10–13), as well as beneficial effects on brain mitochondrial and oxygen metabolism (13–16). We recently extended these findings of improvements in cognition and PFC energy metabolism to older euthymic adults with BD (17, 18). TILS may alleviate some of the cognitive deficits found in BD by facilitating mitochondrial energy production and stabilizing dysfunctional neural networks in the PFC. We have previously studied the cognitive effects of single and repeated TILS administrations for five weekly treatments in older participants with and without BD (17, 19). The effects of TILS were stronger after repeated weekly administrations. For this reason, we chose to examine the effects of repeated administrations in this study.
Our hypothesis was that the neurocognitive deficits found in BD may be ameliorated by weekly TILS treatments. Working memory and attention were assessed with the trail-making task and 2-back task, because these tasks appear sensitive to TILS cognitive effects in individuals with BD (17). Changes in PFC network interactions were measured with functional near-infrared spectroscopy (fNIRS) because NIRS-based optical methods are non-invasive and capable of measuring TILS effects on oxygen metabolism in the PFC of individuals with BD and healthy controls (13, 18). This was an open-label study in which all participants received TILS treatment. It is the first study to explore the effects of repeated TILS on fNIRS network measures in individuals with remitted BD.
All procedures were approved by the University of Texas at Austin (UT Austin) Institutional Review Board and complied with National Institute of Health (NIH) and Declaration of Helsinki guidelines on human research.
Participants were N=29 remitted participants with BD (type I: n=22; type II: n=7), recruited through the Bipolar Disorder Center at Dell Medical School in Austin. The participants comprised n=18 females and n=11 males with an average age of 36 years (range: 19-70 years old). Participants were considered for inclusion if they were: (1) Age 18 years or above; (2) Diagnosed with either Type I or II bipolar disorder; (3) Taking at least one FDA-approved mood stabilizer for at least six weeks; (4) In remission (mood-stabilized, i.e. non-cycling), with a score on the Montgomery-Åsberg Depression Rating Scale (MADRS) ≤ 12 and a score on the Young Mania Rating Scale (YMRS) ≤ 7. Participants who changed or stopped medication partway through the study or showed signs of mood instability were discontinued. A detailed list of all inclusion and exclusion criteria for the study is shown in Supplementary Table 1. Figure 1 shows a CONSORT diagram describing participant enrollment.
Potential participants who indicated interest in the study were screened for inclusion and exclusion criteria by the psychiatry staff at the Bipolar Disorder Center at Dell Medical School at The University of Texas at Austin, who then obtained informed consent and enrolled the participants. All procedures and assessments reported here were conducted at the Gonzalez-Lima Lab at UT Austin. Participants also completed a series of cognitive assessments and MRI scans. The results of these measures will be reported in future manuscripts. Figure 2A summarizes the experimental design.
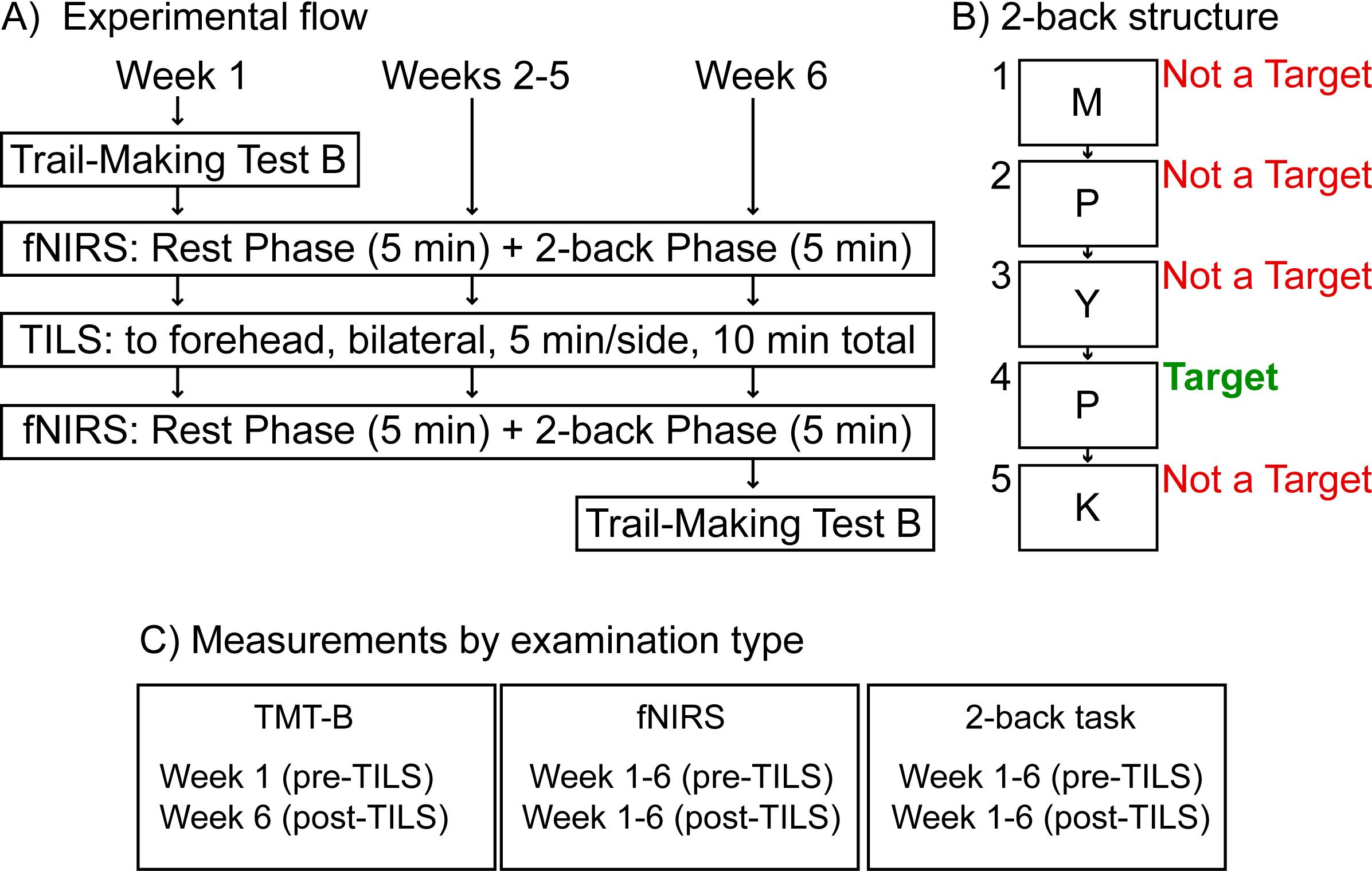
Figure 2. Experimental design. (A) Weekly TILS administration and assessments. (B) Structure of the 2-back cognitive assessment, showing an example of a possible sequence of letters presented onscreen (M, P, Y, P, K) during this task. (C) Measurements by examination type; functional near-infrared spectroscopy (fNIRS); Transcranial infrared laser stimulation (TILS).
Participants received six weeks of TILS treatments, once per week. This weekly TILS treatment was chosen because it has been shown previously to improve cognitive function and modify BOLD-fMRI response in the PFC of cognitively-compromised individuals (19). The TILS device used was FDA-cleared as safe for use in humans, and it was operated in accordance with a standard operating procedure that was approved by the UT Austin Laser Safety Office. All laser operators completed a laser safety class through the Environmental Health and Safety office at UT Austin. All participants and researchers wore protective eyewear throughout each TILS session.
TILS administration consisted of the application of infrared light using a well-collimated, flat-top, continuous-wave laser (HD Laser, Cell Gen Therapeutics, Dallas, TX). The study used the same wavelength (1064 nm), irradiance (250 mW/cm2), cross-sectional beam area (13.6 cm2), total duration (10 minutes), and targeted stimulation sites as in our previous TILS studies with older bipolar adults (17, 18). The target sites were the right and left anterior PFC (frontopolar cortex, Brodmann area 10), with the laser beam centered at Fp1 and Fp2 points corresponding to the standard frontopolar EEG electrode placements. We selected these TILS parameters and points Fp1 and Fp2 as sites of stimulation because these parameters and forehead sites are the same that we have used in previous studies that have shown both cognitive and brain metabolic effects of TILS. Each site received five minutes of TILS, alternating each minute between the two sides (see Figure 3A). The fluence dose (or energy density) was 75 J/cm2 (0.25 W/cm2 x 300 sec) per site on the forehead, with a fluence dose of 150 J/cm2 across the entire forehead. This specific bilateral TILS protocol was selected because it has been previously shown to increase mitochondrial CCO and oxygenated hemoglobin in the PFC of bipolar individuals in a sham-controlled study (18). The present investigation was an open-label study in which all participants received TILS treatment; there was no sham control.
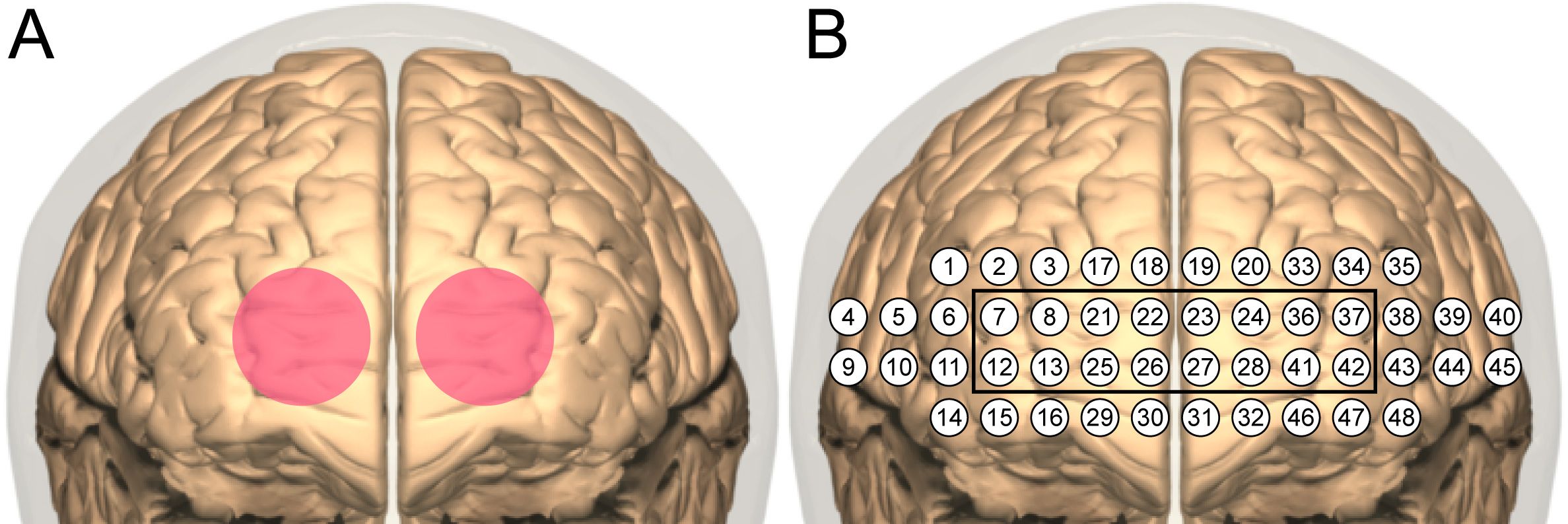
Figure 3. TILS and fNIRS sites. (A) Bilateral stimulation sites (4 cm diameter each) centered on the targeted frontopolar region of PFC. (B) Layout of channels in NIRSIT device. The black square indicates channels sampling the frontopolar region directly stimulated with TILS.
Immediately before the first TILS treatment, participants completed the Trail-Making Test part B (TMT-B). The TMT-B is a “connect-the-dots” task used to assess frontal lobe functions (20) including visual attention and cognitive flexibility. The participant is instructed to connect a series of targets as quickly as possible while still being accurate, alternating between sequential letters and numbers (1, A, 2, B, etc.). Participants completed the TMT-B again immediately after the last TILS treatment. The TMT-B was chosen because performance on this task has been previously shown to improve after TILS in bipolar individuals (17).
Before and after each administration of TILS, network interactions in the PFC were assessed using functional near-infrared spectroscopy (fNIRS). A head-mounted device (NIRSIT: Soterix Medical Inc., Woodbridge, NJ and OBELAB Inc., Seoul, Korea) was used to measure changes in oxygenated hemoglobin concentration across the PFC. This device uses an array of light emitters and photodetectors to spectroscopically sample from 48 channels (see Figure 3B) with a separation of 3 cm between each pair of emitters/detectors that comprised a channel. The device was fixed firmly on the forehead and secured there using a Velcro strap (see Figure 4). Participants confirmed verbally that the headset was securely fastened on the head without being painful. An fNIRS method was used because it has been previously shown to detect TILS-induced changes in oxygenated hemoglobin within the PFC of healthy controls (13). We have previously demonstrated that the increases in concentration of both oxygenated hemoglobin and cytochrome-c-oxidase after TILS are highly correlated (15).
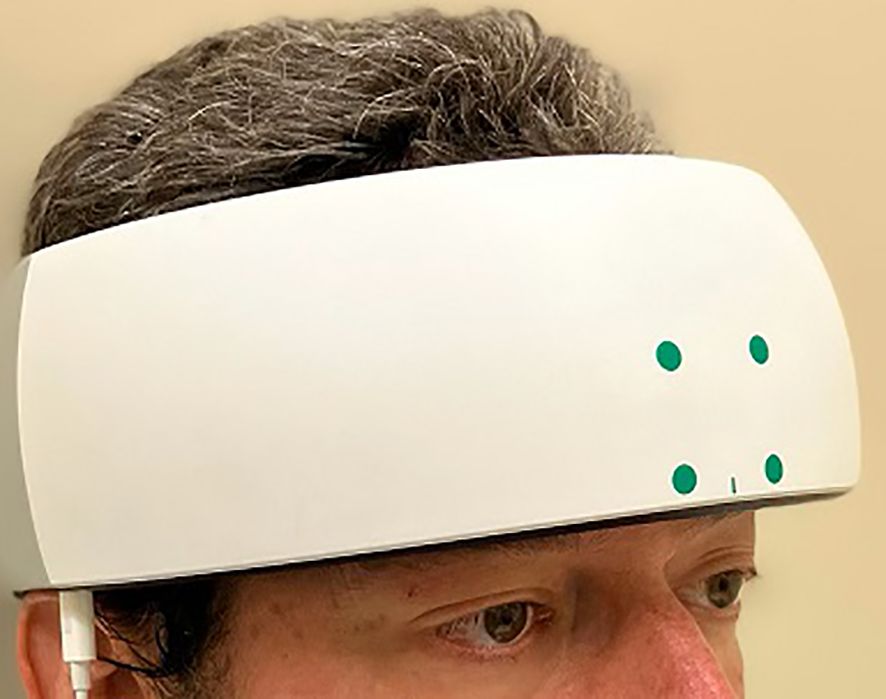
Figure 4. fNIRS apparatus. The NIRSIT near-infrared spectroscopy device (shown on the head of a researcher) was positioned on the forehead, centered on the midsagittal line, and secured with a Velcro strap.
Each fNIRS session lasted approximately 10 minutes and consisted of two phases. During the first five minutes (“Rest Phase”), participants rested quietly. During the last five minutes (“2-back Phase”), participants engaged in the 2-back task, which is a commonly-used measure of working memory and executive control (21) mediated by PFC function (22). Participants were instructed to watch a series of letters presented onscreen, remember which letter was presented two trials back, and only respond if the target letter currently onscreen matched the letter from two trials back. Figure 2B describes the structure of the 2-back task. Figure 2C summarizes each measurement by examination type.
Spectroscopic data gathered with the NIRSIT device was processed using the NIRSIT Analysis Tool provided by OBELAB Inc. The data was processed using standard procedures reported in previous spectroscopic studies of TILS effects in the PFC (13, 15, 16, 18). Briefly, the data was subjected to a band-pass filter with limits of 0.005 Hz to 0.1 Hz to eliminate noise from sources unrelated to the signal from PFC, such as cardiovascular artifacts and respiration. Markers inserted during data acquisition were used to define two epochs for each fNIRS session: the Rest Phase (5 minutes) and the 2-back Phase (5 minutes). Average light intensity from the first five seconds of spectroscopic measurements was defined as the baseline intensity (I0) in the modified Beer-Lambert law, which is a mathematical relationship used to convert changes in light intensity to changes in oxygenated hemoglobin concentration and is commonly used in near-infrared spectroscopy (23). Any channel with a signal-to-noise ratio below 25 was rejected before oxygenated hemoglobin concentration was calculated. For rejection padding, any missing channel was replaced by a backup channel (an alternate pair of emitters/detectors) where possible; otherwise, the brain regional mean was used.
While it is possible to calculate changes in concentration of oxygenated hemoglobin within a single session using fNIRS, it is not possible to calculate absolute concentration, because the initial intensity measurement I0 in the modified Beer-Lambert law is only valid within a given session, and subsequent measurements are specifically dependent on that initial value (13). Therefore, to assess between-session (i.e. weekly) changes, an index of network interactivity was calculated using the within-session concentration changes of oxygenated hemoglobin across all 48 channels. The NIRSIT Analysis Tool software used these concentration changes to generate a 48x48 matrix of pairwise correlation coefficients, representing each pairwise combination of each of the 48 channels sampled by the NIRSIT device. Correlation matrices for the Rest Phase and 2-back Phase were computed separately. Pairwise correlation matrices were used because they are a standard method for analyzing neural network interactions in both animal and human studies (24–26).
To generate a single index of network interactivity for each subject/session/phase, the Fisher Z-transformation was used to convert each 48x48 correlation matrix to a matrix of z-scores. These z-scores were then averaged together, and the subsequent value converted back to a correlation coefficient, which represented the average overall (“all-channels”) network interactivity across the PFC, including every pairwise combination of each channel in the 48-channel array. In addition to this “overall” index of PFC network interactivity, another “stimulation site” index was calculated which focused on the frontopolar stimulation sites in the left and right hemisphere. The same Fisher Z-transformation process was used to obtain this average value for each subject but limited to only those channels defined as “frontopolar” (as seen in Figure 2B) in the NIRSIT Analysis Tool software. Separate values were calculated for right and left frontopolar regions, to assess whether hemispheric laterality played a role in changes in network interactivity due to TILS.
One subject/session was missing from the fNIRS dataset due to an equipment problem. This missing value was replaced using a standard mean imputation approach: data from Week 1 and Week 3 for this individual were averaged together to generate the missing data from Week 2. Corresponding phases were used to impute the missing data: e.g., Week 1, Pre-TILS, Rest Phase was averaged with Week 3, Pre-TILS, Rest Phase to generate Week 2, Pre-TILS, Rest Phase for this subject. One subject had missing 2-back data for Weeks 1 and 2 due to misunderstanding the instructions and pressing the wrong key in response to correct trials. These data were also replaced with a mean imputation, using averaged data from the corresponding time points from all other subjects. These participants received the same number of TILS treatments and cognitive assessments as the other participants, and thus they were treated identically as the rest of the participants in terms of the experimental procedure.
The dependent variables of interest for the cognitive assessments (TMT-B and 2-back) comprised one score reflecting accuracy and another representing speed. For the TMT-B, the dependent variables were number of errors and completion time (seconds). The TMT-B dependent variables were consolidated into a rate correct score, which incorporates both accuracy and completion speed into a single metric (13, 27).
For the 2-back task, the dependent variables were the total number of correct responses (or Hits) and the response time on correct trials (in milliseconds). The dependent variables for the fNIRS data were the “all-channels” average correlation coefficient, reflecting overall network interactions in the PFC, and the frontopolar average correlation coefficients (right and left), representing stimulation site network interactions within the TILS stimulation sites.
The TMT-B data was analyzed using paired t-tests comparing speed and accuracy mean scores at Pre-TILS (Week 1) vs. Post-TILS (Week 6). The 2-back data was analyzed during each week using repeated measures ANOVA with two within-subject variables: PrePost (2 levels: Pre-TILS vs. Post-TILS) and Weeks (6 levels: weeks 1, 2, 3, 4, 5, 6). The fNIRS all-channels network interactivity data was analyzed using repeated measures ANOVA with three within-subject variables: Phase (2 levels: Rest vs. 2-back), PrePost (2 levels: Pre-TILS vs. Post-TILS) and Weeks (6 levels: weeks 1, 2, 3, 4, 5, 6). The fNIRS frontopolar network interactivity data was also analyzed with repeated measures ANOVA but included an additional within-subject variable: Laterality (2 levels: left vs. right).
To test whether participant medication usage had an effect on treatment response to TILS, a series of repeated measures ANOVAs with one within-subject variable [2 levels: Pre-TILS (Week 1) vs. Post-TILS (Week 6)] and one between-subject variable (medication status) was performed on the rate correct score dependent variable. Separate repeated measures ANOVAs were performed using four between-subject variables, corresponding to whether or not a participant was medicated with antipsychotics, antidepressants, ADHD medications, or benzodiazepines (13, 27).
There was no between-subject treatment group variable for these analyses, since all subjects received TILS treatment. The free, open-source statistical package jamovi (28) was used to perform the statistical analyses. The assumption of normality of the TMT-B data in the paired t-tests was checked using the Shapiro-Wilk test (29). The assumption of sphericity of the 2-back and fNIRS data in the repeated measures ANOVAs was checked using Mauchly’s W (30). The Greenhouse-Geisser correction for non-spherical data (31) was used where applicable. A finding was considered statistically significant for p-values below 0.05. Estimates of effect size were calculated as Cohen’s d and partial eta squared (32) for paired t-tests and repeated measures ANOVAs, respectively. For Cohen’s d, a value of 0.2 indicates a small effect size, 0.5 a medium effect size, and 0.8 a large effect size; for partial eta squared, a value of 0.01 indicates a small effect size, 0.06 a medium effect size, and 0.14 a large effect size (33).
Demographic details, the psychiatric comorbidities, and psychotropic medication usage for participants are shown in Table 1. Supplementary Table 2 lists all medications for all participants.
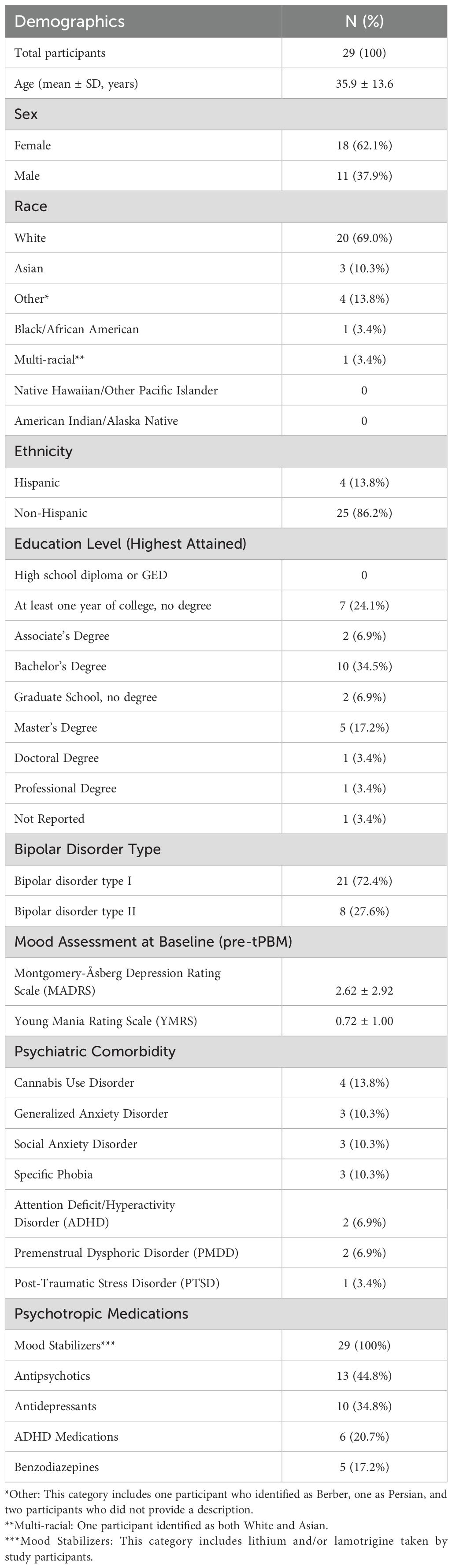
Table 1. Demographic data of participants, psychiatric comorbidities, and psychotropic medication use.
A paired t-test on the rate correct scores on the TMT-B showed a significant effect, t(28)=3.65, p=0.0011, Cohen’s d=0.68, indicating that the participants showed significant improvement on the task. Figure 5 shows this improvement (an increase of 22%) from Week 1 Pre-TILS to Week 6 Post-TILS. The rate correct scores were normally distributed (Shapiro-Wilk test for normality, p>0.05).
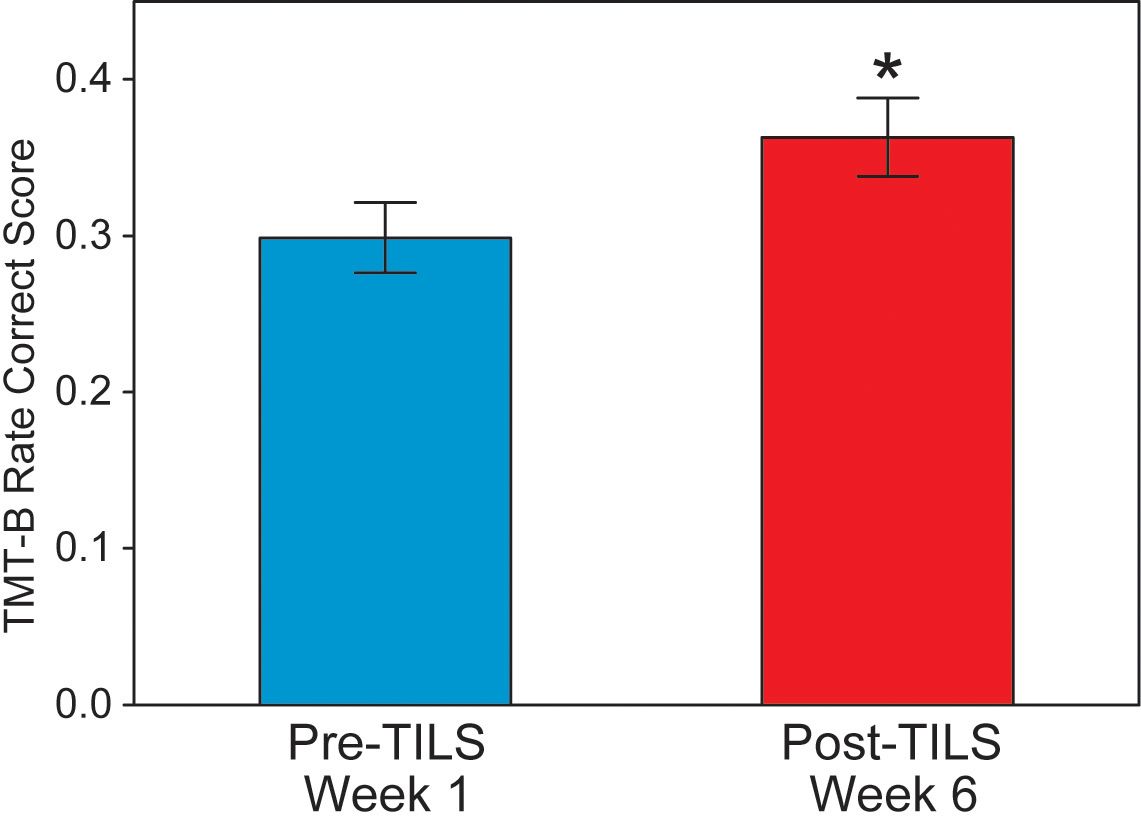
Figure 5. Trail-making test part B results. Rate correct score, before and after 6 weeks of TILS treatment (mean +/- SE). Paired t-test, *p<0.01.
A repeated measures ANOVA on correct trial reaction time during the 2-back task showed a significant main effect of Weeks, F(5,140)=11.32, p<0.001, partial eta squared=0.288, indicating progressively faster speed of performance; as well as a significant main effect of PrePost, F(1,28)=13.06, p=0.001, partial eta squared=0.318, indicating better performance at Post-TILS. There was also a significant interaction of Weeks by PrePost, F(5,140)=3.43, p=0.006, partial eta squared=0.109. Figure 6A shows the significant improvement in reaction time. From Week 1 pre-TILS to Week 6 post-TILS, there was a 22% reduction in reaction time. A test of sphericity (Mauchly’s W) found that the data was not spherical; however, applying the Greenhouse-Geiser sphericity correction resulted in no change in the pattern of significant findings (Weeks: p<0.001; PrePost: p=0.001; Weeks x PrePost: p=0.017). Post-hoc paired t-tests confirmed that while PrePost speed on Week 1 was significantly different (p<0.001), the data converged and was not significant on Week 2 (p=0.104).
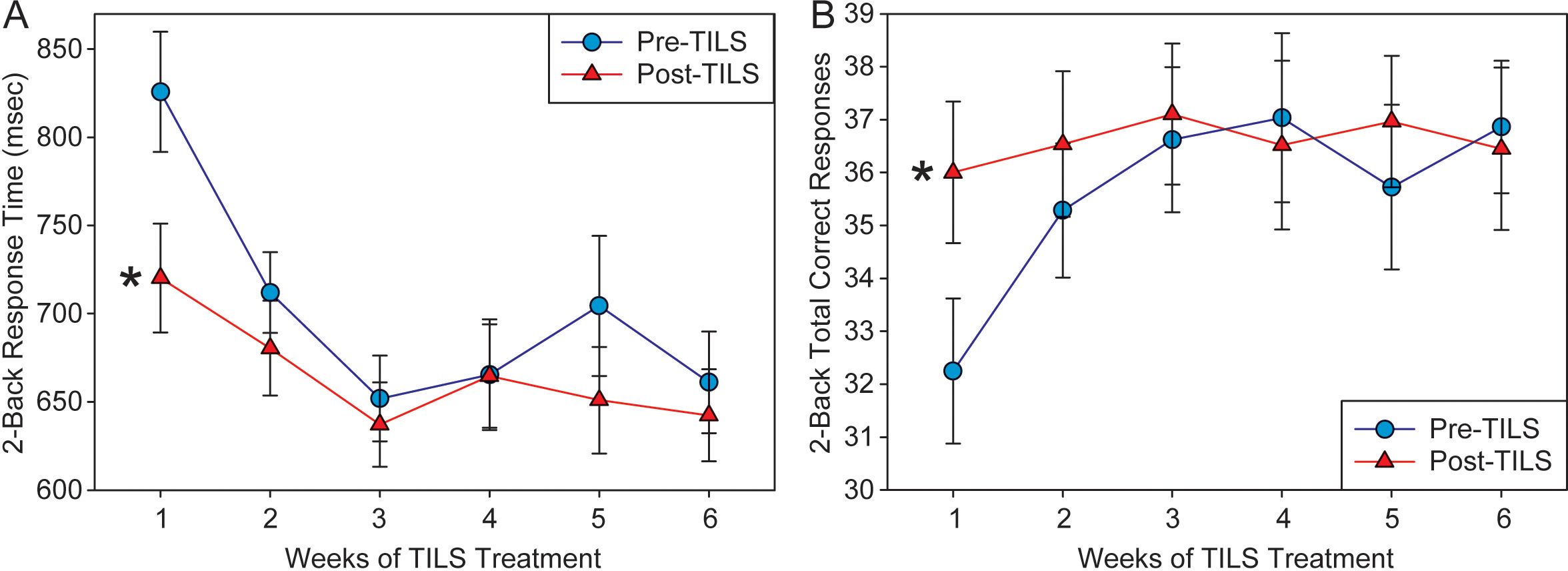
Figure 6. 2-back task results. (A) Response time (in milliseconds) to correct trials in the 2-back task, pre- and post-TILS, across 6 weeks of TILS treatment (mean +/- SE). (B) Number of correct responses on the 2-back task, pre- and post-TILS, across 6 weeks of TILS treatment (mean +/- SE). Repeated measures ANOVA, *p<0.001.
A repeated measures ANOVA on the total number of correct responses in the 2-back test revealed a significant main effect of Weeks, F(5,140)=2.94, p=0.015, partial eta squared=0.095, as well as a significant interaction between Weeks and PrePost, F(5,140)=4.59, p<0.001, partial eta squared=0.141. The main effect of PrePost approached significance, F(1,28)=3.58, p=0.069, partial eta squared=0.113. Figure 6B shows the significant improvement in performance in total correct responses. From Week 1 pre-TILS to Week 6 post-TILS, there was a 13% increase in total correct trials. A test of sphericity (Mauchly’s W) found that the data was not spherical; however, applying the Greenhouse-Geiser sphericity correction resulted in no change in the pattern of significant findings (Weeks: p=0.030; Weeks x PrePost: p=0.003; PrePost: p=0.069). Post-hoc paired t-tests confirmed that while PrePost correct response on Week 1 was significantly different (p<0.001), the data converged and was not significant on Week 2 (p=0.079).
A repeated measures ANOVA on the “all-channels” index of PFC network interactivity, with the within-subject variables Weeks, PrePost, and Phase, revealed a single significant finding: a main effect of Phase, F(1,28)=14.15, p<0.001, partial eta squared=0.336. There was no significant main effect of PrePost (p=0.19) or Weeks (p=0.86), nor any significant variables interactions. Figure 7 shows how the average network correlation across all PFC regions was significantly lower during the 2-back task phase as compared to the rest phase. A test of sphericity (Mauchly’s W) confirmed that this assumption was met for all variables in the repeated measures ANOVA. Post-hoc paired t-tests showed that while Phase was significantly different on Weeks 1-3 (p<0.05), the data converged and Phase was not significant on Week 4 (p=0.146).
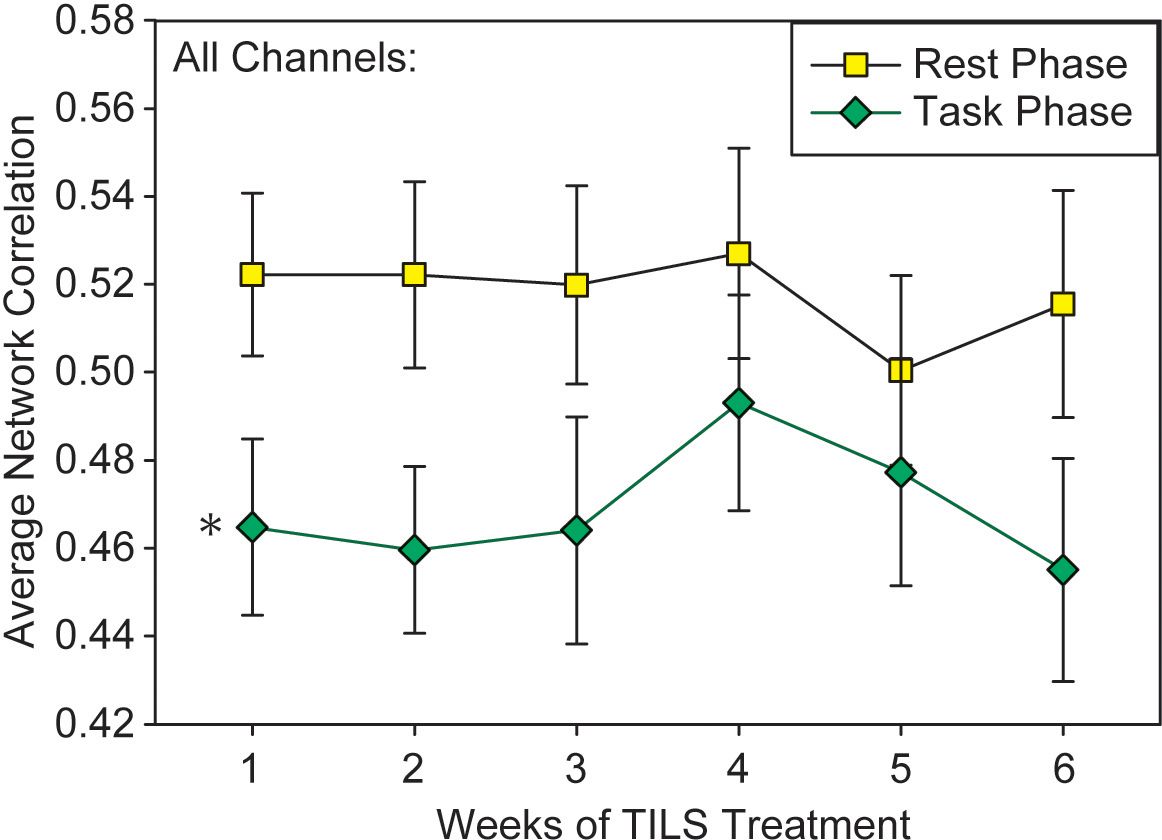
Figure 7. Network interactivity index results. Average pairwise correlations across all frontal regions during the 5-minute rest phase and 5-minute 2-back task (mean +/- SE). Repeated measures ANOVA, *p<0.001.
A repeated measures ANOVA on the index of local network interactivity within the frontopolar stimulation site was conducted with the within-subject variables Weeks, PrePost, Phase, and Laterality. It revealed a significant main effect of Phase, F(1,28)=7.102, p=0.013, partial eta squared=0.20, which was similar to the effect seen in the “all-channels” overall index. It also found a significant main effect of Laterality, F(1,28)=6.164, p=0.019, partial eta squared=0.18, in which the index of network interactivity was reduced in the right frontopolar region as compared to the left (Figure 8). The overall magnitude of the frontopolar site index (Figure 8) was greater than the overall PFC index (Figure 7). A test of sphericity (Mauchly’s W) confirmed that this assumption was met for all variables in the repeated measures ANOVA. Post-hoc paired t-tests showed that while Laterality was significantly different on Week 1 (p=0.037), the data converged and Laterality was not significant on Week 4 (p=0.157).
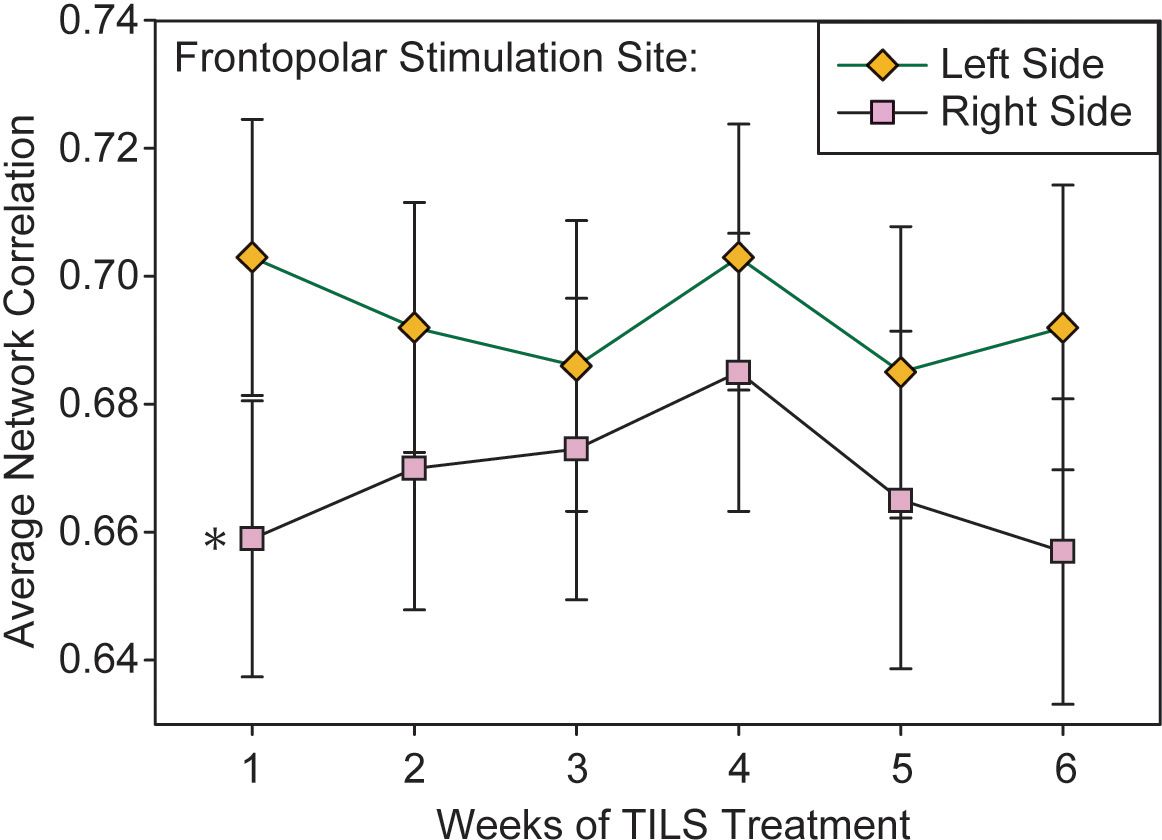
Figure 8. Lateralized effect in frontopolar network interactivity. Average pairwise correlations within the frontopolar region directly stimulated with TILS, as measured in left and right hemispheres. Repeated measures ANOVA, *p<0.05.
Table 2 shows brain-behavior correlations between changes in cognitive function score (pre-post) and changes in fNIRS frontopolar connectivity. The only difference between pre-TILS and post-TILS was a hemispheric laterality observed as an opposite pattern of correlation values between rest (Phase 1) and 2-back (Phase 2) in the right hemisphere, which was not present in the left hemisphere.
Of the four repeated measures ANOVAs assessing whether medication status had an effect on treatment response to TILS, only antipsychotic medication status showed a significant interaction of PrePost by Medication, F(1,27)=7.90, p=0.009, partial eta squared=0.226, with medicated participants showing greater improvement in the TMT-B than unmedicated participants. Figure 9A shows the significant interaction in the rate correct score, and Figure 9B shows a frequency distribution of the exact type of antipsychotic medication prescribed to the n=13 medicated individuals. Some individuals were prescribed more than one type of antipsychotic (all medication prescribed to all participants can be found in Supplementary Table 2).
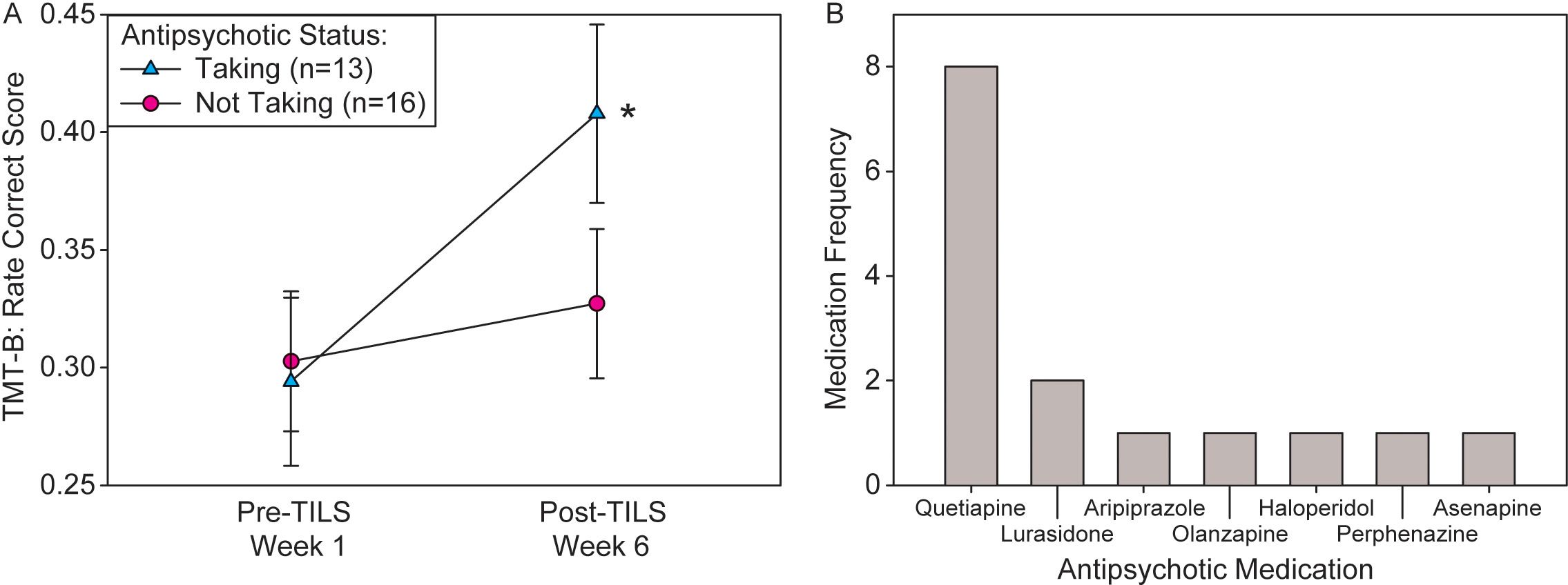
Figure 9. Interaction of antipsychotic medication status and treatment response. (A) Significant interaction of PrePost by Medication on rate correct score (mean +/- SE) in the TMT-B. Participants medicated with antipsychotics showed significantly greater cognitive improvement than unmedicated participants. Repeated measures ANOVA, *p<0.01. (B) Frequency distribution of each specific type of antipsychotic medication prescribed to participants.
Participants reported no adverse effects from the TILS treatment, confirming the safety of this noninvasive intervention in individuals with BD. The cognitive test results showed that in people with remitted BD, TILS was effective at improving cognition with medium to large effect sizes, i.e., enhanced speed and accuracy in tasks reflecting cognitive flexibility, working memory, and attentional control. In the 2-back task, this effect was most pronounced in the acute phase (Week 1) of the experiment, with cognitive scores leveling off in subsequent weeks. This convergence of Pre-TILS and Post-TILS scores was likely due to ceiling effects (in correct responses) and floor effects (in reaction time) in the 2-back task, as well as practice effects. Interpretation of the 2-back results is complicated by the fact that “Pre-TILS” scores in later weeks are also likely benefiting from previous weeks of treatment. Thus, each subsequent “Pre-TILS” score is not purely “Pre,” but also represents a one-week-later follow-up from the previous TILS treatment. These issues are less important for the TMT-B, because it was given only twice (before the first TILS treatment and after the last TILS treatment).
In our previous work (17), older participants with euthymic bipolar disorder showed cognitive benefits from TILS, in assessments of cognitive flexibility, working memory and sustained attention, including the TMT-B. Participants with euthymic bipolar disorder score significantly lower on the TMT-B than healthy controls (34), and in general, 83% of individuals with BD show “serious” cognitive and functional deficits, which represents the highest percentage found in all mood disorders (35). Furthermore, these cognitive deficits are found even in individuals with euthymic BD (4), and therefore, the underlying process impairing cognition in BD is not necessarily related to mood cycling.
Cognitive deficits in BD likely involve mitochondrial dysfunction. Abnormalities in mitochondrial DNA (36), mitochondrial structure (37), mitochondrial function (38), and brain energy metabolism in general (39) are well-established in BD, leading to the hypothesis that mitochondrial impairment plays a causal role in the disease pathology of BD (40). It is possible that the progressive deterioration in cognition found in older adults with euthymic BP (41) is related to the gradual accumulation of oxidative damage with age, with these mitochondrial deficits leading to a kind of accelerated aging effect (42, 43).
This line of evidence implicates mitochondrial function as a therapeutic target in BD. We have previously shown (13, 15, 16) that TILS can result in beneficial increases of oxygenated hemoglobin and oxidized cytochrome-c-oxidase, a key component of the mitochondria and the rate-limiting enzyme for energy production in the electron transport chain (44). This increase in oxidized cytochrome-c-oxidase resulting from TILS is greater in older individuals (16). Our previous sham-controlled study (18) in older adults with euthymic BD demonstrated that a single session of TILS resulted in increases in the concentration of oxygenated hemoglobin and oxidized cytochrome-c-oxidase in PFC as determined by broad-band near-infrared spectroscopy (bbNIRS). But it is not possible to use bbNIRS or fNIRS to assess across-session concentration of chromophores, because these optical methods involve recalibrating the baseline concentration value each session, which precludes calculation of absolute concentrations. Nevertheless, the totality of evidence implies that increased mitochondrial function with concomitant increased brain oxygenation is likely the underlying neurobiological mechanism by which the cognitive benefits seen in the present BD study are made manifest.
The significant reduction in network interactions measured by the correlation analysis of oxygenated hemoglobin changes from the resting phase to the task phase likely reflects neuroplastic changes in PFC, driven by increased cognitive demand of the 2-back task. Shifting from rest to task resulted in an overall decrease in the “all-channels” index, which is consistent with findings from BOLD-fMRI work showing that during the 2-back task, average whole-brain measures of functional connectivity decrease as compared to the resting state (45). Functionally-specialized regions like the PFC serve as discrete information processing units, making specific contributions to cognition. A shift from global to local processing in fNIRS covariance data is similar to that found in studies using electroencephalography (EEG), in which moving from the resting state to active processing of information is accompanied by a shift from synchronized to desynchronized EEG signals (46), as discrete brain regions are recruited to serve their specific functional purpose. However, TILS did not significantly change network interactivity, either acutely (PrePost) or chronically (Weeks), indicating that PFC network interactivity as measured by correlations of oxygenated hemoglobin changes may not be sensitive enough to detect TILS treatment effects or serve as a useful proxy for TILS-induced changes in brain metabolic activity.
The more localized index of network interactivity, which focused on the frontopolar stimulation sites, showed a similar significant main effect of Phase, as well as an effect of hemispheric laterality: right-hemisphere frontopolar cortex showed reduced intra-regional network interactivity than its left-hemisphere counterpart. PFC in general is marked by lateral asymmetries in anatomical connections (47), functional connectivity with other brain regions and networks (48), and functional role in cognition (49), making interpretation of this finding difficult. However, previous TILS studies have found that stimulation of the right PFC is more effective than stimulation of the left PFC at improving cognitive tasks related to attention and working memory in healthy controls and people with increased depressive symptoms, suggesting that the right PFC is more responsive to TILS cognitive effects (50, 51). The present study supports this interpretation because while both frontopolar sites received the same amount of TILS exposure, the right site showed a more pronounced cognitive-task evoked network change. In addition, individuals with BD show greater right-sided functional connectivity between amygdala and PFC in response to emotional faces, suggesting a greater contribution of the right hemisphere to emotional regulation (52, 53). Right hemisphere frontal lobe function is linked to performance on both the TMT-B (54) and the 2-back task (55), so task-related lateralization of function may have also contributed to these findings.
While it is notable that the network interactivity index within frontopolar cortex was larger than the overall PFC index, this is to be expected, as nearby spectroscopy channels tend to be more highly correlated and may sample some of the same tissue as the emitted light travels on an arc-shaped path through the cortex. These nearby channels also likely share some of the same blood vessels supplying the oxygenated hemoglobin that was used to calculate the correlation matrices. However, some of the increased interactivity seen local to the stimulation site may be due to the stimulation itself. We recently found that a single TILS treatment to the entire rat brain resulted in an increase in overall network interconnectivity across the entire brain, as measured by increased numbers of significant inter-regional correlations in cytochrome-c-oxidase enzymatic activity (56). This finding suggests that TILS can induce bioenergetic changes in infrared-stimulated brain tissue, leading to increased neuroplasticity and neuromodulation of functionally coupled neural networks.
Antipsychotic medication status showed a significant interaction with treatment, indicating that it improved treatment response to TILS. The other classes of medication did not show a significant interaction. While the percentages taking antipsychotics and antidepressants were similar (45% vs. 35%), only the antipsychotics showed a significant effect. However, the other medications assessed (ADHD medications, benzodiazepines) were relatively unbalanced in their distributions (see Table 1), which may have affected the statistical power, resulting in negative findings. The proportion of patients taking anti-ADHD medications was higher than the rate of comorbid ADHD (Table 1), because these patients were prescribed anti-ADHD medications off-label by their physicians. Mood stabilizers and antipsychotics are commonly used to treat bipolar disorder and may also help enhance cognitive function. As all participants were on mood stabilizers, no differences would be expected in this group. However, significant improvements in cognitive function may have been observed in those taking antipsychotics.
While specific antipsychotics have a variety of specific receptor interaction profiles, they generally lead to prefrontal cortical activation (57). One common property of second-generation (atypical) antipsychotic is antagonism at serotonin receptors (58). Serotonin antagonism can enhance prefrontal activation either directly (59) or indirectly through dopaminergic mechanisms (60). This process may prime prefrontal cortical regions, leading to a synergistic effect between antipsychotic action and TILS treatment. The most commonly-prescribed antipsychotic in this subject pool, quetiapine, has been shown to increase frontal lobe metabolism during a working memory task (61). Different psychotropic medications are known to have different effects on mitochondrial function (62–64). Quetiapine in particular has been shown to increase brain mitochondrial respiratory chain activity (65).
In addition, the paper-and-pencil trail-making test used here is a psychomotor task, and second-generation antipsychotics can ameliorate negative symptoms of psychosis, such as impaired motor skills (66). Therefore, it is possible that improving mitochondrial function, motor impairment, and prefrontal cortical activation with antipsychotic medication made the TMT-B easier to perform in the medicated participants.
The lack of a sham control group also complicates the interpretation, in terms of assessing how much of the improvement in performance was due to TILS alone vs. practice effects. Considering that the first-week test represents participants’ initial exposure to the 2-back task, it is possible that the results reflect a lack of familiarity rather than a genuine effect. However, we have previously used sham control groups to demonstrate cognitive benefits after TILS (10–13, 67), and the task improvement seen in the current study is consistent with and comparable to these previous findings of cognitive enhancement in neurotypical adults after TILS, so it is likely that at least some of the improvement is due to the TILS treatment. Ultimately, the choice to offer TILS treatment to every participant was based on several reasons, including the extensive time commitment required for the experiment, the difficulty in recruiting this specific population, and an ethical decision to maximize a potential benefit for these individuals. In particular, since significant improvement in cognitive function was observed in participants who continued taking antipsychotics (Figure 9A), it is possible that continued antipsychotic medication contributed to the improvement of cognitive function in remitted BD. We considered only the effects of psychotropic medication, but other factors may also be involved. Therefore, to definitely demonstrate TILS efficacy, a sham-controlled, double-blinded randomized trial should be conducted.
Future directions for this line of research include the analysis and publication of additional data gathered as part of this experiment (results from MRI scans and cognitive assessments). We will also explore using graph theory and structural equation modeling to explore the network properties of fNIRS data more fully, as the makers of the NIRSIT device will be incorporating new features into future updates. It is also possible that photosensitizers such as methylene blue may work synergistically with TILS to improve cognition and brain metabolic activity, over and above TILS treatment alone (68). A major limitation of the current work is the lack of a sham-treated control group; including this in the experimental design would allow the disambiguation of practice effects from TILS effects, as well as the assessment of how the observed lateralized fNIRS effects are related to lateralization of function. Future studies should also examine the effects of TILS in participants with BD experiencing depressed or elevated mood states, which could elucidate whether TILS has mood-state-dependent effects on cognitive performance or neuroplasticity (50). Tasks that involve emotional regulation could be particularly useful in these contexts. Finally, the use of light-emitting diodes (LEDs) will be explored as an alternative mechanism for delivering TILS with lasers, as the use of LEDs would be more practical and economical for daily TILS treatments self-administered by people at home (69).
This work expanded our previous findings of beneficial changes in brain metabolic activity and cognitive enhancement after a single TILS treatment in older adults with BD. Repeated TILS treatments may be effective as a safe, non-invasive, non-pharmacological intervention to improve cognition in people with remitted BD. Further research using randomized sham-controlled trials is supported by the present result and may reveal the potential to ameliorate the cognitive impairment seen in individuals with BD and other psychiatric conditions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Institutional Review Board of the University of Texas at Austin. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
DB: Writing – original draft, Writing – review & editing. RD: Writing – review & editing. JS-R: Writing – review & editing. AB: Writing – review & editing. JA: Writing – review & editing. FG-L: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Baszucki Brain Research Fund - Milken Institute, the Oskar Fischer Project Fund, and Joint Gift Grant to JRCA and FGL.
The authors would like to thank all of the participants for their extensive commitment of both time and effort.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1547230/full#supplementary-material
1. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. (2018) 83:720–1. doi: 10.1016/j.biopsych.2017.09.018
2. Brooks JO, Wang PW, Bonner JC, Rosen AC, Hoblyn JC, Hill SJ, et al. Decreased prefrontal, anterior cingulate, insula, and ventral striatal metabolism in medication-free depressed outpatients with bipolar disorder. J Psychiatr Res. (2009) 43:181–8. doi: 10.1016/j.jpsychires.2008.04.015
3. Adler CM, DelBello MP, Strakowski SM. Brain network dysfunction in bipolar disorder. CNS Spectr. (2006) 11:312–20. doi: 10.1017/S1092852900020800
4. Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. (2006) 93:105–15. doi: 10.1016/j.jad.2006.02.016
5. Miskowiak KW, Seeberg I, Jensen MB, Balanzá-Martínez V, Del Mar Bonnin C, Bowie CR, et al. Randomised controlled cognition trials in remitted patients with mood disorders published between 2015 and 2021: A systematic review by the International Society for Bipolar Disorders Targeting Cognition Task Force. Bipolar Disord. (2022) 24:354–74. doi: 10.1111/bdi.13193
6. Bernabei L, Leone B, Hirsch D, Mentuccia V, Panzera A, Riggio F, et al. Neuromodulation strategies in lifelong bipolar disorder: A narrative review. Behav Sci. (2024) 14:1176. doi: 10.3390/bs14121176
7. Gonzalez-Lima F. Neuroprotection and neurocognitive augmentation by photobiomodulation. In: Opris I, Lebedev A, Casanova, editors. Modern Approaches to Augmentation of Brain Function, Contemporary Clinical Neuroscience. Springer International Publishing, Cham (2021). p. 165–207. doi: 10.1007/978-3-030-54564-2_9
8. Wang X, Gonzalez-Lima F, Liu H. Transcranial infrared laser stimulation. In: Wassermann EM, Peterchev AV, Ziemann U, Lisanby SH, Siebner HR, Walsh V, editors. The Oxford Handbook of Transcranial Stimulation. Oxford, United Kingdom: Oxford University Press (2022). p. 276–98. doi: 10.1093/oxfordhb/9780198832256.013.10
9. Huang L-D, Kao T-C, Sung K-B, Abraham JA. Simulation study on the optimization of photon energy delivered to the prefrontal cortex in low-level-light therapy using red to near-infrared light. IEEE J Select Topics Quantum Electron. (2021) 27:1–10. doi: 10.1109/JSTQE.2021.3051671
10. Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. (2013) 230:13–23. doi: 10.1016/j.neuroscience.2012.11.016
11. Blanco NJ, Maddox WT, Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol. (2017) 11:14–25. doi: 10.1111/jnp.12074
12. Blanco NJ, Saucedo CL, Gonzalez-Lima F. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Memory. (2017) 139:69–75. doi: 10.1016/j.nlm.2016.12.016
13. Holmes E, Barrett DW, Saucedo CL, O’Connor P, Liu H, Gonzalez-Lima F. Cognitive enhancement by transcranial photobiomodulation is associated with cerebrovascular oxygenation of the prefrontal cortex. Front Neurosci. (2019) 13:1129. doi: 10.3389/fnins.2019.01129
14. Tian F, Hase SN, Gonzalez-Lima F, Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med. (2016) 48:343–9. doi: 10.1002/lsm.22471
15. Wang X, Tian F, Reddy DD, Nalawade SS, Barrett DW, Gonzalez-Lima F, et al. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab. (2017) 37:3789–802. doi: 10.1177/0271678X17691783
16. Saucedo CL, Courtois EC, Wade ZS, Kelley MN, Kheradbin N, Barrett DW, et al. Transcranial laser stimulation: Mitochondrial and cerebrovascular effects in younger and older healthy adults. Brain Stimulation. (2021) 14:440–9. doi: 10.1016/j.brs.2021.02.011
17. O’Donnell CM, Barrett DW, Fink LH, Garcia-Pittman EC, Gonzalez-Lima F. Transcranial infrared laser stimulation improves cognition in older bipolar patients: proof of concept study. J Geriatr Psychiatry Neurol. (2022) 35:321–32. doi: 10.1177/0891988720988906
18. O’Donnell CM, Barrett DW, O’Connor P, Gonzalez-Lima F. Prefrontal photobiomodulation produces beneficial mitochondrial and oxygenation effects in older adults with bipolar disorder. Front Neurosci. (2023) 17:1268955. doi: 10.3389/fnins.2023.1268955
19. Vargas E, Barrett DW, Saucedo CL, Huang L-D, Abraham JA, Tanaka H, et al. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med Sci. (2017) 32:1153–62. doi: 10.1007/s10103-017-2221-y
20. Demakis GJ. Frontal lobe damage and tests of executive processing: A meta-analysis of the category test, stroop test, and trail-making test. J Clin Exp Neuropsychol. (2004) 26:441–50. doi: 10.1080/13803390490510149
21. Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. (1958) 55:352–8. doi: 10.1037/h0043688
22. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. (2005) 25:46–59. doi: 10.1002/hbm.20131
23. Delpy DT, Cope M, Zee PVD, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. (1988) 33:1433–42. doi: 10.1088/0031-9155/33/12/008
24. Horwitz B, Soncrant TT, Haxby JV. Covariance analysis of functional interactions in the brain using metabolic and blood flow data. In: Gonzalez-Lima F, Finkenstädt TH, Scheich H, editors. Advances in Metabolic Mapping Techniques for Brain Imaging of Behavioral and Learning Functions. Springer Netherlands, Dordrecht (1992). p. 189–217. doi: 10.1007/978-94-011-2712-7_7
25. McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. (1994) 2:2–22. doi: 10.1002/hbm.460020104
26. Gonzalez-Lima F, McIntosh AR. Analysis of neural network interactions related to associative learning using structural equation modeling. Mathematics Comput Simulation. (1995) 40:115–40. doi: 10.1016/0378-4754(95)00022-X
27. Woltz DJ, Was CA. Availability of related long-term memory during and after attention focus in working memory. Memory Cogn. (2006) 34:668–84. doi: 10.3758/BF03193587
28. The jamovi project. (2024). Available online at: https://www.jamovi.org (Accessed January 26, 2023).
29. Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. (1965) 52:591–611. doi: 10.1093/biomet/52.3-4.591
30. Mauchly JW. Significance test for sphericity of a normal n-variate distribution. Ann Math Statist. (1940) 11:204–9. doi: 10.1214/aoms/1177731915
31. Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. (1959) 24:95–112. doi: 10.1007/BF02289823
32. Cohen J. Statistical power analysis and research results. Am Educ Res J. (1973) 10:225. doi: 10.2307/1161884
33. Cohen J. Statistical Power Analysis of the Behavioral Sciences. 2nd. New York: Academic Press (1988).
34. Hawkins K, Hoffman R, Quinlan D, Rakfeldt J, Docherty N, Sledge W. Cognition, negative symptoms, and diagnosis: a comparison of schizophrenic, bipolar, and control samples. J Neuropsychiatry Clin Neurosci. (1997) 9:81–9. doi: 10.1176/jnp.9.1.81
35. Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. (2005) 62:617. doi: 10.1001/archpsyc.62.6.617
36. Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. (2000) 2:180–90. doi: 10.1034/j.1399-5618.2000.020305.x
37. Cataldo AM, McPhie DL, Lange NT, Punzell S, Elmiligy S, Ye NZ, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol. (2010) 177:575–85. doi: 10.2353/ajpath.2010.081068
38. Clay HB, Daws S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Intl J Devlp Neurosci. (2011) 29:311–24. doi: 10.1016/j.ijdevneu.2010.08.007
39. Zuccoli GS, Saia-Cereda VM, Nascimento JM, Martins-de-Souza D. The energy metabolism dysfunction in psychiatric disorders postmortem brains: focus on proteomic evidence. Front Neurosci. (2017) 11:493. doi: 10.3389/fnins.2017.00493
40. Lam X-J, Xu B, Yeo P-L, Cheah P-S, Ling K-H. Mitochondria dysfunction and bipolar disorder: From pathology to therapy. IBRO Neurosci Rep. (2023) 14:407–18. doi: 10.1016/j.ibneur.2023.04.002
41. Martino DJ, Strejilevich SA, Manes F. Neurocognitive functioning in early-onset and late-onset older patients with euthymic bipolar disorder. Int J Geriat Psychiatry. (2013) 28:142–8. doi: 10.1002/gps.3801
42. Fries GR, Bauer IE, Scaini G, Valvassori SS, Walss-Bass C, Soares JC, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Disord. (2020) 22:498–507. doi: 10.1111/bdi.12876
43. Fries GR, Bauer IE, Scaini G, Wu M-J, Kazimi IF, Valvassori SS, et al. Accelerated epigenetic aging and mitochondrial DNA copy number in bipolar disorder. Transl Psychiatry. (2017) 7:1283. doi: 10.1038/s41398-017-0048-8
44. Gonzalez-Lima F ed. Cytochrome Oxidase in Neuronal Metabolism and Alzheimer’s Disease. Springer US, Boston, MA: Plenum Press (1998). doi: 10.1007/978-1-4757-9936-1
45. Stanley ML, Simpson SL, Dagenbach D, Lyday RG, Burdette JH, Laurienti PJ. Changes in brain network efficiency and working memory performance in aging. PloS One. (2015) 10:e0123950. doi: 10.1371/journal.pone.0123950
46. Ahmed OJ, Cash SS. Finding synchrony in the desynchronized EEG: the history and interpretation of gamma rhythms. Front Integr Neurosci. (2013) 7:58. doi: 10.3389/fnint.2013.00058
47. Li R, Zhang S, Yin S, Ren W, He R, Li J. The fronto-insular cortex causally mediates the default-mode and central-executive networks to contribute to individual cognitive performance in healthy elderly. Hum Brain Mapp. (2018) 39:4302–11. doi: 10.1002/hbm.24247
48. Lopez-Persem A, Roumazeilles L, Folloni D, Marche K, Fouragnan EF, Khalighinejad N, et al. Differential functional connectivity underlying asymmetric reward-related activity in human and nonhuman primates. Proc Natl Acad Sci USA. (2020) 117:28452–62. doi: 10.1073/pnas.2000759117
49. Goel V. Hemispheric asymmetry in the prefrontal cortex for complex cognition, in: Handbook of Clinical Neurology. Elsevier. (2019) pp:179–96. doi: 10.1016/B978-0-12-804281-6.00010-0
50. Disner SG, Beevers CG, Gonzalez-Lima F. Transcranial laser stimulation as neuroenhancement for attention bias modification in adults with elevated depression symptoms. Brain Stimulation. (2016) 9:780–7. doi: 10.1016/j.brs.2016.05.009
51. Zhao C, Li D, Kong Y, Hongyu L, Hu Y, Niu H, et al. Transcranial photobiomodulation enhances visual working memory capacity in humans. Sci Adv. (2022) 8:eabq3211. doi: 10.1126/sciadv.abq3211
52. Almeida JRCD, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. (2009) 66:451–9. doi: 10.1016/j.biopsych.2009.03.024
53. Versace A, Thompson WK, Zhou D, Almeida JRC, Hassel S, Klein CR, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. (2010) 67:422–31. doi: 10.1016/j.biopsych.2009.11.025
54. Kopp B, Rösser N, Tabeling S, Stürenburg HJ, De Haan B, Karnath H-O, et al. Errors on the trail making test are associated with right hemispheric frontal lobe damage in stroke patients. Behav Neurol. (2015) 2015:1–10. doi: 10.1155/2015/309235
55. Salminen T, Forlim CG, Schubert T, Kühn S. Dual n-back training improves functional connectivity of the right inferior frontal gyrus at rest. Sci Rep. (2020) 10:20379. doi: 10.1038/s41598-020-77310-9
56. Wade ZS, Barrett DW, Davis RE, Nguyen A, Venkat S, Gonzalez-Lima F. Histochemical mapping of the duration of action of photobiomodulation on cytochrome c oxidase in the rat brain. Front Neurosci. (2023) 17:1243527. doi: 10.3389/fnins.2023.1243527
57. Liemburg EJ, Knegtering H, Klein HC, Kortekaas R, Aleman A. Antipsychotic medication and prefrontal cortex activation: A review of neuroimaging findings. Eur Neuropsychopharmacol. (2012) 22:387–400. doi: 10.1016/j.euroneuro.2011.12.008
58. Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2003) 27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010
59. Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull. (1997) 23:437–58. doi: 10.1093/schbul/23.3.437
60. Busatto GF, Kerwin RW. Perspectives on the role of serotonergic mechanisms in the pharmacology of schizophrenia. J Psychopharmacol. (1997) 11:3–12. doi: 10.1177/026988119701100102
61. Meisenzahl EM, Scheuerecker J, Zipse M, Ufer S, Wiesmann M, Frodl T, et al. Effects of treatment with the atypical neuroleptic quetiapine on working memory function: a functional MRI follow-up investigation. Eur Arch Psychiatry Clin Neurosci. (2006) 256:522–31. doi: 10.1007/s00406-006-0687-x
62. Hroudova J, Fisar Z. Activities of respiratory chain complexes and citrate synthase influenced by pharmacologically different antidepressants and mood stabilizers. Neuro Endocrinol Lett. (2010) 31:336–42.
63. Holper L, Ben-Shachar D, Mann JJ. Psychotropic and neurological medication effects on mitochondrial complex I and IV in rodent models. Eur Neuropsychopharmacol. (2019) 29:986–1002. doi: 10.1016/j.euroneuro.2019.06.010
64. Gardea-Resendez M, Coombes BJ, Veldic M, Tye SJ, Romo-Nava F, Ozerdem A, et al. Antidepressants that increase mitochondrial energetics may elevate risk of treatment-emergent mania. Mol Psychiatry. (2023) 28:1020–6. doi: 10.1038/s41380-022-01888-x
65. Ignacio Z, Reus G, Abelaira H, Titus S, Carlessi A, Da Luz J, et al. Acute and chronic treatments with quetiapine increase mitochondrial respiratory chain complex activity in the rat brain. CNR. (2015) 12:283–92. doi: 10.2174/1567202612666150603140912
66. Fabrazzo M, Cipolla S, Camerlengo A, Perris F, Catapano F. Second-generation antipsychotics’ Effectiveness and tolerability: A review of real-world studies in patients with schizophrenia and related disorders. JCM. (2022) 11:4530. doi: 10.3390/jcm11154530
67. Zaizar ED, Papini S, Gonzalez-Lima F, Telch MJ. Singular and combined effects of transcranial infrared laser stimulation and exposure therapy on pathological fear: a randomized clinical trial. Psychol Med. (2023) 53:908–17. doi: 10.1017/S0033291721002270
68. Gonzalez-Lima F, Auchter A. Protection against neurodegeneration with low-dose methylene blue and near-infrared light. Front Cell Neurosci. (2015) 9:179. doi: 10.3389/fncel.2015.00179
Keywords: bipolar disorder, transcranial infrared laser stimulation, photobiomodulation, cognitive enhancement, prefrontal cortex stimulation, brain oxygenation
Citation: Barrett DW, Davis RE, Siegel-Ramsay JE, Bichlmeier A, Almeida JRC and Gonzalez-Lima F (2025) Cognitive improvement and prefrontal network interactions in individuals with remitted bipolar disorder after transcranial infrared laser stimulation. Front. Psychiatry 16:1547230. doi: 10.3389/fpsyt.2025.1547230
Received: 18 December 2024; Accepted: 14 January 2025;
Published: 30 January 2025.
Edited by:
Takeshi Terao, Oita University, JapanCopyright © 2025 Barrett, Davis, Siegel-Ramsay, Bichlmeier, Almeida and Gonzalez-Lima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. Gonzalez-Lima, Z29uemFsZXpsaW1hQHV0ZXhhcy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.