
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 18 February 2025
Sec. Neurostimulation
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1478503
 Alwaleed K. Aloufi1,2†
Alwaleed K. Aloufi1,2† Jalal A. Zahhar1,2†
Jalal A. Zahhar1,2† Mahmoud W. Bader1,2
Mahmoud W. Bader1,2 Maher B. Almutairi1,2
Maher B. Almutairi1,2 Abdulqader Alaaldeen1,2
Abdulqader Alaaldeen1,2 Omar E. Hetta1,2
Omar E. Hetta1,2 Abdulaziz M. Gammash1,2
Abdulaziz M. Gammash1,2 Saleh Almuntashiri1,2
Saleh Almuntashiri1,2 Ibrahim S. Binrabaa1,2
Ibrahim S. Binrabaa1,2 Ahmad Alsaleh1,2,3
Ahmad Alsaleh1,2,3 Moayyad AlSalem1,2,3*
Moayyad AlSalem1,2,3*Background: Tourette syndrome (TS) is a neurological disorder characterized by tics, often associated with obsessive-compulsive disorder (OCD). Severe cases may require interventions such as deep brain stimulation (DBS) or repetitive transcranial magnetic stimulation (rTMS).
Methods: A thorough search was performed across PubMed/Medline, Embase, (CENTRAL), and Google Scholar. Studies comparing DBS and rTMS efficacy for TS were included if they reported YGTSS before and after treatment. Two independent reviewers screened the search results, extracted data, and assessed study quality using standardized tools.
Results: 22 studies met the inclusion criteria, with a total of 222 participants. Analysis of RCTs investigating post-intervention rTMS vs baseline showed a statistically insignificant decrease in YGTSS (MD = -5.01, 95% CI: [-10.8, 0.79], P= 0.090) but a statistically significant decrease in YBOCS (MD = -6.6; 95% CI: [-11.64, -1.55], P= 0.010). However, post-intervention rTMS in RCT and non-randomized trials vs baseline showed a significant decrease in YGTSS (MD = -11.6; 95% CI: [-18.25, -4.94], P < 0.001) and YBOCS (MD = -7.5; 95% CI: [-11.85, -3.15], P < 0.001). Post-intervention DBS in RCT and non-RCTs vs baseline showed a significant decrease in YGTSS (MD = -18.29; 95% CI: [-24.93, -11.64], P < 0.001) and YBOCS (MD = -4.76; 95% CI: [-7.30, -2.21], P < 0.001). Analysis of RCTs investigating Post-intervention DBS vs baseline showed a significant decrease in YGTSS (MD = -14.71; 95% CI: [-19.78, -9.63], P <0.001) and YBOCS (MD = -5.04; 95% CI: [-8.28, -1.80], P = 0.002).
Conclusion: Our analysis revealed both DBS and rTMS improved TS and OCD symptoms, however the effect of rTMS on TS in RCTs was insignificant, suggesting DBS stimulation is more effective. Despite this, clinicians may still opt for rTMS before DBS due to its less invasive nature, the limited number of high-quality RCTs, and the lack of studies directly comparing rTMS and DBS.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023386856, identifier CRD42023386856.
Tourette Syndrome (TS) is a neurological disorder characterized by a spectrum of motor and vocal tics - involuntary, repetitive movements or vocalizations (1). The onset of these symptoms generally surfaces between the ages of three and nine (2). The severity of the symptoms is commonly measured using the Yale Global Tic Severity Scale (YGTSS). YGTSS is a widely used assessment tool designed to measure the severity and impact of tics in individuals diagnosed with TS, as recommended by the American Academy of Neurology guideline (3). It comprises several domains, including frequency, intensity, complexity, and impairment related to both motor and vocal tics. Despite significant advancements in neuroscience, the etiology of TS remains vague, with hypotheses revolving around abnormalities in certain brain regions (including the basal ganglia, frontal lobes, and cortex), and the neurotransmitters (dopamine, serotonin, and norepinephrine) that mediate nerve cell communication (4).
The intricate pathology of TS is believed to be multifactorial, involving both genetic predispositions and potential environmental influences (5). Adding layers of complexity to the clinical manifestation and management of the condition, TS is often comorbid with other behavioral disorders such as attention deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) (6), which is assessed using the Yale-Brown Obsessive Compulsive Scale (YBOCS). YBOCS is a standardized assessment tool utilized to measure the severity of OCD symptoms (7, 8). It evaluates the presence and intensity of obsessions and compulsions across several domains, including time spent, distress caused, and interference with daily functioning.
While there is no definitive cure for TS, a multitude of treatment strategies have been adopted to manage the symptoms and improve the quality of life for those affected. These strategies encompass cognitive-behavioral therapy (CBT), pharmacological interventions, deep brain stimulation (DBS), and repetitive transcranial magnetic stimulation (rTMS) (9, 10). Currently the management of Tourette patients consists of the following stages: first, psychological education and social support for mild conditions; second, pharmacological therapy and behavioral intervention; and third, invasive or non-invasive neuromodulation such as DBS or rTMS in severe or refractory cases (11).
Studies that compare the efficacy of DBS and rTMS in TS patients are scarce, mostly due to methodological limitations. Published studies are often conflicting (12–19), thus the development of evidence-based guidance to direct clinical decision-making in the selection of therapeutic regimen for severe or refractory TS is important. Therefore, we aimed to compare the efficacy of DBS and rTMS in TS patients. Our primary objective was to examine change in YGTSS in patients with TS before and after each intervention, and our secondary objectives were to investigate the effect of DBS on different brain areas, and the effect of brain stimulation on OCD symptoms by analyzing the change in YBOCS.
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study protocol was registered in the Prospective Register of Systematic Reviews (PROSPERO) database with registration number CRD42023386856.
Inclusion criteria required that the studies report YGTSS before and after the application of any brain stimulation therapy, including rTMS and DBS, in patients with Tourette Syndrome. These patients must have been diagnosed according to the criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders, the International Classification of Diseases, or the Chinese Classification of Mental Disorders. Only studies published in the English or Arabic languages were considered for inclusion. Studies were excluded if they were conducted on non-human subjects or if they did not report YGTSS. Moreover, studies were excluded if they included in their analysis patients who had initiated or changed their pharmacological treatment dosage within four weeks before receiving brain stimulation therapy.
A thorough search through multiple databases was conducted to identify relevant published papers to our study objectives. Databases searched included PubMed/Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and first 10 pages of google scholar. The search strategy is comprehensively outlined in Supplementary Table S1.
Each article selected was independently reviewed by two authors, with any disagreements being settled either through mutual agreement or by seeking the opinion of a third author. When necessary, further details were requested from the authors of the studies to clarify eligibility criteria. Additionally, the reasons for excluding any articles from the review were documented. Corresponding authors were contacted to provide any missing data when necessary, if we did not receive a response within 1 month, data was verified from other published meta-analyses. The data that were extracted are the following: First Author Name, Year of study publication, Study design, country where the study was conducted, inclusion criteria, exclusion criteria, Sample size for each arm, range of age included in the study, mean age in each arm, gender, duration of intervention in weeks, target area(s) in the brain, frequency used/technique, mean duration of disease, Side effects reported due to intervention, baseline and post intervention YGTSS/YBOCS and standard deviation/standard error.
Two independent reviewers used either the revised Cochrane risk of bias tool for randomized trials (20) or the Risk Of Bias In Non-randomized Studies - of Interventions tool (21) to assess the studies included. Any conflict was resolved by a third author.
Data analysis was conducted using Review Manager (RevMan) 5.4.1. An inverse variance random-effects model was utilized for all analyses. The threshold for statistical significance was established at P ≤ 0.05, ensuring a 95% confidence interval. Statistical heterogeneity was assessed through the I2 and P values derived from the chi-square test. In case the heterogeneity was more than 50%, a sensitivity analysis was conducted. A funnel plot was used to visually assess publication bias. The pooled mean difference of YGTSS and YBOCS were used to assess the change in severity of symptoms in TS and OCD patients, respectively.
We identified 777 studies, of which 209 were excluded due to duplication (Figure 1). A total of 568 studies were screened by title and abstract, of which 488 were considered irrelevant to the stated objective. Further, 80 studies were assessed for eligibility, and 58 studies were excluded after full-text screening. The results in Jackson N. Cagle’s study (22) reported no specific numbers; the study was excluded after receiving no response from the author. The study by Michael S. Okun (23) included patients who were part of a more recently published follow-up study by P. Justin Rossi (24), and was therefore excluded. Lastly, results in Marie-Laure Welter’s study (16) were missing, therefore, the results were selected from the last published systematic review (25) after receiving no response from the author. Finally, 22 studies were included in the review.
The 22 included studies had a total of 222 patients (Table 1). 14 studies were using DBS. Out of the 14 studies using DBS, 12 were randomized controlled trials (RCTs). The remaining 2 studies were non-randomized trials (non-RCTs). Of the 22 included studies, 8 were using rTMS. Of the 8 rTMS studies, 4 were RCT. The remaining 4 studies were non-RCTs The risk of bias assessment showed an overall low to medium risk for randomized studies and high risk for the non-randomized studies (Supplementary Figures S1, S2).
Analysis of RCTs investigating post-intervention rTMS vs baseline showed a statistically insignificant decrease in YGTSS (MD = -5.01, 95% CI: [-10.8, 0.79], P = 0.090, I2 = 0%; Figure 2A). None of the studies showed a significant difference between the rTMS and baseline (Figure 2A). Further combined analysis of RCTs and non-RCTs investigating post-intervention rTMS vs baseline showed a significant decrease in YGTSS after rTMS (MD = -11.60; 95% CI: [-18.25, -4.94], P < 0.001, I2 = 74%; Figure 2B), sensitivity analysis was conducted by removing Kahl et al. (18) which reduced the heterogeneity to 51% (Supplementary Figure S3). Additionally, analysis of sham rTMS studies showed a marginally significant decrease in YGTSS compared to baseline (MD = -4.39; 95% CI: [-8.75, -0.03], P = 0.050, I2 = 0%; Figure 2A). Further analysis of RCTs investigating post-intervention DBS vs baseline showed a significant decrease in YGTSS following DBS (MD = -14.71; 95% CI: [-19.78, -9.63], P < 0.001, I2 = 52%; Figure 2A). Combined analysis of RCTs and non-RCTs investigating post-intervention DBS vs baseline also showed a significant decrease in YGTSS compared to baseline (MD = -18.29; 95% CI: [-24.93, -11.64], P < 0.001, I2 = 76%; Figure 2B), sensitivity analysis did not show a significant change in heterogeneity. Furthermore, analysis of sham DBS studies showed no significant difference in YGTSS compared to baseline (MD = -3.08; 95% CI: [-6.38, 0.23], P = 0.070, I2 = 0%; Figure 2A). Subgroup analysis by location of DBS showed a greater decrease in YGTSS in studies stimulating the thalamic ventrooral region vs studies stimulating the globus pallidus internus (GPi) region (MD = -19.77; 95% CI = [-33.57, -5.97], P = 0.005, vs MD = -16.21; 95% CI: [-24.28, -8.13], P < 0.001; Figure 3). Sensitivity analysis for the GPi studies was performed and showed a decrease in heterogeneity to I2 = 56% after removing Cannon et al. (Supplementary Figure S4). Sensitivity analysis for the thalamic ventrooral studies was performed and showed a decrease in heterogeneity to I2 = 50% after removing Haense et al. (Supplementary Figure S5). On combined analysis of RCTs and non-RCTs, the effect of rTMS on YBOCS showed a significant decrease in YBOCS after rTMS compared to baseline (MD = -7.5; 95% CI: [-11.85, -3.15], P < 0.001, I2 = 0%; Figure 4B); subsequent analysis of RCT studies exclusively also revealed a significant decrease in YBOCS after rTMS compared to baseline (MD = -6.6; 95% CI: [-11.64, -1.55], P = 0.010, I2 = 3%; Figure 4A). Furthermore, combined analysis of RCTs and non-RCTs examining the effect of DBS on YBOCS showed a significant decrease in YBOCS after DBS compared to baseline (MD = -4.76; 95% CI: [-7.30, -2.21], P < 0.001, I2 = 0%; Figure 4B); a further analysis of RCTs alone on the effect of DBS on YBOCS showed a significant decrease in YBOCS after DBS compared to baseline (MD = -5.04; 95% CI: [-8.28, -1.80], P = 0.002, I2 = 0%; Figure 4A). Additional analysis of sham rTMS and sham DBS studies showed no significant difference in YBOCS compared to baseline (Figure 4A). Primary and secondary outcomes showed no publication bias by visual inspection of funnel plot (Supplementary Figures S6–S8) (20, 21).
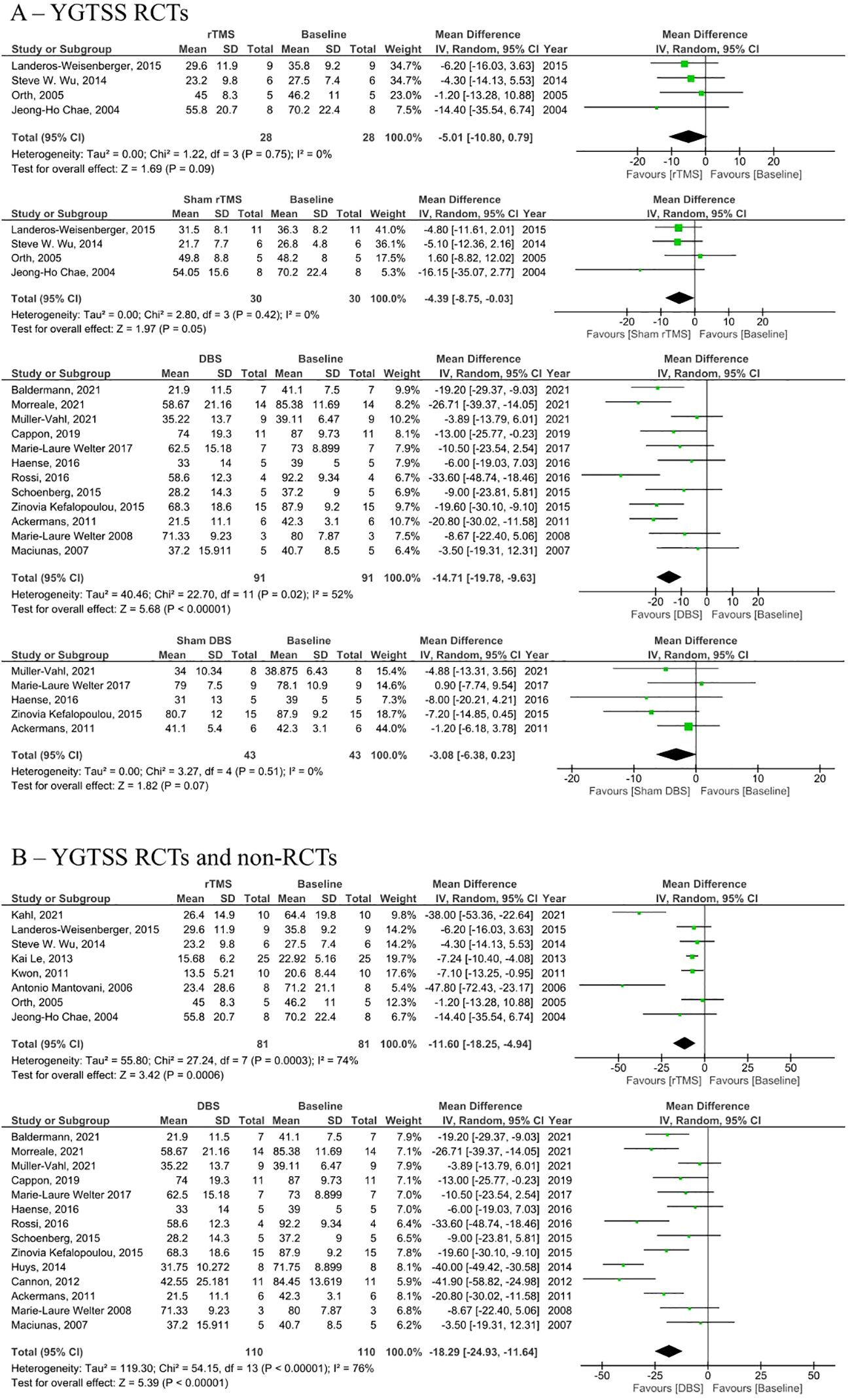
Figure 2. Forest plots comparing YGTSS pre and post rTMS, DBS, and sham stimulation in tourette syndrome patients. (A) YGTSS change in RCTs only, (B) Combined YGTSS change in RCTs and non-RCTs. rTMS, repetitive transcranial magnetic stimulation, DBS, deep brain stimulation, SD, standard deviation, CI, confidence interval.
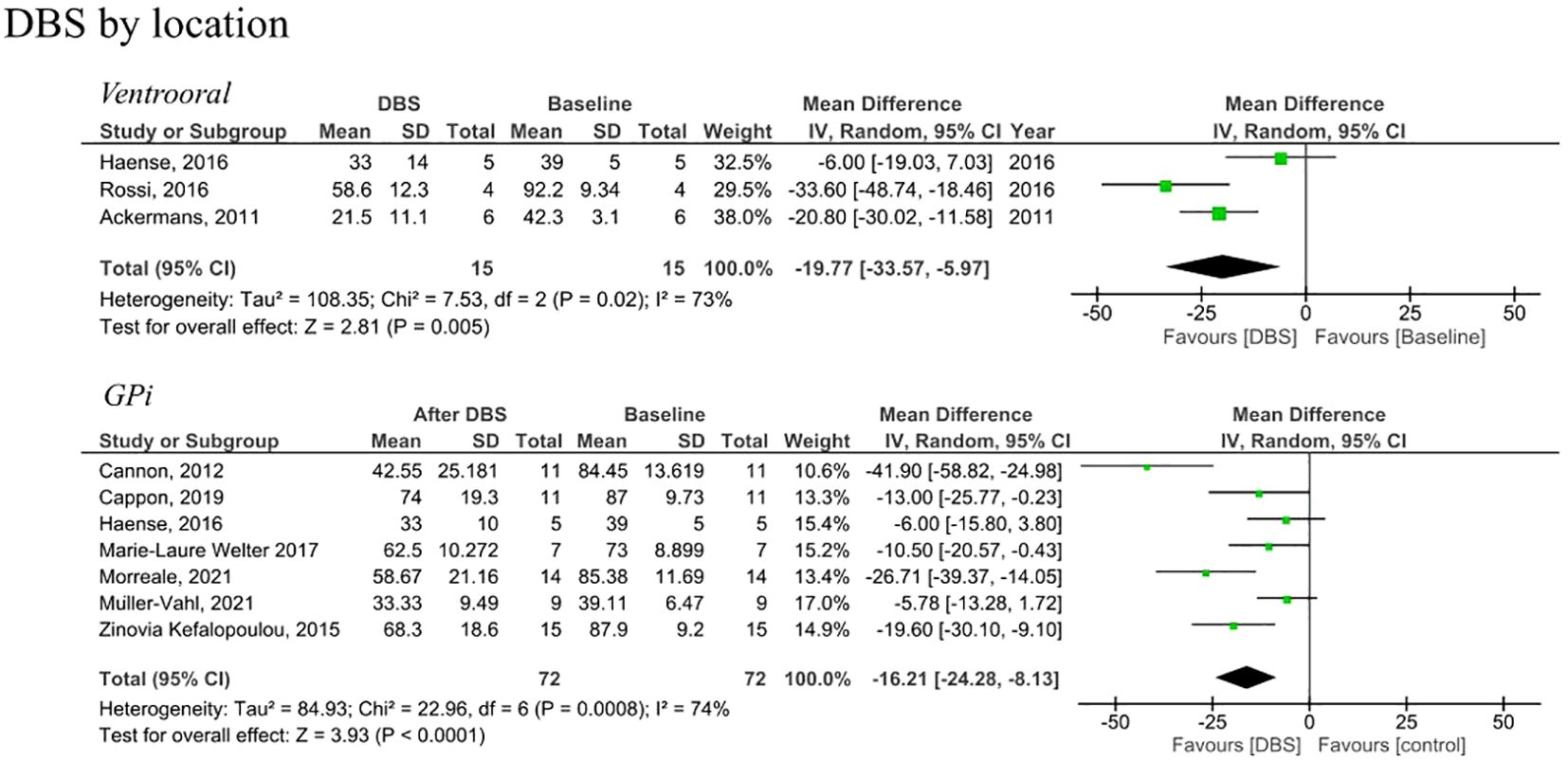
Figure 3. Forest plots comparing YGTSS pre and post DBS in tourette syndrome patients by anatomical location of stimulation. DBS, deep brain stimulation, Gpi, globus pallidus internus, SD, standard deviation, CI, confidence interval.
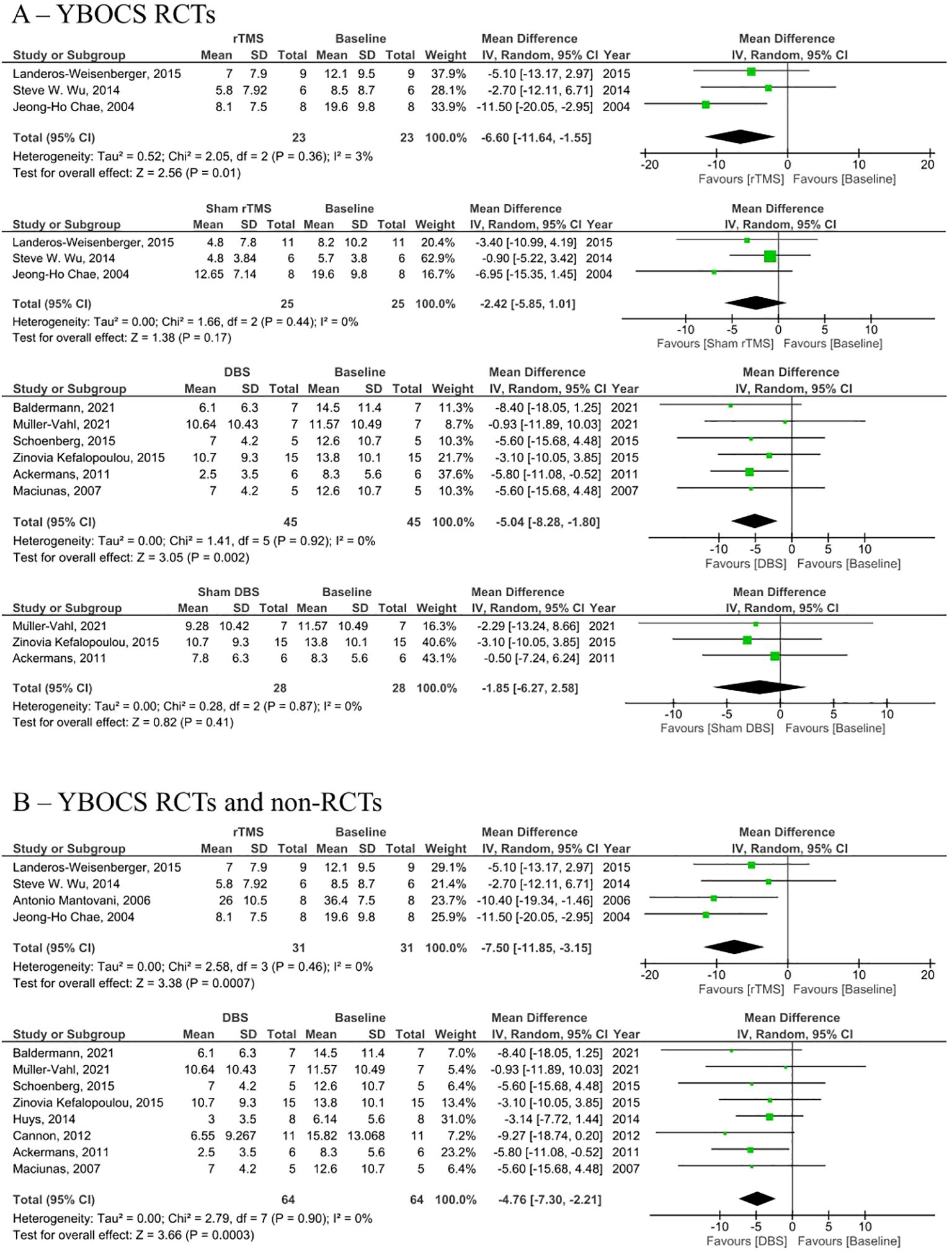
Figure 4. Forest plots comparing YBOCS pre and post rTMS, DBS, and sham stimulation in patients with obsessive-compulsive disorder. (A) YBOCS change in RCTs only, (B) Combined YBOCS change in RCTs and non-RCTs. rTMS, repetitive transcranial magnetic stimulation; DBS, deep brain stimulation; SD, standard deviation; CI, confidence interval. Forest plots comparing YGTSS pre and post rTMS, DBS, and sham stimulation in tourette syndrome patients.
The present study examined the therapeutic efficacy of rTMS and DBS in the treatment of TS and OCD. The results indicate that both rTMS and DBS exhibited effectiveness in improving symptoms in both diseases. Notably, rTMS demonstrated greater efficacy in improving OCD symptoms, while DBS was found to be more effective in TS, as rTMS failed to show effectiveness in the analysis of RCTs.
It is worth noting that few studies have directly compared the efficacy of DBS and rTMS, with most previous investigations primarily focusing on exploring the therapeutic potential of either intervention. Our findings also closely align with the previous meta-analysis by Xiaofeng Lin et al. (25). However, it is important to acknowledge that their study did not include an analysis of sham studies, and subsequent articles incorporating new evidence have been published since then.
Prior studies evaluating the YGTSS have yielded mixed results regarding the effectiveness of rTMS (17–19, 34–38). In our investigation, the YGTSS from RCTs showed no significant difference between the rTMS group and baseline (Figure 2A). However, when non-RCTs were included in the analysis, the rTMS group exhibited a significant decrease in YGTSS compared to the baseline (Figure 2B). This suggests a potential methodological limitation that has not been previously acknowledged in the literature. Furthermore, our analysis of sham rTMS studies demonstrated a marginally significant difference in YGTSS compared to the baseline, which suggests that the effectiveness seen in non-RCTs is possibly due to a placebo effect.
Conversely, DBS demonstrated a significant decrease in YGTSS compared to baseline, with this decrease observed in both RCTs and non-RCTs. Moreover, the analysis of sham DBS studies indicated no significant difference in YGTSS compared to the baseline, providing further evidence that the symptom reduction associated with DBS is attributable to the stimulation rather than the surgical procedure or a placebo effect. Additionally, DBS exhibited a more pronounced decrease in YGTSS in the thalamic ventrooral region compared to the Gpi, although the number of ventrooral studies that we were able to include in the analysis was limited, so this result should be considered with caution.
Furthermore, the YBOCS was assessed following rTMS and DBS interventions, revealing a significant decrease in compared to the baseline. The analysis of sham rTMS and sham DBS studies demonstrated no significant difference in YBOCS compared to the baseline, this also suggests the effectiveness of rTMS and DBS in these patients was not due to a placebo effect.
While both DBS and rTMS have demonstrated efficacy in patients with TS and OCD, it is important to consider the potential side effects associated with these interventions. In several articles, DBS was associated with more severe side effects, including infections, headaches, dizziness, infection, and an increase in tic severity, as indicated in Supplementary Table S2.
There are several limitations to consider in our study. Firstly, none of the included studies directly compared DBS and rTMS, which introduces a significant gap in our understanding of their relative efficacy. The lack of direct comparison is primarily attributed to the limited number of studies available on this topic. Furthermore, the heterogeneity in the frequency and location of stimulation between the studies further complicates the comparison of these interventions. Secondly, while our study includes findings from various trials, it is essential to acknowledge the limited number of high-quality randomized controlled trials (RCTs) available on this subject. The scarcity of such trials restricts the strength of evidence supporting our conclusions. Additionally, the potential for publication bias should be considered, as studies with negative or inconclusive results may be less likely to be published, potentially skewing the available evidence base. Thirdly, it is important to acknowledge that not all studies reported adverse effects associated with the therapies under investigation. This missing data regarding adverse effects introduces a potential bias and limits our comprehensive understanding of the safety profiles of DBS and rTMS. Therefore, caution should be exercised in interpreting the adverse effect data presented in our study. Lastly, the large heterogeneity observed across the included studies can be attributed to various factors. One major contributing factor is the diverse patient characteristics within the study populations. Patients differed significantly in terms of age, comorbidities, and medications used, among other factors. The lack of standardized patient selection criteria and control of confounding variables may have influenced the observed heterogeneity and introduced variability in the treatment outcomes.
It is essential to consider these limitations when interpreting the findings of our study, as they highlight areas for further investigation and underscore the need for more rigorous research to establish the comparative efficacy and safety of DBS and rTMS. Future studies should focus on directly comparing DBS and rTMS in randomized controlled trials to provide robust evidence regarding their relative efficacy and safety. Such research should aim to standardize stimulation protocols, patient selection criteria, and outcome measures to minimize heterogeneity and enhance the comparability of findings. Subgroup analysis including demographic variables should be considered in future studies as they might generate different risk ratios. Additionally, efforts should be made to report adverse effects comprehensively to establish a clearer understanding of the safety profiles of these interventions. Addressing these gaps will contribute significantly to optimizing treatment strategies for tic symptoms and obsessional symptoms.
Our study provides valuable insights into the management of TS and OCD, overall showing the effectiveness of rTMS and DBS in treating both diseases, especially in advanced disease. However, the analysis of rTMS effect on TS showed conflicting results in RCTs vs non-RCTs, likely due to the limited number of RCTs or a placebo effect. It is crucial to consider the limitations of the current literature and the lack of direct comparisons between DBS and rTMS. Further randomized controlled trials comparing both modalities directly are needed.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
AKA: Conceptualization, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. MB: Data curation, Investigation, Writing – original draft, Writing – review & editing, Visualization. MBA: Data curation, Investigation, Writing – original draft, Writing – review & editing. AMA: Data curation, Investigation, Writing – original draft, Writing – review & editing. OH: Data curation, Investigation, Writing – original draft, Writing – review & editing. AG: Data curation, Investigation, Writing – original draft, Writing – review & editing. SA: Data curation, Investigation, Writing – original draft, Writing – review & editing. IB: Writing – original draft, Writing – review & editing, Data curation, Investigation. AA: Resources, Supervision, Writing – original draft, Writing – review & editing. MA: Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1478503/full#supplementary-material
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association, Washington, DC (2013). doi: 10.1176/appi.books.9780890425596
2. Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. J Psychosom Res. (2008) 65:461–72. doi: 10.1016/j.jpsychores.2008.03.006
3. Pringsheim T, Okun MS, Müller-Vahl K, Martino D, Jankovic J, Cavanna AE, et al. Practice guideline recommendations summary: Treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. (2019) 92:896–906. doi: 10.1212/WNL.0000000000007466
4. Singer HS. NEUROBIOLOGY OF TOURETTE SYNDROME. Neurol Clin. (1997) 15:357–79. doi: 10.1016/S0733-8619(05)70318-2
5. Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, et al. Partitioning the heritability of tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PloS Genet. (2013) 9:e1003864. doi: 10.1371/journal.pgen.1003864
6. Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in tourette syndrome. JAMA Psychiatry. (2015) 72:325. doi: 10.1001/jamapsychiatry.2014.2650
7. Scahill L, Riddle MA, Mcswiggin-Hardin M, Ort SI, King RA, Goodman WK, et al. Children’s yale-brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. (1997) 36:844–52. doi: 10.1097/00004583-199706000-00023
8. Lewin AB, De Nadai AS, Park J, Goodman WK, Murphy TK, Storch EA. Refining clinical judgment of treatment outcome in obsessive–compulsive disorder. Psychiatry Res. (2011) 185:394–401. doi: 10.1016/j.psychres.2010.08.021
9. Verdellen C, van de Griendt J, Hartmann A, Murphy T. European clinical guidelines for Tourette Syndrome and other tic disorders. Part III: behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry. (2011) 20:197–207. doi: 10.1007/s00787-011-0167-3
10. Pansaon Piedad JC, Rickards HE, Cavanna AE. What Patients With Gilles de la Tourette Syndrome Should Be Treated With Deep Brain Stimulation and What Is the Best Target? Neurosurgery. (2012) 71:173–92. doi: 10.1227/NEU.0b013e3182535a00
11. Billnitzer A, Jankovic J. Current management of tics and tourette syndrome: behavioral, pharmacologic, and surgical treatments. Neurotherapeutics. (2020) 17:1681–93. doi: 10.1007/s13311-020-00914-6
12. Morreale F, Kefalopoulou Z, Zrinzo L, Limousin P, Joyce E, Foltynie T, et al. Inhibitory Control on a Stop Signal Task in Tourette Syndrome before and after Deep Brain Stimulation of the Internal Segment of the Globus Pallidus. Brain Sci. (2021) 11:461. doi: 10.3390/brainsci11040461
13. Baldermann JC, Kuhn J, Schüller T, Kohl S, Andrade P, Schleyken S, et al. Thalamic deep brain stimulation for Tourette Syndrome: A naturalistic trial with brief randomized, double-blinded sham-controlled periods. Brain Stimul. (2021) 14:1059–67. doi: 10.1016/j.brs.2021.07.003
14. Haense C, Müller-Vahl KR, Wilke F, Schrader C, Capelle HH, Geworski L, et al. Effect of deep brain stimulation on regional cerebral blood flow in patients with medically refractory tourette syndrome. Front Psychiatry. (2016) 7:118. doi: 10.3389/fpsyt.2016.00118
15. Welter M-L, Houeto J-L, Thobois S, Bataille B, Guenot M, Worbe Y, et al. Anterior pallidal deep brain stimulation for Tourette’s syndrome: a randomised, double-blind, controlled trial. Lancet Neurol. (2017) 16:610–9. doi: 10.1016/S1474-4422(17)30160-6
16. Welter M-L, Mallet L, Houeto J-L, Karachi C, Czernecki V, Cornu P, et al. Internal pallidal and thalamic stimulation in patients with tourette syndrome. Arch Neurol. (2008) 65:952–7. doi: 10.1001/archneur.65.7.952
17. Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive–compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. (2005) 9:95. doi: 10.1017/S1461145705005729
18. Kahl CK, Kirton A, Pringsheim T, Croarkin PE, Zewdie E, Swansburg R, et al. Bilateral transcranial magnetic stimulation of the supplementary motor area in children with Tourette syndrome. Dev Med Child Neurol. (2021) 63:808–15. doi: 10.1111/dmcn.14828
19. Orth M, Kirby R, Richardson MP, Snijders AH, Rothwell JC, Trimble MR, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. (2005) 116:764–8. doi: 10.1016/j.clinph.2004.10.003
20. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
21. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
22. Cagle JN, Okun MS, Cernera S, Eisinger RS, Opri E, Bowers D, et al. Embedded human closed-loop deep brain stimulation for tourette syndrome. JAMA Neurol. (2022) 79:1064. doi: 10.1001/jamaneurol.2022.2741
23. Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, et al. A trial of scheduled deep brain stimulation for tourette syndrome. JAMA Neurol. (2013) 70:85. doi: 10.1001/jamaneurol.2013.580
24. Rossi PJ, Opri E, Shute JB, Molina R, Bowers D, Ward H, et al. Scheduled, intermittent stimulation of the thalamus reduces tics in Tourette syndrome. Parkinsonism Relat Disord. (2016) 29:35–41. doi: 10.1016/j.parkreldis.2016.05.033
25. Lin X, Lin F, Chen H, Weng Y, Wen J, Ye Q, et al. Comparison of efficacy of deep brain stimulation, repeat transcranial magnetic stimulation, and behavioral therapy in Tourette syndrome: A systematic review and Bayesian Network Meta-Analysis. Heliyon. (2022) 8:e10952. doi: 10.1016/j.heliyon.2022.e10952
26. Müller-Vahl KR, Szejko N, Saryyeva A, Schrader C, Krueger D, Horn A, et al. Randomized double-blind sham-controlled trial of thalamic versus GPi stimulation in patients with severe medically refractory Gilles de la Tourette syndrome. Brain Stimul. (2021) 14:662–75. doi: 10.1016/j.brs.2021.04.004
27. Cappon D, Beigi M, Kefalopoulou Z, Zrinzo L, Candelario J, Milabo C, et al. Globus pallidal deep brain stimulation for Tourette syndrome: Effects on cognitive function. Parkinsonism Relat Disord. (2019) 69:14–8. doi: 10.1016/j.parkreldis.2019.10.013
28. Schoenberg MR, Maddux BN, Riley DE, Whitney CM, Ogrocki PK, Gould D, et al. Five-months-postoperative neuropsychological outcome from a pilot prospective randomized clinical trial of thalamic deep brain stimulation for tourette syndrome. Neuromodulation: Technol at Neural Interface. (2015) 18:97–104. doi: 10.1111/ner.12233
29. Kefalopoulou Z, Zrinzo L, Jahanshahi M, Candelario J, Milabo C, Beigi M, et al. Bilateral globus pallidus stimulation for severe Tourette’s syndrome: a double-blind, randomised crossover trial. Lancet Neurol. (2015) 14:595–605. doi: 10.1016/S1474-4422(15)00008-3
30. Huys D, Bartsch C, Koester P, Lenartz D, Maarouf M, Daumann J, et al. Motor improvement and emotional stabilization in patients with tourette syndrome after deep brain stimulation of the ventral anterior and ventrolateral motor part of the thalamus. Biol Psychiatry. (2016) 79:392–401. doi: 10.1016/j.biopsych.2014.05.014
31. Cannon E, Silburn P, Coyne T, O’Maley K, Crawford JD, Sachdev PS. Deep brain stimulation of anteromedial globus pallidus interna for severe tourette’s syndrome. Am J Psychiatry. (2012) 169:860–6. doi: 10.1176/appi.ajp.2012.11101583
32. Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. (2011) 134:832–44. doi: 10.1093/brain/awq380
33. Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. (2007) 107:1004–14. doi: 10.3171/JNS-07/11/1004
34. Landeros-Weisenberger A, Mantovani A, Motlagh MG, de Alvarenga PG, Katsovich L, Leckman JF, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe tourette syndrome. Brain Stimul. (2015) 8:574–81. doi: 10.1016/j.brs.2014.11.015
35. Wu SW, Maloney T, Gilbert DL, Dixon SG, Horn PS, Huddleston DA, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. (2014) 7:212–8. doi: 10.1016/j.brs.2013.10.005
36. Le K, Liu L, Sun M, Hu L, Xiao N. Transcranial magnetic stimulation at 1Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. (2013) 20:257–62. doi: 10.1016/j.jocn.2012.01.049
37. Kwon HJ, Lim WS, Lim MH, Lee SJ, Hyun JK, Chae J-H, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. (2011) 492:1–4. doi: 10.1016/j.neulet.2011.01.007
Keywords: Tourette Syndrome, repetitive transcranial magnetic stimulation, deep brain stimulation, Yale Global Tic Severity Scale, obsessive-compulsive disorder, Yale-brown obsessive compulsive scale
Citation: Aloufi AK, Zahhar JA, Bader MW, Almutairi MB, Alaaldeen A, Hetta OE, Gammash AM, Almuntashiri S, Binrabaa IS, Alsaleh A and AlSalem M (2025) Tourette syndrome and brain stimulation therapy: a systematic review and meta-analysis of current evidence. Front. Psychiatry 16:1478503. doi: 10.3389/fpsyt.2025.1478503
Received: 09 August 2024; Accepted: 20 January 2025;
Published: 18 February 2025.
Edited by:
Qiang Zhang, University of Iowa Hospitals and Clinics, United StatesReviewed by:
Shu Wang, Capital Medical University, ChinaCopyright © 2025 Aloufi, Zahhar, Bader, Almutairi, Alaaldeen, Hetta, Gammash, Almuntashiri, Binrabaa, Alsaleh and AlSalem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moayyad AlSalem, RHIubW9heXlhZGFsc2FsZW1AZ21haWwuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.