
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 17 April 2025
Sec. Sleep Disorders
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1434889
This article is part of the Research TopicInteractions Between Diet, Sleep and Musculoskeletal Health: Beyond a Disease-Specific PerspectiveView all articles
Background: Gallstones are a prevalent digestive system disorder with significant health implications. Recent research suggests that sleep disorders, such as insomnia and obstructive sleep apnea hypopnea syndrome, may influence the development of gallstones through various metabolic pathways. Depression, often accompanying sleep disorders, may play a mediating role in this relationship. This study uses data from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) to explore the potential mediating role of depression in the association between sleep disorders and gallstones.
Methods: We analyzed data from 7,868 adults aged 20 and older from NHANES 2017–2020. Gallstones were defined based on self-reported medical diagnoses. Sleep disorders were assessed through self-reported sleep difficulties, and depressive symptoms were measured using the Patient Health Questionnaire-9 (PHQ-9) scale. Logistic regression models evaluated direct associations between sleep disorders, depressive symptoms, and gallstones. Causal mediation analysis further examined the mediating role of depressive symptoms. Finally, subgroup analyses were performed by age, sex, and obesity status.
Results: Both sleep disorders (OR = 2.00; 95% CI, 1.73-2.32; P<0.001) and depressive symptoms (OR = 2.09; 95% CI, 1.70-2.56; P<0.001) were significantly associated with gallstones, with results remaining significant after adjusting for confounders. A significant association was also observed between sleep disorders and depressive symptoms (OR = 5.53; 95% CI, 4.71-6.50; P<0.001). Mediation analysis indicated that depressive symptoms partially mediate the relationship between sleep disorders and gallstones, with an average causal mediation effect (ACME) of 0.00720 (95% CI, 0.00299-0.01220; P<0.001) and an average direct effect (ADE) of 0.0305 (95% CI, 0.0129-0.0488; P<0.001). Depression mediates 18.89% (95% CI, 0.0704-0.4096; P<0.001) of the association between sleep disorders and gallstones. Subgroup analyses showed significant mediation by depressive symptoms in individuals aged 40-59, males, and both obese and non-obese groups (all P<0.05), although no significant mediation was found in females (P>0.05).
Conclusion: This study demonstrates a significant association between sleep disorders and gallstones, with depressive symptoms playing a partial mediating role. Improving depressive symptoms may help reduce the risk of gallstones associated with sleep disorders.
Gallstone disease is a common digestive system disorder, affecting approximately 10% to 20% of adults worldwide, with incidence rates varying by region and population (1). Higher prevalence is observed in Western countries, particularly among women, individuals with obesity, and the elderly (2, 3). The clinical presentation of gallstone ranges from asymptomatic “silent” stones to severe complications such as cholecystitis, pancreatitis, and even an increased risk of cholangiocarcinoma (4, 5). The formation of gallstones is known to be influenced by multiple factors, including age, sex, obesity, metabolic syndrome, dietary habits, genetic predisposition, pregnancy, diabetes, and biliary infections (1, 6, 7). Recently, however, research has begun to explore potential risk factors beyond the traditional ones, particularly those related to metabolism and mental health, such as sleep disorders (8, 9).
Sleep disorders, especially insomnia and obstructive sleep apnea hypopnea syndrome (OSAHS), represent another widespread public health issue affecting the quality of life of millions of people (10). Numerous studies have indicated that sleep disorders are closely linked to various metabolic diseases (e.g., obesity, diabetes), which are known risk factors for gallstone formation (11, 12). Therefore, sleep disorders may influence gallstone formation through multiple metabolic pathways, including insulin resistance, dysregulated lipid metabolism, and weight gain (13–15).
Furthermore, there is a bidirectional relationship between depression and sleep disorders: sleep disorders are both a risk factor for depression and a typical symptom of it (16–18). The relationship between depression and metabolic diseases such as obesity and metabolic syndrome is well-documented, and these metabolic conditions are closely related to gallstone formation (19–21). Thus, depressive symptoms might mediate the relationship between sleep disorders and gallstones by exacerbating metabolic abnormalities and increasing chronic inflammation. However, research on the mediating role of depressive symptoms in the relationship between sleep disorders and gallstones remains limited, with no definitive reports to date.
Therefore, this study aims to use data from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) to systematically explore the potential association between sleep disorders and gallstones, with a focus on evaluating the potential mediating role of depressive symptoms. This study addresses this gap by examining whether depressive symptoms mediate the association between sleep disorders and gallstone disease, offering novel insights into its multifactorial etiology. The specific research questions include: (1) Is there a significant association between sleep disorders and gallstones? (2) Do depressive symptoms mediate this association?
We utilized data from the 2017-2020 NHANES to investigate the relationship between sleep disorders and gallstone risk, and to assess the mediating role of depressive symptoms. NHANES data is maintained by the Centers for Disease Control and Prevention (CDC) and is updated biennially. This study combined data from 2017-2020, and due to the lack of national representativeness for the 2019-2020 data, it was merged with the 2017-2018 cycle data for analysis. More information about the NHANES program can be found on the CDC website.
Participants were included based on the following eligibility criteria: 1) age ≥ 20 years; 2) complete data on sleep disorders, depressive symptoms, and gallstones (18). After rigorous screening, a final cohort of 7,868 eligible participants was identified. (Figure 1)
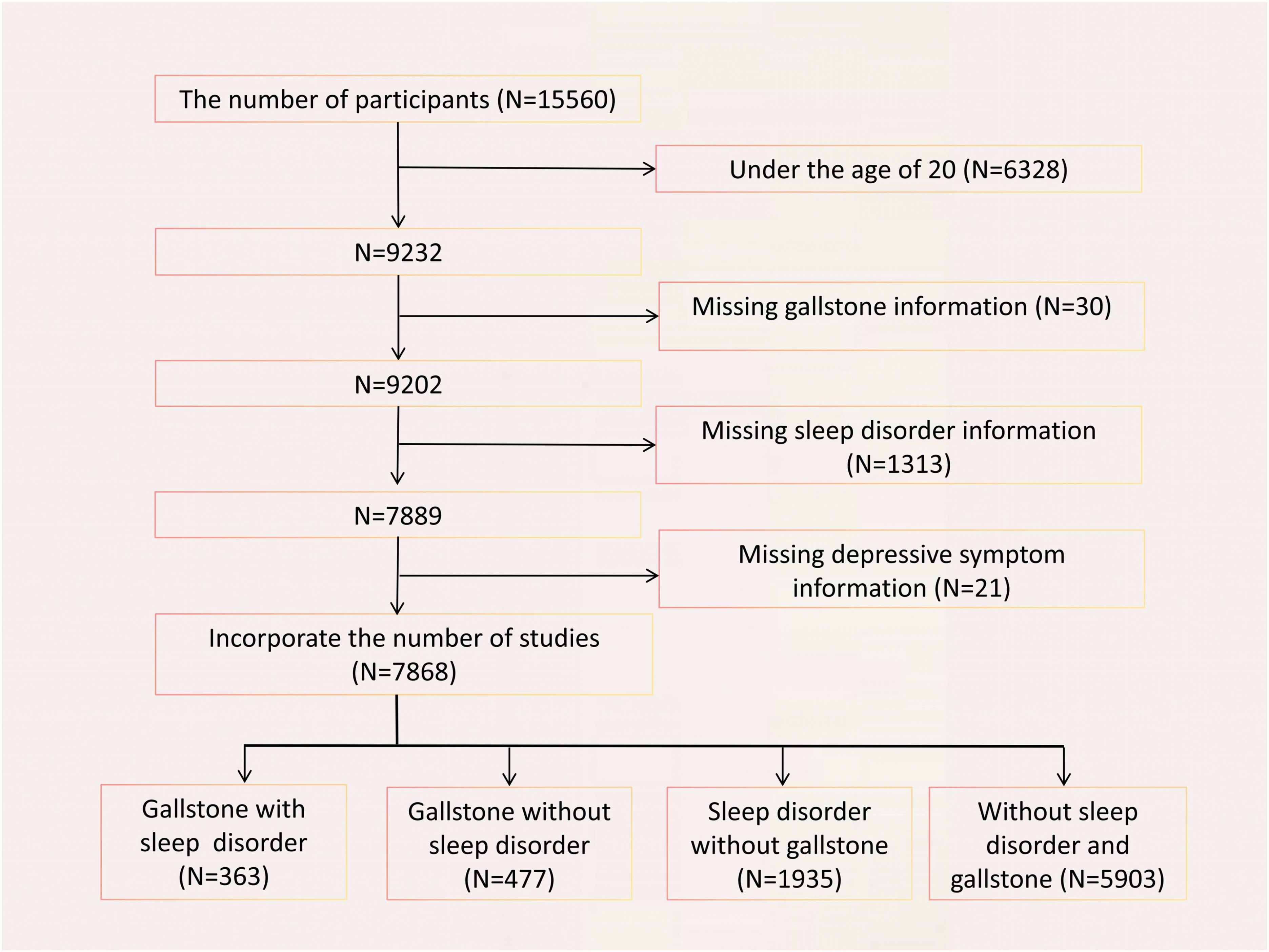
Figure 1. Flowchart of participant selection. A total of 15560 participants were included, and after exclusions, the final number of participan was 7868.
Information on gallstones was obtained from self-reported responses to the question: “MCQ550—Has a doctor ever said you have gallstones?” A response indicating a diagnosis was considered as having gallstones, while a negative response was considered as not having gallstones.
Data on sleep disorders were based on self-reported responses to the question: “SLQ050 - Ever told doctor had trouble sleeping?” Respondents who answered “Yes” were classified as having sleep disorders, while those who answered “No” were classified as not having sleep problems.
Depressive symptoms were assessed using the PHQ-9 scale, which is widely recognized as a reliable and accurate screening tool for depression (22). The PHQ-9 total score ranges from 0 to 27, with higher scores indicating more severe depression. A PHQ-9 score of ≥10 was considered indicative of depressive symptoms, while a score of <10 was considered indicative of no depressive symptoms. Detailed information about the PHQ-9 scale can be found in the NHANES database.
Covariates included in this study were: age (categorized as 20-39 years, 40-59 years, ≥60 years), sex, race, marital status, education level, BMI (with obesity defined as BMI ≥30), smoking status (never, former, current), alcohol consumption (based on 24-hour dietary recall), physical activity (based on the PAQ questionnaire), medication use, and self-reported hypertension, hyperlipidemia, and diabetes status. Specific definitions of some covariates are detailed in Table 1. These variables were selected based on a review of existing literature and their potential impact on the relationship between sleep disorders and gallstones.
First, descriptive statistics of participants’ baseline characteristics were performed. Continuous variables were described using weighted means and standard errors, while categorical variables were presented with observed frequencies and weighted percentages. For normally distributed variables (based on Shapiro-Wilk test results), one-way ANOVA was used for group comparisons; for non-normally distributed variables, Kruskal-Wallis H test was employed. Comparisons of categorical variables were conducted using chi-square tests or Fisher’s exact tests when applicable.
Next, multivariable logistic regression analysis was used to assess the relationship between depressive symptoms, sleep disorders, and gallstones, adjusting for confounders such as age, sex, race, BMI, smoking status, alcohol consumption, physical activity, hypertension, and diabetes. Sensitivity analysis was conducted to evaluate the impact of sleep-related medication on the outcomes.
To further explore the mediating role of depressive symptoms between sleep disorders and gallstone risk, causal mediation analysis was performed. The causal mediation analysis (using Baron and Kenny’s method combined with bootstrap) decomposed the total effect into direct and indirect effects via the mediator. We employed 1,000 bootstrap resamples to enhance the robustness of estimates and reported the average causal mediation effect (ACME), average direct effect (ADE), and total effect. Additionally, the size of the indirect path effect, proportion of mediation effect, and statistical significance (p-value) were presented.
Furthermore, we conducted an item-level analysis of the PHQ-9 scale to examine the causal mediation effects of individual depressive symptoms (scored as 0 or ≥1) on the relationship between sleep disorders and gallstones. Each item score of 0 indicated no symptom, and ≥1 indicated the presence of symptoms, to evaluate the mediation effect of each symptom.
Finally, subgroup analyses were performed to assess the impact of different age groups (20-39 years, 40-59 years, ≥60 years), sex, and obesity status (BMI ≥30) on gallstone risk, and further mediation analysis was conducted for statistically significant subgroups to evaluate whether depressive symptoms mediate the relationship between sleep disorders and gallstone risk within each subgroup.
Baseline demographic characteristics are summarized in Table 2. The study population (n = 7,868) was divided into four groups:”Gallstones with Sleep Disorders,”“Gallstones without Sleep Disorders,”“Sleep Disorders without Gallstones,” and “Neither Gallstones nor Sleep Disorders.” The “Gallstones with Sleep Disorders” group had a mean age of 58.06 years, with 11.3% in the 20-39 age group, 38.0% in the 40-59 age group, and 74.7% female. The mean BMI for this group was 34.65 kg/m².
In comparisons of depressive and sleep disorder groupings, significant differences in PHQ-9 scores were observed among the “Depression with Sleep Disorders,” “Sleep Disorders without Depression,” “Depression without Sleep Disorders,” and “Neither Depression nor Sleep Disorders” groups (mean scores of 6.40 ± 5.32, 3.12 ± 4.28, 5.43 ± 5.20, and 2.27 ± 3.28, respectively; p < 0.001). Participants with gallstones and/or sleep disorders exhibited higher PHQ-9 scores and greater prevalence of depressive symptoms compared to those without either condition.
Table 3 presents the relationships between depressive symptoms, sleep disorders, and gallstones, as well as the association between sleep disorders and depressive symptoms. When gallstones were considered as the outcome variable, the analysis revealed that sleep disorders (OR = 2.00; 95% CI, 1.73-2.32; P<0.001) and depressive symptoms (OR = 2.09; 95% CI, 1.70-2.56; P<0.001) were significantly associated with a higher prevalence of gallstones. After adjusting for age, gender, race/ethnicity, education level, marital status, smoking, alcohol consumption, physical activity, BMI, diabetes status, dyslipidemia status, hypertension status, and use of sleep-related medications, sleep disorders (OR = 1.49; 95% CI, 1.26-1.76; P<0.001) and depressive symptoms (OR = 1.77; 95% CI, 1.39-2.24; P<0.001) remained significantly associated with a higher prevalence of gallstones.
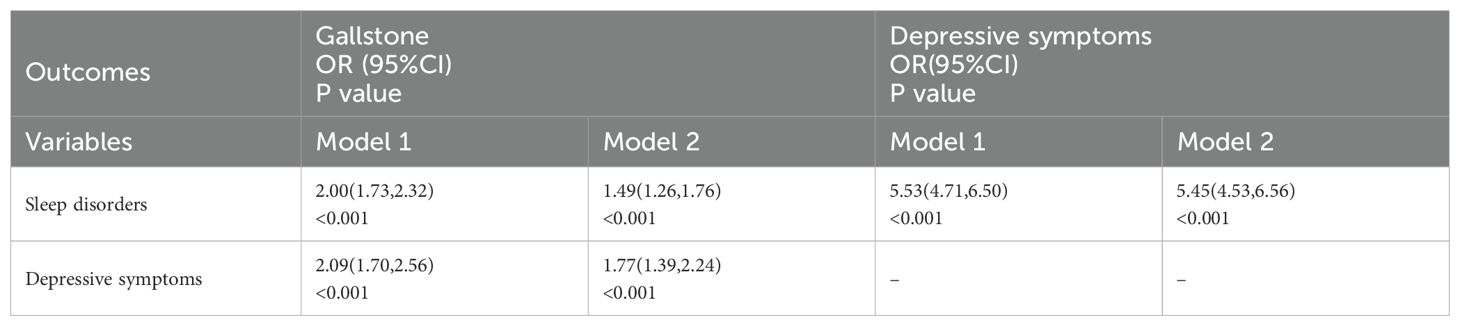
Table 3. Association between depressive symptoms, sleep disorders, and gallstone among adults over 20 years of age in 2017–2020 NHANES.
When depressive symptoms were considered as the outcome variable, sleep disorders (OR = 5.53; 95% CI, 4.71-6.50; P<0.001) were significantly associated with a higher prevalence of depressive symptoms. Even after adjusting for confounding factors, sleep disorders (OR = 5.45; 95% CI, 4.53-6.56; P<0.001) remained significantly associated with a higher prevalence of depressive symptoms.
Additionally, we further investigated the relationships between sleep disorders, depressive symptoms, and gallstones among participants not using sleep-related medications (see Supplementary Table 1). In this subset of the population, sleep disorders (OR = 1.94; 95% CI, 1.67-2.25; P<0.001) and depressive symptoms (OR = 2.09; 95% CI, 1.68-2.54; P<0.001) were significantly associated with a higher prevalence of gallstones. Sleep disorders (OR = 5.37; 95% CI, 4.56-6.33; P<0.001) were also significantly associated with a higher prevalence of depressive symptoms. These relationships remained significant after adjusting for confounding factors.
Table 4 and Figure 2 present the detailed results of the causal mediation analysis. The results indicate that both direct and indirect effects play significant roles in increasing the likelihood of gallstones resulting from sleep disorders. The total effect was 0.03767 (95% CI, 0.0212-0.0533; P < 0.001), with the average causal mediation effect (ACME) being 0.00720 (95% CI, 0.00299-0.01220; P < 0.001) and the average direct effect (ADE) being 0.0305 (95% CI, 0.0129-0.0488; P < 0.001). The proportion of the effect mediated was 0.1889 (95% CI, 0.0704-0.4096; P < 0.001). When confounding factors were not adjusted, the total effect was 0.0725 (95% CI, 0.0569-0.0902; P < 0.001), with the proportion of the mediated effect being 0.13348 (95% CI, 0.0715-0.2228; P < 0.001).
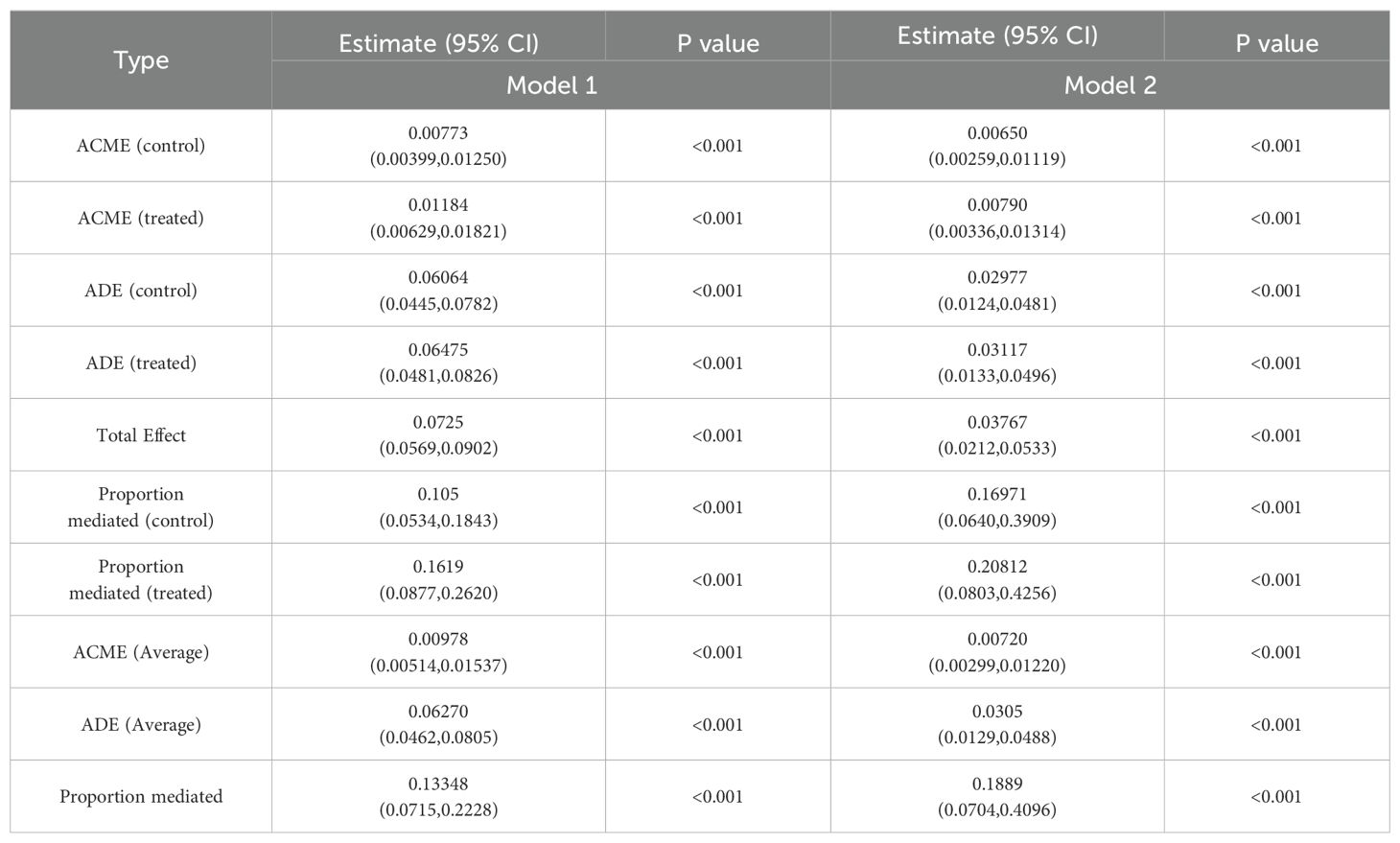
Table 4. Causal mediation analysis of depressive symptoms in the association between sleep disorders and gallstones among adults over 20 years of age in 2017–2020 NHANES.
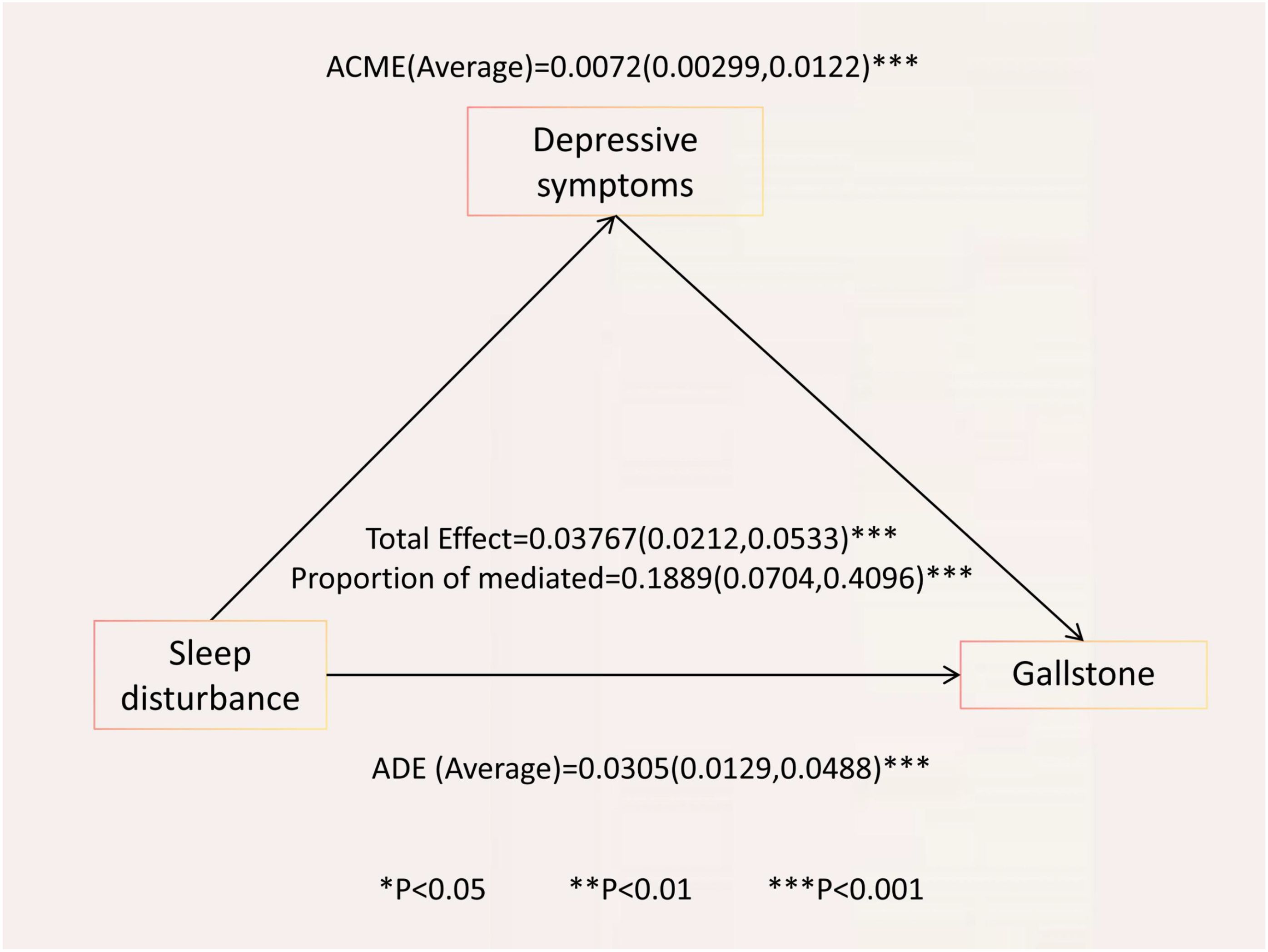
Figure 2. Causal mediation analysis of depressive symptoms in the relationship between sleep disorders and gallstone among adults over 20 years of age in 2017–2020 NHANES. ACME, average causal mediation effect; ADE, average direct effect; Proportion mediated = ACME (Average)/total effect.
We further analyzed the relationship between PHQ-9 item scores, sleep disorders, and gallstones. The results indicated that sleep disorders were significantly associated with higher odds of experiencing depressive symptoms across various PHQ-9 items (OR range: 2.28 to 4.69, all P < 0.05), and that higher PHQ-9 item scores were significantly associated with an increased likelihood of gallstones (OR range: 1.23 to 1.60, all P < 0.05) (Table 5). The causal mediation analysis for sleep disorders and depressive symptoms with respect to gallstones, based on individual PHQ-9 items, is presented in Table 6. Causal mediation effects were observed for all items except for items 1, 2, and 9. Among these, item 3 had the highest average causal mediation effect (ACME) of 0.0091, with a proportion of mediated effect of 0.2424 (95% CI, 0.0874-0.4950; P < 0.001). Items 4 and 7 also showed substantial mediation effects, with ACME values of 0.0055 and 0.00513, and proportions of mediated effect of 0.1458 (95% CI, 0.0429-0.3257; P = 0.006) and 0.13665 (95% CI, 0.0473-0.2880; P = 0.002), respectively.
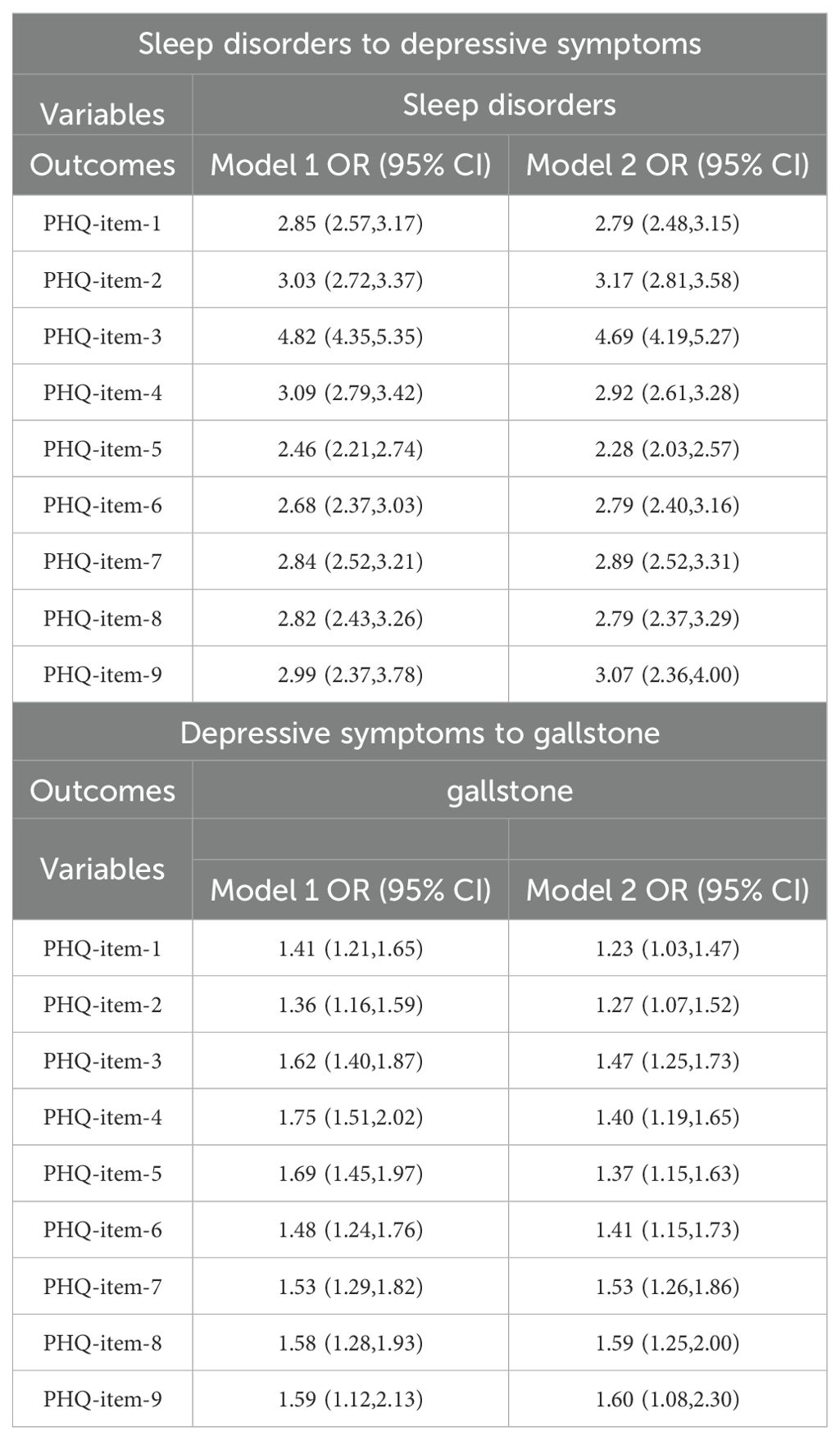
Table 5. Association between each item of the PHQ score and sleep disorders and gallstone among adults over 20 years of age in 2017–2020 NHANES.
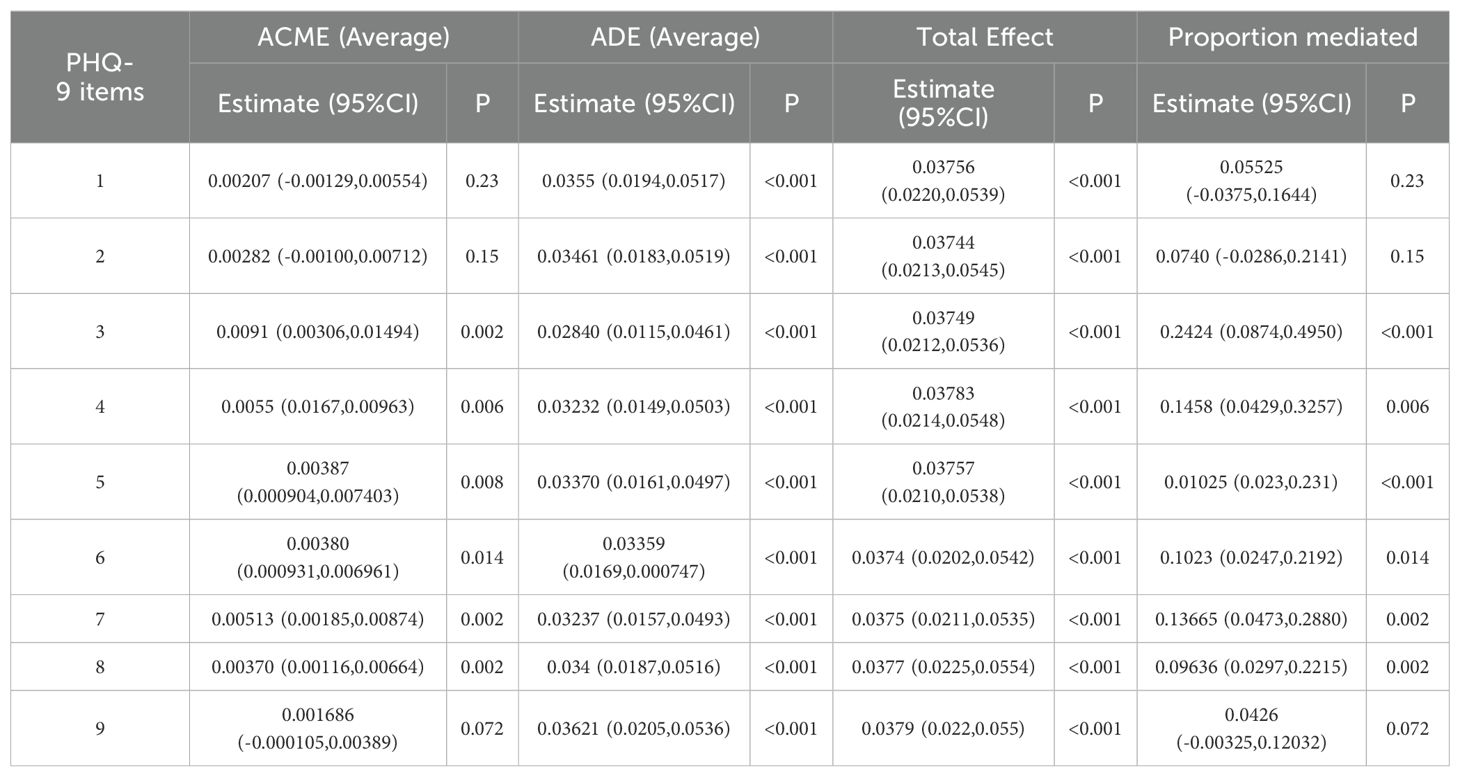
Table 6. Causal mediation analysis of each PHQ item between sleep disorders and gallstone among adults over 20 years of age in 2017–2020 NHANES.
We conducted subgroup analyses based on different age groups, gender, and obesity status to explore the effects of these factors on gallstone risk.
In the age-based subgroups, before adjusting for confounding factors, sleep disorders and depressive symptoms were associated with a higher incidence of gallstones across all age groups, and sleep disorders were also associated with a higher incidence of depressive symptoms (see Supplementary Tables 2-4). However, after adjusting for confounding factors, significant associations between sleep disorders and depressive symptoms with gallstone incidence remained only in the 40-59 years age group, and the association between sleep disorders and depressive symptoms persisted. Causal mediation analysis for the 40-59 years age group showed a total effect of 0.08499 (95% CI, 0.0828-0.0873; P < 0.001), an average causal mediation effect (ACME) of 0.00894 (95% CI, 0.00860-0.00931; P < 0.001), and a proportion of mediated effect of 0.1052 (95% CI, 0.101-0.109; P < 0.001) (see Supplementary Tables 5).
In the gender-based subgroups, both sleep disorders and depressive symptoms were associated with a higher incidence of gallstones, and sleep disorders were also related to a higher incidence of depressive symptoms (see Supplementary Table 6-7). Causal mediation analysis results showed that, among men, the total effect was 0.02908 (95% CI, 0.00825-0.05115; P = 0.002), with an ACME of 0.00792 (95% CI, 0.00288-0.01430; P < 0.001) and a proportion of mediated effect of 0.2692 (95% CI, 0.0914-0.9566; P < 0.001) (see Supplementary Table 8). Among women, the total effect was 0.04849 (95% CI, 0.0223-0.0742; P ≤ 0.001), with an ACME of 0.00589 (95% CI, -0.000368-0.01287; P = 0.068) and a proportion of mediated effect of 0.1191 (95% CI, -0.00877-0.35206; P = 0.068) (see Supplementary Table 9).
In the subgroups based on obesity status, both sleep disorders and depressive symptoms were associated with a higher incidence of gallstones, and sleep disorders were also related to a higher incidence of depressive symptoms (see Supplementary Tables 10, 11). Causal mediation analysis results indicated that, among obese individuals, the total effect was 0.04814 (95% CI, 0.0232-0.0765; P ≤ 0.001), with an ACME of 0.00785 (95% CI, 0.000983-0.01636; P = 0.020) and a proportion of mediated effect of 0.1610 (95% CI, 0.018-0.443; P = 0.020) (see Supplementary Table 12). Among non-obese individuals, the total effect was 0.03623 (95% CI, 0.0170-0.0558; P ≤ 0.001), with an ACME of 0.00683 (95% CI, 0.00202-0.01262; P = 0.004) and a proportion of mediated effect of 0.1820 (95% CI, 0.0527-0.4548; P = 0.004) (see Supplementary Table 13).
This study, based on the 2017-2020 National Health and Nutrition Examination Survey data, explored the relationship between sleep disorders, depressive symptoms, and gallstones. Our findings indicate a significant positive correlation between sleep disorders and the incidence of gallstones. After adjusting for potential confounders (such as age, sex, BMI, alcohol, and physical activity), the risk of gallstones significantly increased among individuals with sleep disorders (OR = 1.49; 95% CI, 1.26-1.76, p < 0.05). Moreover, depressive symptoms partially mediated the association between sleep disorders and gallstones, with approximately 18.89% of the association being mediated by depressive symptoms.
We observed the mediating effect of depressive symptoms in various subgroups, including those aged 40-59, males, and both obese and non-obese groups. However, this mediating effect was not significant among females, which might be due to the potential regulatory role of estrogen in cholesterol metabolism and bile composition.
Sleep disorders can be categorized into several types, such as insomnia disorders, sleep-related breathing disorders, circadian rhythm sleep-wake disorders, hypersomnolence disorders, parasomnias, sleep-related movement disorders, and isolated sleep symptoms (26). However, our study, based on the NHANES sleep disorder questionnaire, could not differentiate between these specific types of sleep disorders, which is a limitation. Despite this, the questionnaire provided a preliminary assessment of generalized sleep disorders. Our results confirm that sleep disorders are an independent risk factor for gallstones, with a significant increase in risk among individuals with sleep disorders (OR = 1.49; 95% CI, 1.26-1.76, p < 0.05). Existing preprint studies indicate a significant association between sleep disorders and the risk of gallstones. These studies show that participants with sleep difficulties were found to have a significantly higher risk of developing gallstones, with adjusted odds ratios ranging from 1.49 to 1.51 (27, 28). However, their study did not explore the potential mediating role of depressive symptoms in the relationship between sleep disorders and gallstones.
Sleep disorders may accelerate gallstone formation through metabolic disturbances, neuroendocrine dysregulation, inflammatory responses, and psychosocial and lifestyle factors. Specifically, sleep disorders, particularly OSAHS, are closely associated with metabolic disturbances, including insulin resistance and metabolic syndrome, which are significant risk factors for gallstones (29, 30). Sleep disorders reduce insulin sensitivity, perturbing bile composition and promoting cholesterol supersaturation in bile, which are key steps in gallstone pathogenesis (2). Additionally, sleep disorders may activate the hypothalamic-pituitary-adrenal (HPA) axis, increasing cortisol secretion and affecting bile metabolism and cholesterol concentration (31). Excess cholesterol secretion is a major cause of cholesterol stone formation, and high cortisol levels may also impact gallbladder contraction, leading to bile stasis and an increased risk of gallstone formation (32). Furthermore, sleep disorders may exacerbate chronic inflammation in the gallbladder, with elevated inflammatory markers such as interleukin-6 (IL-6) and C-reactive protein (CRP) being associated with increased gallstone risk (33, 34). Sleep disorders are often linked with poor lifestyle choices and psychological stress, which may further contribute to gallstone formation by altering dietary habits and increasing smoking and alcohol consumption.
Therefore, we propose a hypothesis: Social psychological factors may act as mediators in the relationship between sleep disorders and gallstones, indirectly affecting the occurrence of gallstones by influencing sleep disorders. Our study further validates that depressive symptoms may play a mediating role between sleep disorders and gallstones. After adjusting for confounders, depressive symptoms significantly increased the risk of gallstones (OR = 1.77; 95% CI, 1.39-2.24, p < 0.05) and enhanced the impact of sleep disorders on gallstone risk. This finding is consistent with previous research results (35–37). Mediation analysis indicates that 18.89% of the association between sleep disorders and gallstones seems to be mediated by depressive symptoms. Subgroup analyses reveal differences in the mediating effect of various PHQ-9 items on sleep disorders and gallstones. Among different age groups, depressive symptoms mediate the relationship between sleep disorders and gallstones primarily in the 40-59 age group, while no significant associations were observed in the 20-39 and ≥60 age groups. Similarly, mediation effects are observed in males, obese, and non-obese groups. These results suggest that improving depressive symptoms in specific populations may reduce the risk of gallstones associated with sleep disorders. The lack of significance in females might be attributed to estrogen’s role in regulating cholesterol metabolism and bile composition, which may reduce the mediating effect of depressive symptoms.
This study is the first to discuss the impact of sleep disorders on gallstones and to reveal the potential role of depressive symptoms in gallstone formation. Clinical practice should incorporate the identification and management of sleep disorders and depressive symptoms as crucial components of gallstones prevention. Early intervention for high-risk groups, particularly those over 40 years old and males, may reduce gallstone incidence. Additionally, our results suggest that clinical attention should extend beyond traditional gallstone risk factors (such as obesity and diet) to include patient sleep and mental health.
Despite providing robust evidence on the association between sleep disorders, depressive symptoms, and gallstones, this study has limitations. Firstly, the cross-sectional nature of the study limits causal inference. Future research should employ longitudinal designs to better determine the temporal sequence and causal pathways between sleep disorders, depressive symptoms, and gallstones. Secondly, self-reported data on sleep disorders and depressive symptoms may be subject to recall and subjective biases. Future studies could use more objective assessment tools, such as polysomnography (PSG) and biomarkers (e.g., cortisol and bile components), to further validate these results. Although we controlled for various potential confounders (e.g., BMI, diet, physical activity), other unconsidered confounders may impact the results, such as genetic susceptibility, long-term medication use (e.g., ursodeoxycholic acid and statins), and other chronic diseases (e.g., non-alcoholic fatty liver disease (NAFLD), chronic hemolytic diseases). Future research should further account for these factors. Finally, sleep disorder data from the questionnaire did not allow for specific classification of sleep disorder types. Future research should consider categorizing different types of sleep disorders for more precise results.
In summary, depressive symptoms and sleep disorders are independently associated with gallstones, and sleep disorders are also associated with a higher risk of depressive symptoms. Depressive symptoms may mediate the relationship between sleep disorders and gallstones. Mediating effects of depressive symptoms on sleep disorders and gallstones were observed in the 40-59 age group, males, and both obese and non-obese populations. Early prevention, detection, and treatment are needed for specific populations. Improving depressive symptoms may reduce the likelihood of gallstones caused by sleep disorders.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Human subjects involved in the studies were granted ethical approval by the Ethical Review Board of the National Center for Health Statistics. The studies were conducted in compliance with local laws and institutional guidelines. Informed consent was obtained from the legal guardians or next of kin of the study participants. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YH: Writing – original draft, Writing – review & editing, Conceptualization, Methodology, Software. RL: Writing – review & editing, Conceptualization, Data curation, Methodology. ZX: Conceptualization, Data curation, Methodology, Software, Validation, Visualization, Writing – review & editing. WC: Conceptualization, Data curation, Methodology, Software, Validation, Visualization, Writing – review & editing. ZL: Conceptualization, Data curation, Methodology, Software, Validation, Visualization, Writing – review & editing. WJ: Conceptualization, Data curation, Methodology, Software, Validation, Visualization, Writing – review & editing. YM: Conceptualization, Data curation, Methodology, Software, Validation, Visualization, Writing – review & editing, Funding acquisition. JH: Conceptualization, Data curation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors wish to extend their gratitude to the participants and researchers of the National Health and Nutrition Examination Survey for their contributions to the study, and they also express their appreciation to NHANES for providing the essential, open-source data that were utilized in this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1434889/full#supplementary-material
1. Sun H, Warren J, Yip J, Ji Y, Hao S, Han W, Ding Y, et al. Factors influencing gallstone formation: A review of the literature. Biomolecules. (2022) 12:550. doi: 10.3390/biom12040550
2. Cortés VA, Barrera F, Nervi F. Pathophysiological connections between gallstone disease, insulin resistance, and obesity. Obes reviews: an Off J Int Assoc Study Obes. (2020) 21:e12983. doi: 10.1111/obr.12983
3. Song Y, Ma Y, Xie FC, Jin C, Yang X-B, Yang X, et al. Age, gender, geographic and clinical differences for gallstones in China: a nationwide study. Ann Trans Med. (2022) 10:735. doi: 10.21037/atm-21-6186
4. Gallaher JR, Charles A. Acute cholecystitis: A review. Jama. (2022) 327:965–75. doi: 10.1001/jama.2022.2350
5. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet (London England). (2015) 386:85–96. doi: 10.1016/S0140-6736(14)60649-8
6. Schwulst SJ, Son M. Management of gallstone disease during pregnancy. JAMA Surg. (2020) 155:1162. doi: 10.1001/jamasurg.2020.3683
8. Chen CH, Lin CL, Hsu CY, Kao C-H. Risk of gallstones in patients with obstructive sleep apnea: a nationwide observational cohort study. Sleep breathing = Schlaf Atmung. (2019) 23:355–62. doi: 10.1007/s11325-018-1696-5
9. Yan W, Zhou J, Jiang M, Kong Y, Qin H, Qi Y, et al. Obstructive sleep apnea and 19 gastrointestinal diseases: a Mendelian randomization study. Front Psychiatry. (2024) 15:1256116. doi: 10.3389/fpsyt.2024.1256116
10. Humer E, Pieh C, Brandmayr G. Metabolomics in sleep, insomnia and sleep apnea. Int J Mol Sci. (2020) 21:7244. doi: 10.3390/ijms21197244
11. Chaput JP, Mchill AW, Cox RC, Broussard JL, Dutil C, da Costa BGG, et al. The role of insufficient sleep and circadian misalignment in obesity. Nat Rev Endocrinol. (2023) 19:82–97. doi: 10.1038/s41574-022-00747-7
12. Kurnool S, Mccowen KC, Bernstein NA, Malhotra A. Sleep apnea, obesity, and diabetes - an intertwined trio. Curr Diabetes Rep. (2023) 23:165–71. doi: 10.1007/s11892-023-01510-6
13. Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. (2018) 84:56–66. doi: 10.1016/j.metabol.2018.02.010
14. He C, Shen W, Chen C, Wang Q-H, Lu Q, Shao W, et al. Circadian rhythm disruption influenced hepatic lipid metabolism, gut microbiota and promoted cholesterol gallstone formation in mice. Front Endocrinol (Lausanne). (2021) 12:723918. doi: 10.3389/fendo.2021.723918
15. Muscogiuri G, Barrea L, Annunziata G, Di Somma C, Laudisio D, Colao A, et al. Obesity and sleep disturbance: the chicken or the egg? Crit Rev Food Sci Nutr. (2019) 59:2158–65. doi: 10.1080/10408398.2018.1506979
16. Crouse JJ, Carpenter JS, Song YJC, Hockey SJ, Naismith SL, Grunstein RR, et al. Circadian rhythm sleep-wake disturbances and depression in young people: implications for prevention and early intervention. Lancet Psychiatry. (2021) 8:813–23. doi: 10.1016/S2215-0366(21)00034-1
17. Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med Rev. (2014) 18:521–9. doi: 10.1016/j.smrv.2014.03.006
18. Zhou W, Sun L, Zeng L, Wan L. Mediation of the association between sleep disorders and cardiovascular disease by depressive symptoms: An analysis of the National health and Nutrition Examination Survey (NHANES) 2017-2020. Prev Med Rep. (2023) 33:102183. doi: 10.1016/j.pmedr.2023.102183
19. Milaneschi Y, Simmons WK, Van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
20. Pistis G, Milaneschi Y, Vandeleur CL, Lasserre AM, Penninx BWJH, Lamers F, et al. Obesity and atypical depression symptoms: findings from Mendelian randomization in two European cohorts. Trans Psychiatry. (2021) 11:96. doi: 10.1038/s41398-021-01236-7
21. Zhang M, Chen J, Yin Z, Wang L, Peng L. The association between depression and metabolic syndrome and its components: a bidirectional two-sample Mendelian randomization study. Trans Psychiatry. (2021) 11:633. doi: 10.1038/s41398-021-01759-z
22. Costantini L, Pasquarella C, Odone A, Colucci ME, Costanza A, Serafini G, et al. Screening for depression in primary care with Patient Health Questionnaire-9 (PHQ-9): A systematic review. J Affect Disord. (2021) 279:473–83. doi: 10.1016/j.jad.2020.09.131
23. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. (2020) 30:160–4. doi: 10.1016/j.tcm.2019.05.003
24. American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:: S15–s33. doi: 10.2337/dc21-S002
25. Wierzbicki AS, Kim EJ, Esan O, Ramachandran R. Hypertriglyceridaemia: an update. J Clin Pathol. (2022) 75:798–806. doi: 10.1136/jclinpath-2021-207719
26. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. (2014) 146:1387–94. doi: 10.1378/chest.14-0970
27. Liu X, Huang Y, Huang Y, Lin CC, Xu B, Zeng YL, et al. Association of trouble sleeping with increased risk of gallstone disease in U.S. In: Adults: A cross-sectional study of NHANES 2017-2020, 25 june 2024, PREPRINT. doi: 10.21203/rs.3.rs-4516566/v1]2024
28. Xu C, Song Z, Bian X-H, Li CC. Association between sleep and gallstone disease in US adult: A population- based study, 27 August 2024, PREPRINT (Version 1) available at Research Square. doi: 10.21203/rs.3.rs-4263605/v1]2024.
29. Koren D, Taveras EM. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism. (2018) 84:67–75. doi: 10.1016/j.metabol.2018.04.001
30. Wang X, Zhao C, Feng H, Li GH, He L, Yang L, et al. Associations of insomnia with insulin resistance traits: A cross-sectional and mendelian randomization study. J Clin Endocrinol Metab. (2023) 108:e574–e82. doi: 10.1210/clinem/dgad089
31. Liyanarachchi K, Ross R, Debono M. Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Pract Res Clin Endocrinol Metab. (2017) 31:459–73. doi: 10.1016/j.beem.2017.10.011
32. Chen Y, Kong J, Wu S. Cholesterol gallstone disease: focusing on the role of gallbladder. Lab investigation; J Tech Methods Pathol. (2015) 95:124–31. doi: 10.1038/labinvest.2014.140
33. Yin J, Gong R, Zhang M, Ding L, Shen T, Cai YY, et al. Associations between sleep disturbance, inflammatory markers and depressive symptoms: Mediation analyses in a large NHANES community sample. Prog Neuropsychopharmacol Biol Psychiatry. (2023) 126:110786. doi: 10.1016/j.pnpbp.2023.110786
34. Wright KP Jr., Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain behavior Immun. (2015) 47:24–34. doi: 10.1016/j.bbi.2015.01.004
35. Wang B, Xiong Y, Li R, Zhang S. Depression increases the risk of gallstone: A cross-sectional study and Mendelian randomization analysis. J Affect Disord. (2024) 362:606–14. doi: 10.1016/j.jad.2024.07.119
36. Li J, Zhang J, Kong B, Chen L, Yuan J, He M, et al. Abdominal obesity mediates the causal relationship between depression and the risk of gallstone disease: retrospective cohort study and Mendelian randomization analyses. J psychosomatic Res. (2023) 174:111474. doi: 10.1016/j.jpsychores.2023.111474
Keywords: gallstones, sleep disorders, depression, national health and nutrition examination survey, causal mediation analysis
Citation: Hou Y, Li R, Xu Z, Chen W, Li Z, Jiang W, Meng Y and Han J (2025) Depressive symptoms mediate the association between sleep disorders and gallstone disease: a causal mediation analysis of NHANES 2017–2020. Front. Psychiatry 16:1434889. doi: 10.3389/fpsyt.2025.1434889
Received: 11 September 2024; Accepted: 26 March 2025;
Published: 17 April 2025.
Edited by:
Xiwei Fan, Central South University, ChinaReviewed by:
Ivan Šoša, University of Rijeka, CroatiaCopyright © 2025 Hou, Li, Xu, Chen, Li, Jiang, Meng and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Meng, bXkxNzZAMTI2LmNvbQ==; Jianli Han, aGpsMTM4MDM0NTY1NDVAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.