- 1Country Department of Psychiatry, Renmin Hospital of Wuhan University, Wuhan, China
- 2Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, China
Objective: This study aimed to investigate the independent or synergistic effects of evening chronotype and poor sleep quality on cognitive impairment in patients with major depressive disorder (MDD).
Methods: A cross-sectional study was conducted on 249 individuals diagnosed with MDD, recruited from the Mental Health Center of Renmin Hospital of Wuhan University. Chronotype preference was assessed using the reduced Horne and Ostberg Morningness - Eveningness Questionnaire (rMEQ), while sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI). Cognitive function was evaluated through the Digit Symbol Substitution Test (DSST), defining impairment as a DSST score ≤ 56 (the lowest quartile of the cohort). Univariate analysis and logistic regression models were employed to explore the factors associated with cognitive impairment, focusing on the potential interactive effects of evening chronotype and poor sleep quality.
Results: Of the 249 subjects recruited, about 41% were classified as evening chronotype. These individuals exhibited poorer sleep quality and more severe depressive symptoms compared to non-evening chronotype (p < 0.01). Univariate analysis revealed that first episode status, Hamilton Depression Rating Scale (HAMD-17) scores, evening chronotype, and poor sleep quality were significantly associated with cognitive impairment (p < 0.05). Multivariate logistic regression analysis further demonstrated that the co-existence of evening chronotype and poor sleep quality significantly increased the likelihood of cognitive impairment (adjusted odds ratio [AdjOR] = 2.65, 95% confidence interval [CI] = 1.09–6.45, p < 0.05).
Conclusion: Our findings suggest that evening chronotype, poor sleep quality, and their interaction are important contributors to cognitive impairment in patients with MDD, alongside the severity of depression and first episode status. These results emphasize the need for integrated approaches targeting circadian rhythm disruptions and sleep disturbances in the treatment of cognitive dysfunction in MDD.
1 Introduction
Major depressive disorder (MDD) is a disabling psychiatric condition that has been associated with impairments across emotional, functional, and cognitive deficits, along with executive functions, language, and working memory dysfunctions (1). Clinically, MDD patients frequently present with cognitive complaints, mainly encompassing difficulties in thinking, concentration, and decision-making. Cognitive dysfunction in MDD spans various areas such as memory, executive function, processing speed, and attention, with a notable impairment in executive functions (2, 3). These cognitive impairments significantly influence treatment outcomes, rehabilitation processes, quality of life, and social activities of MDD patients, and are particularly associated with a poorer response to medication (4) and psychosocial dysfunction (5). In patients with MDD, circadian disruption and poor sleep quality are prevalent, exacerbating depressive symptoms and cognitive dysfunction (6, 7). Nonetheless, limited studies have evaluated the impacts of circadian rhythm and sleep disturbances on cognitive dysfunction in MDD.
Patients with MDD commonly experience altered circadian rhythms, sleep disturbances, and mood changes with circadian cycle. Individual circadian preference, reflecting behavioral preferences for sleep-wake and daily activities, varies significantly among individuals. Chronotypes are commonly classified as morningness, intermediate and eveningness preferences. Irregularities in circadian rhythms and sleep patterns have been frequently observed not just during acute episodes but also during the prodromal and remission phases of depression (8). Even minor shifts in the circadian phase of sleep can profoundly affect sleep quality and duration (9–11). Disturbances in circadian rhythms and sleep are increasingly recognized as playing crucial roles in the pathophysiology of mood disorders (12).
The severity of depression in MDD patients has been linked to circadian misalignment (13). The evening chronotype could be proposed as a potential vulnerability factor for depression, while the morning chronotype may be protective (14, 15). Evening preference has been reported to be associated with an increased risk of MDD and various other psychiatric symptoms or disorders, including poor sleep, anxiety, bipolar disorder and seasonal affective disorder (16, 17). Notably, recent research indicates that in patients with fibromyalgia, higher eveningness, greater severity of diurnal dysrhythmia, lower wakeability, and poorer sleep quality predict a lack of response to SNRI antidepressants (18). Additionally, poor sleep quality has been shown to exacerbate affective symptoms, foster negative cognitive biases, and diminish sustained attention to non-affective stimuli (15, 19). Importantly, poor sleep quality has been identified as a significant predictor for the onset of major depression (20). Sleep quality in patients with MDD may mediate a significant association between evening preference and depressive symptoms (15).
There is a potentially complex relationship between cognitive impairment, sleep disturbance, and chronotype. A meta-analysis reported that sleep disorders were associated with poorer cognitive performance in general and multiple specific cognitive domains (21). Both severely impaired sleep quality and evening preference contribute to cognitive impairment in MDD patients (22). Furthermore, studies have revealed a link between circadian preference and cognitive deficits. For example, patients with delayed sleep-wake phase disorder often show pronounced attention problems, especially in the morning and when forced to wake early (23). Additionally, depressed patients with evening preference self-report more domains of cognitive impairments compared to those with morning and intermediate types (24). Historical studies in this area did not systematically account for variables like sleep quality or current mood states together, which could influence cognitive performance in MDD. Sleep disturbances and evening preference are associated with non-remission of major depression (25). However, the impact of sleep quality and whether sleep quality and chronotype preferences on cognitive impairment in MDD was poorly reported.
Currently cognitive assessment tools are often time-consuming and intricate. Variability in research findings regarding cognitive performance in MDD patients can be attributed to differing test timings, the specific neuropsychological tasks employed, and diverse study designs. Although a range of cognitive measures are utilized in randomized controlled trials for MDD therapeutic interventions, the Digit Symbol Substitution Test (DSST) stands out as the only cognitive endpoint available as a test for cognitive dysfunction in the studies assessed (26). DSST is a ‘pencil and paper’ cognitive test that evaluates several of the most impaired aspects of cognitive function in MDD, such as components of executive function, processing speed, attention, and working memory.
Therefore, rhythm and sleep disturbances are prevalent in MDD, while both circadian rhythm disruptions and poor sleep quality are involved in the onset and outcome of depression, their independent or synergistic effects on cognitive deficits have been reported rarely. The study aims to characterize the clinical features of depressed patients based on the eveningness - morningness chronotype. Furthermore, we investigate the association between self-reported diurnal preference, sleep quality and cognitive performance as measured by the DSST in patients with depression.
2 Methods
2.1 Participants
This study was part of a larger Chinese “Early-warning System and Comprehensive Intervention for Depression” (ESCID) project from April 2019 to July 2020. The study targeted outpatients and inpatients aged 18 - 39 years with Major Depressive Disorder (MDD) from the Mental Health Center of Renmin Hospital of Wuhan University. Diagnosis was confirmed by two experienced psychiatrists using the Mini-International Neuropsychiatric Interview (27). All clinicians evaluating the scales were all strictly trained for consistency. Participants met the diagnostic criteria for major depressive disorder of the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5), underwent questionnaire and routine laboratory screening. Patients with a history of bipolar disorders, schizophrenia spectrum disorders, intellectual disability, dementia, and alcohol or substance misuse/dependence were excluded. Shift workers and subjects who were unable to provide valid informed consent were excluded. Additionally, those with severe physical illness or craniocerebral trauma that rendered them unable to complete the clinical interview were also excluded.
All participants were informed of the purpose of the study and agreed to participate before the survey, and the Ethics Committee of Renmin Hospital of Wuhan University approved the study protocol (approval number: WDRY2020-K191).
2.2 Socio-demographical and clinical characteristics
Age, gender, ethnicity, place of residence, marital status, educational status, employment status, body mass index (BMI), and age-on-set were recorded. The ethnicities were all Chinese, divided into Han group and non-Han group (including Hui, Yao, Mongol, Tujia, Buyi, etc.). Place of residence was categorized as urban and rural. Marital status was categorized as married, single/divorced. Educational status was grouped into high school graduate and below, or college graduate and higher. Employment status was categorized as employed and unemployed.
2.3 Severity of depressive symptoms and current mood
HAMD-17 is a 17-item replacement scale used to assess the severity of depressive symptom (28). Each question has a score of 0 - 2 or 0 - 4, with a total score of 0 - 52. Higher total scores indicate more severe depressive symptoms. The scores are interpreted as follows: 0 - 7 for no depressive symptoms, 8 - 17 for mild depressive symptoms, 18 - 24 for moderate depressive symptoms, and 25 - 52 for severe depressive symptoms.
2.4 Measure of chronotype preference
The reduced version of Horne and Ostberg Morningness Eveningness Questionnaire (rMEQ) was used to measure chronotype preference (29). The questionnaire yields scores ranging from 4 to 24, with common reference categories to the following cut - offs: evening type (score < 12), intermediate type (score 12 - 17) and morning type (score > 17). The rMEQ has been validated for use in ethnic Chinese individuals and demonstrates robust psychometric properties (30). Due to the limited number of morning-type samples, we divided the samples into two groups: evening type and non-evening type.
2.5 Sleep quality assessment
The Pittsburgh Sleep Quality Index (PSQI) is a self-rated questionnaire that assesses subject sleep quality and disturbances over a one-month period (31). The 18 items of the PSQI form a seven-component score ranging from 0 to 3, which can be summarized as a general score. The total score ranges from 0 - 21, with higher scores indicating worse sleep quality. For subgroup analysis, a PSQI total score > 10 was defined as significantly impaired sleep quality, referring to previous literature (22).
2.6 Cognitive impairment evaluation
Cognitive impairment was defined as a DSST score falling with lowest quartile. The score is the total number of correct symbols drawn within 2 minutes, with one point given for a correct response and a maximum score of 133. Since there is no well-defined threshold of DSST score for detecting cognitive impairment, the lowest unweighted quartile in the study population (DSST ≤ 56) was used to define cognitive impairment or low cognitive function, consistent with methods previously used (32, 33). Subjects with DSST scores > 56 were considered not to be cognitively impaired.
2.7 Statistical analysis
Continuous variables were expressed as mean (M) ± standard deviation (SD), while categorical variables were expressed as percentages. The normality of data distributions was assessed using the Kolmogorov-Smirnov test. Differences between groups for normally distributed variables were analyzed using the t-test, while the chi-squared and Kruskal-Wallis tests were employed for categorical variables and non-normally distributed variables, respectively. Variables demonstrating significance (p < 0.05) in the univariate analysis were further evaluated using logistic regression models. Binary logistic regression models were established to explore the effects of both evening chronotype and impaired sleep quality on cognitive impairment, sequentially adjusting for variables such as severity of depression (HAMD-17 total scores > 17) and first episode status (Model 2 and 3), as well as age and gender (Model 4). The strength of the association was measured by adjusted odds ratios (Adj OR) with 95% confidence intervals (CI). A p value of less than 0.05 was considered as statistical significance. SPSS Statistic 26.0 was used for all the data analyses.
3 Results
3.1 Study population characteristics by chronotype
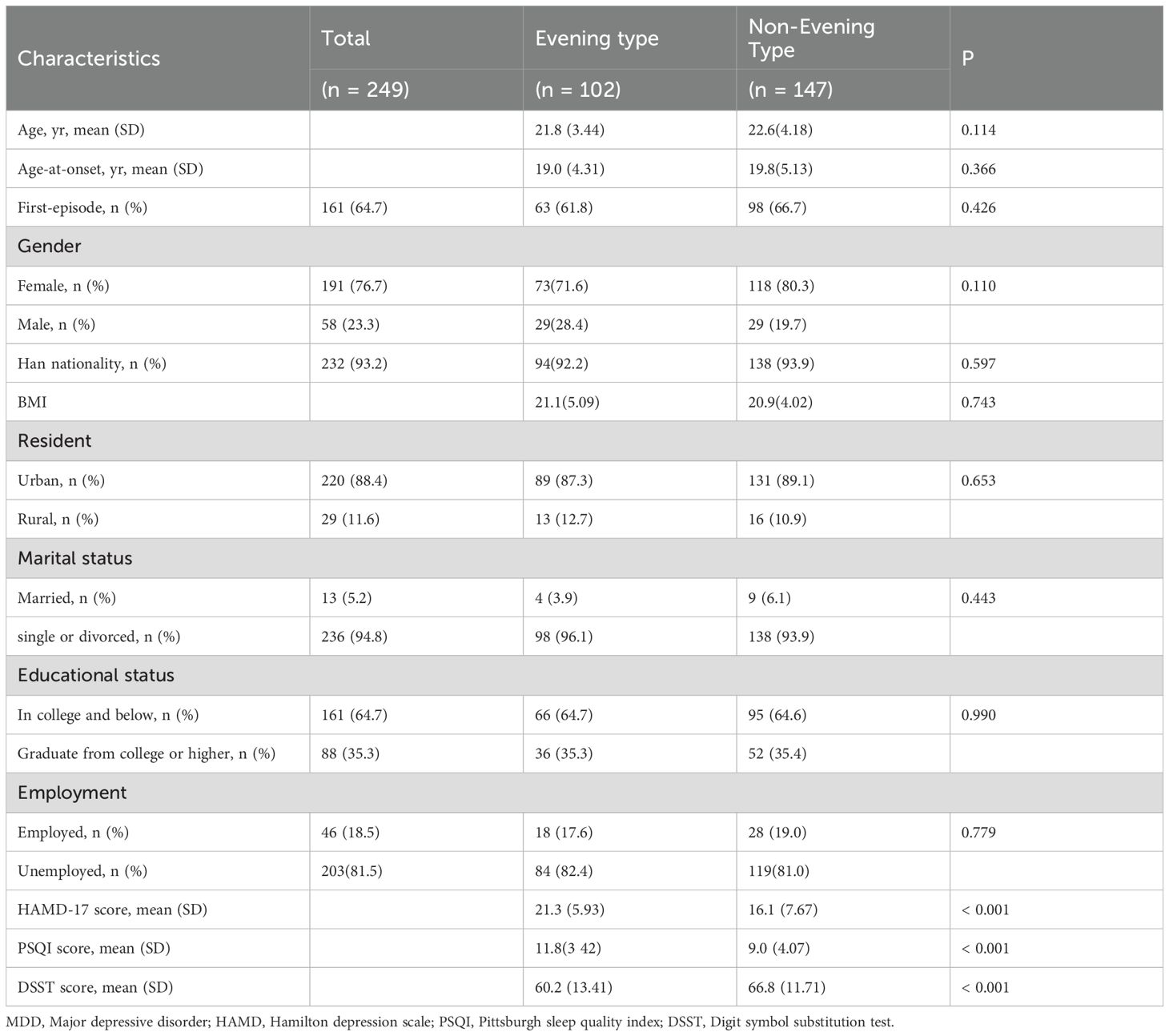
A total of 249 participants were eligible for the present study and were grouped by diurnal preference based on a split of the rMEQ scores. They were labeled as evening preference and non-evening preference, respectively. Table 1 lists a detailed comparison of the socio-demographic and clinical characteristics between two groups. Both groups were comparable regarding age, gender, ethnicity, BMI, place of residence, marital status, education, and employment status. However, the evening preference group exhibited significantly higher HAMD-17 total scores, poorer sleep quality, and lower DSST scores compared to the non-evening preference group.
3.2 Participant characteristics by cognitive function status
The characteristics of the study participants, stratified by cognitive function status, were detailed in Table 2. Of the participants, 67 (26.9%) were defined as having cognitive impairment with an unweighted DSST score ≤ 56. Compared to subjects without cognitive impairment, those with cognitive impairment had significantly higher scores on the depression scale, poorer sleep quality, and a greater likelihood of being classified as having an evening chronotype.
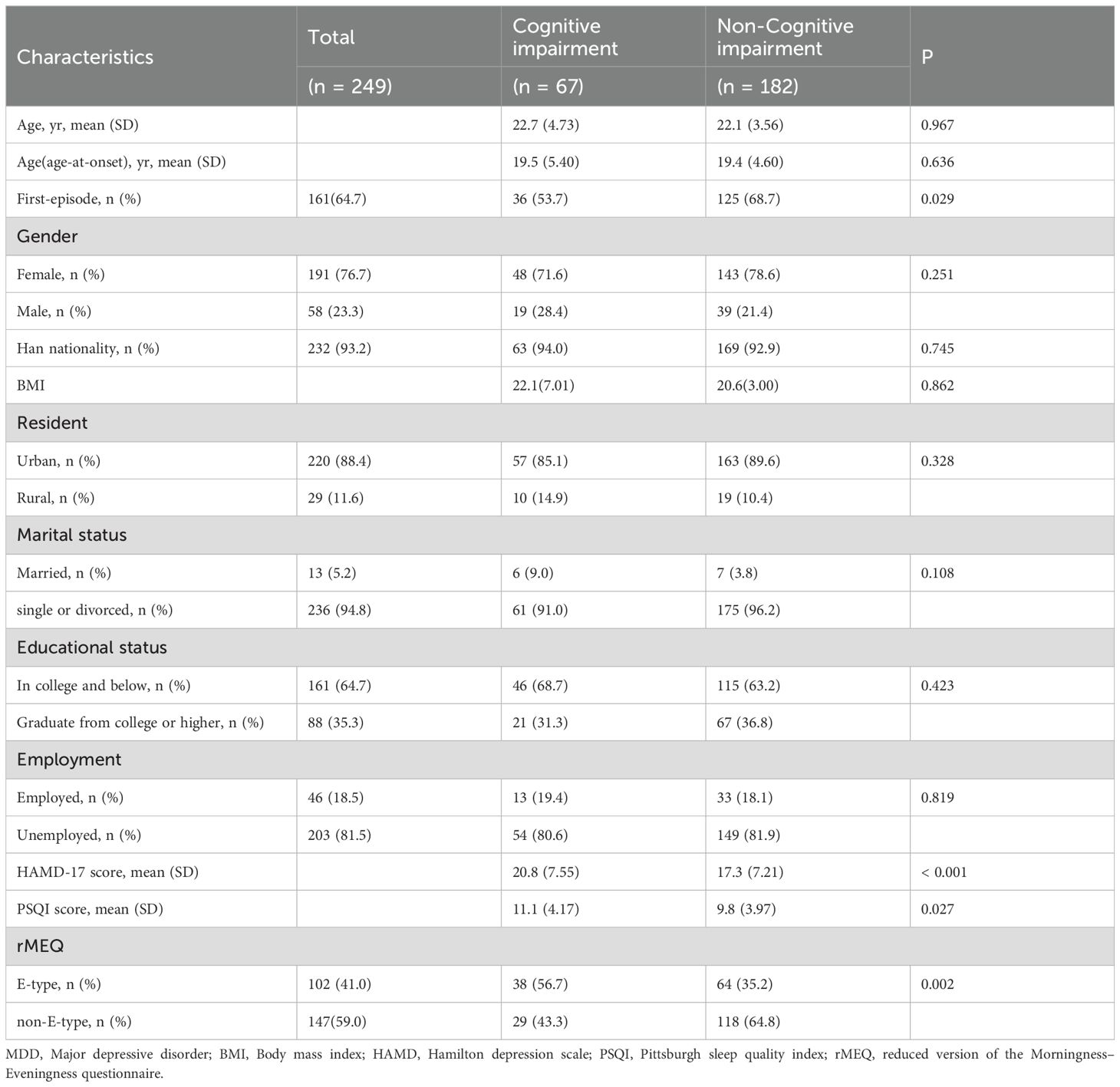
Table 2. Comparisons of demographic features, sleep characteristics and psychological correlates according to cognitive impairment.
3.3 Current depression severity and cognitive functioning in patients with MDD: subgroup analysis of diurnal preference and sleep quality
In order to investigate the association of diurnal preference and sleep quality with cognitive performance in MDD patients, we carried out a subgroup analysis by further dividing the sample according to the severity of sleep quality impairment (PSQI ≤ 10vs. > 10). The sample was categorized into four groups: “non-evening type with non-impaired sleep quality” (n=94), “non-evening type with impaired sleep quality” (n=53), “evening type with non-impaired sleep quality” (n=38), and “evening type with impaired sleep quality” (n=64). The proportion of patients with more severe depression (HAMD-17 total scores > 17 points) was generally higher among those MDD patients with impaired sleep quality regardless of diurnal preference (“non-evening type + non-impaired sleep quality” vs “non-evening type + impaired sleep quality” vs “evening type + non-impaired sleep quality” vs “evening type + impaired sleep quality”: 27.7% vs 73.6% vs 57.9% vs 90.6%; linear by linear association: χ² = 66.9, p < 0.001; Figure 1A). Similarly, the prevalence of relatively cognitive impairment, as measured by the DSST, was 17.0%, 24.5%, 23.7%, and 45.3% in the four groups, respectively (linear by linear association: χ² = 16.0, p = 0.001; Figure 1B).
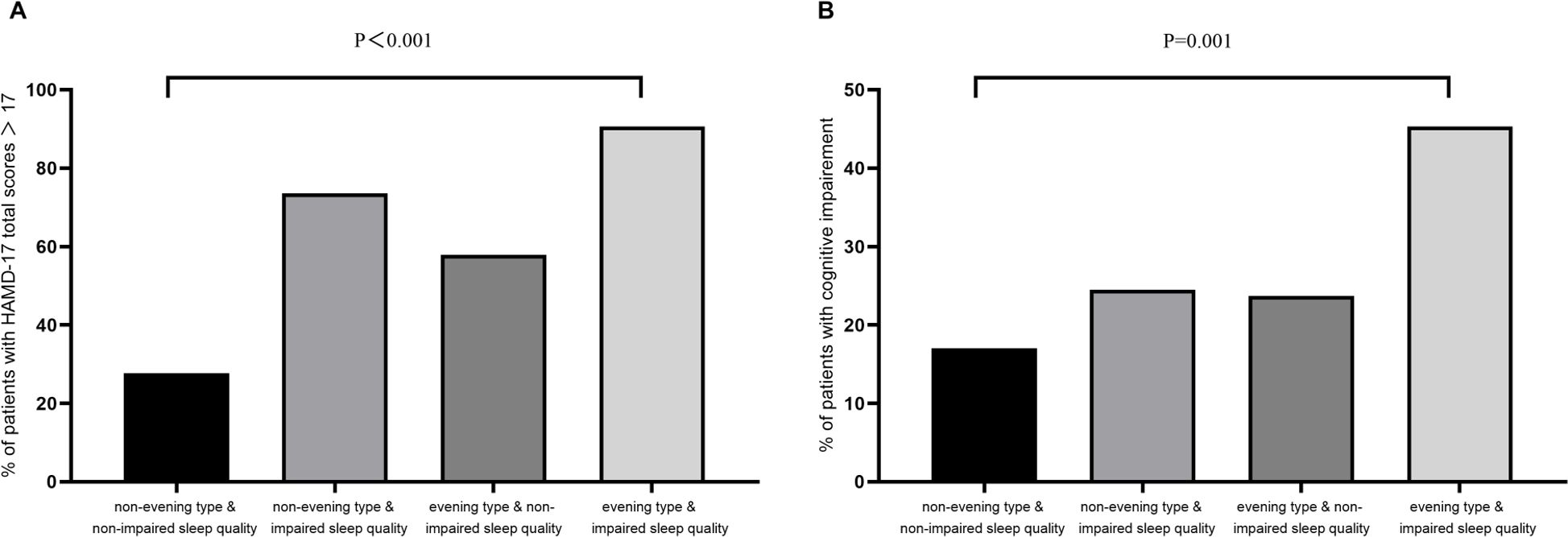
Figure 1. Subgroup analysis of diurnal preference and sleep quality. (A) Presence of sleep quality and evening type in relation to moderate to severe depression measured by HAMD-17 in MDD; (B) Presence of sleep quality and evening type in relation to impaired cognition measured by DSST in MDD. MDD, Major depressive disorder; HAMD, Hamilton depression scale; DSST, Digit symbol substitution test.
3.4 The associations of evening type and impaired sleep quality with cognitive impairment in MDD
As shown in Table 3, compared with “non-impaired sleep quality and non-evening type” group, individuals categorized as “evening type and impaired sleep quality” demonstrated an increased risk of cognitive impairment (OR = 4.03, 95% CI = 1.95-8.37, p < 0.001). After further adjustment for variables, including the severity of depression, first episode, age, gender, education, and BMI, the correlation was relatively weakened, but the results were still statistically significant (Model 4: AdjOR = 2.65, 95% CI = 1.09-6.45, p < 0.05). Conversely, no significant differences in cognitive impairment were observed between the other two groups and the reference group (p > 0.05). Additionally, it is worth noting that more severe depression, as measured by HAMD-17, was independently associated with cognitive impairment in all adjustment models (Model 4: AdjOR = 2.48, 95% CI = 1.15–5.39, p = 0.021). Furthermore, compared with first episode depressive disorder, patients with recurrent depression had a higher risk of more severe cognitive impairment in both models 3 and 4 (Model 4: AdjOR = 1.97, 95% CI = 1.05–3.67, p = 0.034).
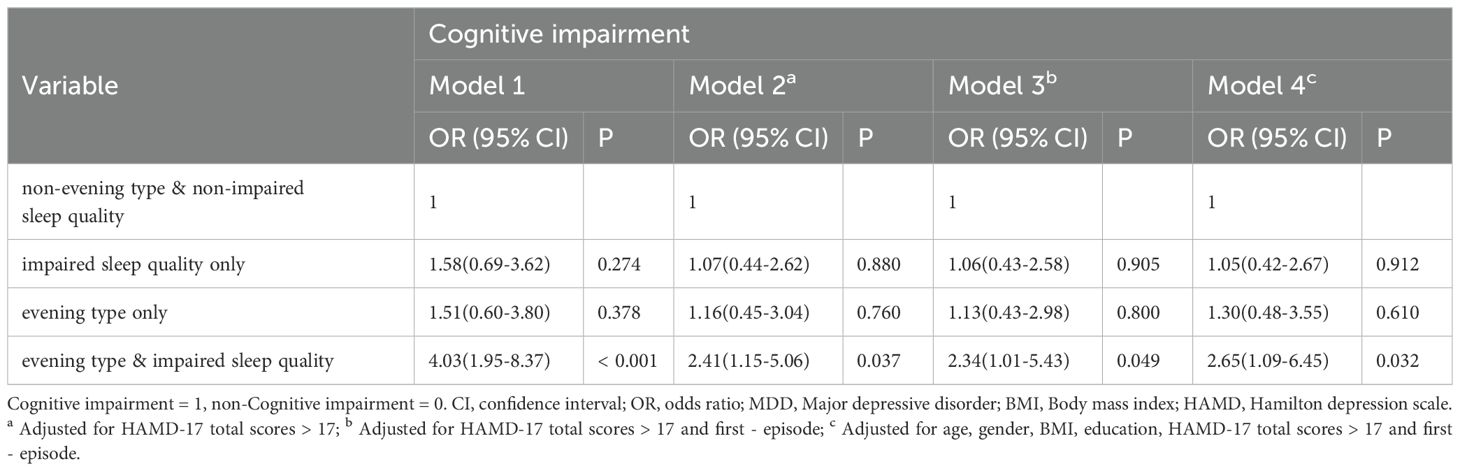
Table 3. Association of evening preference and sleep quality and cognitive impairment measured in MDD.
4 Discussion
The current study investigated the association of chronotype and poor sleep quality with cognitive impairment in MDD. Our results revealed a high prevalence of severe depressive symptoms and cognitive impairment among those with evening chronotype and poor sleep quality. Specifically, we observed that evening preference and poor sleep quality could contribute to cognitive dysfunction, while the co-existence of these two conditions could confer a greater risk in MDD.
In line with previous research (15, 34), we found that evening preference was common among patients with MDD, especially those with concomitant poor sleep quality. Approximately 41% of the participants in the study sample were classified as evening type. They demonstrated poorer sleep quality when compared to those who were non-evening types. The finding was consistent with existing literature, which suggests that individuals with evening chronotypes have been found to show greater sleep-wake irregularities, wakefulness distress and greater sensitivity to poor sleep quality than those with other chronotypes (11). Moreover, evening-type patients with sleep disorders have a higher probability of irregular sleep habits and sleep-related dysfunctional cognitions, which in turn may perpetuate evening-type sleep disorders (35, 36). The high prevalence of evening preference comorbid sleep disturbance emphasizes the importance of considering circadian factors when assessing and managing sleep and mood problems in patients with MDD.
Our study did not observe significant differences in age, gender and other variables among the depressed individuals. Similarly, earlier research has found no substantial difference between sociodemographic characteristics including education, gender, marital status, income, and sleep quality. These demographic variables have no significant influence on the direct and indirect relationship between sleep quality-mediated chronotype preference and depressive symptoms in MDD (15).
Recent research indicates that the cognitive performance of Chinese patients with MDD is influenced by a range of clinical characteristics, including age, age of onset, depression severity, years of education, and sleep disturbances (37). Contrary to these findings, our study did not identify the influence of sociodemographic factors such as age, age of onset and educational attainment on neurocognitive impairment in MDD. Our findings do suggest, though, that cognitive impairment may be more related to the recurrence of MDD (38). The variability in results across different studies may be attributed to the diversity of the samples of patients with MDD, which can vary in terms of current depressive symptom severity, psychotropic medication use, age, comorbidities, and assessment tools employed (3, 37, 39). There is evidence that higher educational attainment can serve as a protective factor against cognitive dysfunction in MDD and might even compensate for cognitive deficits that have already occurred (40, 41). In our study, the participants were generally young and mostly students or working individuals, which might have partially compensated for their cognitive impairment. This underscores the importance of considering educational levels in the clinical evaluation of MDD patients, as higher levels of education appear to be associated with better cognitive function, regardless of depression severity (42).
Existing evidence suggests that improving cognitive function with the use of antidepressants is often associated with alleviations in the severity of depressive symptoms (43). Our study similarly found that cognitive impairment was independently related to the severity of major depressive disorder, aligning with meta-analyses (44) reporting that depression severity was associated with cognitive performance in episodic memory, executive function, and processing speed, explaining psychomotor slowing as a feature of more severe depression that may lead to impairments in the cognitive aspects of patients with MDD. It is important to note that MDD patients often show significant impairments in tasks requiring episodic memory and mental flexibility compared to healthy adults and those with mild depression, the latter two groups exhibiting similar neurocognitive performance (45).
However, inconsistencies exist in the literature regarding the extent to which depression severity impacts cognitive performance. McDermott (44) highlighted that these discrepancies might be due to the varying efficacy of neuropsychological tests in the cognitive field used, as well as differences in sample characteristics among studies. The cognitive deficits observed in learning and memory, executive function, processing speed, and attention and concentration in the DSST tests are frequently documented in MDD patients undergoing major depressive episodes. The area of cognitive dysfunction is broad in MDD and is associated with depressed mood, advocating that combined conventional antidepressant treatments to improve depressed mood can help facilitate cognitive improvements so far (46). However, improvements in depressive mood do not necessarily translate into cognitive enhancements, reinforcing the notion that the depression severity remains a significant risk factor for cognitive-related dysfunction in MDD.
While numerous studies have documented the effects of sleep disturbance and evening preference on depressive symptoms and cognitive function, few have investigated these factors simultaneously. In our study, a combination of clinical interviews and objective measures was employed to confirm the diagnosis of sleep and depressive disorders. Our findings revealed that cognitive performance was negatively influenced by evening preference, poor sleep quality and the severity of depressive symptoms, which was supported by prior research suggesting circadian rhythms and sleep quality affect cognition regardless of current depressive mood (22). The study further demonstrated that the co-occurrence of evening preference and impaired sleep quality independently elevates the risk of cognitive impairment in MDD. This association remained significant even after adjusting for potential confounders such as age and sex, further validating the combined impact of circadian preference and sleep on neuropsychological outcomes in MDD.
Furthermore, existing evidence suggests that the multifactorial nature of sleep disorders can significantly influence overall life satisfaction. For instance, studies have shown a clear relationship between pain severity, life satisfaction, and sleep quality in individuals with temporomandibular disorders (47). Additionally, a polysomnographic study has revealed that sleep quality is intricately linked to orofacial pain and headache complaints (48). Such findings indicate that psychological factors, such as depression, can exacerbate sleep disturbances, thereby affecting life satisfaction. Therefore, improving sleep quality may alleviate cognitive deficits in patients with major depressive disorder and enhance their overall health and life satisfaction, reinforcing the importance of integrated treatment strategies that address both sleep and emotional health.
Poor sleep quality, a prevalent biological symptom of MDD, was found to be intricately connected with circadian preference, thereby impacting neuropsychological performance. This study corroborated earlier findings indicating that both evening chronotype and poor sleep quality were independently and directly associated with higher depression severity in patients with depressive syndromes (49). Moreover, both sleep disturbances and evening preference were strongly associated with non-remission of MDD (25), implying that sleep quality and circadian rhythms might play unique roles in the development and maintenance of depression. The complex interrelations among circadian preference, sleep disturbances, severity of depression, and cognition highlight the need for further longitudinal and interventional studies to elucidate their intricate dynamics and causality. Severe sleep disturbances combined with an evening preference may act as a “double whammy” on brain function, exacerbating the cognitive deficits observed in MDD patients. Therefore, comprehensive systematic identification and assessment of sleep and circadian preferences should be integral to the daily clinical management of depression, as this may help and accelerate the functional recovery of patients.
Our study primarily demonstrated that evening preference, impaired sleep quality, and the severity of depression collectively exacerbate cognitive impairment in MDD. These findings, while requiring further validation in larger samples, underscore the clinical significance of understanding the neurobiological mechanisms underlying these associations. Chronobiological interventions and cognitive-behavioral therapy targeting sleep disturbances may offer promising avenues for improving cognition in MDD patients. Previous research has indicated that evening chronotype and poor sleep quality are common in MDD patients and are associated with cognitive dysfunction, social impairment, and lower quality of life (50). This suggests that clinicians should pay special attention to sleep-wake cycle disturbances when assessing cognitive function in MDD patients.
However, this study has certain limitations. The small sample size limited the robustness of our cognitive assessment and constrained the application of the usual three chronotypes classification analysis. Additionally, the cultural background of our sample, rooted in Chinese values of introversion, implicitness, and inclusiveness, may have influenced the way individuals self-regulate and absorb stress, potentially affecting our findings. Moreover, as a cross-sectional study, the data presented weak causal inferences, highlighting the need for future longitudinal studies to explore the connections between the remission of sleep disorders and cognitive impairment in MDD. A significant limitation is the lack of a control group composed of healthy volunteers, which restricts the ability to effectively compare cognitive function, chronotype preferences, and sleep quality. Furthermore, evidence suggests that cognitive deficits in MDD may persist even after mood symptoms improve, supporting the state, trait, and scar hypotheses (51). This further emphasizes the complexity of neurocognitive deficits in depression. Therefore, systematic identification and assessment of sleep and circadian preferences should be integral to the daily clinical management of depression, as this may help accelerate the functional recovery of patients. Finally, it may be necessary to consider the impact of pharmacological treatments and employ more specific measurement tools, such as polysomnography and circadian biomarker monitoring, to evaluate sleep effects in MDD patients more accurately.
This study highlights the prevalence of cognitive impairment in MDD patients and its close association with specific clinical factors, including first-episode status, severity of depression, circadian preference, and sleep quality. Additionally, our findings suggest that the co-existence of evening preference and poor sleep quality significantly increases the risk of cognitive impairment, highlighting these conditions as independent risk factors. These results emphasize the necessity for clinicians to pay special attention to circadian rhythms and sleep quality when assessing and treating patients with MDD. Targeted interventions addressing these factors could mitigate cognitive impairment in individuals suffering from major depression, advocating for a holistic treatment strategy that considers both the psychiatric and neurocognitive dimensions for improved patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Renmin Hospital of Wuhan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LW: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. YH: Methodology, Writing – original draft. LY: Software, Supervision, Writing – original draft. NZ: Validation, Writing – original draft. SM: Investigation, Project administration, Software, Writing – original draft. ZN: Resources, Validation, Writing – original draft. WW: Validation, Writing – original draft. EZ: Investigation, Writing – original draft. SX: Data curation, Writing – original draft. SW: Data curation, Writing – original draft. DX: Resources, Writing – original draft. MH: Writing – review & editing. ZL: Conceptualization, Project administration, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (grant number: U21A20364) and the National Key R&D Program of China (grant number: 2018YFC1314600).
Acknowledgments
The authors would like to thank the patients with depression and their families for participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Darcet F, Gardier AM, Gaillard R, David DJ, Guilloux J-P. Cognitive dysfunction in major depressive disorder. a translational review in animal models of the disease. Pharmaceuticals. (2016) 9:9. doi: 10.3390/ph9010009
2. Carvalho AF, Miskowiak KK, Hyphantis TN, Kohler CA, Alves GS, Bortolato B, et al. Cognitive dysfunction in depression - pathophysiology and novel targets. CNS Neurol Disord Drug Targets. (2014) 13:1819–35. doi: 10.2174/1871527313666141130203627
3. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. (2013) 139:81–132. doi: 10.1037/a0028727
4. Groves SJ, Douglas KM, Porter RJ. A systematic review of cognitive predictors of treatment outcome in major depression. Front Psychiatry. (2018) 9:382. doi: 10.3389/fpsyt.2018.00382
5. Cambridge OR, Knight MJ, Mills N, Baune BT. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: a systematic review. Psychiatry Res. (2018) 269:157–71. doi: 10.1016/j.psychres.2018.08.033
6. Chen RF, Cai Y, Zhu ZH, Hou WL, Chen P, Wang J, et al. Sleep disorder as a clinical risk factor of major depression: associated with cognitive impairment. Asian J Psychiatry. (2022) 76:103228. doi: 10.1016/j.ajp.2022.103228
7. Taillard J, Sagaspe P, Philip P, Bioulac S. Sleep timing, chronotype and social jetlag: Impact on cognitive abilities and psychiatric disorders. Biochem Pharmacol. (2021) 191:114438. doi: 10.1016/j.bcp.2021.114438
8. Srinivasan V, Pandi-Perumal SR, Trakht I, Spence DW, Hardeland R, Poeggeler B, et al. Pathophysiology of depression: role of sleep and the melatonergic system. Psychiatry Res. (2009) 165:201–14. doi: 10.1016/j.psychres.2007.11.020
9. Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on Its circadian phase. Science. (1980) 210:1264–7. doi: 10.1126/science.7434029
10. Lazar AS, Santhi N, Hasan S, Lo JC-Y, Johnston JD, Von Schantz M, et al. Circadian period and the timing of melatonin onset in men and women: predictors of sleep during the weekend and in the laboratory. J Sleep Res. (2013) 22:155–9. doi: 10.1111/jsr.12001
11. Ong JC, Huang JS, Kuo TF, Manber R. Characteristics of insomniacs with self-reported morning and evening chronotypes. J Clin Sleep Med. (2007) 03:289–94. doi: 10.5664/jcsm.26801
12. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. (2008) 23:571–85. doi: 10.1002/hup.964
13. Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. (2009) 168:259–61. doi: 10.1016/j.psychres.2009.04.009
14. Gaspar-Barba E, Calati R, Cruz-Fuentes CS, Ontiveros-Uribe MP, Natale V, De Ronchi D, et al. Depressive symptomatology is influenced by chronotypes. J Affect Disord. (2009) 119:100–6. doi: 10.1016/j.jad.2009.02.021
15. Selvi Y, Boysan M, Kandeger A, Uygur OF, Sayin AA, Akbaba N, et al. Heterogeneity of sleep quality in relation to circadian preferences and depressive symptomatology among major depressive patients. J Affect Disord. (2018) 235:242–9. doi: 10.1016/j.jad.2018.02.018
16. Müller MJ, Haag A. The concept of chronotypes and its clinical importance for depressive disorders. Int J Psychiatry Med. (2018) 53:224–40. doi: 10.1177/0091217417749787
17. Zou H, Zhou H, Yan R, Yao Z, Lu Q. Chronotype, circadian rhythm, and psychiatric disorders: Recent evidence and potential mechanisms. Front Neurosci. (2022) 16:811771. doi: 10.3389/fnins.2022.811771
18. Krupa AJ, Chrobak AA, Sołtys Z, Korkosz M, Nowakowski J, Dudek D, et al. Chronobiological variables predict non-response to serotonin and noradrenaline reuptake inhibitors in fibromyalgia: a cross-sectional study. Rheumatol Int. (2024) 44:1987–95. doi: 10.1007/s00296-024-05650-0
19. Gobin CM, Banks JB, Fins AI, Tartar JL. Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention. J Sleep Res. (2015) 24:535–42. doi: 10.1111/jsr.12302
20. Selvi Y, Aydin A, Boysan M, Atli A, Agargun MY, Besiroglu L. Associations between chronotype, sleep quality, suicidality, and depressive symptoms in patients with major aepression and healthy controls. Chronobiol Int. (2010) 27:1813–28. doi: 10.3109/07420528.2010.516380
21. Wardle-Pinkston S, Slavish DC, Taylor DJ. Insomnia and cognitive performance: a systematic review and meta-analysis. Sleep Med Rev. (2019) 48:101205. doi: 10.1016/j.smrv.2019.07.008
22. Cabanel N, Schmidt A-M, Fockenberg S, Brückmann KF, Haag A, Müller MJ, et al. Evening preference and poor sleep independently affect attentional-executive functions in patients with depression. Psychiatry Res. (2019) 281:112533. doi: 10.1016/j.psychres.2019.112533
23. Solheim B, Olsen A, Kallestad H, Langsrud K, Bjorvatn B, Gradisar M, et al. Cognitive performance in DSWPD patients upon awakening from habitual sleep compared with forced conventional sleep. J Sleep Res. (2019) 28:e12730. doi: 10.1111/jsr.12730
24. Coleman MY, Cain SW. Eveningness is associated with greater subjective cognitive impairment in individuals with self-reported symptoms of unipolar depression. J Affect Disord. (2019) 256:404–15. doi: 10.1016/j.jad.2019.05.054
25. Chan JWY, Lam SP, Li SX, Yu MWM, Chan NY, Zhang J, et al. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. (2014) 37:911–7. doi: 10.5665/sleep.3658
26. Baune BT, Brignone M, Larsen KG. A network meta-analysis comparing effects of various antidepressant classes on the digit symbol substitution test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int J Neuropsychopharmacol. (2018) 21:97–107. doi: 10.1093/ijnp/pyx070
27. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59:22–33.
28. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
29. Adan A, Almirall H. Horne & Östberg morningness-eveningness questionnaire: a reduced scale. Pers Individ Differ. (1991) 12:241–53. doi: 10.1016/0191-8869(91)90110-W
30. Carciofo R, Du F, Song N, Qi Y, Zhang K. Age-related chronotype differences in Chinese, and reliability assessment of a reduced version of the Chinese morningness–eveningness questionnaire. Sleep Biol Rhythms. (2012) 10:310–8. doi: 10.1111/j.1479-8425.2012.00577.x
31. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
32. Chen SP, Bhattacharya J, Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. (2017) 135:963–70. doi: 10.1001/jamaophthalmol.2017.2838
33. Yen F-S, Wang S-I, Lin S-Y, Chao Y-H, Wei JC-C. The impact of heavy alcohol consumption on cognitive impairment in young old and middle old persons. J Transl Med. (2022) 20:155. doi: 10.1186/s12967-022-03353-3
34. Lemoine P, Zawieja P, Ohayon MM. Associations between morningness/eveningness and psychopathology: an epidemiological survey in three in-patient psychiatric clinics. J Psychiatr Res. (2013) 47:1095–8. doi: 10.1016/j.jpsychires.2013.04.001
35. Li SX, Chan NY, Man Yu MW, Lam SP, Zhang J, Yan Chan JW, et al. Eveningness chronotype, insomnia symptoms, and emotional and behavioural problems in adolescents. Sleep Med. (2018) 47:93–9. doi: 10.1016/j.sleep.2018.03.025
36. Adan A, Fabbri M, Natale V, Prat G. Sleep beliefs scale (SBS) and circadian typology. J Sleep Res. (2006) 15:125–32. doi: 10.1111/j.1365-2869.2006.00509.x
37. Zhu N, Tong J, Pei Y, Zhang J, Sun X. Factors associated with objective and subjective cognitive impairment in Chinese patients with acute major depressive disorder. BMC Psychiatry. (2023) 23:348. doi: 10.1186/s12888-023-04857-y
38. Lin J, Su Y, Shi C, Liu Q, Wang G, Wei J, et al. Neurocognitive profiles of patients with first-episode and recurrent depression: a cross-sectional comparative study from China. J Affect Disord. (2021) 286:110–6. doi: 10.1016/j.jad.2021.02.068
39. Cui F, Liu Q, Lv X, Leonhart R, Tian H, Wei J, et al. Severe sleep disturbance is associated with executive function impairment in patients with first-episode, treatment-naïve major depressive disorders. BMC Psychiatry. (2021) 21:1–11. doi: 10.1186/s12888-021-03194-2
40. James TA, Weiss-Cowie S, Hopton Z, Verhaeghen P, Dotson VM, Duarte A. Depression and episodic memory across the adult lifespan: A Meta-Analytic Review. Psychol Bull. (2021) 147:1184–214. doi: 10.1037/bul0000344
41. Venezia RG, Gorlyn M, Burke AK, Oquendo MA, Mann JJ, Keilp JG. The impact of cognitive reserve on neurocognitive performance in major depressive disorder. Psychiatry Res. (2018) 270:211–8. doi: 10.1016/j.psychres.2018.09.031
42. McLaren ME, Szymkowicz SM, Kirton JW, Dotson VM. Impact of Education on memory deficits in subclinical depression. Arch Clin Neuropsychol. (2015) 30:387–93. doi: 10.1093/arclin/acv038
43. Pan Z, Park C, Brietzke E, Zuckerman H, Rong C, Mansur RB, et al. Cognitive impairment in major depressive disorder. CNS Spectr. (2019) 24:22–9. doi: 10.1017/S1092852918001207
44. McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. (2009) 119:1–8. doi: 10.1016/j.jad.2009.04.022
45. Airaksinen E, Larsson M, Lundberg I, Forsell Y. Cognitive functions in depressive disorders: evidence from a population-based study. Psychol Med. (2004) 34:83–91. doi: 10.1017/s0033291703008559
46. Knight MJ, Baune BT. Cognitive dysfunction in major depressive disorder. Curr Opin Psychiatry. (2018) 31:26–31. doi: 10.1097/YCO.0000000000000378
47. Seweryn P, Orzeszek SM, Waliszewska-Prosół M, Jenča A, Osiewicz M, Paradowska-Stolarz A, et al. Relationship between pain severity, satisfaction with life and the quality of sleep in Polish adults with temporomandibular disorders. Dent Med Probl. (2023) 60:609–17. doi: 10.17219/dmp/171894
48. Orzeszek S, Martynowicz H, Smardz J, Wojakowska A, Bombała W, Mazur G, et al. Assessment of sleep quality in patients with orofacial pain and headache complaints: a polysomnographic study. Dent Med Probl. (2024) 61:549–62. doi: 10.17219/dmp/177008
49. Müller MJ, Kundermann B, Cabanel N. Eveningness and poor sleep quality independently contribute to self-reported depression severity in psychiatric inpatients with affective disorder. Nord J Psychiatry. (2016) 70:329–34. doi: 10.3109/08039488.2015.1112832
50. Takaesu Y, Kanda Y, Nagahama Y, Shiroma A, Ishii M, Hashimoto T, et al. Delayed sleep-wake rhythm is associated with cognitive dysfunction, social dysfunction, and deteriorated quality of life in patients with major depressive disorder. Front Psychiatry. (2022) 13:1022144. doi: 10.3389/fpsyt.2022.1022144
Keywords: major depressive disorder, cognitive impairment, evening chronotype, sleep quality, digit symbol substitution test
Citation: Wang L, Huo Y, Yao L, Zhang N, Ma S, Nie Z, Wang W, Zhou E, Xu S, Weng S, Xiang D, Hu M and Liu Z (2024) Association of evening chronotype, sleep quality and cognitive impairment in patients with major depressive disorder. Front. Psychiatry 15:1494032. doi: 10.3389/fpsyt.2024.1494032
Received: 10 September 2024; Accepted: 23 October 2024;
Published: 18 November 2024.
Edited by:
Mieszko Wieckiewicz, Wroclaw Medical University, PolandReviewed by:
Marcin Siwek, Jagiellonian University Medical College, Krakow, PolandAnna Paradowska-Stolarz, Wroclaw Medical University, Poland
Copyright © 2024 Wang, Huo, Yao, Zhang, Ma, Nie, Wang, Zhou, Xu, Weng, Xiang, Hu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongchun Liu, emNsaXU2QHdodS5lZHUuY24=
 Li Wang
Li Wang Yingchao Huo1
Yingchao Huo1 Nan Zhang
Nan Zhang Simeng Ma
Simeng Ma Zhaowen Nie
Zhaowen Nie Maolin Hu
Maolin Hu Zhongchun Liu
Zhongchun Liu