- 1Department of Psychiatry, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 2Department of Basic Medical Science, Neuroscience and Sense Organs, University of Bari ‘Aldo Moro’, Bari, Italy
- 3Department of Medicine and Surgery, Libera Università Mediterranea Giuseppe Degennaro, Casamassima, Italy
- 4Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, Section of Psychiatry, University of Genoa, Genoa, Italy
- 5Department of Neuroscience, Section of Psychiatry, University of Turin, Turin, Italy
- 6Department of Biotechnological and Applied Clinical Sciences, Section of Psychiatry, University of L’Aquila, L’Aquila, Italy
Background: The development of neuroimaging biomarkers in patients with schizophrenia (SCZ) requires a refined clinical characterization. A limitation of the neuroimaging literature is the partial uptake of progress in characterizing disease-related features, particularly negative symptoms (NS) and cognitive impairment (CI). In the present study, we assessed NS and CI using up-to-date instruments and investigated the associations of abnormalities in brain resting-state (rs)-activity with disease-related features.
Methods: Sixty-two community-dwelling SCZ subjects participated in the study. Multiple regression analyses were performed with the rs-activity of nine regions of interest as dependent variables and disease-related features as explanatory variables.
Results: Attention/vigilance deficits were negatively associated with dorsal anterior cingulate rs-activity and, together with depression, were positively associated with right dorsolateral prefrontal cortex rs-activity. These deficits and impairment of Reasoning/problem-solving, together with conceptual disorganization, were associated with right inferior parietal lobule and temporal parietal junction rs-activity. Independent of other features, the NS Expressive Deficit domain was associated with the left ventral caudate, while the Motivational Deficit was associated with the dorsal caudate rs-activity.
Conclusion: Neurocognitive deficits and the two negative symptom domains are associated with different neural markers. Replications of these findings could foster the identification of clinically actionable biomarkers of poor functional outcomes.
1 Introduction
Schizophrenia is a complex and heterogeneous mental disorder in terms of pathophysiology, clinical presentation, and functional outcome (1–5). This disorder greatly impacts several aspects of functional outcome, such as social, vocational and independent living skills (6–14). Among illness-related aspects, negative symptoms and cognitive impairments, seem to represent the major predictors of a poor functional outcome, more than positive symptoms, disorganization and depression (7, 9, 12, 15–21). Negative symptoms are core features of schizophrenia, they often remain stable through the different phases of the illness largely contributing to the disability of the patients (15, 22–27). They cluster into two domains: the Motivational Deficit, which includes avolition, anhedonia and asociality, and the Expressive Deficit, which includes blunted affect and alogia (22, 24, 28–30). Although not part of the diagnostic criteria, neurocognitive dysfunctions are present in most subjects with schizophrenia (SCZ) and in their non-affected relatives and have a significant impact on daily functioning (31–35). Different neurocognitive domains, such as attention, speed of processing, working memory, visuospatial learning and memory, verbal learning and memory, reasoning and problem solving and executive functions, are impaired in patients with schizophrenia (36).
Several studies investigated brain alterations that might underlie different clinical features of schizophrenia. Functional magnetic resonance imaging (fMRI) during resting has been widely used to gather valuable information on the brain activity and connectivity when the brain does not perform any task (37–40).
Negative symptoms are linked to different alterations in brain activity and connectivity within several areas and circuits (28, 41–44). The Motivational Deficit domain seems to be related to brain alterations in pathways implied in different aspects of motivation, which are often impaired in subjects affected by schizophrenia. These pathways mainly involve brain areas within the “motivational value system or reward” and the “motivational salience” circuits (28)). In particular the Motivational Deficit domain has been found to be associated with dysfunctions of the resting-state functional connectivity within the right ventral putamen-medial orbitofrontal cortex pathway (45), the cingulo-opercular pathway (46), the left dorsal caudate-dorsolateral prefrontal cortex pathway (47), the precuneus (48), and the medial prefrontal and temporal pathways (49), as well as with with altered functional connectivity between the ventral tegmental area and the right ventro-lateral prefrontal cortex, the bilateral insular cortex, and the right lateral occipital complex (50).
The Expressive Deficit domain seems to be associated with alterations in neurodevelopmental processes (22, 51–53). Very few rs-fMRI studies investigated the neural correlates of the Expressive Deficit domain, showing that abnormalities in fronto-polar cortex functional connectivity could be associated with this domain (54, 55). Brain areas most probably involved in the pathophysiology of this domain are the cortical motor areas, the ventrolateral prefrontal cortex, the rostral anterior cingulate cortex, the amygdala, and the basal ganglia (41).
Neurocognitive impairments have been regarded as the result of the effect of different alterations in cortico–cerebellar–thalamic circuits involved in neurodevelopment, neuronal maturation and neuroplasticity (56–58). Recent meta-analyses reported that the impairment in neurocognitive performance in schizophrenia is correlated with the decreased resting-state activity of different neural networks, such as the Default Mode Network (DMN), the visual network, the salience network (including the left amygdala, left insula, bilateral inferior frontal gyrus, and right anterior cingulate cortex), and some other brain areas, such as the supplementary motor area and the putamen (59, 60). Most studies included in these meta-analyses measured cognitive functions by using assessment tools that do not separately and systematically evaluate all the cognitive domains found impaired in schizophrenia. Actually, different neurocognitive domains seem to be related with different brain alterations. For instance, working memory deficits seem to be associated with a functional dysconnectivity between the left and right Central Executive Networks, the visuospatial sketchpad (right ventro-lateral prefrontal cortex and intraparietal sulcus) and the phonologic Loop (left ventro-lateral prefrontal cortex and temporo-parietal junction) (61); deficits in speed of processing are associated with resting-state connectivity in the bilateral postcentral gyri, paracentral lobule (62), the right superior frontal gyrus and left postcentral gyrus (63).
Resting state fMRI abnormalities have also been investigated in relationship with other psychopathological dimensions of SCZ.
The severity of positive symptoms has been associated with an increased cerebral blood flow (CBF) in frontotemporal-parietal regions, posterior cingulate gyrus, lingual gyrus (64) and in subcortical regions such as the lenticular nucleus (i.e., pallidum and putamen), caudate, striatum, and hippocampus (65). These symptoms have also been associated with aberrant functional connectivity in the DMN, frontotemporal and auditory networks in SCZ (66).
Severity of disorganization in previous fMRI resting-state studies has been reported in association with the hyperactivity of the language network, i.e., the inferior frontal gyrus, superior temporal gyrus and inferior parietal lobe (67, 68). Auditory verbal hallucinations have also been associated with abnormalities in these areas (69, 70), suggesting a key role of the language network in the pathophysiology of positive symptoms and disorganization. Moreover, conceptual disorganization in schizophrenia has been reported to be strongly associated with a widespread brain dysconnectivity at rest (66, 71), involving the right lingual gyrus, left precuneus, left middle temporal gyrus, left posterior superior temporal sulcus, and right fusiform gyrus (67, 71–73).
To our knowledge, no study investigated the specific neural correlates of depressive symptoms in schizophrenia using rs-fMRI. Only one study using task-based fMRI showed a positive association of the activity of left thalamus, putamen, globus pallidus, insular lobe, inferior frontal gyrus, middle frontal gyrus and precentral gyrus, with depressive symptoms severity in schizophrenia (74).
Despite the large number of studies investigating the resting-state neural activity in schizophrenia, drawing clear conclusions about the neurobiological abnormalities associated to specific psychopathological dimensions of schizophrenia is still difficult. This could be mainly linked to: 1) the different conceptualization of the psychopathological dimensions across studies; 2) the presence of confounding factors, such as the impact of antipsychotic treatments on the neural activity of SCZ.
Our study aimed to improve the knowledge on the neural pathways underling the complex clinical presentation of schizophrenia investigating the relationships between illness-related features and abnormalities in rs brain activity. We hypothesized that deficits in different neurocognitive domains, evaluated through a comprehensive battery of standardized tests, and different aspects of psychopathology, characterized by using up-to-date instruments based on most recent conceptualizations, could identify distinct alterations of rs brain activity.
2 Methods
2.1 Participants
Subjects participating in the current study were from the same cohort as a previously published study conducted by Giordano et al. (37).
Sixty-six SCZ were enrolled across five Italian university psychiatric clinics that joined the Italian Network for Research on Psychoses (NIRP), as detailed in Giordano et al. (37).
The inclusion criterion was a diagnosis of schizophrenia according to DSM-IV, confirmed by the Structured Clinical Interview for DSM IV-Patient version (SCID-I-P). Exclusion criteria were: (a) a history of head injury resulting in loss of consciousness; (b) a history of moderate-to-severe intellectual disability or neurological diseases; (c) a history of alcohol and/or substance abuse in the previous six months; (d) current pregnancy or breastfeeding; (e) an inability to provide informed consent; and (f) treatment modifications and/or hospitalization due to symptom exacerbation in the previous three months.
All subjects were requested to provide a written informed consent to take part in the study after receiving a thorough description of the study’s procedures. These procedures were in line with the Helsinki Declaration of 1975, as updated in 2008, and to the ethical requirements of the relevant national and institutional committees on human experimentation. This study was approved by the Ethics Committee of the Università degli Studi della Campania “Luigi Vanvitelli”— Azienda Ospedaliera Universitaria “Luigi Vanvitelli”, A.O.R.N. “Ospedali dei Colli” and by the Ethics Committees of the involved collaborating institutions.
2.2 Psychopathological assessment
In the present study, the Positive and Negative Syndrome Scale (PANSS) (75) was used to assess positive, negative, and disorganization dimensions. In particular, the positive dimension was calculated according to Wallwork and colleagues (76) by adding up the scores of the items “delusions” (P1), “hallucinatory behavior” (P3), “grandiosity” (P5), and “unusual thought” (G9) and the disorganization dimension was assessed by the PANSS item “conceptual disorganization” (P2), in order to prevent overlap with cognitive impairment (7).
Negative symptoms were assessed using the Italian version of the Brief Negative Symptom Scale (BNSS) (77, 78). The BNSS is a scale developed according to the recent conceptualization of negative symptoms, in line with the NIMH-MATRICS Consensus Statement on Negative Symptoms (30). It explores all the domains of the negative construct, including avolition, anhedonia, asociality, blunted affect, and alogia, plus an additional aspect, “distress”, which evaluates the lack of normal experience of distressing and unpleasant emotions (30). The scale includes 13 items and 6 subscales (5 negative symptom subscales that include anhedonia, asociality, avolition, blunted affect, and alogia, and the control subscale that includes distress). The ratings for each item range from absent (0) to moderate (3) to extremely severe (6). In the present study, the “distress” subscale was subtracted from the overall score to calculate the negative symptom total score (78). The Motivational Deficit domain was obtained by adding the scores of the subscales of anhedonia, asociality, and avolition, and the Expressive Deficit domain was obtained by adding the scores of the alogia and blunted affect subscales.
We also used the Calgary Depression Scale for Schizophrenia (CDSS) to evaluate depression (79).
For all these evaluations, higher scores indicated more severe symptoms.
Neurocognitive functions were assessed using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) (80, 81). This battery evaluates six neurocognitive domains: (a) processing speed (PS); (b) attention and vigilance (A/V); (c) working memory (WM); (d) verbal learning (SLe); (e) visual learning (VLe); (f) reasoning and problem solving (RPS). Raw scores on the MCCB were standardized to T-scores, corrected for age and gender, based on the Italian normative sample of community participants (82). For cognitive domains including more than one measure a summary score for the domain was calculated by summing the T-scores of the tests included in that domain and then standardizing the sum to a T-score. The same standardization procedure was adopted for the Neurocognitive Composite score. For all domains and composite scores, a T value of 50 is the normative mean and 10 is the SD. The lower the T value the more impaired the performance on the considered domain.
2.3 MRI data acquisition and pre-processing and ROI selection
Information regarding resting-state (rs) fMRI data acquisition and pre-processing, as well as regarding ROI selection procedure, are reported in the Supplementary Materials. In the present study we selected the ROIs whose rs activity differed between SCZ and healthy controls (HC) in the study by Giordano and colleagues (37). The ROIs were the following: dorsolateral prefrontal cortex (DLPFC), the inferior parietal lobule (IPL), the temporo-parietal junction (TPJ), the dorsal anterior cingulate cortex (dACC), the ventral caudate (vCa), and the dorsal caudate (dCa). Coordinates and size of these ROIs are summarized in Supplementary Materials (Supplementary Table S1).
2.4 Statistical analyses
Demographic continuous variables were reported as mean ± standard deviation (SD), while categorical variables were reported as frequencies.
We conducted separate stepwise multivariate regression analyses to explore potential illness-related variables with the highest associations with the rs-fMRI activity of those brain area that we previously found to differ between SZs and HCs. Specifically, we tested nine different regression models, one for each ROI that differed between SCZ and HCs (right IPL, right TPJ, right DLPFC, right and left dACC, right and left vCa, and right and left dCA). For each regression model, we used the rs activity of the ROI as dependent variables and PANSS positive dimension and disorganization, BNSS motivational deficit, BNSS expressive deficit, CDSS total score and the six MCCB neurocognitive domains (PS, A/V, WM, SLe, VLe and RPS) as independent variables. We could not include in the regression models the chlorpromazine equivalent dose, since we had this information only for a part of the participants. Therefore, to rule out the possible effects of pharmacological therapy on our results, we conducted Pearson’s correlations between chlorpromazine equivalent dose and the variables which had been included in the regression models.
The Statistical Package for the Social Sciences (IBM SPSS Statistics), Version 25, was used to conduct the statistical analyses.
3 Results
3.1 Sample characteristics
62 subjects with schizophrenia were included in the present analysis. The mean age of the sample was 37.92 ± 10.58 years. Participants had absent or mild positive symptoms and disorganization, and mild to moderate severe negative symptoms. Overall, patients showed deficits in all neurocognitive domains. Almost all patients were treated with antipsychotics, mostly second-generation drugs. Table 1 shows the demographic and clinical characteristics of the study sample.
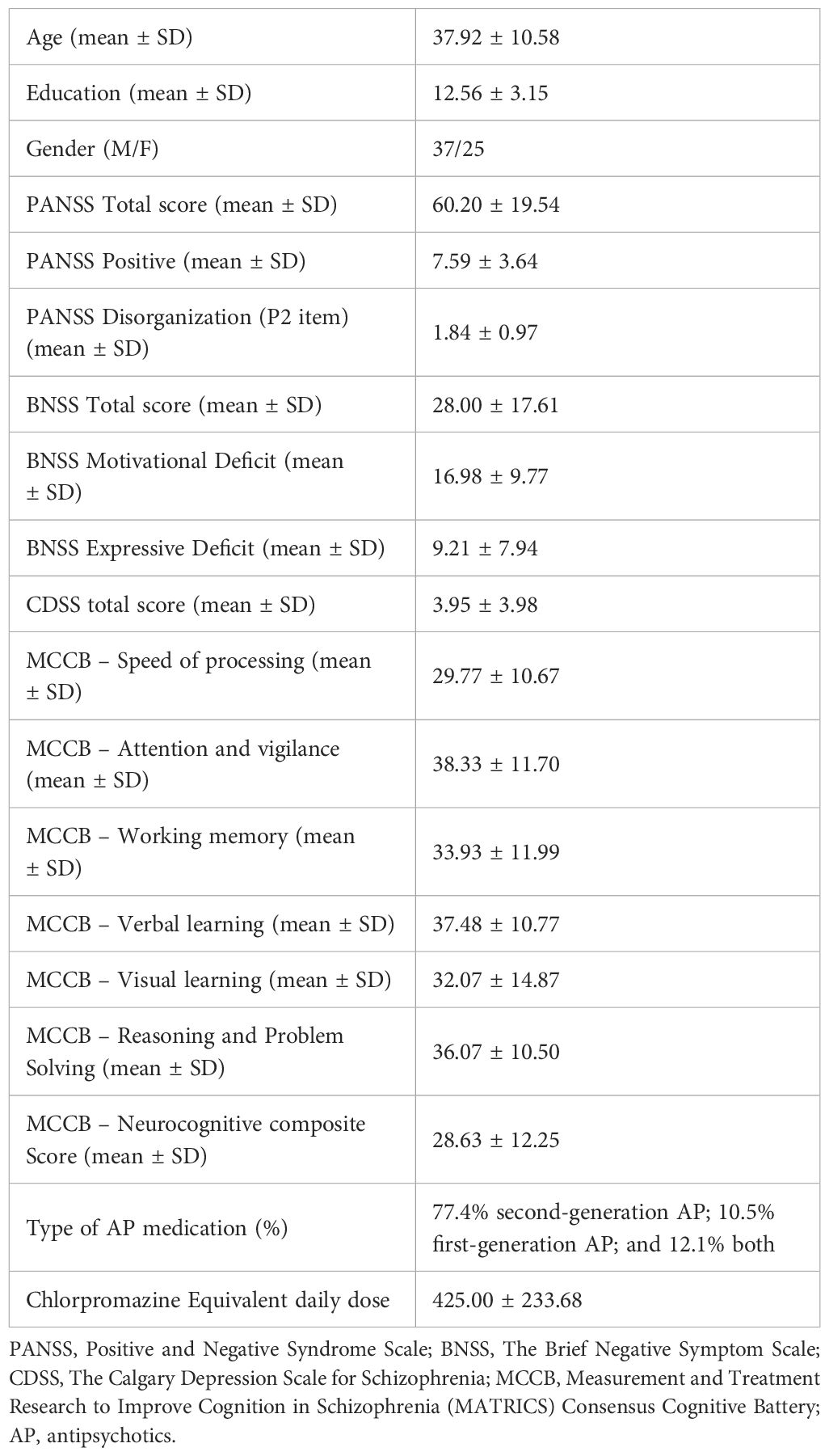
Table 1 Sociodemographic and clinical characteristics of patients with schizophrenia included in the study (N=62).
3.2 Regression analyses
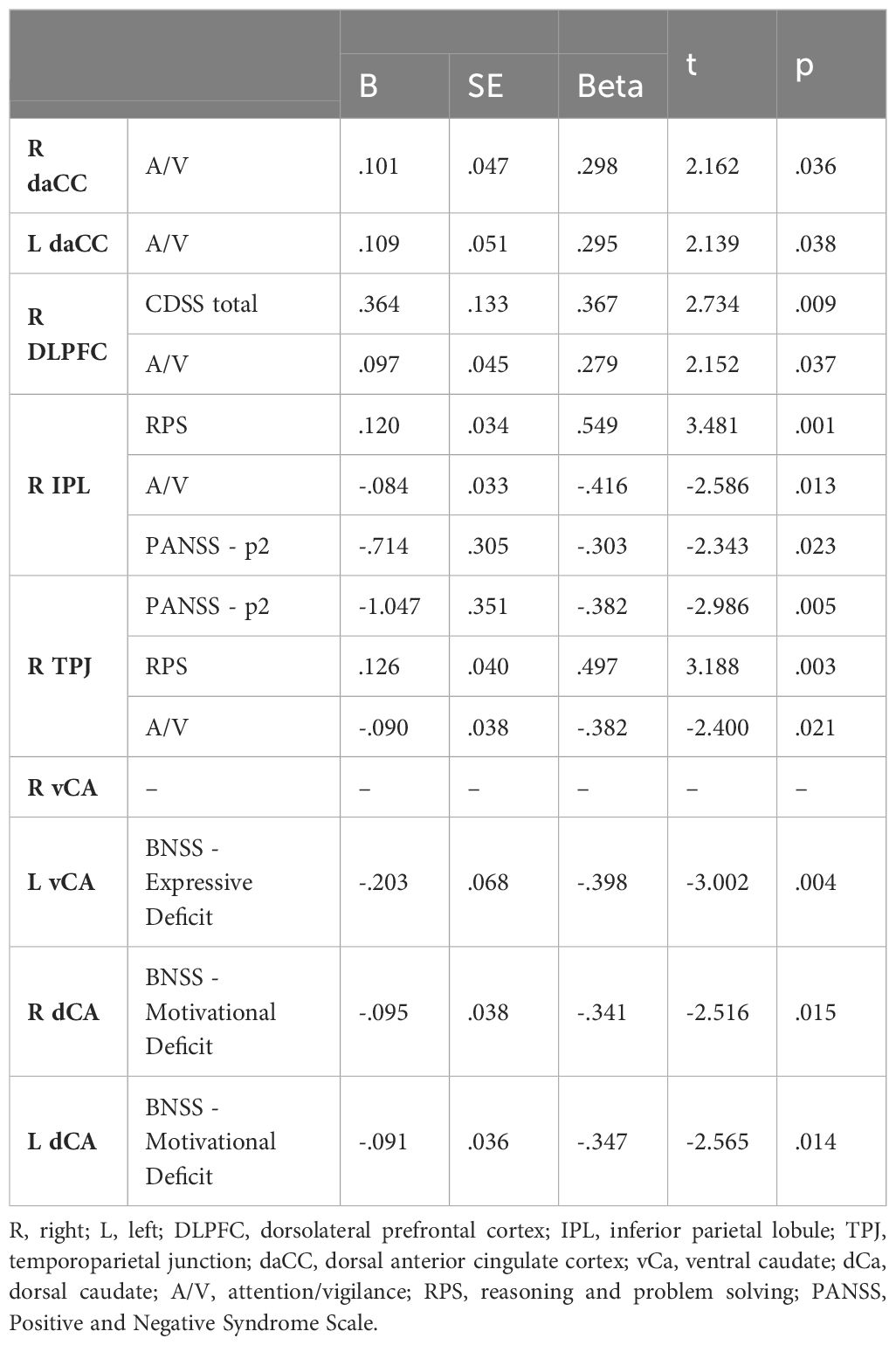
We found that neurocognitive functions and negative symptom domains were associated with alterations of the rs-fMRI activity in several brain regions.
A reduced rs-fMRI activity in the bilateral dACC was associated with a worse performance in A/V domain (right dACC: β = 0.298, p = 0.036; left dACC: β = 0.295, p = 0.038), independently from psychopathology and deficits in other cognitive domains. Furthermore, a reduced rs-fMRI activity in the right DLPFC was associated with a worse performance in the A/V domain (β =0.279, p =0.037) and a reduced severity of depressive symptoms (β = 0.367, p = 0.009). An increased rs-fMRI activity in the right IPL and TPJ was associated with a worse performance in A/V (IPL: β = -0.416, p = 0.013; TPJ: β = -0.382, p = 0.021), better performance in reasoning and problem-solving (IPL: β = 0.549, p = 0.001; TPJ: β = 0.497, p = 0.003), and a reduced severity of the conceptual disorganization (IPL: β = -0.303, p = 0.023; TPJ: β = -0.382, p = 0.005). As to the relationships between rs-fMRI activity and negative symptom domains, a reduced rs-fMRI activity in the left vCA was associated with a higher severity of the Expressive Deficit (β = -0.398, p = 0.004); a reduced ars-fMRI activity of the bilateral dCA was associated with a higher severity of the Motivational Deficit (right: β = -0.341, p = 0.015; left: β = -0.347, p = 0.014).
The results of regression analyses are reported in Table 2.
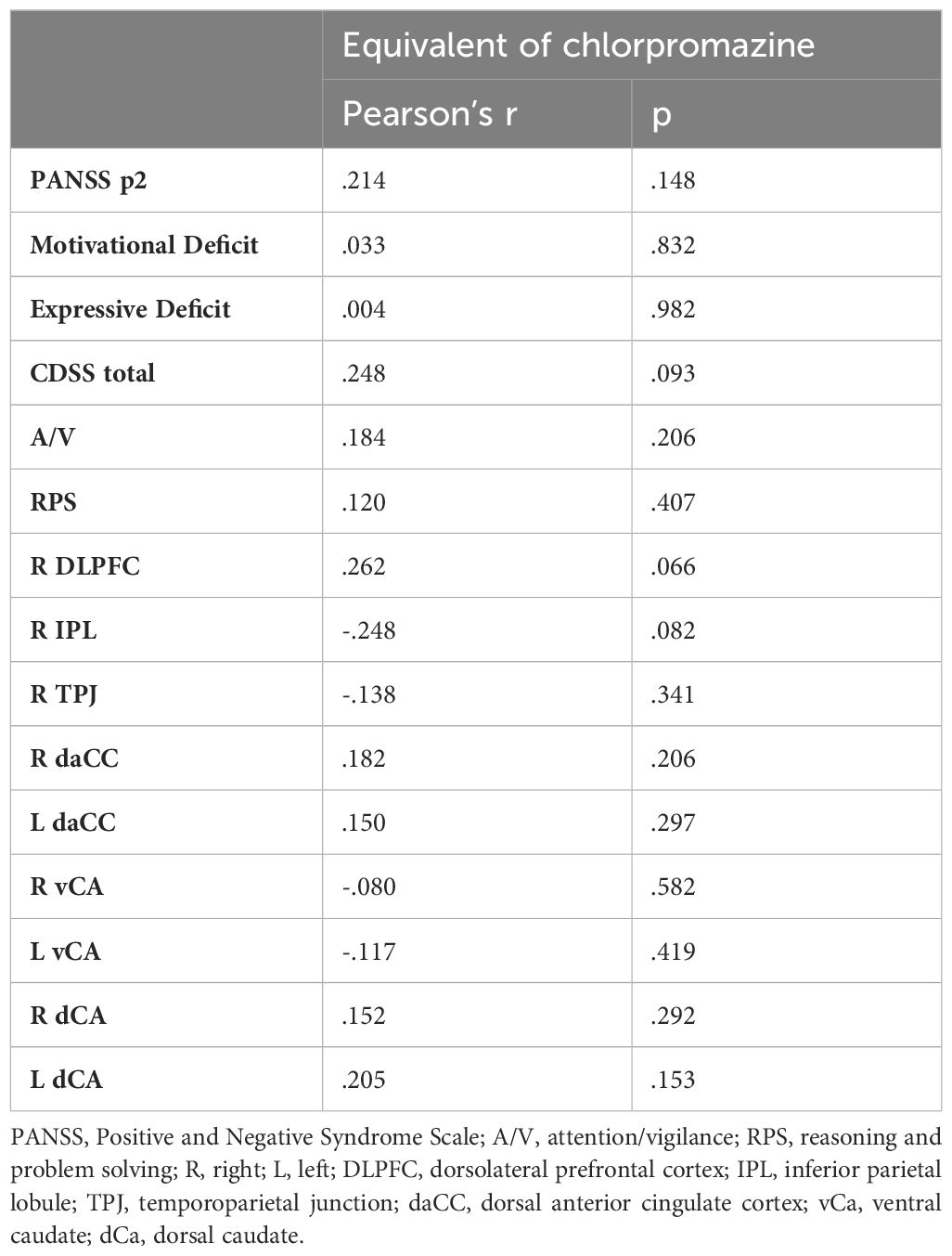
3.3 Control analysis
The chlorpromazine equivalent dose was available only for 50 subjects (81% of the sample). We found that the chlorpromazine equivalent dose did not correlate with any of the variables included in the regression models (Table 3).
4 Discussion
The current study aimed to investigate the relationships between the illness-related features of schizophrenia and abnormalities in resting-state brain activity.
The results of our study highlighted that positive symptoms were not associated with the rs brain activity in the selected ROIs, while cognitive impairment and negative symptoms had the highest number of associations. The lack of associations between positive symptoms and rs brain activity should be interpreted in the light of the characterization of our sample which is mainly composed by chronic patients, with absent or mild positive symptoms, but moderate to severe negative symptoms and cognitive deficits.
Our results concerning the associations of negative symptoms and cognitive deficits with alterations of rs-fMRI activity in different ROIs are in line with recent literature reporting that, although these two aspects are often associated in chronic patients, they represent separate constructs subtended by alterations of different neural pathways (83).
Among neurocognitive deficits, impairment in the A/V domain was related to the reduced rs-activity of the right DLPFC and bilateral daCC, and the increased activity of the right TPJ and IPL. The direct relationship between A/V and the activity of the right DLPFC and bilateral daCC is in line with the majority of previous literature results (84–87), although a few studies did not report this association (88, 89). Moreover, the DLPFC and the daCC are strongly related to each other, forming nodes in several networks, and they are involved in different cognitive processes, particularly in the A/V (84, 87, 90–93). While the activity of the bilateral daCC was predicted only by cognitive dysfunctions, the reduced activity of the right DLPFC was associated also with a lower severity of depressive symptoms. This result could be interpreted in the light of a previous study that investigated the specific neural correlates of depressive symptoms in schizophrenia using a task-based fMRI (74). This study showed that the activation of the middle frontal gyrus (which roughly corresponds to the DLPFC), as well as the inferior and precentral gyri, the left thalamus, putamen, globus pallidus and insular lobe in response to fearful expressions during an implicit affect processing task (74) was associated with the severity of depressive symptoms. However, drawing conclusion on the relationship between depressive symptoms in schizophrenia and DLPFC is difficult since the literature on the topic is scarce (74). Therefore, further studies are needed, in order to replicate these findings.
Furthermore, deficits in the A/V, together with deficits in RPS, and conceptual disorganization were associated with the rs activity of the right TPJ and the right IPL. The inverse relationship between the activity of these regions and attention in SCZs is in line with previous findings showing that, in these patients, lower levels of attention were associated to increased activity of the right TPJ and right IPL, and to a reduced connectivity between the right TPJ and other brain regions such as posterior cingulate cortex or DLPFC (94–97). Evidence from neuroimaging studies suggested that the right TPJ and IPL are connected to each other and represent nodes in several brain networks, such as default mode network and the ventral attention network (98). This pathway is involved in a broad range of functions, such as the maintaining and reorienting of attention, reasoning and problem solving, memory, executive functions and social cognition (98–105). Moreover, the activity of IPL and TPJ is strongly lateralized (98). Indeed, in line with our findings, the literature suggests that the A/V functions are sustained by the right lateralized activity of the IPL and TPJ, while inconsistent findings are reported on the RPS functions, which are reported either to be subtended by lateralized left brain activity (106, 107), either not-lateralized brain activity (108–111). Furthermore, according to previous studies (67, 112, 113), we found that the rs hyperactivity in the right IPL and TPJ was associated also with a better performance in RPS and less severe conceptual disorganization. This finding might suggest a partial overlap between disorganization and some cognitive functions, such as A/V and RPS, as reported in some studies (114–119).
In our study the severity of negative symptoms was related to the rs hypoactivity of the caudate. In particular, the Expressive Deficit was associated to the activity of the right ventral caudate, while the Motivational Deficit to the bilateral dorsal caudate. This finding, in line with the literature, supports the hypothesis that the two negative symptom domains might show different neurophysiological correlates (22, 37, 50, 51, 120, 121). Particularly, the relationship between the severity of the Expressive Deficit domain and the resting-state hypoactivity of the left vCa in SCZs was already showed in our previous study (37), but it is in contrast with the literature on topic, that reported associations between the ventral caudate hypoactivity and the Motivational Deficit domain or the negative symptoms belonging to it (42, 122–133). To our knowledge only one rs-fMRI study already reported an association between alterations of the caudate activity and the severity of Expressive Deficit (134), without differentiating the dorsal and the ventral part of the nucleus. On the other side, we found an inverse association between the severity of Motivational Deficit domain and the rs activity of bilateral dorsal caudate. Our findings confirm the results of previous studies which reported the same correlation pattern in SCZs (37, 43, 135), thus supporting the hypothesis of dysfunctions within the motivational patwhay. Indeed, the dorsal caudate is part of the dorsal striatum and it is involved in the motivational value system. Furthermore, this brain region is engaged in coding associations between actions/stimuli and outcomes in goal-directed behaviors and in selecting actions based on their currently predicted reward value (136).
It is important to acknowledge some limitations that may affect the generalizability and reproducibility of the study findings. First, the sample size was relatively small, and included only clinically stable, treated subjects, thus limiting the possibility of generalizing the results. Therefore, further studies with larger samples, including drug-naïve subjects, are needed to replicate these findings. In addition, although we performed control analyses investigating the effect of chlorpromazine equivalent dose on the activity of the ROIs and the clinical features involved in the analyses, we could not include this variable into the regression models since we had this information only for a part of the participants. Therefore, additional studies including drug-naïve subjects are needed to confirm our findings. Another limitation is the reliance solely on cross-sectional data. Longitudinal designs offer advantages in enhancing the power of associations between brain measures and clinical and cognitive variables, as they allow for the observation of changes over time within the same individuals. Future research could benefit from incorporating additional timepoints per subject to strengthen the observed relationships (137). Additionally, our study was limited to resting-state fMRI data. Resting-state fMRI could overcome issues related to the study of task-related activation/functional connectivity that might result in specious findings due to the poor intellectual capacities or memory impairments frequently present in subjects with schizophrenia. However, task-based fMRI has been shown to yield stronger predictions of behavior, particularly cognition, and incorporating such paradigms in future research may provide a more comprehensive understanding of the neural correlates of negative and cognitive symptoms (138). Therefore, future studies should consider using both resting-state and task-based fMRI to improve the robustness and generalizability of the findings.
5 Conclusions
In conclusion, the results of the present study highlight that a detailed and comprehensive assessment of psychopathology and cognitive performance is a crucial step to improve our knowledge about neural correlate of schizophrenia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Università degli Studi della Campania”Luigi Vanvitelli”—Azienda Ospedaliera Universitaria “Luigi Vanvitelli”, A.O.R.N. “Ospedali dei Colli” (protocol code 202, 10 March 2020). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. PP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. GG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. LF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AM: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AP: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. GB: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. MA: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. PR: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AR: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AB: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. SG: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. MM: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Group members of Italian Network for Research on Psychoses
Antonio Melillo (University of Campania “Luigi Vanvitelli”, Naples), Cecilia Riccardi, Cristiana Montemagni, Daria Pietrafesa, Edoardo Caporusso, Eleonora Merlotti, Elisa Del Favero (University of Turin), Francesca Pacitti, Francesco Brando, Giuseppe Piegari, Marco Papalino, Martino Belvedere Murri, Noemi Sansone, Paola Bucci, Pietro Calcagno, Raffaella Romano (University of Bari), Rodolfo Rossi, Simone Cattedra (University of Genoa), Valentina Socci (University of L’Aquila), Vitalba Calia.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the PRIN 2017 project from the Italian Minister of Education, University and Research “Factors influencing real-life functioning of people with a diagnosis of schizophrenia: a four-year follow-up multicenter study” (grant number: 2017M7SZM8).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1458624/full#supplementary-material
References
1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1211–59. doi: 10.1016/s0140-6736(17)32154-2
2. Correll CU, Solmi M, Croatto G, Schneider LK, Rohani-Montez SC, Fairley L, et al. Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry. (2022) 21:248–71. doi: 10.1002/wps.20994
3. Sass L. Subjectivity, psychosis and the science of psychiatry. World Psychiatry. (2022) 21:165–6. doi: 10.1002/wps.20986
4. Mesholam-Gately RI, Johnston D, Keshavan MS. What’s in the name “schizophrenia”? A clinical, research and lived experience perspective. World Psychiatry. (2023) 22:156–7. doi: 10.1002/wps.21033
5. McCutcheon RA, Pillinger T, Efthimiou O, Maslej M, Mulsant BH, Young AH, et al. Reappraising the variability of effects of antipsychotic medication in schizophrenia: a meta-analysis. World Psychiatry. (2022) 21:287–94. doi: 10.1002/wps.20977
6. Galderisi S, Rucci P, Kirkpatrick B, Mucci A, Gibertoni D, Rocca P, et al. Interplay among psychopathologic variables, personal resources, context-related factors, and real-life functioning in individuals with schizophrenia: A network analysis. JAMA Psychiatry. (2018) 75:396–404. doi: 10.1001/jamapsychiatry.2017.4607
7. Galderisi S, Rucci P, Mucci A, Rossi A, Rocca P, Bertolino A, et al. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry. (2020) 19:81–91. doi: 10.1002/wps.20700
8. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. (2012) 17:1228–38. doi: 10.1038/mp.2012.23
9. Mucci A, Galderisi S, Gibertoni D, Rossi A, Rocca P, Bertolino A, et al. Factors associated with real-life functioning in persons with schizophrenia in a 4-year follow-up study of the Italian network for research on psychoses. JAMA Psychiatry. (2021) 78:550–9. doi: 10.1001/jamapsychiatry.2020.4614
10. Giordano GM, Bucci P, Mucci A, Pezzella P, Galderisi S. Gender differences in clinical and psychosocial features among persons with schizophrenia: A mini review. Front Psychiatry. (2021) 12:789179. doi: 10.3389/fpsyt.2021.789179
11. Watson D, Levin-Aspenson HF, Waszczuk MA, Conway CC, Dalgleish T, Dretsch MN, et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry. (2022) 21:26–54. doi: 10.1002/wps.20943
12. Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. (2014) 13:275–87. doi: 10.1002/wps.20167
13. Fusar-Poli P, Estradé A, Stanghellini G, Venables J, Onwumere J, Messas G, et al. The lived experience of psychosis: a bottom-up review co-written by experts by experience and academics. World Psychiatry. (2022) 21:168–88. doi: 10.1002/wps.20959
14. Schäfer SK, Thomas LM, Lindner S, Lieb K. World Health Organization’s low-intensity psychosocial interventions: a systematic review and meta-analysis of the effects of Problem Management Plus and Step-by-Step. World Psychiatry. (2023) 22:449–62. doi: 10.1002/wps.21129
15. Galderisi S, Mucci A, Dollfus S, Nordentoft M, Falkai P, Kaiser S, et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur Psychiatry. (2021) 64:e23. doi: 10.1192/j.eurpsy.2021.11
16. Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry. (2012) 11:73–9. doi: 10.1016/j.wpsyc.2012.05.004
17. Ventura J. Computer-based virtual reality assessment of functional capacity in primary psychosis. World Psychiatry. (2022) 21:464–5. doi: 10.1002/wps.21024
18. Vita A, Gaebel W, Mucci A, Sachs G, Barlati S, Giordano GM, et al. European Psychiatric Association guidance on treatment of cognitive impairment in schizophrenia. Eur Psychiatry. (2022) 65:e57. doi: 10.1192/j.eurpsy.2022.2315
19. Galderisi S. Promoting schizophrenia research in Europe: the contribution of the European Group for Research in Schizophrenia. World Psychiatry. (2023) 22:486–7. doi: 10.1002/wps.21100
20. Brady LS, Larrauri CA. Accelerating Medicines Partnership(®) Schizophrenia (AMP(®) SCZ): developing tools to enable early intervention in the psychosis high risk state. World Psychiatry. (2023) 22:42–3. doi: 10.1002/wps.21038
21. Dragioti E, Radua J, Solmi M, Gosling CJ, Oliver D, Lascialfari F, et al. Impact of mental disorders on clinical outcomes of physical diseases: an umbrella review assessing population attributable fraction and generalized impact fraction. World Psychiatry. (2023) 22:86–104. doi: 10.1002/wps.21068
22. Giordano GM, Caporusso E, Pezzella P, Galderisi S. Updated perspectives on the clinical significance of negative symptoms in patients with schizophrenia. Expert Rev Neurother. (2022) 22:541–55. doi: 10.1080/14737175.2022.2092402
23. Maj M, van Os J, De Hert M, Gaebel W, Galderisi S, Green MF, et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry. (2021) 20:4–33. doi: 10.1002/wps.20809
24. Correll CU, Schooler NR. Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. (2020) 16:519–34. doi: 10.2147/NDT
25. Starzer M, Hansen HG, Hjorthøj C, Albert N, Nordentoft M, Madsen T. 20-year trajectories of positive and negative symptoms after the first psychotic episode in patients with schizophrenia spectrum disorder: results from the OPUS study. World Psychiatry. (2023) 22:424–32. doi: 10.1002/wps.21121
26. Siskind D, Yung A. After the acute crisis - engaging people with psychosis in rehabilitation-oriented care. World Psychiatry. (2022) 21:246–7. doi: 10.1002/wps.20970
27. Ostuzzi G, Bertolini F, Tedeschi F, Vita G, Brambilla P, Del Fabro L, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry. (2022) 21:295–307. doi: 10.1002/wps.20972
28. Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. (2018) 5:664–77. doi: 10.1016/S2215-0366(18)30050-6
29. Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. (2017) 16:14–24. doi: 10.1002/wps.20385
30. Kirkpatrick B, Fenton WS, Carpenter WT Jr., Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. (2006) 32:214–9. doi: 10.1093/schbul/sbj053
31. Vita A, Gaebel W, Mucci A, Sachs G, Erfurth A, Barlati S, et al. European Psychiatric Association guidance on assessment of cognitive impairment in schizophrenia. Eur Psychiatry. (2022) 65:e58. doi: 10.1192/j.eurpsy.2022.2316
32. Perrottelli A, Giordano GM, Brando F, Giuliani L, Pezzella P, Mucci A, et al. Unveiling the associations between EEG indices and cognitive deficits in schizophrenia-spectrum disorders: A systematic review. Diagnost (Basel). (2022) 12:2193. doi: 10.3390/diagnostics12092193
33. McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. (2023) 28:1902–18. doi: 10.1038/s41380-023-01949-9
34. Keshavan MS, Eack SM. Cognitive enhancement interventions are effective for schizophrenia: why not provide them early? World Psychiatry. (2023) 22:326–7. doi: 10.1002/wps.21091
35. Giordano GM, Brando F, Pezzella P, De Angelis M, Mucci A, Galderisi S. Factors influencing the outcome of integrated therapy approach in schizophrenia: A narrative review of the literature. Front Psychiatry. (2022) 13:970210. doi: 10.3389/fpsyt.2022.970210
36. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. (2004) 72:41–51. doi: 10.1016/j.schres.2004.09.009
37. Giordano GM, Pezzella P, Giuliani L, Fazio L, Mucci A, Perrottelli A, et al. Resting-state brain activity dysfunctions in schizophrenia and their associations with negative symptom domains: an fMRI study. Brain Sci. (2023) 13:83. doi: 10.3390/brainsci13010083
38. Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: A meta-analysis of resting-state functional connectivity. Schizophr Bull. (2018) 44:168–81. doi: 10.1093/schbul/sbx034
39. Abi-Dargham A, Moeller SJ, Ali F, DeLorenzo C, Domschke K, Horga G, et al. Candidate biomarkers in psychiatric disorders: state of the field. World Psychiatry. (2023) 22:236–62. doi: 10.1002/wps.21078
40. Berk M. Biomarkers in psychiatric disorders: status quo, impediments and facilitators. World Psychiatry. (2023) 22:174–6. doi: 10.1002/wps.21071
41. Bègue I, Kaiser S, Kirschner M. Pathophysiology of negative symptom dimensions of schizophrenia - Current developments and implications for treatment. Neurosci Biobehav Rev. (2020) 116:74–88. doi: 10.1016/j.neubiorev.2020.06.004
42. Kirschner M, Hager OM, Bischof M, Hartmann MN, Kluge A, Seifritz E, et al. Ventral striatal hypoactivation is associated with apathy but not diminished expression in patients with schizophrenia. J Psychiatry Neurosci. (2016) 41:152–61. doi: 10.1503/jpn.140383
43. Mucci A, Dima D, Soricelli A, Volpe U, Bucci P, Frangou S, et al. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol Med. (2015) 45:1765–78. doi: 10.1017/S0033291714002943
44. Mucci A, Galderisi S, Amodio A, Dierks T. Neuroimaging and psychopathological domains: achievements and perspectives. In: Galderisi S, DeLisi L, Borgwardt S, editors. Neuroimaging of Schizophrenia and Other Primary Psychotic Disorders. Springer, Cham. (2019), 57–155. doi: 10.1007/978-3-319-97307-4_2
45. Shukla DK, Chiappelli JJ, Sampath H, Kochunov P, Hare SM, Wisner K, et al. Aberrant frontostriatal connectivity in negative symptoms of schizophrenia. Schizophr Bull. (2019) 45:1051–9. doi: 10.1093/schbul/sby165
46. Tu PC, Hsieh JC, Li CT, Bai YM, Su TP. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. Neuroimage. (2012) 59:238–47. doi: 10.1016/j.neuroimage.2011.07.086
47. Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. (2013) 70:1143–51. doi: 10.1001/jamapsychiatry.2013.1976
48. Forlim CG, Klock L, Bächle J, Stoll L, Giemsa P, Fuchs M, et al. Reduced resting-state connectivity in the precuneus is correlated with apathy in patients with schizophrenia. Sci Rep. (2020) 10:2616. doi: 10.1038/s41598-020-59393-6
49. Abram SV, Wisner KM, Fox JM, Barch DM, Wang L, Csernansky JG, et al. Fronto-temporal connectivity predicts cognitive empathy deficits and experiential negative symptoms in schizophrenia. Hum Brain Mapp (2017) 38(3):1111–24. doi: 10.1002/hbm.23439
50. Giordano GM, Koenig T, Mucci A, Vignapiano A, Amodio A, Di Lorenzo G, et al. Neurophysiological correlates of Avolition-apathy in schizophrenia: A resting-EEG microstates study. NeuroImage Clin. (2018) 20:627–36. doi: 10.1016/j.nicl.2018.08.031
51. Giordano GM, Brando F, Perrottelli A, Di Lorenzo G, Siracusano A, Giuliani L, et al. Tracing links between early auditory information processing and negative symptoms in schizophrenia: an ERP study. Front Psychiatry. (2021) 12:790745. doi: 10.3389/fpsyt.2021.790745
52. Giordano GM, Pezzella P, Quarantelli M, Bucci P, Prinster A, Soricelli A, et al. Investigating the relationship between white matter connectivity and motivational circuits in subjects with deficit schizophrenia: A diffusion tensor imaging (DTI) study. J Clin Med. (2021) 11:61. doi: 10.3390/jcm11010061
53. Klingberg T, Judd N, Sauce B. Assessing the impact of environmental factors on the adolescent brain: the importance of regional analyses and genetic controls. World Psychiatry. (2022) 21:146–7. doi: 10.1002/wps.20934
54. Mingoia G, Wagner G, Langbein K, Maitra R, Smesny S, Dietzek M, et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res. (2012) 138:143–9. doi: 10.1016/j.schres.2012.01.036
55. Hare SM, Ford JM, Mathalon DH, Damaraju E, Bustillo J, Belger A, et al. Salience-default mode functional network connectivity linked to positive and negative symptoms of schizophrenia. Schizophr Bull. (2019) 45:892–901. doi: 10.1093/schbul/sby112
56. Gourion D, Goldberger C, Olie JP, Lôo H, Krebs MO. Neurological and morphological anomalies and the genetic liability to schizophrenia: a composite phenotype. Schizophr Res. (2004) 67:23–31. doi: 10.1016/S0920-9964(03)00099-9
57. Tripathi A, Kar SK, Shukla R. Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clin Psychopharmacol Neurosci. (2018) 16:7–17. doi: 10.9758/cpn.2018.16.1.7
59. Picó-Pérez M, Vieira R, Fernández-Rodríguez M, De Barros MAP, Radua J, Morgado P. Multimodal meta-analysis of structural gray matter, neurocognitive and social cognitive fMRI findings in schizophrenia patients. Psychol Med. (2022) 52:614–24. doi: 10.1017/S0033291721005523
60. Gong J, Wang J, Luo X, Chen G, Huang H, Huang R, et al. Abnormalities of intrinsic regional brain activity in first-episode and chronic schizophrenia: a meta-analysis of resting-state functional MRI. J Psychiatry Neurosci. (2020) 45:55–68. doi: 10.1503/jpn.180245
61. Wylie KP, Harris JG, Ghosh D, Olincy A, Tregellas JR. Association of working memory with distributed executive control networks in schizophrenia. J Neuropsychiatry Clin Neurosci. (2019) 31:368–77. doi: 10.1176/appi.neuropsych.18060131
62. Wang J, Kochunov P, Sampath H, Hatch KS, Ryan MC, Xue F, et al. White matter brain aging in relationship to schizophrenia and its cognitive deficit. Schizophr Res. (2021) 230:9–16. doi: 10.1016/j.schres.2021.02.003
63. Li X, Liu Q, Chen Z, Li Y, Yang Y, Wang X, et al. Abnormalities of regional brain activity in patients with schizophrenia: A longitudinal resting-state fMRI study. Schizophr Bull. (2023) 49:1336–44. doi: 10.1093/schbul/sbad054
64. Pinkham A, Loughead J, Ruparel K, Wu WC, Overton E, Gur R, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. (2011) 194:64–72. doi: 10.1016/j.pscychresns.2011.06.013
65. Percie du Sert O, Unrau J, Gauthier CJ, Chakravarty M, Malla A, Lepage M, et al. Cerebral blood flow in schizophrenia: A systematic review and meta-analysis of MRI-based studies. Prog Neuropsychopharmacol Biol Psychiatry. (2023) 121:110669. doi: 10.1016/j.pnpbp.2022.110669
66. Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. (2010) 117:21–30. doi: 10.1016/j.schres.2010.01.001
67. Cavelti M, Kircher T, Nagels A, Strik W, Homan P. Is formal thought disorder in schizophrenia related to structural and functional aberrations in the language network? A systematic review of neuroimaging findings. Schizophr Res. (2018) 199:2–16. doi: 10.1016/j.schres.2018.02.051
68. Cavelti M, Winkelbeiner S, Federspiel A, Walther S, Stegmayer K, Giezendanner S, et al. Formal thought disorder is related to aberrations in language-related white matter tracts in patients with schizophrenia. Psychiatry Res Neuroimaging. (2018) 279:40–50. doi: 10.1016/j.pscychresns.2018.05.011
69. Ćurčić-Blake B, Ford JM, Hubl D, Orlov ND, Sommer IE, Waters F, et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog Neurobiol. (2017) 148:1–20. doi: 10.1016/j.pneurobio.2016.11.002
70. Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. (2004) 61:658–68. doi: 10.1001/archpsyc.61.7.658
71. Stein F, Gruber M, Mauritz M, Brosch K, Pfarr JK, Ringwald KG, et al. Brain structural network connectivity of formal thought disorder dimensions in affective and psychotic disorders. Biol Psychiatry. (2023) 95(7):629–38. doi: 10.1016/j.biopsych.2023.05.010
72. Kircher T, Bröhl H, Meier F, Engelen J. Formal thought disorders: from phenomenology to neurobiology. Lancet Psychiatry. (2018) 5:515–26. doi: 10.1016/S2215-0366(18)30059-2
73. Kircher TT, Bulimore ET, Brammer MJ, Williams SC, Broome MR, Murray RM, et al. Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophr Res. (2001) 50:27–40. doi: 10.1016/S0920-9964(00)00042-6
74. Kumari V, Peters E, Guinn A, Fannon D, Russell T, Sumich A, et al. Mapping depression in schizophrenia: A functional magnetic resonance imaging study. Schizophr Bull. (2016) 42:802–13. doi: 10.1093/schbul/sbv186
75. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
76. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. (2012) 137:246–50. doi: 10.1016/j.schres.2012.01.031
77. Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. (2011) 37:300–5. doi: 10.1093/schbul/sbq059
78. Mucci A, Galderisi S, Merlotti E, Rossi A, Rocca P, Bucci P, et al. The Brief Negative Symptom Scale (BNSS): Independent validation in a large sample of Italian patients with schizophrenia. Eur Psychiatry. (2015) 30:641–7. doi: 10.1016/j.eurpsy.2015.01.014
79. Addington J, Shah H, Liu L, Addington D. Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophr Res. (2014) 153:64–7. doi: 10.1016/j.schres.2013.12.014
80. Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. (2008) 165:203–13. doi: 10.1176/appi.ajp.2007.07010042
81. Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. (2008) 165:214–20. doi: 10.1176/appi.ajp.2007.07010043
82. Mucci A, Galderisi S, Green MF, Nuechterlein K, Rucci P, Gibertoni D, et al. Familial aggregation of MATRICS Consensus Cognitive Battery scores in a large sample of outpatients with schizophrenia and their unaffected relatives. Psychol Med. (2018) 48:1359–66. doi: 10.1017/S0033291717002902
83. Giordano GM, Perrottelli A, Mucci A, Di Lorenzo G, Altamura M, Bellomo A, et al. Investigating the relationships of P3b with negative symptoms and neurocognition in subjects with chronic schizophrenia. Brain Sci. (2021) 11:1632. doi: 10.3390/brainsci11121632
84. Woodcock EA, Wadehra S, Diwadkar VA. Network profiles of the dorsal anterior cingulate and dorsal prefrontal cortex in schizophrenia during hippocampal-based associative memory. Front Syst Neurosci. (2016) 10:32. doi: 10.3389/fnsys.2016.00032
85. Choi JW, Jeong BS, Kim JW. Dysfunction of the left dorsolateral prefrontal cortex is primarily responsible for impaired attentional processing in schizophrenia. Psychiatry Investig. (2008) 5:52–9. doi: 10.4306/pi.2008.5.1.52
86. MacDonald AW 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. (2005) 162:475–84. doi: 10.1176/appi.ajp.162.3.475
87. Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, et al. Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res. (2003) 123:1–15. doi: 10.1016/S0925-4927(03)00019-2
88. Huang ML, Khoh TT, Lu SJ, Pan F, Chen JK, Hu JB, et al. Relationships between dorsolateral prefrontal cortex metabolic change and cognitive impairment in first-episode neuroleptic-naive schizophrenia patients. Med (Baltimore). (2017) 96:e7228. doi: 10.1097/MD.0000000000007228
89. Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. (2007) 64:1356–66. doi: 10.1001/archpsyc.64.12.1356
90. MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. (2000) 288:1835–8. doi: 10.1126/science.288.5472.1835
91. Zhang L, Gläscher J. A brain network supporting social influences in human decision-making. Sci Adv. (2020) 6:eabb4159. doi: 10.1126/sciadv.abb4159
92. Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, Roesch MR. Attention for learning signals in anterior cingulate cortex. J Neurosci. (2011) 31:18266–74. doi: 10.1523/JNEUROSCI.4715-11.2011
93. Kerns JG, Cohen JD, MacDonald AW 3rd, Johnson MK, Stenger VA, Aizenstein H, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. (2005) 162:1833–9. doi: 10.1176/appi.ajp.162.10.1833
94. Wible CG. Hippocampal temporal-parietal junction interaction in the production of psychotic symptoms: a framework for understanding the schizophrenic syndrome. Front Hum Neurosci. (2012) 6:180. doi: 10.3389/fnhum.2012.00180
95. Penner J, Osuch EA, Schaefer B, Théberge J, Neufeld RWJ, Menon RS, et al. Temporoparietal junction functional connectivity in early schizophrenia and major depressive disorder. Chronic Stress (Thousand Oaks). (2018) 2:2470547018815232. doi: 10.1177/2470547018815232
96. Ojeda N, Ortuño F, Arbizu J, López P, Martí-Climent JM, Peñuelas I, et al. Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Hum Brain Mapp. (2002) 17:116–30. doi: 10.1002/hbm.10055
97. Carter RM, Bowling DL, Reeck C, Huettel SA. A distinct role of the temporal-parietal junction in predicting socially guided decisions. Science. (2012) 337:109–11. doi: 10.1126/science.1219681
98. Igelström KM, Graziano MSA. The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia. (2017) 105:70–83. doi: 10.1016/j.neuropsychologia.2017.01.001
99. Numssen O, Bzdok D, Hartwigsen G. Functional specialization within the inferior parietal lobes across cognitive domains. Elife. (2021) 10:e63591. doi: 10.7554/eLife.63591
100. Ahmad N, Zorns S, Chavarria K, Brenya J, Janowska A, Keenan J. Are we right about the right TPJ? A review of brain stimulation and social cognition in the right temporal parietal junction. Symmetry. (2021) 13:2219. doi: 10.3390/sym13112219
101. Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci. (2003) 4:26–36. doi: 10.1038/nrn1005
102. Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. (2007) 11:30–6. doi: 10.1016/j.tics.2006.10.011
103. Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. (2009) 47:1434–48. doi: 10.1016/j.neuropsychologia.2008.11.033
104. Igelström KM, Webb TW, Graziano MS. Neural processes in the human temporoparietal cortex separated by localized independent component analysis. J Neurosci. (2015) 35:9432–45. doi: 10.1523/JNEUROSCI.0551-15.2015
105. Igelström KM, Webb TW, Kelly YT, Graziano MS. Topographical organization of attentional, social, and memory processes in the human temporoparietal cortex. eNeuro. (2016) 3:ENEURO.0060-16.2016. doi: 10.1523/ENEURO.0060-16.2016
106. Chochon F, Cohen L, Moortele P, Dehaene S. Differential contributions of the left and right inferior parietal lobules to number processing. J Cogn Neurosci. (1999) 11:617–30. doi: 10.1162/089892999563689%
107. Federico G, Reynaud E, Navarro J, Lesourd M, Gaujoux V, Lamberton F, et al. The cortical thickness of the area PF of the left inferior parietal cortex mediates technical-reasoning skills. Sci Rep. (2022) 12:11840. doi: 10.1038/s41598-022-15587-8
108. Desco M, Navas-Sanchez FJ, Sanchez-González J, Reig S, Robles O, Franco C, et al. Mathematically gifted adolescents use more extensive and more bilateral areas of the fronto-parietal network than controls during executive functioning and fluid reasoning tasks. Neuroimage. (2011) 57:281–92. doi: 10.1016/j.neuroimage.2011.03.063
109. Vickery TJ, Jiang YV. Inferior parietal lobule supports decision making under uncertainty in humans. Cereb Cortex. (2008) 19:916–25. doi: 10.1093/cercor/bhn140%
110. Newman SD, Carpenter PA, Varma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. (2003) 41:1668–82. doi: 10.1016/S0028-3932(03)00091-5
111. Huang F, Tang S, Sun P, Luo J. Neural correlates of novelty and appropriateness processing in externally induced constraint relaxation. Neuroimage. (2018) 172:381–9. doi: 10.1016/j.neuroimage.2018.01.070
112. Müller VI, Cieslik EC, Laird AR, Fox PT, Eickhoff SB. Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization. Front Hum Neurosci. (2013) 7:268. doi: 10.3389/fnhum.2013.00268
113. Bor J, Brunelin J, d’Amato T, Costes N, Suaud-Chagny M-F, Saoud M, et al. How can cognitive remediation therapy modulate brain activations in schizophrenia?: An fMRI study. Psychiatry Res: Neuroimaging. (2011) 192:160–6. doi: 10.1016/j.pscychresns.2010.12.004
114. Vignapiano A, Koenig T, Mucci A, Giordano GM, Amodio A, Altamura M, et al. Disorganization and cognitive impairment in schizophrenia: New insights from electrophysiological findings. Int J Psychophysiol. (2019) 145:99–108. doi: 10.1016/j.ijpsycho.2019.03.008
115. Ventura J, Wood RC, Jimenez AM, Hellemann GS. Neurocognition and symptoms identify links between facial recognition and emotion processing in schizophrenia: Meta-analytic findings. Schizophr Res. (2013) 151:78–84. doi: 10.1016/j.schres.2013.10.015
116. Bell MD, Lysaker PH, Milstein RM, Beam-Goulet JL. Concurrent validity of the cognitive component of schizophrenia: Relationship of PANSS scores to neuropsychological assessments. Psychiatry Res. (1994) 54:51–8. doi: 10.1016/0165-1781(94)90064-7
117. Bryson G, Bell M, Greig T, Kaplan E. Internal consistency, temporal stability and neuropsychological correlates of three cognitive components of the Positive and Negative Syndrome Scale (PANSS). Schizophr Res. (1999) 38:27–35. doi: 10.1016/S0920-9964(99)00004-3
118. Dominguez Mde G, Viechtbauer W, Simons CJ, van Os J, Krabbendam L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol Bull. (2009) 135:157–71. doi: 10.1037/a0014415
119. Agurto C, Norel R, Wen B, Wei Y, Zhang D, Bilgrami Z, et al. Are language features associated with psychosis risk universal? A study in Mandarin-speaking youths at clinical high risk for psychosis. World Psychiatry. (2023) 22:157–8. doi: 10.1002/wps.21045
120. Kaiser S, Lyne J, Agartz I, Clarke M, Mørch-Johnsen L, Faerden A. Individual negative symptoms and domains – Relevance for assessment, pathomechanisms and treatment. Schizophr Res. (2017) 186:39–45. doi: 10.1016/j.schres.2016.07.013
121. Mørch-Johnsen L, Smelror RE, Andreou D, Barth C, Johannessen C, Wedervang-Resell K, et al. Negative symptom domains are associated with verbal learning in adolescents with early onset psychosis. Front Psychiatry. (2021) 12:825681. doi: 10.3389/fpsyt.2021.825681
122. Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, et al. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front Psychol. (2015) 6:1280. doi: 10.3389/fpsyg.2015.01280
123. Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. (2010) 67:902–11. doi: 10.1016/j.biopsych.2009.10.020
124. Leroy A, Amad A, D’Hondt F, Pins D, Jaafari N, Thomas P, et al. Reward anticipation in schizophrenia: A coordinate-based meta-analysis. Schizophr Res. (2020) 218:2–6. doi: 10.1016/j.schres.2019.12.041
125. Moran EK, Culbreth AJ, Kandala S, Barch DM. From neuroimaging to daily functioning: A multimethod analysis of reward anticipation in people with schizophrenia. J Abnorm Psychol. (2019) 128:723–34. doi: 10.1037/abn0000461
126. Nielsen MO, Rostrup E, Wulff S, Bak N, Broberg BV, Lublin H, et al. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. (2012) 69:1195–204. doi: 10.1001/archgenpsychiatry.2012.847
127. Schneider K, Michels L, Hartmann-Riemer MN, Burrer A, Tobler PN, Stämpfli P, et al. Cerebral blood flow in striatal regions is associated with apathy in patients with schizophrenia. J Psychiatry Neurosci. (2019) 44:102–10. doi: 10.1503/jpn.170150
128. Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, et al. Neural correlates of reward processing in schizophrenia–relationship to apathy and depression. Schizophr Res. (2010) 118:154–61. doi: 10.1016/j.schres.2009.11.007
129. Stepien M, Manoliu A, Kubli R, Schneider K, Tobler PN, Seifritz E, et al. Investigating the association of ventral and dorsal striatal dysfunction during reward anticipation with negative symptoms in patients with schizophrenia and healthy individuals. PloS One. (2018) 13:e0198215. doi: 10.1371/journal.pone.0198215
130. Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. (2009) 34:1567–77. doi: 10.1038/npp.2008.214
131. Waltz JA, Schweitzer JB, Ross TJ, Kurup PK, Salmeron BJ, Rose EJ, et al. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. (2010) 35:2427–39. doi: 10.1038/npp.2010.126
132. Waltz JA, Xu Z, Brown EC, Ruiz RR, Frank MJ, Gold JM. Motivational deficits in schizophrenia are associated with reduced differentiation between gain and loss-avoidance feedback in the striatum. Biol Psychiatry Cognit Neurosci Neuroimaging. (2018) 3:239–47. doi: 10.1016/j.bpsc.2017.07.008
133. Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA, et al. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull. (2014) 40:1328–37. doi: 10.1093/schbul/sbu026
134. Stip E, Fahim C, Liddle P, Mancini-Marïe A, Mensour B, Bentaleb LA, et al. Neural correlates of sad feelings in schizophrenia with and without blunted affect. Can J Psychiatry. (2005) 50:909–17. doi: 10.1177/070674370505001405
135. Morris RW, Quail S, Griffiths KR, Green MJ, Balleine BW. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol Psychiatry. (2015) 77:187–95. doi: 10.1016/j.biopsych.2014.06.005
136. Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. (2000) 20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000
137. Makowski C, Nichols TE, Dale AM. Quality over quantity: powering neuroimaging samples in psychiatry. Neuropsychopharmacology. (2024). doi: 10.1038/s41386-024-01893-4
Keywords: cognitive impairments, negative symptoms, expressive deficit domain, motivational deficit domain, biomarkers, resting-state fMRI
Citation: Giuliani L, Pezzella P, Giordano GM, Fazio L, Mucci A, Perrottelli A, Blasi G, Amore M, Rocca P, Rossi A, Bertolino A, Galderisi S and Maj M (2024) Illness-related variables and abnormalities of resting-state brain activity in schizophrenia. Front. Psychiatry 15:1458624. doi: 10.3389/fpsyt.2024.1458624
Received: 02 July 2024; Accepted: 17 July 2024;
Published: 06 August 2024.
Edited by:
Massimo Tusconi, University of Cagliari, ItalyReviewed by:
Serdar M. Dursun, University of Alberta, CanadaCristiano Chaves, Queen’s University, Canada
Copyright © 2024 Giuliani, Pezzella, Giordano, Fazio, Mucci, Perrottelli, Blasi, Amore, Rocca, Rossi, Bertolino, Galderisi and Maj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Maria Giordano, Z2l1bGlhbWdpb3JkYW5vQGdtYWlsLmNvbQ==
 Luigi Giuliani
Luigi Giuliani Pasquale Pezzella
Pasquale Pezzella Giulia Maria Giordano
Giulia Maria Giordano Leonardo Fazio
Leonardo Fazio Armida Mucci
Armida Mucci Andrea Perrottelli
Andrea Perrottelli Giuseppe Blasi
Giuseppe Blasi Mario Amore
Mario Amore Paola Rocca
Paola Rocca Alessandro Rossi
Alessandro Rossi Alessandro Bertolino
Alessandro Bertolino Silvana Galderisi
Silvana Galderisi Mario Maj1
Mario Maj1