- 1Department of Neurosurgery Intensive Care Unit, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 2Department of Neurology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 3Department of Rehabilitation Medicine, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 4Department of Functional Neurology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 5Department of Psychology and Sleep Medicine, The Second Hospital of Anhui Medical University, Hefei, China
- 6Department of Interventional Neurology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 7Department of Neurosurgery, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
Background: The diagnosis of major depressive disorder (MDD) mainly depends on subjective clinical symptoms, without an acceptable objective biomarker for the clinical application of MDD. Inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4) showed a high specificity as biomarker for the diagnosis and treatment of MDD. The present study aimed to investigate differences in plasma ITIH4 in two different aged MDD patients and underlying pathological mechanisms of plasma ITIH4 in the occurrence and development of MDD.
Methods: Sixty-five adult MDD patients, 51 adolescent MDD patients, and 64 healthy controls (HCs) were included in the present study. A 14-days’ antidepressive treatment was conducted in all MDD patients. Psychological assessments were performed and plasma ITIH4 and astrocyte-related markers were detected for all participants.
Results: (1) Plasma levels of ITIH4 in adult MDD patients were significantly higher than adolescent MDD patients and HCs, and significantly increased plasma ITIH4 levels was observed in adolescent MDD patients compared with HCs (2). There were positive correlations between plasma ITIH4 levels and 24-item Hamilton Depression Scale (HAMD-24) scores and plasma glial fibrillary acidic protein (GFAP) levels in MDD patients, however, plasma ITIH4 levels were significantly correlated with age just in adult MDD patients (3). Plasma ITIH4 showed area under the curve values of 0.824 and 0.729 to differentiate adult MDD patients and adolescent MDD patients from HCs, respectively (4). There was significant decrease in plasma levels of ITIH4 between before and after antidepressive treatment in adult MDD patients, but not in adolescent MDD patients (5). Changed value of ITIH4 levels were correlated with the changed value of GFAP levels and changed rate of HAMD-24 scores in adult MDD patients following antidepressive treatment.
Conclusion: Plasma ITIH4 may be potential plasma biomarkers of MDD with age-related specificity, which was associated with depressive symptoms astrocyte-related pathologic changes, and antidepressive treatment efficacy.
Introduction
Major depressive disorder (MDD) is the most common mental disorder, with an increasing yearly prevalence (1, 2). The primary clinical symptoms of MDD include a depressed mood, anhedonia, and suicidal thoughts (3). Although the precise pathological mechanism of MDD is still unclear, several hypotheses are proposed to explain the underlying basis of MDD, including the monoamine hypothesis, the hypothalamic-pituitary-adrenal axis dysfunction, neuroinflammatory, the genetic and epigenetic anomaly (4). Currently, the diagnosis of MDD mainly depends on subjective recognition of the clinical symptoms, without objective diagnostic evidence (5–7). Therefore, identification of effective biomarker for the clinical application of MDD is essential. Blood-based indicators could be used as convenient tools to improve the accuracy of clinical diagnosis in MDD, such as brain-derived neurotrophic factor (8, 9). However, the widely accepted objective biomarker for the diagnosis of MDD is still lacking. Meanwhile, dissatisfactory accuracy of identification is also the common issue for researches of biomarkers in MDD, which may be affected by the individual characteristics, e.g., sex (10, 11). Exploration of potential influencing factors of biomarkers is essential for the utilization of them in individualized diagnosis and treatment of MDD.
Inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4) is a 120 kDa plasma glycoprotein, belonging to the liver-restricted serine protease inhibitor family (12). ITIH4, as an acute phase protein, can play a key role of anti-inflammation in neuroinflammation (13, 14). Two studies of proteomic analysis found that ITIH4 levels was significantly elevated in the serum protein profiling of MDD patients when compared to health controls (15, 16). Our previous study firstly revealed the plasma ITIH4 was a diagnostic biomarker of MDD with high specificity and consistent expression of ITIH4 were verified between plasma and the post-mortem brain tissue in MDD patients (17). Another study based on post-mortem brain tissue found that ITIH4 gene expression was up-regulated in the prefrontal cortices of MDD patients using DNA microarray (18). Moreover, genetic variants of the ITIH4 genes were identified to be associated with the higher risk of suicidal attempts in mood disorders (19). These evidences support that ITIH4 may be involved in the pathology of MDD and peripheral ITIH4 may be used as a valuable diagnostic indicator of MDD. Furthermore, in another of our studies, plasma ITIH4 levels were significantly reduced at the end of repetitive transcranial magnetic stimulation treatment as compared to before treatment (20). Hence, plasma ITIH4 may be also a potential index for evaluating the effect of antidepressive treatment. However, all previous studies were performed in adult MDD patients, and whether individual characteristics, e.g., sex, age, can affect the levels of plasma ITIH4 is unclear.
In the present study, we aimed to assess differences in plasma ITIH4 among HCs, adult MDD patients, and adolescent MDD patients. Potential associations between plasma ITIH4 levels and clinical characteristics of MDD patients were also explored. Additionally, the clinical value of plasma ITIH4 in the treatment of antidepressive drugs in MDD patients was thoroughly assessed.
Materials and methods
Study cohorts
There were two cohorts were included in the present study. Cohort one included 65 adult MDD patients and 64 healthy controls (HCs), and was recruited from the Affiliated Wuxi People’s Hospital of Nanjing Medical University. Cohort two consisted of 51 adolescent MDD patients recruited from the Second Affiliated Hospital at Anhui Medical University.
All participants or their legal guardians provided informed consent. The ethical approval was obtained from the Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (approval number: KY22082). The study was also approved by the Ethics Committees of the Second Hospital of Anhui Medical University (approval number: SL-YX2024-022).
Inclusion and exclusion criteria of participants
The inclusion criteria for MDD patients included the following: (1) first episode outpatients or inpatients; (2) patients that were drug-naïve or drug-free (i.e., free of antidepressive treatment longer than 4 weeks prior to the beginning of the study); (3) no family history of psychosis. Meanwhile, adult MDD patients were aged 18 to 55 years, and adolescent MDD patients were aged 12 to 17 years. Furthermore, HCs had no history of DSM-V Axis I disorders, mental disorders or severe physical disease.
Participants fulfilling any of the following criteria were excluded: (1) organic lesions of the central nervous system or neurodegenerative disorders, including stroke or Alzheimer’s disease; (2) secondary mental disorders, e.g., those caused by the severe physical illness; (3) other types of psychiatric disorders other than MDD, e.g., schizophrenia; (4) abuse and dependence of alcohol or drugs; (5) significant physical disorder (e.g., endocrine disease, autoimmune disease, or impaired function of the liver or kidneys); or (6) any type of tumor or cerebral trauma.
Treatment
All MDD patients, including adult and adolescent ones, obtained a 14-days’ antidepressive treatment. Only one kind of the selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors was used as the primary antidepressant throughout the treatment. Furthermore, one kind of the atypical antipsychotics was additionally used when single antidepressant provided a poor efficiency, e.g., aripiprazole, quetiapine. Detailed information of drugs was displayed in Supplementary Material. Notably, because assessment of antidepressive treatment efficacy was not the major goal of this study, the current study was designed as an observational study rather than a randomized control trail.
Psychological assessments
The interviews of participants were completed by a trained researcher according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-V) (21). Meanwhile, the psychological assessments, including the 24-item Hamilton Depression Scale (HAMD-24) (22) and Self-Rating Depression Scale (SDS) (23), were also conducted to evaluate the depressive symptoms. The psychological assessments were performed for all MDD patients before and after treatment.
Plasma sampling
After overnight fasting, peripheral venous blood was collected into EDTA-coated tubes. Within 30 minutes of collection, the blood samples were centrifuged at 1000 × g at 4°C for 10 minutes, subsequently plasma was aspirated and stored at –80°C until required for analysis. All MDD patients provided plasma samples before treatment, however, after the 14-days’ treatment, just 30 MDD patients provided plasma samples in adult MDD group and adolescent MDD group, respectively.
Enzyme-linked immunosorbent assay analyses
The concentrations of plasma ITIH4, glial fibrillary acidic protein (GFAP), and S100beta protein (S100β) were measured in triplicate, using the commercial ELISA kits (FineTest, Wuhan, China; Catalog Number: EH1525 for ITIH4, EH0410 for GFAP, and EH0543 for S100β) in accordance with the manufacturer’s protocols. The concentration of protein in each plate was calculated according to standard curves and dilution factors. The inter- and intra-assay coefficients of variation were < 5%.
Statistical analysis
Data were analyzed using SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to evaluate the normal distribution of the data. Categorical variables were analyzed using a chi-squared test. Continuous variables using a one-way ANOVA (Bonferroni correction for post-hoc test) when normally distributed, otherwise using a Kruskal-Wallis H test (Nemenyi correction for post-hoc test). The paired t-test was used for the comparison of changes of variables before and after intervention. Correlation analysis was performed to determine the relationships between two variables. Line regression analysis was used for the multivariate analysis. Receiver operating characteristic (ROC) curves were used to calculate the area under the curve (AUC) for determining the diagnostic accuracy of the plasma indices. The Youden index (24) was used to assess optimal values of sensitivity and specificity. Significance was defined as p < 0.05 (two tailed).
Results
Characteristics of participants
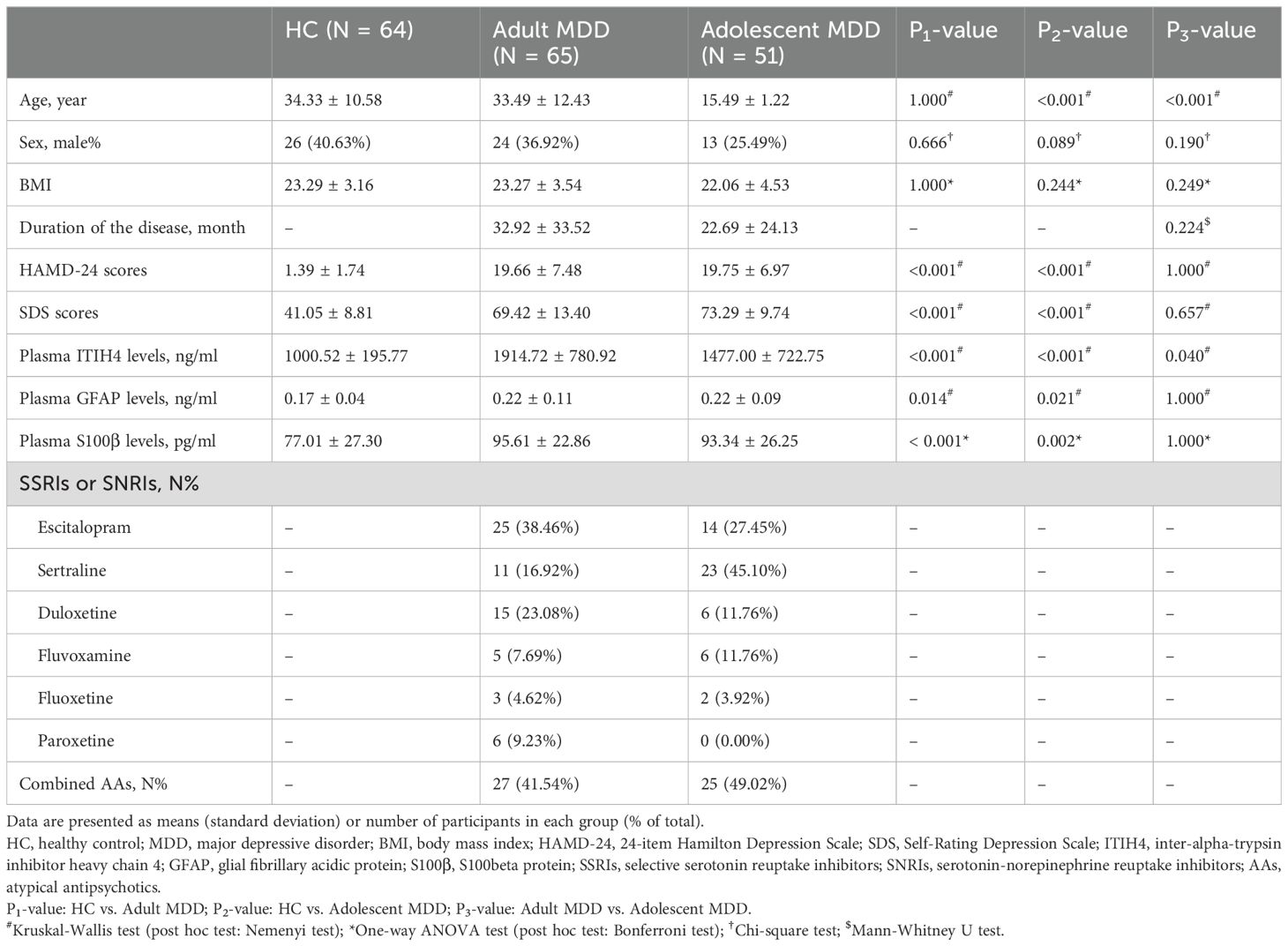
The demographic and clinical characteristics of participants across the three groups are summarized in Table 1. No significant differences were observed among these three groups in terms of sex and body mass index. In addition, there were there was no significant difference in duration of the disease between adult MDD and adolescent MDD groups. However, there was significant difference in age between adolescent MDD patients and HCs or adult MDD patients.
Compared with HCs, adult MDD patients or adolescent MDD patients showed significantly increased HAMD-24 scores, SDS scores, plasma GFAP levels, and plasma S100β levels, however, no significant difference was found between adult MDD and adolescent MDD groups regarding these characteristics (Table 1). Furthermore, plasma ITIH4 levels were significantly different between each two of these groups (Table 1), and adult MDD patients exhibited the highest levels of plasma ITIH4, followed by adolescent MDD patients.
Association of plasma ITIH4 with demographic and neuropsychological characteristics and plasma indices in MDD patients
As shown in Table 2, an elevated plasma ITIH4 level was correlated with higher age, HAMD-24 scores, plasma GFAP and S100β levels in adult MDD patients. The positive correlations between plasma ITIH4 and HAMD-24 scores and plasma levels of GFAP were also observed in adolescent MDD patients (Table 2). However, no significant correlation was observed between plasma levels of ITIH4 and age of the participants in adolescent MDD group.
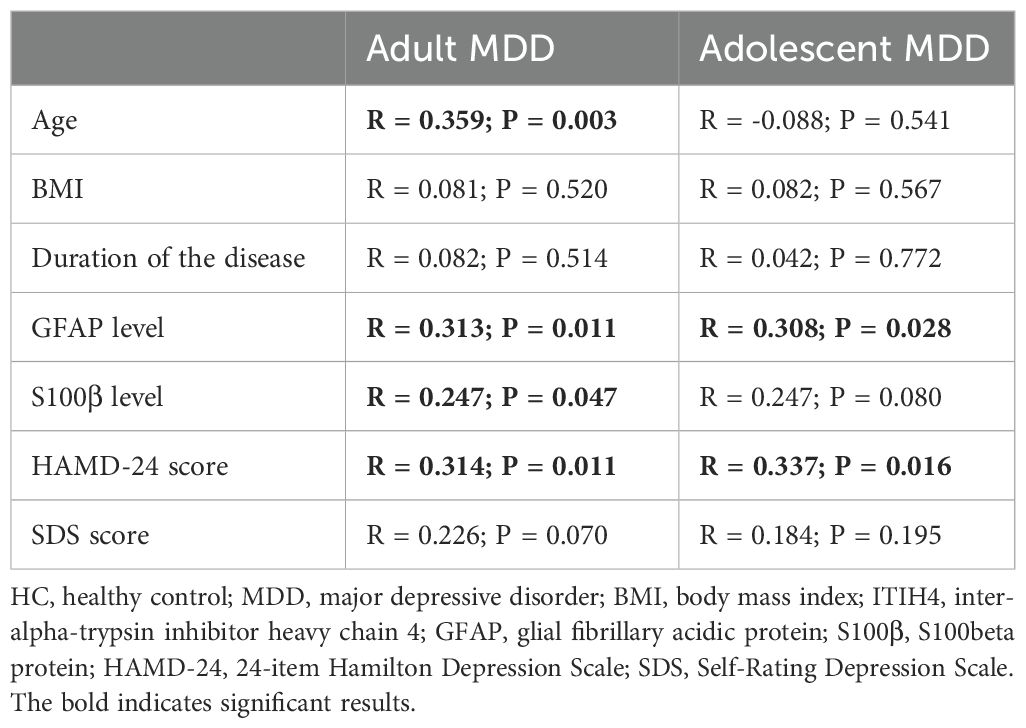
Table 2. Correlation analyses of ITIH4 levels with clinical characteristics and plasma indices levels in adult MDD participants and adolescent MDD participants.
Further multivariate analysis indicated that plasma ITIH4, plasma GFAP, and age had significant and interactive effects on the HAMD-24 scores in adult MDD patients (Table 3). However, in the adolescent MDD group, just plasma ITIH4 could significantly affect the HAMD-24 scores using line regression analysis (Table 3).
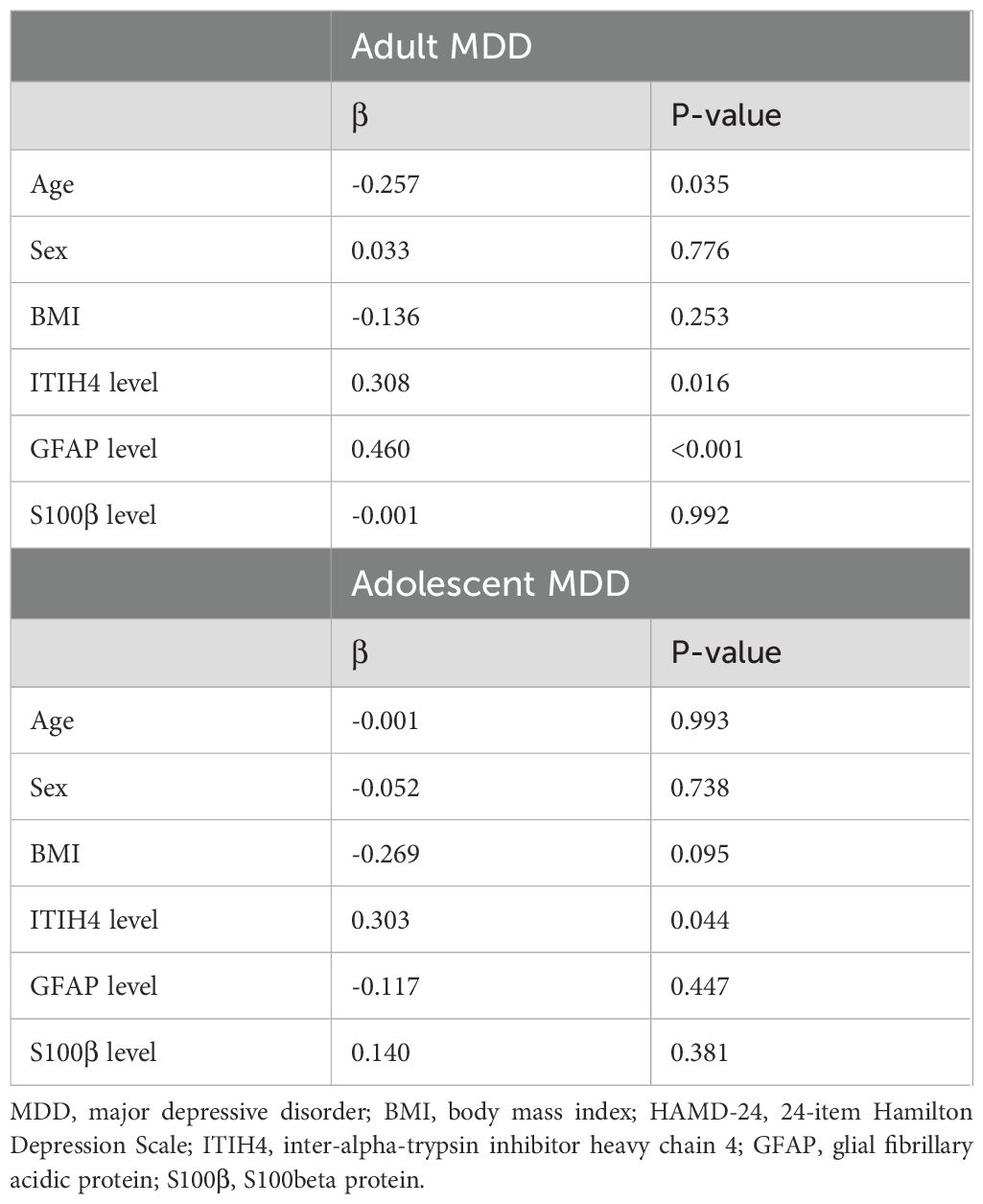
Table 3. Line regression analysis for the association of HAMD-24 scores with the demographic characteristics and plasma indices levels in two MDD groups.
Diagnostic performance of plasma ITIH4
ROC curve analysis indicated that plasma ITIH4 exhibited an AUC value of 0.824 for differentiating adult MDD patients from HCs (specificity = 98.44%, sensitivity = 69.23%; Figure 1). Meanwhile, plasma ITIH4 also could provide an acceptable AUC value > 0.700 to identify adolescent MDD patients from HCs (AUC = 0.729; specificity = 98.44%, sensitivity = 54.23%; Figure 1). However, for distinguishing adult MDD patients from adolescent MDD patients, plasma ITIH4 just showed an AUC value of 0.646 (specificity = 68.63%, sensitivity = 58.46%; Figure 1).

Figure 1. ROC curve analysis of plasma ITIH4. (A) Adult MDD vs. HC. (B) Adolescent MDD vs. HC. (C) Adult MDD vs. adolescent MDD. ROC, receiver operating characteristic; MDD, major depressive disorder; HC, healthy control; AUC, area under the curve.
Influence of antidepressive treatment
After 14-days’ antidepressive treatment, both adult MDD patients and adolescent MDD patients showed significantly reduced HAMD-24 and SDS scores as compared to assessment scores before treatment (Supplementary Table 1). Meanwhile, compared with before treatment, plasma GFAP also displayed a significantly decreased levels in both adult MDD and adolescent MDD patients after treatment (Supplementary Table 2).
In addition, in the adult MDD group, plasma levels of ITIH4 had a significantly decrease after antidepressive treatment when compared to before treatment (Figure 2A). However, there was only a trend in the decrease in the adolescent MDD group before and after treatment (Figure 2A).
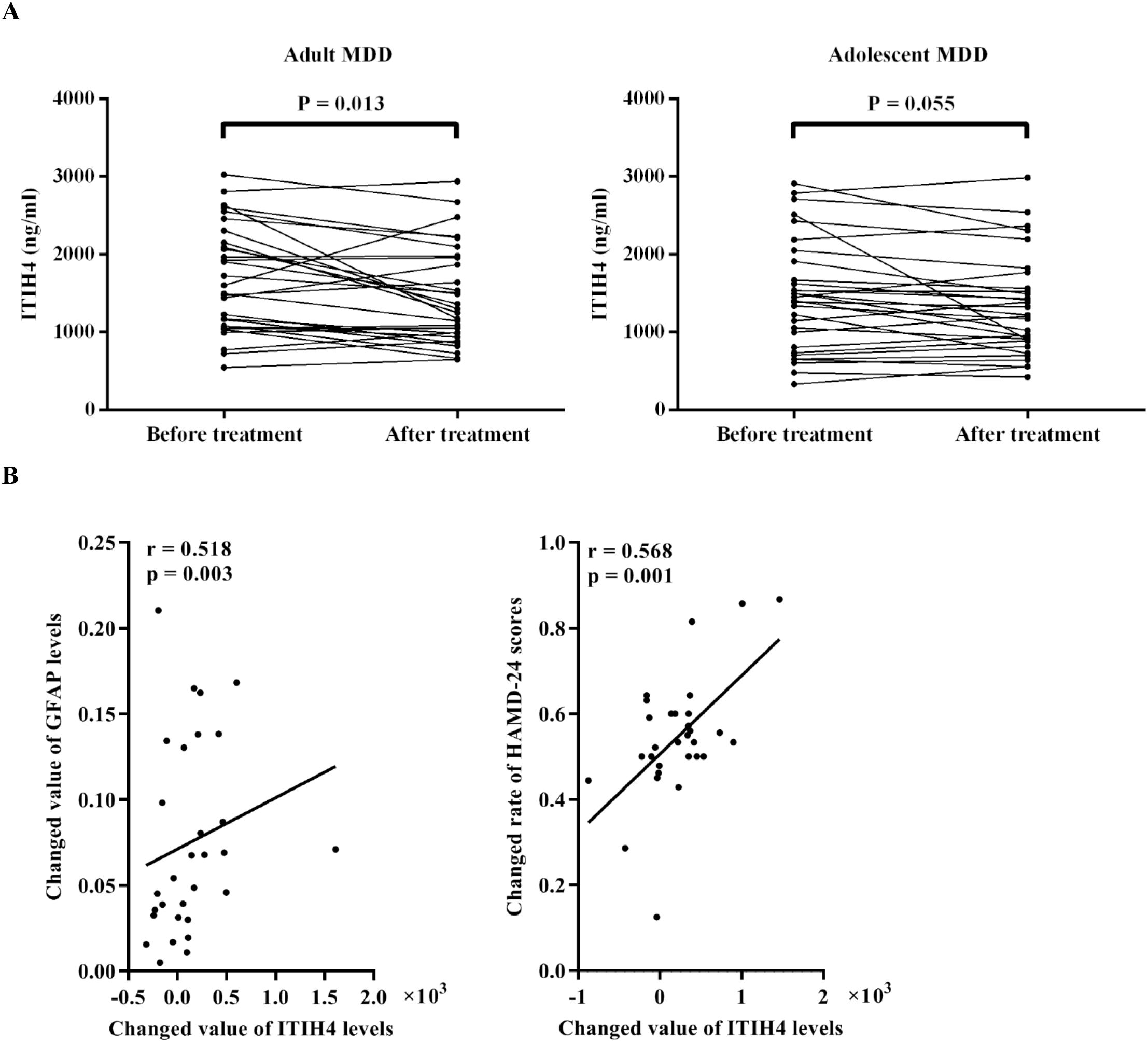
Figure 2. Effect of antidepressive treatment in MDD patients. (A) The change of levels of plasma ITIH4 following antidepressive treatment in adult MDD patients and adolescent MDD patients. (B) Associations of change of plasma ITIH4 levels with change of plasma GFAP levels and HAMD-24 scores in adult MDD patients. MDD, major depressive disorder; ITIH4, inter-alpha-trypsin inhibitor heavy chain 4; GFAP, glial fibrillary acidic protein; HAMD-24, 24-item Hamilton Depression Scale.
Further correlation analyses showed that there was a positive correlation between changed value of ITIH4 levels and changed value of GFAP levels in adult MDD patients (annotation: changed value of level = before treatment level – after treatment level) (Figure 2B). Meanwhile, in adult MDD patients, the changed rate of HAMD-24 scores was positively correlated with the changed value of plasma ITIH4 levels after treatment (annotation: changed rate of score = [before treatment score – after treatment score]/before treatment score) (Figure 2B). However, no any correlation was detected in the adolescent MDD group.
Discussion
The main findings of the present study are as follows: (1) there was significantly increased levels of ITIH4 in plasma samples of MDD patients compared with HCs, and the significant increase in plasma ITIH4 was observed in adult MDD patients compared with adolescent MDD patients; (2) levels of plasma ITIH4 was positively correlated with HAMD-24 scores and plasma GFAP levels in MDD patients, but the significant correlation between plasma ITIH4 levels and age just was found in adult MDD patients; (3) plasma ITIH4 could provide a good performance to differentiate either adult MDD patients or adolescent MDD patients from HCs; (4) plasma levels of ITIH4 significantly changed following antidepressive treatment in adult MDD patients, but not in adolescent MDD patients; (5) the change in plasma ITIH4 following antidepressive treatment reflected the change in depressive symptoms following treatment in adult MDD patients. Taken together, plasma ITIH4 may be meaningfully associated with depressive symptomatology and therapeutic effect in MDD patients, which showed an obvious age-specific performance.
In consistent with our previous study (17), this study also found significant increase in plasma ITIH4 levels in adult MDD patients as compared to HCs. Meanwhile, the present study firstly found that plasma ITIH4 levels in adolescent MDD patients were significantly higher than HCs but significantly lower than adult MDD patients, which indicated that the plasma levels of ITIH4 showed significant changes in multiple types of MDD when compared to HCs, and these changes displayed an age-specific performance in MDD. Furthermore, our association analyses showed that there was positive correlation between plasma ITIH4 levels and age in adult MDD patients not in adolescent MDD patients, which suggested that age had a key effect on the plasma levels of ITIH4 in adult MDD. However, a smaller age span (i.e., 12-17 years old) used in adolescent MDD patients might be a primary cause to affect the results in the association analysis. Moreover, the interaction between plasma ITIH4 levels and age could be associated with the HAMD-24 scores of adult MDD patients, further suggesting the depressive symptoms could be affected by the plasma ITIH4 levels and age. Although age of onset has been determined as an important element to impact on symptomatology of MDD (25), many pathological differences in MDD may are dependent on the age, e.g., brain gray matter volume and cortical thickness (26) and urinary metabolite expression (27). In the current clinical practice, the same diagnostic criteria of MDD are used in adults and adolescents, which seems to imply the age-independent presentation of the disorder, however, there are explicit aetiological differences between adolescent and adult MDD (28, 29). The differences in plasma ITIH4 expression in the present study between adolescent MDD and adult MDD might indicate a different progress of some molecular changes in the development of adolescent and adult MDD. Additionally, in the present study, our findings provide clinical evidence for the diagnosis of MDD using plasma ITIH4. Plasma ITIH4 provided the great performance for identifying MDD (either adult MDD or adolescent MDD) patients from HCs. However, for distinguishing adult MDD patients from adolescent MDD patients, plasma ITIH4 just provided an inadequate diagnostic power. Therefore, we successfully identified different plasma ITIH4 levels in adolescent and adult MDD patients, which was helpful for developing age-specific diagnostic method for MDD.
The present study found that effective antidepressive drug could change the levels of plasma ITIH4 in adult MDD, which was similar to our previous study (20). However, for adolescent MDD patients, the changes of plasma ITIH4 levels were not significant between before and after treatment. Previous study revealed that differences in treatment influence in adolescent and adult MDDs may be associated with differences in depression symptom profiles, especially vegetative symptoms (30). Our findings further indicated that the potential ITIH4-related pathophysiological mechanisms might be involved in the age-related differences in effect sizes of antidepressant in MDD (31, 32). Additionally, in adult MDD patients, there was a positive correlation between the changed value of plasma ITIH4 levels and the changed rate of HAMD-24 scores following treatment, suggesting the change in plasma ITIH4 could be associated with the therapeutic effect of antidepressive treatment. Hence, plasma ITIH4 might have a potential clinical value for the antidepressive treatment, in particular for adult MDD patients.
Astrocytes play a key role in regulating synaptic and intracellular levels of neurotransmitters and neuroinflammatory processes, and animal and human postmortem studies have demonstrated prominent astrocyte pathology in the pathogenesis of MDD (33, 34). For investigating the possible mechanism of ITIH4 in the pathophysiology of MDD, we detected two robust fluid markers for reflecting astrocyte reactivity, i.e., GFAP and S100β, both of which has generally been found to be increased in MDD (35–38). In the present study, there were positive correlations between plasma GFAP and ITIH4 in both adult and adolescent MDD patients and positive correlations between plasma S100β and ITIH4 in adult MDD patients. Meanwhile, line regression analyses also indicated that potential interaction of plasma GFAP and ITIH4 levels could affect the scores of HAMD-24 in adult MDD patients. These findings suggested that ITIH4-related pathological mechanisms in MDD might be associated with activation of astrocytes (39, 40). Furthermore, through the 14-days’ antidepressive treatment, the change of plasma ITIH4 and GFAP showed a significant correlation in adult MDD patients. Previous studies indicated that the alteration of GFAP expression after antidepressive therapy implied the improvement of the number and morphology of astrocytes in MDD (33, 41, 42). Therefore, the influence of antidepressive treatment on the change of plasma ITIH4 might be associated with the reversibility of change of astrocytes in MDD.
In the present study, plasma ITIH4 was recommended is an acceptable biomarker for the diagnosis of adult or adolescent MDD from HCs. However, this indicator was not splendid biomarker for distinguishing adult MDD patients from adolescent MDD ones. Furthermore, high specificity and low sensitivity of plasma ITIH4 leaded to this indicator just showed moderate diagnostic power for MDD. Our findings provided valuable evidence supported that plasma ITIH4 as a great potential biomarker to combine other markers for establishing the clinically diagnostic model in MDD.
However, the present study has several limitations: (1) the expression of the plasma ITIH4 has not been validated in patients with geriatric depression; (2) the same antidepressant drug is not used during treatment in each MDD patient; (3) animal experiments is lacking to determine the change of astrocytes when ITIH4 expression was up-regulated or down-regulated; (4) due to some patients rejected to provided blood sample secondly at the end of treatment, the change of plasma ITIH4 in MDD before and after treatment needed to further be verified. Thus, further researches, including expanding verification population of MDD patients with multiple ages, strict intervening measures for antidepressive treatment, and depressive-like animal model experiments, are currently ongoing.
Conclusion
The present study demonstrated that plasma ITIH4 levels were significantly increased in MDD patients as compared to HCs, and adult MDD patients showed significantly higher plasma ITIH4 levels than adolescent MDD patients, suggesting the increase of plasma ITH4 in MDD patients might exhibit an age-related change. Meanwhile, in MDD patients, plasma ITIH4 showed obvious age-specific characteristic in associations of depressive symptomatology, astrocyte-related molecular changes, and effect of antidepressive treatment. The present findings may enhance clinical evaluations for MDD and assist clinicians in promoting earlier detection and individualized treatment in MDD individuals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The ethical approval was obtained from the Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (approval number: KY22082). The study was also approved by the Ethics Committees of the Second Hospital of Anhui Medical University (approval number: SL-YX2024-022). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PH: Writing – original draft. XL: Writing – original draft. MZ: Writing – original draft. HW: Writing – review & editing. JW: Writing – review & editing. XZ: Writing – review & editing. CJ: Writing – review & editing. FG: Writing – review & editing. YS: Writing – review & editing. GZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the WuXi Municipal Health Commission (No. Q202222), National Natural Science Foundation of China (No. 82301715), Jiangsu province double-creation doctoral talent plan (No. JSSCBS20221995), General Program of Wuxi Medical Center, Nanjing Medical University (WMCHL202304), and Wuxi Taihu Lake Talent Plan, Supports for Leading Talents in Medical and Health Profession (No. 2020THRC-DJ-SNW).
Acknowledgments
The authors would like to thank all participants in the present study for their cooperation and relevant medical staff (e.g., Mr. Guangjun Xi, Mrs. Yan Han, Mrs. Qianqian Gao, Mrs. Xuemei Ma, and CJ) from the Affiliated Wuxi People’s Hospital of Nanjing Medical University for their assistance in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1449202/full#supplementary-material
References
2. Marx W, Penninx B, Solmi M, Furukawa TA, Firth J, Carvalho AF, et al. Major depressive disorder. Nat Rev Dis Primers. (2023) 9:44. doi: 10.1038/s41572-023-00454-1
3. Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. (2012) 379:1045–55. doi: 10.1016/s0140-6736(11)60602-8
4. Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. (2024) 9:30. doi: 10.1038/s41392-024-01738-y
5. Shi Y, Wang Q, Song R, Kong Y, Zhang Z. Non-coding RNAs in depression: Promising diagnostic and therapeutic biomarkers. EBioMedicine. (2021) 71:103569. doi: 10.1016/j.ebiom.2021.103569
6. Shi Y, Zhang L, He C, Yin Y, Song R, Chen S, et al. Sleep disturbance-related neuroimaging features as potential biomarkers for the diagnosis of major depressive disorder: A multicenter study based on machine learning. J Affect Disord. (2021) 295:148–55. doi: 10.1016/j.jad.2021.08.027
7. Shi Y, Zhang L, Wang Z, Lu X, Wang T, Zhou D, et al. Multivariate machine learning analyses in identification of major depressive disorder using resting-state functional connectivity: A multicentral study. ACS Chem Neurosci. (2021) 12:2878–86. doi: 10.1021/acschemneuro.1c00256
8. Shi Y, Luan D, Song R, Zhang Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: A systematic review and meta-analysis. Eur Neuropsychopharmacol. (2020) 41:40–51. doi: 10.1016/j.euroneuro.2020.09.633
9. Dadkhah M, Jafarzadehgharehziaaddin M, Molaei S, Akbari M, Gholizadeh N, Fathi F. Major depressive disorder: Biomarkers and biosensors. Clin Chim Acta. (2023) 547:117437. doi: 10.1016/j.cca.2023.117437
10. Poulter MO, Du L, Weaver ICG, Palkovits M, Faludi G, Merali Z, et al. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. (2008) 64:645–52. doi: 10.1016/j.biopsych.2008.05.028
11. Seney ML, Chang LC, Oh H, Wang X, Tseng GC, Lewis DA, et al. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front Psychiatry. (2013) 4:104. doi: 10.3389/fpsyt.2013.00104
12. Noh CK, Kim SS, Kim DK, Lee HY, Cho HJ, Yoon SY, et al. Inter-alpha-trypsin inhibitor heavy chain H4 as a diagnostic and prognostic indicator in patients with hepatitis B virus-associated hepatocellular carcinoma. Clin Biochem. (2014) 47:1257–61. doi: 10.1016/j.clinbiochem.2014.05.002
13. Shi X, Ohta Y, Liu X, Shang J, Morihara R, Nakano Y, et al. Acute anti-inflammatory markers ITIH4 and AHSG in mice brain of a novel alzheimer's disease model. J Alzheimers Dis. (2019) 68:1667–75. doi: 10.3233/jad-181218
14. Zhang J, Hu J, Zhao W. Longitudinal change of serum inter-α-trypsin inhibitor heavy chain H4 and its relation with inflammation, disease recurrence, and mortality in acute ischemic stroke patients. Tohoku J Exp Med. (2023) 259:221–7. doi: 10.1620/tjem.2022.J116
15. Lee J, Joo EJ, Lim HJ, Park JM, Lee KY, Park A, et al. Proteomic analysis of serum from patients with major depressive disorder to compare their depressive and remission statuses. Psychiatry Investig. (2015) 12:249–59. doi: 10.4306/pi.2015.12.2.249
16. Wang Q, Su X, Jiang X, Dong X, Fan Y, Zhang J, et al. iTRAQ technology-based identification of human peripheral serum proteins associated with depression. Neuroscience. (2016) 330:291–325. doi: 10.1016/j.neuroscience.2016.05.055
17. Shi Y, Song R, Wang L, Qi Y, Zhang H, Zhu J, et al. Identifying Plasma Biomarkers with high specificity for major depressive disorder: A multi-level proteomics study. J Affect Disord. (2020) 277:620–30. doi: 10.1016/j.jad.2020.08.078
18. Tochigi M, Iwamoto K, Bundo M, Sasaki T, Kato N, Kato T. Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neurosci Res. (2008) 60:184–91. doi: 10.1016/j.neures.2007.10.010
19. Amare AT, Schubert KO, Klingler-Hoffmann M, Cohen-Woods S, Baune BT. The genetic overlap between mood disorders and cardiometabolic diseases: a systematic review of genome wide and candidate gene studies. Transl Psychiatry. (2017) 7:e1007. doi: 10.1038/tp.2016.261
20. Song R, Shi Y, Li X, Zhu J, Zhang H, Li K, et al. Potential of antithrombin III as a biomarker of antidepressive effect in major depressive disorder. Front Psychiatry. (2021) 12:678384. doi: 10.3389/fpsyt.2021.678384
21. Uher R, Payne JL, Pavlova B, Perlis RH. Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress Anxiety. (2014) 31:459–71. doi: 10.1002/da.22217
22. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
23. Zung WW. A SELF-RATING DEPRESSION SCALE. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
24. Youden WJ. Index for rating diagnostic tests. Cancer. (1950) 3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3
25. Cui L, Wang Y, Cao L, Wu Z, Peng D, Chen J, et al. Age of onset for major depressive disorder and its association with symptomatology. J Affect Disord. (2023) 320:682–90. doi: 10.1016/j.jad.2022.09.096
26. Myoraku A, Lang A, Taylor CT, Scott Mackin R, Meyerhoff DJ, Mueller S, et al. Age-dependent brain morphometry in Major Depressive disorder. NeuroImage Clin. (2022) 33:102924. doi: 10.1016/j.nicl.2021.102924
27. Chen JJ, Xie J, Li WW, Bai SJ, Wang W, Zheng P, et al. Age-specific urinary metabolite signatures and functions in patients with major depressive disorder. Aging (Albany NY). (2019) 11:6626–37. doi: 10.18632/aging.102133
28. Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research review: the role of cytokines in depression in adolescents: a systematic review. J Child Psychol Psychiatry. (2013) 54:816–35. doi: 10.1111/jcpp.12080
29. Zhang E, Hauson AO, Pollard AA, Meis B, Lackey NS, Carson B, et al. Lateralized grey matter volume changes in adolescents versus adults with major depression: SDM-PSI meta-analysis. Psychiatry Res Neuroimaging. (2023) 335:111691. doi: 10.1016/j.pscychresns.2023.111691
30. Rice F, Riglin L, Lomax T, Souter E, Potter R, Smith DJ, et al. Adolescent and adult differences in major depression symptom profiles. J Affect Disord. (2019) 243:175–81. doi: 10.1016/j.jad.2018.09.015
31. Hauenstein EJ. Depression in adolescence. J Obstet Gynecol Neonatal Nurs. (2003) 32:239–48. doi: 10.1177/0884217503252133
32. Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: A systematic review and meta-analysis. JAMA Psychiatry. (2017) 74:1011–20. doi: 10.1001/jamapsychiatry.2017.2432
33. Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. (2013) 14:1225–36. doi: 10.2174/13894501113149990156
34. O'Leary LA, Mechawar N. Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans. Glia. (2021) 69:2077–99. doi: 10.1002/glia.23994
35. Fang Y, Xiao SF, Zhang SY, Qiu Q, Wang T, Li X. Increased plasma S100β Level in patients with major depressive disorder. CNS Neurosci Ther. (2016) 22:248–50. doi: 10.1111/cns.12517
36. Güleş E, Iosifescu DV, Tural Ü. Plasma neuronal and glial markers and anterior cingulate metabolite levels in major depressive disorder: A pilot study. Neuropsychobiology. (2020) 79:214–21. doi: 10.1159/000505782
37. Steinacker P, Al Shweiki MR, Oeckl P, Graf H, Ludolph AC, Schönfeldt-Lecuona C, et al. Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. J Psychiatr Res. (2021) 144:54–8. doi: 10.1016/j.jpsychires.2021.09.012
38. Zhang J, Liu D, Xiang J, Yang M. Combining glial fibrillary acidic protein and neurofilament light chain for the diagnosis of major depressive disorder. Anal Chem. (2024) 96:1693–9. doi: 10.1021/acs.analchem.3c04825
39. Xu SX, Xie XH, Yao L, Wang W, Zhang H, Chen MM, et al. Human in vivo evidence of reduced astrocyte activation and neuroinflammation in patients with treatment-resistant depression following electroconvulsive therapy. Psychiatry Clin Neurosci. (2023) 77:653–64. doi: 10.1111/pcn.13596
40. Sánchez-Juan P, Valeriano-Lorenzo E, Ruiz-González A, Pastor AB, Rodrigo Lara H, López-González F, et al. Serum GFAP levels correlate with astrocyte reactivity, post-mortem brain atrophy and neurofibrillary tangles. Brain. (2024) 147:1667–79. doi: 10.1093/brain/awae035
41. Jansson L, Wennström M, Johanson A, Tingström A. Glial cell activation in response to electroconvulsive seizures. Prog Neuropsychopharmacol Biol Psychiatry. (2009) 33:1119–28. doi: 10.1016/j.pnpbp.2009.06.007
Keywords: major depressive disorder, inter-alpha-trypsin inhibitor heavy chain 4, glial fibrillary acidic protein, astrocyte, antidepressive treatment
Citation: He P, Lu X, Zhong M, Weng H, Wang J, Zhang X, Jiang C, Geng F, Shi Y and Zhang G (2024) Plasma alpha-trypsin inhibitor heavy chain 4 as an age-specific biomarker in the diagnosis and treatment of major depressive disorder. Front. Psychiatry 15:1449202. doi: 10.3389/fpsyt.2024.1449202
Received: 14 June 2024; Accepted: 26 August 2024;
Published: 11 September 2024.
Edited by:
Marcin Siwek, Jagiellonian University, PolandCopyright © 2024 He, Lu, Zhong, Weng, Wang, Zhang, Jiang, Geng, Shi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaojia Zhang, Z2ppYXpoYW5nQDE2My5jb20=; Feng Geng, cHN5Z2VuZ2ZlbmdAMTI2LmNvbQ==; Yachen Shi, eWFjaGVuX3NoaUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Ping He1,2†
Ping He1,2† Feng Geng
Feng Geng Yachen Shi
Yachen Shi Gaojia Zhang
Gaojia Zhang