- 1Department of Psychiatry and Biological Psychiatry Laboratory, Jiangxi Mental Hospital & Affiliated Mental Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 2The 3rd Clinical Medical College, Jiangxi Medical College, Nanchang University, Nanchang, China
- 3Department of Neurology, The Second Clinical Medical College, The Second Affiliated Hospital, Fujian Medical University, Quanzhou, China
- 4Nanchang City Key Laboratory of Biological Psychiatry, Jiangxi Provincial Clinical Research Center on Mental Disorders, Jiangxi Mental Hospital, Nanchang, China
- 5Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 6Shanghai Pudong New Area Mental Health Center, School of Medicine, Tongji University, Shanghai, China
Objective: Auditory hallucinations are the most frequently occurring psychotic symptom in schizophrenia. Continuous theta burst stimulation (cTBS) has been used as an adjuvant treatment for auditory hallucinations. This meta-analysis focused on randomized controlled clinical trials (RCTs) to assess the efficacy of adjuvant cTBS on auditory hallucinations in schizophrenia.
Methods: We performed a comprehensive search of four international databases from their inception to January 14, 2024, to identify relevant RCTs that assessed the effects of adjuvant cTBS on auditory hallucinations. The key words included “auditory hallucinations”, “continuous theta burst stimulation” and “transcranial magnetic stimulation”. Inclusion criteria included patients with auditory hallucinations in schizophrenia or schizoaffective disorder. The Revised Cochrane risk-of-bias tool for randomized trials (RoB1) were used to evaluate the risk of bias and the Review Manager Software Version 5.4 was employed to pool the data.
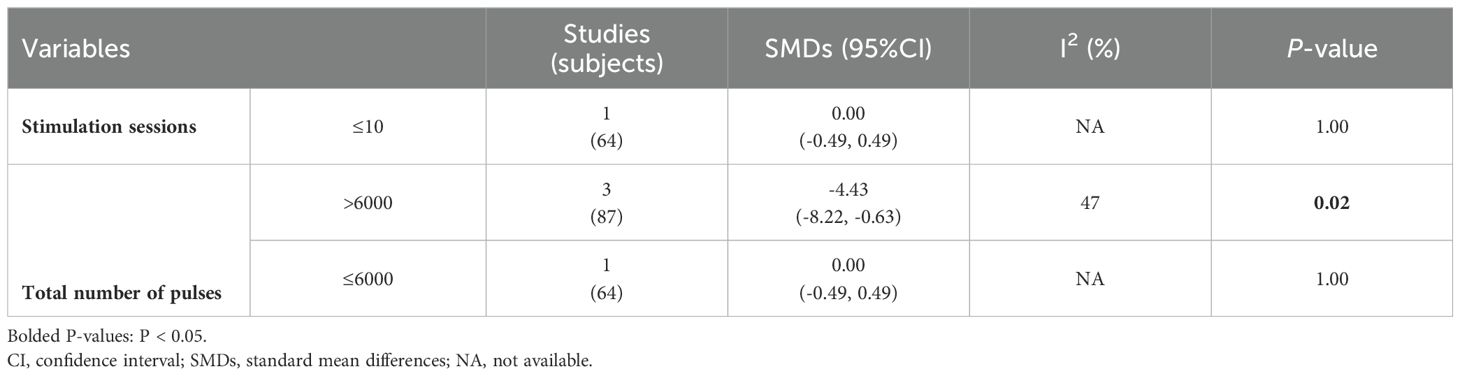
Results: A total of 4 RCTs involving 151 patients with auditory hallucinations were included in the analysis. The Cochrane risk of bias of these studies presented “low risk” in all items. Preliminary analysis showed no significant advantage of adjuvant cTBS over sham stimulation in reducing hallucinations [4 RCTs, n = 151; SMD: -0.45 (95%CI: -1.01, 0.12), P = 0.13; I2 = 61%]. Subgroup analysis revealed that patients treated with adjuvant cTBS for more than 10 stimulation sessions and total number of pulses more than 6000 [3 RCTs, n = 87; SMD: -4.43 (95%CI: -8.22, -0.63), P = 0.02; I2 = 47%] had a statistically significant improvement in hallucination symptoms. Moreover, the rates of adverse events and discontinuation did not show any significant difference between the cTBS and sham group.
Conclusions: Although preliminary analysis did not revealed a significant advantage of adjuvant cTBS over sham stimulation, subgroup analysis showed that specific parameters of cTBS appear to be effective in the treatment of auditory hallucinations in schizophrenia. Further large-scale studies are needed to determine the standard protocol of cTBS for treating auditory hallucinations.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024534045.
1 Introduction
Auditory hallucination refers to the perception of another person’s voice in the absence of any external stimuli (1), usually occurring in individuals with mental illness. It is estimated that approximately 60-80% of schizophrenia patients (2) and 40% of major depression patients experience auditory hallucinations (3, 4). These hallucinations can be extremely distressing and painful, particularly when they are abusive, demeaning, or commanding in nature (5). Chronic hallucinations would impair patients’ emotional perception and increase the risk of suicidal behaviors or violence (6, 7). Although current antipsychotic medications are effective in managing auditory hallucinatory symptoms, up to 30% of the patients cannot receive any relief (8).
Brain connectivity dysfunction is thought to be the basis of auditory hallucinations (9). In 1998, Karl J proposed that alterations in prefrontal and temporal cortex connectivity were responsible for hallucinations (10). Subsequent studies, using brain imaging techniques, showed that there was increased activity in the frontotemporal lobe regions of patients with auditory hallucinations (11, 12). Abnormal connectivity between the auditory cortex and other cortical regions was also found in patients with auditory verbal hallucinations (13, 14). Based on the ‘brain connectivity dysfunction’ hypothesis, nonpharmacological treatment strategies that regulate brain activity, such as electroconvulsive therapy (ECT) (15) and non-invasive brain stimulation techniques (16), have been introduced to increase the therapeutic efficiency of antipsychotics (APs) on hallucinations.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulation technique that has been widely recommended for the treatment of neuropsychiatric disorders (17–19). Studies have shown that rTMS can alleviate auditory hallucinations by influencing cortical excitability, and its therapeutic effect is affected by the frequency and site of stimulation (20, 21). Normally, low frequency rTMS (LF; < 5Hz) results in persistent alterations in the inhibitory activity of target regions, whereas high frequency rTMS (HF; ≥ 5Hz) tends to have an excitatory effect (22). The temporo-parietal junction (TPJ) is a connection of the primary (PAC) and secondary (SAC) auditory cortices and showed overactivity during episodes of auditory verbal hallucinations (23). Low-frequency rTMS can reduce the intensity of auditory hallucinations by reducing the activity of TPJ (24). Hoffman et al. also reported a decrease in the intensity of auditory hallucinations after applying 1 Hz TMS to the left TPJ (25). However, despite the initial enthusiasm generated by positive studies, the efficacy of inhibitory rTMS in the treatment of auditory hallucinations in schizophrenia remains controversial. The latest meta-analysis on the subject, by Guttesen et al. (26), did not show superiority over placebo. Furthermore, the conventional rTMS protocols still have certain practical limitations, such as modest and short-lasting effects, and a delayed time-to-response. To enhance the therapeutic effects of rTMS, novel types of rTMS such as theta burst stimulation (TBS) have been developed.
TBS is a stimulation pattern that imitates the natural activity of the brain during learning tasks (27). It delivers three biphasic pulses at a frequency of 50 Hz in bursts separated by 200 ms at a frequency of 5 Hz. This stimulation protocol has several advantages over traditional TMS. It offers a longer-lasting ability to modulate cortical excitability, shorter stimulation session, and greater efficacy at lower stimulation intensities (28, 29). Continuous TBS (cTBS), a form of TBS that produces 20 minutes of suppression with 20 seconds of stimulation, is a quicker and potentially more effective technique to reduce cortical hyperactivity. Preliminary findings have suggested that cTBS therapy may yield positive results in improving auditory hallucination and minimizing adverse consequences (30–33). Among them, one randomized controlled trials (RCTs) of 50 patients showed that active cTBS treatment exhibited an odds ratio of 5.6 for auditory hallucinations response compared with sham stimulation (32). However, Koops S et al. reported that adjuvant cTBS treatment in patients with auditory hallucinations did not show an advantage over sham stimulation (34). Given the results of studies on the efficacy of cTBS are controversial, we here conducted a meta-analysis of RCTs of adjuvant cTBS in patients with auditory hallucinations, aiming to assess the clinical efficacy and safety of adjuvant cTBS in the treatment of auditory hallucinations.
2 Method
This systematic review was registered with PROSPERO (CRD42024534045) [see Supplementary Materials for the PROSPERO register].
2.1 Eligibility criteria
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (35) and utilized the PICOS acronym to determine inclusion criteria. The participants were patients with auditory hallucinations in schizophrenia or schizoaffective disorder, and the intervention involved active cTBS plus APs versus sham or placebo stimulation plus APs. The included subjects did not respond sufficiently to at least one antipsychotic medication administered for a minimum of 6 weeks or longer at the highest acceptable dosage. During cTBS and sham stimulation therapy, patients received the same dose and type of antipsychotic medication as before treatment. The primary outcome measure is the improvement in auditory hallucination symptoms as assessed by standardized scales such as the Auditory Hallucination Rating Scale (AHRS) or Psychotic Symptom Rating Scales-Auditory Hallucination (PSYRST-AH) at the end of cTBS treatment. Secondary outcomes include the rate of adverse events as measured by the Global Index of Safety (GIS), changes in other psychotic symptoms as assessed by the Positive and Negative Symptom Scale (PANSS), and discontinuation due to any reason. This study included only RCTs evaluating the efficacy and safety of cTBS in the treatment of auditory hallucinations, and did not include animal studies, observational studies, and review articles on cTBS in the treatment of auditory hallucinations. When there was overlapping data across multiple published articles, only articles with complete data were included in the analysis.
2.2 Search strategy
Two researchers independently searched for relevant RCTs across four major international databases, including PubMed, EMBASE, Web of Science, and the Cochrane Library, from their inception dates up to January 14, 2024. The search terms used were (Transcranial Magnetic Stimulation OR continuous theta burst stimulation OR theta burst stimulation OR theta-burst stimulations OR theta burst transcranial magnetic stimulation OR transcranial theta burst stimulation OR TBS OR cTBS) AND (hallucinations OR Auditory Hallucination, Verbal) OR (Verbal Auditory Hallucination OR Verbal Auditory Hallucinations OR Auditory Hallucinations, Verbal OR Auditory Hallucination OR Auditory Hallucinations OR Phonism OR Voice).
2.3 Data extraction
Two investigators (Ye S-Y, Chen C-N) collaborated in the study selection and data extraction procedures. They employed a predetermined form to gather pertinent information, and in the event of any discrepancies, they resolved them through discussions and consultation with a senior researcher (Wei B). Furthermore, the first and/or corresponding authors were contacted when deemed necessary to obtain clarification or missing data.
2.4 RoB of included studies and certainty of overall evidence
Two researchers (Zhan J-Q, Li Y-H) individually evaluated the quality of all the studies included using the Cochrane Collaboration’s bias risk assessment tool (ROB1.0) (36). This tool examines five areas, such as randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The tool assigns a risk of bias as “high risk”, “low risk”, or “some concerns” for each study. In case of any disagreements, the entire review team discussed and resolved the issue. Similarly, the two examiners (Zhan J-Q, Li Y-H), carried out an independent evaluation of the overall strength of evidence for each meta-analytic outcome using the GRADE system (37, 38).
2.5 Data analysis
In this meta-analysis, we employed the Review Manager Software Version 5.4 (Cochrane IMS, Oxford, United Kingdom) to examine the efficacy and safety of cTBS treatment. To analyze continuous outcomes, we calculated standardized mean differences (SMDs) with 95% confidence intervals (CIs), while risk ratios (RRs) with 95% CIs were used for dichotomous data. Following the recommendation of previous study (39), we utilized the random-effects model to generate all meta-analytic outcomes. We evaluated heterogeneity among studies using Cochrane’s Q and I2 test. If the P-value was less than 0.1 and the I2-value was greater than 50%, it indicated significant study heterogeneity (40). The publication bias was assessed through visual funnel plot inspection and the Egger test (41), if there were at least 10 eligible RCTs in the meta-analysis (42). As the result of preliminary analysis was negative, we did not further calculate the failed-safe number. We established a significance level of P < 0.05 for this meta-analysis.
3 Results
3.1 Literature search and study selection
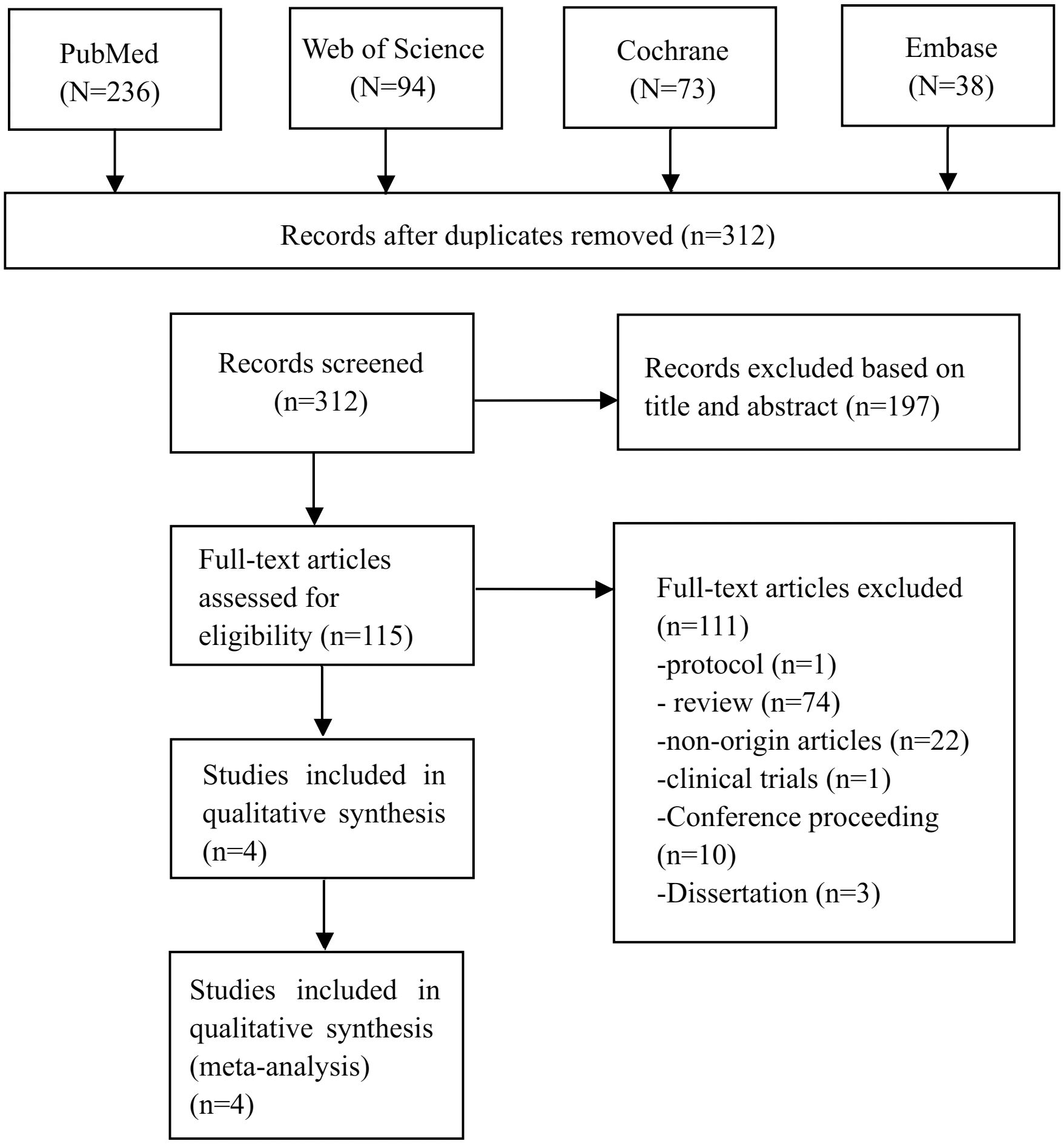
As depicted in Figure 1, a total of 441 publications were sourced from English databases. After applying the inclusion criteria, 4 research trials (n = 151) (30–32, 34) were identified as eligible for the meta-analysis.
3.2 Study characteristics
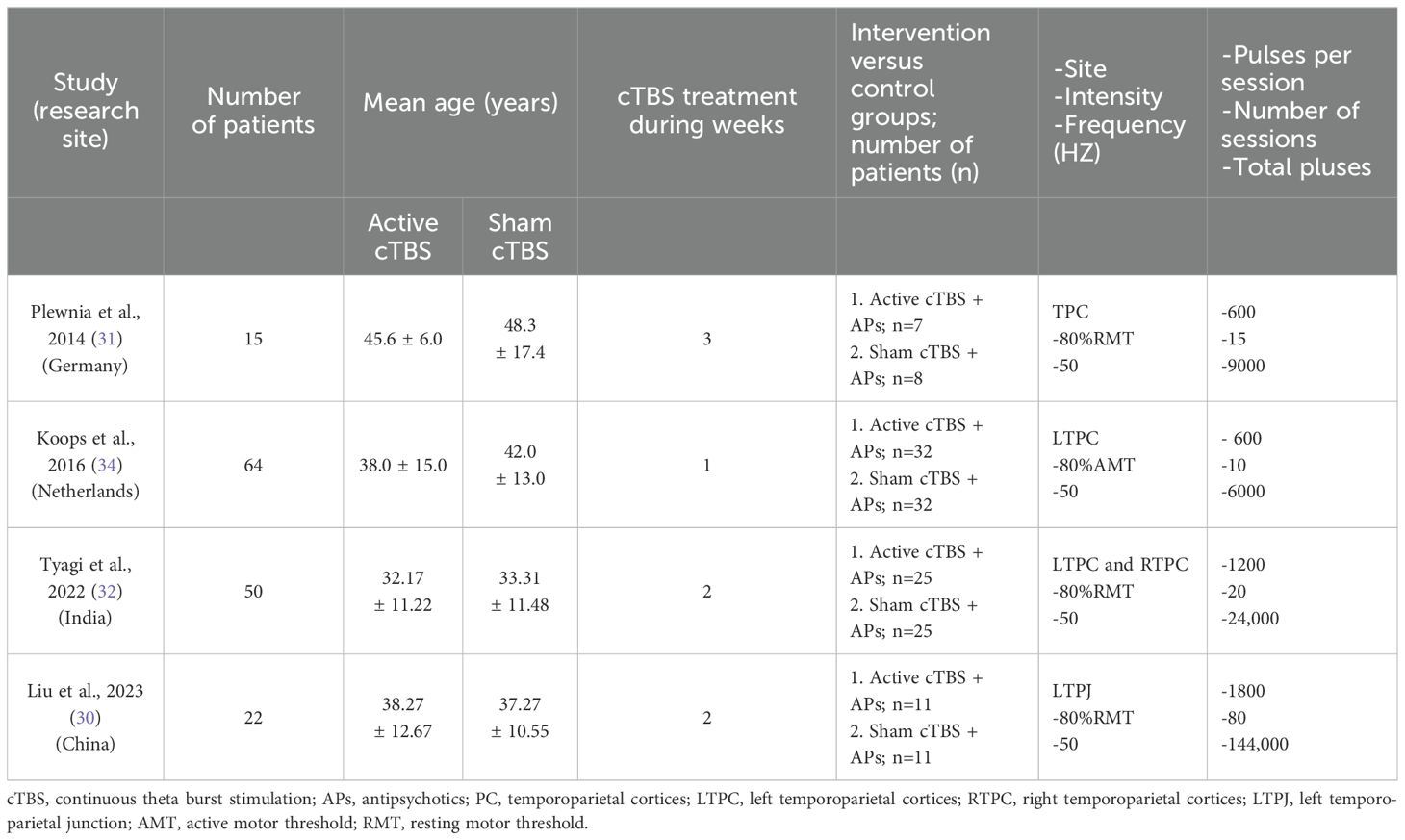
The Table 1 provides an overview of the features of the RCTs that were included in the meta-analysis. Four RCTs were performed to compare the efficiency of active cTBS plus APs (n = 75) and sham cTBS plus APs (n = 76). The average age of patients was 37.78 (standard deviation, SD = 13.51). These four RCTs were conducted in different countries, with one each in China (n = 22), Germany (n = 15), India (n = 50) and Netherlands (n = 64). The duration of cTBS treatment ranged from 1 to 3 weeks, and the treatment sessions ranged from 10 to 80. The magnetic stimulus targeted the temporoparietal cortex.
3.3 RoB of included studies and certainty overall evidence
Supplementary Table 1 summarize the Cochrane risk of bias. The random sequence generation and allocation concealment, performance bias, attrition bias and reporting bias in all four RCTs (4/4, 100%) were rated at “low risk”. Additionally, the overall evidence level for the four meta-analytic outcomes was rated as moderate (100%, 4/4) according to the GRADE approach, as shown in Supplementary Table 2.
3.4 The improvement of auditory hallucination symptoms
Preliminary analysis showed that in four RCTs involving 151 participants, there was no significant difference in improvement of auditory hallucination symptoms between the active cTBS and the sham group, as assessed by AHRS and PSYRAT-AH [SMD = -0.45 (95% CI: -1.01, 0.12), I2 = 61%; P = 0.13; Figure 2]. After one study with an outlier effect size was removed, the statistical analysis remained stable. However, subgroup analyses found that when stimulation session > 10 and the total number of pulses > 6000 [3 RCTs, n = 87; SMD: -4.43 (95%CI: -8.22, -0.63), P = 0.02; I2 = 47%], active cTBS produced a significant improvement of auditory hallucination symptoms in patients as compared to sham stimulation (Table 2). The frequency of cTBS stimulation, whether 2 sessions/day or more, did not show an advantage over the sham group (P > 0.05).
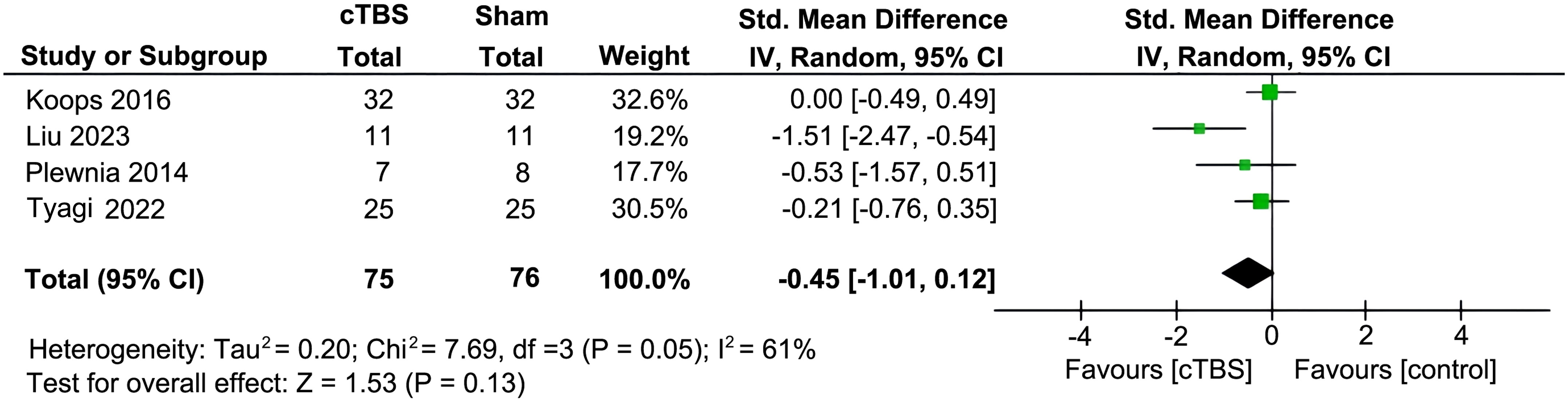
Figure 2. Forest plot for auditory hallucinations as measured by the Auditory Hallucination Rating Scale (AHRS) and the Psychotic Symptom Rating Scales-Auditory Hallucination (PSYRST-AH) post cTBS treatment.
3.5 Psychotic symptoms and discontinuation
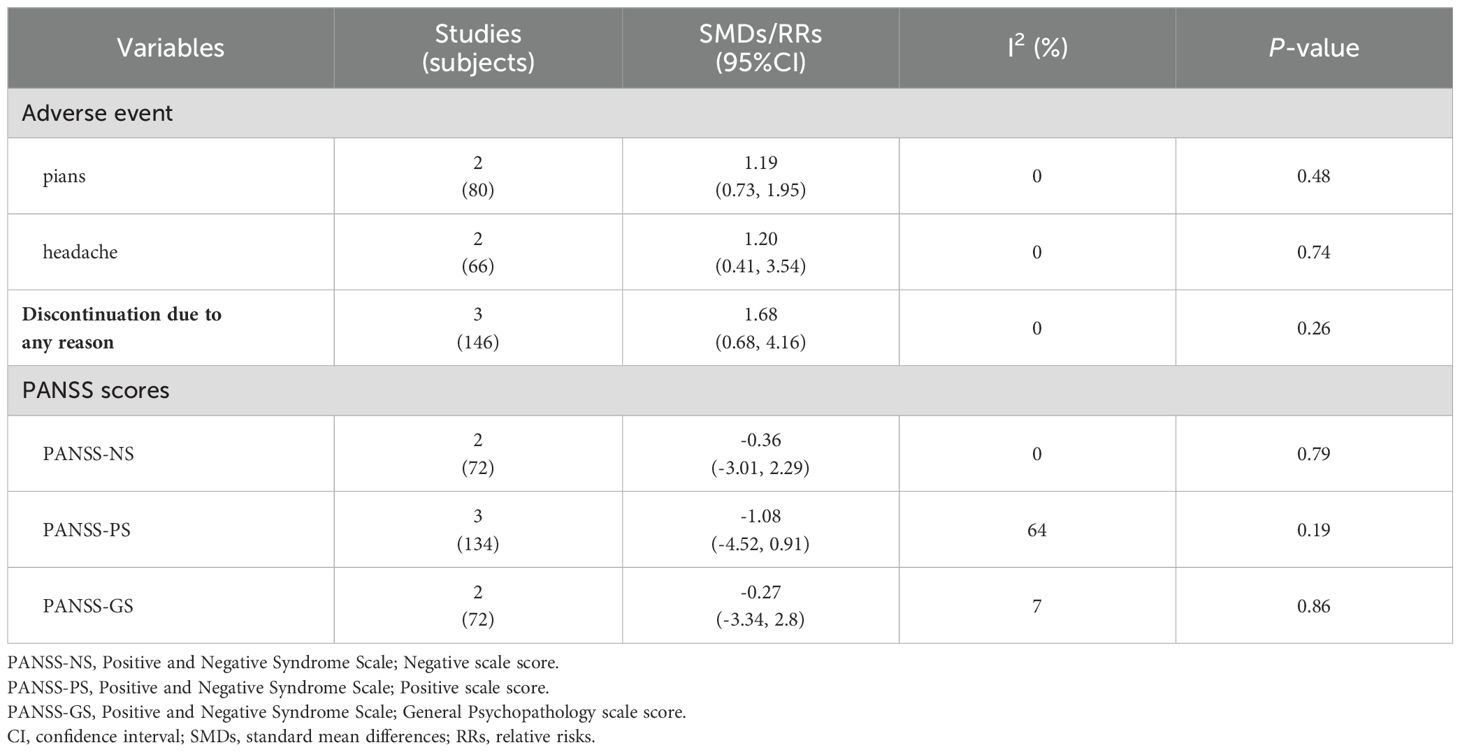
Although adjuvant cTBS treatment alleviated the auditory hallucinations of patients, it did not improve the total psychopathological symptoms compared to the sham group, as measured by the Positive and Negative Symptom Scale (PANSS-P/N/G) [PANSS-P: 3 RCTs, n = 134, SMD = -1.08 (95% CI: -4.52, 0.91), P = 0.19; I2 = 64%; PANSS-N: 2 RCTs, n = 72; SMD = -0.36 (95% CI: -3.01, 2.29), P = 0.79; I2 = 0%; PANSS-G: 2 RCTs, n = 72; SMD = -0.27 (95% CI: -3.34, 2.8), P = 0.86; I2 = 7%] (Table 3).
Only three RCTs reported adverse events. There was no significant difference between the cTBS and the sham group regarding the occurrence of pians [2 RCTs, n = 80; RR = 1.19 (95% CI: 0.73, 1.95), P = 0.48; I2 = 0%] and headaches [2 RCTs, n = 66; RR = 1.20 (95% CI: 0.41, 3.54), P = 0.74; I2 = 0%] (Table 3). Similarly, no difference between the active cTBS and the sham group was observed regarding discontinuation for any reason [3 RCTs, n = 146; RR = 1.68 (95% CI: 0.68, 4.16), P = 0.26; I2 = 0%] (Table 3).
3.6 Publication bias
Four RCTs were included in this meta-analysis, however, we were unable to meet the criteria for the Egger test, which requires a minimum of ten studies. As a result, we did not evaluate the potential for publication bias for the primary outcome in this analysis.
4 Discussion
To the best of our knowledge, this is the first meta-analysis to evaluate the efficacy and safety of cTBS in the treatment of auditory hallucinations. We show that adjuvant cTBS treatment with stimulation sessions > 10 and a total number of pluses > 6000 could significantly improve auditory hallucination symptoms in patients. In addition, cTBS treatment did not increase adverse events and discontinuation to any reason, suggesting that cTBS is safe and well-tolerated for treating auditory hallucinations.
As a form of rTMS, TBS has been utilized to modulate neural network abnormalities associated with neuropsychiatric disorders (43, 44). TBS can be delivered either continuously (cTBS), which has an inhibitory effect, or intermittently (iTBS) in 2-second trains separated by 8-second intervals, which is considered excitatory (28). Previous study has shown that LF-rTMS targeting TPJ, a brain region closely associated with auditory hallucinations, can reduce the intensity of auditory hallucinations in individuals experiencing psychotic symptoms (25). Therefore, cTBS therapy targeting TPJ is theoretically also effective for auditory hallucinations. Four RCTs have been conducted to study the efficacy and safety of adjuvant cTBS treatment for auditory hallucinations. Meta-analysis of these RCTs revealed that there was no significant advantage of active cTBS over sham in reducing auditory hallucination symptoms in the total of 151 participants. Considering that the effectiveness of TBS depends on various parameters, such as stimulation time per session, stimulus intensity, stimulation session, and stimulus site, thus we further analyzed the efficacy of cTBS for the treatment of auditory hallucinations under different conditions. We found that when stimulation session > 10 and a total number of pluses > 6000, cTBS treatment could significantly improve the auditory hallucination symptoms in patients. This finding demonstrates that appropriate parameters determine the clinical efficacy of cTBS for the treatment of auditory hallucinations.
Increased pulse number of TBS can have a greater cumulative effect on cortical excitability and functional connectivity (45). A greater number of sessions and higher total pulse dose appear to produce superior clinical efficacy (46, 47). Furthermore, it has been suggested that amelioration of auditory hallucinations might only occur gradually after prolonged treatment (48). One of these RCTs studied in the meat-analysis, in which the participants received TB-rTMS or placebo treatment twice a day for 5 consecutive days (10 sessions), showed that improvement of auditory verbal hallucinations did not differ significantly between the TB-rTMS and the placebo group as measured with both the PSYRST-AH and the AHRS (34). We postulate that the negative results in this RCT might be attributable to the limited treatment session and period. Compared to this study (34), the other three studies administered more treatment sessions over a more extended period (30–32). Specifically, Liu et al., adopted a high-dose accelerated 1800-pulse cTBS (cTBS1800) protocol, which consists of a total of 80 cTBS1800 sessions during two consecutive weeks (5 days on, 2 days off), and found a significant difference between the active cTBS and sham group in terms of improvement of auditory hallucinations (30). An increase in the number and intensity of cTBS has also been shown to improve treatment efficacy in clinical trials of major depressive disorder, where the dose-effect of cTBS has been identified (49). Therefore, it is necessary to explore appropriate cTBS stimulation parameters to ensure that it is safe and well tolerated in patients with auditory hallucinations and has therapeutic properties in the future.
It should be noted that the included studies targeted the left (and right for one study) temporo-parietal junction, based simply on the median zone between T3 and P3 of the 10-20 EEG system. This approach has been criticized for not accounting for inter-individual variability and the variability of the zones whose hyperactivity is associated with hallucinations. It has been suggested that the use of neuronavigation based on structural or functional targets would be more effective (50, 51). However, the subgroup analysis of this meta-analysis found that cTBS targeting temporo-parietal junction based on the median zone between T3 and P3 of the EEG system with specific parameters has a superior therapeutic effect than sham stimulation, indicating that neuronavigation-based targeting approaches may not be necessary for cTBS therapy. Of course, if the conditions are available, the use of neuronavigation technology will be more conducive to the efficacy of TBS in the treatment of auditory hallucinations.
The AHRS has perfect psychometric properties for detecting the changes in auditory hallucinations, and is therefore recommended for the measurement of auditory hallucination symptoms in schizophrenia (52). Other scales, such P3 item in the PANSS and PSYRST-AH, are also used to measure auditory hallucinations, but they appear to be less reliable for detecting the changes in hallucination symptoms. Previous studies have also reported that the use of different scales can lead to differences in measures of auditory hallucinations symptoms (53). In this meta-analysis, only 2 RCTs (32, 34) adopted AHRS to evaluate auditory hallucinations, thus the standardized data might be biased against primary outcomes. Hence, it is suggested that future studies should consistently use AHRS to obtain more credible and reliable results.
It is imperative to note several limitations in the present meta-analysis. Firstly, all four RCTs included had relatively small sample sizes, which limited the statistical power of the study. Secondly, the concomitant use of antipsychotics was neither standardized nor consistently reported in the included studies, which precludes the exploration of potential confounding effects of clinical medications. Thirdly, some secondary outcomes were analyzed based on a small number of studies, which also reduced the likelihood of detecting significant findings. Lastly, the current literature search strategy may have selection bias since the search was limited to English-language database.
In this meta-analysis, we show that adjuvant cTBS treatment can produce a therapeutic effect on auditory hallucinations and this treatment appears to be safe and well-tolerated in patients. Given the important impact of different parameters such as stimulation session and number of pulses on the efficacy of cTBS, multicenter RCTs with a larger sample size are needed to determine an appropriate and standard cTBS protocol for the treatment of auditory hallucinations in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
SY: Software, Methodology, Investigation, Formal analysis, Data curation, Writing – original draft. CC: Project administration, Investigation, Funding acquisition, Writing – original draft. BW: Project administration, Investigation, Writing – original draft. JZ: Methodology, Formal analysis, Data curation, Writing – review & editing. YL: Methodology, Data curation, Writing – review & editing. CZ: Project administration, Methodology, Investigation, Writing – review & editing. JH: Project administration, Methodology, Investigation, Writing – review & editing. YY: Supervision, Project administration, Methodology, Investigation, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by grants from the National Natural Science Foundation of China (82271557, 82060258) and the Jiangxi Provincial Clinical Research Center Projects (2020BCG74002). It was also supported by the Natural Science Foundation of Fujian Province (2023J01104), the Joint Funds for the Innovation of Science and Technology of Fujian Province (2023Y9255) and the Science and Technology Bureau of Quanzhou (2023C005YR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1446849/full#supplementary-material
References
1. Northoff G, Qin P. How can the brain's resting state activity generate hallucinations? A 'resting state hypothesis' of auditory verbal hallucinations. Schizophr Res. (2011) 127:202–14. doi: 10.1016/j.schres.2010.11.009
2. Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. (1991) 17:27–49. doi: 10.1093/schbul/17.1.27
3. Baethge C, Baldessarini RJ, Freudenthal K, Streeruwitz A, Bauer M, Bschor T. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. (2005) 7:136–45. doi: 10.1111/j.1399-5618.2004.00175.x
4. Zhuo C, Ji F, Lin X, Tian H, Wang L, Xu Y, et al. Common and distinct brain functional alterations in pharmacotherapy treatment-naïve female borderline personality disorder patients with and without auditory verbal hallucinations: a pilot study. Eur Arch Psychiatry Clin Neurosci. (2021) 271:1149–57. doi: 10.1007/s00406-020-01102-5
5. Daalman K, Boks MP, Diederen KM, De Weijer AD, Blom JD, Kahn RS, et al. The same or different? A phenomenological comparison of auditory verbal hallucinations in healthy and psychotic individuals. J Clin Psychiatry. (2011) 72:320–5. doi: 10.4088/JCP.09m05797yel
6. McCarthy-Jones S, Trauer T, Mackinnon A, Sims E, Thomas N, Copolov DL. A new phenomenological survey of auditory hallucinations: evidence for subtypes and implications for theory and practice. Schizophr Bull. (2014) 40:231–5. doi: 10.1093/schbul/sbs156
7. Shan P, Zhuo C, Ma X, Sang H, Zhong B, Lin X, et al. Treatment of auditory verbal hallucinations with atypical antipsychotics in healthy individuals: an artificially controlled post-treatment report. J Int Med Res. (2020) 48:300060519875830. doi: 10.1177/0300060519875830
8. Shergill SS, Murray RM, McGuire PK. Auditory hallucinations: a review of psychological treatments. Schizophr Res. (1998) 32:137–50. doi: 10.1016/S0920-9964(98)00052-8
10. Friston KJ. The disconnection hypothesis. Schizophr Res. (1998) 30:115–25. doi: 10.1016/S0920-9964(97)00140-0
11. Lennox BR, Park SB, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. (2000) 100:13–20. doi: 10.1016/S0925-4927(00)00068-8
12. Gromann PM, Tracy DK, Giampietro V, Brammer MJ, Krabbendam L, Shergill SS. Examining frontotemporal connectivity and rTMS in healthy controls: implications for auditory hallucinations in schizophrenia. Neuropsychology. (2012) 26:127–32. doi: 10.1037/a0026603
13. Horga G, Fernández-Egea E, Mané A, Font M, Schatz KC, Falcon C, et al. Brain metabolism during hallucination-like auditory stimulation in schizophrenia. PloS One. (2014) 9:e84987. doi: 10.1371/journal.pone.0084987
14. Amico F, O'hanlon E, Kraft D, Oertel-Knchel V, Clarke M, Kelleher I, et al. Functional connectivity anomalies in adolescents with psychotic symptoms. PloS One. (2017) 12:e0169364. doi: 10.1371/journal.pone.0169364
15. Kaliora SC, Zervas IM, Papadimitriou GN. [Electroconvulsive therapy: 80 years of use in psychiatry]. Psychiatriki. (2018) 29:291–302. doi: 10.22365/jpsych
16. Darmani G, Bergmann TO, Butts Pauly K, Caskey CF, De Lecea L, Fomenko A, et al. Non-invasive transcranial ultrasound stimulation for neuromodulation. Clin Neurophysiol. (2022) 135:51–73. doi: 10.1016/j.clinph.2021.12.010
17. Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol. (2020) 131:474–528. doi: 10.1016/j.clinph.2019.11.002
18. McClintock SM, Reti IM, Carpenter LL, Mcdonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. (2018) 79:16cs10905. doi: 10.4088/JCP.16cs10905
19. Ikeda T, Kurosawa M, Morimoto C, Kitayama S, Nukina N. Multiple effects of repetitive transcranial magnetic stimulation on neuropsychiatric disorders. Biochem Biophys Res Commun. (2013) 436:121–7. doi: 10.1016/j.bbrc.2013.03.017
20. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol. (2002) 5:73–103. doi: 10.1017/S1461145702002791
21. Bai Z, Zhang J, Fong KNK. Effects of transcranial magnetic stimulation in modulating cortical excitability in patients with stroke: a systematic review and meta-analysis. J Neuroeng Rehabil. (2022) 19:24. doi: 10.1186/s12984-022-00999-4
22. Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. (1998) 15:333–43. doi: 10.1097/00004691-199807000-00005
23. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. (2011) 168:73–81. doi: 10.1176/appi.ajp.2010.09101522
24. Xie Y, He Y, Guan M, Wang Z, Zhou G, Ma Z, et al. Low-frequency rTMS treatment alters the topographical organization of functional brain networks in schizophrenia patients with auditory verbal hallucination. Psychiatry Res. (2022) 309:114393. doi: 10.1016/j.psychres.2022.114393
25. Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated "voices. Biol Psychiatry. (1999) 46:130–2. doi: 10.1016/S0006-3223(98)00358-8
26. Guttesen LL, Albert N, Nordentoft M, Hjorthøj C. Repetitive transcranial magnetic stimulation and transcranial direct current stimulation for auditory hallucinations in schizophrenia: Systematic review and meta-analysis. J Psychiatr Res. (2021) 143:163–75. doi: 10.1016/j.jpsychires.2021.09.001
27. Rounis E, Huang YZ. Theta burst stimulation in humans: a need for better understanding effects of brain stimulation in health and disease. Exp Brain Res. (2020) 238:1707–14. doi: 10.1007/s00221-020-05880-1
28. Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. (2005) 45:201–6. doi: 10.1016/j.neuron.2004.12.033
29. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. (2015) 32:182–92. doi: 10.1002/da.22335
30. Liu X, Xu L, Gong J, Ye Q, Jin G, Zhou D. The effect of high-dose accelerated continuous theta burst stimulation (cTBS) treatment on auditory verbal hallucinations (AVH): A pilot study. Psychiatry Res. (2023) 326:115337. doi: 10.1016/j.psychres.2023.115337
31. Plewnia C, Zwissler B, Wasserka B, Fallgatter AJ, Klingberg S. Treatment of auditory hallucinations with bilateral theta burst stimulation: a randomized controlled pilot trial. Brain stimulation. (2014) 7:340–1. doi: 10.1016/j.brs.2014.01.001
32. Tyagi P, Dhyani M, Khattri S, Tejan V, Tikka SK, Garg S. Efficacy of intensive bilateral Temporo-Parietal Continuous theta-burst Stimulation for Auditory VErbal hallucinations (TPC-SAVE) in schizophrenia: A randomized sham-controlled trial? Asian J Psychiatry. (2022) 74:103176. doi: 10.1016/j.ajp.2022.103176
33. Chithra U, Samantaray S, Kumar V, K. R, Maity K, E N, et al. Add-on accelerated continuous theta burst stimulation (a-cTBS) over the left temporoparietal junction for the management of persistent auditory hallucinations in schizophrenia: A case series. Brain Stimul. (2022) 15:1511–2. doi: 10.1016/j.brs.2022.11.005
34. Koops S, Dellen EV, Schutte MJL, Nieuwdorp W, Neggers SFW, Sommer IEC. Theta burst transcranial magnetic stimulation for auditory verbal hallucinations: negative findings from a double-blind-randomized trial. Schizophr Bull. (2016) 42:250–7. doi: 10.1093/schbul/sbv100
35. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
36. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
37. Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. (2003) 60:49–56. doi: 10.1001/archpsyc.60.1.49
38. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
39. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
40. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
41. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
42. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. (2011) 343:d4002. doi: 10.1136/bmj.d4002
43. Shinn AK, Hurtado-Puerto AM, Roh YS, Ho V, Hwang M, Cohen BM, et al. Cerebellar transcranial magnetic stimulation in psychotic disorders: intermittent, continuous, and sham theta-burst stimulation on time perception and symptom severity. Front Psychiatry. (2023) 14:1218321. doi: 10.3389/fpsyt.2023.1218321
44. Noda Y. Toward the establishment of neurophysiological indicators for neuropsychiatric disorders using transcranial magnetic stimulation-evoked potentials: A systematic review. Psychiatry Clin Neurosci. (2020) 74:12–34. doi: 10.1111/pcn.12936
45. Nettekoven C, Volz LJ, Kutscha M, Pool EM, Rehme AK, Eickhoff SB, et al. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. (2014) 34:6849–59. doi: 10.1523/JNEUROSCI.4993-13.2014
46. Yip AG, George MS, Tendler A, Roth Y, Zangen A, Carpenter LL. 61% of unmedicated treatment resistant depression patients who did not respond to acute TMS treatment responded after four weeks of twice weekly deep TMS in the Brainsway pivotal trial. Brain Stimul. (2017) 10:847–9. doi: 10.1016/j.brs.2017.02.013
47. Avery DH, Isenberg KE, Sampson SM, Janicak PG, Lisanby SH, Maixner DF, et al. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry. (2008) 69:441–51. doi: 10.4088/JCP.v69n0315
48. Eberle MC, Wildgruber D, Wasserka B, Fallgatter AJ, Plewnia C. Relief from chronic intractable auditory hallucinations after long-term bilateral theta burst stimulation. Am J Psychiatry. (2010) 167:1410. doi: 10.1176/appi.ajp.2010.10070988
49. Chistyakov AV, Rubicsek O, Kaplan B, Zaaroor M, Klein E. Safety, tolerability and preliminary evidence for antidepressant efficacy of theta-burst transcranial magnetic stimulation in patients with major depression. Int J Neuropsychopharmacol. (2010) 13:387–93. doi: 10.1017/S1461145710000027
50. Jardri R, Lucas B, Delevoye-Turrell Y, Delmaire C, Delion P, Thomas P, et al. An 11-year-old boy with drug-resistant schizophrenia treated with temporo-parietal rTMS. Mol Psychiatry. (2007) 12:320. doi: 10.1038/sj.mp.4001968
51. Jardri R, Pins D, Thomas P. A case of fMRI-guided rTMS treatment of coenesthetic hallucinations. Am J Psychiatry. (2008) 165:1490–1. doi: 10.1176/appi.ajp.2008.08040504
52. Dondé C, Haesebaert F, Poulet E, Mondino M, Brunelin J. Validation of the french version of the auditory hallucination rating scale in a sample of hallucinating patients with schizophrenia. Can J Psychiatry. (2020) 65:237–44. doi: 10.1177/0706743719895641
Keywords: continuous theta burst stimulation (cTBS), auditory hallucinations, treatment, meta-analysis, non-invasive brain stimulation
Citation: Ye S-Y, Chen C-N, Wei B, Zhan J-Q, Li Y-H, Zhang C, Huang J-J and Yang Y-J (2024) The efficacy and safety of continuous theta burst stimulation for auditory hallucinations: a systematic review and meta-analysis of randomized controlled trials. Front. Psychiatry 15:1446849. doi: 10.3389/fpsyt.2024.1446849
Received: 10 June 2024; Accepted: 30 July 2024;
Published: 19 August 2024.
Edited by:
Zezhi Li, Guangzhou Medical University, ChinaReviewed by:
Noomane Bouaziz, EPS Ville Evrard, FranceBao-Liang Zhong, Wuhan Mental Health Center, China
Copyright © 2024 Ye, Chen, Wei, Zhan, Li, Zhang, Huang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan-Jian Yang, eXVhbmppbXlhbmdAeWVhaC5uZXQ=; Jing-Jing Huang, ampodWFuZ19hdHRAMTYzLmNvbQ==; Chen Zhang, emhhbmdjaGVuNjQ1QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shi-Yi Ye
Shi-Yi Ye Chun-Nuan Chen
Chun-Nuan Chen Bo Wei
Bo Wei Jin-Qiong Zhan1,4
Jin-Qiong Zhan1,4 Chen Zhang
Chen Zhang Yuan-Jian Yang
Yuan-Jian Yang