
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 09 October 2024
Sec. Perinatal Psychiatry
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1443352
This article is part of the Research Topic Psychiatric Illness Across the Menstrual Cycle View all 10 articles
 Lindsay R. Standeven1*†‡
Lindsay R. Standeven1*†‡ Mira Bajaj2*†‡
Mira Bajaj2*†‡ Kathleen McEvoy3
Kathleen McEvoy3 Dalar Shirinian4
Dalar Shirinian4 Kristin Voegtline5
Kristin Voegtline5 Lauren M. Osborne5
Lauren M. Osborne5 Jennifer L. Payne4
Jennifer L. Payne4 Liisa Hantsoo1
Liisa Hantsoo1Background: Premenstrual Syndrome (PMS) and Premenstrual Dysphoric Disorder (PMDD), collectively known as Premenstrual Disorders (PMDs), cause significant distress and functional impairment, and premenstrual exacerbation (PME) affects a large proportion of women with psychiatric diagnoses. Childhood trauma is one factor that may contribute to PMD/PME risk. This study examines the relationship between childhood trauma and PMDs, PME, and non-PMD psychiatric illness.
Methods: This study is a secondary analysis of data from a prospective cohort. Participants completed self-assessments on childhood trauma using the Childhood Traumatic Event Scale (CTE-S) and on premenstrual symptoms using the Premenstrual Symptoms Screening Tool (PSST). Psychiatric diagnoses were assessed through structured clinical interviews. Participants were divided into four groups based on their PSST scores and psychiatric illness status: (1) Premenstrual Disorders (PMDs; moderate to severe PMS and PMDD), (2) PME, (3) psychiatric controls (PC; individuals with psychiatric illness but no significant premenstrual symptoms), and (4) healthy controls (HC; individuals with no psychiatric illness and no significant premenstrual symptoms). Statistical analyses, including ANOVA, Tukey’s HSD test, Fisher’s exact test, and logistic regression, were conducted to examine differences among the groups.
Results: Data from 391 participants were analyzed. Participants with PME and PC reported a higher quantity and severity of childhood traumatic events compared to HCs (p <.05). There was a weak but significant correlation between childhood trauma and premenstrual symptom burden across all groups (R = .18, p <.001). Within-group analysis revealed moderate correlations between childhood trauma and premenstrual symptoms driven by the PMD group (R = .42, p = .01).
Conclusions: The findings underscore the impact of childhood traumatic events on mental health and premenstrual symptoms and highlight the need for additional research to explore the underlying mechanisms linking childhood trauma to the continuum of premenstrual disorders, to improve the efficacy of trauma-focused interventions for affected individuals.
On average, women menstruate for 40 years of their lives, and approximately 80% experience physical and/or mood symptoms in the premenstrual week. While most women experience mild premenstrual symptoms that do not affect quality of life or functioning, an estimated 20-40% of women experience premenstrual syndrome (PMS) and 5-8% meet criteria for Premenstrual Dysphoric Disorder (PMDD). Premenstrual Syndrome (PMS) is characterized by recurrent physical and/or emotional symptoms occurring in the late luteal phase (roughly the week prior to the onset of menses); symptoms remit with the start of menstruation (1). Although these symptoms can affect daily functioning and quality of life, the affective symptoms that occur do not reach threshold for a depressive disorder, and some women have physical symptoms only. PMDD, on the other hand, is a mood disorder characterized by functional impairment secondary to affective changes in the late luteal phase. Collectively, these premenstrual syndromes (PMS and PMDD), often thought of as a continuum of Premenstrual Disorders (PMDs), contribute to significant physical and psychic burden across a woman’s reproductive lifespan.
In addition to the PMDs, there is Premenstrual Exacerbation (PME) of psychiatric disorders. PME occurs in women with psychiatric disorders who experience a significant worsening in psychiatric symptoms during the week preceding their menses. PME of affective disorders is common. Recent retrospective and prospective studies have indicated PME prevalence rates ranging from 33.7% to 68.5% in women with major depressive disorder and 44-68% among those with bipolar depression (2–7). PME is associated with increased morbidity of psychiatric illness, including decreased general functioning, shorter remission times, more severe symptoms, and higher rates of treatment resistance. Like the PMDs, PME is thought to involve abnormal sensitivity to ovarian hormone fluctuations, although specific mechanisms remain unclear and could vary based on primary diagnosis (2).
Numerous biopsychosocial factors have been proposed as potential contributors to PMDs, including genetic predisposition, hormone sensitivity, neurotransmitter alterations, and inflammatory processes (8–14). Amidst these potential contributors, the influence of psychosocial stress, particularly trauma or adverse childhood experiences (ACEs), has emerged as a contributor to the risk for and severity of PMDs (2, 3, 15). ACEs refer to potentially traumatic events or experiences occurring in childhood or adolescence that fall into one of three general categories and can include one or many of the following: abuse (physical, emotional or sexual), neglect (physical or emotional) and household challenges (substance abuse, mental illness, domestic violence, parental separation or divorce, incarceration) (15). Several studies have found an association between the number of ACEs and the development and severity of PMDs. In a large cross-sectional analysis of nearly 12,000 participants, Yang et al. (2022) found a positive linear association between the number of ACEs experienced and probability of PMDs, with those experiencing >4 ACEs having a higher likelihood of having PMDD (7). Another study observed a similar correlation where the number and severity of premenstrual symptoms increased with increasing levels of childhood trauma (16). In a recent study, more childhood adversity was associated with increasing negative affect and greater stress response (as measured by cortisol) as patients progressed from the follicular to the luteal phase among women with PMDD (17).
Other research has focused on whether specific types of traumatic or adverse childhood experiences are associated with higher risk for or severity of PMDs. For example, some studies found a significant association between childhood emotional and physical abuse and later risk for PMDs (18–22). Yet other investigations pointed to sexual abuse during childhood and adolescence as posing the highest risk for PMDs, and particularly for PMDD (23). Conversely, others failed to find an association between sexual abuse and PMDs (24). Some studies found higher rates of PMDD among women with higher levels of childhood adversity independent of category (i.e. physical abuse, sexual abuse, emotional abuse, and neglect). Together, these conflicting results may stem from differences in study methodology, varying definitions of PMDs (i.e., whether specifically studying PMDD versus PMS), limited sample sizes, and a scarcity of studies focused on the stressor timing (childhood versus lifetime adversity) in relation to the onset or severity of PMDs.
Despite the known connection between childhood trauma and increased risk for various mental health conditions (25, 26), including PMDs, only a few studies have explored the relationship between childhood trauma or adversity and PME. One such study by Koci et al. (2007) assessed the impact of childhood sexual and physical abuse on subsequent symptoms of PMS and mood symptoms throughout the menstrual cycle and discovered that both types of abuse were elevated among women experiencing “premenstrual magnification” of mood symptoms and among women with persistently heightened mood symptoms across the menstrual cycle, irrespective of premenstrual changes (23). Although this study underscores a connection between trauma and the development of both mood symptoms and PME, its applicability is constrained by the absence of formal psychiatric evaluations.
To date, significant gaps persist in our understanding of the correlations between PMDs (as well as PME) and the timing (e.g. childhood exposure), type, and severity of traumatic events; thus the present study aims to assess these relationships within a cohort of women who underwent thorough psychiatric assessment and premenstrual symptom evaluation. We defined participant groups by their primary diagnosis and premenstrual symptoms (1) PMDs (2), PME (3), psychiatric controls (PC) (4), healthy controls (HC), and evaluated associations with the frequency, nature, and timing of childhood traumatic events. We hypothesized that individuals with PMDs would have more childhood trauma (in both quantity and severity) compared to those with PME, psychiatric illness alone, and controls.
This is a secondary analysis of data from The Prospective Study of Pregnant Women, an observational cohort of pregnant individuals aged 18 and older with and without mood disorders at The Johns Hopkins University School of Medicine (27, 28). Exclusion criteria included current active suicidal ideation, medical instability, and active substance use disorders. Upon enrollment in the study, participants filled out a variety of study questionnaires, including demographic information, reproductive history including age of menarche, and self-assessments focusing on trauma and menstrual cycle symptoms: the Childhood Traumatic Event Scale (CTE-S), which gauges the history of childhood trauma, and the Premenstrual Symptoms Screening Tool (PSST), which collects retrospective assessments of mood and physical premenstrual symptoms. Past and current psychiatric diagnoses were determined with a Structured Clinical Interview for DSM-IV Disorders (SCID) (29) conducted by a trained research assistant and confirmed through clinical interview by a psychiatrist using DSM-IV criteria. Any discrepancy in diagnosis was reconciled through review by the study psychiatrists and research assistant. Data were collected and securely stored using the REDCap (Research Electronic Data Capture) tool hosted at Johns Hopkins University (30, 31). This study was approved by the Institutional Review Board of The Johns Hopkins University.
Childhood Traumatic Events Scale (CTE-S): The Childhood Traumatic Events Scale (CTE-S) is a self-report questionnaire used to assess exposure to different types of trauma prior to the age of 17. This scale (32) assesses whether participants experienced six different types of trauma: 1) death of a close friend or family member, 2) major upheaval between parents (divorce, separation, etc.), 3) traumatic sexual experience, 4) physical violence, 5) extreme illness or injury, 6) “other major upheaval.” For each type of trauma, participants are first asked whether they experienced it prior to the age of 17 or not. If they endorse experiencing it, they are asked further questions about their age at the time of trauma, the severity of how traumatic this event was (Likert scale 1 to 7), and how much they confided in others (Likert scale 1 to 7). For each participant, the sum of total number of traumatic events experienced and the total severity of traumatic events was calculated.
Premenstrual Symptoms Screening Tool (PSST): The Premenstrual Symptoms Screening Tool (PSST) is a questionnaire that translates DSM-IV criteria for Premenstrual Dysphoric Disorder (PMDD) into a rating scale with degrees of severity (33). The questionnaire assesses the severity of 14 different premenstrual symptoms and degree of interference in five areas of life using a Likert scale (1 to 4, “Not at All” to “Severe”). The PSST stratifies participants into those with no to mild premenstrual symptoms, moderate-to-severe premenstrual symptoms or PMS, and likely PMDD.
Structured Clinical Interview for DSM-IV Disorders (SCID): The Structured Clinical Interview for DSM-IV Disorders (SCID) is a semi-structured interview guide for making DSM-IV diagnoses. The SCID for Axis I assesses for current and lifetime mood disorders, psychotic disorders, substance use disorders, anxiety disorders, and eating disorders through questions that map onto DSM-IV diagnostic criteria (29).
Of the four-hundred and nine participants enrolled in the parent study as of June 2020, 18 participants were missing either PSST or CTE data, resulting in a final sample of N = 391 participants used for this analytic cohort. Participants were stratified into 4 groups based on their level of premenstrual distress (as measured by their PSST category) and their history of psychiatric illness as follows: 1) Premenstrual Exacerbation (PME) – those with moderate-to-severe premenstrual symptoms on PSST and either a current mood or anxiety disorder on the SCID or those on medication for a lifetime mood or anxiety disorder; 2) Premenstrual Disorders (PMDs) – those who met “moderate-to-severe PMS” or “PMDD” on the PSST, but did not have current mood/anxiety disorder nor medication treatment for a lifetime mood/anxiety disorder; 3) Controls with psychiatric illness [Psychiatric Controls (PC)] – those with none to mild premenstrual symptoms on the PSST and either a current mood/anxiety disorder on the SCID or medication treatment for a lifetime mood/anxiety disorder and 4) Healthy Controls (HC) – those with none to mild premenstrual symptoms, no current mood/anxiety disorder and no medication treatment for a lifetime mood/anxiety disorder.
Demographics were summarized by group (HC, PC, PMD, PME). Categorical variables were summarized with N (%) and group-level differences were tested using Fisher’s Exact Tests. Analysis of variance (ANOVA) was used to test for group-level differences in the CTE trauma measurements (number of events experienced, severity of events experienced). Adjusted models controlling for demographic variables were conducted to control for baseline differences. For post-hoc analysis of any significant ANOVA findings, Tukey’s HSD test was used to test for pairwise comparisons between groups (34). Fisher’s exact test was used to test for univariate differences in type of trauma experienced by group and to evaluate for differences in experience of prepubertal trauma, with prepubertal trauma being defined as trauma experienced before 2 years prior to menarche. Multinomial logistic regression with trauma type as the outcome was used to test for group level differences in types of trauma experienced, adjusting for relevant demographic covariates.
Exploratory correlation analyses assessed the relationship between the total PSST score (sum of all items) and CTE-S trauma measurements (number of events experienced, severity of events experienced) in order to explore the relationship between childhood trauma and premenstrual symptoms using continuous as opposed to categorical groupings.
Exploratory analyses assessed for differences in total PSST scores between the groups using ANOVA, to see if groups with the same categorical level of premenstrual distress (e.g. PME and PMD; PC and HC) experienced the same quantitative level of premenstrual symptoms.
Significance was evaluated at a level of p <.05. As this was largely an exploratory hypothesis-generating study, we did not correct for multiple comparisons in order to not miss associations worthy of future study in larger and more rigorous samples. Analyses were completed using R version 3.6.2 (35).
N=217 participants were classified as HC, 102 PC, 34 PMD, and 38 PME. Table 1 summarizes the participant demographics overall and by group. The groups differed in their distribution of race (p = .040), education level (p <.001), and income level (p <.001). The groups were otherwise similar in terms of age, ethnicity, and employment status.
136 participants (62.7%) in the HC group, 74 participants (72.5%) in PC group, 26 participants (76.5%) in PMD, and 31 participants (81.6%) in PME experienced trauma prior to age 17.
One-way ANOVA revealed a significant difference in number of traumatic events reported between groups [F(3,387) = 4.46, p = .004]. Tukey’s HSD test for multiple comparisons revealed that this was driven by a significant difference between HC and PC (adjusted difference = 0.37, 95% CI = 0.03 – 0.71, p = .025) and a significant difference between HC and PME (adjusted difference = 0.53, 95% CI = 0.03 – 1.02, p = .033), such that HCs reported fewer traumatic events compared to the PC and PME groups (Figure 1A). This difference between groups remained significant after controlling for medication use, race, income, and education [F(3,353) = 5.27, p = .001]. No other pairwise comparisons were significantly different (p’s >.35).
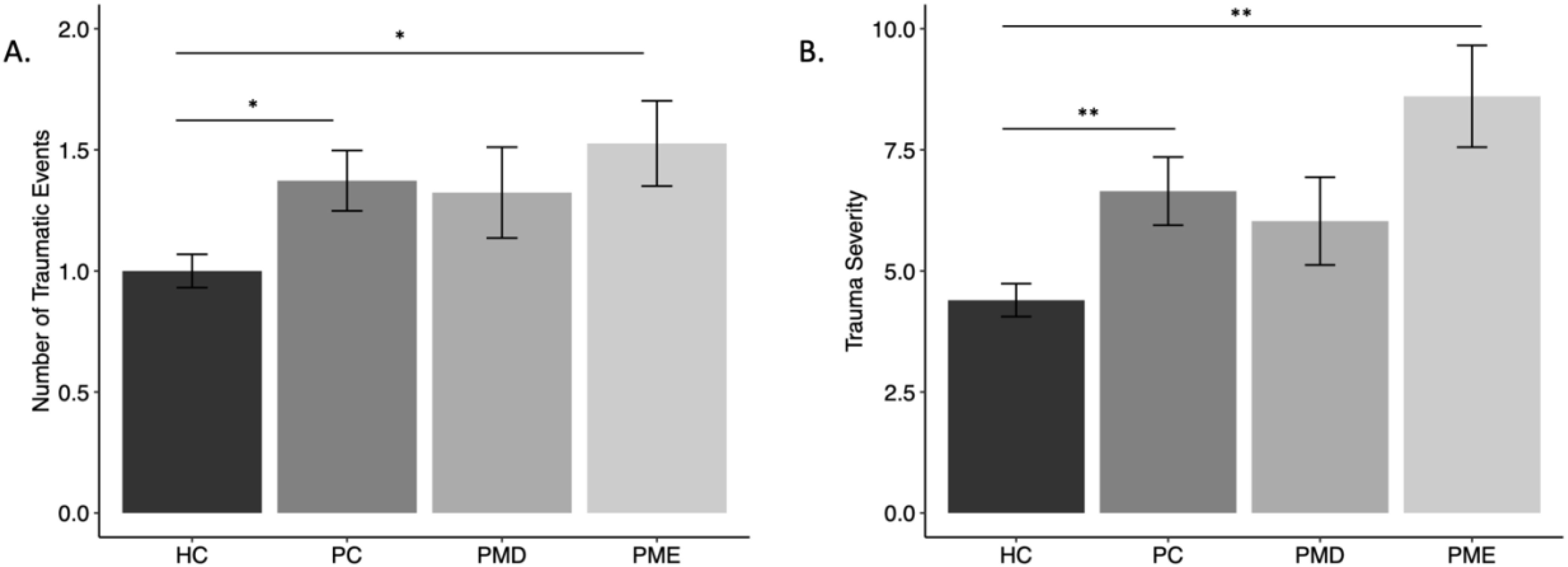
Figure 1. Mean measures of childhood trauma by group. [(A) number of traumatic events, (B) severity of events] by group. Error bars represent standard error. HC, Healthy Controls; PC, Psychiatric Controls (Controls with Psychiatric Illness); PMD, premenstrual disorder; PME, premenstrual exacerbation. * = p <.05; ** = p <.01.
There was a significant difference in the severity of reported traumatic events among groups (F(3,387) = 7.67, p <.001). Tukey’s HSD test for multiple comparisons revealed that differences in severity scores were driven by a significant difference between HC and PC (adjusted difference = 2.25, 95% CI = 0.45 – 4.05, p = .007) and a significant difference between HC and PME (adjusted difference = 4.21, 95% CI = 1.58 – 6.84, p = .003), such that HCs reported a lower severity of traumatic events compared to the PC and PME groups (Figure 1B). This group difference remained significant after controlling for medication use, race, income, and education [F(3,353) = 3.81, p = .010]. No other pairwise comparisons were significantly different (p’s >.23).
Table 2 depicts the frequency of types of traumas reported within each group. Fisher’s exact test revealed a significant difference between groups, with more participants in the PC and PME groups reporting a history of sexual abuse (p = .020) and “other” trauma (p = .008), but no significant differences across other trauma types. However, multinomial logistic regression models revealed that there was no significant group difference in experience of sexual abuse once controlling for medication use, race, income, and education (ps >.15), but that those who earned higher income had lower odds of experiencing sexual abuse in childhood compared to those making $50,000 or less (Table 3). Participants who earned between $50,000 and $100,000 (OR = 0.39, 95% CI = 0.15 - 0.99, p = .047) and those making $100,000 or more (OR = 0.20, 95% CI = 0.07 - 0.57, p = .003) had lower odds of experiencing sexual abuse in comparison to those making $50,000 or less. Additionally, when controlling for medication use, race, income, and education, there was no longer a group-level difference or any significant demographic predictors of experiencing other types of trauma (Table 3).
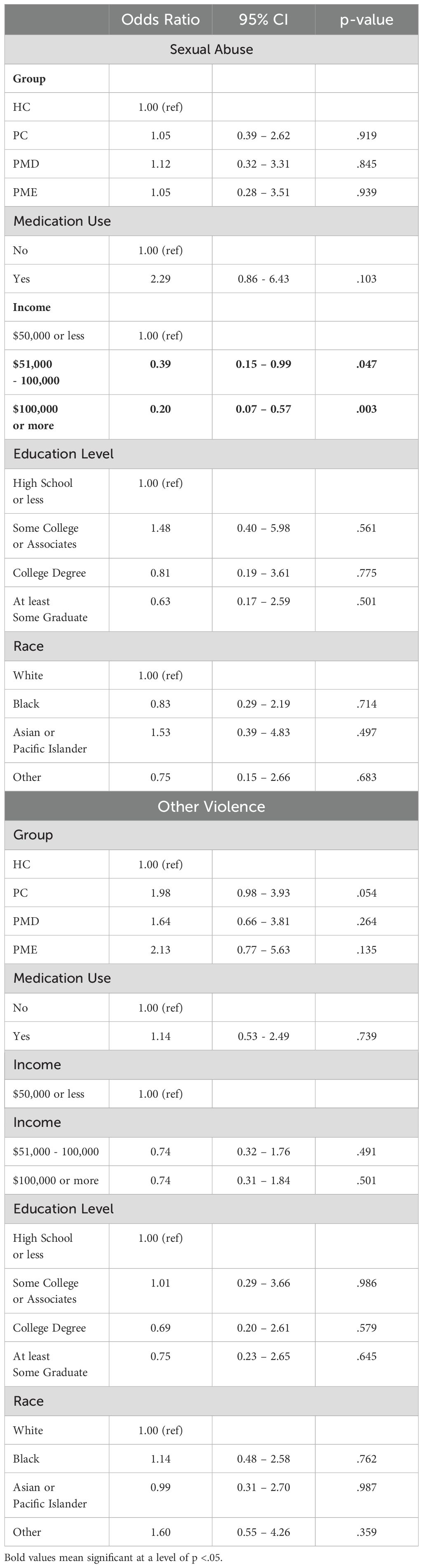
Table 3. Multinomial logistic regression for trauma type outcomes (sexual abuse and other types of trauma).
61 participants (28.1%) in the HC group, 30 participants (29.4%) in PC group, 17 participants (50%) in PMD, and 16 participants (42.1%) in PME experienced prepubertal trauma. Fisher’s Exact Test showed that there was not a significant difference between groups in number of individuals with prepubertal trauma exposure (p = .125).
Across groups, there was a weak but significant positive correlation between number of childhood traumatic events and total PSST score (R = .18, p <.001), see Figure 2A. Upon testing for correlations within each group, the association was significant within the PMD group (R = .42, p = .013), but not the PME (R = .20, p = .234), HC (R = .05, p = .495), or PC groups (R = .13, p = .181).
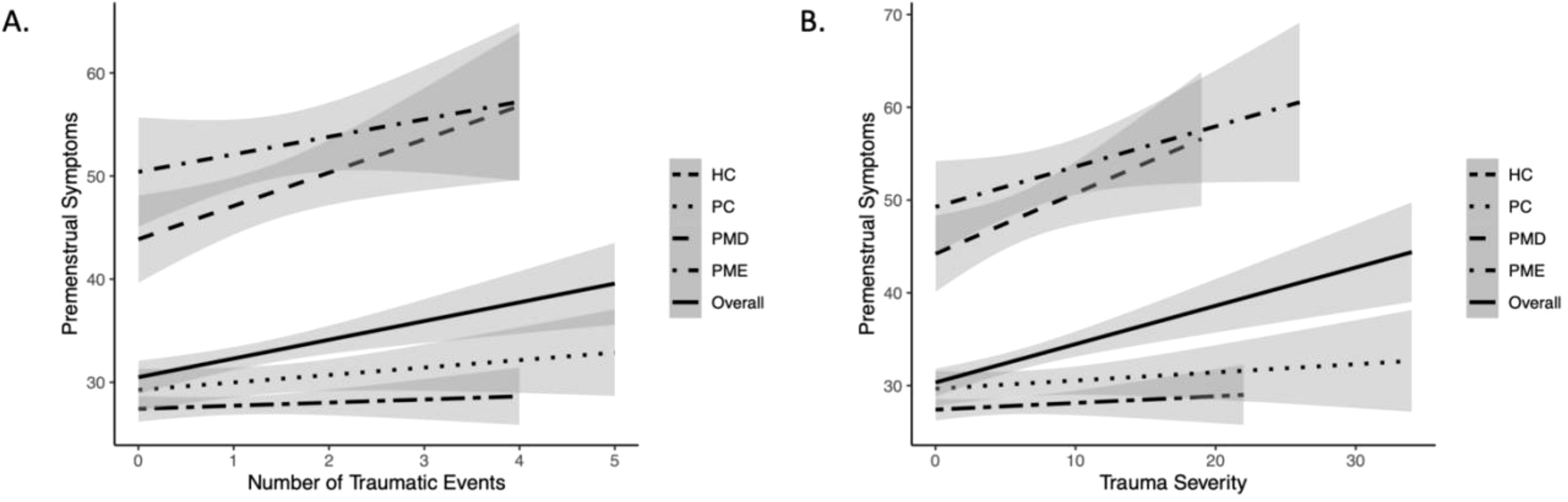
Figure 2. Association between measures of childhood trauma and premenstrual symptoms overall and within each group. [(A) number of traumatic events, (B) severity of events] and premenstrual symptoms (measured by PSST) by group. HC, Healthy Controls; PC, Psychiatric Controls (Controls with Psychiatric Illness); PMD, premenstrual disorder; PME, premenstrual exacerbation.
Across groups, there was a weak but significant positive correlation between the severity of childhood traumatic events and total PSST score (R = .22, p <.001), see Figure 2B.
Correlation analyses within each group revealed a significant association within the PMD group (R = .41, p = .015), but not the PME (R = .30, p = .066), HC (R = .05, p = .422), or PC groups (R = .09, p = .348).
Exploratory analyses were run to assess whether premenstrual symptoms (as measured by the total PSST score) differed between the PME and PMD groups (Table 4). ANOVA examining PSST scores revealed a significant difference by group (F(3,387) = 177.9, p <.001) Post-hoc analyses revealed a significant difference between the PME and PMD groups (adjusted difference = 4.85, 95% CI = 0.28 – 9.43, p = .033), such that those in the PME group experienced higher levels of premenstrual symptoms compared to the PMD group, but there was no difference between the HC and PC groups (adjusted difference = 1.95, 95% CI = - 0.38 – 4.27, p = .136). All other pairwise comparisons (PMD-HC, PMD-PC, PME-HC PME-PC) were significantly different from each other (p <.001). After controlling for medication use, race, income, and education level, there was a significant group effect (F(3,353) = 175.0, p <.001), but no longer a significant difference between the PME and PMD groups in post-hoc analyses (adjusted difference = 4.10, 95% CI = - 0.43 – 8.64, p = .092).
The current study examined the relationships among PMDs, PME, psychiatric illness, and childhood trauma experiences. We found that the experience of childhood trauma (number of traumatic events and severity of traumatic events) differed by group; participants with PME, and participants with a current or lifetime mood/anxiety disorder (PC), had a significantly higher number of traumatic events and greater severity of trauma compared to HCs. It is notable, however, that our study did not find significant differences in exposure to specific types of traumatic events after confounding for variables. A childhood history of sexual abuse, for example, have been previously associated with PMDs (18, 36, 37). This discrepancy is likely a result of differences in the questionnaires used across studies. For example, most data evaluating early childhood trauma and adversity as it relates to PMDs have utilized the ACE Questionnaire (ACE-Q) (38) or the Childhood Trauma Questionnaire (CTQ) scales. Indeed, both the ACE-Q and CTQ specifically ask questions about emotional, physical, and sexual abuse and/or neglect. Physical and emotional abuse, as well as emotional neglect, have all been specifically associated with the development of PMDs (7, 39, 40). Since the CTE-S does not ask about emotional abuse or neglect, however, our results may have failed to show any differences in trauma type in the PMDs group for this reason. Additionally, sexual abuse has been associated with higher rates of developing PMDD, but due to the limited number of participants who met criteria for PMDD (n=6) in this study, we were underpowered to evaluate this difference. It is interesting, however, that the specific types of traumatic events measured by the CTE-S (e.g., divorce, death of family/friend, illness/injury, or being a victim of violence) were not associated with PMD status but do appear to be associated with the risk for psychiatric illness more generally. Regarding premenstrual symptom severity, we found a weak but significant correlation between childhood trauma (number of traumatic events and severity of trauma experience) and higher premenstrual symptom burden. Within-group analysis suggested that this association was driven by correlations within the PMD group but not the other groups. Indeed, this finding is supported by other studies, which also found a correlation between the frequency and intensity of traumatic experiences and the corresponding severity of premenstrual symptoms (16, 18, 23). Although we did not detect a significant pairwise difference between PMDs and HCs in premenstrual symptom severity, it is worth highlighting that the data (see Figure 1) show a similar pattern for both PMD and PME patients. In both cases the frequency and severity of trauma were higher relative to HCs. Future studies with larger sample sizes, particularly among those with PMD, may observe statistically significant effects. We also found that those with PME experienced quantitatively higher levels of premenstrual symptoms on the PSST compared to the PMD group, despite both groups scoring in the moderate to severe range on PSST. However, after controlling for medication use, race, income, and education level, the initial difference in PSST scores between the PME and PMD groups was no longer statistically significant in post-hoc analyses. Together, this suggests that socioeconomic and demographic factors may partially account for the observed differences in elevated premenstrual symptom severity observed in the PME group. Another potential explanation is that the PME group may have represented a more psychiatrically ill population (as indicated by the increased presence of medication use). In this case those with PME may be experiencing ongoing symptoms of anxiety and depression throughout the month that (by definition) that worsen in the premenstrual period. Particularly, if the psychiatric symptoms experienced outside of the premenstrual time are already moderate to severe, it may be that the premenstrual worsening represents a time of additional suffering on an already stressed individual. Additionally, it is known that individuals experiencing depression have a subjectively enhanced experience of both physical and psychic suffering, which may further intensify the experience of physical and emotional premenstrual symptoms (41–43). To date, there are no known studies that have evaluated (qualitatively nor quantitatively) the symptom trajectory, characteristics, or severity of symptoms among individuals with PME across the menstrual cycle. Larger studies will be needed to determine whether there are differences in the subjective experience of premenstrual symptoms based on primary diagnosis and sociodemographic factors. It will be interesting to expand on the current findings to elucidate if higher PSST scores are associated with worse baseline mood or anxiety symptoms, or if PSST scores are independent of overall psychiatric severity outside of the premenstrual window.
Together, our results show that while PME and psychiatric diagnosis were associated with more frequent and more severe trauma exposure than in healthy controls, PMDs categorically were not associated with greater trauma exposure. Instead, within the PMD group, the number and severity of traumatic events was associated with greater premenstrual symptom load, which was not true in the other groups. Thus, it appears that trauma exposure increases risk for psychiatric diagnosis and PME generally, but among those with PMDs, it is the premenstrual symptoms that are more severe with greater exposure to childhood trauma. These results extend the expanding realm of research concerning the role of childhood adversity in reproductive affective disorders. Indeed, a history of trauma has been linked to most reproductive psychiatric illnesses, including PMDs, postpartum depression, and perimenopausal depression (44, 45). However, it is unknown the mechanisms by which childhood trauma would increase risk for reproductive affective disorders, which are characterized by sensitivity to gonadal hormone fluctuations. Recent data have suggested that the interplay between the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes, particularly the modulation of the HPA axis by gonadal hormones such as estrogen and progesterone and their neuroactive steroid metabolites, may underlie the elevated prevalence of mood disorders observed among women (45–48). Particularly relevant to women, who experience higher levels of childhood adversity than males, early life adversity may prime the HPA axis, resulting in more sensitivity to the natural gonadal hormone fluctuations across the reproductive life span. Intriguingly, recent research has demonstrated distinct profiles in cortisol function across the menstrual cycle among individuals with PMS and PMDD. In a study examining the intersection of childhood adversity and PMDs, women with PMDD and a history of abuse had greater premenstrual mood symptoms and higher cortisol levels than those with no abuse history, as they transitioned from the gonadal hormone milieu of the follicular phase to the luteal phase of the menstrual cycle (15). To date, there have been no specific investigation into HPA-HPG axis function in PME; future studies might discern whether the HPA axis profiles across the menstrual cycle among individuals with PME resemble those of individuals with PMS, PMDD, or MDD. In addition, gonadal hormone interaction with the serotonergic and GABAergic systems have been an important focus in exploring potential biological contributors to PMD risk (10, 15).
It’s important to consider some important limitations when interpreting our findings. Firstly, our study assessed childhood trauma based on participants’ retrospective self-reports, which naturally introduces a potential for recall bias. In addition to the challenge of recall bias, retrospective assessments limit the evaluation of any confounding variables that may have affected the outcome assessed. For example, the PSST asks retrospectively about premenstrual physical and mood symptoms, but we did not prospectively assess mood symptoms across the menstrual cycle. Additionally, while our study created groupings based on current PMD and psychiatric status to examine group differences in past ACEs, we acknowledge that the reverse approach could also be informative, i.e. grouping by ACE status (49, 50); however, we employed ANOVA evaluation to specifically observe differences across groups as we could not evaluate directionality in this retrospective analysis.
Although our study relied on validated measures to evaluate early life trauma, the CTE-S is not as widely used as the ACE-Q or CTQ, limiting generalizability of this study, and does not contain some of the key trauma domains (e.g. physical or emotional abuse/neglect) that have been previously associated with PMDs. The lack of information about emotional abuse and neglect may have limited our findings and ability to discern differences between our participant groups. Additionally, due to large differences in our sample sizes between groups and the relatively small number of participants with PMDD, our study may have been underpowered to recognize more subtle trends among groups. Further, the CTE-S only assesses trauma experienced in childhood and adolescence, and thus we were not able to account for the potential impact of stress or trauma experienced post-adolescence on PMD/PME risk.
Despite these limitations, our research contributes to an emerging field that confirms connections among childhood trauma, premenstrual symptoms, and psychiatric disorders. Our study uniquely focuses on evaluating the impact of trauma specifically on premenstrual symptoms and particularly among individuals with PME, a condition that has received insufficient attention in research and clinical practice. Our findings reveal that individuals with PME experience more severe premenstrual symptoms compared to those with PMD, underscoring the importance of further investigation, clinical assessment, and intervention for this specific patient group. Future studies focused on exploring the link between trauma and PME may provide valuable insights into the underlying mechanisms connecting early life trauma or adversity to reproductive psychiatric disorders. Additionally, exploring the hormonal and neurobiological pathways involved in PME (particularly differences in the HPA and HPG axes), in comparison to those with mood disorders alone or in comparison to PMDs, may help elucidate the biological bases of these illnesses and fuel investigations to identify potential biological targets for intervention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Johns Hopkins University Institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LS: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing, Formal analysis. KM: Writing – original draft, Writing – review & editing. KV: Formal analysis, Methodology, Supervision, Writing – review & editing. DS: Writing – review & editing, Conceptualization, Formal analysis. LO: Supervision, Writing – review & editing. JP: Supervision, Writing – review & editing, Project administration. LH: Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. NIMH R01 MH104262 and R01 MH112704 (PI JP); NIMH K23 MH110607 (PI LO).
JP has research funding from NIMH, Janssen Pharmaceuticals and Myriad. She has served as a consultant to SAGE Therapeutics, Biogen, Merck, Brii Biologics, Pure Tech, Dionysus Health, and Flo Health. She has founders stock in Dionysus Health. She has two patents: “Epigenetic Biomarkers of Postpartum Depression” and “Epigenetic Biomarkers of PMDD and SSRI Response.”
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin: no 15: premenstrual syndrome. Obstet Gynecol. (2000) 95:1–9.
2. Kuehner C, Nayman S. Premenstrual exacerbations of mood disorders: findings and knowledge gaps. Curr Psychiatry Rep. (2021) 23:78. doi: 10.1007/s11920-021-01286-0
3. Miller MN, Miller BE. Premenstrual exacerbations of mood disorders. Psychopharmacol Bull. (2001) 35:135–49.
4. Nolan LN, Hughes L. Premenstrual exacerbation of mental health disorders: a systematic review of prospective studies. Arch Womens Ment Health. (2022) 25:831–52. doi: 10.1007/s00737-022-01246-4
5. Payne J. Bipolar disorder in women with premenstrual exacerbation. Am J Psychiatry. (2011) 168:344–6. doi: 10.1176/appi.ajp.2011.11010171
6. Payne JL, Roy PS, Murphy-Eberenz K, Weismann MM, Swartz KL, McInnis MG, et al. Reproductive cycle-associated mood symptoms in women with major depression and bipolar disorder. J Affect Disord. (2007) 99:221–9. doi: 10.1016/j.jad.2006.08.013
7. Yang Q, Þórðardóttir EB, Hauksdóttir A, Aspelund T, Jakobsdóttir J, Halldorsdottir T, et al. Association between adverse childhood experiences and premenstrual disorders: a cross-sectional analysis of 11,973 women. BMC Med. (2022) 20:60. doi: 10.1186/s12916-022-02275-7
8. Hantsoo L, Grillon C, Sammel M, Johnson R, Marks J, Epperson CN. Response to sertraline is associated with reduction in anxiety-potentiated startle in premenstrual dysphoric disorder. Psychopharmacol (Berl). (2021) 238:2985–97. doi: 10.1007/s00213-021-05916-6
9. Miller KN, Standeven L, Morrow AL, Payne JL, Epperson CN, Hantsoo L. GABAergic neuroactive steroid response to sertraline in premenstrual dysphoric disorder. Psychoneuroendocrinology. (2024) 160:106684. doi: 10.1016/j.psyneuen.2023.106684
10. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. (2017) 174:6–7. doi: 10.1176/appi.ajp.2016.16101144
11. Jarosz AC, El-Sohemy A. Association between vitamin D status and premenstrual symptoms. J Acad Nutr Diet. (2019) 119:115–23. doi: 0.1016/j.jand.2018.06.014
12. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review . Available online at: https://pubmed.ncbi.nlm.nih.gov/10334745/ (Accessed August 29, 2024).
13. Bertone-Johnson ER, Ronnenberg AG, Houghton SC, Nobles C, Zagarins SE, Takashima-Uebelhoer BB, et al. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum Reprod. (2014) 29:1987–94. doi: 10.1093/humrep/deu170
14. Gold EB, Wells C, Rasor MO. The association of inflammation with premenstrual symptoms. J Womens Health (Larchmt). (2016) 25:865–74. doi: 10.1089/jwh.2015.5529
15. Hantsoo L, Payne JL. Towards understanding the biology of premenstrual dysphoric disorder: From genes to GABA. Neurosci Biobehav Rev. (2023) 149:105168. doi: 10.1016/j.neubiorev.2023.105168
16. Azoulay M, Reuveni I, Dan R, Goelman G, Segman R, Kalla C, et al. Childhood trauma and premenstrual symptoms: the role of emotion regulation. Child Abuse Negl. (2020) 108:104637. doi: 10.1016/j.chiabu.2020.104637
17. Nayman S, Schricker IF, Reinhard I, Kuehner C. Childhood adversity predicts stronger premenstrual mood worsening, stress appraisal and cortisol decrease in women with Premenstrual Dysphoric Disorder. Front Endocrinol (Lausanne). (2023) 14:1278531. doi: 10.3389/fendo.2023.1278531
18. Bertone-Johnson ER, Whitcomb BW, Missmer SA, Manson JE, Hankinson SE, Rich-Edwards JW. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: A longitudinal study. J Womens Health (Larchmt). (2014) 23:729–39. doi: 10.1089/jwh.2013.4674
19. Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. (2007) 26:201–13. doi: 10.1037/0278-6133.26.2.201
20. Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA, et al. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. (2003) 65:849–56. doi: 10.1097/01.PSY.0000088593.38201.CD
21. Soydas EA, Albayrak Y, Sahin B. Increased childhood abuse in patients with premenstrual dysphoric disorder in a Turkish sample: a cross-sectional study. Prim Care Companion CNS Disord. (2014) 16. doi: 10.4088/PCC.14m01647
22. Eisenlohr-Moul TA, Rubinow DR, Schiller CE, Johnson JL, Leserman J, Girdler SS. Histories of abuse predict stronger within-person covariation of ovarian steroids and mood symptoms in women with menstrually related mood disorder. Psychoneuroendocrinology. (2016) 67:142–52. doi: 10.1016/j.psyneuen.2016.01.026
23. Koci A, Strickland O. Relationship of adolescent physical and sexual abuse to perimenstrual symptoms (Pms) in adulthood. Issues Ment Health Nursing. (2007) 28:75–87. doi: 10.1080/01612840600996281
24. Ito K, Doi S, Isumi A, Fujiwara T. Association between childhood maltreatment history and premenstrual syndrome. Int J Environ Res Public Health. (2021) 18:781. doi: 10.3390/ijerph18020781
25. Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry. (2010) 197:378–85. doi: 10.1192/bjp.bp.110.080499
26. Afifi TO, Enns MW, Cox BJ, Asmundson GJG, Stein MB, Sareen J. Population attributable fractions of psychiatric disorders and suicide ideation and attempts associated with adverse childhood experiences. Am J Public Health. (2008) 98:946–52. doi: 10.2105/AJPH.2007.120253
27. Voegtline K, Payne JL, Standeven LR, Sundel B, Pangtey M, Osborne LM. Using the penn state worry questionnaire in the peripartum. J Women’s Health. (2021) 30:1761–8. doi: 10.1089/jwh.2020.8669
28. Standeven LR, Osborne LM, Betz JF, Yenokyan G, Voegtline K, Hantsoo L, et al. Allopregnanolone and depression and anxiety symptoms across the peripartum: an exploratory study. Arch Womens Ment Health. (2022) 25:521–6. doi: 10.1007/s00737-021-01186-5
29. Glasofer D, Brown A, Riegel M. Structured clinical interview for DSM-IV (SCID). (2015). pp. 1–4. (Singapore: Springer).
30. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J BioMed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
31. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J BioMed Inform. (2009) 42:377–81 doi: 10.1016/j.jbi.2008.08.010.
32. Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Soc Sci Med. (1988) 26:327–32. doi: 10.1016/0277-9536(88)90397-8
33. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. (2003) 6:203–9. doi: 10.1007/s00737-003-0018-4
34. The Tukey multiple comparison test: 1953–1976. Available online at: https://psycnet.apa.org/record/1978-20170-001 (Accessed August 29, 2024).
35. R Core Team. R: A language and environment for statistical ## computing. Vienna, Austria: R Foundation for Statistical Computing (2021). Available at: https://www.R-project.org/.
36. Golding JM, Taylor DL, Menard L, King MJ. Prevalence of sexual abuse history in a sample of women seeking treatment for premenstrual syndrome. J Psychosom Obstet Gynaecol. (2000) 21:69–80. doi: 10.3109/01674820009075612
37. Schmidt MR, Narayan AJ, Atzl VM, Rivera LM, Lieberman AF. Childhood maltreatment on the adverse childhood experiences (ACEs) scale versus the childhood trauma questionnaire (CTQ) in a perinatal sample. J Aggression Maltreatment Trauma. (2020) 29:38–56. doi: 10.1080/10926771.2018.1524806
38. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. (1998) 14:245–58. doi: 10.1016/S0749-3797(98)00017-8
39. Carvalho Silva R, Oliva F, Barlati S, Perusi G, Meattini M, Dashi E, et al. Childhood neglect, the neglected trauma. A systematic review and meta-analysis of its prevalence in psychiatric disorders. Psychiatry Res. (2024) 335:115881. doi: 10.1016/j.psychres.2024.115881
40. Kulkarni J, Leyden O, Gavrilidis E, Thew C, Thomas EHX. The prevalence of early life trauma in premenstrual dysphoric disorder (PMDD). Psychiatry Res. (2022) 308:114381. doi: 10.1016/j.psychres.2021.114381
41. Amaro-Díaz L, Montoro CI, Fischer-Jbali LR, Galvez-Sánchez CM. Chronic pain and emotional stroop: A systematic review. J Clin Med. (2022) 11:3259. doi: 10.3390/jcm11123259
42. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. (2003) 163:2433–45. doi: 10.1001/archinte.163.20.2433
43. Latthe P, Mignini L, Gray R, Hills R, Khan K. Factors predisposing women to chronic pelvic pain: systematic review. BMJ. (2006) 332:749–55. doi: 10.1136/bmj.38748.697465.55
44. Epperson CN, Sammel MD, Bale TL, Kim DR, Conlin S, Scalice S, et al. Adverse childhood experiences and risk for first-episode major depression during the menopause transition. J Clin Psychiatry. (2017) 78:e298–307. doi: 10.4088/JCP.16m10662
45. Hantsoo L, Jagodnik KM, Novick AM, Baweja R, di Scalea TL, Ozerdem A, et al. The role of the hypothalamic-pituitary-adrenal axis in depression across the female reproductive lifecycle: current knowledge and future directions. Front Endocrinol. (2023) 14:1295261/full. doi: 10.3389/fendo.2023.1295261/full
46. Hamidovic A, Davis J, Soumare F. Blunted cortisol response to acute psychosocial stress in women with premenstrual dysphoric disorder. Int J Neuropsychopharmacol. (2024) 27:pyae015. doi: 10.1093/ijnp/pyae015
47. Klusmann H, Schulze L, Engel S, Bücklein E, Daehn D, Lozza-Fiacco S, et al. HPA axis activity across the menstrual cycle - a systematic review and meta-analysis of longitudinal studies. Front Neuroendocrinol. (2022) 66:100998. doi: 10.1016/j.yfrne.2022.100998
48. Barone JC, Ho A, Osborne LM, Eisenlohr-Moul TA, Morrow AL, Payne JL, et al. Luteal phase sertraline treatment of premenstrual dysphoric disorder (PMDD): Effects on markers of hypothalamic pituitary adrenal (HPA) axis activation and inflammation. Psychoneuroendocrinology. (2024) 169:107145. doi: 10.1016/j.psyneuen.2024.107145
49. Hantsoo L, Duffy KA, Sammel M, Johnson RL, Kim D, Grillon C, et al. Enduring impact of childhood adversity: Affective modulation of acoustic startle response during pregnancy and postpartum. Physiol Behav. (2023) 258:114031. doi: 10.1016/j.physbeh.2022.114031
Keywords: childhood trauma, premenstrual syndrome, premenstrual exacerbation (PME), Premenstrual Dysphoric Disorder (PMDD), adverse childhood experiences, menstrual cycle
Citation: Standeven LR, Bajaj M, McEvoy K, Shirinian D, Voegtline K, Osborne LM, Payne JL and Hantsoo L (2024) The link between childhood traumatic events and the continuum of premenstrual disorders. Front. Psychiatry 15:1443352. doi: 10.3389/fpsyt.2024.1443352
Received: 03 June 2024; Accepted: 06 September 2024;
Published: 09 October 2024.
Edited by:
Hong Wang Fung, Hong Kong Metropolitan University, Hong Kong SAR, ChinaReviewed by:
Peter Yee-Lap To, University of Warwick, United KingdomCopyright © 2024 Standeven, Bajaj, McEvoy, Shirinian, Voegtline, Osborne, Payne and Hantsoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lindsay R. Standeven, TGluZHNheS5TdGFuZGV2ZW5AY3VhbnNjaHV0ei5lZHU=; Mira Bajaj, bWJhamFqM0BtZ2guaGFydmFyZC5lZHU=
†Present addresses: Lindsay R. Standeven, Department of Psychiatry, University of Colorado School of Medicine, Aurora, CO, United States
Mira Bajaj, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, United States
‡These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.