- 1Department of Psychosomatic Medicine, the First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
- 2School of Medicine, Ningbo University, Ningbo, Zhejiang, China
- 3Department of Psychiatry, Affiliated Kangning Hospital of Ningbo University, Ningbo, Zhejiang, China
- 4Department of Psychiatry, Ningbo Kangning Hospital, Ningbo, Zhejiang, China
Introduction: Little was known about the relationship between sleep disturbances and depressive and anxiety disorders, as well as the efficacy of treatment regimens.
Methods: During 2021 to 2023, a total of 417 participants were screened by Hamilton Depression Rating Scale (HAMD-17) and Hamilton Anxiety Rating Scale (HAMA-14) for psychological status, and Pittsburgh sleep quality index (PSQI) assessment. 409 participants were finally enrolled, of which 188 (45.97%) were suffered from sleep disorders. All participants were received polysomnography (PSG) followed by six-week pharmacological treatment of escitalopram and zopiclone, and finally assessed by HAMD-17 and HAMA-14 for treatment efficacy.
Result: PSG monitoring indicated that participants with depression experienced prolonged rapid eye movement sleep latency (REMSL) and increased wakefulness after sleep onset (WASO) (P=0.030 and P=0.002, respectively). Those with anxiety disorders demonstrated a significantly higher percentage of non-rapid eye movement sleep (NREM%) and reduced WASO (P=0.013 and P=0.001, respectively). After six-weeks pharmacological treatment, participants with or without sleep disorders exhibited with similar efficacy outcomes of depression and anxiety disorders (P>0.05). However, every point of PSQI increment at baseline would decrease 0.78 and 0.85 times of probability of effective pharmacological treatment of depression and anxiety disorders. Moreover, participants with both effective outcomes of depression and anxiety disorders were found significant shorter sleep onset latency (SOL) (P<0.001).
Discussion: The insights gained underscore the necessity of considering sleep disturbances in enhancing the overall effectiveness of pharmacological treatments for depression and anxiety disorders.
1 Introduction
Major depressive disorder (MDD) constitutes a widespread mood disorder, comprising a spectrum of conditions characterized by depressed mood and accompanied by cognitive and behavioral changes that significantly impair social functioning. According to World Health Organization (WHO) data, an estimated 280 million people worldwide are afflicted with depression, representing approximately 3.8% of the global population. Globally, adults with depression comprise about 5% of the total adult population. Patients afflicted with depressive disorders commonly experience various degrees of sleep disturbances, which consequently can aggravate the symptoms of the disorder. Between 50% and 90% of individuals with depressive disorders exhibit sleep disturbances, primarily characterized by difficulties in initiating or maintaining sleep. Research indicates that chronic insomnia heightens the risk of relapse in patients with newly diagnosed depressive disorders (1), sleep deprivation elevates the risk of postpartum depression, and poor sleep quality correlates with increased severity of depressive disorders (2).
The etiology of depressive disorders remains elusive. Current understanding suggests that depressive disorders are associated with neurotransmitter function and homeostatic imbalances, with prevailing hypotheses including the serotonin, dopamine, and norepinephrine system hypotheses. Recent research has demonstrated that biorhythm disorders, which constitute significant clinical features and pathophysiological mechanisms, are widespread among patients with depressive disorders (3–7). Biorhythm disorders are intimately linked to the onset, symptomatology, prognosis, and recurrence of depressive disorders (8–10).
Despite the frequent clinical manifestations of biorhythm disorders in patients with depressive disorders, the clinical evaluation of biorhythms is not routinely conducted (11). Although recent advancements in brain imaging, such as functional magnetic resonance imaging (fMRI), have offered new insights into the diagnosis and treatment of depressive disorders, establishing a precise correlation between imaging changes and depressive symptoms remains challenging. Furthermore, the sensitivity and specificity of these findings often fail to meet the rigorous demands of clinical diagnosis. Consequently, there is an imperative need to develop objective tools for the assessment of depressive disorders. The aim of this study is to develop a sleep rhythm evaluation system and to investigate the impact of sleep disturbances on the treatment efficacy and prognosis of depressive disorders.
Research from the Sequenced Treatment Alternatives to Relieve Depression (STAR-D) initiative has demonstrated that depression patients with characteristics such as nocturnal biorhythms, sleep rhythm disturbances, daytime hypersomnia, and daytime or seasonal affective changes typically exhibit more severe conditions, poorer treatment outcomes, increased suicide risks, greater residual symptoms, and reduced recovery rates (12–14). An investigation into the correlation between the severity of biorhythm disturbances and depressive disorders found that increased severity of rhythm disturbances is directly correlated with heightened depression severity (15).
This research primarily seeks to elucidate the relationship between sleep disturbances and symptom severity in patients with depressive and anxiety disorders, as well as the efficacy of pharmacological treatment. With the use of polysomnography (PSG), this study also explore the impact of abnormal sleep patterns on pharmacological treatment depression and anxiety.
2 Materials and methods
2.1 Participant recruitment
Between January 2021 and December 2023, 417 participants enrolled in our hospital’s Department of Psychosomatic Medicine. Inclusion criteria for participants were as follows: aged between 18 and 60 years; met the ICD-10 diagnostic criteria for depressive or anxiety disorders; scored greater than 7 points on the Hamilton Depression Rating Scale (HAMD-17) or Hamilton Anxiety Rating Scale (HAMA-14); Exclusion criteria were as follows: bipolar disorder, obsessive-compulsive disorder, post-traumatic stress disorder, restless legs syndrome, sleep apnea-hypopnea syndrome, rapid eye movement sleep behavior disorder, serious organic diseases, and alcohol or drug dependence. The ethics committee of our hospital has approved this study, and informed consent was obtained from all patients or their families.
2.2 Interventions and procedures
After evaluations of age, medical histories, HAMD-17, HAMA-14, and Pittsburgh Sleep Quality Index (PSQI), eligible participants underwent overnight polysomnography. Then participants in both groups received an initial treatment of escitalopram 10 mg/day and zopiclone 7.5 mg/night, with the option to increase the escitalopram dosage to 20 mg/day after two weeks. The treatment course lasted for six weeks, during which HAMD-17 and HAMA-14 were assessed at the end of the treatment period. Study flowchart is illustrated in Figure 1.
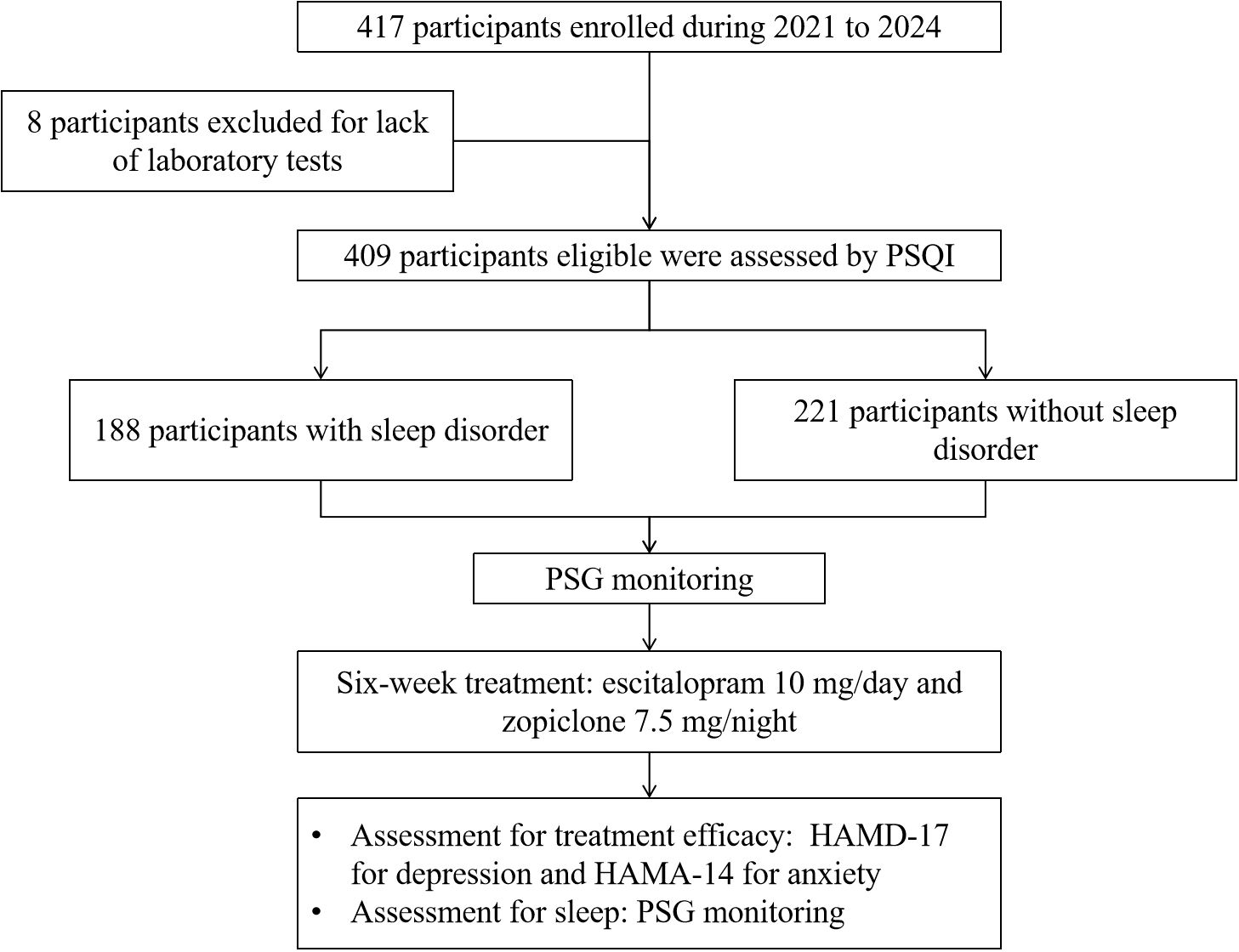
Figure 1. Flowchart of this study. HAMD-17, Hamilton Depression Rating Scale; HAMA-14, Hamilton Anxiety Rating Scale; PSG, polysomnography; PSQI, Pittsburgh sleep quality index.
2.3 Assessments
2.3.1 Sleep rhythm assessment
Sleep disturbances were evaluated using the Pittsburgh sleep quality index (PSQI) scale, where scores ranged as follows: 0-5 denoting normal sleep quality; 6-10 suggesting a mild sleep disorder; 11-15 indicating a moderate sleep disturbance; 16-20 reflecting a severe sleep disorder; and 21 representing a very severe sleep disorder. The analysis specifically categorized sleep patterns into three types: nocturnal, intermediate, and early morning. Anxiety disorders were assessed with the HAMA-14, with scoring interpreted as follows: less than 7 indicating no anxiety, 8-13 suggesting possible anxiety, 14-20 confirming anxiety, 21-28 indicating significant anxiety, and 29 or higher denoting severe anxiety. Depressive disorders were evaluated using the HAMD-17 scale, with scores categorized as follows: 0-7 indicating no depression, 8-17 suggesting mild depression, 18-24 denoting moderate depression, and 25 or greater indicating severe depression.
2.3.2 Polysomnography monitoring
Before any pharmacological treatment for depression and/or anxiety, PSG monitoring was conducted on all participants to exclude disorders such as SAHS, RLS, and rapid eye movement sleep behavior disorder. Sleep parameters including total sleep time (TST), wakefulness after sleep onset (WASO), percentage of stage 1 sleep (N1%), percentage of stage 2 sleep (N2%), percentage of stage 3 sleep (N3%), sleep onset latency (SOL), rapid eye movement sleep latency (REMSL), and percentage of REM sleep (REM%) were monitored.
2.3.3 Laboratory tests
All participants were received laboratory tests from blood samples at baseline, which include triiodothyronine (T3), tetraiodothyronine (T4), free triiodothyronine (FT3), free tetraiodothyronine (FT4), thyroid stimulating hormone (TSH), thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TGAb), growth hormone (GH), follicle stimulating hormone (FSH), luteinizing hormone (LH), and prolactin (PRL) for endocrinological assessments, cancer antigen 125 (CA125), cancer antigen 199 (CA199), alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA) as tumor biomarkers, and white blood cell (WBC) count, hypersensitive C-reaction protein (hs-CRP), smooth muscle antibody (SMA), anti-mitochondrial antibody (AMA), and anti-nuclear antibody (ANA) for immunological evaluations.
2.3.4 Evaluation of efficacy
Treatment efficacy is evaluated based on the rate of score reduction of HAMA-14 or HAMD-17 scale. The criteria are as follows: a reduction rate of over 75% is considered as recovered; 50%-75% as significant improved; 25%-49% as improved; and less than 25% as ineffective. The score reduction rate is calculated using the formula: (pre-treatment score−post-treatment score)/pre-treatment score ×100%. The total effectiveness rate is calculated as: (number of recovered cases + number of significant improved cases + number of improved cases)/total number of cases × 100%.
2.4 Statistical analysis
For quantitative data, a Shapiro-Wilk test for normality was first conducted. Normal quantitative data were described using mean and standard deviation (SD), and examined by t-tests for two-group comparisons. Quantitative data without normality were described using median and interquartile range (P50, P25, P75). Wilcoxon rank-sum test was used for comparisons between two groups, and the Kruskal-Wallis tests were used for comparisons among multiple groups. For categorical data without order, frequencies and proportions (%) are used to represent the data, and chi-square tests are used for comparisons between two or multiple groups. For categorical data with order, rank-sum tests were used for examinations between groups. Logistic regressions were used to examined the associations between depression and anxiety treatment effectiveness and PSQI, HAMA-14, and HAMD-17 scores at baseline. P<0.05 was deemed to be significant. All analysis were performed in Stata 16.0.
3 Results
3.1 Baseline characteristics
Baseline characteristics of the study participants are summarized in Table 1. This study initially enrolled a total of 417 participants. Eight participants lacking blood tests were excluded, resulting in a final cohort of 409. The mean age, height, weight, and BMI of participants were 48.41 ± 0.95 years, 64.15 ± 0.50 cm, 59.56 ± 0.57 kg, and 21.97 ± 3.42 kg/m2, respectively. Majority of participants were female (70.90%) and married (72.37%). Among all participants, 6.11% of them were diabetic, 17.85% suffered from hypertension, 9.54% found having histories of thyroid diseases, and 9.29% diagnosed with hyperlipidemia. After HAMD-17 and HAMA-14 evaluation, 55.26% and 40.83% of participants were diagnosed with depression and anxiety disorders respectively.
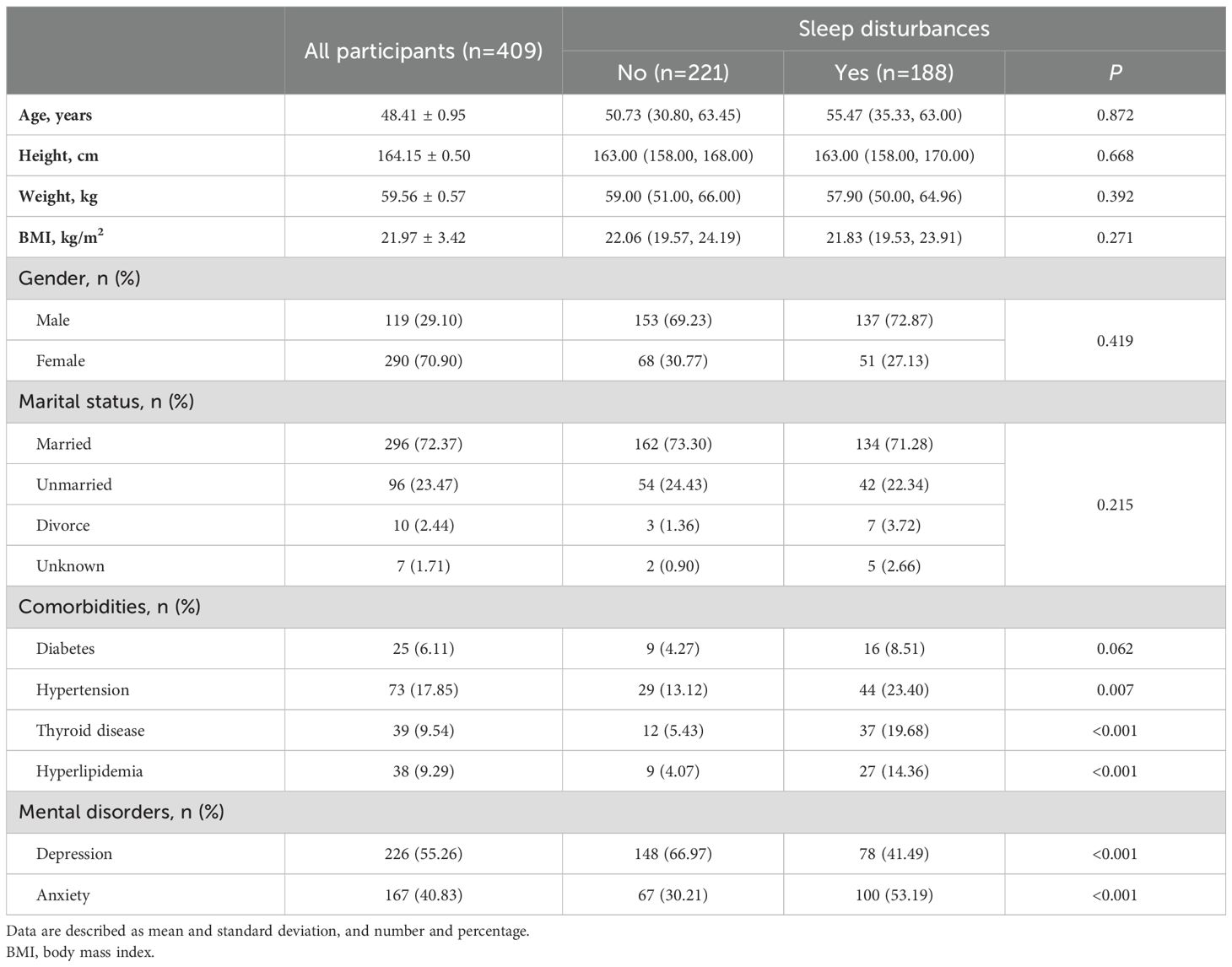
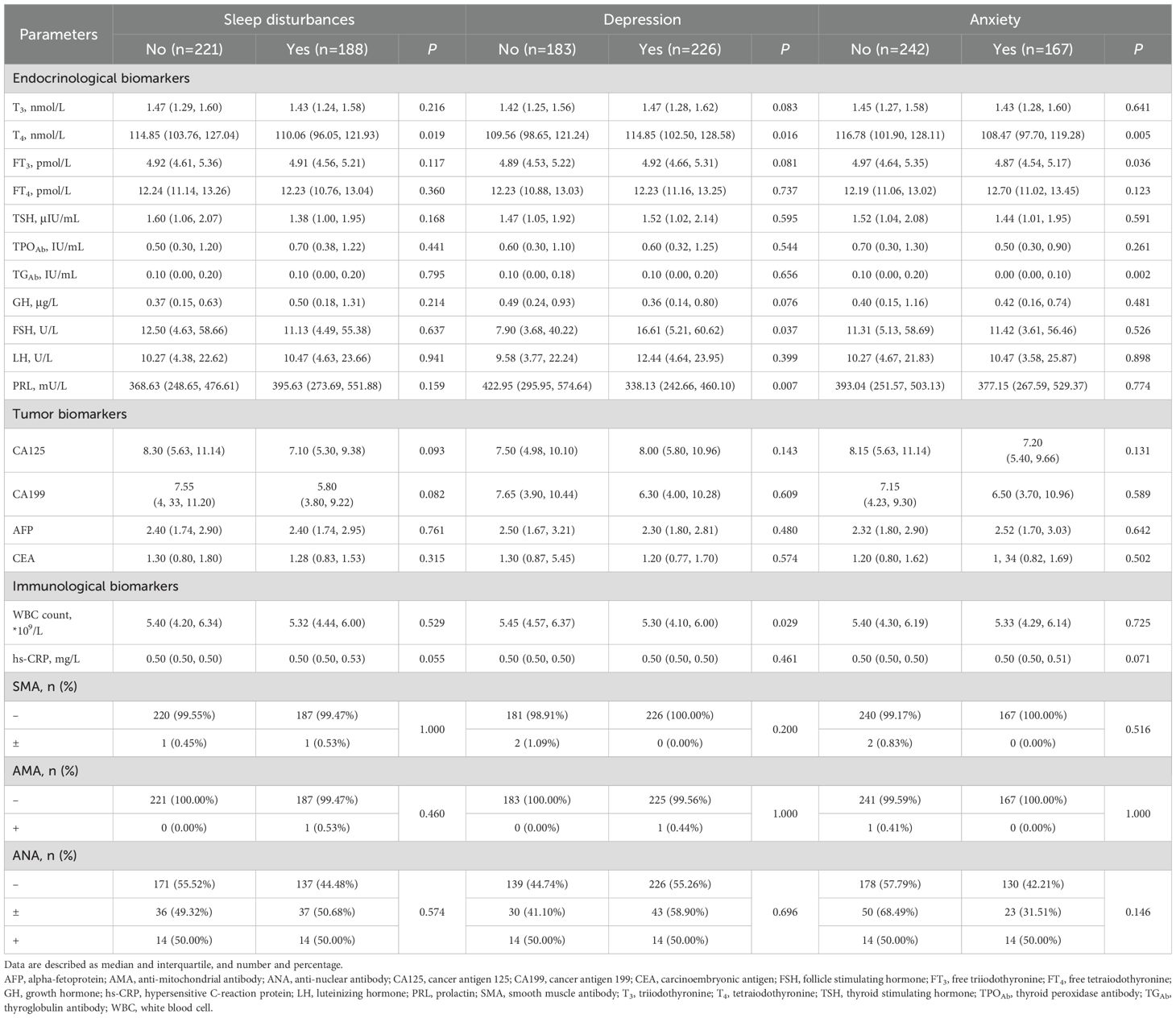
Table 1. Baseline demographics and comorbidities in all participants and participants with or without sleep disturbances.
3.2 Sleep disturbances analysis
No differences were observed in demographics including age, height, BMI, gender and marital status between participants with and without sleep disturbances (P>0.05). Prevalence of diabetes was found no significant difference in participants with and without sleep disturbances (8.51% vs. 4.27%, P=0.062, Table 1). However, hypertension (23.40% vs. 13.12%, P=0.007), thyroid disease (19.68% vs. 5.43%, P<0.001), and hyperlipidemia (14.36% vs. 4.07%, P<0.001) were more prevalent in participants with sleep disturbances than those without them (Table 1).
As shown in Table 1, significantly less participants with sleep disturbances, compared with those without sleep disturbances, were diagnosed with depression disorders (41.49% vs. 66.97%, P<0.001). While it was contrary to the distribution of diagnosis of anxiety disorders (30.21% vs. 53.19%, P<0.001).
Results of laboratory tests are summarized in Table 2. Significant differences were observed in the levels of tetraiodothyronine (T4) between participants with and without sleep disturbances (median of 110.06 nmol/L vs. 114.85 nmol/L, P=0.019). For those diagnosed with depression disorders, significant differences were noted in the levels of T4 (median of 114.85 nmol/L vs. 109.56 nmol/L,P=0.016), follicle stimulating hormone (FSH) (median of 16.61 U/L vs. 7.90 U/L, P=0.037), and white blood cell count (WBC) (median of 5.30 *109/L vs. 5.45 *109/L, P=0.029), when compared to participants both with and without these disorders. In the comparison of participants with and without anxiety disorders, significant differences were identified in the levels of T4 (median of 108.47 nmol/L vs. 116.78 nmol/L, P=0.005), free triiodothyronine (FT3) (median of 4.87 pmol/L vs. 4.97 pmol/L, P=0.036), and thyroglobulin antibody (TGAb) (median of 0.00 IU/mL vs. 0.10 IU/mL, P=0.002).
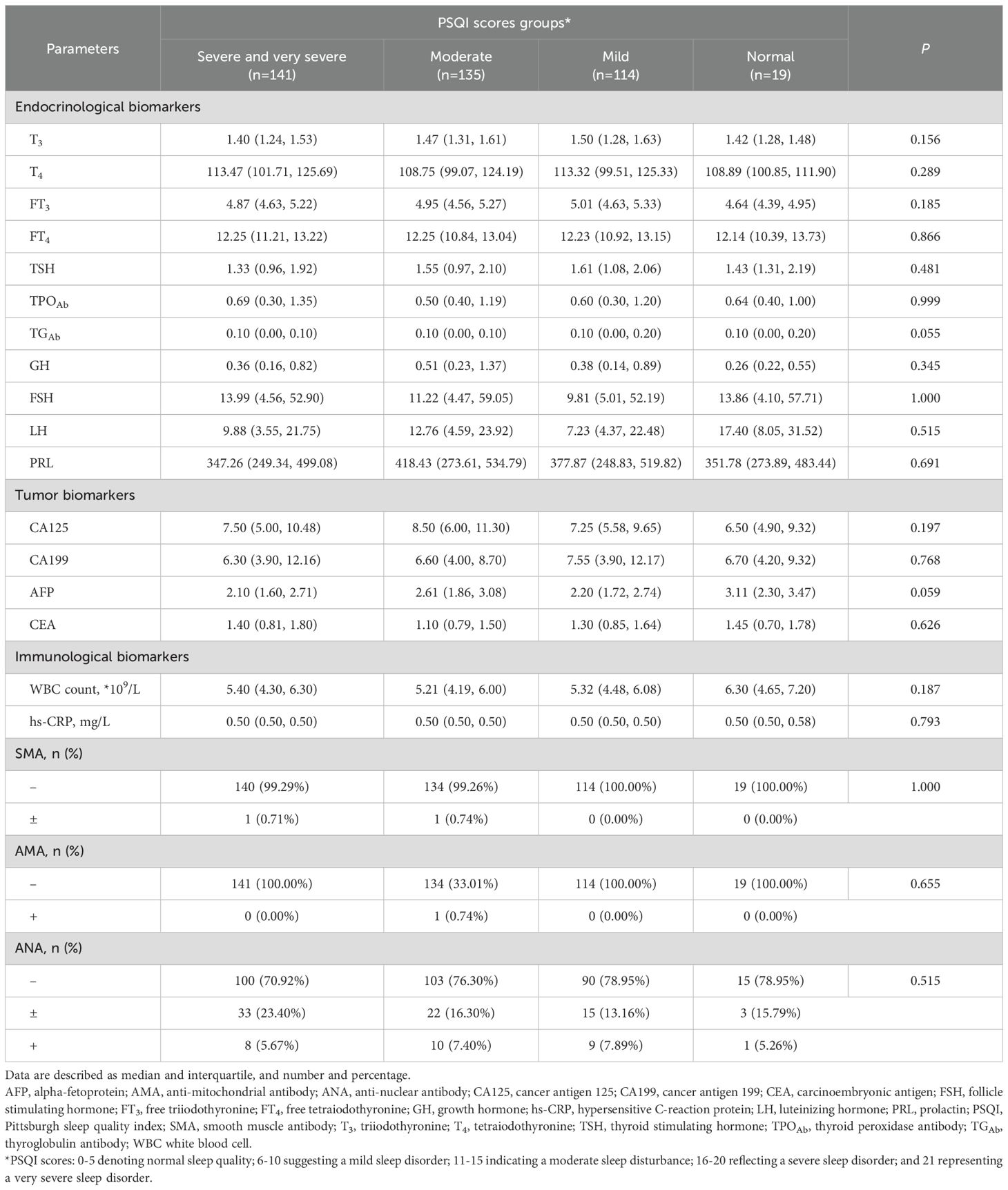
The PSQI examination results are summarized in Table 3. No significant differences of laboratory tests results across PSQI scores groups were observed (P>0.05).
3.3 Polysomnography results
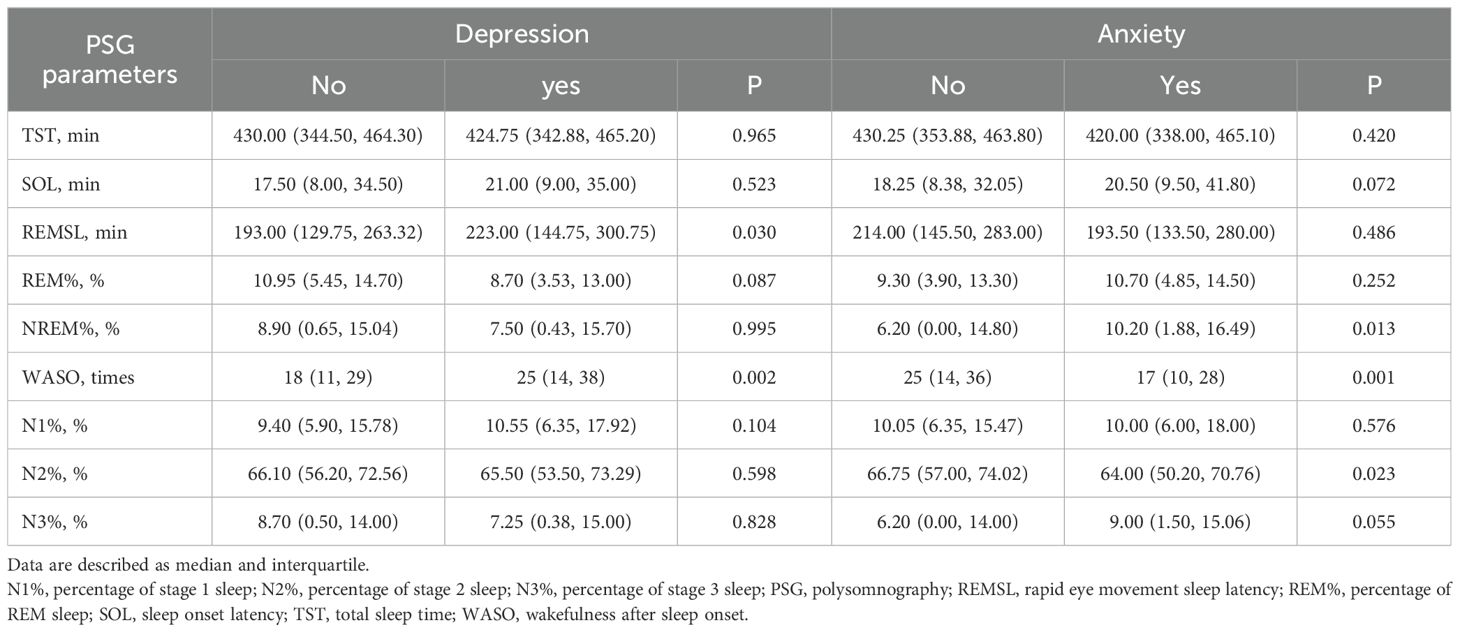
Results of PSG monitoring results from participants diagnosed with depression and anxiety are detailed in Table 4. PSG monitoring demonstrated significant longer in REMSL (median of 223 min vs. 193 min, P=0.030) and WASO (median of 25 times vs. 18 times, P=0.002) in participants with depression disorders than their counterparts. For those with anxiety disorders, significant higher NREM% were found, compared to those without anxiety disorders (median of 10.20% vs. 6.20%, P=0.013). Additionally, the WASO (median of 17 times vs. 25 times, P=0.001) was significantly less, and N2% (median of 64.00% vs. 66.75%, P=0.023) was significantly lower in participants with anxiety compared to those without anxiety disorders.
Figure 2 illustrates the comparative analysis of sleep cycles among different populations. It was observed that individuals with anxiety disorders exhibited longer SOL, and extended REM and N3 sleep stages, while their N1 and N2 stages were shorter compared to those without anxiety disorders. In contrast, individuals with depressive disorders demonstrated prolonged SOL and increased durations of N1 and N3 sleep stages, but reduced REM duration compared to those without depressive disorders.
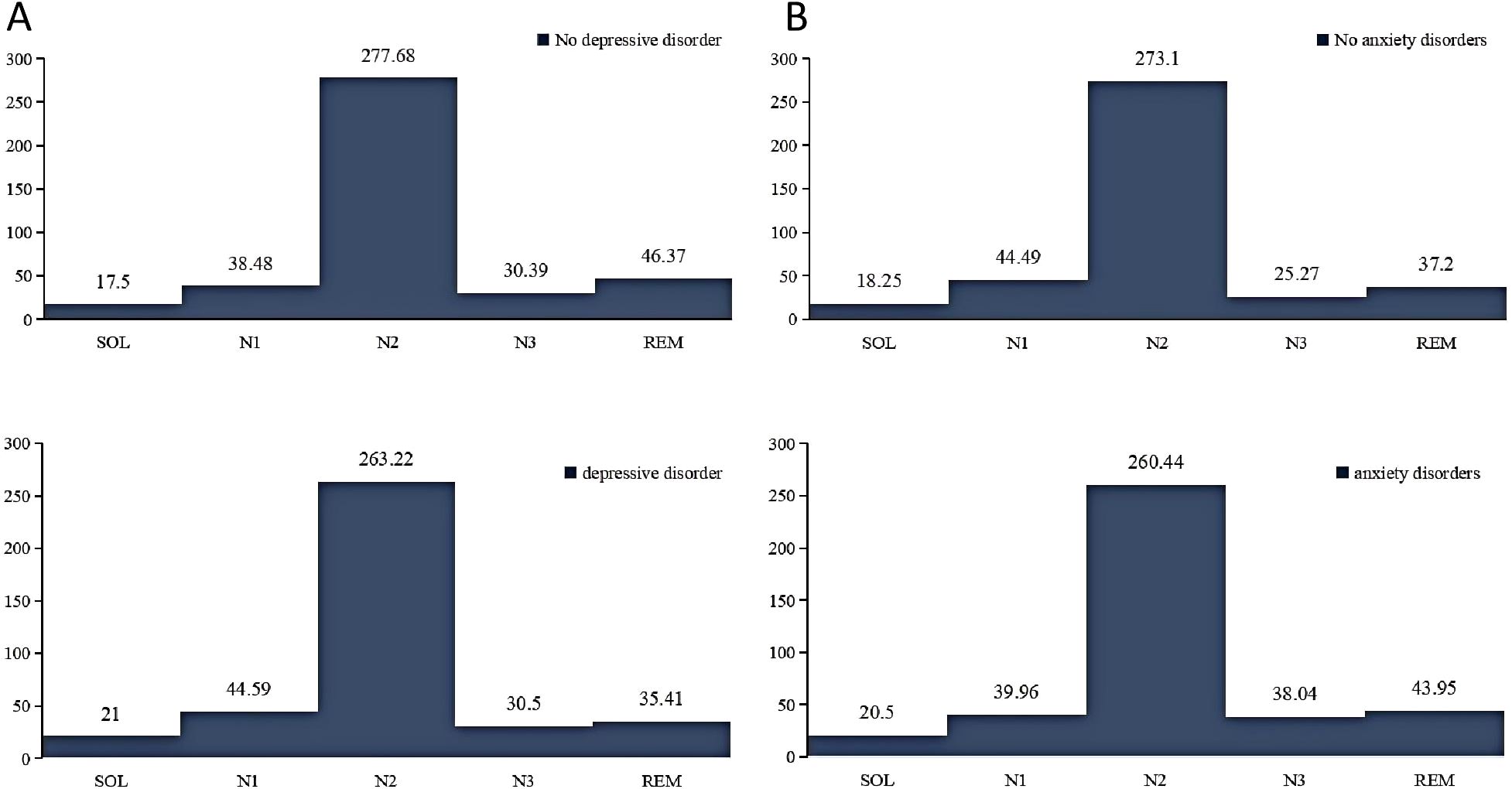
Figure 2. Comparison of sleep cycles in different mental disorders. (A), sleep cycles in participants with and without depression. (B), sleep cycles in participants with and without anxiety. N1, stage 1 sleep; N2, stage 2 sleep; N3, stage 3 sleep; REM, rapid eye movement; SOL, sleep onset latency.
3.4 Relationship between sleep disturbances and treatment outcomes
There was no significant difference after six-week pharmacological treatment outcomes of neither depression (P=0.904) nor anxiety (P=0.509) between the two groups (Table 5). In the group without sleep disturbances, 36 participants were recovered from depression by assessment of HAMD-17 scale, 77 were significant improved, 49 improved, and 59 showed ineffective. Conversely, in the group with sleep disturbances, 21 participants were recovered, 67 significantly improved, 44 improved, and 56 showed ineffective. Outcomes of anxiety for participants with or without sleep disturbances were as follows: recovered (34 participants vs. 33 participants), significantly improved (68 vs. 65), improved (47 vs. 75), ineffective (39 vs. 48).

Table 5. Comparison of clinical efficacy of six-week treatment between participants with and without sleep disturbances.
Table 6 presents the baseline PSG results by treatment outcomes of depression and anxiety. Patients with depression were categorized into effective and ineffective treatment groups based on their therapeutic responses. Participants with effective outcomes of both depression (median of 17.50 min vs. 30.00 min, P<0.001) and anxiety (median of 17.00 min vs. 34.50 min, P<0.001) demonstrated a significantly shorter SOL than the ineffective group. REMSL was significantly longer in participants with effective anxiety treatment outcomes compared to the ineffective group (median of 216.75 min vs. 187.25 min, P=0.034).
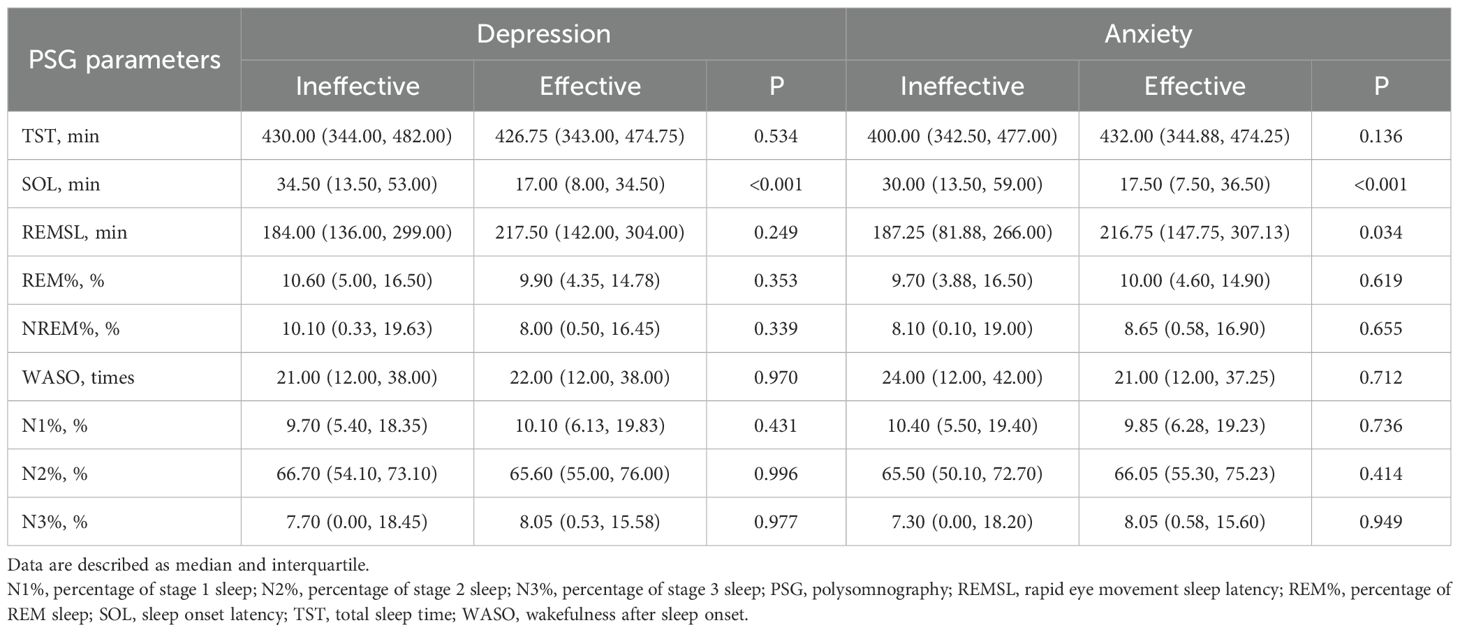
Table 6. Comparison of polysomnography parameters by six-week treatment outcomes of depression and anxiety.
Associations between effective treatment and severity of symptoms of sleep disturbances, depression, and anxiety at baseline were examined by Logistic regressions analysis in Table 7. PSQI scores at baseline were negatively associated with effective treatment of both depression and anxiety with the odds ratio (OR) of 0.78 (95% of confidence interval [CI]: 0.71-0.84, P<0.001) and 0.85 (95% CI: 0.79-0.91, P<0.001), respectively, which meant every point of PSQI increment at baseline would decrease 0.78 and 0.85 times of probability of effective treatment of depression and anxiety disorders. HAMD-17 (OR: 1.19, 95% CI:1.12-1.27, P<0.001) and HAMA-14 (OR: 1.17, 95% CI:1.11-1.25, P<0.001) scores were respectively associated with effective treatment of depression and anxiety. However, no significant correlations were found neither between HAMD-17 and effective treatment of anxiety, nor HAMA-14 and depression.
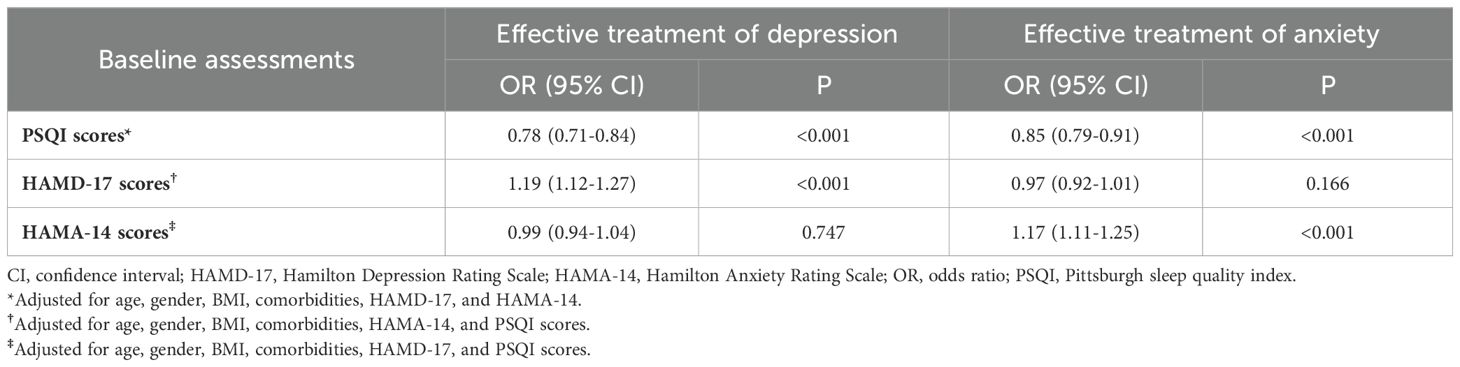
Table 7. Associations between effective treatment and severity of symptoms of sleep disturbances, depression, and anxiety at baseline.
4 Discussion
The current study include 409 participants diagnosed with depression and/or anxiety disorders. comorbidities, endocrinological, tumor and immunological biomarkers were compared in participants with or without sleep disturbances, depression, and anxiety disorders. Moreover, relationships between sleep disturbances and depression as well as anxiety disorders were studied, as well as the influence of sleep disturbances and PSG monitoring results on depression and/or anxiety disorders pharmacological treatment. We found that baseline status of sleep disturbances had no impact to pharmacological treatment outcomes of both depression and anxiety disorders, which were, however, associated with PSQI scores and SOL by PSG monitoring at baseline.
Depressive symptoms are associated with a significant disease burden in adults and can contribute to premature mortality (16). This study indicates a robust association between sleep disturbances and various psychosomatic disorders, including behavioral disorders, substance disorders, and particularly anxiety disorders (17–20). Prior research has demonstrated that both PSG and multi-sensor trackers are effective in monitoring sleep patterns, although PSG is found inadequate for classifying the severity of sleep apnea in infants (21–23). Consequently, this study used PSG to monitor sleep patterns in adults diagnosed with depression and anxiety disorders. It was found that adults with depressive disorders exhibited greater difficulties in initiating sleep and had extended durations of REM sleep latency (REMSL) and frequent wake after sleep onset (WASO) compared to healthy controls (24–26). Jorge Mendoza’s research demonstrated that circadian rhythm disruptions in mood disorder patients could serve as crucial biomarkers for the prevention and treatment of depression (27).
This study sought to investigate the potential contributory role of sleep disturbances and depression in cancer risk by analyzing levels of CA125, CA199, AFP, and CEA. However, the results indicated no association between depression and these biomarker levels in cases of sleep disturbances like previous studies (28, 29). Other studies have revealed that inadequate or prolonged sleep, insomnia symptoms, and nocturnal phenotypes elevate the risk of lung cancer. Additionally, variations in sleep duration, the use of sleep aids, and insomnia manifest differently in women’s breast tissues, potentially exacerbating difficulties in sleep initiation among cancer patients (28, 29). Research has identified sleep disturbances as a cause for alterations in inflammatory factors, thereby elevating hs-CRP levels, a finding that is consistent with other studies (30, 31). Treatment options for sleep rhythm disorders encompass light therapy and techniques to counteract early rhythm phase delays (32–34). We recommend increasing physical activity to enhance mental health, as supported by previous findings (35, 36).
5 Limitation
Some participants in the study had incomplete test results, which were treated as missing data. Despite prior training, maintaining consistency in the investigators’ expertise level throughout the survey implementation proved challenging. This study was conducted over three years, and the investigators’ experience may deepened, potentially influencing assessment of scales and resulting in bias of the outcomes.
6 Conclusion
Findings from PSG revealed that participants with depression and anxiety disorders exhibited distinct sleep patterns, such as prolonged REMSL and altered WASO, which differed significantly from those without these disorders. Notably, effective pharmacological treatment outcomes were associated with significantly shorter SOL. These results revealed the importance of integrating sleep management into therapeutic strategies for mental disorders. The insights gained underscore the necessity of considering sleep disturbances in enhancing the overall effectiveness of pharmacological treatments for depression and anxiety disorders.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Research Ethics Committee at the First Affiliated Hospital of Ningbo University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QZ: Conceptualization, Writing – original draft. MT: Conceptualization, Methodology, Writing – review & editing. YJ: Data curation, Project administration, Writing – review & editing. YH: Data curation, Investigation, Writing – original draft. ZL: Formal analysis, Project administration, Writing – original draft. DW: Data curation, Writing – original draft. YM: Data curation, Writing – review & editing. PM: Data curation, Formal analysis, Writing – review & editing. JT: Investigation, Writing – review & editing. ZZ: Funding acquisition, Supervision, Validation, Writing – review & editing. LR: Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Zhejiang Provincial Medical and Health Science and Technology Project (2021KY978, 2024KY349, 2024KY1498, 2023KY1062, 2023RC258). Ningbo Key Research and Development Project (2023Z197, 2022Z145). Hospital level project of the First Affiliated Hospital of Ningbo University (XGZ-2304).
Acknowledgments
We would like to thank all the authors in the citation for their contributions to similar studies and the financial support provided by Zhejiang Provincial Medical and Health Science and Technology Project. Thanks to the First Hospital of Ningbo University for its convenience and assistance in research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sakurai H, Suzuki T, Yoshimura K, Mimura M, Uchida H. Predicting relapse with individual residual symptoms in major depressive disorder: a reanalysis of the STAR*D data. Psychopharmacol (Berl). (2017) 234:2453–61. doi: 10.1007/s00213-017-4634-5
2. Gallaher KGH, Slyepchenko A, Frey BN, Urstad K, Dørheim SK. The role of circadian rhythms in postpartum sleep and mood. Sleep Med Clin. (2018) 13:359–74. doi: 10.1016/j.jsmc.2018.04.006
3. Lunsford-Avery JR, Gonçalves BDSB, Brietzke E, Bressan RA, Gadelha A, Auerbach RP, et al. Adolescents at clinical-high risk for psychosis: Circadian rhythm disturbances predict worsened prognosis at 1-year follow-up. Schizophr Res. (2017) 189:37–42. doi: 10.1016/j.schres.2017.01.051
4. Satyanarayanan SK, Su H, Lin YW, Su KP. Circadian rhythm and melatonin in the treatment of depression. Curr Pharm Des. (2018) 24:2549–55. doi: 10.2174/1381612824666180803112304
5. Scott MR, McClung CA. Circadian rhythms in mood disorders. Adv Exp Med Biol. (2021) 1344:153–68. doi: 10.1007/978-3-030-81147-1_9
6. Jones SG, Benca RM. Circadian disruption in psychiatric disorders. Sleep Med Clin. (2015) 10:481–93. doi: 10.1016/j.jsmc.2015.07.004
7. Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. (2011) 378:621–31. doi: 10.1016/S0140-6736(11)60095-0
8. Brydges CR, Bhattacharyya S, Dehkordi SM, Milaneschi Y, Penninx B, Jansen R, et al. Metabolomic and inflammatory signatures of symptom dimensions in major depression. Brain Behav Immun. (2022) 102:42–52. doi: 10.1016/j.bbi.2022.02.003
9. Bahk Y-C, Han E, Lee S-H. Biological rhythm differences and suicidal ideation in patients with major depressive disorder. J Affect Disord. (2014) 168:294–7. doi: 10.1016/j.jad.2014.07.001
10. Berdynaj D, Boudissa SN, Grieg MS, Hope C, Mahamed SH, Norbury R. Effect of chronotype on emotional processing and risk taking. Chronobiol Int. (2016) 33:406–18. doi: 10.3109/07420528.2016.1146739
11. Alston M, Cain SW, Rajaratnam SMW. Advances of melatonin-based therapies in the treatment of disturbed sleep and mood. Handb Exp Pharmacol. (2019) 253:305–19. doi: 10.1007/164_2018_139
12. Murray JM, Sletten TL, Magee M, Gordon C, Lovato N, Bartlett DJ, et al. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. (2017) 1:40(1). doi: 10.1093/sleep/zsw002
13. Chinese Academy of Depressive Disorders. Clinical practice guidelines and recommendations on major depressive disorder with biological rhythm disturbance. Chin J Psychiatry. (2019) 52:110–6. doi: 10.3760/cma.j.issn.1006-7884.2019.02.002
14. Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. (2016) 25:131–43. doi: 10.1111/jsr.12371
15. Lee Y, Field JM, Sehgal A. Circadian rhythms, disease and chronotherapy. J Biol Rhythms. (2021) 36:503–31. doi: 10.1177/07487304211044301
16. Janaway BM, Kripalani M. Early intervention for depression in young people: a blind spot in mental health care. Lancet Psychiatry. (2019) 6:283. doi: 10.1016/S2215-0366(19)30089-6
17. Li Z, Li Y, Chen L, Chen P, Hu Y. Prevalence of depression in patients with hypertension: A systematic review and meta-analysis. Med (Baltimore). (2015) 94:e1317. doi: 10.1097/MD.0000000000001317
18. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. (2019) 23:2324–32. doi: 10.1111/jcmm.14170
19. Nauha L, Farrahi V, Jurvelin H, Jämsä T, Niemelä M, Kangas M, et al. Comparison and agreement between device estimated and self-reported sleep periods in adults. Ann Med. (2023) 55:2191001. doi: 10.1080/07853890.2023.2191001
20. Murray G, Gottlieb J, Hidalgo MP, Etain B, Ritter P, Skene DJ, et al. Measuring circadian function in bipolar disorders: Empirical and conceptual review of physiological, actigraphic, and self-report approaches. Bipolar Disord. (2020) 22:693–710. doi: 10.1111/bdi.12963
21. Pesonen AKL. The validity of a new consumer-targeted wrist device in sleep measurement: an overnight comparison against polysomnography in children and adolescents. J Clin Sleep Med. (2018) 14:585–91. doi: 10.5664/jcsm.7050
22. de Zambotti M, Rosas L, Colrain IM, Baker FC. The sleep of the ring: comparison of the ŌURA sleep tracker against polysomnography. Behav Sleep Med. (2019) 17:124–36. doi: 10.1080/15402002.2017.1300587
23. Daftary AS, Jalou HE, Shively L, Slaven JE, Davis SD. Polysomnography reference values in healthy newborns. J Clin Sleep Med. (2019) 15:437–43. doi: 10.5664/jcsm.7670
24. Melo MCA, Abreu RLC, Linhares Neto VB, de Bruin PFC, de Bruin VMS. Chronotype and circadian rhythm in bipolar disorder: a systematic review. Sleep Med Rev. (2017) 34:46–58. doi: 10.1016/j.smrv.2016.06.007
25. Robillard R, Carpenter JS, Rogers NL, Fares S, Grierson AB, Hermens DF, et al. Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl Psychiatry. (2018) 8:213. doi: 10.1038/s41398-018-0255-y
26. Crouse JJ, Carpenter JS, Song YJC, Hockey SJ, Naismith SL, Grunstein RR, et al. Circadian rhythm sleep–wake disturbances and depression in young people: implications for prevention and early intervention. Lancet Psychiatry. (2021) 8:813–23. doi: 10.1016/S2215-0366(21)00034-1
27. Mendoza J. Circadian insights into the biology of depression: Symptoms, treatments and animal models. Behav Brain Res. (2019) 376:112186. doi: 10.1016/j.bbr.2019.112186
28. Zhou T, Wang Z, Qiao C, Wang S, Hu S, Wang X, et al. Sleep disturbances and the risk of lung cancer: a meta-epidemiological study. BMC Cancer. (2023) 23:884. doi: 10.1186/s12885-023-11392-2
29. Büttner-Teleagă A, Kim YT, Osel T, Richter K. Sleep disorders in cancer-A systematic review. Int J Environ Res Public Health. (2021) 18:11696. doi: 10.3390/ijerph182111696
30. Tong HH, Li JR, Feng Y, Li SW, Qiu H, Hong JF. The association between sleep disturbance and proinflammatory markers in patients with cancer: a meta-analysis. Cancer Nurs. (2023) 46:91–8. doi: 10.1097/NCC.0000000000001055
31. Chang S-L, Durocher F, Diorio C. Sleep quality traits correlate with inflammatory markers in the breast tissue of women. Cytokine. (2022) 160:156028. doi: 10.1016/j.cyto.2022.156028
32. Carpenter JS, Robillard R, Hermens DF, Naismith SL, Gordon C, Scott EM, et al. Sleep-wake profiles and circadian rhythms of core temperature and melatonin in young people with affective disorders. J Psychiatr Res. (2017) 94:131–8. doi: 10.1016/j.jpsychires.2017.07.007
33. Bellivier F, Geoffroy PA, Etain B, Scott J. Sleep- and circadian rhythm-associated pathways as therapeutic targets in bipolar disorder. Expert Opin Ther Targets. (2015) 19:747–63. doi: 10.1517/14728222.2015.1018822
34. Hickie IB, Naismith SL, Robillard R, Scott EM. Manipulating the sleep-wake cycle and circadian rhythms to improve clinical management of major depression. BMC Med. (2013) 11:79. doi: 10.1186/1741-7015-11-79
35. Esaki Y, Takeuchi I, Tsuboi S, Fujita K, Iwata N, Kitajima T. A double-blind, randomized, placebo-controlled trial of adjunctive blueblocking glasses for the treatment of sleep and circadian rhythm in patients with bipolar disorder. Bipolar Disord. (2020) 22:739–48. doi: 10.1111/bdi.12912
Keywords: sleep disturbances, depressive and anxiety disorders, polysomnography, antidepressant therapy, treatment efficacy
Citation: Zhang Q, Tong M, Ji Y, Hou Y, Lou Z, Wu D, Mi Y, Miu P, Tian J, Zhu Z and Ruan L (2024) The impact of sleep disturbances on treatment efficacy and prognosis in patients with depressive and anxiety disorders. Front. Psychiatry 15:1432538. doi: 10.3389/fpsyt.2024.1432538
Received: 16 May 2024; Accepted: 18 September 2024;
Published: 14 October 2024.
Edited by:
Marcin Siwek, Jagiellonian University Medical College, PolandReviewed by:
Gniewko Więckiewicz, Medical University of Silesia, PolandColin K. Drummond, Case Western Reserve University, United States
Copyright © 2024 Zhang, Tong, Ji, Hou, Lou, Wu, Mi, Miu, Tian, Zhu and Ruan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenzhen Zhu, emh1emhlbnpoZW4xOTg5QDE2My5jb20=; Liemin Ruan, MTM4MDU4NjkxNjJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Qingyu Zhang
Qingyu Zhang Maoqing Tong
Maoqing Tong Yunxin Ji1
Yunxin Ji1 Yanbin Hou
Yanbin Hou Zongze Lou
Zongze Lou Danjuan Wu
Danjuan Wu Jiaxin Tian
Jiaxin Tian Liemin Ruan
Liemin Ruan