
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 30 September 2024
Sec. Schizophrenia
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1432407
 Xing Peng1,2,3
Xing Peng1,2,3 Yu-shen Ding2,3
Yu-shen Ding2,3 Bo Ren4
Bo Ren4 Xi-xi Zhao2,3
Xi-xi Zhao2,3 Fei-fei Wang2,3
Fei-fei Wang2,3 Jie Zhao5
Jie Zhao5 Yuan-yuan Zhang6
Yuan-yuan Zhang6 Xiu-jun Zhang1*
Xiu-jun Zhang1* Fu-chun Zhou2,3*
Fu-chun Zhou2,3* Chuan-yue Wang2,3
Chuan-yue Wang2,3Objective: The aim of this study was to investigate whether a potential moral cognitive impairment (failure in understanding moral rules) exists in patients with schizophrenia (SCZ) and to explore the effect of childhood trauma (CT) on moral cognition in a group of patients with SCZ.
Methods: A total of 99 patients with SCZ and 102 healthy controls (HCs) were included in this study. The Childhood Trauma Questionnaire-Short Form (CTQ) was administered to assess childhood trauma experiences in both groups, while the Moral Identity Measure (MIM) and the Moral Foundations Questionnaire (MFQ) were applied for a comparative evaluation of moral cognition across the two groups. The Positive and Negative Syndrome Scale (PANSS) was administered to assess the psychopathology.
Results: Patients with schizophrenia had significantly greater CTQ scores than HCs (42.77 ± 13.50 vs. 29.11 ± 4.25, t=9.697, p<0.001). The prevalence of childhood trauma (χ2 = 58.452, p<0.001) and history of aggressive behaviors (χ2 = 23.565, p=0.001) among patients with SCZ were greater than that among HCs. In addition, the scores of moral cognition (MIM: 61.82 ± 15.12 vs. 70.88 ± 8.87, p=0.001; MFQ: 87.24 ± 22.30 vs. 112.62 ± 23.42, p=0.045) in the SCZ group was lower than that in the HC group after controlling for the influence of CT covariates. The MFQ score was negatively correlated with the CTQ score, the emotional abuse (EA) score, the physical abuse (PA) score and the physical neglect (PN) score in SCZ patients. Among HCs, the MFQ score was positively correlated with the CTQ score, as well as with the dimensions of physical abuse (PA) and emotional Neglect (EN). Multiple linear regression analyses revealed that impaired moral cognition performance was significantly predicted by the CTQ score (beta=-0.235, p=0.034, 95% CI -0.743 to -0.031) in patients with SCZ but was significantly predicted by years of education (beta=-0.392, p<0.001, 95% CI -4.783 to -1.876), alcohol use (beta=0.210, p=0.023, 95% CI 2.191 to 29.399) and the CTQ score (beta=0.184, p=0.046, 95% CI 0.019 to 1.928) in HCs. CTQ moderated the effect of SCZ on MFQ (B = 0.516); Simple tests revealed that the group effect on the MFQ was B=12.306 at the lower level(-1SD) and B = 54.089 at the higher level(+1SD) of the CTQ scores.
Conclusions: SCZ patients exhibit impaired moral cognition. The contribution of CT to the presence of moral cognitive impairments seems to be independent of psychopathology.
Schizophrenia (SCZ) is a chronic and debilitating mental disorder (1, 2) that is often accompanied by various cognitive and social functional impairments (3–7).
The “moral cognition” involves the mental processes related to moral reasoning, judgment, and decision-making (8, 9). Moral cognition is a complex process influenced by a wide range of interpersonal, intrapersonal, social, cultural, and biological factors (10–12). Understanding this multifaceted nature of moral development is important for comprehending the subtleties inherent in ethical decision-making and behavior. Moral Cognitive impairment refers to the deficits in understanding and applying moral principles, assessing the moral value of actions, and making moral decisions. This includes challenges in comprehending moral norms, recognizing moral emotions, understanding, and formulating moral decisions. Moral cognition relies on the proper functioning of various brain regions and neural networks, and is closely related to certain neurocognitive processes, such as executive functions and decision-making (13). Moral cognition is also a part of social cognition (6) and heavily influenced by an individual’s understanding and interpretation of social norms, values, and contextual cues (12).
The functional magnetic resonance imaging (fMRI) offering insights into the neural correlates of moral cognition (14, 15). Compared to nonpsychopaths, psychopaths exhibit decreased activity in the ventromedial prefrontal cortex and anterior temporal cortex when differentiating between moral and nonmoral pictures (15). Individuals with SCZ often present with deficits in executive functions (16), social cognition (17), and emotional processing (7, 18)—key components of the moral cognition framework (19). The prefrontal cortex (20) and temporal lobes (21), regions pivotal for moral reasoning, are notably affected in SCZ, leading to challenges in integrating emotional and cognitive information to make ethical judgments.
Whether patients with SCZ experience moral cognitive impairment has long been a topic of controversy in previous research (20, 22–27). As early as the 19th century, attention was drawn to specific subtypes of schizophrenia (then termed “hebephrenia”) and was described as a disorder associated with difficulties in understanding moral guidelines and proper moral behavior (28). Clinically, some of patients with SCZ exhibit a lack of empathy, superficial emotional experiences, indifference to the well-being of others, and a lack of awareness of their immoral behavior (29). In a study examining moral dilemmas from various perspectives, patients with SCZ took longer to make moral judgements from a third-person perspective, indicating obstacles in moral reasoning (24). This delay in moral reasoning was linked to decreased empathy and a diminished aversion to immoral behavior (24). Additionally, patients with SCZ demonstrated a more utilitarian approach in moral reasoning, which was significantly associated with their high interpersonal conflict (24). Compared with healthy controls (HCs), patients with SCZ showed differences in brain activation patterns during moral judgement in an fMRI study. Specifically, patients with SCZ showed reduced activation in the right hippocampus and increased activity in the superior and inferior frontal gyri (20). These factors may contribute to difficulties in social interaction and moral decision-making, which ultimately impact social functioning.
However, some researchers argue that the evidence to support the claim of a moral cognitive impairment in patients with SCZ is insufficient. In a behavioral study, patients with SCZ and their first-degree relatives made comparable utilitarian decisions in a moral judgement task, with no significant differences compared to HCs (20). Similarly, this conclusion was consistent with a study involving 23 patients with SCZ and 32 HCs, which revealed no significant difference in the tolerance for immoral behavior during moral judgement (23). The juxtaposition of these divergent research outcomes underscores the imperative for further investigations into the domain of moral cognition pertinent to individuals afflicted with schizophrenia.
Childhood trauma (CT) is considered a potential influencing factor that could affect moral cognition in people with SCZ (30). Previous studies have shown that CT not only diminishes emotional bonding between healthy controls (HCs) and their parents (particularly the mother), but also impairs cognitive functions related to moral reasoning, such as theory of mind (ToM) (31), as well as precipitates the decline in cognitive function in late adulthood (32). As suggested in Chemtob’s survival mode theory (33), when people have PTSD, they see even ambiguous situations as threatening, which activates a survival mode process in their brains that results in anger/violence and takes precedence over other, non-violent cognitive functions. Even moral behaviors, such as non-violence in situations that do not warrant it, can be overridden by the brain processing information as threatening due to such trauma history (33). Therefore, CT may play a potential moderator role in the relationship between SCZ and moral reasoning.
The aim of this study was to investigate whether a potential impairment in the moral cognition of patients with schizophrenia (SCZ) exists and to determine whether CT influences moral cognition in patients with SCZ. Accordingly, we propose the following hypotheses: (1) An impairment in the moral cognition of patients with schizophrenia is present, and (2) CT may serves as a moderator or mediator in the relationship between SCZ and the presence of moral cognitive deficits.
From March 2021 through June 2023, a total of 99 inpatients and outpatients with SCZ were recruited from Beijing Anding Hospital, Capital Medical University. Additionally, 102 HCs were enrolled from the local community and matched with the patients on sociodemographic characteristics. The patients were from the “Early Psychosis Cohort Study of Beijing Anding Hospital”, and the HCs were from a study of North China University of Science and Technology. The two studies were approved by the Ethics Committees of Beijing Anding Hospital and North China University of Science and Technology, respectively. All participants in the study provided written informed consent.
The inclusion criteria for patients with schizophrenia were as follows: (1) had a diagnosis of schizophrenia in accordance with the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), which was determined using the Mini International Neuropsychiatric Interview (MINI, 7.0.2) (34); (2) had a minimum of 9 years of education; (3) were native Chinese speakers; and (4) were aged between 13 and 50 years. The exclusion criteria for patients were as follows: (1) had received neuromodulation treatments such as transcranial magnetic stimulation (TMS) or transcranial alternating/direct current stimulation (tACS/tDCS) within the past three months; (2) had ever suffered from another mental illness or serious physical illness; (3) were extremely excited, impulsive, or had risks of self-injury or harming others and were unable to cooperate with the experimenter; and (4) had a history of substance abuse; (5) women who were pregnant or lactating.
The inclusion criteria for healthy controls were as follows: (1) had a minimum of 9 years of education, (2) were native Chinese speakers, (3) were aged between 13 and 50 years. The exclusion criteria were as follows: (1) individuals had a history of any mental illness, substance abuse or a family history of mental disorders as determined by the negative results from the MINI (7.0.2); (2) individuals with severe neurological disorders or medical conditions (such as a history of brain trauma or infection, brain tumor, cerebrovascular diseases, epilepsy, etc.); (3) women who were pregnant or lactating; and (4) individuals who had previously participated in similar studies.
A self-designed questionnaire was used to collect sociodemographic information from participants, including age, sex, years of education, history of aggressive behavior, smoking status, and alcohol consumption.
The Mini International Neuropsychiatric Interview (MINI) was developed to serve as a concise, structured diagnostic interview for screening major psychiatric disorders. It has been extensively employed in diverse environments such as clinical and research settings. Its validity and reliability have been examined through various studies, demonstrating that the MINI bears comparable psychometric properties to other structured diagnostic instruments, with the distinct advantage of requiring a significantly shorter administration time (34).
In addition, basic clinical data such as the duration of illness and antipsychotic usage was also collected from patients’ electronic medical records. The Chinese version of the Positive and Negative Syndrome Scale (PANSS) (35) was administered to assess the severity of psychopathology in patients. The patients’ psychopathology was assessed by the treating psychiatrists using the Positive and Negative Syndrome Scale (PANSS). All the raters have completed the training program to ensure good inter-rater reliability.
The 28-item Childhood Trauma Questionnaire-Short Form (CTQ) was employed for the assessment of CT. The CTQ is a retrospective self-report scale suitable for individuals aged 12 and older (36). The scale utilizes a 5-point Likert scale consisting of “1 = Never”, “2 = Rarely”, “3 = Sometimes”, “4 = Often” and “5 = Always”. The CTQ comprises 5 subscales organized into 2 principal categories (abuse/neglect) and 5 minor categories. The abuse dimension includes emotional abuse (EA), physical abuse (PA), and sexual abuse (SA), while the neglect dimension includes emotional neglect (EN) and physical neglect (PN), with each subscale consisting of 5 items. The scale provides detailed documentation of potentially traumatic events that may have occurred during childhood.
Based on a previous study (37), the specific threshold scores for different dimensions of the CTQ are defined as follows: EA-13, PA-10, SA-8, EN-15, and PN-10. Individuals who reached or exceeded the threshold on any of the CTQ subdimensions were considered to have a history of childhood trauma in the present study.
The Moral Identity Measure (MIM) and the Moral Foundations Questionnaire (MFQ) were employed in this study to assess participants’ moral cognition.
The MIM (38) originally consisted of 10 items. However, it has been subsequently revised to incorporate a total of 16 items. The revised MIM utilizes a 5-point Likert scale ranging from 1 (completely disagree) to 5 (completely agree). Higher scores on the MIM indicate a greater level of moral identity among the participants (39).
The original MFQ (40) encompassed five dimensions and a total of 32 items: ① Harm/Care, ② Fairness/Reciprocity, ③ In-group/Loyalty, ④ Authority/Respect, and ⑤ Purity/Sanctity. Each dimension comprises six items, including two validation items designed to evaluate participants’ response accuracy. Considering cultural differences and given that the participants in this study were all Chinese and often considered “Civilization/In-civilization” to be an important indicator of morality, the Chinese scholar Zhao Ying-nan (41) incorporated a “Civilization/In-civilization” dimension based on the relationship between civilization and moral cognition. This dimension consisted of six items and was derived from guidelines provided by the Chinese Civilization website (http://www.wenming.cn/) and the “Beijing Municipal Regulations on the Promotion of Civilized Behavior”. The final Chinese version of the Moral Foundations Questionnaire (MFQ) comprised a total of six dimensions and 38 items. The modified scale displayed good reliability and validity, with an internal consistency coefficient (Cronbach’s α) of 0.93. Additionally, Cronbach’s α for each dimension exceeded 0.6, indicating good internal consistency of the Chinese version of the MFQ. A 6-point Likert scale ranging from 0 (strongly disagree) to 5 (strongly agree) was used to rate the responses on the MFQ. Higher scores on the MFQ indicated a greater degree of moral cognition among the participants.
Statistical analyses were performed using SPSS software (version 22.0, SPSS Inc., Chicago, IL, USA). For between-group differences in demographics and scores on the CTQ, MIM, and MFQ between SCZ and HC, categorical data were analyzed using the chi-square test, whereas independent-sample t-tests and analysis of covariance (ANCOVA) were applied for continuous variables. Pearson’s correlation analyses were used to examine the relationships among sociodemographic variables and CTQ, MFQ, and MIM scores if the data met the assumptions; otherwise, point-biserial correlation analysis was performed. Multiple linear regression analyses were conducted to explore the independent predictors of moral cognition in patients with schizophrenia as well as HCs. In the regression analyses, the MFQ scores were entered as the dependent variable, and all variables that showed significant correlations with the MFQ were entered as independent variables. The moderating effect analysis was performed to explore the conditional effects of group membership (Group 1: SCZ; Group 2: HC) on MFQ, moderated by CTQ. The analysis involved a series of regression models to evaluate the main and interactive effects of group and trauma on the outcome variable. The initial model estimated the main effects of group and CTQ on MFQ. Subsequent models included the interaction term (Group × CTQ) to assess the conditional effects. The group and CTQ were centered to reduce multicollinearity and improve the interpretability of the interaction. The results were considered statistically significant if p < 0.05 according to a two-tailed test. Bonferroni adjustment was applied to the exploratory multiple between-group comparisons.
No significant differences were observed between the two groups of participants with regard to sex, age, years of education, smoking status or alcohol consumption. However, a significant difference was found between the two groups in terms of a history of aggressive behavior and childhood trauma (see Table 1).
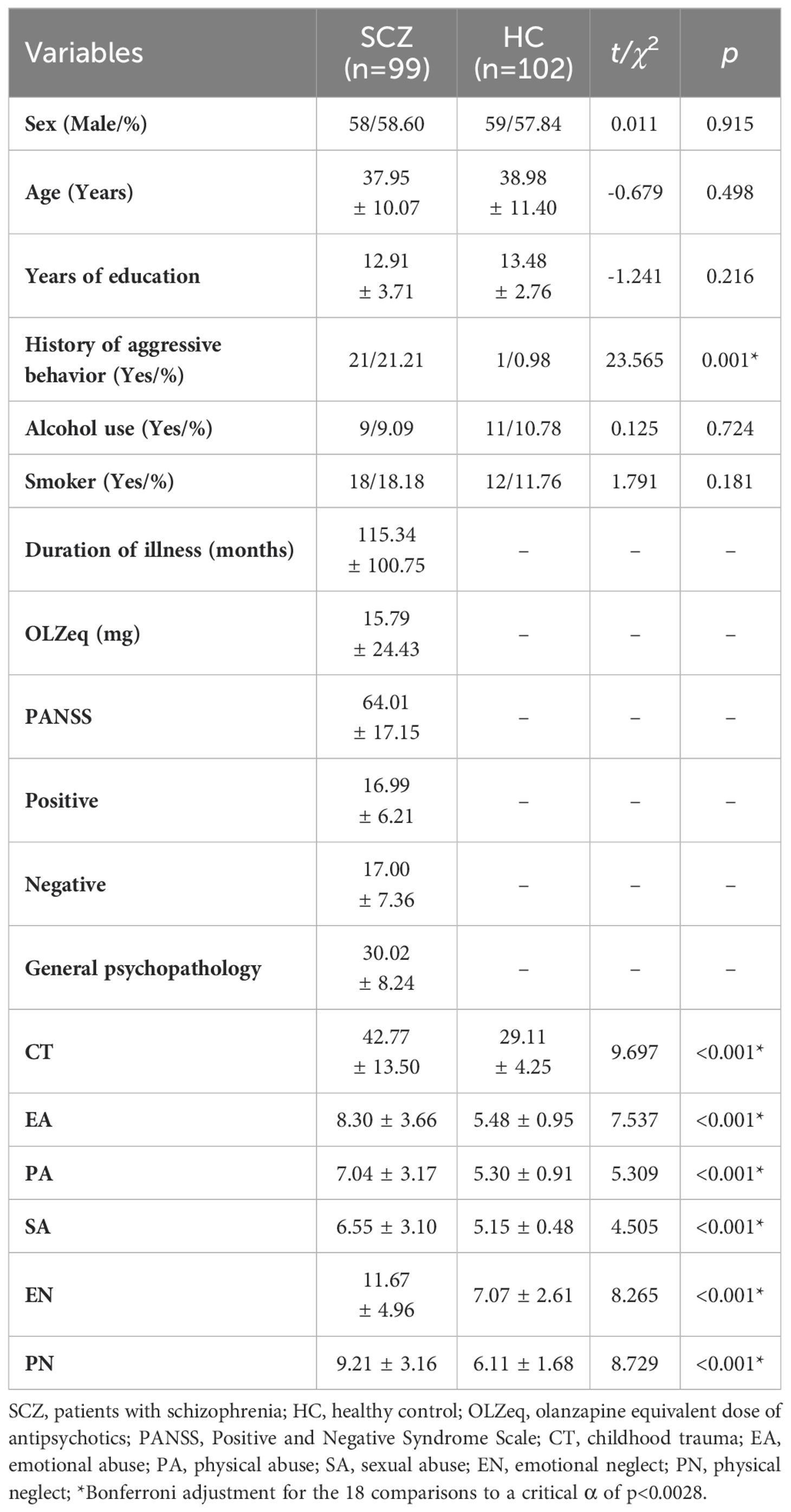
Table 1. Sociodemographic characteristics and childhood trauma among the two groups of participants ( ± S).
The CT score of the SCZ group was significantly higher than that of the HC group (t = 9.697, p < 0.001), as were the scores for each dimension of CTQ, with all p values less than 0.001. The percentage of patients with SCZ (53.53%) who experienced CT was significantly greater than that of HCs (3.92%) (χ2 = 58.452, p < 0.001). Similar results were observed for other dimensions of CTQ, and all p values for these dimensions were below the threshold of 0.001 (see Table 1; Figure 1; Supplementary Table 1; Supplementary Figure 1).

Figure 1. Comparison of childhood trauma in schizophrenia patients and healthy controls. SCZ, patients with schizophrenia; HC, healthy controls; CT, childhood trauma; EA, emotional abuse; PA, physical abuse; SA, sexual abuse; EN, emotional neglect; PN, physical neglect; ***p<0.001.
The SCZ group scored lower than the HC group on the MIM (t=-5.202, p<0.001) as well as on the MFQ (t =-7.862, p <0.001) (see Table 2; Figure 2).
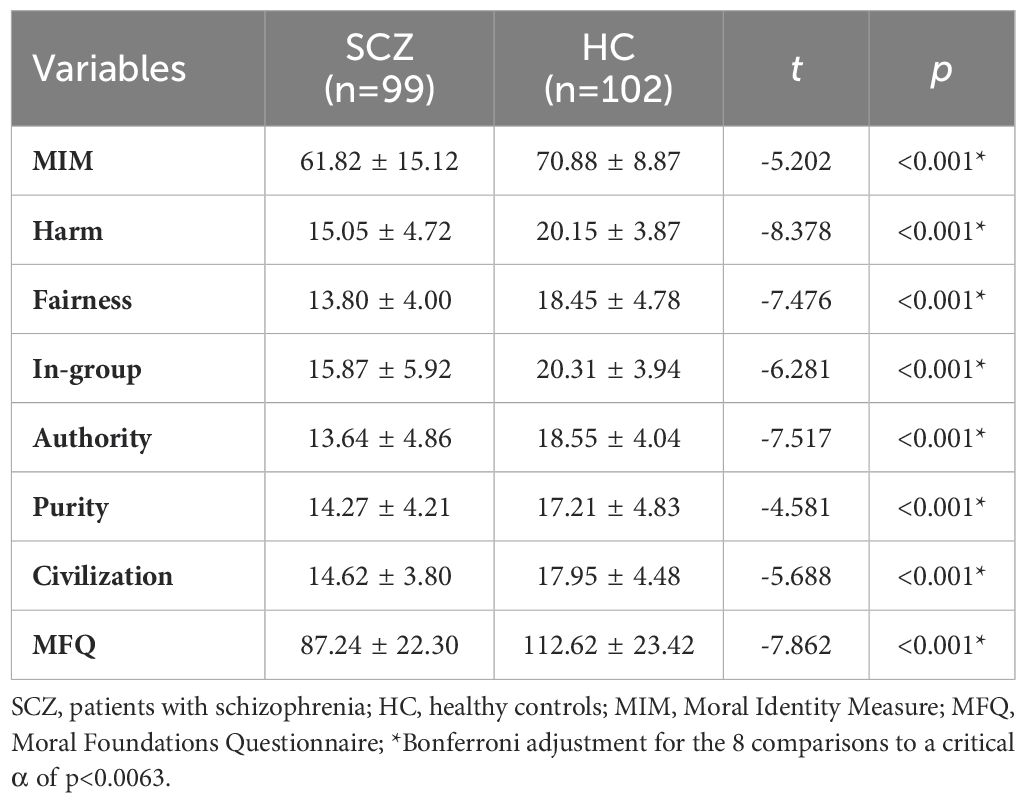
Table 2. Comparison of the MFQ and MIM scores between patients with schizophrenia and healthy controls ( ± S).
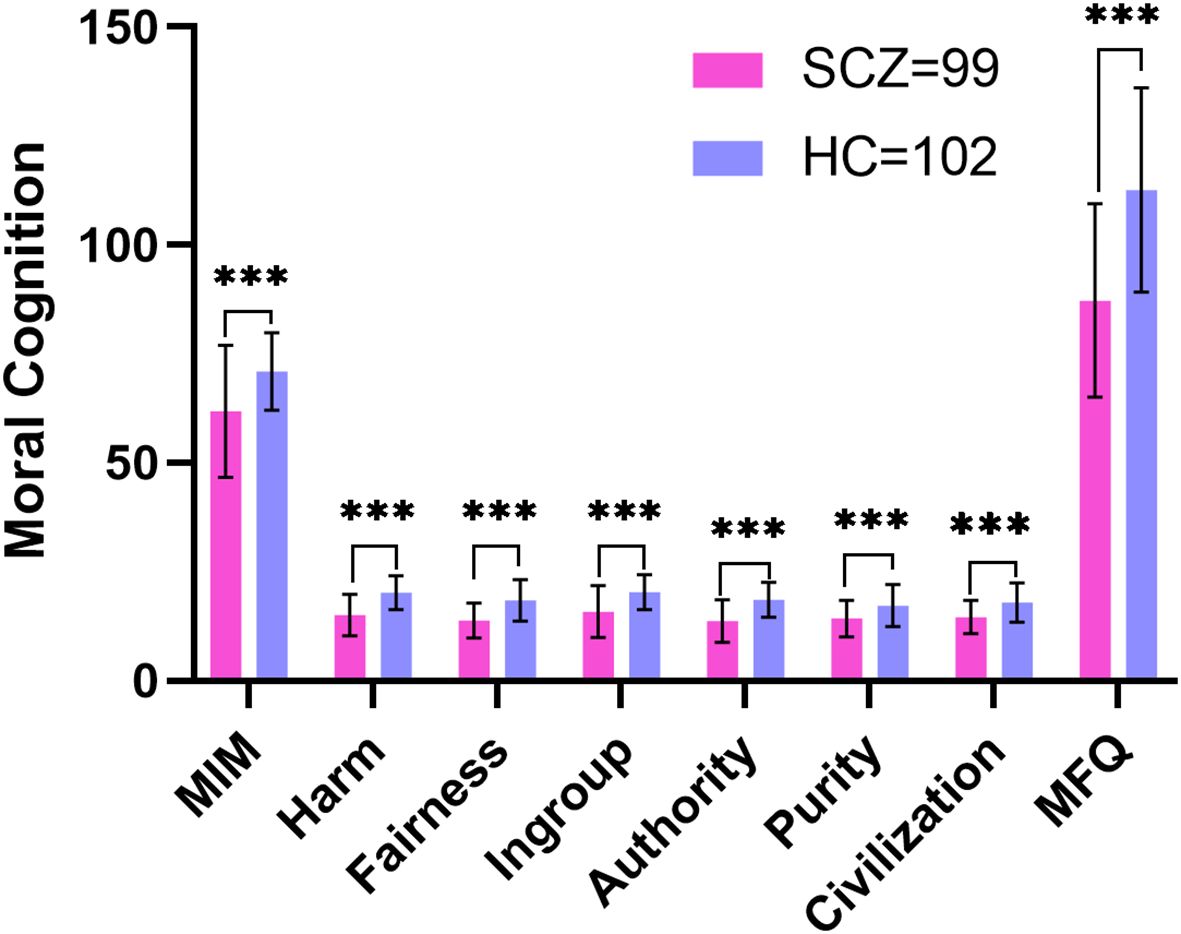
Figure 2. Comparison of the MFQ and MIM scores between patients with schizophrenia and healthy controls. SCZ, patients with schizophrenia; HC, healthy controls; MFQ, Moral Foundations Questionnaire; MIM, Moral Identity Measure; ***p<0.001.
Taking MIM and MFQ scores as dependent variables respectively, the group as the independent variable, and CT as the covariate, ANCOVA was conducted to explore whether differences in moral cognition persisted between the two groups.
The moral cognition of patients with schizophrenia (MIM: 61.82 ± 15.12 vs. 70.88 ± 8.87, F= 11.964, p=0.001; MFQ: 87.24 ± 22.30 vs. 112.62 ± 23.42, F=4.073, p=0.045) remained lower than that of HC.
SCZ: MFQ is negatively correlated with CTQ (r=-0.247, p=0.014), as well as EA (r=-0.238, p=0.018), PA (r=-0.208, p=0.039) and PN (r=-0.198, p=0.050) in patients with schizophrenia. Scores for the subdimensions of the MFQ had similar negative correlations with scores for the subdimensions of the CTQ, with r values ranging from -0.302 to -0.212 and p values ranging from 0.005 to 0.035.
HCs: The MFQ score was significantly positively correlated with the CTQ scores (r=0.267, p=0.007), as well as with scores for the dimensions of PA (r=0.202, p=0.042) and EN (r=0.203, p=0.040) among HCs. The scores for subdimensions of the MFQ were also positively correlated with those of the CTQ, with r values ranging from 0.196 to 0.299 and p values ranging from 0.002 to 0.049 (see Figure 3; Supplementary Tables 2, 3).
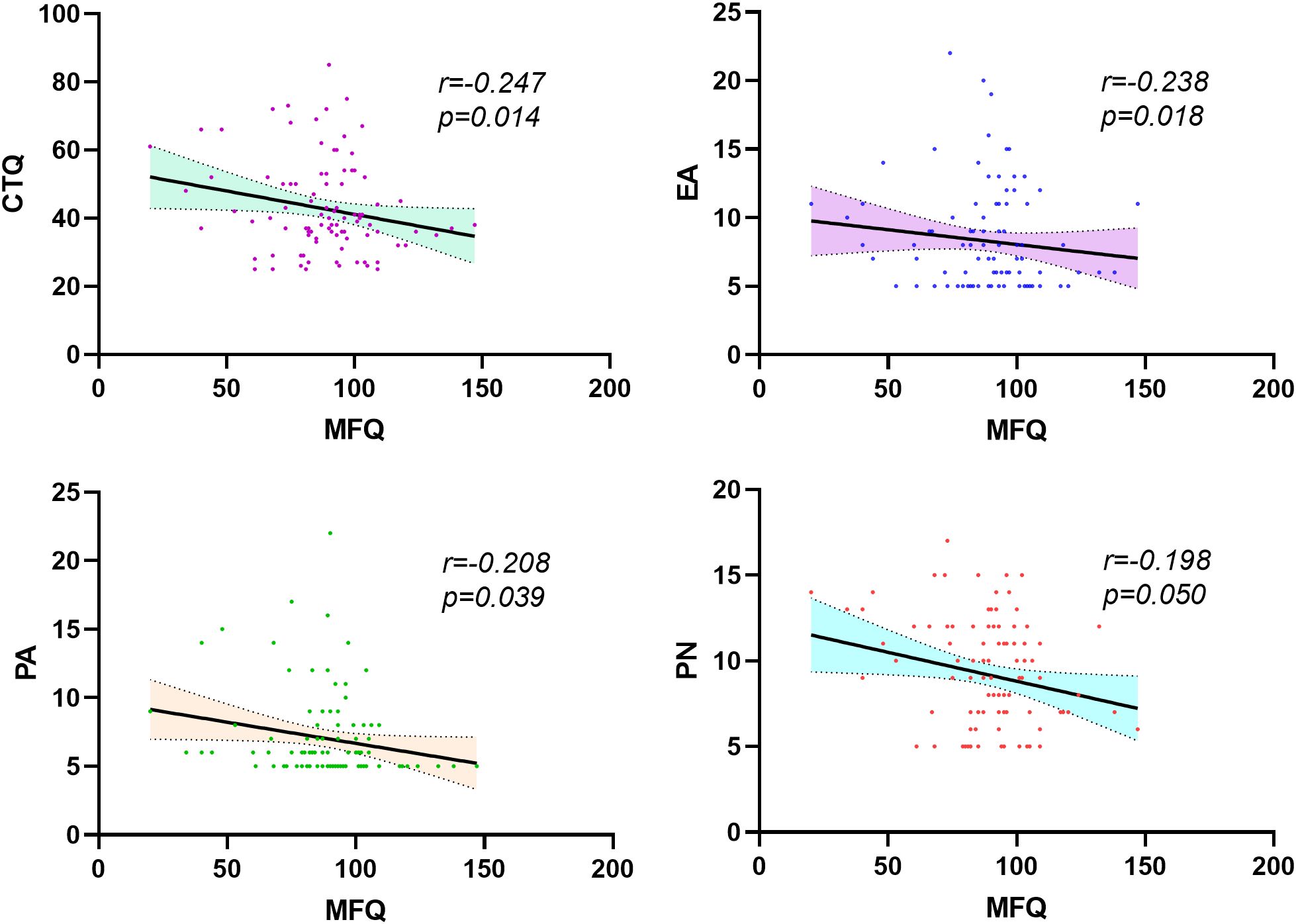
Figure 3. Correlation analysis of MFQ and CTQ scores and their subdimensions in patients with schizophrenia. CTQ, Childhood Trauma Questionnaire; EA, emotional abuse; PA, physical abuse; PN, physical Neglect.
SCZ: In the stepwise linear regression analyses, the MFQ score was entered as the dependent variable, and the duration of illness and CTQ scores were entered as independent variables. Higher MFQ score was significantly predicted by the CTQ score (beta=-0.235, p=0.034, 95% CI -0.743 to -0.031) in SCZ patients.
HCS: In the stepwise linear regression analyses, the MFQ score was entered as the dependent variable, and years of education, alcohol use, and CTQ scores were entered as independent variables. A higher MFQ score was significantly correlated with years of education (beta=-0.392, p <0.001, 95% CI -4.783 to -1.876), alcohol consumption (beta=0.210, p=0.023, 95% CI 2.191 to 29.399) and the CTQ score (beta=0.184, p=0.046, 95% CI 0.019 to 1.928) in HCs (see Table 3).
The model summary indicated a significant fit for the regression model, with R2 = 0.288, and F = 26.542, p < 0.001. The main effects of MFQ predicted by CTQ was not significance (B = 0.516, SE = 0.266, t = 1.939, p = 0.054, 95% CI -0.009 to 1.042). In contrast, the main effect of group was significant (B = 1.823, SE = 0.526, t = 3.463, p <0.001, 95% CI 0.785 to 2.861).
The interaction between group and CTQ was significant (B = 1.823, SE = 0.526, t = 3.463, p = 0.001, 95% CI 0.785 to 2.861), indicating that the effect of group on MFQ varied depending on the level of CTQ. Simple tests revealed that the group effect on the MFQ was B =12.306 at the lower level (-1SD) and B = 54.089 at the higher level (+1SD) of the CTQ scores; The conditional effects of group at different levels of CTQ are presented in Figure 4.
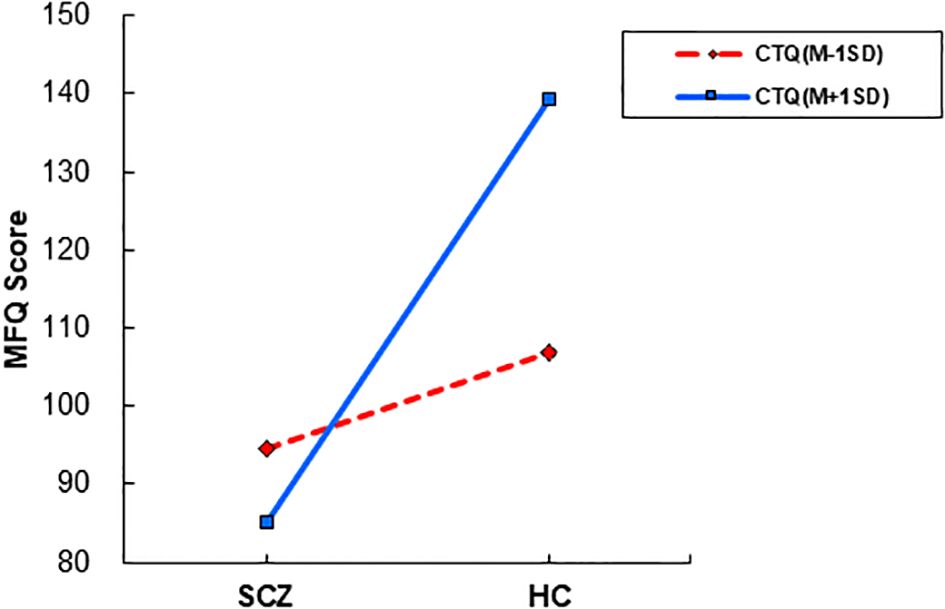
Figure 4. The interactive effects of childhood trauma on moral cognition moderation. SCZ, patients with schizophrenia; MFQ, Moral Foundations Questionnaire.
To our knowledge, this is the first study to investigate the effect of CT on moral cognition in schizophrenia patients. The results confirmed our hypotheses. Compared to healthy controls, patients with schizophrenia reported experiencing a higher level of childhood trauma, and were more likely to exhibit moral cognitive impairments. The current study’s results indicated that individuals with schizophrenia experienced a higher rate of childhood trauma compared to their healthy counterparts, which is consistent with previous findings (42–46).
The results of this research confirmed the hypothesis that the moral cognition of patients with schizophrenia is impaired, as evidenced by their lower scores on both the MIM and MFQ and analysis of covariance. As early as the 1980s, research indicated that adolescents with SCZ scored significantly lower on Kohlberg’s Moral Judgement Interview (MJI) than HCs did, and their teachers also reported that the morality of schizophrenia adolescents differed both qualitatively and quantitatively from that of typical adolescents (22). Additionally, in clinical settings, a subset of patients with schizophrenia exhibits a deficiency in empathy, superficial emotional responses, and insufficient awareness of immoral actions (29). These individuals have significant impairments in comprehending moral norms and behaviors, which are specifically characterized by a slowed pace of moral reasoning, a diminished capacity to generate feelings of aversion towards immoral conduct, and a reduction in empathetic abilities (25, 47). Moreover, their behaviors often tend to be egocentric, with a concurrent decrease in prosocial actions (47). Another study involving 45 individuals diagnosed with schizophrenia also reported that this population tends to use stricter evaluation criteria when assessing social transgressions and takes more time in making moral judgements during the process of testing moral permissibility (48). A slower moral judgement is associated with lower empathic abilities, while the tendency to harshly condemn social transgressions is related to poorer perspective-taking and decision-making abilities (48). Research also indicates that in third-person scenarios, patients with schizophrenia tend to exhibit utilitarian characteristics in their moral judgements that differ from those of the general population, and this utilitarian tendency is significantly correlated with their level of interpersonal conflict (24). These findings consistently reveal an impairment in the moral cognition of patients with schizophrenia, which aligns with the results of the present study.
According to Kohlberg’s theory of moral cognitive development (49), the capacity for moral cognition in humans evolves progressively with age. Individuals’ understanding of morality, which encompasses self-awareness, empathy for others, recognition of collective interests, and comprehension of social norms and moral concepts, is subject to ongoing transformation (49). Through continuous learning and imitation, these cognitive processes and experiences are internalized, forming the individual’s own moral standards. Childhood represents a critical period for the development of moral cognition (50). However, childhood trauma, such as physical or sexual abuse, significantly disrupts this normal developmental process. This disruption can have a profound impact on an individual’s moral cognition at these crucial stages.
Bandura’s social learning theory (51) suggests that children, during their moral cognition developmental phase, observe and emulate the behaviors of others, thereby learning and internalizing a spectrum of behavioral patterns that are both directly and indirectly applied to themselves. This encompasses behaviors of a moral transgression, such as physical assault, verbal insult, and sexual abuse. The process of learning and internalization can lead to the formation of detrimental habits in children’s moral cognition, thereby obstructing the natural progression of their moral development. Research indicates that exposure to physical aggression in childhood can lead to a greater likelihood of aggressive behavior in adulthood (52, 53). As the results of the aforementioned studies show, children may learn and adopt violent actions through observation and imitation, which can be both directly experienced and witnessed indirectly with respect to themselves. This learning process can potentially lead to the formation of detrimental moral cognition habits, thereby obstructing the natural progression of moral development.
Furthermore, consistent with Bowlby’s principles on attachment (54), children establish a close attachment relationship with their intimate caregivers, especially their mothers, during childhood that is based on a “trusted” and “secure” “internal working model”, which is crucial for individuals to engage in healthy interactions with others in adulthood. However, childhood trauma disrupts this “internal working model”, leading to difficulties in forming intimate relationships with others. As a result, individuals may manifest psychological defenses and are inclined to exhibit behavior that is deemed ‘immoral’, particularly in an aggressive and violent manner, as a means to manage what they perceive as ‘dangerous’ and ‘unsafe’ interpersonal relationships. As described in Novaco and Chemtob’s survival mode theory (33), when individuals are confronted with danger from the real world or perceive potential threats, such as experiencing severe physical abuse, they tend to process such information in a limited way and significantly increase their alertness, thereby losing the ability to self-monitor. Consequently, they may perceive ambiguous situations as potential threats, even if these situations do not necessitate anger or violent behavior, that is, moral action (i.e., non-violence in situations that do not warrant it) can be overridden by the brain processing information as threatening due to trauma history (33). This result is consistent with the findings in this study that patients with schizophrenia have a greater history of aggressive behavior than healthy controls.
According to revictimization theory (55–58), individuals who have experienced CT, particularly emotional and sexual abuse (55, 57, 58), are more likely to become victims of further victimization or exhibit certain aggressive behaviors. Traumatic experiences in childhood can have a profound impact on the psychological development of individuals, leading to an increased likelihood of experiencing emotional problems such as anxiety, depression, irritability, and posttraumatic stress disorder(PTSD) in adulthood (18, 59, 60). Moreover, one study has shown that CT may increase the tendency to be irritable in patients with schizophrenia, either directly or indirectly, through the misidentification of others’ emotions, which can make them more susceptible to emotional problems and even aggressive behavior (59). Additionally, the pervasive cognitive deficits observed in schizophrenia can be attributed not only to the condition itself but also to compromised decision-making abilities, a prevalent challenge for patients with SCZ that stems from struggles with executive functions and is tied to deficits within the salience network. Both the salience network, responsible for recognizing stimuli that may be of interest or pose a threat, and the frontoparietal network (16), which facilitates attention, decision-making, and action, exhibit impairments in patients with SCZ. The reactivity of the salience network to ambiguous stimuli is heightened by trauma, suggesting a neurological basis for the difficulties that patients with SCZ may experience in responding fittingly to moral dilemmas (33). Consequently, individuals who have suffered CT may be more inclined toward behavior that is considered immoral, a tendency stemming from the complex interplay between emotional dysregulation and impaired social cognition, akin to the negative correlation observed in this study between patients’ childhood trauma and moral cognition.
Some scholars also believe that CT may also affect individual neurodevelopment (42, 61, 62) and have proposed the traumatic neurodevelopmental (TN) model of schizophrenia (63, 64). This model argues that traumatic events have a similar impact on developing brains as do biological abnormalities found in individuals with SCZ (64). The brain inherently connects these physiological alterations to the realm of psychological functioning, underscoring the intricate relationship between brain chemistry and moral cognition (65). Moreover, the TN model also suggests that the abnormal secretion of dopamine, norepinephrine, serotonin, and other neurotransmitters resulting from CT is strongly linked to increased aggression in individuals (66–69).
In our study, we also observed a positive correlation between the moral cognition scores of the HC and their experiences of CT. This correlation may be attributed to the individuals’ enhanced psychological resilience, which allows them to modulate their moral sensitivity in response to adverse childhood events such as trauma or bullying. This hypothesis is supported by an Italian study involving 581 healthy primary school students, which found that children who are victims of bullying and trauma exhibit higher moral sensitivity (70).
In conclusion, SCZ patients exhibit impaired moral cognition. The contribution of CT to the presence of moral cognitive impairments seems to be independent of psychopathology. The findings provide a novel perspective on SCZ and the impact of CT on moral cognitive development in adulthood, laying the groundwork for raising public awareness of CT and reducing stigma with schizophrenia.
Several limitations of this study should be acknowledged. First, the self-assessment and retrospective nature of the questionnaire used to assess moral cognition and childhood trauma experiences may have introduced bias. A combination of self report and behavioral experiments would be a better approaches for the study in the future. Second, the small sample size of our study may have reduced statistical power, affecting the robustness of our findings. Increasing the sample size in future research is essential to confirm our conclusions with greater confidence. Third, the inclusion of both adolescents and adults in our sample may have influenced the results due to potential cognitive differences between these age groups. Future studies should consider the impact of age-related cognitive differences on the outcomes. Fourth, the study primarily focused on the subjective aspect of moral cognition in schizophrenia, which may limit the objectivity compared to those using objective measures such as neuroimaging and clinical electroencephalography. Fifth, moral cognition is a complex process influenced by a wide range of interpersonal, intrapersonal, social, cultural, and biological factors, but it is not feasible to control for all these factors within the current study design.
Last but not least, the study only included schizophrenia patients from a single hospital, which may limit the generalizability of the results due to potential sampling bias. Multicenter studies with larger and more diverse samples are necessary to enhance the validity and applicability of the findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study has received ethical approval from both the Medical Ethics Committee of North China University of Science and Technology and the Ethics Committee of Beijing Anding Hospital, Capital Medical University. Additionally, all participating volunteers and their immediate family members have provided informed consent, and their signatures have been obtained to confirm their willingness to participate in the study.
XP: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Y-SD: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation. BR: Writing – review & editing, Writing – original draft, Methodology, Investigation. X-XZ: Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation, Funding acquisition. F-FW: Writing – review & editing, Writing – original draft, Data curation. JZ: Investigation, Writing – review & editing, Writing – original draft, Data curation. Y-YZ: Writing – review & editing, Writing – original draft, Data curation. X-JZ: Investigation, Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization. F-CZ: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization. C-YW: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was sponsored by National Natural Science Foundation of China (Grant No. 82171501), Early Psychosis Cohort Program of Beijing Anding Hospital (Grant No. ADDL-03) National Natural Science Foundation of China (Grant No.82201701), Beijing Municipal Hospital Research and Development Project (Grant No. PX2021068), Beijing Hospitals Authority Clinical medicine Development of special funding support (Grant No. ZLRK202335), the Ministry of Science and Technology of China, National Key R&D Program of China (Grant No. 2023YFC2506800) and STI2030-Major Projects (Grant No. 2021ZD0200701). The study design, data collection, analysis, interpretation, manuscript writing, and decision to submit for publication were independent of any influence from the funding sources.
The authors wish to express their gratitude to the volunteers who participated in this study. They would also like to extend thanks to Dr Fang Dong, Dr Qi-jing Bo, and all the doctors involved in recruiting the participants of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1432407/full#supplementary-material
1. Jauhar S, Johnstone M, Mckenna PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/S0140-6736(21)01730-X
3. Dziwota E, Stepulak M, Włoszczak-Szubzda A, Olajossy M. Social functioning and the quality of life of patients diagnosed with schizophrenia. Ann Agric Environ Med. (2018) 25:50–5. doi: 10.5604/12321966.1233566
4. Gebreegziabhere Y, Habatmu K, Mihretu A, Cella M, Alem A. Cognitive impairment in people with schizophrenia: an umbrella review. Eur Arch Psychiatry Clin Neurosci. (2022) 272:1139–55. doi: 10.1007/s00406-022-01416-6
5. Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. (2015) 16:620–31. doi: 10.1093/schbul/sbn045
6. Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. (2019) 18:146–61. doi: 10.1002/wps.20624
7. Okruszek A, Wichniak A, Jarkiewicz M, Schudy A, Gola M, Jednoróg K, et al. Social and nonsocial affective processing in schizophrenia — An ERP study. Int J Psychophysiol. (2016) 107:54–62. doi: 10.1016/j.ijpsycho.2016.06.007
8. Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. (2004) 44:389–400. doi: 10.1016/j.neuron.2004.09.027
9. Kvaran T, Sanfey AG. Toward an integrated neuroscience of morality: the contribution of neuroeconomics to moral cognition. Top Cognit Sci. (2010) 2:579–95. doi: 10.1111/j.1756-8765.2010.01086.x
10. Sarlo M, Lotto L, Manfrinati A, Rumiati R, Gallicchio G, Palomba D. Temporal dynamics of cognitive–emotional interplay in moral decision-making. J Cogn Neurosci. (2012) 24:1018–29. doi: 10.1162/jocn_a_00146
11. Siegel JZ, Crockett MJ. How serotonin shapes moral judgment and behavior. Ann N Y Acad Sci. (2013) 1299:42–51. doi: 10.1111/nyas.12229
12. Wischniewski J, Brune M. Moral reasoning in schizophrenia: an explorative study into economic decision making. Cognit Neuropsych. (2011) 16:348–63. doi: 10.1080/13546805.2010.539919
13. Greene JD. The rise of moral cognition. Cognition. (2015) 135:39–42. doi: 10.1016/j.cognition.2014.11.018
14. Fede SJ, Borg JS, Nyalakanti PK, Harenski CL, Cope LM, Sinnott-Armstrong W, et al. Distinct neuronal patterns of positive and negative moral processing in psychopathy. Cognit Affect Behav Neurosci. (2016) 16:1074–85. doi: 10.3758/s13415-016-0454-z
15. Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. J Abnorm Psychol. (2010) 119:863–74. doi: 10.1037/a0020979
16. Kandilarova S, Stoyanov DS, Paunova R, Todeva-Radneva A, Aryutova K, Maes M. Effective connectivity between major nodes of the limbic system, salience and frontoparietal networks differentiates schizophrenia and mood disorders from healthy controls. J Pers Med. (2021) 11:1110. doi: 10.3390/jpm11111110
17. Rodriguez V, Aas M, Vorontsova N, Trotta G, Gadelrab R, Rooprai NK, et al. Exploring the interplay between adversity, neurocognition, social cognition, and functional outcome in people with psychosis: A narrative review. Front Psychiatry. (2021) 12. doi: 10.3389/fpsyt.2021.596949
18. Cancel A, Comte M, Boutet C, Schneider, Rousseau P, Boukezzi S, et al. Childhood trauma and emotional processing circuits in schizophrenia: A functional connectivity study. Schizophr Res. (2017) 184:69–72. doi: 10.1016/j.schres.2016.12.003
19. Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Sci (American Assoc Advance Science). (2001) 293:2105–8. doi: 10.1126/science.1062872
20. de Achával D, Villarreal MF, Salles A, Bertomeu MJ, Costanzo EY, Goldschmidt M, et al. Activation of brain areas concerned with social cognition during moral decisions is abnormal in schizophrenia patients and unaffected siblings. J Psychiatr Res. (2013) 47:774–82. doi: 10.1016/j.jpsychires.2012.12.018
21. Sellaro R, Güroǧlu B, Nitsche MA, van den Wildenberg WP, Massaro V, Durieux J, et al. Increasing the role of belief information in moral judgments by stimulating the right temporoparietal junction. Neuropsychologia. (2015) 77:400–8. doi: 10.1016/j.neuropsychologia.2015.09.016
22. Benson AL. Morality of schizophrenic adolescents. J Abnorm Psychol. (1980) 89:674–7. doi: 10.1037//0021-843x.89.5.674
23. Kronbichler L, Stelzig-Schöler R, Lenger M, Weber S, Pearce BG, Reich LA, et al. Preserved intention understanding during moral judgments in schizophrenia. PloS One. (2021) 16:e0251180. doi: 10.1371/journal.pone.0251180
24. Mcguire J, Brüne M, Langdon R. Outcome-focused judgements of moral dilemmas in schizophrenia. Conscious Cogn. (2017) 52:21–31. doi: 10.1016/j.concog.2017.04.004
25. Mcguire J, Langdon R, Brüne M. Moral cognition in schizophrenia. Cognit Neuropsych. (2014) 19:495–508. doi: 10.1080/13546805.2014.928195
26. O’reilly K, O’connell P, Corvin A, O'Sullivan D, Coyle C, Mullaney R, et al. Moral cognition and homicide amongst forensic patients with schizophrenia and schizoaffective disorder: A cross-sectional cohort study. Schizophr Res. (2018) 193:468–9. doi: 10.1016/j.schres.2017.07.026
27. O’reilly K, O’connell P, O’sullivan D, Corvin A, Sheerin J, O'Flynn P, et al. Moral cognition, the missing link between psychotic symptoms and acts of violence: a cross-sectional national forensic cohort study. BMC Psychiatry. (2019) 19:408. doi: 10.1186/s12888-019-2372-4
28. Kahlbaum D. On heboidophrenia. History Psychiatry. (2002) 13:201–8. doi: 10.1177/0957154x0201305006
29. Decety J, Chen C, Harenski CL, Kiehl KA. Socioemotional processing of morally-laden behavior and their consequences on others in forensic psychopaths. Hum Brain Mapp. (2015) 36:2015–26. doi: 10.1002/hbm.22752
30. Békés V, Szabó D, Lévay EE, Salgó E, Unoka Z. Moral injury and shame mediate the relationship between childhood trauma and borderline personality disorder, PTSD, and complex PTSD symptoms in psychiatric inpatients. J Pers Disord. (2023) 37:406–23. doi: 10.1521/pedi.2023.37.4.406
31. Waikamp V, Serralta FB, Ramos-Lima LF, Zatti C, Freitas LHM. Relationship between childhood trauma, parental bonding, and defensive styles and psychiatric symptoms in adult life. Trends Psychiatry Psychother. (2021) 43:225–34. doi: 10.47626/2237-6089-2020-0086
32. Lin L, Cao B, Chen W, Li J, Zhang Y, Guo VY. Association of adverse childhood experiences and social isolation with later-life cognitive function among adults in China. JAMA Netw Open. (2022) 5:e2241714. doi: 10.1001/jamanetworkopen.2022.41714
33. Novaco RW, Chemtob CM. Anger and combat-related posttraumatic stress disorder. J Trauma Stress. (2002) 15:123–32. doi: 10.1023/a:1014855924072
34. Pettersson A, Modin S, Wahlström R, Af Winklerfelt Hammarberg S, Krakau I. The Mini-International Neuropsychiatric Interview is useful and well accepted as part of the clinical assessment for depression and anxiety in primary care: a mixed-methods study. BMC Fam Pract. (2018) 19:19. doi: 10.1186/s12875-017-0674-5
35. Wu BJ, Lan TH, Hu TM, Lee SM, Liou JY. Validation of a five-factor model of a Chinese Mandarin version of the Positive and Negative Syndrome Scale (CMV-PANSS) in a sample of 813 schizophrenia patients. Schizophr Res. (2015) 169:489–90. doi: 10.1016/j.schres.2015.09.011
36. Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. (2003) 27:169–90. doi: 10.1016/s0145-2134(02)00541-0
37. Shannon C, Douse K, Mccusker C, Feeney L, Barrett S, Mulholland C. The association between childhood trauma and memory functioning in schizophrenia. Schizophr Bull. (2011) 37:531–7. doi: 10.1093/schbul/sbp096
38. Aquino K, Reed AN. The self-importance of moral identity. J Pers Soc Psychol. (2002) 83:1423–40. doi: 10.1037/0022-3514.83.6.1423
39. Pechorro P, Barroso R, Poiares C, Oliveira JP, Torrealday O. Validation of the Buss-Perry Aggression Questionnaire-Short Form among Portuguese juvenile delinquents. Int J Law Psychiatry. (2016) 44:75–80. doi: 10.1016/j.ijlp.2015.08.033
40. Haidt J. The new synthesis in moral psychology. Science. (2007) 316:998–1002. doi: 10.1126/science.1137651
41. Zhao Y. Morality in everyday life: research on the moral foundations of the chinese. JiLin University, JiLin, China. (2021).
42. Asmal L, Kilian S, du Plessis S, Scheffler F, Chiliza B, Fouche JP, et al. Childhood trauma associated white matter abnormalities in first-episode schizophrenia. Schizophr Bull. (2019) 45:369–76. doi: 10.1093/schbul/sby062
43. Rokita KI, Dauvermann MR, Mothersill D, Holleran L, Holland J, Costello L, et al. Childhood trauma, parental bonding, and social cognition in patients with schizophrenia and healthy adults. J Clin Psychol. (2021) 77:241–53. doi: 10.1002/jclp.23023
44. Rokita KI, Holleran L, Dauvermann MR, Mothersill D, Holland J, Costello L, et al. Childhood trauma, brain structure and emotion recognition in patients with schizophrenia and healthy participants. Soc Cognit Affect Neurosci. (2020) 15:1336–50. doi: 10.1093/scan/nsaa160
45. Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: A meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. (2012) 38:661–71. doi: 10.1093/schbul/sbs050
46. Vila-Badia R, Butjosa A, Del Cacho N, Serra-Arumí C, Esteban-Sanjusto M, Ochoa S, et al. Types, prevalence and gender differences of childhood trauma in first-episode psychosis. What is the evidence that childhood trauma is related to symptoms and functional outcomes in first episode psychosis? A systematic review. Schizophr Res. (2021) 228:159–79. doi: 10.1016/j.schres.2020.11.047
47. Johnson DL. THE MORAL JUDGMENT OF SCHIZOPHRENICS. J nervous Ment Dis. (1960) 130:278–85. doi: 10.1097/00005053-196004000-00002
48. Mcguire J, Brüne M, Langdon R. Judgment of moral and social transgression in schizophrenia. Compr Psychiatry. (2017) 76:160–8. doi: 10.1016/j.comppsych.2017.04.008
49. Kohlberg L. Stages and aging in moral developmentsome speculations. Gerontologist (1973) 13(4):497–502. doi: 10.1093/geront/13.4.497
50. Ma HK. The moral development of the child: an integrated model. Front Public Health. (2013) 1:57. doi: 10.3389/fpubh.2013.00057
51. Bobo doll experiment | Description, Methodology, Results, & Fact (2023). Available online at: https://www.britannica.com/event/Bobo-doll-experiment (Accessed September 22, 2024).
52. Chong LS, Gordis E, Hunter L, Amoh J, Strully K, Appleton AA, et al. Childhood violence exposure and externalizing behaviors: A systematic review of the role of physiological biomarkers. Psychoneuroendocrinology. (2022) 145:105898. doi: 10.1016/j.psyneuen.2022.105898
53. Verrill A. The relationship between childhood abuse and aggressive behavior in adulthood. J Interdiscip Undergrad Res. (2018) 10.
54. Altschul S. Attachment and loss, vol. 3. Loss, sadness and depression. By john bowlby. J Am Psychoanal Assoc. (1984) 32:216–8. doi: 10.1177/000306518403200125
55. Culatta E, Clay-Warner J, Boyle KM, Oshri A. Sexual revictimization: A routine activity theory explanation. J Interpers Violence. (2020) 35:2800–24. doi: 10.1177/0886260517704962
56. Pittenger SL, Pogue JK, Hansen DJ. Predicting sexual revictimization in childhood and adolescence: A longitudinal examination using ecological systems theory. Child Maltreat. (2018) 23:137–46. doi: 10.1177/1077559517733813
57. Walker HE, Wamser-Nanney R. Revictimization risk factors following childhood maltreatment: A literature review. Trauma Violence Abuse. (2023) 24:2319–32. doi: 10.1177/15248380221093692
58. Wöller W. Trauma repetition and revictimization following physical and sexual abuse. Fortschr Neurol Psychiatr. (2005) 73:83–90. doi: 10.1177/1077559517733813
59. Bilgi MM, Taspinar S, Aksoy B, Oguz K, Coburn K, Gonul AS. The relationship between childhood trauma, emotion recognition, and irritability in schizophrenia patients. Psychiatry Res. (2017) 251:90–6. doi: 10.1016/j.psychres.2017.01.091
60. Powers A, Fani N, Cross D, Ressler KJ, Bradley B. Childhood trauma, PTSD, and psychosis: Findings from a highly traumatized, minority sample. Child Abuse Negl. (2016) 58:111–8. doi: 10.1016/j.chiabu.2016.06.015
61. Gentner MB, Leppert M. Environmental influences on health and development: nutrition, substance exposure, and adverse childhood experiences. Dev Med Child Neurol. (2019) 61:1008–14. doi: 10.1111/dmcn.14149
62. Miguel PM, Pereira LO, Silveira PP, Meaney MJ. Early environmental influences on the development of children’s brain structure and function. Dev Med Child Neurol. (2019) 61:1127–33. doi: 10.1111/dmcn.14182
63. Longden E, Read J. Social adversity in the etiology of psychosis: A review of the evidence. Am J Psychother. (2016) 70:5–33. doi: 10.1176/appi.psychotherapy.2016.70.1.5
64. Read J, Perry BD, Moskowitz A, Connolly J. The contribution of early traumatic events to schizophrenia in some patients: A traumagenic neurodevelopmental model. Psych: Interperson Biol Process. (2001) 64:319–45. doi: 10.1521/psyc.64.4.319.18602
65. Reese M, Bryant D, Ethridge L. Biomarkers for moral cognition: Current status and future prospects for neurotransmitters and neuropeptides. Neurosci Biobehav Rev. (2020) 113:88–97. doi: 10.1016/j.neubiorev.2020.03.009
66. Coccaro EF, Lee R, Mccloskey M. Norepinephrine function in personality disorder: plasma free MHPG correlates inversely with life history of aggression. CNS Spect. (2003) 8:731–6. doi: 10.1017/s1092852900019106
67. Ryding E, Lindström M, Träskman-Bendz L. The role of dopamine and serotonin in suicidal behaviour and aggression. Prog Brain Res. (2008) 172:307–15. doi: 10.1016/s0079-6123(08)00915-1
68. Soyka M. Neurobiology of aggression and violence in schizophrenia. Schizophr Bull. (2011) 37:913–20. doi: 10.1093/schbul/sbr103
69. Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv Genet. (2011) 75:151–69. doi: 10.1016/b978-0-12-380858-5.00005-8
Keywords: childhood trauma, moral cognition, schizophrenia, CTQ, MFQ
Citation: Peng X, Ding Y-s, Ren B, Zhao X-x, Wang F-f, Zhao J, Zhang Y-y, Zhang X-j, Zhou F-c and Wang C-y (2024) The effect of childhood trauma on moral cognition in patients with schizophrenia. Front. Psychiatry 15:1432407. doi: 10.3389/fpsyt.2024.1432407
Received: 14 May 2024; Accepted: 10 September 2024;
Published: 30 September 2024.
Edited by:
Tianhong Zhang, Shanghai Jiao Tong University, ChinaReviewed by:
Wei Zheng, Guangzhou Medical University, ChinaCopyright © 2024 Peng, Ding, Ren, Zhao, Wang, Zhao, Zhang, Zhang, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fu-chun Zhou, ZnJhbmtjaG93QGNjbXUuZWR1LmNu; Xiu-jun Zhang, emh4akBuY3N0LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.