- 1Department of Internal Medicine, Shaheed Mohtarma Benazir Bhutto Medical College Lyari, Karachi, Pakistan
- 2Department of Internal Medicine, Dow University of Health Sciences, Karachi, Pakistan
- 3Department of Internal Medicine, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, United States
Introduction: Major depressive disorder (MDD), postpartum depression (PPD), and insomnia are neuropsychological conditions in which zuranolone is used to improve symptoms and prognosis of the disorder. This meta-analysis aimed to determine the efficacy of zuranolone in comparison to other drugs used for treating these conditions.
Methods: This meta-analysis included patients aged between 18 and 75 years who were diagnosed with major depressive disorder and postpartum depression with or without insomnia and were administered zuranolone for treatment. Only randomized controlled trials (RCTs) were included, and animal studies were excluded. The databases used were PubMed, Scopus, Cochrane, and Clinicaltrials.gov, with MeSH terms and relevant keywords for (Zuranolone) and (Depression). The Cochrane risk of bias tool was used for quality assessment.
Results: The meta-analysis included eight RCTs that analyzed data from 2031 patients. The meta-analysis revealed statistically significant changes in the Hamilton Depression Rating Scale (HAM-D), Montgomery-Åsberg Depression Rating Scale (MADRS), Hamilton Anxiety Rating Scale (HAM-A), and treatment-emergent adverse effects (TEAE) scores in the PPD subgroup. HAM-D and TEAEs scores were also significant in the MDD subgroup, but the changes in the MADRS, HAM-A, and Bech-6 scores were insignificant. Serious adverse events were insignificant in all subgroups.
Conclusion: Meta-analysis found a significant improvement in depressive symptoms with zuranolone treatment, especially on day 15. This suggests that zuranolone is a promising therapeutic option for patients with MDD and PPD with or without insomnia.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=459554, identifier CRD42023459554.
Introduction
Major Depressive Disorder (MDD) is a psychiatric condition characterized by persistent feelings of sadness and hopelessness, often accompanied by other symptoms such as changes in appetite, sleep disturbances, loss of interest in activities, and cognitive impairments. According to the World Health Organization, MDD is one of the most prevalent mental disorders worldwide, affecting over 300 million people of all ages (1). In the United States, it is estimated that 8.9 million adults had MDD in 2021, making it a leading cause of disability (1–3). The exact cause of MDD is not fully understood, but it is believed to be a result of complex interactions between various risk factors (4). Another mood disorder that can present with depressed mood is postpartum depression (PPD), which affects approximately 15% of new mothers after childbirth (5, 6). Factors that increase the risk of PPD include a history of depression, exposure to violence, obesity, and poor sleep quality after childbirth (7). Both MDD and PPD can also manifest as insomnia, defined as difficulty falling asleep or maintaining sleep (8). It is estimated that 10% of the global adult population suffers from chronic insomnia, while an additional 20% experience occasional symptoms (9, 10). Insomnia is closely associated with depression, and it is believed to exacerbate symptoms of depression and increase the risk of self-harm or suicide (11). Depression can also lead to insomnia by reducing the latency of REM sleep, which disrupts the body’s natural sleep-wake cycle (12). There are several options for treating MDD, such as benzodiazepines, Selective Serotonin Reuptake Inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors (SNRIs). Benzodiazepines promote the release of the neurotransmitter gamma-aminobutyric acid (GABA), which inhibits nerve activity in the brain. SSRIs decrease the reuptake of serotonin, resulting in the alleviation of MDD symptoms (13–15). SNRIs inhibit the uptake of monoamines within the synaptic cleft, which ultimately leads to improvement (16). Mirtazapine is beneficial in treating MDD by blocking presynaptic alpha 2 adrenergic receptors (17).
Another class of drugs, known as neuroactive steroids, has gained significant attention in addressing these conditions. Brexanolone, in its intravenous form, and allopregnanolone are essential in treating PPD by modulating gamma-aminobutyric acid (GABAa) receptors, resulting in reduced anxiety levels and improved sleep quality (18, 19). While these drugs have demonstrated promise in treating MDD, they also have numerous adverse effects. Benzodiazepines, for instance, can cause cognitive impairment, hyperexcitability, insomnia, anxiety, and seizures upon withdrawal. In contrast, approximately 30% of patients show no response to selective serotonin reuptake inhibitors (SSRIs) (20, 21). Zuranolone, a synthetic neuroactive steroid, increases GABA release by positive allosteric modulation of GABA receptors, showing potential for treating MDD and PPD (22–24). A study by Maximos et al. revealed that zuranolone improved PPD symptoms in adults and had beneficial effects on their insomnia symptoms (23). Another study demonstrated that zuranolone effectively relieved insomnia symptoms in females with anxiety and PPD (25).
Our systematic review and meta-analysis aim to determine whether zuranolone is a more effective treatment option for patients with PPD and MDD, with or without insomnia. This information can help physicians identify alternative treatment strategies for patients with MDD and PPD who may not respond to or experience severe side effects from conventional treatments. This study can prove beneficial for patients with MDD and PPD in assessing the efficacy of zuranolone in treating these disorders in individuals with concurrent insomnia symptoms.
Methods
Protocol and registration
This systematic review and meta-analysis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26). Prior to the initiation of the study, it was registered in the International Prospective Register of Systematic Reviews (PROSPERO) registry under the credentials CRD42023459554 (27).
Search strategy
An extensive search of electronic databases, including PubMed, Scopus, Clinicaltrials.gov, PsychINFO, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL), was performed from inception until September 2023. No restrictions were imposed on time or sample size during the literature search. The following keywords were used in the search strategy along with relevant MeSH terms and Boolean operators: “Zuranolone” OR “SAGE-217” AND “depression” OR “major depressive disorder” AND “Postpartum depression.” The detailed search strategy is presented in Supplementary Table 1. Furthermore, the bibliometrics of the published articles on related topics and conference proceedings were examined to ensure that no articles were overlooked.
Study selection
All articles retrieved from the search results were entered into Rayyan.ai, an online systematic review management platform (28). After removing duplicates, two investigators (S. A. and M.B.A. S.) independently screened the remaining studies in accordance with the specified inclusion and exclusion criteria. The initial screening process involved evaluating titles and abstracts to identify potentially eligible studies, which were then followed by a full-text assessment to ensure relevance based on our predetermined criteria. Any conflicts or disagreements were resolved through a consensus process or with the assistance of a third reviewer (L. M.). Studies were selected based on the following Population, Intervention, Control, and Outcomes (PICO) criteria: randomized clinical trials with parallel groups, population aged 18–75 years diagnosed with major depressive disorder (MDD) or postpartum depression (PPD) with or without insomnia, and intervention with zuranolone for treatment compared to a control group receiving placebo. We included studies reporting outcomes such as changes from baseline in the Hamilton Depression Rating Scale (HAM-D), Montgomery–Åsberg Depression Rating Scale (MADRS), and Hamilton Anxiety Rating Scale (HAM-A). Non-randomized trials, quasi-randomized trials, animal experiments, chemistry or cell line studies, conference abstracts, comments, editorials, review articles, meta-analyses, case reports, and case series were excluded, as were publications that reported duplicate data or were written in languages other than English.
Data extraction
Data extraction was performed by two authors: (A. R. and S.R.) In the event of disagreement, a third reviewer, M. A., was consulted to reach a consensus. Information on the title, author, year of publication, and demographics, including age, sex, and sample size in each group, was extracted meticulously. Moreover, relevant characteristics, such as the HAM-D score, dosage, length of treatment, status and degree of treatment-resistant depression, treatment strategy, adverse events, and general methodological details, including study arms and crossover trials, were also included. Data on treatment outcomes, including the post-HAM-D score, post Bech-6 score, MADRS, and post-HAM-A scores, were extracted from the included studies.
Quality assessment
Two independent authors, R. K. and A. R., assessed the risk of bias in the included studies using the Cochrane Risk of Bias (RoB 2.0) tool for randomized controlled trials (RCTs) (29). The RoB 2.0 addressed five specific domains: (1) Bias arising from the randomization process; (2) Bias due to deviations from the intended intervention; (3) Bias due to missing outcome data; (4) Bias in the measurement of the outcome; and (5) Bias in the selection of the reported results. This tool was applied to each included study, and the studies were classified as having a high, moderate, or low risk of bias based on the RoB classification. A third review author, H. A. U. R., was consulted to resolve any disagreements concerning the ROB assessment.
Statistical analysis
Statistical analysis was conducted using a random-effects model to pool the Mean Differences (MDs) and Risk Ratios (RRs) for continuous and dichotomous outcomes, respectively, with 95% Confidence Intervals (CIs). The pooled results were graphically represented in forest plots, and the I2 statistic was used to assess the heterogeneity across studies. An I2 statistic of > 50% was considered significant, indicating high heterogeneity, whereas a value of less than 50% indicated low heterogeneity. The I2 values were interpreted according to the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions. Statistical significance was set at P < 0.05. Funnel plots were used to visually assess publication bias, and subgroup analyses were performed to identify sources of heterogeneity in clinical outcomes. The subgroups included patients with 1) major depressive disorder, 2) postpartum depression, and 3) Insomnia in patients with major depressive disorder or postpartum depression. Statistical analysis was conducted using Review Manager (RevMan, Version 5.4.1; The Cochrane Collaboration, Copenhagen, Denmark).
Results
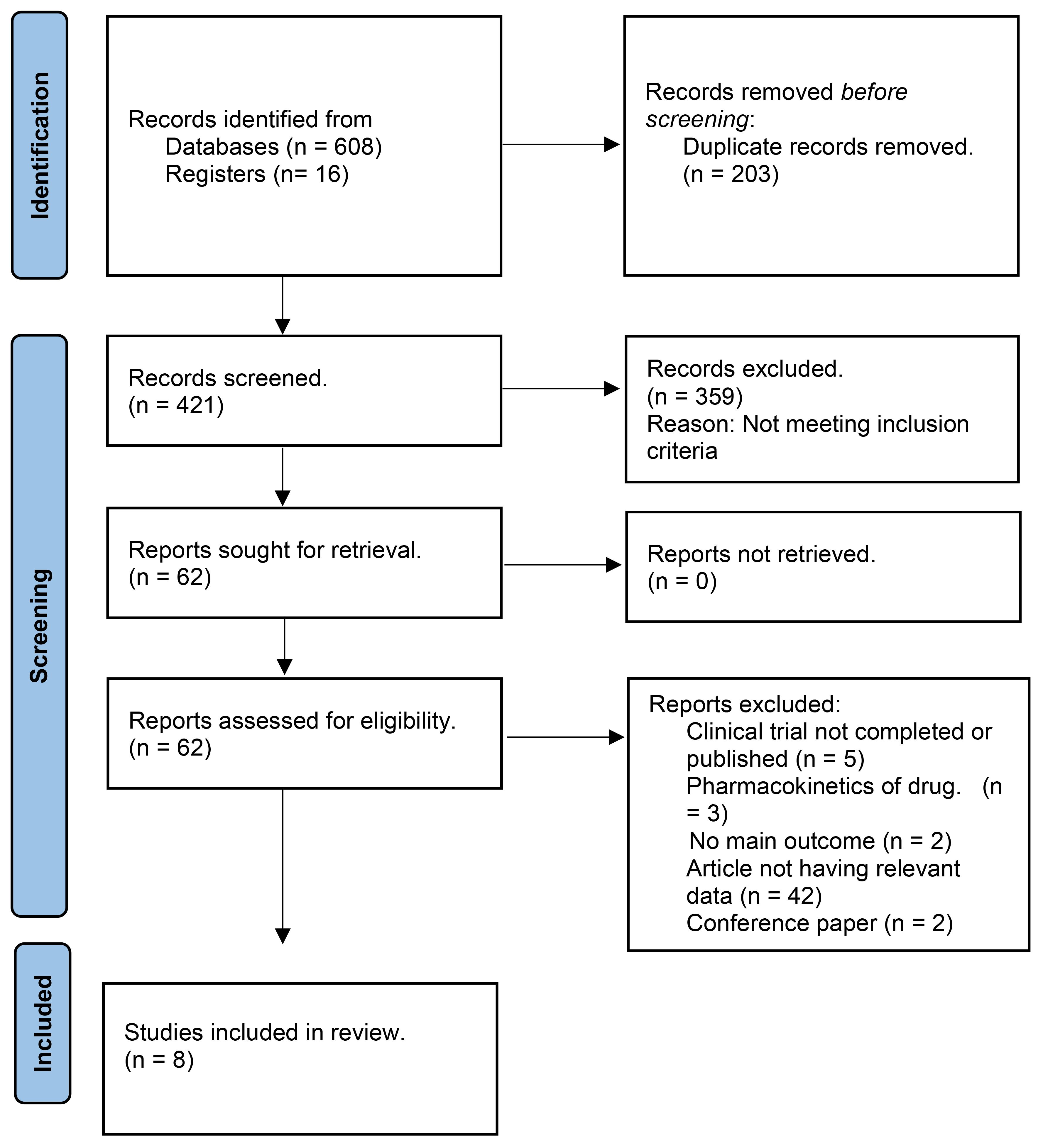
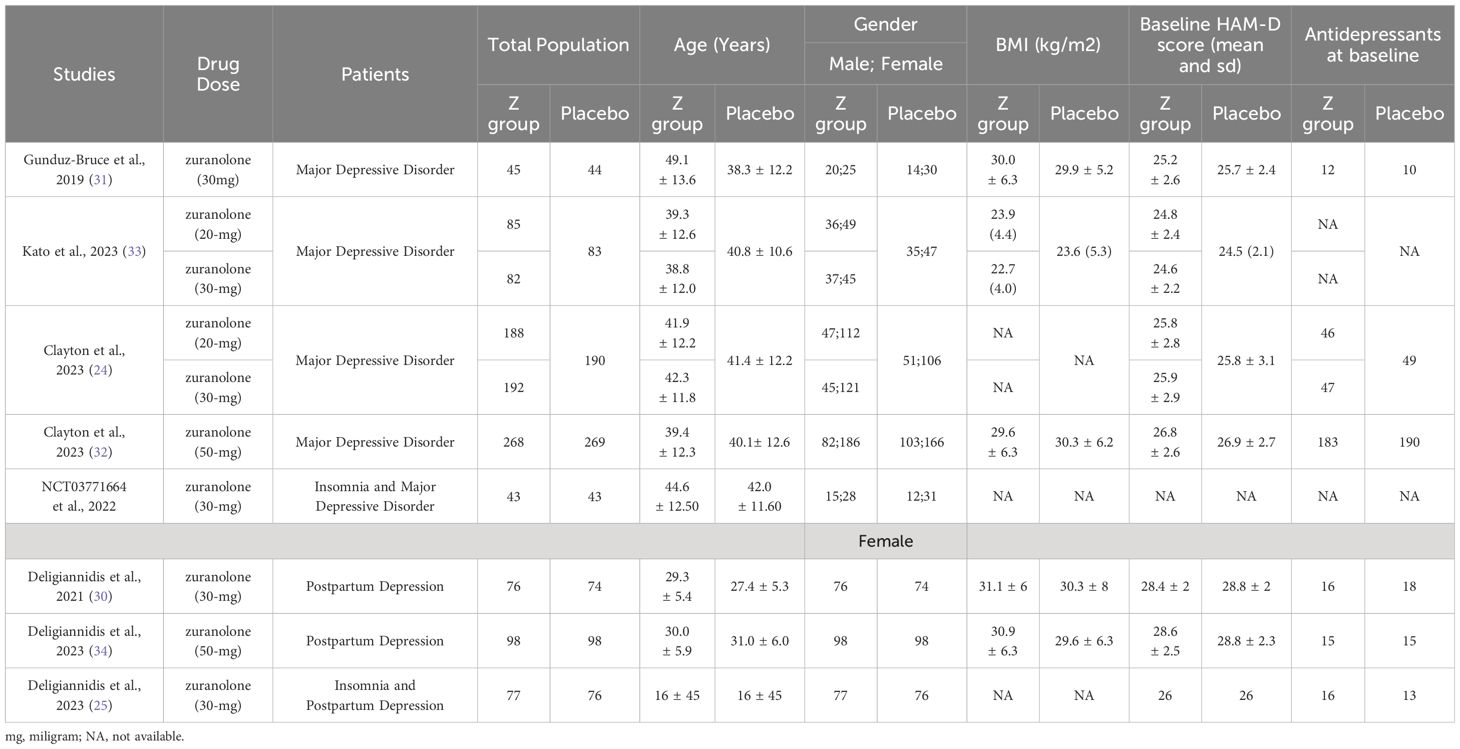
A total of 624 studies were obtained, following which 8 RCTs were selected for inclusion in our review after excluding duplicates, reviews, and ineligible articles through Primary and Secondary screening (24, 25, 30–35). The PRISMA flowchart (Figure 1) provides a summary of the literature screening process. Our systematic review and meta-analysis included 8 studies that reported data on 2,031 patients. The characteristics of the included studies are presented in Table 1, which also indicates that all studies included had a low-to-moderate risk of bias. A detailed quality assessment of the included studies is illustrated in Figures 2A, B and detailed reasons are provided in Supplementary Table 2.
Primary outcome
Change from baseline in HAM-D score
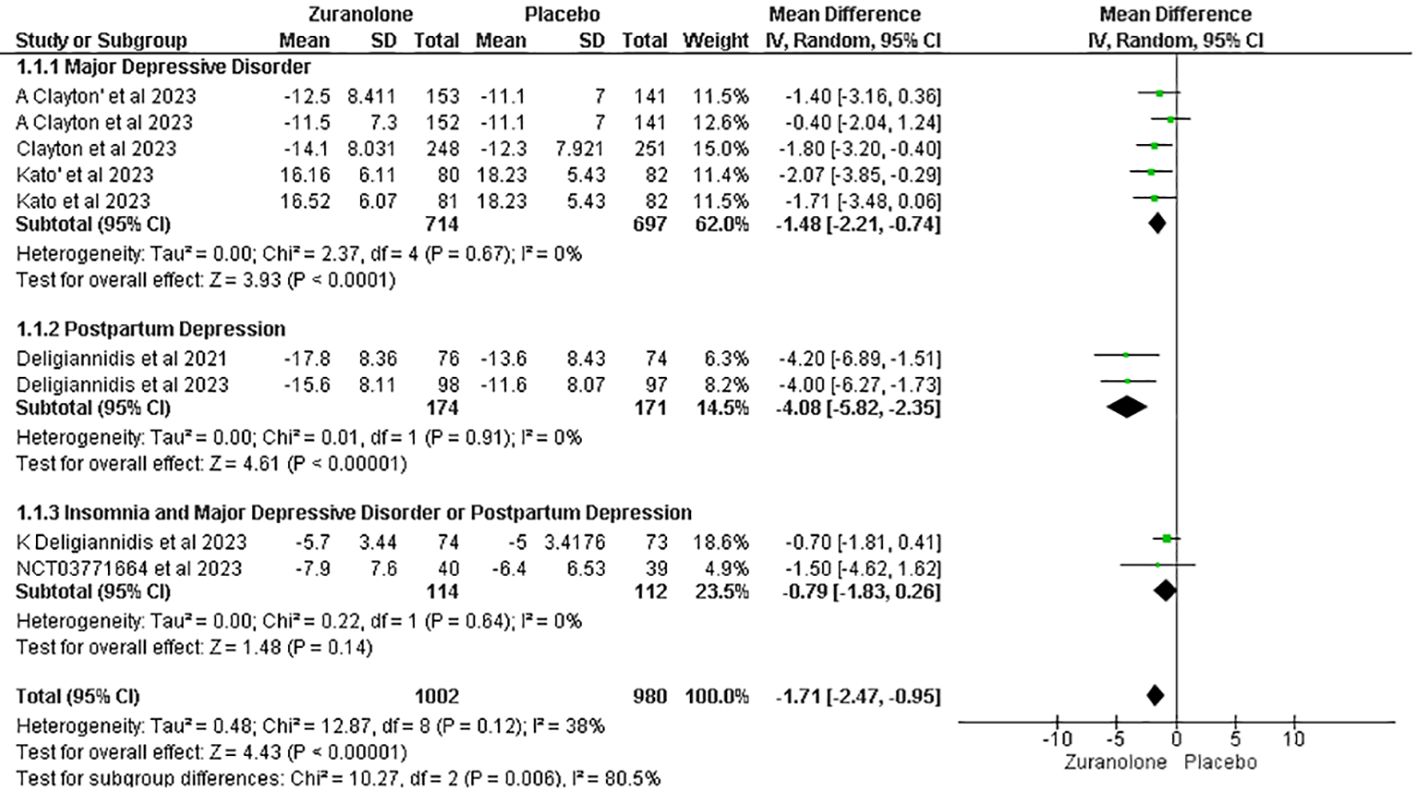
Seven studies were assessed to determine the relationship between changes in HAM-D scores and the use of zuranolone. The results indicated a significant decrease in the HAM-D score in zuranolone consumers compared to the placebo group (MD: -1.71; 95% CI= [-2.47, -0.95]; p<0.00001; I2 = 38%). A consistent finding was observed in the subgroup analysis for the MDD (MD: -1.48; 95% CI [-2.21, -0.74]; p<0.0001; I2 = 0%) and PPD subgroup (MD: -4.08; 95% CI [-5.82, -2.35]; p<0.00001; I2 = 0%), except for patients with insomnia and MDD or PPD (MD: -0.79; 95% CI [-1.83, 0.26]; p=0.14; I2 = 0%) (Figure 3).
Secondary outcomes
Change from baseline in MADRS score
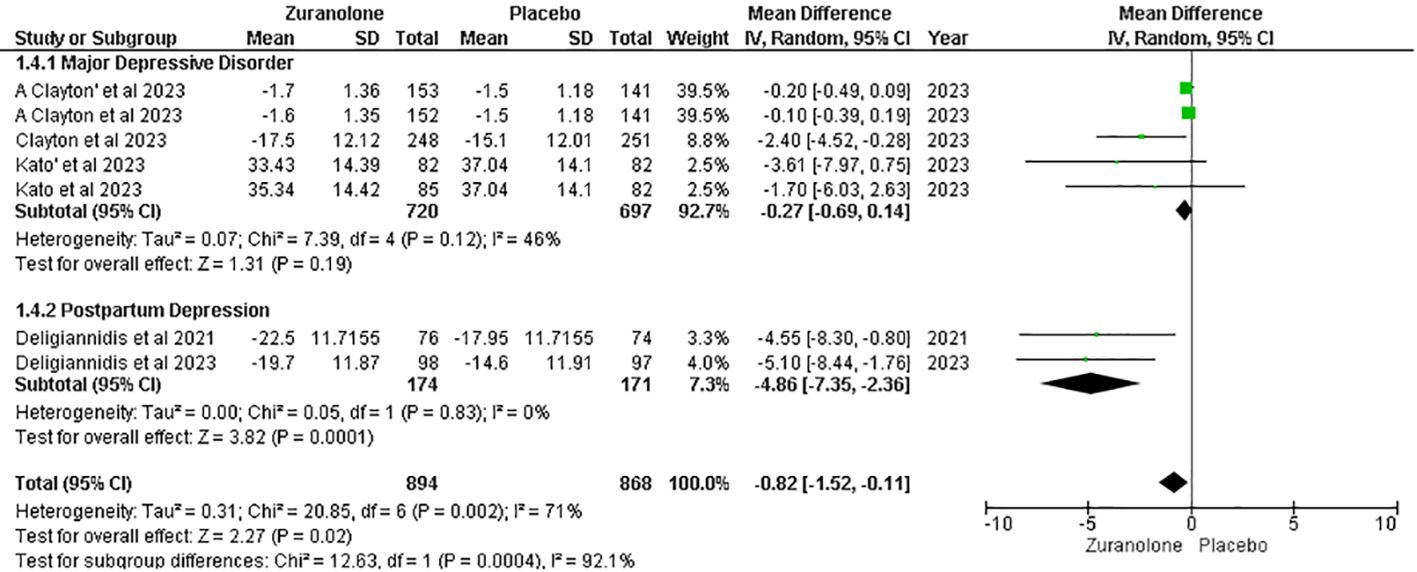
Five studies were evaluated to determine the impact of zuranolone on MADRS scores, which demonstrated a decrease in MADRS scores compared to placebo (MD=-0.82; 95% CI= [-1.52, -0.11]; p=0.02; I2 = 71%). A subgroup analysis revealed significant results within the PPD subgroups (MD=-4.86; 95% CI= [-7.35, -2.36]; p=0.0001; I2 = 0). However, no significant findings were observed in the MDD subgroup (MD= -0.27; 95% CI= [-0.69, 0.14]; p=0.12; I2 = 46%) (Figure 4).
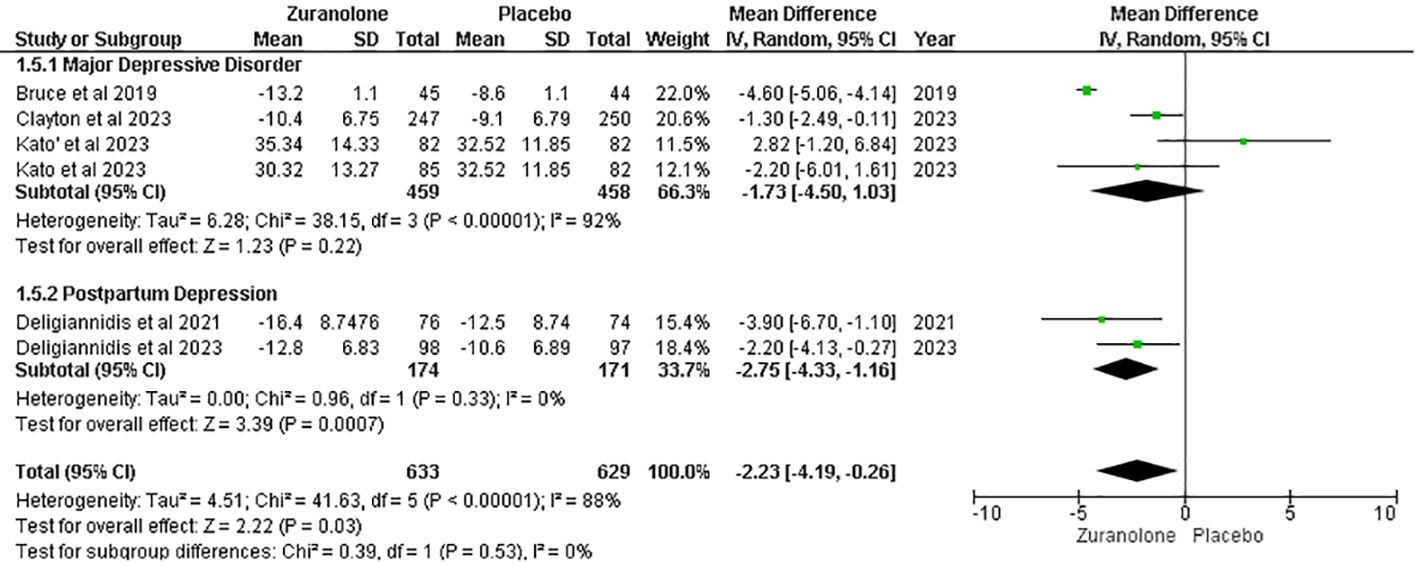
Change from baseline in HAM-A score
Five studies were evaluated to determine the impact of zuranolone on HAM-A scores, which showed a significant overall reduction in HAM-A scores in patients administered zuranolone compared with placebo (MD=-2.23; 95% CI= [-4.19, -0.26]; p=0.03; I2 = 88%). A subgroup analysis showed significant results in the PPD subgroup (MD -2.75; 95% CI= [-4.33, -1.16]; p=0.0007; I2 = 0%). However, no significant reduction was observed in the MDD subgroup (MD=-1.73; 95% CI= [-4.50, 1.03]; p=0.22; I2 = 92%) (Figure 5).
The leave-one-out sensitivity analysis showed that the reduction in the HAM-A score was affected by a single study by Bruce et al. (12). Removing that study resulted in a significant reduction in I2 values (p = 0.09; I2 = 50%) and overall effect [MD = −1.65, 95% CI (−3.17, −0.14), p= 0.03] (Supplementary Figure 1).
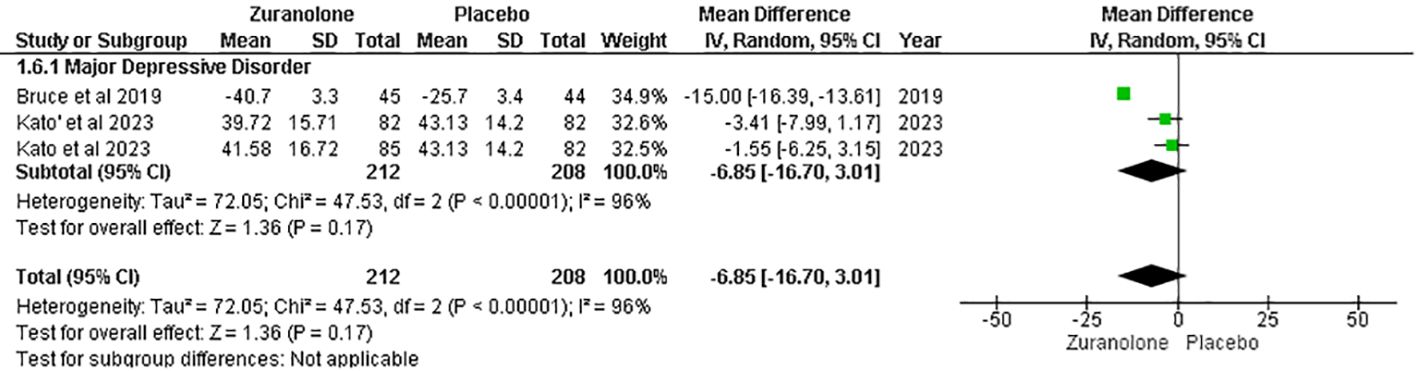
Change from baseline in Bech-6 score
Two studies were analyzed for the impact of Zuranolone on the Bech-6 score. The results showed a non-significant difference between zuranolone and placebo in reducing the Bech-6 score (MD=-6.85; 95% CI= [-16.70, 3.01]; p=0.17; I2 = 96%). (Figure 6) The leave-one-out sensitivity analysis indicated that the Bech-6 score was influenced by a single study, i.e., Bruce et al. (12). When this study was removed, the I2 values showed a significant reduction in overall effect [MD = −2.50, 95% CI (−5.78, 0.78), p = 0.13] (Supplementary Figure S2).
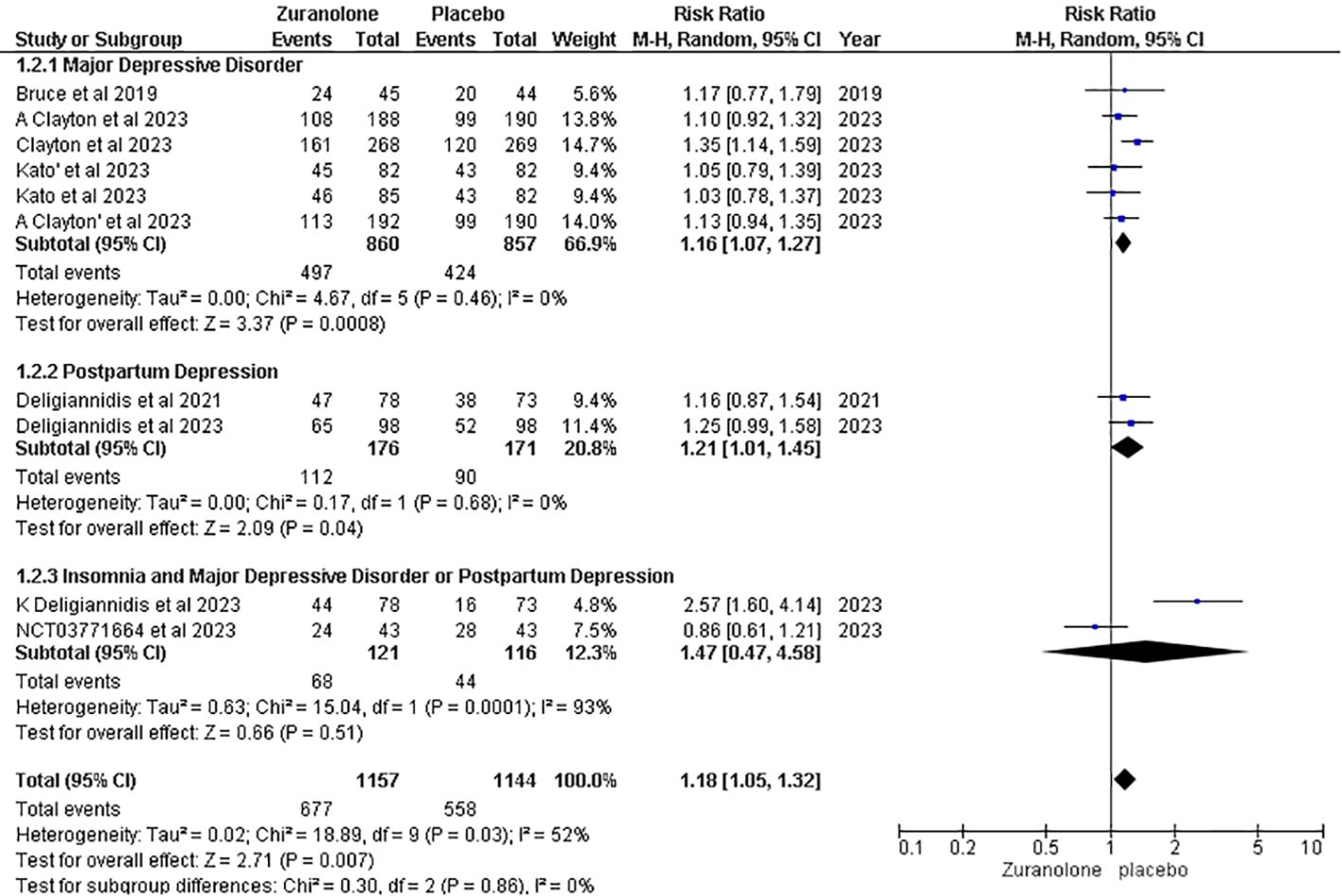
Treatment-emergent adverse effects
Eight studies were included for the outcome of TEAEs, and the overall pooled result demonstrated that placebo was associated with fewer TEAEs than Zuranolone. (RR: 1.18, 95% CI [1.05, 1.32], p=0.007; I2 = 52%). After conducting a subgroup analysis, it was found that TEAEs were significantly higher in the Zuranolone group than in the placebo group for MDD and PPD. (RR: 1.16, 95% CI [1.16, 1.27]; p=0.0008, I2 = 0%) and (RR: 1.21, 95% CI [1.01, 1.45]; p=0.04; I2 = 0%) respectively. However, the insomnia subgroup and MDD or PPD did not show a significant association. (RR: 1.47, 95% CI [0.47, 4.58]; p=0.51; I2 = 52%) (Figure 7).
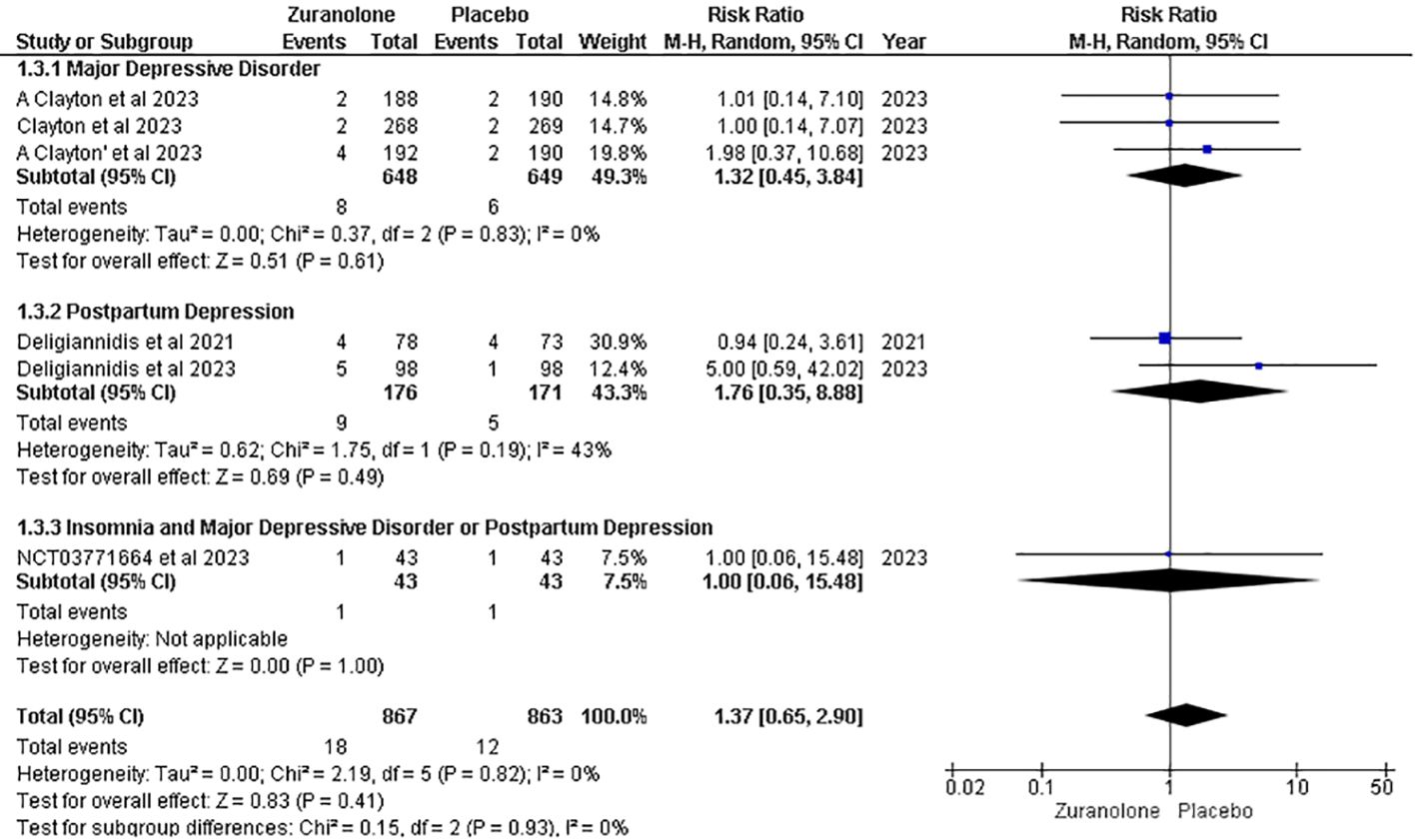
Serious adverse effects
Five studies were included in the analysis of serious adverse effects (AEs), and the overall pooled result showed no significant association between the groups (RR, 1.37; 95% CI [0.65, 2.90], p=0.41; I2 = 0%). Following a subgroup analysis, insignificant findings were revealed for the MDD (RR: 1.32; 95% CI [0.45, 3.84]; p = 0.61; I2 = 0%), PD (RR: 1.76; 95% CI [0.35, 8.88]; p = 0.49; I2 = 43%), and insomnia with MDD or PD (RR: 1.00; 95% CI [0.06, 15.48]; p = 1.00) groups (Figure 8).
Meta-regression
We assessed mean age, female sex percentage, and body mass index (BMI) as potential covariates that could affect the effect size on our primary outcome and change from baseline in the HAM-D score. Female sex percentage and BMI showed a non-significant association; however, mean age was found to have a significant effect on the HAM-D score. The results are as follows: mean age, Coeff: 0.0270, p=0.0099; female sex percentage, Coeff: -0.0050, p=0.0563; BMI, Coeff: -0.0034, p=0.8616 (Supplementary Figures 3A–C).
Discussion
The purpose of this meta-analysis was to investigate the safety and efficacy of zuranolone in the treatment of postpartum depression, insomnia, and major depressive disorders. Our analysis included eight studies comparing zuranolone with a placebo and focused on six outcomes (24, 25, 30–35). The primary outcome measure was the change in the Hamilton Depression Rating Scale, while the secondary outcomes included the Montgomery Asberg Depression Rating Scale, Hamilton Anxiety Scale, Bech Scale, treatment-emergent side effects, and serious adverse effects. Although our primary outcome and some secondary outcomes showed significant improvements favoring zuranolone, these two outcomes did not reach statistical significance. However, the overall results favored drug use. However, due to the low certainty of the evidence, we approached the findings cautiously, emphasizing the need for additional studies to strengthen the reliability of the results. In summary, our meta-analysis suggests potential benefits of zuranolone in the treatment of postpartum depression, major depressive disorder, and insomnia. Specifically, we observed improvements in HAM-D scores on day 15 with zuranolone compared with placebo, along with reductions in HAM-A and MADRS scores. Nevertheless, zuranolone users experienced mild treatment-related adverse effects.
Depression has been treated with a variety of medications, each presenting a unique balance between efficacy and safety considerations (20). These medications are among the most prescribed for depression, but their efficacy can be limited in certain cases. Tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) are effective but have notable side effects and carry the risk of toxic reactions if overdosed (36). In contrast, newer drugs such as ketamine have shown promise in treating treatment-resistant depression and reducing suicidal behavior (37). However, concerns regarding its long-term use and potentially severe side effects have tempered enthusiasm. Psilocybin, another emerging option, appears promising for alleviating depression, anxiety, and insomnia (12). However, limited research has hampered broader acceptance (38). These concerns and the quest for more effective and safer treatments culminated in the development of zuranolone, which received FDA approval in 2023 (39). Zuranolone, which acts on GABA receptors, stands out for its rapid onset of action and efficacy in relieving depressive symptoms (6, 40). It helps in the orientation of neuronal circuits and organizes brain function, which is specifically linked to mood and behavior (41).
However, there are concerns regarding its long-term use and safety in the context of MDD; consequently, it has not been approved for use in treating MDD (42, 43). Studies have assessed the efficacy of zuranolone under various conditions. For example, in postpartum depression, significant improvements in the scores of the Hamilton Rating Scale for Depression (HAM-D), the Montgomery-Åsberg Depression Rating Scale (MADRS), and the Hamilton Rating Scale for Anxiety (HAM-A) were observed within a short treatment duration of 14 days, although accompanied by notable adverse effects (34, 44). Additionally, zuranolone has demonstrated benefits in individuals with major depressive disorder by enhancing neural circuits and overall brain function (45). It has also been effective in addressing insomnia in conjunction with postpartum depression, leading to improvements in sleep quality (46, 47). However, the use of zuranolone is associated with side effects, including a higher risk compared with a placebo, as indicated in certain studies (34, 46). Concerns regarding the potential suicide risk and impairment in daily activities have also been raised. Nevertheless, conflicting findings exist, with some studies reporting no significant increase in suicidal ideation, although noting other adverse effects (48). These complexities underscore the ongoing need for comprehensive research and cautious consideration of the benefits and risks of zuranolone in clinical practice.
The levels of zuranolone in the blood were dependent on the dose, with the highest levels observed in the 30 mg dosage group. These dosages were chosen based on safety and tolerability assessments from a prior phase 1 trial involving healthy Japanese individuals, including older adults, where the 30 mg dose showed promising results. Interestingly, both healthy Japanese and White adults had plasma exposure levels that were comparable to those of zuranolone. Furthermore, a phase 2 study conducted in the US on individuals with MDD revealed a significant reduction in depressive symptoms with a 30 mg daily dose of zuranolone over 14 days without encountering any serious safety or tolerability concerns (33, 49). Additionally, a meta-analysis showed that increasing the dose of zuranolone correlated with symptom improvement, although this was accompanied by an increased risk of side effects (34). This finding was supported by another study that demonstrated the dose-dependent efficacy of zuranolone while noting a non-significant association between higher doses and severe adverse effects, consistent with our study’s observations (50). These results were favorable when zuranolone was used for 14 days; however, when the drug was used for a similar duration throughout the year, it led to mild to moderate side effects, which were more common in patients who used 30 mg compared with those who took 50 mg (51).
Limitations
Despite the insights provided by our study, it has several limitations. First, the limited availability of recent studies resulted in small sample sizes, which could compromise the reliability and accuracy of our findings. Second, insufficient data prevented us from considering preexisting medical conditions that may affect drug efficacy and safety. Lastly, we could not explore the potential interactions between the drug and pretreatment medications, which could influence its efficacy.
Conclusion
In summary, this meta-analysis was conducted to evaluate the effectiveness of zuranolone in treating MDD and PPD with or without insomnia. The results unequivocally showed a statistically significant improvement in depressive symptoms with the use of zuranolone, which was particularly pronounced on day 15. These findings underscore the potential of zuranolone as a promising therapeutic option for individuals with MDD and PPD with or without insomnia. However, further randomized controlled trials and observational studies are required to gain a deeper understanding of the efficacy of zuranolone in treating MDD and PPD with or without insomnia. It would be highly beneficial if future research included a larger sample size and information about new scales and outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
AR: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. SA: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Data curation, Conceptualization. MBAS: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Investigation. SLM: Writing – review & editing, Writing – original draft, Validation, Resources, Project administration, Methodology, Investigation, Conceptualization. RK: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Investigation. MA: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. SR: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. SBA: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Data curation, Conceptualization. HAUR: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Conceptualization. FD: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Methodology, Investigation, Formal analysis. MA: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Project administration, Methodology, Investigation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1425295/full#supplementary-material.
References
1. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2. doi: 10.1038/nrdp.2016.65
2. Trivedi MH. Major depressive disorder in primary care: strategies for identification. J Clin Psychiatry. (2020) 81. doi: 10.4088/JCP.UT17042BR1C
3. Zhdanava M, Pilon D, Ghelerter I, Chow W, Joshi K, Lefebvre P, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. (2021) 82. doi: 10.4088/JCP.20m13699
5. Stewart DE, Vigod SN. Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annu Rev Med. (2019) 70:183–96. doi: 10.1146/annurev-med-041217-011106
6. Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. (2009) 200:357–64. doi: 10.1016/j.ajog.2008.11.033
7. Zhao X, Zhang Zh. Risk factors for postpartum depression: An evidence-based systematic review of systematic reviews and meta-analyses. Asian J Psychiatr. (2020) 53. doi: 10.1016/j.ajp.2020.102353
8. Kroska EB, Stowe ZN. Postpartum depression: identification and treatment in the clinic setting. Obstet Gynecol Clin North Am. (2020) 47:409–19. doi: 10.1016/j.ogc.2020.05.001
9. Morin CM, Jarrin DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. (2022) 17:173–91. doi: 10.1016/j.jsmc.2022.03.003
10. Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. (2016) 5:780. doi: 10.4103/2249-4863.201153
11. Nutt DJ, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. (2008) 10:329–36. doi: 10.31887/DCNS.2008.10.3/dnutt
12. Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. (1992) 49:651–68. doi: 10.1001/archpsyc.1992.01820080059010
13. Davidson JRT. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. (2010) 71 Suppl E1. doi: 10.4088/JCP.9058se1c.04gry
15. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. (1998) 51:215–35. doi: 10.1016/S0165-0327(98)00221-3
16. Fanelli D, Weller G, Liu H. New serotonin-norepinephrine reuptake inhibitors and their anesthetic and analgesic considerations. Neurol Int. (2021) 13:497–509. doi: 10.3390/neurolint13040049
18. Kargbo RB. Neurosteroids and postpartum depression: the mechanism, efficacy, and approval of brexanolone and zurzuvae. ACS Med Chem Lett. (2023) 14:1326–8. doi: 10.1021/acsmedchemlett.3c00388
20. Vgontzas AN, Kales A, Bixler EO. Benzodiazepine side effects: role of pharmacokinetics and pharmacodynamics. Pharmacology. (1995) 51:205–23. doi: 10.1159/000139363
22. Cutler AJ, Mattingly GW, Maletic V. Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder. Transl Psychiatry. (2023) 13. doi: 10.1038/s41398-023-02514-2
23. Maximos B, Deligiannidis K. Effect of zuranolone on insomnia symptoms in patients with postpartum depression in the SKYLARK study. AJOG. (2023). SMBAJ of, 2023 Undefined. doi: 10.1016/j.ajog.2022.11.488
24. Clayton AH, Lasser R, Nandy I, Sankoh AJ, Jonas J, Kanes SJ. Zuranolone in major depressive disorder: results from MOUNTAIN-A phase 3, multicenter, double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. (2023) 84. doi: 10.4088/JCP.22m14445
25. Deligiannidis KM, Citrome L, Huang MY, Acaster S, Fridman M, Bonthapally V, et al. Effect of zuranolone on concurrent anxiety and insomnia symptoms in women with postpartum depression. J Clin Psychiatry. (2023) 84. doi: 10.4088/JCP.22m14475
26. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. bmj. (2024). doi: 10.31222/osf.io/v7gm2
27. Dikeç G, Özer D. Protocol registration and reporting of systematic review and meta-analyses published in psychiatric and mental health nursing journals: A descriptive study. Issues Ment Health Nurs. (2023). doi: 10.1080/01612840.2023.2212768
29. Higgins J, Altman D, Gøtzsche P, Bmj PJ. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. bmj. (2011). doi: 10.1136/bmj.d5928
30. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, et al. Effect of zuranolone vs placebo in postpartum depression: A randomized clinical trial. JAMA Psychiatry. (2021) 78:951–9. doi: 10.1001/jamapsychiatry.2021.1559
31. Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. (2019) 381:903–11. doi: 10.1056/NEJMoa1815981
32. Clayton AH, Lasser R, Parikh SV, Iosifescu DV, Jung JA, Kotecha M, et al. Zuranolone for the treatment of adults with major depressive disorder: A randomized, placebo-controlled phase 3 trial. Am J Psychiatry. (2023) 180:676–84. doi: 10.1176/appi.ajp.20220459
33. Kato M, Nakagome K, Baba T, Sonoyama T, Okutsu D, Yamanaka H, et al. Efficacy and safety of zuranolone in Japanese adults with major depressive disorder: A double-blind, randomized, placebo-controlled, phase 2 clinical trial. Psychiatry Clin Neurosci. (2023) 77:497–509. doi: 10.1111/pcn.13569
34. Deligiannidis KM, Meltzer-Brody S, Maximos B, Peeper EQ, Freeman M, Lasser R, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. (2023) 180:668–75. doi: 10.1176/appi.ajp.20220785
35. A study to evaluate the safety, tolerability, and efficacy of SAGE-217 compared to placebo in adult participants with comorbid major depressive disorder (MDD) and insomnia - study results. ClinicalTrials.gov. (2023).
36. Medicines used in depressive disorders - Pharmacological Treatment of Mental Disorders in Primary Health Care. NCBI Bookshelf, 49–70 .
37. Serafini G, Howland R, Rovedi F, Girardi P, Amore M. The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol. (2014) 12:444–61. doi: 10.2174/1570159X12666140619204251
38. Raison CL, Sanacora G, Woolley J, Heinzerling K, Dunlop BW, Brown RT, et al. Single-dose psilocybin treatment for major depressive disorder: A randomized clinical trial. JAMA. (2023) 330:843–53. doi: 10.1001/jama.2023.14530
39. FDA approves first oral treatment for postpartum depression. FDA (2024). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression.
40. Hoffmann E, Nomikos GG, Kaul I, Raines S, Wald J, Bullock A, et al. SAGE-217, A novel GABAA receptor positive allosteric modulator: clinical pharmacology and tolerability in randomized phase I dose-finding studies. Clin Pharmacokinet. (2020) 59:111–20. doi: 10.1007/s40262-019-00801-0
41. Biogen and sage therapeutics announce FDA accepts filing of new drug application and grants priority review of zuranolone in the treatment of major depressive disorder and postpartum depression. Biogen. (2024).
42. FDA documents hint at ‘uphill battle’ for broad approval of Sage’s depression drug. BioPharma Dive. (2024).
43. FDA approves ZURZUVAE™ (zuranolone), the first and only oral treatment approved for women with postpartum depression, and issues a complete response letter for major depressive disorder. Biogen. (2024).
45. Carvalho T. New depression drug zuranolone one step closer to FDA ruling. Nat Med. (2023) 29:1032–3. doi: 10.1038/d41591-023-00032-8
46. Nashwan AJ, Rehan ST, Imran L, Abbas SG, Khan SF. Exploring the clinical potentials of zuranolone in managing postpartum depression: A new therapeutic horizon. Prog Neuropsychopharmacol Biol Psychiatry. (2024) 132. doi: 10.1016/j.pnpbp.2024.110983
47. Bullock A, Gunduz-Bruce H, Zammit GK, Qin M, Li H, Sankoh AJ, et al. A phase 1 double-blind, placebo-controlled study of zuranolone (SAGE-217) in a phase advance model of insomnia in healthy adults. Hum Psychopharmacol. (2022). doi: 10.1002/hup.2806
49. Althaus AL, Ackley MA, Belfort GM, Gee SM, Dai J, Nguyen DP, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. (2020) 181. doi: 10.1016/j.neuropharm.2020.108333
50. Ahmad A, Rafeh Awan A, Nadeem N, Shahid Javed A, Farooqi M, Daniyal M, et al. Zuranolone for treatment of major depressive disorder: a systematic review and meta-analysis. Front Neurosci. (2024) 18. doi: 10.3389/fnins.2024.1361692/full
51. Cutler AJ, Mattingly GW, Kornstein SG, Aaronson ST, Lasser R, Zhang H, et al. Long-term safety and efficacy of initial and repeat treatment courses with zuranolone in adult patients with major depressive disorder: interim results from the open-label, phase 3 SHORELINE study. J Clin Psychiatry. (2023) 85. doi: 10.4088/JCP.23m14845
Keywords: zuranolone, major depressive disorder, postpartum depression, insomnia, meta-analysis
Citation: Raja A, Ahmed S, Basit Ali Siddiqui M, Lamiya Mir S, Kumar R, Ahmed M, Raja S, Bin Amin S, Alim Ur Rahman H, Deepak F and Asghar MS (2024) Evaluating the safety and efficacy of zuranolone in the management of major depressive disorder and postpartum depression, with or without concurrent insomnia: a rigorous systematic review and meta-analysis. Front. Psychiatry 15:1425295. doi: 10.3389/fpsyt.2024.1425295
Received: 29 April 2024; Accepted: 10 June 2024;
Published: 05 July 2024.
Edited by:
Ali Saffet Gonul, Ege University, TürkiyeReviewed by:
Abdulqadir J. Nashwan, Hamad Medical Corporation, QatarAwais Nazir, King Edward Medical University, Pakistan
Copyright © 2024 Raja, Ahmed, Basit Ali Siddiqui, Lamiya Mir, Kumar, Ahmed, Raja, Bin Amin, Alim Ur Rahman, Deepak and Asghar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adarsh Raja, YWRhcnNoYnVkaHdhbmkwMUBnbWFpbC5jb20=
 Adarsh Raja
Adarsh Raja Saboor Ahmed
Saboor Ahmed Muhammad Basit Ali Siddiqui
Muhammad Basit Ali Siddiqui Syeda Lamiya Mir
Syeda Lamiya Mir Rakesh Kumar
Rakesh Kumar Muhammad Ahmed
Muhammad Ahmed Sandesh Raja
Sandesh Raja Shafin Bin Amin
Shafin Bin Amin Hafsah Alim Ur Rahman
Hafsah Alim Ur Rahman Fnu Deepak
Fnu Deepak Muhammad Sohaib Asghar
Muhammad Sohaib Asghar