- 1Department of Neurology, The First Affiliated Hospital of Hainan Medical University, Haikou, China
- 2Department of Psychology, The Brain Hospital of Guangxi Zhuang Autonomous Region, Liuzhou, China
- 3Department of Psychology, Chongqing Jiangbei Mental Health Center, Chongqing, China
- 4The Affiliated Brain Hospital, Guangzhou Medical University, Guangzhou, China
- 5Key Laboratory of Neurogenetics and Channelopathies of Guangdong Province and the Ministry of Education of China, Guangzhou Medical University, Guangzhou, China
Objectives: The purpose of this systematic review of randomized controlled trials (RCTs) was to evaluate the effectiveness, safety, and tolerability of psilocybin in adult patients with major depressive disorder (MDD).
Methods: A systematic search (up to September 14, 2023) was conducted for RCTs that examined the efficacy, safety, and tolerability of psilocybin in physically healthy adult patients with MDD. Three independent researchers extracted data from publications where the primary outcome was a change in depressive symptoms, and key secondary outcomes were changes in anxiety symptoms and suicidal ideation, discontinuation rates for any reason, and adverse drug reactions (ADRs).
Results: Five RCTs with 472 adult patients with MDD on psilocybin (n = 274) and controls (n = 198) were included. Two of the five RCTs (40%) reported mixed results, while the other three (60%) found that psilocybin had a beneficial effect on MDD treatment. Four RCTs (80%) assessing the anxiolytic effects of psilocybin for treating MDD found that psilocybin was significantly more effective than the control group in improving anxiety symptoms. Psilocybin was more effective than the control group in improving suicidal ideation in one out of five RCTs. Discontinuation rates were similar for any reason between the psilocybin group (2–13%) and the control group (4–21%) (P > 0.05). Four RCTs (80%) reported ADRs in detail. The most common ADR in both groups was headache.
Conclusion: Psilocybin was effective in improving depressive symptoms in over half of the included studies and reduced anxiety symptoms in patients with MDD. The long-term efficacy and safety of psilocybin for MDD treatment needs to be further investigated in large RCTs.
1 Introduction
Major depressive disorder (MDD) is a highly prevalent condition in society (1) and is characterized by severe, persistent, unremitting depression, anhedonia, feelings of powerlessness, and guilt (2). MDD can lead to disability and is associated with an increased risk of mortality (3). The most common pharmacological treatments for MDD are selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, and other related drugs that selectively target neurotransmitters (4). A meta-analysis of 21 common antidepressant drugs in adults with MDD found that all were more effective than the placebo in improving depressive symptom severity, but the effect sizes were small (5). These drugs have a delayed onset of action, requiring weeks to months of treatment, high side effect rates, high relapse rates, and chronic dosing (6). Therefore, new treatments that are more effective and work faster to improve depressive symptoms are needed.
Novel pharmacological interventions, such as ketamine/esketamine (7) or psilocybin (8), have shown positive results in treating patients with MDD and have the potential to provide better protection. Treatment of depression with single or multiple infusions of ketamine is safe and effective (9, 10). In addition, multiple infusions of ketamine have cumulative and sustained antidepressant effects (9). A recent systematic review found that esketamine and psilocybin were effective in reducing depression symptoms and after overcoming some limitations, could be regarded as possible novel antidepressants (11). Compared to psilocybin, ketamine has a higher potential for addiction and toxic effects (12, 13), such as ulcerative cystitis (14).
Psilocybin is a naturally occurring psychoactive alkaloid that acts as an unselective agonist of many serotonergic receptors, particularly 5-hydroxytryptamine 2A (5-HT2A) (15). Studies have increasingly shown that psilocybin can be effective in treating mood disorders and reducing anxiety and depression symptoms (16). However, the findings of RCTs (8, 17–20) of psilocybin examining the efficacy and tolerability of psilocybin in patients with MDD have been inconsistent. Previous systematic reviews and meta-analyses have examined the efficacy and tolerability of psilocybin in patients with MDD. Some of these reviews included both primary and secondary depression (21–23), while others focused only on secondary depression, such as patients with life-threatening cancer (24, 25). For example, a recent meta-analysis found that psychedelics were significantly more effective than a placebo in reducing anxiety and depression among patients with cancer or other life-threatening diseases (24).
To date, no published systematic reviews have examined the efficacy and safety of psilocybin, focusing solely on physically healthy adults with MDD. Thus, we conducted this systematic review to examine the antidepressant, anxiolytic, and anti-suicidal effects and tolerability of psilocybin as an adjunctive treatment for physically healthy patients with primary MDD.
2 Methods
2.1 Data sources and search strategy
Three investigators, LJL, ZMS, and YM, independently, searched six online databases, including PubMed, Cochrane Library, PsycINFO, EMBASE, Chinese Journal Net, and WanFang databases, from the time the databases were launched until September 14, 2023. The following search terms were used: (“psilocybin” [Mesh] OR psilocybin OR psilocybine OR psilocibin OR psiloc*) AND (“depressive disorder” [Mesh] OR “depression”[Mesh] OR depress* OR dysthymi* OR adjustment disorder* OR mood disorder* OR affective disorder OR affective symptoms) AND (random* OR placebo OR control). The reference lists of the included trials (8, 17–20) and relevant review articles (16, 23, 26, 27) were manually searched to find any additional trials. Although the study protocol was not registered, this systematic review followed the PRISMA guidelines (Supplementary Table 1).
2.2 Study criteria and data extraction
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al., 2009). Studies were selected and assessed for inclusion according to the following PICOS criteria. Participants: Physically healthy patients (≥ 18 years old) diagnosed with MDD according to international diagnostic criteria. Intervention versus Comparison: adjunctive psilocybin (e.g., psychological support plus psilocybin) versus control (e.g., psychological support plus placebo) groups. Outcomes: The primary outcome was changes in depressive symptoms as measured by standardized scales (e.g., the Montgomery-Åsberg Depression Rating Scale [MADRS] (28), 17-item Hamilton Depression Rating Scale [HAMD-17] (29), Beck Depression Inventory [BDI] (30), 9-item Patient Health Questionnaire [PHQ-9] (31), or 16-item Quick Inventory of Depressive Symptomatology-Self Report [QIDS-SR-16] (32)). Key secondary outcomes were changes in anxiety symptoms as measured by standardized scales (e.g., the Hamilton Anxiety Rating Scale [HAMA] (33), the Spielberger’s Trait Anxiety Inventory [STAI] (34)) and suicidal ideation as measured by standardized scales (e.g., the Colombia-Suicidality Severity Rating Scale [C-SSRS] (35), the Suicidal Ideation Attributes Scale [SIDAS] (36), the Food and Drug Administration Classification Algorithm of Suicide Assessment 2012 [FDA-CASA 2012] (37)), the rates of discontinuation for any reason, and adverse drug reactions (ADRs). Study design: Published single-blind or double-blind RCTs focusing on the efficacy, safety, and tolerability of adjunctive psilocybin in physically healthy patients with MDD. Studies focusing on patients with chronic and serious physical illnesses, such as cancer (38–41), or healthy volunteers (42–46) were excluded. Only studies with the most complete data were included (8, 17, 18) when there were multiple publications based on the same dataset. Additionally, as previously recommended (47), open-label studies (48–51) were excluded. This systematic review did not include any review articles, retrospective studies, or case reports/series.
Three investigators, LJL, ZMS, and YM, independently extracted data from each included study. The three investigators (LJL, ZMS, and YM) discussed any discrepancies in data entry and consulted the senior author (WZ) when necessary. We contacted the first and/or corresponding author to obtain missing information when necessary.
2.3 Quality assessment
Three investigators LJL, ZMS, and YM, independently assessed the quality of each included RCT using the Jadad scale (52) and Cochrane Risk of Bias (53). As previously reported (54), RCTs were considered high quality if the Jadad score was ≥ 3.
3 Results
3.1 Literature search
Figure 1 shows that 816 articles were initially identified from the six databases mentioned above. After removing 219 duplicates, 563 records were excluded based on their title and abstract. Finally, 34 articles were screened for full text. Five RCTs (8, 17–20) met the inclusion and exclusion criteria for this systematic review.
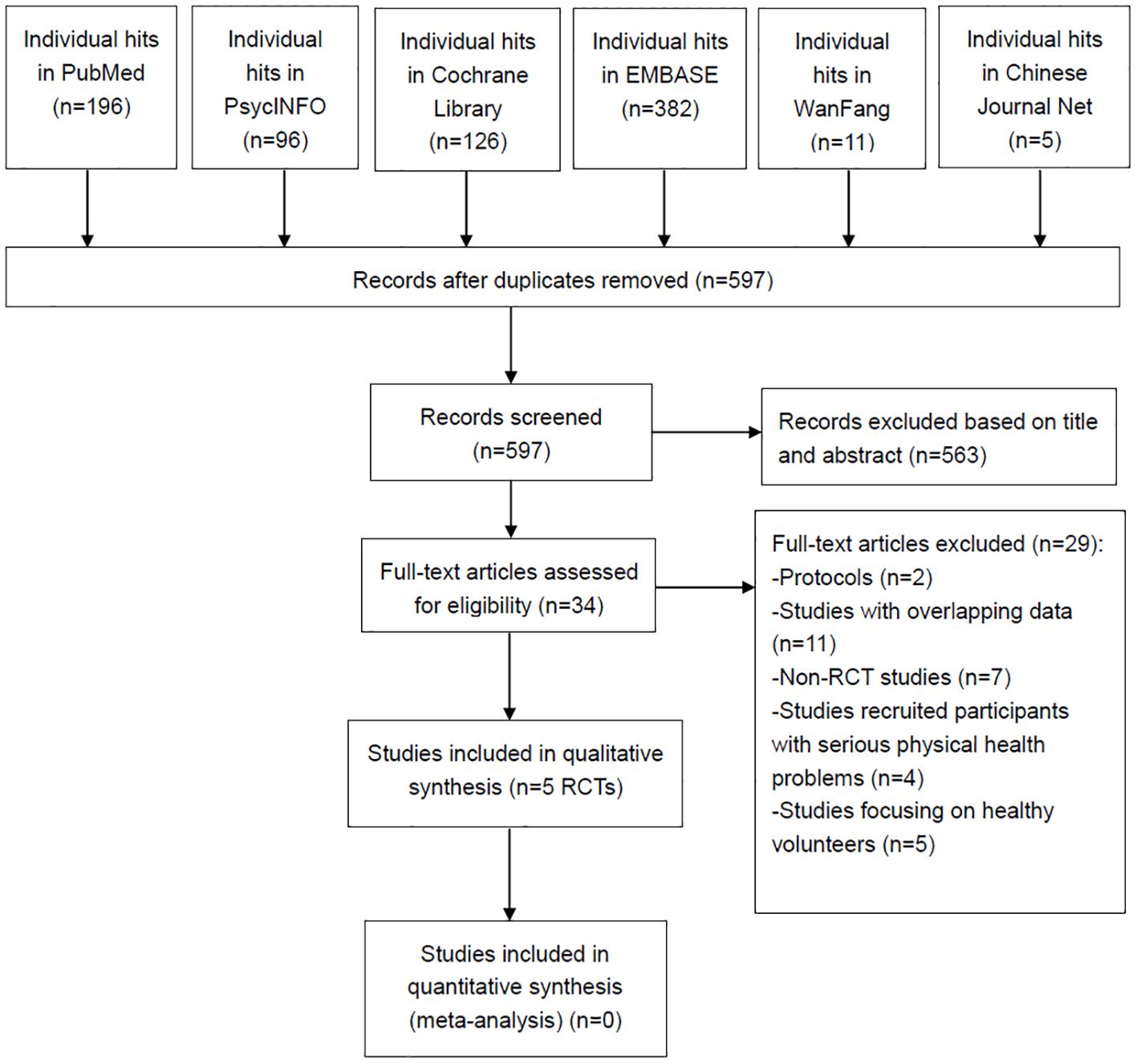
Figure 1. PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCTs, randomized controlled trials.
3.2 Patient and study characteristics
Table 1 summarizes the characteristics of each RCT. The study samples had a mean age ranging from 36.8 to 41.2 years, with 48.4% of patients being male. Approximately 16% of the patients had used psilocybin or other psychedelics prior to this study. In two RCTs (8, 17), patients were given two separate doses of psilocybin (session 1: 25mg and session 2: 25 mg in Carhart-Harris et al.’s study (8); session 1: 20 mg/70 kg and session 2: 30 mg/70 kg in Davis et al.’s study (17)). In the remaining RCTs (18–20), patients were given a single dose of psilocybin (0.215 mg/kg or 1–25 mg). The RCTs included in the study varied in duration from 2 to 12 weeks.
3.3 Quality assessment
Table 1 shows that all the RCTs included in this study met the criteria for high quality. The Jadad score ranged from 3 (1 RCT) (17) to 5 (4 RCTs) (8, 18–20). As shown in Figure 2, all RCTs were assessed as having a low risk of bias for selection, detection, attrition, and reporting.
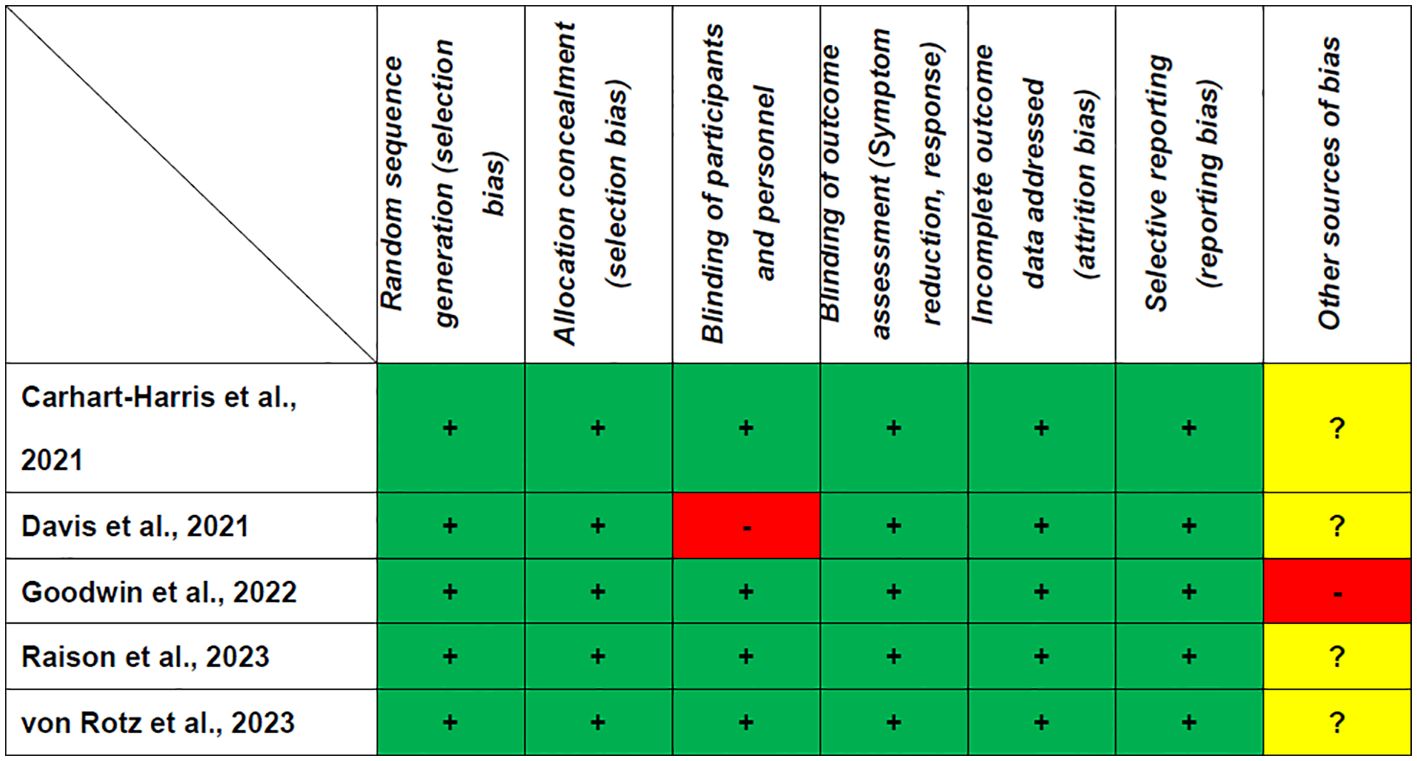
Figure 2. Cochrane risk of bias. +: Low risk of bias; -: High risk of bias; ?: Unclear risk of bias.
3.4 Clinical efficacy
3.4.1 Changes in depressive symptoms
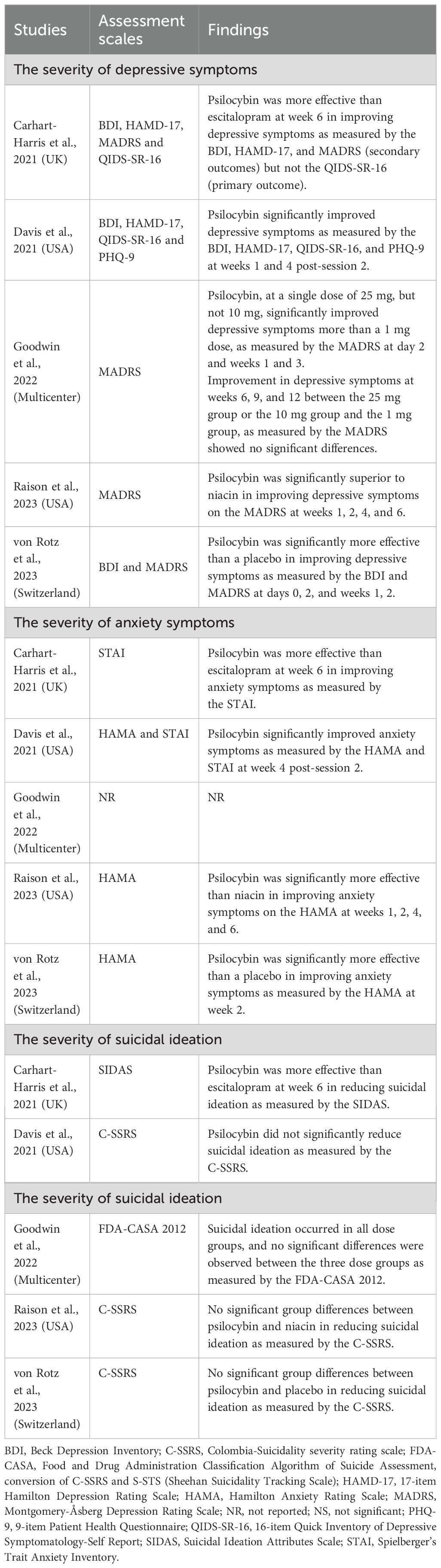
Table 2 shows that two RCTs (40%, 2/5) (8, 18) reported mixed findings, while three RCTs (60%, 3/5) (17, 19, 20) found a positive effect of psilocybin in treating MDD. Specifically, one RCT (8) found that psilocybin was more effective than escitalopram in improving depressive symptoms as measured by the BDI, HAMD-17, and MADRS, but not the QIDS-SR-16 at week 6. Another RCT (18) found that a single dose of 25 mg of psilocybin, but not 10 mg, significantly improved depressive symptoms more than a 1 mg dose, as measured by the MADRS on day 2 and weeks 1 and 3. The remaining three RCTs (17, 19, 20) found that psilocybin significantly improved depressive symptoms, as measured by the HAMD-17, MADRS, QIDS-SR-16, PHQ-9, or BDI, and that the effects lasted from 2 to 6 weeks (Table 2).
3.4.2 Changes in anxiety symptoms
Four RCTs (80%) (8, 17, 19, 20) assessed the anxiolytic effects of psilocybin in treating MDD. Among these RCTs, it was found that psilocybin was significantly better than the control group at improving anxiety symptoms, as measured by the HAMA and/or STAI (Table 2).
3.4.3 Changes in suicidal ideation
All included RCTs (8, 17–20) assessed the anti-suicidal effects of psilocybin in treating MDD. Only one RCT (20%) (8) found that psilocybin was more effective than escitalopram in reducing suicidal ideation as measured by the SIDAS, while the other four RCTs (80%) (17–20) found that psilocybin did not significantly reduce suicidal ideation as measured by the C-SSRS (three RCTs) (17, 19, 20) and FDA-CASA 2012 (one RCT) (18) compared to the control group (Table 2).
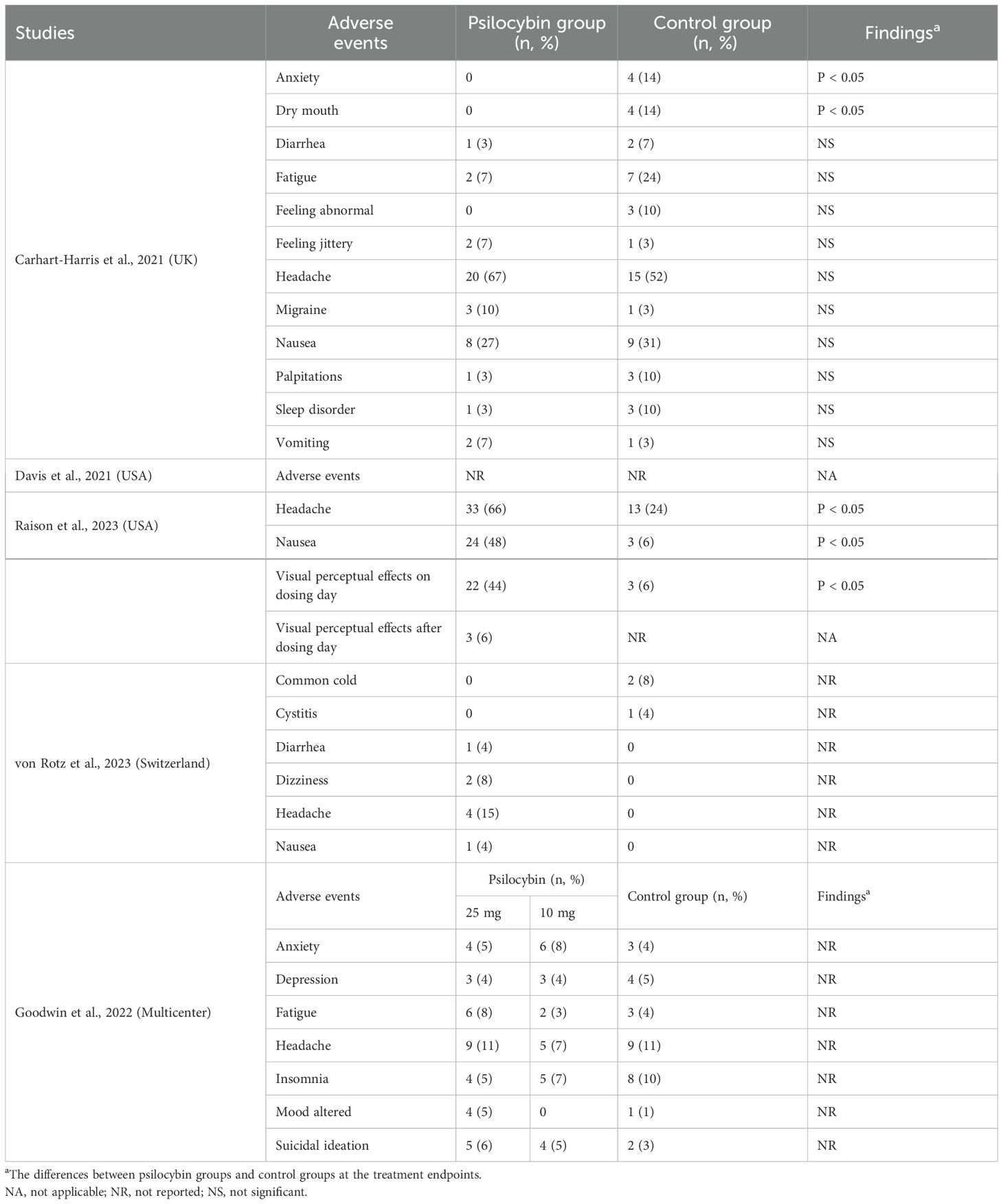
3.5 Discontinuation rate and adverse events
Table 3 summarizes the discontinuation rates for any reason and adverse events. All the included RCTs (8, 17–20) reported a discontinuation rate for any reason. The psilocybin group had discontinuation rates ranging from 2–13%, while the control group had discontinuation rates ranging from 4–21%. Three RCTs (60%, 3/5) (8, 18, 19) reported adverse events in both groups. Four RCTs reported adverse events in detail (80%) (8, 18–20). Headache was the most common adverse event in both groups (Table 4).
4 Discussion
To the best of our knowledge, this systematic review of five RCTs (8, 17–20) is the first to investigate the efficacy and safety of psilocybin in 472 physically healthy adults with MDD. The main findings of this systematic review were as follows: (1) 60% of the included RCTs found that psilocybin was more effective than the control group in treating depressive symptoms. However, the remaining two RCTs found inconsistent results; (2) patients with MDD treated with psilocybin showed significant improvement in anxiety symptoms compared to the control group; (3) the effectiveness of psilocybin over the control group in reducing suicidal ideation was only found in one RCT (8); and (4) the rates of any adverse events and discontinuation due to any reason were similar in both groups. The most frequent adverse event was headache in both groups.
While two RCTs (40%) (8, 18) reported mixed results, the other RCTs (60%) (17, 19, 20) found that psilocybin was significantly more effective than the placebo in improving depressive symptoms. The inconsistent results of psilocybin in the treatment of MDD can be attributed to the methodological heterogeneity of the included studies. The studies included different doses of psilocybin (1–25 mg or 0.215 mg/kg) and psychological support with different components. It is therefore difficult to distinguish between the effects of psilocybin alone and those arising from psychological support in this systematic review. Furthermore, participants across RCTs demonstrate heterogeneity in this systematic review. For example, Goodwin et al. exclusively enrolled patients with treatment-resistant depression (TRD) (18), while another study by Raison et al. (19) reported that 12.5% of participants had TRD. individuals with TRD have been associated with heightened severity and prolonged duration of illness, increased disability, and an elevated risk of suicide (55). Therefore, the proportion of patients with TRD was reported in only 3 RCTs (60%, 3/5), making it difficult to compare the efficacy and safety of psilocybin in patients with TRD with that in non-TRD patients. Patients with TRD were associated with smaller hippocampal volume compared to non-TRD patients (56). It is therefore justified to investigate the comparative efficacy and safety of psilocybin in patients with TRD versus non-TRD in the future.
Evidence exists that psilocybin-assisted therapy has substantial antidepressant effects for at least 12 months after acute intervention (57). For example, an open-label clinical trial found that marked reductions in depressive symptoms were observed after just two psilocybin treatment sessions and remained significant six-month after treatment in patients with TRD (58). Similar to ketamine and esketamine at sub-anesthetic doses (7), psilocybin has a rapid onset of antidepressant effects in MDD (20). For example, Von Rotz et al. reported a response rate of 69% 48 h after treatment (20), which was similar to the response rate of 71% for ketamine 24 h after treatment (59). A systematic review found that depressive symptoms can be rapidly and permanently reduced with the use of esketamine and psilocybin (11). However, it is unclear whether psilocybin has more rapid and sustained antidepressant effects compared to ketamine/esketamine. Interestingly, 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), a naturally occurring tryptamine used for spiritual and recreational purposes, can alleviate depression and anxiety subjectively (60). Future RCTs should investigate the efficacy and safety of 5-MeO-DMT in patients with MDD or anxiety.
How psilocybin mediates its antidepressant and psychedelic effects is currently unknown (15). The antidepressant effects of psilocybin are believed to be caused by changes to the serotonergic system, particularly through activation of 5-HT2A receptors and subsequent changes in gene expression (15). Moreover, psilocybin has the potential to indirectly modulate the dopaminergic and glutamatergic systems, as well as interact with various low-affinity receptors (15). A functional magnetic resonance imaging (fMRI) study indicated that psilocybin therapy has a distinct antidepressant mechanism, revealing that higher-order functional networks, which are rich in 5-HT2A receptors, exhibited increased functional connectivity and flexibility following psilocybin treatment, whereas no such effects were observed with escitalopram (61). Psilocybin affects not only neurochemical systems, but also neural circuitry and critical brain regions associated with MDD, such as the amygdala and the default mode network.
In this systematic review, 80% of the included RCTs assessed the anxiolytic effects of psilocybin in treating MDD and consistently found that psilocybin was significantly more effective than the control in improving anxiety symptoms, which supports the results of a recent meta-analysis (16). Anxious depression is common (approximately 45.1-81.0%) in MDD (62). Anxious depression is associated with worse treatment outcomes, lower quality of life, and lower well-being than non-anxious depression (63). Interestingly, in contrast to non-anxious depressed patients, anxious depressed patients showed weaker antianhedonic (62) and antidepressant responses (64) to ketamine. Similarly, a recent study found that esketamine had a greater anti-suicidal effect on adolescents with non-anxious MDD than on those with anxious MDD (65). However, it is unclear whether psilocybin is more effective or safer for patients with anxious or non-anxious depression. Although all included RCTs assessed the anti-suicidal effects of psilocybin in the treatment of MDD, only 20% of the included studies found that psilocybin was more effective than the control group in improving suicidal ideation. Taken together, psilocybin did not appear to be effective in reducing suicidal ideation.
The most common adverse effect of psilocybin in patients with MDD was headache, although it was generally well tolerated with mild to moderate adverse events. Furthermore, adverse events related to psilocybin are generally limited to the acute dosing period (19). A recent meta-analysis revealed that a headache, the most prevalent adverse event associated with psilocybin use, is transient and does not have lasting effects (27). Notably, this meta-analysis identified significant dose-response relationships for various side effects of psilocybin, such as nausea and headache (27). Additionally, psilocybin produces acute perceptual and subjective effects in healthy volunteers (42) and is effective and safe for treating depressive symptoms in patients with other diagnoses, such as life-threatening cancer (39). A previous RCT found that psilocybin may reduce depression and anxiety in cancer patients with life-threatening diagnoses and symptoms of depression and/or anxiety (39). Overall, psilocybin is safe and well-tolerated. Studies should focus on determining the optimal dose of psilocybin to reduce depression scores while minimizing side effects.
This systematic review has several limitations. First, although we conducted a comprehensive systematic search, the number of included studies and the sample size were relatively small for qualitative synthesis. Second, a meta-analysis could not be performed because of the significant heterogeneity among the included RCTs. Third, psilocybin was usually administered with psychological support in the included studies, making it difficult to assess its isolated effects in treating MDD. Finally, the five RCTs focused on psilocybin for adult MDD, limiting the generalizability of the results to depression in other age groups.
5 Conclusion
Psilocybin was effective in improving depressive symptoms in over half of the included studies and reduced anxiety symptoms in patients with MDD. Further large-scale RCTs should investigate the long-term efficacy, safety, and tolerability of psilocybin for MDD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LL: Writing – original draft, Conceptualization, Data curation, Formal analysis. YM: Conceptualization, Data curation, Writing – review & editing. ZS: Investigation, Methodology, Writing – original draft. XH: Project administration, Supervision, Validation, Writing – review & editing. YN: Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. HW: Formal analysis, Methodology, Supervision, Writing – review & editing. XY: Investigation, Supervision, Validation, Writing – review & editing. WZ: Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82101609), the Science and Technology Program of Guangzhou (2023A03J0839, 2023A03J0436), Science and Technology Planning Project of Liwan District of Guangzhou (202201012), National Clinical Key specialty construction project ((2023) 33), The Natural Science Foundation Program of Guangdong (2023A1515011383, 2024A1515012578), the Science and Technology Program of Guangzhou (202206010077), Guangzhou Municipal Key Discipline in Medicine (2021-2023), Guangzhou Municipal Key Discipline in Medicine (2021-2023), Guangzhou Science and Technology Plan Project (2023A03J0827), Guangzhou Traditional Chinese Medicine and Integrated Traditional Chinese and Western Medicine Science and Technology Project (20232A010014), Guangzhou High-level Clinical Key Specialty, Department of Emergency Medicine of National clinical key specialty, Guangzhou Research-oriented Hospital, Hainan Provincial Natural Science Foundation of China (821QN0987), and Hainan Province Clinical Medical Center (2021). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1416420/full#supplementary-material
Supplementary Table 1 | The PRISMA Checklist. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit: www.prisma-statement.org.
References
1. Canli D, Karasar B. Predictors of major depressive disorder: the need for social approval and self-esteem. Alpha Psychiatry. (2021) 22:38–42. doi: 10.5455/apd.97683
2. Li Y, Yu Y, Yin Y, Hu X, Wu S. Regions with altered degree centrality and their functional connectivity in first-episode drug-naive major depressive disorder: a resting-state functional magnetic resonance imaging study. Alpha Psychiatry. (2023) 24:217–25. doi: 10.5152/alphapsychiatry.2023.231191
3. Baldessarini RJ, Forte A, Selle V, Sim K, Tondo L, Undurraga J, et al. Morbidity in depressive disorders. Psychother psychosomatics. (2017) 86:65–72. doi: 10.1159/000448661
4. Gartlehner G, Dobrescu A, Chapman A, Toromanova A, Emprechtinger R, Persad E, et al. Nonpharmacologic and pharmacologic treatments of adult patients with major depressive disorder: a systematic review and network meta-analysis for a clinical guideline by the american college of physicians. Ann Intern Med. (2023) 176:196–211. doi: 10.7326/M22-1845
5. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. doi: 10.1016/S0140-6736(17)32802-7
6. Blackburn TP. Depressive disorders: treatment failures and poor prognosis over the last 50 years. Pharmacol Res Perspect. (2019) 7:e00472. doi: 10.1002/prp2.472
7. Huang XB, Zheng W. Ketamine and electroconvulsive therapy for treatment-refractory depression. Alpha Psychiatry. (2023) 24:244–6. doi: 10.5152/alphapsychiatry.2023.231358
8. Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. Trial of psilocybin versus escitalopram for depression. New Engl J Med. (2021) 384:1402–11. doi: 10.1056/NEJMoa2032994
9. Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: A randomized controlled trial. Am J Psychiatry. (2019) 176:401–9. doi: 10.1176/appi.ajp.2018.18070834
10. Zheng W, Zhou YL, Liu WJ, Wang CY, Zhan YN, Li HQ, et al. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. J Psychiatr Res. (2018) 106:61–8. doi: 10.1016/j.jpsychires.2018.09.013
11. Psiuk D, Nowak EM, Dycha N, Łopuszańska U, Kurzepa J, Samardakiewicz M. Esketamine and psilocybin—the comparison of two mind-altering agents in depression treatment: systematic review. Int J Mol Sci. (2022) 23(19):11450. doi: 10.3390/ijms231911450
12. Gable RS. Acute toxic effects of club drugs. J psychoactive Drugs. (2004) 36:303–13. doi: 10.1080/02791072.2004.10400031
13. Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE. The abuse potential of medical psilocybin according to the 8 factors of the controlled substances act. Neuropharmacology. (2018) 142:143–66. doi: 10.1016/j.neuropharm.2018.05.012
14. Morgan CJ, Curran HV. Ketamine use: a review. Addiction. (2012) 107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x
15. Ling S, Ceban F, Lui LMW, Lee Y, Teopiz KM, Rodrigues NB, et al. Molecular mechanisms of psilocybin and implications for the treatment of depression. CNS Drugs. (2022) 36:17–30. doi: 10.1007/s40263-021-00877-y
16. Hodge AT, Sukpraprut-Braaten S, Strayhan RC. The efficacy of psilocybin in the treatment of depression and anxiety: A meta-analysis. Curr Psychiatry Res Rev. (2023) 19:95–106. doi: 10.2174/2666082218666220513142002
17. Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry. (2021) 78:481–9. doi: 10.1001/jamapsychiatry.2020.3285
18. Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. New Engl J Med. (2022) 387:1637–48. doi: 10.1056/NEJMoa2206443
19. Raison CL, Sanacora G, Woolley J, Heinzerling K, Dunlop BW, Brown RT, et al. Single-dose psilocybin treatment for major depressive disorder: a randomized clinical trial. Jama. (2023) 330:843–53. doi: 10.1001/jama.2023.14530
20. von Rotz R, Schindowski EM, Jungwirth J, Schuldt A, Rieser NM, Zahoranszky K, et al. Single-dose psilocybin-assisted therapy in major depressive disorder: a placebo-controlled, double-blind, randomised clinical trial. EClinicalMedicine. (2023) 56:101809. doi: 10.1016/j.eclinm.2022.101809
21. Goldberg SB, Pace BT, Nicholas CR, Raison CL, Hutson PR. The experimental effects of psilocybin on symptoms of anxiety and depression: a meta-analysis. Psychiatry Res. (2020) 284:112749. doi: 10.1016/j.psychres.2020.112749
22. Leger RF, Unterwald EM. Assessing the effects of methodological differences on outcomes in the use of psychedelics in the treatment of anxiety and depressive disorders: a systematic review and meta-analysis. J Psychopharmacol. (2022) 36:20–30. doi: 10.1177/02698811211044688
23. Li NX, Hu YR, Chen WN, Zhang B. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. (2022) 296:26–34. doi: 10.1016/j.jad.2021.09.041
24. Sicignano D, Snow-Caroti K, Hernandez AV, White CM. The impact of psychedelic drugs on anxiety and depression in advanced cancer or other life-threatening disease: a systematic review with meta-analysis. Am J Clin Oncology: Cancer Clin Trials. (2023) 46:236–45. doi: 10.1097/COC.0000000000000998
25. Yu CL, Yang FC, Yang SN, Tseng PT, Stubbs B, Yeh TC, et al. Psilocybin for end-of-life anxiety symptoms: a systematic review and meta-analysis. Psychiatry Invest. (2021) 18:958–67. doi: 10.30773/pi.2021.0209
26. Ko K, Kopra EI, Cleare AJ, Rucker JJ. Psychedelic therapy for depressive symptoms: a systematic review and meta-analysis. J Affect Disord. (2023) 322:194–204. doi: 10.1016/j.jad.2022.09.168
27. Perez N, Langlest F, Mallet L, De Pieri M, Sentissi O, Thorens G, et al. Psilocybin-assisted therapy for depression: a systematic review and dose-response meta-analysis of human studies. Eur Neuropsychopharmacol. (2023) 76:61–76. doi: 10.1016/j.euroneuro.2023.07.011
28. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. (1979) 134:382–9. doi: 10.1192/bjp.134.4.382
29. Hamilton M. A rating scale for depression. J neurology neurosurgery Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
30. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. (1996) 67:588–97. doi: 10.1207/s15327752jpa6703_13
31. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Internal Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
32. Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. (2003) 54:573–83. doi: 10.1016/s0006-3223(02)01866-8
33. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
34. Metzger RL. A reliability and validity study of the State-Trait Anxiety Inventory. J Clin Psychol. (1976) 32:276–8. doi: 10.1002/(ISSN)
35. Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. (2011) 168:1266–77. doi: 10.1176/appi.ajp.2011.10111704
36. Van Spijker BA, Batterham PJ, Calear AL, Farrer L, Christensen H, Reynolds J, et al. The suicidal ideation attributes scale (sidas): community-based validation study of a new scale for the measurement of suicidal ideation. Suicide Life Threat Behav. (2014) 44:408–19. doi: 10.1111/sltb.12084
37. Sheehan DV, Alphs LD, Mao L, Li Q, May RS, Bruer EH, et al. Comparative validation of the S-STS, the ISST-Plus, and the C-SSRS for assessing the suicidal thinking and behavior FDA 2012 suicidality categories. Innov Clin Neurosci. (2014) 11:32–46.
38. Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. (2011) 68:71–8. doi: 10.1001/archgenpsychiatry.2010.116
39. Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol (Oxford England). (2016) 30:1181–97. doi: 10.1177/0269881116675513
40. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol (Oxford England). (2016) 30:1165–80. doi: 10.1177/0269881116675512
41. Ross S, Agin-Liebes G, Lo S, Zeifman RJ, Ghazal L, Benville J, et al. Acute and sustained reductions in loss of meaning and suicidal ideation following psilocybin-assisted psychotherapy for psychiatric and existential distress in life-threatening cancer. ACS Pharmacol Trans Sci. (2021) 4:553–62. doi: 10.1021/acsptsci.1c00020
42. Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology. (2011) 218:649–65. doi: 10.1007/s00213-011-2358-5
43. Kraehenmann R, Schmidt A, Friston K, Preller KH, Seifritz E, Vollenweider FX. The mixed serotonin receptor agonist psilocybin reduces threat-induced modulation of amygdala connectivity. NeuroImage Clin. (2016) 11:53–60. doi: 10.1016/j.nicl.2015.08.009
44. Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. (2012) 72:898–906. doi: 10.1016/j.biopsych.2012.04.005
45. Szigeti B, Kartner L, Blemings A, Rosas F, Feilding A, Nutt DJ, et al. Self-blinding citizen science to explore psychedelic microdosing. eLife. (2021) 10. doi: 10.7554/eLife.62878
46. Mallaroni P, Mason NL, Reckweg JT, Paci R, Ritscher S, Toennes SW, et al. Assessment of the acute effects of 2C-B vs. psilocybin on subjective experience, mood, and cognition. Clin Pharmacol Ther. (2023) 114:423–33. doi: 10.1002/cpt.2958
47. Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. (2009) 373:31–41. doi: 10.1016/s0140-6736(08)61764-x
48. Shnayder S, Ameli R, Sinaii N, Berger A, Agrawal M. Psilocybin-assisted therapy mediates psycho-social-spiritual change in cancer patients as assessed by the NIH-HEALS. J Affect Disord. (2023) 323:592–7. doi: 10.1016/j.jad.2022.11.046
49. Lyons T, Carhart-Harris RL. More realistic forecasting of future life events after psilocybin for treatment-resistant depression. Front Psychol. (2018) 9:1721. doi: 10.3389/fpsyg.2018.01721
50. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol (Oxford England). (2018) 32:811–9. doi: 10.1177/0269881117748902
51. Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. (2016) 3:619–27. doi: 10.1016/S2215-0366(16)30065-7
52. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
53. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
54. Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges LV, et al. Are the clinical effects of homeopathy placebo effects? a meta-analysis of placebo-controlled trials. Lancet. (1997) 350:834–43. doi: 10.1016/s0140-6736(97)02293-9
55. Alshehri AS, Algarni AM, Almahdi HAM, Asiri AHH, Asiri HYM, Alsulami AAH, et al. Study to determine the epidemiology of treatment-resistant depression among the Saudi Arabian population: A cross-sectional study. J Educ Health promotion. (2023) 12:425. doi: 10.4103/jehp.jehp_809_23
56. Abdallah CG, Jackowski A, Sato JR, Mao X, Kang G, Cheema R, et al. Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur Neuropsychopharmacol. (2015) 25:1082–90. doi: 10.1016/j.euroneuro.2015.04.025
57. Gukasyan N, Davis AK, Barrett FS, Cosimano MP, Sepeda ND, Johnson MW, et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: prospective 12-month follow-up. J Psychopharmacol (Oxford England). (2022) 36:151–8. doi: 10.1177/02698811211073759
58. Carhart-Harris RL, Bolstridge M, Day CMJ, Rucker J, Watts R, Erritzoe DE, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology. (2018) 235:399–408. doi: 10.1007/s00213-017-4771-x
59. Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. (2006) 63:856–64. doi: 10.1001/archpsyc.63.8.856
60. Davis AK, So S, Lancelotta R, Barsuglia JP, Griffiths RR. 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. Am J Drug Alcohol Abuse. (2019) 45:161–9. doi: 10.1080/00952990.2018.1545024
61. Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. (2022) 28:844–51. doi: 10.1038/s41591-022-01744-z
62. Zheng W, Yang XH, Gu LM, Tan JQ, Zhou YL, Wang CY, et al. Antianhedonic effects of serial intravenous subanaesthetic ketamine in anxious versus nonanxious depression. J Affect Disord. (2022) 313:72–6. doi: 10.1016/j.jad.2022.06.081
63. Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. (2008) 165:342–51. doi: 10.1176/appi.ajp.2007.06111868
64. Chen MH, Lin WC, Wu HJ, Bai YM, Li CT, Tsai SJ, et al. Efficacy of low-dose ketamine infusion in anxious vs nonanxious depression: revisiting the Adjunctive Ketamine Study of Taiwanese Patients with Treatment-Resistant Depression. CNS Spectr. (2021) 26:362–7. doi: 10.1017/s1092852920001194
Keywords: psilocybin, major depressive disorder, systematic review, efficacy, randomized controlled trial
Citation: Li L-J, Mo Y, Shi Z-M, Huang X-B, Ning Y-P, Wu H-W, Yang X-H and Zheng W (2024) Psilocybin for major depressive disorder: a systematic review of randomized controlled studies. Front. Psychiatry 15:1416420. doi: 10.3389/fpsyt.2024.1416420
Received: 12 April 2024; Accepted: 16 August 2024;
Published: 23 September 2024.
Edited by:
Alina Wilkowska, Medical University of Gdansk, PolandReviewed by:
Aleksander Kwaśnyy, Medical University of Gdansk, PolandMariusz Stanisław Wiglusz, Medical University of Gdansk, Poland
Copyright © 2024 Li, Mo, Shi, Huang, Ning, Wu, Yang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zheng, emhlbmd3ZWkwNzAyQDE2My5jb20=
†These authors have contributed equally to this work
 Li-Juan Li
Li-Juan Li Yu Mo2†
Yu Mo2† Zhan-Ming Shi
Zhan-Ming Shi Xing-Bing Huang
Xing-Bing Huang Yu-Ping Ning
Yu-Ping Ning Hua-Wang Wu
Hua-Wang Wu Xin-Hu Yang
Xin-Hu Yang Wei Zheng
Wei Zheng