- 1Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Epidemiology and Data Science, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
- 3Institute for Brain Disorders, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 4Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
Objective: This study aimed to summarize and assess the certainty of evidence of non-pharmacological interventions (NPIs) on the depressive outcomes in people with mild cognitive impairment (MCI) based on published systematic reviews (SRs).
Method: Databases including PubMed, EMBASE, PsycINFO, the Cochrane Database of Systematic Reviews, CNKI, CBM, Wanfang and VIP database were searched from their inception to June 6, 2023. The methodological quality of the SRs was evaluated using the AMSTAR2 tool, and the quality of evidence was assessed using the Grading of Recommendation Assessment, Development and Evaluation (GRADE) framework.
Results: Twelve eligible SRs were included. Three SRs focused on cognitive interventions (general, computer-based, cognitive stimulation/rehabilitation), six reviews on physical activity (Tai Chi, exercise therapy, dance), three on psychosocial interventions including cognitive behavioral therapy (CBT), mindfulness-based intervention (MBI) and type not specified, one on music therapy, and one on health education; moreover, there were two SRs on multimodal NPIs. One Cochrane SR was rated as moderate quality, while the others were rated as low quality according to AMSTAR2. The overlap between primary studies of included SRs (a total of 51 studies) was 1.8%, indicating slight overlap. General cognitive interventions (SMD=-0.25, 95% CI [−0.46, −0.04], GRADE: moderate) and computer-based cognitive interventions (narrative evidence) showed potential benefits in improving depression. Exercise therapy showed consistency between two SRs in benefiting depressive symptoms of MCI (SMD=-0.33, 95% CI [−0.56, −0.10], GRADE: Low; SMD=−0.37, 95% CI [-0.64, -0.10], GRADE: Low). Dance (SMD=−0.37, 95% CI [-1.11, 0.38], GRADE: Low), CBT (SMD=0.03,95% CI [-0.18, 0.24], GRADE: Moderate), MBI (SMD=0.29, 95% CI [0.00, 0.57], GRADE: Very Low) and health education (SMD=-0.12, 95% CI [−0.44, 0.20], GRADE: Low) did not show significant difference compared to control group in improving depressive symptoms, while the effectiveness of Tai Chi, music therapy and multimodal NPIs showed inconsistency across different studies.
Conclusion: Cognitive interventions (general or computer-based) and exercise therapy (a type of physical activity) show preliminary potential to improve depressive symptoms, while others do not show significant effects or relate to confused effects. Further methodologically rigorous and adequately powered primary studies are necessary for each of these NPIs, with reporting on the components of the interventions clearly in MCI patients.
1 Introduction
Mild cognitive impairment (MCI) represents a critical transitional phase between healthy aging and dementia, affecting approximately 15.56% of individuals aged 50 years and older globally (1, 2). Depression, a common comorbidity in MCI, complicates the condition, affecting up to 32% of those with MCI (3–5). The presence of depression in MCI patients is particularly concerning, as it significantly heightens the risk of progression to dementia. A comprehensive meta-analysis of 32 studies involving over 62,000 participants suggests that depression nearly doubles the risk of developing dementia (6). This review also noted a trend toward smaller effect sizes in studies with longer follow-ups, indicating that depression may play a prodromal role in dementia. Therefore, effective management strategies for depression in MCI are crucial, underscoring the need for interventions aimed at alleviating depressive symptoms to potentially slow the progression toward dementia.
While antidepressants are a standard treatment for depression, their effectiveness in preventing cognitive decline and dementia in MCI is not well-established. The World Health Organization (WHO) has highlighted the lack of sufficient evidence to support the use of antidepressants for reducing the risk of cognitive decline and/or dementia in this population (7). Given these challenges, non-pharmacological interventions recommended by the WHO emerge as important alternatives (7).
Currently, various non-pharmacological interventions, such as psychological therapies (e.g., cognitive behavioral therapy, problem-solving therapy, interpersonal therapy, and behavioral activation), are used to improve depressive symptoms in individuals with MCI. However, their effectiveness remains uncertain. Although several systematic reviews have assessed these interventions, their findings are inconsistent. For instance, one review suggests that cognitive interventions are effective in alleviating depressive symptoms (8), while another one by Xu et al. reports them as ineffective (9). These discrepancies may result from methodological differences, sample characteristics, or varying applicability of interventions across patient populations. Such conflicting results contribute to the challenges faced by patients and physicians in selecting appropriate treatments.
Therefore, a comprehensive overview of systematic reviews is needed to integrate and compare findings, providing a more reliable evidence base to guide clinical practice and decision-making. Our study aims to fill this gap by conducting an overview of systematic reviews on non-pharmacological interventions for depression in MCI. By doing so, it is expected to provide a clear evidence-based understanding of these non-pharmacological interventions’ effectiveness, aiding in the informed management of depression in MCI and potentially mitigating progression to dementia.
2 Methods
2.1 Eligibility criteria
2.1.1 Type of studies
Systematic reviews and meta-analyses were included, with no language limitation and no restrictions on the study design of primary studies. Conference abstracts were excluded.
2.1.2 Population
Patients included in the systematic reviews should have been diagnosed with MCI using Petersen criteria (10), the National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria (11); or clear diagnostic criteria based on neuropsychological testing was also accepted if well described. Patients diagnosed with dementia were excluded. If both MCI and dementia patients were involved, only systematic reviews with separate analyses on MCI patients were included.
2.1.3 Interventions
Non-pharmacological intervention is any type of healthcare intervention which is not primarily based on medications. It included, but not limited to, physical activity, cognitive interventions, and music therapy. The intervention groups did not include pharmacological therapies, such as antidepressants, unless such therapies were administered equally to both the intervention and control groups.
2.1.4 Comparison
The comparison focused on placebo, no treatment, treatment as usual, and pharmacological therapies.
2.1.5 Outcomes
The primary outcome was the change in depression or depressive symptoms assessed using scales such as the Beck Depression Inventory (BDI), Cornell Depression Score (CDS), Cornell Scale for Depression in Dementia (CSDD), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), Montgomery-Asberg Depression Scale (MADRS), and Patient Health Questionnaire-9 (PHQ-9).
2.2 Literature search
Electronic databases including PubMed, EMBASE, PsycINFO, Cochrane Database of Systematic Reviews, CNKI, CBM, Wanfang and the VIP database were systematically searched from inception to June 6, 2023. The detailed search strategy is provided in the Supplementary Table 1.
2.3 Study selection and data collection
After excluding duplicate publications, two authors screened the titles and abstracts, followed by the full texts, independently for study eligibility based on the inclusion criteria. Disagreements were resolved through discussion or consultation with a third author.
Two authors independently extracted the data of included systematic reviews using a specifically designed extraction form. The details of author, date of review, intervention and comparator, number of participants, diagnosis criteria, outcomes examined, meta-analysis or narrative results were extracted. All discrepancies were resolved through discussion or consultation with a third author.
2.4 Assessment of methodological quality of included reviews
The methodological quality of eligible reviews was appraised by two authors independently using AMSTAR2 tool (12). Disagreements were resolved through discussion or consultation with a third author.
AMSTAR2 includes 16 items with 7 critical items (protocol registered before commencement of the review, adequacy of the literature search, justification for excluding individual studies, risk of bias from individual studies being included in the review, appropriateness of meta-analytical methods, consideration of risk of bias when interpreting the results of the review, assessment of presence and likely impact of publication bias) (12). Items will be recorded as ‘Yes’, ‘No’, ‘Partial Yes’, ‘Includes only NRSI’, ‘Includes only RCTs’ or ‘No meta-analysis conducted’. Reviews with no or one non-critical weakness are rated as high quality. Reviews with more than one non-critical weakness are rated as moderate quality. Reviews with one critical flaw with or without non-critical weaknesses are rated as low quality.
2.5 Assessment of certainty of evidence
Two authors independently appraised the certainty of evidence from meta-analyses using the GRADE approach. Any disagreement was resolved by discussion or consultation with a third author.
In the GRADE system, the certainty of evidence is classified as: high, moderate, low, or very low. Evidence based on randomized controlled trials begins as high-quality. Five reasons (study limitation, imprecision, inconsistency of result, indirectness of evidence and reporting bias) may decrease confidence in the evidence (13).
2.6 Data synthesis
A narrative analysis was conducted to summarize the results from the included reviews. Meta-analysis was not performed due to the heterogeneity in study designs among the primary studies. Corrected covered area (CCA) index was assessed to quantify the degree of overlap between systematic reviews to be pooled in an overview (14). CCA values were categorized as representing slight (0-5%), moderate (6-10%), high (11-15%), or very high (>15%) overlap (15). CCA was calculated for the total number of included systematic reviews and for individual non-pharmacological interventions with more than two systematic reviews. Subgroup analyses will be performed regarding amnestic versus non-amnestic MCI, MCI due to Alzheimer’s disease versus due to other conditions when data available for the purpose. The effects of various non-pharmacological interventions were presented in forest plots using Python version 3.15 (Python Software Foundation) and the Matplotlib library (16). The plots were based on the meta-analysis results of the included studies.
3 Results
3.1 Search results
A total of 3,391 reviews were identified. After excluding duplicates, 2569 references were screened through titles and abstracts, of these 36 full-texts were read. Totally, 12 systematic reviews were included. The detailed selection process is illustrated in Figure 1.
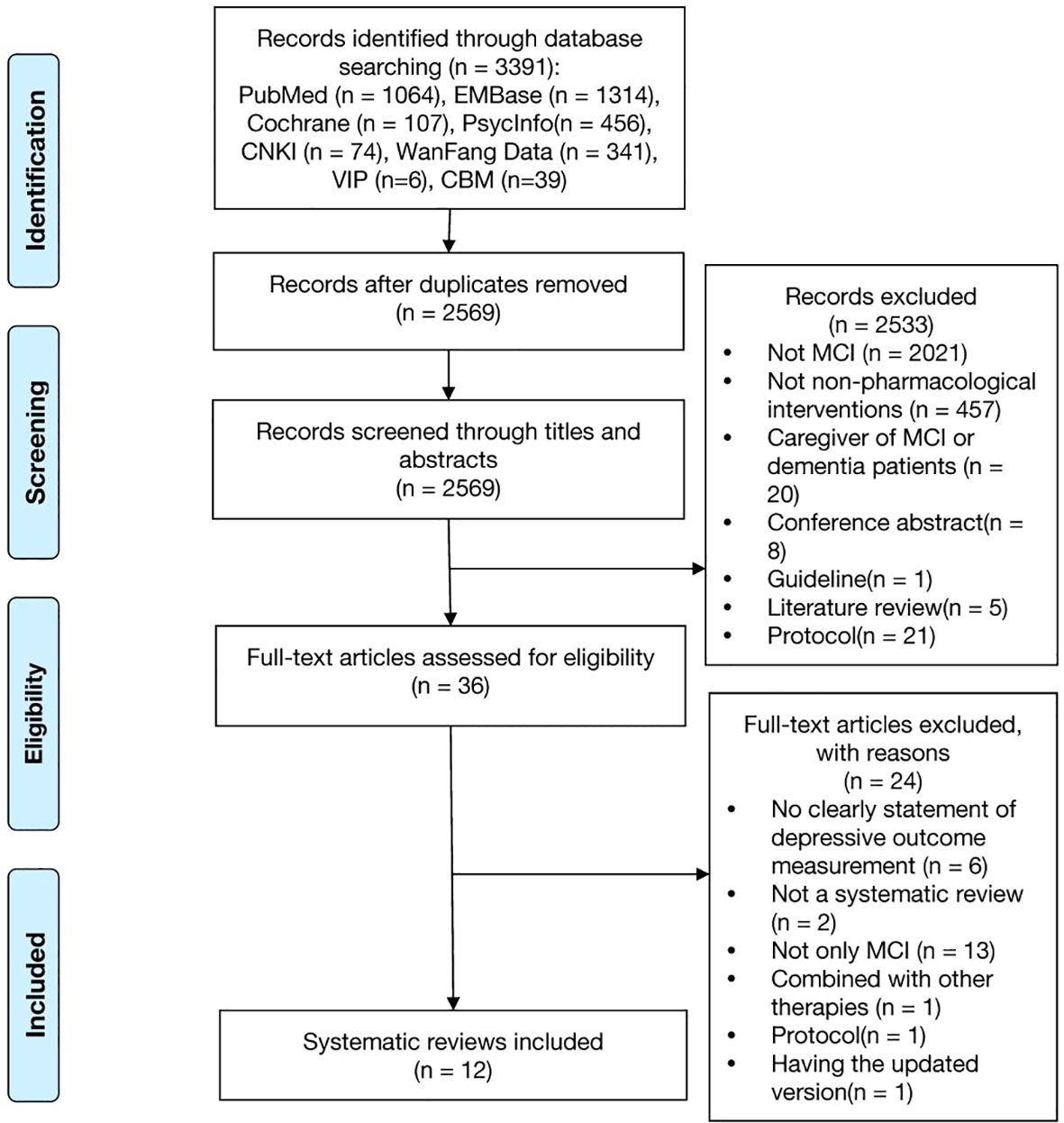
Figure 1. Flow diagram of the systematic review identification process. A lot of studies were excluded during the title and abstract screening because the keywords we used in search strategy (Supplementary Table 1) were not specific, such as, cognitive dysfunction, cognitive disorder, neurocognitive disorder, cognitive decline, mental deterioration, which lead to excluding many studies were not specific on MCI. And we also didn’t set any keywords on non-pharmacological interventions to obtain as much study as possible, which also lead to excluding many studies were not specific on non-pharmacological interventions. In Supplementary Table 2 there were all 24 out of 36 papers excluded after full-text reading, leaving 12 papers included.
3.2 Study characteristics
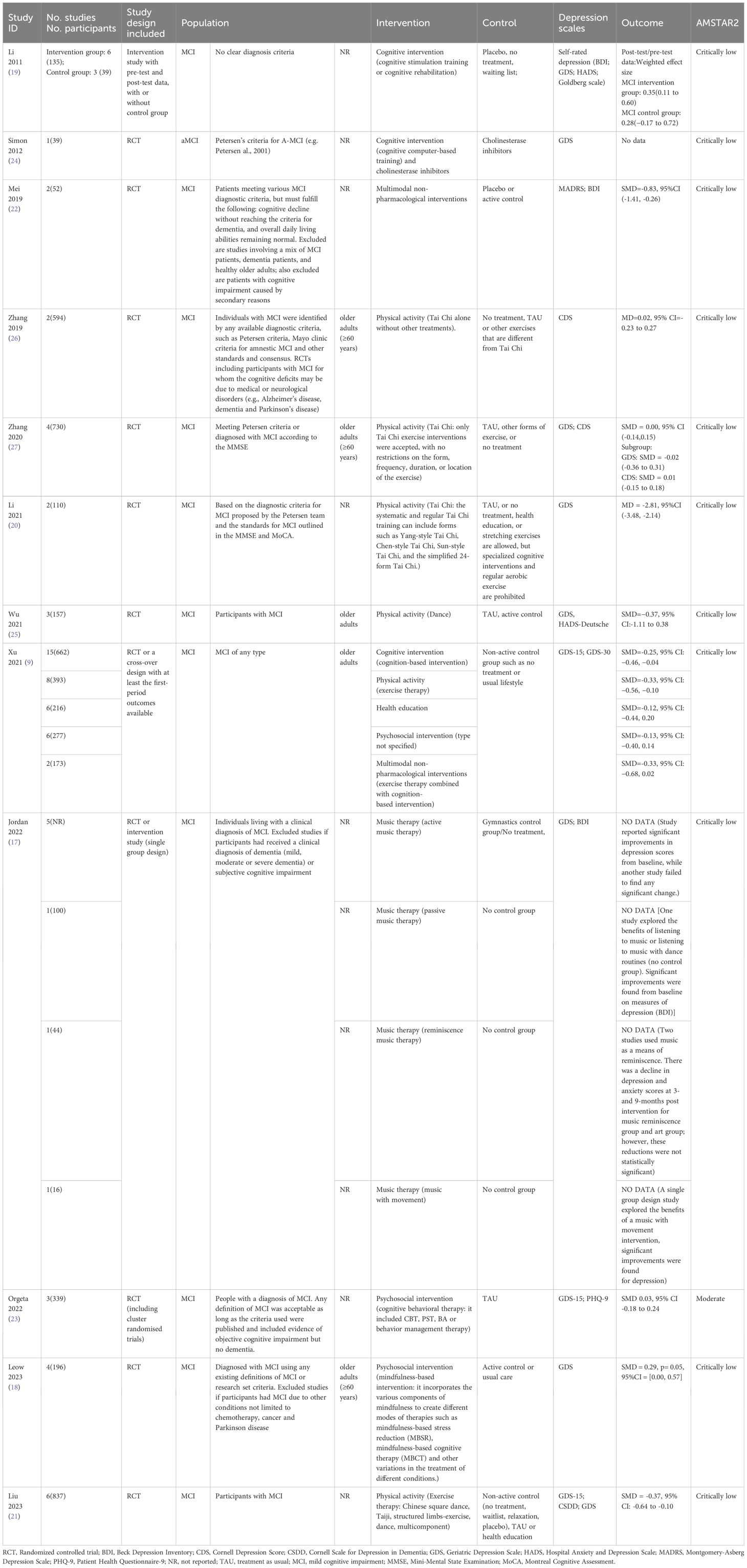
Among the 12 systematic reviews (9, 17–27), 9 reviews included only RCTs (18, 20–27), one review included pre-test and post-test data from both the intervention and control groups (19), one included RCTs or a cross-over design (9), and one included RCTs or observational studies (17). Only one review focused on a specific subtype, amnestic MCI (24), while the others investigated MCI without specifying the subtypes. Some studies, such as Zhang et al. (26) and Li et al. (20), explicitly used Petersen criteria or other established definitions of MCI. However, several studies, including Li et al. (19)and Wu et al. (25), did not specify the exact diagnostic criteria employed. Three reviews focused on cognitive interventions (9, 19, 24), six reviews on physical activities (9, 20, 21, 25–27), three on psychosocial interventions (9, 18, 23), one on music therapy (17), one on health education (9); moreover, there were two on multimodal non-pharmacological interventions (9, 22). None of the included systematic reviews required the presence of depressive symptoms as an inclusion criterion; instead, depressive symptoms were evaluated as an outcome. Most reviews (10/12) used GDS as the measurement index of depression (9, 17–21, 23–25, 27), while three reviews used BDI (17, 19, 22), 2 used CDS (26, 27), 2 used HADS (19, 25), 1 used Goldberg scale (19), 1 used PHQ-9 (23), and 1 used CSDD (21). The characteristics of included reviews are detailed in Table 1.
Ten systematic reviews conducted meta-analyses, while two performed narrative analysis (17, 24). The overlap between primary studies (a total of 51 studies) was 1.8%, indicating slight overlap. The detailed results of overlap are presented in the Supplementary Table 3.
3.3 Quality assessment
One Cochrane systematic review was rated as moderate quality (23), while the other systematic reviews were rated as low quality according to AMSTAR2. The main reasons for the low quality were low scores on two critical items, item 2 and item 7. The certainty of evidence for 14 meta-analyses in 9 systematic reviews was assessed using GRADE with most rated as low or very low certainty; only two were graded as moderate certainty. The detailed results of AMSTAR and GRADE are presented in Supplementary Tables 4, 5.
3.4 Outcomes
Results of the outcomes are presented in Table 1 and Figure 2.
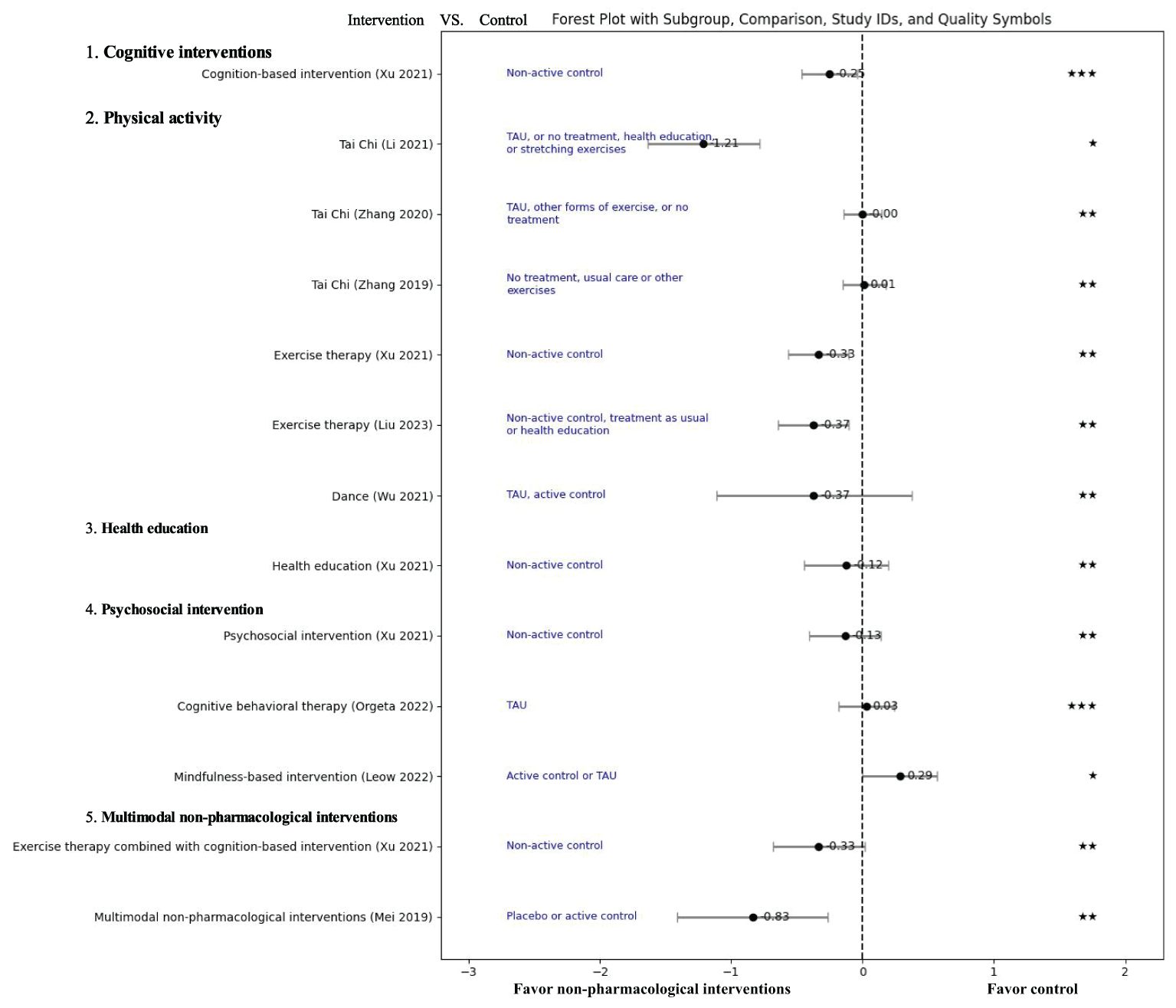
Figure 2. Effect of non-pharmacological treatments on the depressive outcomes in patients with mild cognitive impairment. TUA, Treatment as usual; SMD, Standardized mean difference; GRADE score: “High”: “★★★★”, “Moderate”: “★★★”, “Low”: “★★”, “Very Low”: “★”.
3.4.1 Cognitive interventions
Cognitive intervention focuses on guided practice on tasks target at specific cognitive functions, such as memory, attention, or problem-solving, including but not limited to playing online games or completing cognitive tasks in alignment with a training regimen, playing video games that require visuospatial reasoning, and engaging in novel activities such as art, and music (28, 29). Three systematic reviews on cognitive interventions were included, covering a total of 24 primary studies, with an overlap of 2.1% (9, 19, 24).
Two systematic reviews favored cognitive intervention than the control group in improving depression for MCI patients. Xu et al. focused on cognition-based intervention, but not specifically clarified the content of the intervention (9). The analysis showed that cognitive intervention significantly improved depressive symptoms compared to a non-activity group (SMD=-0.25, 95% CI [−0.46, −0.04], 15 RCTs, 662 participants, GRADE: Moderate) (9). Simon et al. explored the cognitive intervention focusing on cognitive computer-based training (24). This review found that cognitive computer-based training combined with cholinesterase inhibitors was more effective than cholinesterase inhibitors alone in improving depression based on one RCT with 39 participants (24).
Li et al. focused on cognitive stimulation, training or rehabilitation, and conducted a meta-analysis using pre-test and post-test data, which found a small effect of cognitive intervention on self-rated depression, but the effect did not reach statistical significance between intervention (weighted effect size =0.35, 95% CI [0.11, 0.60], 6 studies, 135 participants) and control groups (weighted effect size =0.28, 95% CI [-0.17, 0.72], 3 studies, 39 participants) (Q=0.08, P=0.78) (19).
3.4.2 Physical activity
Physical activity refers to any bodily movement produced by skeletal muscles that requires energy expenditure, including Tai Chi, exercise therapy and dance (7). Six systematic reviews assessed PA (9, 20, 21, 25–27), of which three were about Tai Chi (20, 26, 27), two about exercise therapy (9, 21) and one about dance (25).
3.4.2.1 Tai Chi
Tai Chi is a traditional fitness art from China. Two systematic reviews found no difference between Tai Chi group and control group (MD=0.02, 95% CI [-0.23, 0.27], P=0.89, I2 = 0%, 2 RCTs, 594 participants, GRADE: Low; SMD=0,00 95% CI [-0.14, 0.15], P=0.95, I2 = 0%, 4 RCTs, 730 participants, GRADE: Low) (26, 27). However, Li et al. found that Tai Chi can significantly improve GDS score (MD=-2.81,95%CI [-3.48, -2.14], P<0.001, I2 = 45%, 2RCTs, 110 participants, GRADE: Very low) (20). The overlap between primary studies in 3 systematic reviews (a total of 5 studies) was 30%.
3.4.2.2 Exercise therapy
Exercise therapy is a planned, structured, repetitive and purposeful form of PA, including various types of joint functional training, plyometric training, and aerobic training (30). Two systematic reviews demonstrated that exercise therapy can improve depressive symptoms significantly compared to non-active control groups (SMD=-0.33, 95% CI [−0.56, −0.10], 8 RCTs, 393 participants, GRADE: Low; SMD=−0.37, 95% CI [-0.64, -0.10], P=0.007, I2 = 68%, 6 RCTs, 837 participants, GRADE: Low) (9, 21). The overlap between primary studies (a total of 26 studies) was 7.69%.
3.4.2.3 Dance
Dance is a universal cultural expression that transcends time and provides valuable physical activity, extremely advantageous together with music therapy facilitating emotional bonding and enhancing adherence in older adults (31, 32). A meta-analysis by Wu et al. in 2021 found no significant difference in depression scores between the dance group and control group (SMD=−0.37, 95% CI [-1.11, 0.38], P=0.34, I2 = 80%, 3 RCTs, 157 participants, GRADE: Low) (25).
3.4.3 Psychosocial intervention
Psychosocial intervention refers to planned step-by-step processes aimed at influencing the psychological activities, personality traits, or psychological problems based on psychological theories, with the goal of achieving desired changes (7). Three systematic reviews have examined psychosocial interventions (9, 18, 23), with two focusing specifically on cognitive behavioral therapy (23) and mindfulness-based intervention (18), and one exploring the effect of psychosocial intervention type not specified.
3.4.3.1 Cognitive behavioral therapy
Cognitive behavioral therapy is a psychosocial intervention aimed at improving mental health by developing strategies to change negative thoughts and beliefs, thus adapting to adverse emotions and behaviors (33). Orgeta et al. conducted a systematic review which revealed no significant difference between cognitive behavioral therapy and no treatment (treatment as usual) or non-specific psychosocial activities for MCI with depression (SMD=0.03,95% CI [-0.18, 0.24], P=0.77, I2 = 0%, 3 RCTs, 339 participants, GRADE: Moderate) (23).
3.4.3.2 Mindfulness-based intervention
Mindfulness-based intervention was originally developed for mindfulness-based intervention stress reduction program, which help individuals improve their overall well-being (34, 35). In 2023, Leow et al. conducted a meta-analysis on MBI for treating depression, and the results favored the control group receiving psychoeducation, health education programs, cognitive training and treatment as usual (SMD=0.29, 95% CI [0.00, 0.57], P=0.05, I2 = 0%, 4 RCTs, 196 participants, GRADE: Very Low) (18).
3.4.3.3 Psychosocial intervention type not specified
The meta-analysis focusing on psychosocial intervention type not specified revealed no significant difference in depression scores between the psychosocial intervention group and the non-active control group (SMD=-0.13, 95% CI [−0.40, 0.14], 6 RCTs, 277 participants, GRADE: Low) (9).
3.4.4 Music therapy
Music therapy involves various activities designed to improve mood and motivation, whether through listening to selected recordings or live performances by a music therapist, or through actively participating in the creation of music (36, 37). Jordan et al. reported the effects of four types of music therapies (passive, active, reminiscence, and music with movement) on depression in MCI patients through a narrative analysis (17). Lack of quantitative data from this included narrative review, we were unable to present meta-analyses with GRADE assessments of music therapy on depression in MCI patients; instead, a narrative description was provided.
Passive music therapy involves listening to pre-recorded or live music performed by a therapist, that is, music listening. The review showed that passive music therapy can significantly improve depression according to two single-group studies.
Active music therapy refers to actively participating in the creation of music, that is, music playing. Two RCTs on active music therapy yielded contradictory results on depression when assessed by different scales: one study showed a significant reduction of active music therapy than gymnastics in depression when assessed by GDS scores, while another study found no significant difference between active music therapy and no intervention in depression when assessed by BDI scores.
Reminiscence music therapy refers to using music to evoke memories and emotions from the past, that is, music listening purpose to evoke memories. The narrative review did not show significant improvement in depression after reminiscence music therapy.
Music with movement combines music with physical movement or dance. The review showed significant improvement in depression in favor of music with movement.
3.4.5 Health education
Health education involves educational activities and processes that help individuals and groups gain health knowledge, establish health concepts, and adopt beneficial health behaviors through information dissemination and behavioral interventions (38, 39). A meta-analysis found that health education cannot significantly improve depression compared to a non-active control group (SMD=-0.12, 95% CI [−0.44, 0.20], 6 RCTs, 216 participants, GRADE: Low) (9).
3.4.6 Multimodal non-pharmacological interventions
Multimodal non-pharmacologic interventions involve the use of two or more non-pharmacologic therapies. Two systematic reviews have examined multimodal non-pharmacologic interventions (9, 22, 40).
Xu et al. compared an exercise therapy plus cognitive intervention to non-active control, and the meta-analysis showed no significant difference in improving depression between the two groups (MD=-0.33, 95% CI [−0.68, 0.02], 2 RCTs, 173 participants, GRADE: Low) (9).
Mei et al. compared multimodal non-pharmacologic interventions, including cognitive intervention, physical activity, and stress management, to placebo or positive intervention (22). The meta-analysis found a significant improvement in depression score for multimodal non-pharmacologic interventions (2 RCTs, 52 participants, SMD=-0.83,95% CI [-1.41, -0.26], P=0.005, I2 = 0%, GRADE: Low).
4 Discussion
4.1 Key findings
Our comprehensive review of 12 systematic reviews, spanning from 2011 to 2023, highlights the diverse landscape of six types of non-pharmacological interventions targeting at depressive symptoms in MCI. These interventions include cognitive interventions (general, computer-based, cognitive rehabilitation/stimulation), physical activity (Tai Chi, exercise therapy, dance), psychosocial interventions (cognitive behavioral therapy, mindfulness-based intervention), music therapy, health education, and multimodal non-pharmacological interventions, each demonstrating varying levels of effectiveness. Specifically, cognitive interventions (general or computer-based) and exercise therapy (a type of physical activity) show preliminary potential to improve depressive symptoms. In contrast, dance (a type of physical activity), health education, and psychosocial interventions do not show significant effects based on the systematic reviews included. The effectiveness of Tai Chi (a type of physical activity), music therapy and multimodal non-pharmacological interventions showed inconsistency across studies. The strength of the evidence is rated as low to very low for all non-pharmacological interventions, except for that on cognitive intervention (general) and cognitive behavior therapy (a psychosocial intervention).
4.2 Strengths and weaknesses of the review
This study undertook a comprehensive overview on non-pharmacological interventions for depression in MCI, with no language restrictions. This effort not only responds to the WHO’s call for evidence-based practices in managing depression in non-specialized health settings, but also addresses the critical implications of depression on cognitive health in aging populations. We included systematic reviews of any types of primary studies as well as narrative reviews to obtain this complete evidence map of all potential non-pharmacological interventions at systematic review level. And this study can provide information of inconsistent results from different systematic reviews on the same topic. We assessed the outcomes on depression by the GRADE to determine the information on the strength of evidence. We critically appraised the included systematic reviews by AMSTAR2 tool to provide levels of methodological quality of included evidence. The quality assessments facilitate the readers interpreting the effects of non-pharmacological interventions with reference to the quality of the supporting evidence, and thus making the clinical decisions more rationally. To avoid the potential duplicate use of primary studies results and the ensuing false precision in the analysis, we have calculated the CCA to evaluate the overlap of primary studies across the systematic reviews. The observed low CCA (1.8%) indicates minimal overlap among the original studies, suggesting that our conclusions are based on a diverse set of primary studies. Lastly, this study only included MCI patients considering the heterogeneity among the population of healthy population, MCI, and dementia.
The review has limitations as well. Most of these systematic reviews acknowledged the poor quality of the included primary trials, which is also reflected in GRADE scores. Heterogeneity exists among the included systematic reviews due to variations in diagnostic criteria, study design of included primary studies, definition of interventions, comparison groups, scales to assess depressive symptoms, which complicates data interpretation and affects the reliability and generalizability of our findings. For example, a systematic review exploring the effect of active music therapy revealed inconsistent findings on the improvements of depressive symptoms, that is, being effective as assessed by GDS while ineffective assessed by BDI (17). Inconsistent measurements of depressive symptoms hinder the integration and comparison of the effect of non-pharmacological interventions.
4.3 Comparison to current recommendations
From this comprehensive overview of systematic reviews, there is some evidence of improvement in depression by non-pharmacological interventions including cognitive interventions (general or computer-based) and physical activity (exercise therapy). Our review aligns with previous recommendations on cognitive interventions by MCI guidelines and consensus statements. Petersen et al. stated that clinicians may recommend cognitive interventions for MCI patients (41); and Kandiah et al. recommended that the management strategy should at least include cognitive training (42). The recommendations mainly focus on cognitive training due to its potential in improving the overall cognitive function and functions of multiple cognitive domains (43). Our study provided certain evidence for supporting the use of cognitive interventions, general or computer-based, in improving depressive symptoms of MCI patients; but the importance of dissecting and clarifying the content of cognitive interventions is stressed to further understand the potential varied impacts. Previous guidelines and consensus statements have recommended physical activity in management of MCI: physical activity may be recommended to adults with MCI to reduce the risk of cognitive decline (7); and clinicians should recommend regular exercise (twice/week) as part of an overall approach to management (41). Physical activity is also recommended involving aerobic exercise for dementia from the perspective of improving cognitive outcomes (44). However, the recommendation of physical activity related to improvement of depression remains unclear. Besides exercise therapy, our overview obtained evidence on Tai Chi and Dance in management of depression in MCI, but the conclusions from systematic reviews were inconsistent or negative (20, 25–27). The guideline also mentioned that the current recommendation about physical activity is conditional based on low quality of evidence (7).
Besides the cognitive interventions and physical activity (exercise therapy), a previous analysis of 13 available MCI clinical practice guidelines recommended non-pharmacological interventions such as dietary and nutritional interventions, acupuncture, and counseling (2). In our overview of systematic reviews, there is no evidence found of dietary and nutritional interventions, and acupuncture on depression in MCI. However, dietary interventions and acupuncture have shown potential benefits for cognitive function in MCI population (45, 46). Xu et al. revealed no significant effect of health education on depression in MCI (9). However, Petersen et al. recommended that clinicians should counsel patients and families to discuss long-term planning topics such as advance directives, driving safety, finances, and estate planning (41). Health education and counseling usually come together but perhaps with slight difference. Health education is to equip patients with accurate and right knowledge, while counseling helps them to apply that knowledge by changing their attitudes and behaviors.
Despite the findings in this overview indicating that psychosocial interventions, such as cognitive behavioral therapy and mindfulness-based interventions, did not result in significant improvements in depressive symptoms in MCI patients, this does not negate their potential values. According to the WHO mhGAP Intervention Guide, psychosocial treatments recommended for adults with moderate to severe depressive disorder include psychoeducation, addressing psychosocial stressors, reactivating social networks, brief psychological treatments, and offering regular follow-up (47). While the WHO also notes the current lack of sufficient evidence for these interventions in managing depression and reducing the risk of cognitive decline/dementia, yet emphasizes their importance for other benefits without advising against their use (7). Psychosocial interventions may still be considered valuable in therapeutic strategies for MCI patients, potentially due to offering benefits beyond direct symptom relief such as enhancing overall well-being and quality of life.
4.4 Relevance to clinical practice and research
The analyses in our overview mostly obtain the general effect of non-pharmacological interventions on depressive outcomes of MCI, however, clinical decisions often depend on individual patient characteristics. In need of individually based clinical practice guidelines, it is necessary to report analyses on specific MCI subgroups by identifying whether there is consistency of the effect of non-pharmacological interventions among different patient groups. In view of its value to both patients and clinicians, we planned to perform subgroup analyses regarding different clinical types of MCI, such as amnestic versus non-amnestic MCI, MCI due to Alzheimer’s disease versus due to other conditions. However, only one included SR explicitly addressed patients with amnestic MCI while the others did not specify the type (24). We were unable to conclude on the effect of non-pharmacological interventions regarding different types of MCI currently. As no specific accepted test and cutoff score by the guidelines for the diagnosis of MCI (2), researches on the difference between different types of MCI mostly focus on how to differentiate one from another in diagnosis (48), but few discussed their potential varied responses to certain interventions. For the effectiveness evaluation studies in future, it is advocated to design more specifically on population of MCI, thereby drawing clearer pictures of the overall treatment effect of non-pharmacological interventions across certain MCI subgroups, and perhaps providing some patients with its benefits and protecting others from its harm. Moreover, researches comparing the effect of certain non-pharmacological intervention between different schemes (e.g. long-term versus short-term, high- versus low-frequency) are also expected to find the ‘optimal component’ of this intervention in playing the acts on depressive symptoms of MCI population.
Cognitive decline or dementia is a rapidly growing global public health issue. Except for age, several potentially modifiable risk factors and medical conditions are associated with the increased risk, meaning that the prevention of cognitive decline is possible through a public health approach, including the implementation of key interventions target at different risk factors and comorbidities (7). Non-pharmacological interventions have shown potential benefits for medical conditions related to cognitive decline (e.g. diabetes, hypertension) as well as dietary risk factors (e.g. smoking cessation) of MCI (49–51). Even though the implementation of non-pharmacological interventions in MCI patients for managing depression needs more robust evidence, clinicians may recommend these interventions from the perspective of reducing risk factors of cognitive decline and commodities. The patients with MCI could at least consider the convenient non-pharmacological interventions such as changing physical inactivity and unhealthy diets.
5 Conclusion
This overview highlights the diverse landscape of non-pharmacological interventions, including cognitive interventions, physical activity, psychosocial interventions, music therapy, health education, and multimodal non-pharmacological interventions, for depressive symptoms in MCI. Cognitive interventions (general or computer-based) and exercise therapy (a type of physical activity) show preliminary potential to improve depressive symptoms, while others do not show significant effects or relate to confused effects. Further methodologically rigorous and adequately powered primary studies are necessary for each of these non-pharmacological interventions, with reporting on the components of the interventions clearly in MCI patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
CY: Conceptualization, Writing – original draft. YL: Writing – original draft. CZ: Writing – review & editing. HZ: Formal analysis, Writing – review & editing. ZF: Conceptualization, Formal analysis, Writing – review & editing. LZ: Writing – review & editing. FL: Writing – review & editing. LY: Validation, Visualization, Writing – review & editing. DX: Validation, Visualization, Writing – review & editing. CQ: Validation, Visualization, Writing – review & editing. LH: Formal analysis, Validation, Writing – review & editing. ZH: Formal analysis, Writing – review & editing. LN: Validation, Writing – review & editing. SN: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021A05505).
Acknowledgments
During the preparation of this work, the author(s) used Chat Generative Pre-trained Transformer 3.5 (ChatGPT 3.5) to improve readability and language. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1415113/full#supplementary-material
References
1. Anderson ND. State of the science on mild cognitive impairment (MCI). CNS Spectrums. (2019) 24:78–87. doi: 10.1017/S1092852918001347
2. Chen Y, Liang N, Li X, Yang S, Wang Y, Shi N-N. Diagnosis and treatment for mild cognitive impairment: A systematic review of clinical practice guidelines and consensus statements. Front Neurol. (2021) 12:719849. doi: 10.3389/fneur.2021.719849
3. Shahnawaz Z, Reppermund S, Brodaty H, Crawford J, Draper B, Trollor J, et al. Prevalence and characteristics of depression in mild cognitive impairment: the Sydney Memory and Ageing Study. Acta psychiatrica Scandinavica. (2013) 127:394–402. doi: 10.1111/acps.2013.127.issue-5
4. Gabryelewicz T, Styczynska M, Pfeffer A, Wasiak B, Barczak A, Luczywek E, et al. Prevalence of major and minor depression in elderly persons with mild cognitive impairment–MADRS factor analysis. Int J Geriatric Psychiatry. (2004) 19:1168–72. doi: 10.1002/gps.v19:12
5. Ismail Z, Elbayoumi H, Fischer CE, Hogan DB, Millikin CP, Schweizer T, et al. Prevalence of depression in patients with mild cognitive impairment: A systematic review and meta-analysis. JAMA Psychiatry. (2017) 74:58–67. doi: 10.1001/jamapsychiatry.2016.3162
6. Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer Report 2014-Dementia and Risk Reduction: An analysis of protective and modifiable risk factors. London: Alzheimer’s Disease International (2014). 99 p.
7. WHO. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization (2019). 78 p.
8. Wang X, Zhang Y, Yu H, Yang F, Li Y, Li Y. Efect of computerized cognitive training in elderly with mild cognitive impairment: a meta-analysis. Clin Focus. (2019) 34:843–9. doi: 10.3969/j.issn.1004-583X.2019.09.017
9. Xu Z, Sun W, Zhang D, Chung VC-H, Wong SY-S. Comparative effectiveness of non-pharmacological interventions for depressive symptoms in mild cognitive impairment: Systematic review with network meta-analysis. Aging Ment Health. (2021) 26:2129–35. doi: 10.1080/13607863.2021.1998356
10. Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. (2005) 62:1160–3; discussion 7. doi: 10.1001/archneur.62.7.1160
11. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. (2011) 7:270–9. doi: 10.1016/j.jalz.2011.03.008
12. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
13. Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. (2009) 64:669–77. doi: 10.1111/j.1398-9995.2009.01973.x
14. Lunny C, Pieper D, Thabet P, Kanji S. Managing overlap of primary study results across systematic reviews: practical considerations for authors of overviews of reviews. BMC Med Res Methodol. (2021) 21:140. doi: 10.1186/s12874-021-01269-y
15. Pieper D, Antoine S-L, Mathes T, Neugebauer E, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. (2014) 67:368–75. doi: 10.1016/j.jclinepi.2013.11.007
16. Hunter JD. Matplotlib: A 2D graphics environment. Computing Sci Engineering. (2007) 9:90–5. doi: 10.1109/MCSE.2007.55
17. Jordan C, Lawlor B, Loughrey D. A systematic review of music interventions for the cognitive and behavioural symptoms of mild cognitive impairment (non-dementia). J Psychiatr Res. (2022) 151:382–90. doi: 10.1016/j.jpsychires.2022.04.028
18. Leow Y, Rashid NLBA, Klainin-Yobas P, Zhang Z, Wu XV. Effectiveness of mindfulness-based interventions on mental, cognitive outcomes and neuroplastic changes in older adults with mild cognitive impairment: A systematic review and meta-analysis. J Advanced Nursing. (2023) 79:4489–505. doi: 10.1111/jan.v79.12
19. Li H, Li J, Li N, Li B, Wang P, Zhou T. Cognitive intervention for persons with mild cognitive impairment: A meta-analysis. Ageing Res Rev. (2011) 10:285–96. doi: 10.1016/j.arr.2010.11.003
20. Li W, Xiang Q, Fan T. A Meta-analysis of efficacy of Tai Chi on cognitive function in patients with mild cognitive impairment. Clin J Chin Med. (2021) 13:129–36. doi: 10.3969/j.issn.1674-7860.2021.10.045
21. Liu X, Wang G, Cao Y. The effectiveness of exercise on global cognitive function, balance, depression symptoms, and sleep quality in patients with mild cognitive impairment: A systematic review and meta-analysis. Geriatric Nursing. (2023) 51:182–93. doi: 10.1016/j.gerinurse.2023.03.013
22. Mei X, Zhao X, Wang Y, Wang L, Cao H. Efficacy of multimodal nonpharmacological interventions in mild cognitive impairment: A meta-analysis. Chin J Evidence-Based Med. (2019) 19:180–8. doi: 10.7507/1672-2531.201803015
23. Orgeta V, Leung P, del-Pino-Casado R, Qazi A, Orrell M, Spector AE, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Systematic Rev. (2022) 4:CD009125. doi: 10.1002/14651858.CD009125.pub3
24. Simon SS, Yokomizo JE, Bottino CMC. Cognitive intervention in amnestic mild cognitive Impairment: A systematic review. Neurosci Biobehav Rev. (2012) 36:1163–78. doi: 10.1016/j.neubiorev.2012.01.007
25. Wu VX, Chi Y, Lee JK, Goh HS, Chen DYM, Haugan G, et al. The effect of dance interventions on cognition, neuroplasticity, physical function, depression, and quality of life for older adults with mild cognitive impairment: A systematic review and meta-analysis. Int J Nurs Stud. (2021) 122:104025. doi: 10.1016/j.ijnurstu.2021.104025
26. Zhang Q, Hu J, Wei L, Cao R, Ma R, Song H, et al. Effects of traditional Chinese exercise on cognitive and psychological outcomes in older adults with mild cognitive impairment: A systematic review and meta-analysis. Med (Baltimore). (2019) 98:e14581. doi: 10.1097/MD.0000000000014581
27. Zhang Q, Song H, Cao R, Sun X, Jin Y. Effects of Tai Chi on cognitive function for aged with Mild Cognitive Impairment: a Meta-analysis. Chinese Nursing Manag. (2020) 20:865–71. doi: 10.1097/MD.0000000000014581
28. Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. (2013) 2013:Cd003260. doi: 10.1002/14651858.CD003260.pub2
29. Bahar-Fuchs A, Martyr A, Goh AM, Sabates J, Clare L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev. (2019) 3:Cd013069. doi: 10.1002/14651858.CD013069.pub2
30. Bigarella LG, Ballotin VR, Mazurkiewicz LF, Ballardin AC, Rech DL, Bigarella RL, et al. Exercise for depression and depressive symptoms in older adults: an umbrella review of systematic reviews and Meta-analyses. Aging Ment Health. (2022) 26:1503–13. doi: 10.1080/13607863.2021.1951660
31. Wu C, Xiong H, Zheng J, Wang X. Dance movement therapy for neurodegenerative diseases: A systematic review. Front Aging Neurosci. (2022) 14:975711. doi: 10.3389/fnagi.2022.975711
32. Menezes AC, Drumond G, Shigaeff N. Dance therapy and cognitive impairment in older people: A review of clinical data. Dementia e Neuropsychologia. (2022) 16:373–83. doi: 10.1590/1980-5764-dn-2021-0103
33. Pettitt RM, Brown EA, Delashmitt JC, Pizzo MN. The management of anxiety and depression in pediatrics. Cureus. (2022) 14:e30231. doi: 10.7759/cureus.30231
34. Pérez V, Menéndez-Crispín EJ, Sarabia-Cobo C, de Lorena P, Fernández-Rodríguez A, González-Vaca J. Mindfulness-based intervention for the reduction of compassion fatigue and burnout in nurse caregivers of institutionalized older persons with dementia: A randomized controlled trial. Int J Environ Res Public Health. (2022) 19:11441. doi: 10.3390/ijerph191811441
35. Yang J, Du Y, Shen H, Ren S, Liu Z, Zheng D, et al. Mindfulness-based movement intervention to improve sleep quality: A meta-analysis and moderator analysis of randomized clinical trials. Int J Environ Res Public Health. (2022) 19:10284. doi: 10.3390/ijerph191610284
36. Bacus IP, Mahomed H, Murphy A-M, Connolly M, Neylon O, O’Gorman C. Play, art, music and exercise therapy impact on children with diabetes. Irish J Med Science. (2022) 191:2663–8. doi: 10.1007/s11845-021-02889-5
37. Rennie C, Irvine DS, Huang E, Huang J. Music therapy as a form of nonpharmacologic pain modulation in patients with cancer: A systematic review of the current literature. Cancers (Basel). (2022) 14:4416. doi: 10.3390/cancers14184416
38. Anitha CT, Akter K, Mahadev K. An overview of public health education in South Asia: Challenges and opportunities. Front Public Health. (2022) 10:909474. doi: 10.3389/fpubh.2022.909474
39. Ashish J, Ashruti B, Mansi G, Ashoo G, Rani SS, Vikas MI. The current state of public health education in India: A scoping review. Front Public Health. (2022) 10:970617. doi: 10.3389/fpubh.2022.970617
40. Wang X, Wang H, Ye Z, Ding G, Li F, Ma J, et al. The neurocognitive and BDNF changes of multicomponent exercise for community-dwelling older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Aging (Albany NY). (2020) 12:4907–17. doi: 10.18632/aging.102918
41. Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
42. Kandiah N, Chan YF, Chen C, Dasig D, Dominguez J, Han SH, et al. Strategies for the use of Ginkgo biloba extract, EGb 761(®), in the treatment and management of mild cognitive impairment in Asia: Expert consensus. CNS Neurosci Ther. (2021) 27:149–62. doi: 10.1111/cns.13536
43. Jia J. Writing group of Chinese expert consensus of cognitive training, and Chinese medical doctor association neurologist branch cognitive disorders professional committee. Chin Expert consensus Cogn training Natl Med J China. (2019) 99:4–8. doi: 10.3760/cma.j.issn.0376-2491.2019.01.002
44. Ismail Z, Black SE, Camicioli R, Chertkow H, Herrmann N, Laforce R Jr., et al. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement. (2020) 16:1182–95. doi: 10.1002/alz.12105
45. McGrattan AM, McEvoy CT, McGuinness B, McKinley MC, Woodside JV. Effect of dietary interventions in mild cognitive impairment: a systematic review. Br J Nutr. (2018) 120:1388–405. doi: 10.1017/S0007114518002945
46. Yin Z, Li Y, Jiang C, Xia M, Chen Z, Zhang X, et al. Acupuncture for mild cognitive impairment: A systematic review with meta-analysis and trial sequential analysis. Front Neurol. (2022) 13:1091125. doi: 10.3389/fneur.2022.1091125
47. WHO. mhGAP Intervention Guide for mental, neurological and substance use disorders in non-specialized health settings Version 2.0. Geneva: World Health Organization (2019). 173 p.
48. Kim J-G, Kim H, Hwang J, Kang SH, Lee C-N, Woo J, et al. Differentiating amnestic from non-amnestic mild cognitive impairment subtypes using graph theoretical measures of electroencephalography. Sci Rep. (2022) 12:6219. doi: 10.1038/s41598-022-10322-9
49. Lee D, Lee H, Shin Y, Park G. Effectiveness of non-pharmacological interventions for adolescents with type 1 diabetes in the last five years: A systematic review and meta-analysis. Asian Nurs Res. (2024) 18:51–9. doi: 10.1016/j.anr.2024.01.008
50. Nian T, Guo K, Liu W, Deng X, Hu X, Xu M, et al. Non-pharmacological interventions for smoking cessation: analysis of systematic reviews and meta-analyses. BMC Med. (2023) 21:378. doi: 10.1186/s12916-023-03087-z
Keywords: mild cognitive impairment, depression, non-pharmacological interventions, music therapy, exercise therapy, cognitive interventions, psychosocial intervention, health education
Citation: Yaxin C, Lijiao Y, Zhao C, Ziteng H, Fuqiang Z, Zhenhong L, Luda F, Yixiang L, Xiangwei D, Qianzi C, Huizhen L, Haili Z, Ning L and Nannan S (2025) Effects of non-pharmacological interventions on the depressive outcomes in people with mild cognitive impairment: an overview of systematic reviews. Front. Psychiatry 15:1415113. doi: 10.3389/fpsyt.2024.1415113
Received: 12 April 2024; Accepted: 23 December 2024;
Published: 15 January 2025.
Edited by:
Francesco Panza, University of Bari Aldo Moro, ItalyReviewed by:
Daniele Corbo, University of Brescia, ItalyNatalia Roberto Herrero, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), Spain
Copyright © 2025 Yaxin, Lijiao, Zhao, Ziteng, Fuqiang, Zhenhong, Luda, Yixiang, Xiangwei, Qianzi, Huizhen, Haili, Ning and Nannan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Ning, bGlhbmduaW5nMjI5QDE2My5jb20=; Shi Nannan, MTM4MTE4MzkxNjRAdmlwLjEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Chen Yaxin1,2†
Chen Yaxin1,2† Yan Lijiao
Yan Lijiao Chen Zhao
Chen Zhao Hu Ziteng
Hu Ziteng Liu Zhenhong
Liu Zhenhong Feng Luda
Feng Luda Che Qianzi
Che Qianzi Li Huizhen
Li Huizhen Zhang Haili
Zhang Haili Liang Ning
Liang Ning Shi Nannan
Shi Nannan