- 1First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Gastroenterology Department, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Gastrointestinal and Hernia Surgery, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
Background: Alcohol consumption, depression, and chronic diarrhea are all public health issues of concern, with irreversible consequences for individual health and significant economic burdens on health care systems. Previous studies have shown that depression increases the risk of developing chronic diarrhea, but few studies have explored whether alcohol consumption has an effect on the relationship between depression and chronic diarrhea.
Objective: To explore the effect of alcohol consumption on the relationship between depression and chronic diarrhea.
Methods: 12,538 adults (≥20 years) in NHANES from 2005-2010 were analyzed. Participants were stratified according to drinking status, and differences between the risk of depression and chronic diarrhea among participants who drank alcohol or not were assessed using multiple regression analysis and likelihood ratio tests.
Results: In this cross sectional, after adding possible confounders, the prevalence of depression with chronic diarrhea was higher in the drinking population than in the non-drinking population (OR,2.34, 95%CI:1.84-2.98 and 1.26, 95%CI:0.85-1.86), with a likelihood ratio test of P=0.024.
Conclusion: Our findings suggest that there is a significant association between depression and chronic diarrhea and that alcohol consumption may increase the correlation between depression and chronic diarrhea. However, these findings require further prospective studies to provide more evidence.
Introduction
Depression is a type of illness caused by various factors, with low mood as the main symptom. It is characterized by loss of interest, feelings of guilt, difficulty concentrating, decreased appetite, suicidal thoughts, and other cognitive, behavioral, and social functional abnormalities (1). According to World Health Organization (WHO) statistics, approximately 350 million people worldwide are currently suffering from depression. In the United States, the 12-month and lifetime prevalence rates of depression are 10.4% and 20.6%, respectively, with a higher incidence among females than males (2–4). In both developed and developing countries, depression is the second leading cause of the global disease burden (5, 6). Depression can increase the risk of developing diabetes, heart disease, hypertension, obesity, cancer, cognitive impairments, and Alzheimer’s disease (7–13). Some studies suggest that the occurrence of chronic diarrhea is closely associated with depression (14, 15). The all-cause mortality rate for depression is around 10%. If accompanied by other comorbidities, the risk of mortality increases by 60-80%. Studies have shown that eliminating depression entirely could lead to a 10% decrease in mortality rate (1, 16, 17).
Diarrhea is defined as passing loose or liquid stools three or more times a day and/or having a stool weight exceeding 200 grams (18). The distinction between chronic diarrhea and acute diarrhea mainly depends on the duration: 4 weeks is a commonly used dividing line (19). In global statistics, the prevalence of chronic diarrhea is approximately 3-20% (20). Chronic diarrhea does not increase the risk of mortality in patients, but the detection rates of organic diseases such as colonic polyps and ulcerative colitis are significantly higher compared to other populations (21), This greatly impacts human health and quality of life, while also causing a substantial economic burden on society.
In our perception, individuals with depression often engage in alcohol abuse, and research has indeed confirmed the correlation between the two (22, 23). However, it is still unknown whether there is a change in the risk of diarrhea among individuals with depression who consume alcohol. Therefore, we are conducting a study to investigate the impact of alcohol consumption on the relationship between depression and diarrhea.
Methods
Study design and data source
This study was designed as a cross-sectional analysis using data from the National Health and Nutrition Examination Survey (NHANES) database (https://www.cdc.gov/nchs/nhanes/index.htm), which is collected by the National Center for Health Statistics (NCHS). NHANES gathers valuable health statistics that are utilized by public health officials, legislators, and doctors to formulate effective health policies, develop health programs and services, and enhance knowledge about national health concerns. The survey findings will aid in determining the prevalence of major diseases and risk factors associated with them. Additionally, the information will be used to assess nutritional status and its correlation with health promotion and disease prevention. Data in NHANES were de-identified and the study was approved by the National Ethical Review Board for Research in Health Statistics (ERB) (https://www.cdc.gov/nchs/nhanes/irba98.htm). This study was conducted in accordance with relevant guidelines and regulations (https://www.cdc.gov/nchs/data_access/restrictions.htm).
Study population
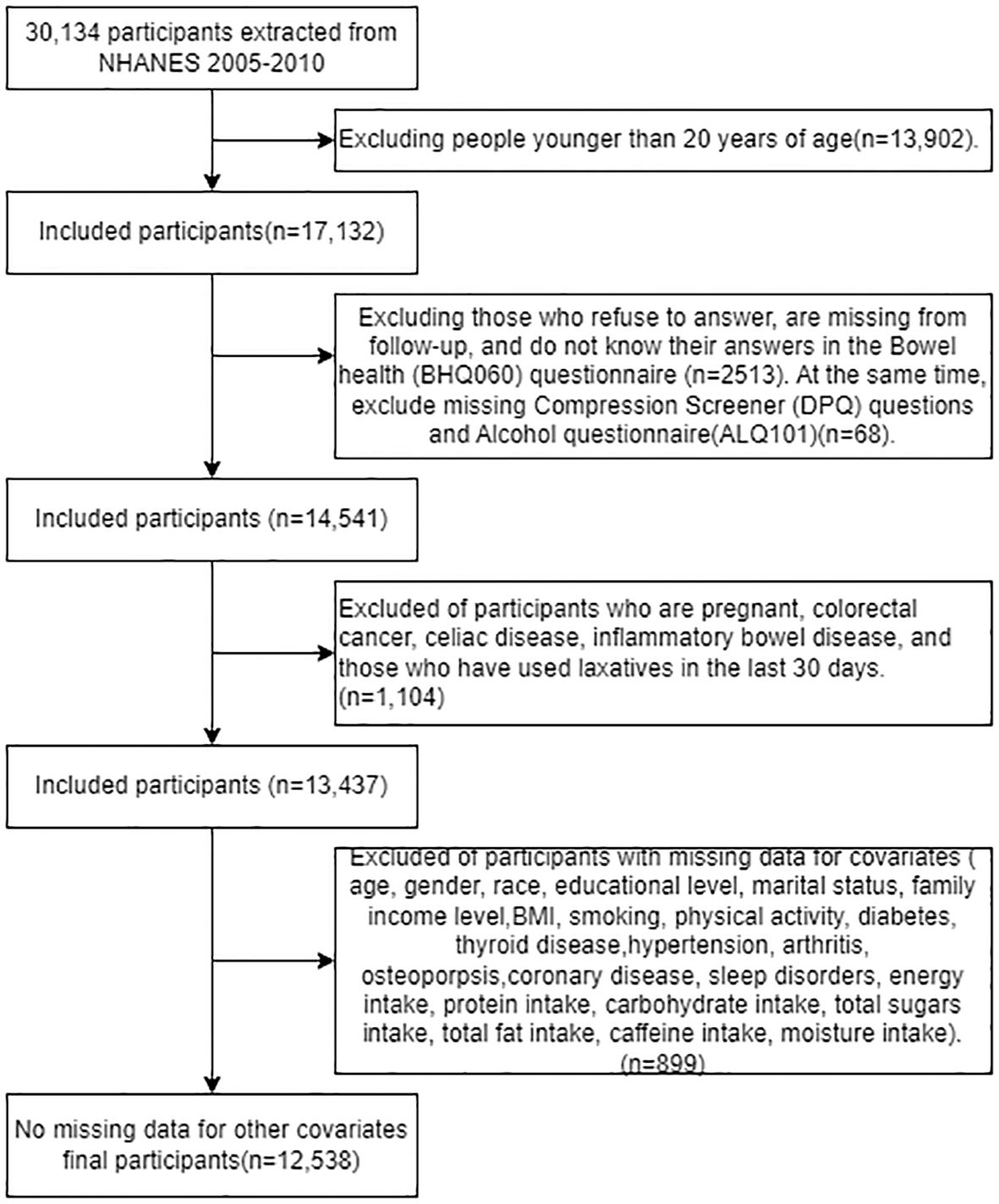
Participants were drawn from NHANES records from mid-2005-2010, as these were the only three cycles in which the bowel health portion of the Mobile Examination Center (MEC) interview provided personal interview data on fecal incontinence and bowel function in adults aged 20 years and older (24). We extracted data from 20,134 participants in the NHANES dataset from 2005 to 2010; among them, 17,132 adults (≥20 years old) completed the gastrointestinal health questionnaire. Excluding invalid information from the gastrointestinal health questionnaire, depression screening questionnaire, and alcohol use questionnaire (n=2581), as well as excluding pregnant individuals, patients with colorectal cancer, individuals with abdominal diseases, those suffering from inflammatory bowel disease, and those who had used laxatives in the past 30 days (n=1104). After excluding participants with missing covariate data (n=899), 12,538 participants were included in our analysis, the exclusion criteria flowchart is shown in Figure 1.
Alcohol use question and definition of drinkers
The Alcohol Use Questionnaire (ALQ) was administered in the MEC using a computer-assisted personal interview system only to medical examiners aged 20 years and older. On the questionnaire, those who consumed at least 12 alcoholic beverages in a year and answered “yes” were identified as drinkers. Among them, the number of people defined as drinking alcohol is 9102, and the number of people who do non-drinking is 3436.
Depression screener and definition of depression
Depression is measured by the Patient Health Questionnaire (PHQ), a self-reported assessment based on nine signs and symptoms of depression based on The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), and is a tool for the initial diagnosis of depression, referred to in NHANES as the Depression Screening Questionnaire (DPQ). The nine symptom questions on the DPQ are scored on a scale from “0” (not at all) to “3” (almost every day.) The PHQ is most commonly used in primary care settings and has been shown to be a reliable and valid tool for the diagnosis of depression (25, 26). A score of 10 or greater has been well validated and is commonly used to define depression in clinical studies (25, 26).
Bowel health questionnaire and definition of chronic diarrhea
The Bowel Health Questionnaire was completed in the MEC using a computer-assisted personal interview system. Subjects were presented with a card with a colorful picture and a description of the Bristol Stool Form Scale (BSFS) types (Type 1 to Type 7) and were asked to answer the “number corresponding to the usual or most common type of stool”. Subjects were classified as having chronic diarrhea only if they thought their usual or most common stool type was Bristol Stool Form Scale type 6 (fluffy edges, pasty) or type 7 (watery, no solid pieces) (14, 27).
Data collection
Data collection based on demographics characteristics(age, gender, race, marital status, education level, family income); lifestyle characteristics (smoking, sleep disorder, physical activity); dietary status (protein intake, energy intake, carbohydrate intake, dietary fiber intake, total fat intake, caffeine intake, moisture intake) and comorbidity(hypertension, diabetes, arthritis, coronary disease, thyroid disease, osteoporosis).
The demographic characteristics contained age, gender (male or female), race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), marital status (married, widowed/divorced and never married), education level (<12th grad and high school or above) and family income (< 20,000$ or ≥ 20,000$).
The lifestyle characteristics included smoking, physical activity and sleep disorders. Smokers are defined as those who smoke more than 100 cigarettes in their lifetime. Physical activities is divided into vigorous activities and moderate activities. The vigorous activities were defined by doing any vigorous activities for at least 10 minutes that caused heavy sweating, or large increases in breathing or heart rate over the past 30 days. The moderate physical activity was defined by doing moderate activities for at least 10 minutes that cause only light sweating or a slight to moderate increase in breathing or heart rate over the past 30 days. Participants who are informed by doctors or other health professionals of sleep disorders are defined as having sleep disorders.
Energy intake, protein intake, carbohydrate intake, total sugar intake, dietary fiber intake, total fat intake, caffeine intake, and moisture intake were collected during the first 24 hours prior to the interview through dietary recall interviews conducted at the Dietary Survey Center.
Definitions of hypertension, diabetes, arthritis, coronary heart disease, thyroid disease, and osteoporosis were taken from the Medical Condition Questionnaire, in which a doctor or other healthcare professional asked: Have you ever been told by a doctor or healthcare professional that you have a related condition? Respondents who answered “yes” were considered to have the disease.
Statistical analysis
Data are expressed as mean± standard deviation(SD) for continuous variable and as frequency or percentage for categorical variables. The t-test was used to analyze the normal distribution and the Kruskal-Wallis test to analyze the skewed distribution in continuous variables. We used univariate and multivariate binary logistic regression to analyze the association between depression and chronic diarrhea. In multivariate logistic regression, we showed unadjusted models. Model I adjusted gender, age, race, education level, marital status, family income; Model II adjusted gender, age, race, education level, marital status, family income, smoking, BMI, high intensity exercise, moderate intensity exercise, sleep disorders, hypertension, diabetes, arthritis, coronary disease, thyroid disease, osteoporosis; Model III adjusted gender, age, race, education level, marital status, family income, smoking, BMI, high intensity exercise, moderate intensity exercise, sleep disorders, hypertension, diabetes, arthritis, coronary disease, thyroid disease, osteoporosis, energy intake, protein intake, carbohydrate intake, total sugars intake, dietary fiber intake, total fat intake, caffeine intake, moisture intake. We compared the risk of depression and chronic diarrhea between people who drank alcohol and those who did not, multivariate logistic regression analyzed subgroups according to drinking status, examined interactions between subgroups by likelihood ratios, and demonstrated trends between subgroups using smoothed curve fitting. Finally, we performed multivariate logistic regression for depression and chronic diarrhea separately using alcohol consumption as the dependent variable to verify the stability of the results. All analyses were performed using the statistical package R 4.3.2 (http://www.R-project.org), and P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
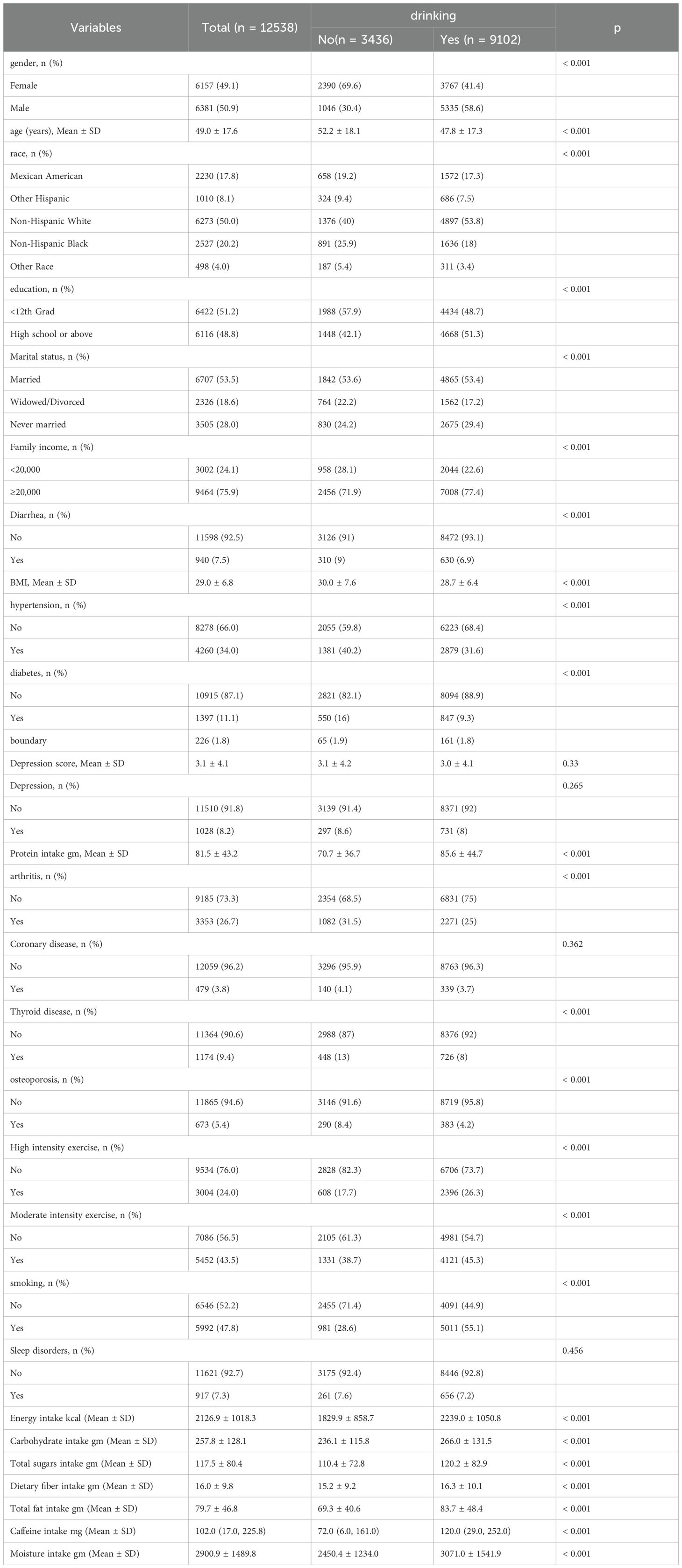
Table 1 presents a comparison of baseline demographic and clinical characteristics between the drinking group and the non-drinking group. Compared to the non-drinking group, drinking group was more common in males, younger individuals, non-Hispanic white individuals, those with higher education and income, married individuals, and mostly smokers. Additionally, the alcohol-consuming group had higher intakes of alcohol, protein, sugar, fiber, fat, caffeine, and water. Furthermore, we also included baseline demographic information for individuals with and without depression, as well as those with and without diarrhea, in Supplementary Tables 1 and 2.
Depression affects the risk of developing diarrhea
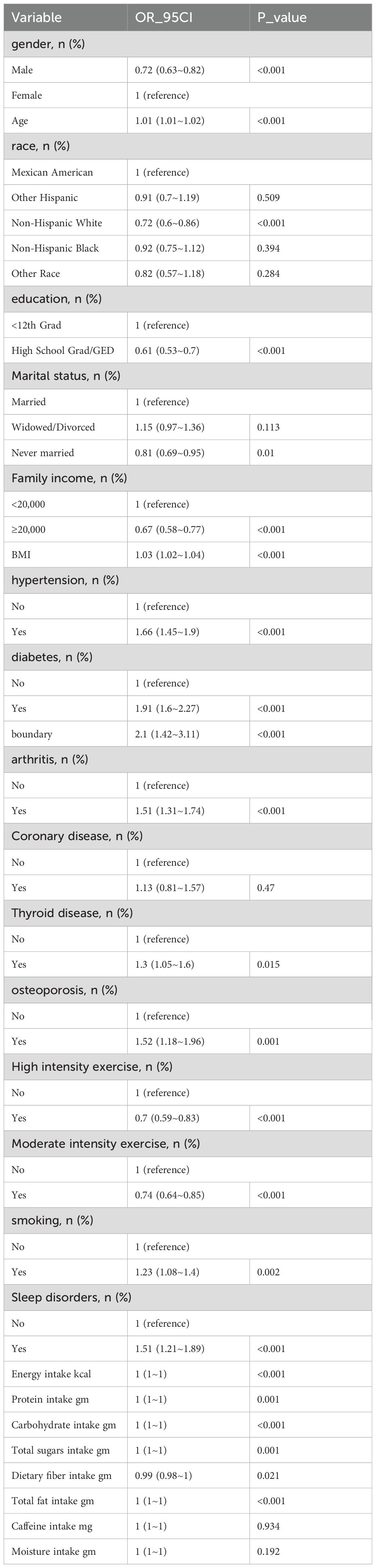
The univariate analysis in Table 2 shows that gender, age, race, education level, marital status, income level, BMI, hypertension, diabetes, arthritis, coronary heart disease, thyroid disorders, osteoporosis, physical activity level, smoking, sleep disorders, and dietary fiber intake are associated with chronic diarrhea.
Table 3 presents the results of the logistic regression analysis. In the unadjusted model, the risk of developing diarrhea in the depressed population is 2.36 times that of the non-depressed population (95% CI, 1.96-2.85); after adjusting for baseline variables (gender, age, race, education level, marital status, household income), the results remain similar (OR, 2.18; 95% CI, 1.79-2.64). After adjusting for other potential confounding factors (BMI, high intensity exercise, moderate intensity exercise, sleep disorders, hypertension, diabetes, arthritis, coronary disease, thyroid disease, osteoporosis, energy intake, protein intake, carbohydrate intake, total sugars intake, dietary fiber intake, total fat intake, caffeine intake, moisture intake) using a multivariable regression model, the risk of diarrhea in the depressed population was 1.94 times that of the non-depressed population (95% CI, 1.58-2.37). When depression was transformed into different levels of severity based on the scores, a significant relationship was observed in different adjusted models (P < 0.05). Additionally, we analyzed depression scores as a continuous variable, and the results remained stable.
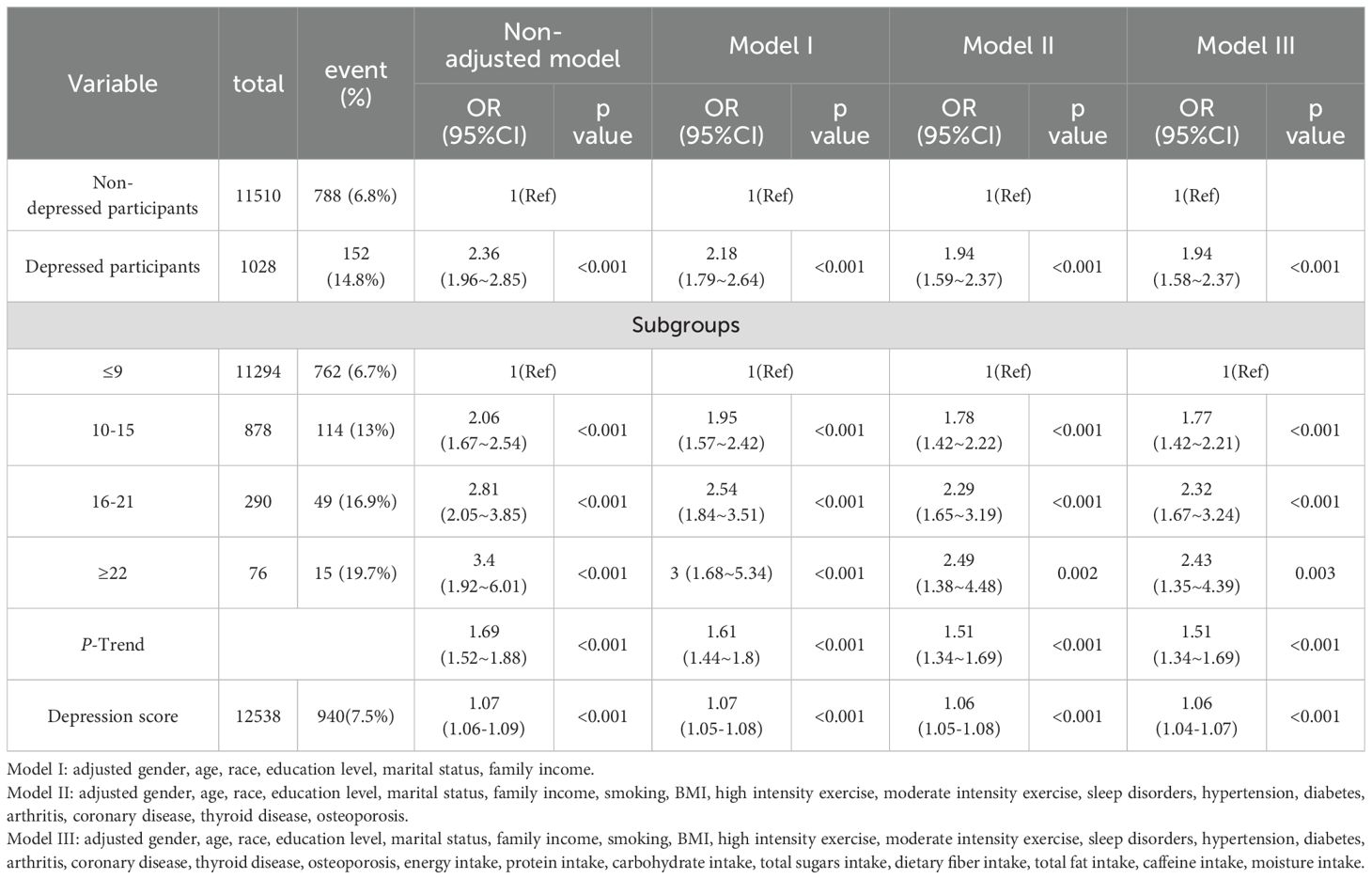
Table 3. Weighted odds ratios (95% confidence interval) for chronic diarrhea and different levels of depression in different models.
The association between depression and the risk of diarrhea in populations based on drinking
Figure 2 illustrates the difference in depression questionnaire scores between the alcohol-consuming and non-alcohol-consuming populations. Among the alcohol-consuming group, the depression scores were similar between the diarrhea and non-diarrhea subgroups (2 vs 2, P < 0.05); however, in the non-alcohol-consuming group, the diarrhea subgroup had higher depression scores compared to the non-diarrhea subgroup (1 vs 2.5, P < 0.05).
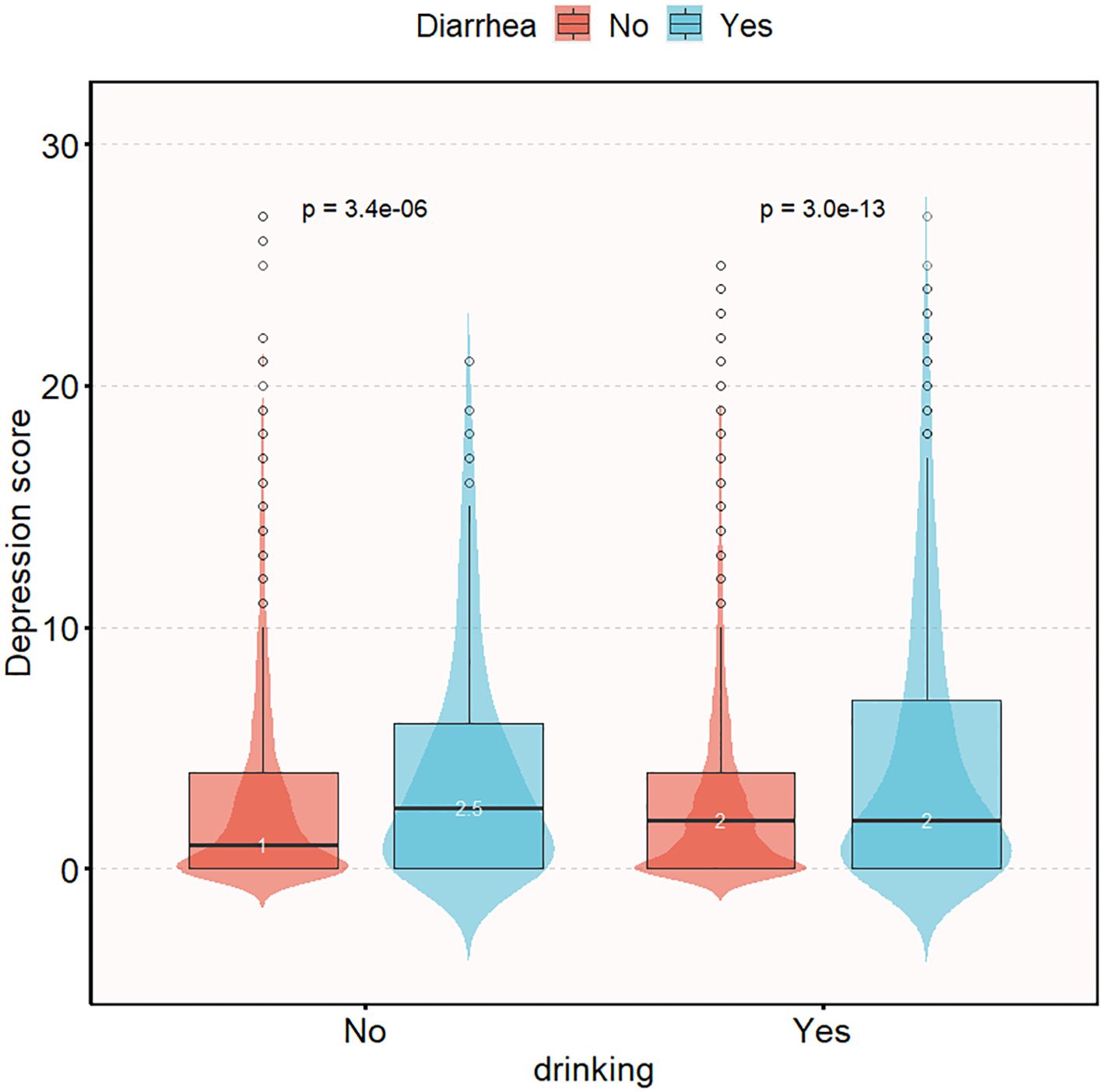
Figure 2. Distribution of depression questionnaire scores in chronic diarrhea population grouped according to drinking status. Adjusted gender, age, race, education level, marital status, family income, smoking, BMI, high intensity exercise, moderate intensity exercise, sleep disorders, hypertension, diabetes, arthritis, coronary disease, thyroid disease, osteoporosis, energy intake, protein intake, carbohydrate intake, total sugars intake, dietary fiber intake, total fat intake, caffeine intake, moisture intake.
Table 4 illustrates that after adjusting for multiple confounding factors (gender, age, race, education level, marital status, family income, smoking, BMI, high intensity exercise, moderate intensity exercise, sleep disorders, hypertension, diabetes, arthritis, coronary disease, thyroid disease, osteoporosis, energy intake, protein intake, carbohydrate intake, total sugars intake, dietary fiber intake, total fat intake, caffeine intake, moisture intake), the risk of chronic diarrhea in the depressed population compared to the non-depressed population is 2.34 in the alcohol-consuming group (95% CI, 1.84-2.98; P < 0.05). However, in the non-alcohol-consuming group, there was no significant interaction between depression and chronic diarrhea (OR: 1.26; 95% CI, 0.85-1.86; P > 0.05). Compared to the non-drinking population, the probability of chronic diarrhea occurring in patients with depression is 2.34 times higher in the drinking population than in non-depressed patients (95% CI: 1.84-2.98); this interaction remained significant when depression was transformed into different levels of severity based on scores (likelihood ratio test, P = 0.043). Finally, analyzing depression scores as a continuous variable showed that the risk of diarrhea in the depressed population was lower in the non-alcohol-consuming group compared to the alcohol-consuming group (likelihood ratio test, P = 0.024).
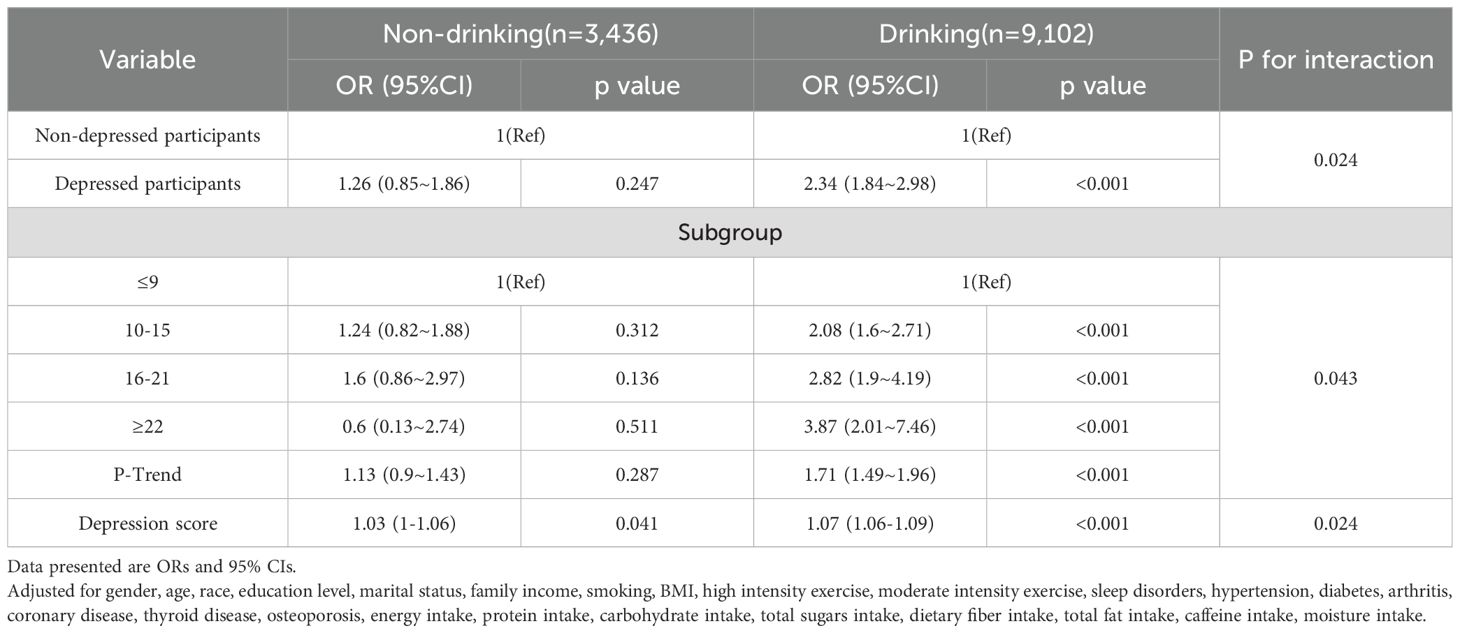
Table 4. Interaction between depression levels and chronic diarrhea in patients with alcohol consumption.
The smooth fitting curve of depression scores and the risk of chronic diarrhea
Finally, after adjusting for potential confounding factors (gender, age, race, education level, marital status, family income, smoking, BMI, high intensity exercise, moderate intensity exercise, sleep disorders, hypertension, diabetes, arthritis, coronary disease, thyroid disease, osteoporosis, energy intake, protein intake, carbohydrate intake, total sugars intake, dietary fiber intake, total fat intake, caffeine intake, moisture intake), we conducted a smooth fitting curve analysis of depression scores and the risk of chronic diarrhea. In Figure 3, we grouped individuals based on alcohol consumption and found that when depression scores were low, alcohol consumption had little effect on the relationship between depression and chronic diarrhea. However, as depression scores increased, individuals who consumed alcohol had a higher risk of developing diarrhea.
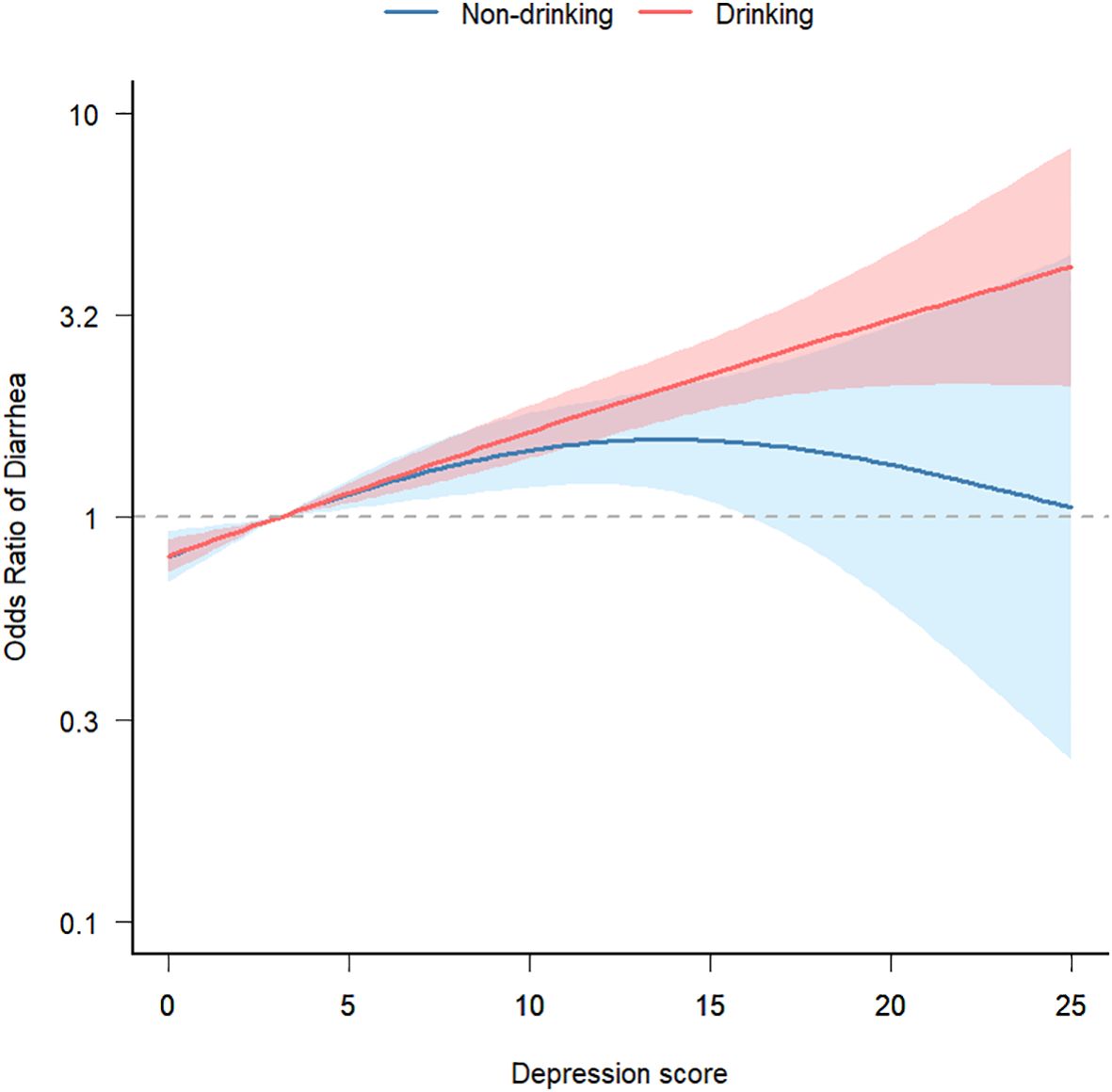
Figure 3. Smooth fit curve of depression score and risk of chronic diarrhea. Adjusted gender, age, race, education level, marital status, family income, smoking, BMI, high intensity exercise, moderate intensity exercise, sleep disorders, hypertension, diabetes, arthritis, coronary disease, thyroid disease, osteoporosis, energy intake, protein intake, carbohydrate intake, total sugars intake, dietary fiber intake, total fat intake, caffeine intake, moisture intake.
Sensitivity analysis
To control for the independent effects of alcohol consumption on depression and chronic diarrhea, we conducted separate multiple regression analyses using alcohol consumption as a variable for depression and chronic diarrhea. The results indicated that in the unadjusted model, alcohol consumption had a protective effect against chronic diarrhea; however, this association lost significance after adjusting for confounding factors. Interestingly, in the logistic regression between alcohol consumption and depression, the unadjusted model showed a protective effect of alcohol consumption on depression, which remained non-significant. What’s intriguing is that this result flipped in models I and II, where the risk of developing depression in the alcohol-consuming group increased by 18%(P=0.038) and 25%(P=0.005) compared to the non-alcohol-consuming group. However, in model III, the significance disappeared (P=0.273). We believe that some of the confounding factors were strongly correlated with depression or had strong negative correlations, leading to these results. Supplementary Tables 3 and 4 display the results of the multiple logistic regression analyses between alcohol consumption and depression, as well as chronic diarrhea.
Discussions
Based on a cross-sectional analysis of U.S. adults (≥20 years) from NHANES(2005-2010), we found that after adjusting for potential important confounding factors, depression is positively associated with the risk of chronic diarrhea occurrence. This result is consistent with several previous studies (14, 15). The association between functional gastrointestinal disorders and mental illnesses is well known to most clinical practitioners. A cross-sectional survey conducted by Wang found a significant correlation between fecal incontinence and depression, with depression increasing the risk of fecal incontinence (28). Gorard (29), in a study involving 42 individuals in a general psychiatric outpatient clinic, measured oral-cecal transit time using lactulose hydrogen breath testing and whole gut transit time with an ingestion of a non-absorbable marker followed by abdominal X-ray imaging. The results indicated a relationship between anxiety patients and increased bowel frequency, showing that emotions can influence intestinal motility. An analysis by Mate also found a high prevalence of irritable bowel syndrome (IBS) associated with psychological disorders (30). Depression often coexists with alcohol abuse, and when we included alcohol consumption in the analysis, we found that drinking increased the risk of developing diarrhea in individuals with depression. This association remained stable even after adjusting for multiple confounding factors.
Psychological stress has significant effects on intestinal sensitivity, motility, secretion, permeability, and mucosal immune activation (31). The mechanism behind the increased risk of diarrhea in patients with depression may be related to the brain-gut axis and visceral hypersensitivity. The brain-gut axis combines the activity of the autonomic and enteric nervous systems with central nervous system regulation, with its components communicating bidirectionally, cooperatively, and antagonistically to regulate the host’s physiological equilibrium. The bidirectional regulation between the brain and gut through this axis is known as brain-gut interaction (32, 33). As an important neurotransmitter in the brain-gut axis, 5-HT can regulate gastrointestinal motility, visceral sensation, and mucosal secretion. Research has shown that elevated serum 5-HT is associated with diarrhea-predominant irritable bowel syndrome, indicating a close relationship between elevated serum 5-HT and this subtype of irritable bowel syndrome (34).
According to the Global Burden of Disease Study findings, alcohol consumption has been identified as a major factor contributing to the burden of disease and death (35). A survey (36) from China found that alcohol consumption is significantly positively correlated with depressive symptoms, similar to previous research (37, 38). After adjusting for possible confounding factors, the relationship between alcohol consumption and depression was not significant, in contrast to previous research. Meanwhile, we also analyzed the relationship between alcohol consumption and chronic diarrhea, which remained non-significant after controlling for confounders. However, our final analysis revealed that among alcohol consumers, the risk of chronic diarrhea was higher in individuals with depression.
In this study, we examined the association between depression and chronic diarrhea in both alcohol-consuming and non-alcohol-consuming populations after adjusting for relevant variables. Our study has limitations. Firstly, a cross-sectional design does not allow for causal inference. Secondly, we cannot rule out the possibility that the observed associations may be due to unmeasured confounding factors. Lastly, some variables were based on self-reporting, which could lead to issues such as misunderstanding of questions or recall bias. Despite these limitations, our study has some merits. Firstly, our study population consisted of a large, representative sample of American adults. Secondly, aside from exploring the association between depression and chronic diarrhea, we also stratified the analysis based on alcohol consumption. Lastly, we used smooth curve fitting to assess the relationship between depression and chronic diarrhea in alcohol-consuming and non-alcohol-consuming populations.
Conclusion
In conclusion, this study found an association between depression and chronic diarrhea, with alcohol consumption increasing this association. This may alert individuals with depression to take precautions to avoid the occurrence of chronic diarrhea. Our study provides some clinical insights but further prospective research is needed to provide more evidence.
The impact on research and practice
Our findings hold significant implications for public health policy and clinical practice. In terms of public health policy, it is crucial to increase awareness about the impact of alcohol consumption on mental and digestive health. Public awareness campaigns should be enhanced to highlight the dangers of drinking, and efforts to implement alcohol control policies should be strengthened, particularly among vulnerable populations such as individuals with depression. In clinical practice, healthcare professionals should pay close attention to the drinking habits of patients with depression and incorporate alcohol management strategies into their treatment plans. For patients exhibiting symptoms of chronic diarrhea, especially those with a history of alcohol consumption and depression, doctors should consider alcohol as a potential contributing factor and conduct comprehensive assessments and treatments accordingly.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
The studies involving humans were approved by NCHS Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YW: Data curation, Formal analysis, Visualization, Writing – original draft. XL: Validation, Writing – review & editing. ZC: Supervision, Writing – review & editing. YZ: Funding Acquisition, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1393546/full#supplementary-material
References
1. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder’, Nature Reviews. Dis Primers. (2016) 1(2):16065. doi: 10.1038/nrdp.2016.65
2. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. (2013) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
3. Kessler RC, Sampson NA, Berglund P, Gruber MJ, Al-Hamzawi A, Andrade L, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. (2015) 24:210–26. doi: 10.1017/S2045796015000189
4. Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. (2018) 75(4):336–46. doi: 10.1001/jamapsychiatry.2017.4602
5. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London England). (2015) 386:743–800. doi: 10.1016/S0140-6736(15)60692-4
6. König H, König H-H, Konnopka A. The excess costs of depression: a systematic review and meta-analysis. Epidemiol Psychiatr Sci. (2019) 29:e30. doi: 10.1017/S2045796019000180
7. Glassman AH. Depression and cardiovascular comorbidity. Dialogues Clin Neurosci. (2007) 9:9–17. doi: 10.31887/DCNS.2007.9.1/ahglassman
8. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. psychol Med. (2014) 44:2029–40. doi: 10.1017/S0033291713002535
9. Moulton CD, Pickup JC, Ismail K. ‘The link between depression and diabetes: the search for shared mechanisms’, The Lancet. Diabetes Endocrinol. (2015) 3:461–71. doi: 10.1016/S2213-8587(15)00134-5
10. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
11. Wang Y-H, Li J-Q, Shi J-F, Que J-Y, Liu J-J, Lappin JM, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. (2020) 25:1487–99. doi: 10.1038/s41380-019-0595-x
12. Cai Y, Chen M, Zhai W, Wang C. Interaction between trouble sleeping and depression on hypertension in the NHANES 2005-2018. BMC Public Health. (2022) 22:481. doi: 10.1186/s12889-022-12942-2
13. Harerimana NV, Liu Y, Gerasimov ES, Duong D, Beach TG, Reiman EM, et al. Genetic evidence supporting a causal role of depression in alzheimer’s disease. Biol Psychiatry. (2022) 92:25–33. doi: 10.1016/j.biopsych.2021.11.025
14. Ballou S, Katon J, Singh P, Rangan V, Lee HN, McMahon C, et al. Chronic diarrhea and constipation are more common in depressed individuals. Clin Gastroenterol Hepatology: Off Clin Pract J Am Gastroenterological Assoc. (2019) 17:2696–703. doi: 10.1016/j.cgh.2019.03.046
15. Singh P, Lee H-N, Rangan V, Ballou S, Lembo J, Katon J, et al. Similarities in clinical and psychosocial characteristics of functional diarrhea and irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatology: Off Clin Pract J Am Gastroenterological Assoc. (2020) 18:399–405.e1. doi: 10.1016/j.cgh.2019.08.020
16. Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW, et al. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. (2014) 171:453–62. doi: 10.1176/appi.ajp.2013.13030325
17. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. (2015) 72:334–41. doi: 10.1001/jamapsychiatry.2014.2502
18. Thomas PD, Forbes A, Green J, Howdle P, Long R, Playford R, et al. Guidelines for the investigation of chronic diarrhoea, 2nd edition. Gut. (2003) 52 Suppl 5:v1–15. doi: 10.1136/gut.52.suppl_5.v1
19. Camilleri M, Sellin JH, Barrett KE. Pathophysiology, evaluation, and management of chronic watery diarrhea. Gastroenterology. (2017) 152:515–532.e2. doi: 10.1053/j.gastro.2016.10.014
20. Chu C, Rotondo-Trivette S, Michail S. Chronic diarrhea. Curr Problems Pediatr Adolesc Health Care. (2020) 50:100841. doi: 10.1016/j.cppeds.2020.100841
21. Xu F, Dai N. Long-term symptom development in functional bowel disease and the effect of lactose-restricted diet on symptoms. Chin J Gastroenterol. (2017) 37:673–8.
22. Zhong B-L, Xu Y-M, Xie W-X, Lu J, Yu W-B, Yan J, et al. ‘Alcohol drinking in chinese methadone-maintained clients: A self-medication for depression and anxiety? J Addict Med. (2019) 13:314–21. doi: 10.1097/ADM.0000000000000500
23. Kim AJ, Sherry SB, Nealis LJ, Mushquash A, Lee-Baggley D, Stewart SH, et al. ‘Do symptoms of depression and anxiety contribute to heavy episodic drinking? A 3-wave longitudinal study adult Community members’ Addictive Behaviors 130. (2022) p:107295. doi: 10.1016/j.addbeh.2022.107295
24. Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. (2000) 43:9–16. doi: 10.1007/BF02237236
25. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. (1999) 282:1737–44. doi: 10.1001/jama.282.18.1737
26. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Internal Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
27. Sommers T, Mitsuhashi S, Singh P, Hirsch W, Katon J, Ballou S, et al. Prevalence of chronic constipation and chronic diarrhea in diabetic individuals in the United States. Am J Gastroenterol. (2019) 114:135–42. doi: 10.1038/s41395-018-0418-8
28. Wang Y, Li N, Zhou Q, Wang P. Fecal incontinence was associated with depression of any severity: insights from a large cross-sectional study. Int J Colorectal Dis. (2023) 38:271. doi: 10.1007/s00384-023-04563-x
29. Gorard DA, Gomborone JE, Libby GW, Farthing MJ. Intestinal transit in anxiety and depression. Gut. (1996) 39:551–5. doi: 10.1136/gut.39.4.551
30. Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. (2014) 264:651–60. doi: 10.1007/s00406-014-0502-z
31. Raskov H, Burcharth J, Pommergaard H-C, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. (2016) 7:365–83. doi: 10.1080/19490976.2016.1218585
32. Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
33. Dowling LR, Strazzari MR, Keely S, Kaiko GE. Enteric nervous system and intestinal epithelial regulation of the gut-brain axis. J Allergy Clin Immunol. (2022) 150:513–22. doi: 10.1016/j.jaci.2022.07.015
34. Cooper N, Stasi R, Cunningham-Rundles S, Cesarman E, McFarland JG, Bussel JB. Platelet-associated antibodies, cellular immunity and FCGR3a genotype influence the response to rituximab in immune thrombocytopenia. Br J Haematology. (2012) 158:539–47. doi: 10.1111/j.1365-2141.2012.09184.x
35. Rehm J, Imtiaz S. A narrative review of alcohol consumption as a risk factor for global burden of disease. Subst Abuse Treatment Prevention Policy. (2016) 11:37. doi: 10.1186/s13011-016-0081-2
36. Ma D, Lv Y, Liu H, Wu Z, Ma Z, Liu B. Effects of drinking behavior on depressive symptoms in college students: the mediating role of anxiety symptoms. J Air Force Med Univ. (2023), 1–6.
37. McHugh RK, Weiss RD. Alcohol use disorder and depressive disorders. Alcohol research: Curr Rev. (2019) 40:arcr.v40.1.01. doi: 10.35946/arcr.v40.1.01
Keywords: drinking, depression, diarrhea, NHANES, cross-sectional study
Citation: Wang Y, Li X, Cao Z and Zhou Y (2024) The impact of alcohol consumption on the relationship between depression and chronic diarrhea: a cross-sectional study analysis on NHANES (2005-2010). Front. Psychiatry 15:1393546. doi: 10.3389/fpsyt.2024.1393546
Received: 29 February 2024; Accepted: 30 July 2024;
Published: 30 August 2024.
Edited by:
Wulf Rössler, Charité University Medicine Berlin, GermanyReviewed by:
Junzhi Chen, Guangdong Provincial People’s Hospital, ChinaJesús Cebrino, University of Seville, Spain
Copyright © 2024 Wang, Li, Cao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
 Yongsen Wang1,2
Yongsen Wang1,2 Xiaotong Li
Xiaotong Li