
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 10 July 2024
Sec. Personality Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1392605
This article is part of the Research TopicReviews in Psychiatry 2023: Personality DisordersView all 10 articles
Background: Atrial fibrillation (AF) is one of the most common form of arrhythmia. Previous studies have shown a link between AF and mental illness. However, the causal relationship between mental illness and AF remains unclear. The purpose of this study was to investigate the bidirectional causal relationship between borderline personality disorder (BPD) and AF.
Method: We used the bidirectional Two-sample Mendelian randomization (TSMR) method to evaluate the causal relationship between BPD and AF. Instrumental variables associated with BPD were derived from a genome-wide association study involving 214,816 Europeans (2,637 cases and 212,179 controls). We then obtained atrial fibrillation data from the GWAS meta-analysis (60,620 cases and 970,216 controls). The TSMR analyses were performed in five methods, namely fixed-effect inverse-variance weighted (IVW) method、random-effect IVW method, MR Egger regression method, Weighted median method and Simple mode method. Several sensitivity analyses are used to test the robustness of positive results.
Results: The fixed-effect inverse-variance weighted model [Odds ratio (OR), 1.033, 95% confidence interval (CI), 1.011-1.056, P = 0.0031], random-effect inverse-variance weighted model (OR, 1.033; 95%CI, 1.005-1.062; P = 0.0191) and Weighted median (OR, 1.034; 95%CI, 1.002-1.068; P = 0.0394) all showed that genetically predicted BPD was associated with an increased risk of AF. Sensitivity analysis using other MR Methods, including the MR-Egger intercept, MR-Presso method, and leave-one-out analyses, showed that the results were robust. In reverse MR analysis, there was no causal relationship of AF on BPD.
Conclusion: Our study provides a causal relationship between BPD and AF. This means that patients with BPD should be monitored for the occurrence of AF. Early screening and proper management of BPD may show anti-arrhythmic benefits.
Atrial fibrillation (AF) is one of the most common arrhythmias in clinic. It is characterized by rapid and disordered atrial electrical activity. AF can be divided into first diagnosed AF, paroxysmal AF, persistent AF, and permanent AF (1). AF can make people experience a variety of uncomfortable symptoms such as panic, fatigue, sweating, which seriously affect their quality of life (2). The purpose of AF treatment is to prevent thromboembolic complications, restore and maintain sinus rhythm, and control ventricular rate during AF (3). The most important thing in the treatment of AF is to actively search for the primary disease and inducing factors, and make corresponding treatment. Studies have shown that male sex, advancing age, and Caucasian ancestry are important risk factors for developing AF (4). Controllable risk factors for AF include smoking (5), alcohol use (6), high blood pressure (7), diabetes (8), obesity (9), obstructive sleep apnea (10), and a sedentary lifestyle (11), among other factors. Previous studies have shown that AF and psychiatric disorders are inextricably linked. It is important to note that patients with AF often exhibit one or more negative emotions, such as anxiety, depression, reactive instability, and high neuroticism under the influence of severe stressors (12). It has been demonstrated that depression significantly increases the cumulative incidence of atrial fibrillation (from 1.92% to 4.44% at 10 years), and 20-40% of AF patients are found to have high levels of depression (13). Similarly, Kim et al. found that depression was associated with a significantly increased risk and cumulative incidence of new-onset AF (14). A meta-analysis of cohort studies demonstrated that anxiety was independently associated with an increased risk of recurrence of AF after catheter ablation (adjusted relative risk, 2.3; 95%CI, 1.710-3.260; P < 0.001) (15). One study demonstrated that the level of anxiety before coronary artery bypass surgery is an important factor in the development of AF after surgery (16). Studies have shown that severe psychological distress (35%) and suicidal ideation (20%) were prevalent in the tertiary population with AF (17).
Mental illness causes serious social and economic burden, and it is difficult to accurately identify its pathogenesis and risk factors (18). Due to the complex pathogenesis of mental diseases, the reverse causality between risk factors and mental diseases is easily confused, which makes MR analysis become an important tool in the study of mental diseases. A previous review of 50 MR articles showed a causal relationship between a particular psychiatric disorder and its causative factors (19). This review is divided into four groups based on the mental disorders assessed: schizophrenia, major depressive disorder, attention deficit and hyperactivity disorder, and autism spectrum disorder or other mental disorders. BPD, though one of the screening criteria in this review, was not included in the target article. It can also be seen that the number of MR articles on BPD is relatively small. To some extent, our paper can fill the gap in the study of MR of BPD. Borderline personality disorder (BPD), also known as emotionally unstable personality disorder, is characterized by widespread instability in interpersonal relationships, emotional regulation, impulse control, and self-image management (20). Patients with BPD often suffer from depression, anxiety, impulsivity, anger and other negative emotions, and even have self-harm and suicidal tendencies (21). Emotional instability is the main characteristic of BPD. BPD is a serious mental disorder that can have a significant impact on an individual’s quality of life, mental health and social interactions. We hypothesize that there may be a causal relationship between BPD and AF.
Mendelian Randomization (MR) is a data analysis technique used to evaluate causal inference in epidemiological studies. In MR analysis, genetic variants are used as Instrumental Variables (IVs) to estimate the causal relationship between the exposure factor of interest and the outcome of concern (22). We conducted a two-sample bidirectional MR study to estimate the causal relationship between BPD and AF. This paper expounds the causal relationship between BPD and AF, and provides the basis for early prevention and detection of arrhythmia in patients with BPD.
In this study, bidirectional two-sample Mendelian randomization (TSMR) was used to evaluate the causal relationship between BPD and AF.
The summary data for BPD were derived from a recently published genome-wide association study (GWAS) that included 2,637 patients with BPD and 212,179 control patients (https://gwas.mrcieu.ac.uk/datasets/finn-b-F5_EMOPER/). We used the most recent GWAS meta-analysis of AF by Nielsen et al., which included 60,620 patients with AF and 970,216 controls of European ancestry (https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST006414/). The analysis pooled six contributing studies [The Michigan Genomics Initiative (MGI), deCODE, the Nord-Trøndelag Health Study (HUNT), DiscovEHR, UK Biobank, and the AFGen Consortium] (23). Detailed information about the data sources is shown in Table 1.
In MR analysis, three core assumptions must be satisfied in order to obtain valid results (24), as shown in Figure 1. Specifically, multiple genetic variants must satisfy: (1) the correlation assumption: IVs are closely related to exposure; (2) the independence assumption: IVs are not associated with other confounding factors; (3) the exclusion assumption: IVs only affect the outcome through the exposure.
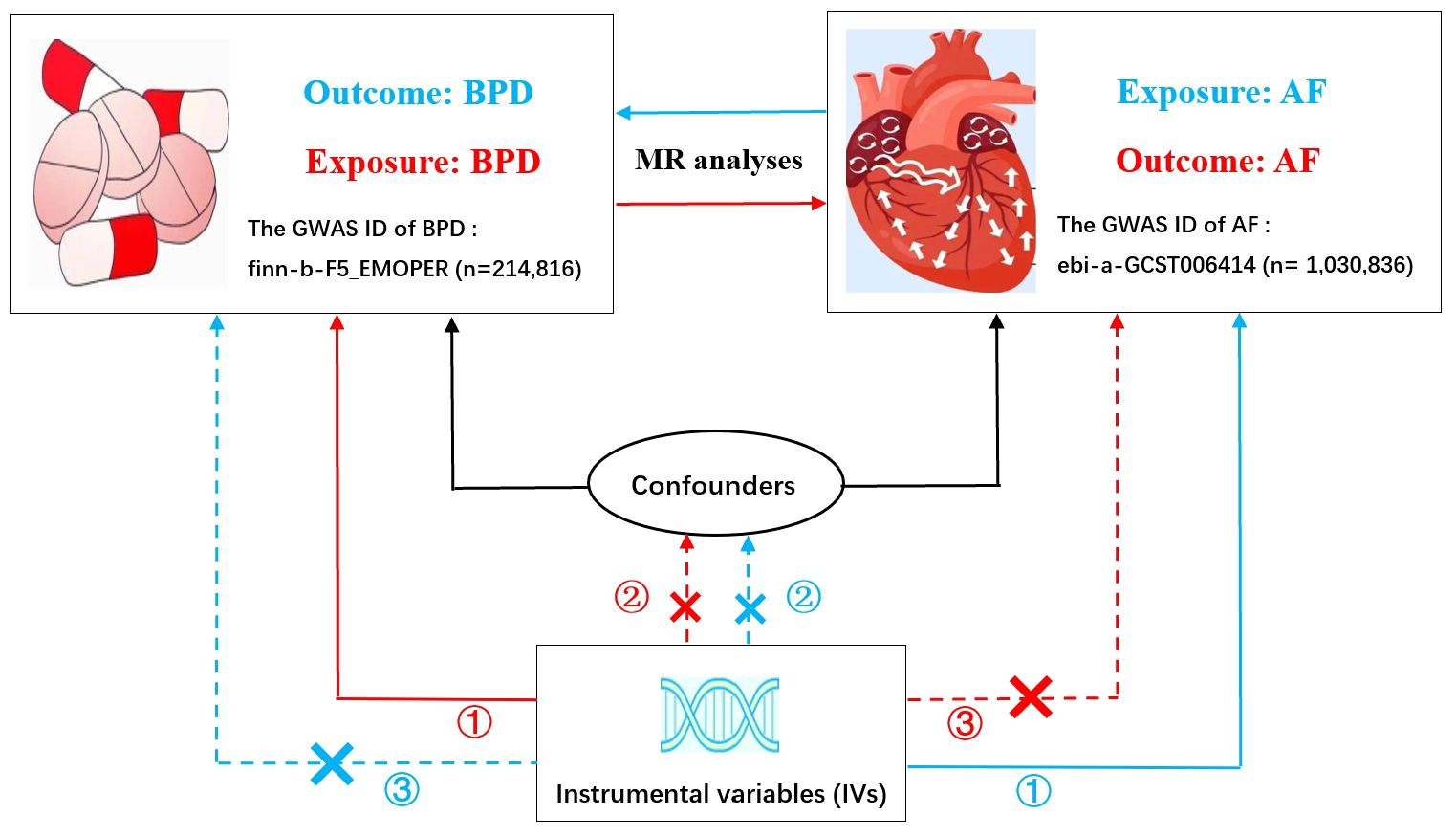
Figure 1 Bidirectional Mendelian randomization model of BPD and AF. (The red line represents BPD as exposure and AF as outcome; The blue line represents AF as exposure and BPD as outcome.) BPD, borderline personality disorder; AF, atrial fibrillation; GWAS, genome-wide association study. Three crucial hypotheses of the Mendelian randomization study: ① the correlation assumption; ② the independence assumption; ③ the exclusion assumption.
In this study, in order to obtain more screening data, we used P < 5×10-6 as the criterion for screening SNPs for BPD at the genome-wide level (25). In the genome range, 16 gene loci were identified that were significantly associated with BPD. Similarly, we found 111 independent genetic loci for AF that achieved genome-wide significance (P < 5×10-8). Linkage disequilibrium (LD) refers to a nonrandom association between allele of different loci, measured using two parameters, r2 and kb. The LD window is set to 10000kb, r2 > 0.001 to ensure the independence of the selected genetic variation. F-statistic of genetic variation 10, the genetic tool is considered to be effective, that is, it is not affected by weak instrument bias (26). The calculation formula is: F=R²*(N-2)/(1-R²). R²=2*(1-MAF)*MAF*β². R² is the degree of variation explained by each SNP, EAF is the minor allele frequency, β is the beta coefficient associated with exposure, and N is the total sample size. Prior to MR analysis, we performed effective allelic comparisons to remove all SNPs with palindromic structures. This gives us a standard instrumental variable that conforms to MR analysis.
Two-sample Mendelian randomization (TSMR) method was used in this study. TSMR analysis methods include fixed-effect inverse-variance weighted (IVW) method、random-effect IVW method, MR Egger regression method, Weighted median method and Simple mode method. IVW is the most important method. Heterogeneity among genetic variants was examined by heterogeneity statistics. By analyzing the p-value of the Q test of Cochrane, when P > 0.05, there was no significant heterogeneity. Through the MR-Egger intercept test, the horizontal multiplicity of the data can be detected, and the robustness of the results can be evaluated (27). An outlier test examines whether there are SNPS that differ significantly to further reduce this level of multiplicity. Finally, the leave-one-out method is used to determine whether the selected SNPs is reliable and stable. The results were described by odd ratio (OR) and 95% confidence interval (CI). The overall process of MR Analysis in this study is shown in Figure 2. In our study, R software package “TwosampleMR” was used for MR Analysis.
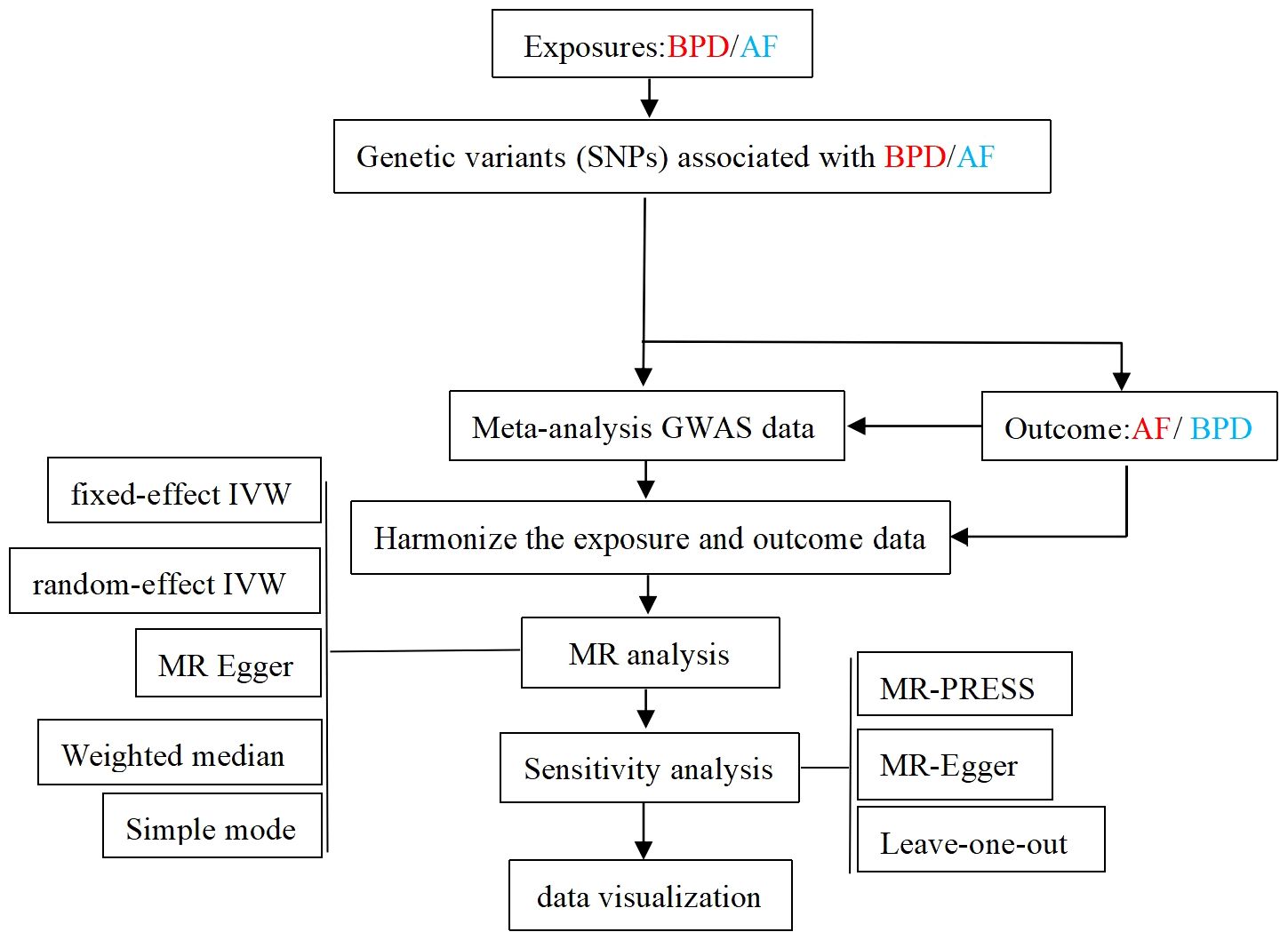
Figure 2 Flow chart of the overall design of the MR Analytical framework for this study. BPD, borderline personality disorder; AF, atrial fibrillation; GWAS, genome-wide association study.
SNPs for BPD and AF were derived from European studies involving both men and women. We selected 16 SNPs that met the criteria when BPD was used as exposure. We got 111 SNPs when AF was the exposure factor. Details can be found in the Supplementary Material.
Our findings suggest that BPD is associated with an increased risk of AF. An observed P < 0.05 is considered significant evidence of causality. IVW is the main method of our research. Based on the random-effects model IVW approach, we found that an increase in BPD determined by the one standard deviation (SD) gene was causally associated with a 3.3% increase in the relative risk of AF (N = 16 SNPs; OR, 1.033; 95%CI, 1.005-1.062; P = 0.0191). By IVW method of fixed effect model (OR, 1.033; 95%CI, 1.011-1.056; P = 0.0031) and Weighted median analysis (OR, 1.034; 95%CI, 1.002-1.068; P = 0.0394),We also found a causal relationship between BPD and AF risk. The specific causal relationship between BPD and AF is shown in Figure 3. The standard forest plot shows the effect size of each SNP and its 95% confidence interval (CI) (Figure 4A). The leave-one-out method was used for sensitivity analysis to evaluate the reliability of the results (Figure 4B). Each point in the scatter plot corresponds to a SNP, showing the association between this genetic variation and BPD and AF (Figure 4C). Funnel plot is used to detect heterogeneity among genetic variants (Figure 4D).
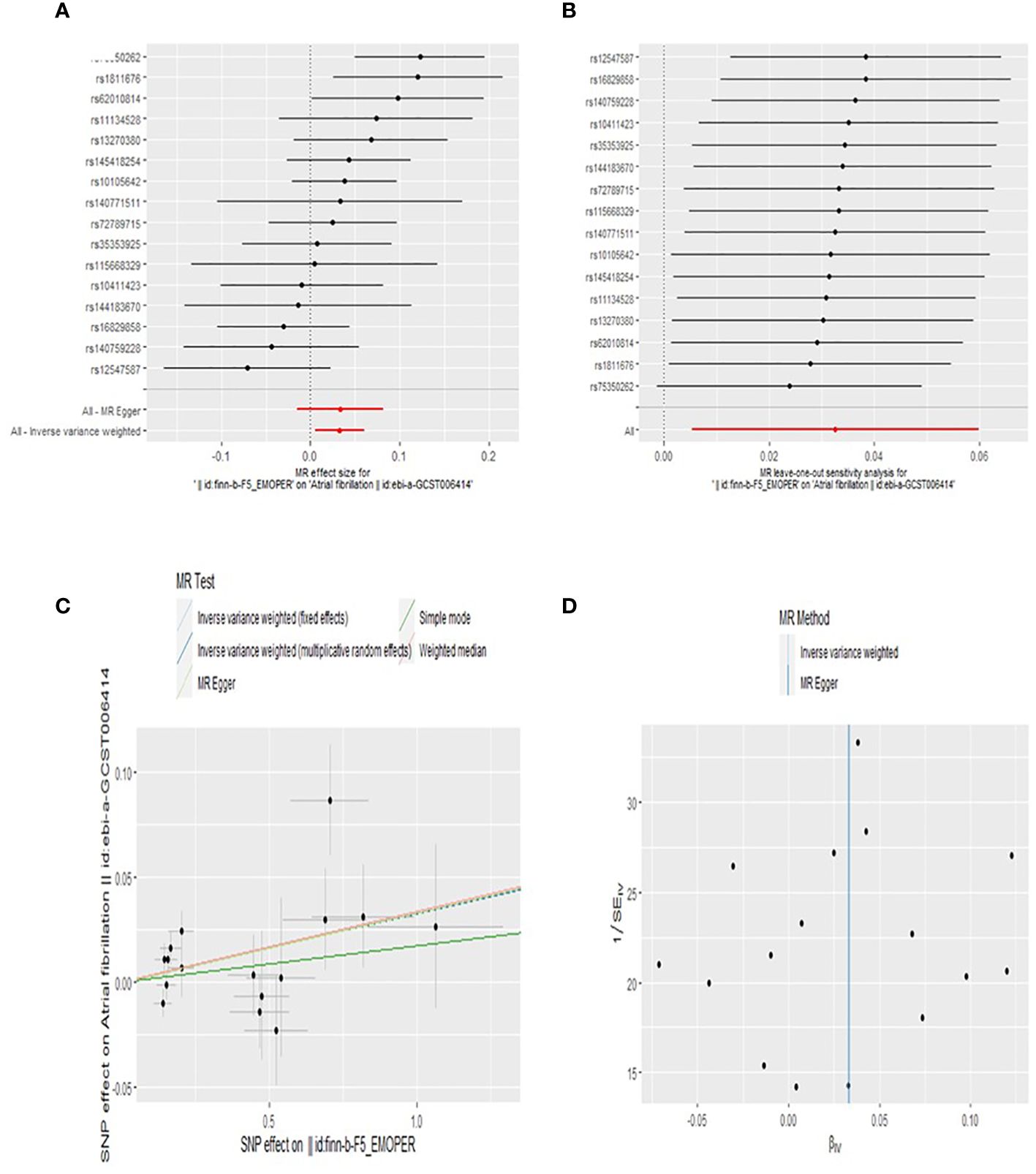
Figure 4 Forest plot (A), sensitivity analysis (B), scatter plot (C), and funnel plot (D) of the effect of BPD on AF levels.
In reverse MR analysis, none of the five MR methods supported a causal relationship between AF genetic susceptibility and BPD (Figure 5). Details of MR Estimates and sensitivity analyses can be found in Figure 6.
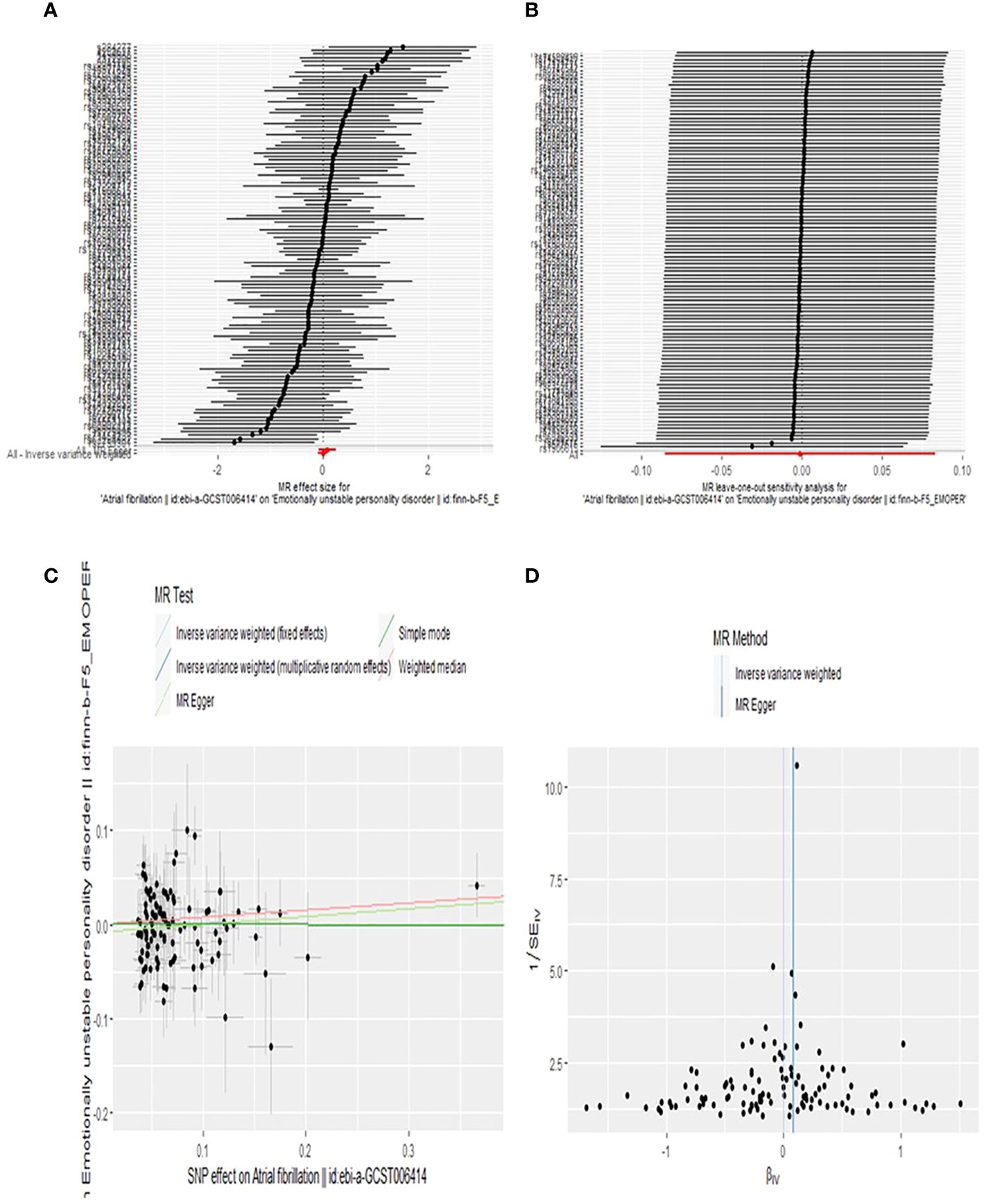
Figure 6 Forest plot (A), sensitivity analysis (B), scatter plot (C), and funnel plot (D) of the effect of AF on BPD levels.
We used mr_pleiotropy_test as the core analysis method to detect horizontal pleiotropy. The intercept term of the MR-Egger method was statistically tested. If there was no statistical difference, that is, P > 0.05, horizontal pleiotropy could not be considered. We also used the MR-PRESSO global test to detect horizontal pleiotropy. The leave-one-out method was applied to remove each SNP one by one, and then the meta effect of the remaining SNPs was calculated to observe whether the results would change significantly after the removal of a particular SNP. In the MR study of AF as an outcome, the sensitivity analysis is detailed in Table 2. Sensitivity analysis of AF as exposure in MR study is detailed in Supplementary Materials.
To our knowledge, this is the first MR analysis to assess the causal relationship between BPD and the risk of AF. We have two new findings in European populations. On the one hand, our study suggests a causal relationship between genetic susceptibility to BPD and an increased risk of AF. On the other hand, there is no evidence to support a causal relationship between AF and the risk of BPD. However, due to certain limitations of MR studies, these results must be interpreted with caution.
AF is one of the most common persistent arrhythmia. Mental disorders such as anxiety, depression and stress may activate the autonomic nervous system, which may be closely related to the development of AF. Previous studies have systematically and prospectively revealed that negative emotions such as stress, sadness, anger, and anxiety can induce AF, while happiness has a protective effect on AF (28). A systematic review noted that AF imposes significant psychosocial burdens on individuals, including depression and anxiety, as well as impaired quality of life in people with AF (29). A meta-analysis of 2017,276 participants (222,253 with anxiety disorders) from 46 cohorts showed that anxiety was not associated with AF [relative risk(RR), 1.27; 95%CI, 0.90-1.80] (30). Studies have confirmed that 20-40% of AF patients are found to have high levels of depression. Depression significantly increased the 10-year cumulative incidence of AF (from 1.92% to 4.44%) (13). A descriptive study involving 126 patients undergoing coronary artery bypass grafting (CABG) found that the mean trait anxiety scale score of patients with postoperative atrial fibrillation was 40.2 ± 7.8, with a statistically significant difference (16). A meta-analysis involving 5,329,908 participants clearly indicated that anxiety increased the risk of AF by 10% [hazard ratios(HRs) 1.10; 95%CI, 1.02-1.19; P = 0.013; N = 235,599 in 6 studies]. Anger increased the risk of AF by 15% (HR, 1.15; 95%CI 1.04-1.26; P = 0.04, N = 21,791 in 3 studies). Depression increased the risk of AF by 25% (HR, 1.25; 95%CI, 1.12-1.39; P < 0.001; N = 5,160,247 in 6 studies). Work stress increased the risk of AF by 18% (HR, 1.18; 95%CI,1.05-1.32; P = 0.004; N = 51,664 in 4 studies) (31). At the same time, many studies have shown how to influence the negative emotions of patients with AF to improve the prognosis and improve the quality of life of patients. Randomized studies in Australia have reported that improvement in psychological symptoms of anxiety and depression can be observed in patients with symptomatic AF treated with catheter ablation (32). Lakkireddy et al. have shown that yoga therapy improves depression, anxiety, blood pressure and resting heart rate, as well as quality of life in patients with paroxysmal AF (33). While several past observational studies have found a link between mental disorders and AF, the findings have been inconsistent. A large population survey suggests otherwise, that symptoms of depression and anxiety are not associated with an increased incidence of AF (34).
As mentioned above, there have been numerous studies that have linked AF to negative emotions such as depression and anxiety or psychiatric disorders, but the specific nature of their relationship is unclear. The current study also could not shed light on a key question: whether the mental disorder occurred before or after AF, or whether they affected each other at the same time. BPD, centered on emotional dysregulation, can be composed of many symptoms such as depression, anxiety, stress, and suicidal tendencies (20, 35, 36). The multiple manifestations of these unstable personality traits are similar to many mental disorders of AF. Our study specifically identified BPD as an exposure factor and concluded that BPD may predate and lead to AF.
Several studies have shown that patients with BPD exhibit fewer Respiratory sinus arrhythmia (RSA) than healthy individuals, a result that can be explained by reduced parasympathetic activity in BPD patients (37–39). We found that no experts had conducted systematic studies on the relationship between BPD and other arrhythmias. Our study was a MR study of the causal relationship between BPD and AF at the genetic level. It has been documented that inflammation is a driver of AF (40). Similarly, some experts believe that BPD may exhibit a pro-inflammatory state (41). Inflammation may be a mediating factor between BPD and AF. The TSMR analysis we performed was sufficient to validate the most direct causal relationship between BPD and AF, without further exploring how this causal relationship is affected step by step.
Finally, it should be noted that in the course of our study, we used a more relaxed threshold (P < 5×10-6) to select instrumental variables for BPD. Although this improves the statistical efficiency, it is more likely to introduce multi-effect instrumental variables. Although we performed multiple sensitivity analyses, each SNP independently affected BPD and AF, which reduced the reliability of the results.
To our knowledge, our study is the first to use MR Analysis to explore the causal relationship between BPD and AF. TSMR analysis has several advantages (1): Compared with traditional observational studies, MR Method reduces the influence of confounding factors and reverse causality (2); We have strictly identified the SNPs selection to reduce the sampling bias; (3) The data we used are all from European populations, which reduces the influence of population stratification to a certain extent.
Our study also has some limitations: (1) Due to the lack of individual information in the samples, we could not stratify the analysis of age, and AF subtypes; (2) BPD is more common in women, and we did not stratify our study subjects by gender;(3) The samples we studied were all of European ancestry, and the conclusions cannot be generalized to all populations; (4) Not all data employed in the GWAS diagnosed BPD patients according to Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD) criteria, together with the lack of adjustment for comorbidities, it is difficult to be sure that the results are specific to BPD and not just common to psychiatric disorders in general.
Our TSMR analysis provides genetic evidence of a causal relationship between BPD and an increased risk of AF. On the contrary, no causal relationship of AF on BPD risk was observed. Our study enhances the current understanding of the role of psychiatric disorders in AF. Our study provides evidence to support early prevention of arrhythmias in patients with BPD. Further research is now needed to explore strategies for detecting and treating BPD.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
WZ: Methodology, Supervision, Writing – review & editing, Validation. ZW: Conceptualization, Data curation, Methodology, Writing – original draft. HH: Formal analysis, Investigation, Project administration, Writing – review & editing. YS: Resources, Software, Writing - original draft. QW: Project administration, Supervision, Validation, Writing – original draft. MX: Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1392605/full#supplementary-material
1. Hammond-Haley M, Providencia R, Lambiase PD. Temporal pattern/episode duration-based classification of atrial fibrillation as paroxysmal vs. persistent: is it time to develop a more integrated prognostic score to optimize management? Europace. (2018) 20:f288–98. doi: 10.1093/europace/eux178
2. Mitamura H. [Pathophysiology and clinical manifestations of atrial fibrillation]. Nihon rinsho. Japanese J Clin Med. (2013) 71:23–8.
3. Kirchhof P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet (London England). (2017) 390:1873–87. doi: 10.1016/s0140-6736(17)31072-3
4. Zhang J, Johnsen SP, Guo Y, Lip GYH. Epidemiology of atrial fibrillation: geographic/ecological risk factors, age, sex, genetics. Cardiac electrophysiol clinics. (2021) 13:1–23. doi: 10.1016/j.ccep.2020.10.010
5. Watanabe I. Smoking and risk of atrial fibrillation. J Cardiol. (2018) 71:111–2. doi: 10.1016/j.jjcc.2017.08.001
6. Voskoboinik A, Prabhu S, Ling LH, Kalman JM, Kistler PM. Alcohol and atrial fibrillation: A sobering review. J Am Coll Cardiol. (2016) 68:2567–76. doi: 10.1016/j.jacc.2016.08.074
7. Naccache S, Ben Kilani M, Tlili R, Ben Ameur Y, Boujnah MR. Atrial fibrillation and hypertension: State of the art. La Tunisie medicale. (2017) 95:455–60.
8. Wang A, Green JB, Halperin JL, Piccini JP. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:1107–15. doi: 10.1016/j.jacc.2019.07.020
9. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. (2017) 70:2022–35. doi: 10.1016/j.jacc.2017.09.002
10. Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Lévy P, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: A review. JAMA Cardiol. (2018) 3:532–40. doi: 10.1001/jamacardio.2018.0095
11. Gallagher C, Middeldorp ME, Hendriks JM, Lau DH, Sanders P. Lifestyle as a risk factor for atrial fibrillation. Cardiac electrophysiol clinics. (2021) 13:263–72. doi: 10.1016/j.ccep.2020.11.013
12. Troshina DV, Volel BA, Syrkina EA. [Stress-induced atrial fibrillation]. Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova. (2019) 119:6–13. doi: 10.17116/jnevro20191190116
13. Manolis TA, Manolis AA, Apostolopoulos EJ, Melita H, Manolis AS. Depression and atrial fibrillation in a reciprocal liaison: a neuro-cardiac link. Int J Psychiatry Clin practice. (2023) 27:397–415. doi: 10.1080/13651501.2023.2248214
14. Kim YG, Lee KN, Han KD, Han KM, Min K, Choi HY, et al. Association of depression with atrial fibrillation in South Korean adults. JAMA Net Open. (2022) 5:e2141772. doi: 10.1001/jamanetworkopen.2021.41772
15. Du H, Yang L, Hu Z, Zhang H. Anxiety is associated with higher recurrence of atrial fibrillation after catheter ablation: A meta-analysis. Clin Cardiol. (2022) 45:243–50. doi: 10.1002/clc.23753
16. Alkan Kayhan S, Güner E, Hanedan MO, Topal Çolak E, Mataraci İ. Relationship between preoperative anxiety and atrial fibrillation after coronary artery bypass graft surgery. J Nurs Res: JNR. (2022) 30:e187. doi: 10.1097/jnr.0000000000000473
17. Walters TE, Wick K, Tan G, Mearns M, Joseph SA, Morton JB, et al. Psychological distress and suicidal ideation in patients with atrial fibrillation: prevalence and response to management strategy. J Am Heart Assoc. (2018) 7:e005502. doi: 10.1161/jaha.117.005502
18. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. (2015) 72:334–41. doi: 10.1001/jamapsychiatry.2014.2502
19. Saccaro LF, Gasparini S, Rutigliano G. Applications of Mendelian randomization in psychiatry: a comprehensive systematic review. Psychiatr Genet. (2022) 32:199–213. doi: 10.1097/ypg.0000000000000327
20. Mendez-Miller M, Naccarato J, Radico JA. Borderline personality disorder. Am Family physician. (2022) 105:156–61.
21. Kaplan B, Yazici Gulec M, Gica S, Gulec H. The association between neurocognitive functioning and clinical features of borderline personality disorder. Rev Bras psiquiatria (Sao Paulo Brazil: 1999). (2020) 42:503–9. doi: 10.1590/1516-4446-2019-0752
22. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol: JASN. (2016) 27:3253–65. doi: 10.1681/asn.2016010098
23. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50:1234–9. doi: 10.1038/s41588-018-0171-3
24. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
25. Lv WQ, Lin X, Shen H, Liu HM, Qiu X, Li BY, et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J cachexia sarcopenia muscle. (2021) 12:1860–70. doi: 10.1002/jcsm.12788
26. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084
27. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
28. Lampert R, Jamner L, Burg M, Dziura J, Brandt C, Liu H, et al. Triggering of symptomatic atrial fibrillation by negative emotion. J Am Coll Cardiol. (2014) 64:1533–4. doi: 10.1016/j.jacc.2014.07.959
29. Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. (2006) 119:448. doi: 10.1016/j.amjmed.2005.10.057
30. Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, Hunn BH. Meta-analysis of anxiety as a risk factor for cardiovascular disease. Am J Cardiol. (2016) 118:511–9. doi: 10.1016/j.amjcard.2016.05.041
31. Wu H, Li C, Li B, Zheng T, Feng K, Wu Y. Psychological factors and risk of atrial fibrillation: A meta-analysis and systematic review. Int J Cardiol. (2022) 362:85–92. doi: 10.1016/j.ijcard.2022.05.048
32. Al-Kaisey AM, Parameswaran R, Bryant C, Anderson RD, Hawson J, Chieng D, et al. Atrial fibrillation catheter ablation vs medical therapy and psychological distress: A randomized clinical trial. Jama. (2023) 330:925–33. doi: 10.1001/jama.2023.14685
33. Lakkireddy D, Atkins D, Pillarisetti J, Ryschon K, Bommana S, Drisko J, et al. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA My Heart Study. J Am Coll Cardiol. (2013) 61:1177–82. doi: 10.1016/j.jacc.2012.11.060
34. Feng T, Malmo V, Laugsand LE, Strand LB, Gustad LT, Ellekjær H, et al. Symptoms of anxiety and depression and risk of atrial fibrillation-The HUNT study. Int J Cardiol. (2020) 306:95–100. doi: 10.1016/j.ijcard.2019.11.107
35. Stone MH. Borderline personality disorder: clinical guidelines for treatment. Psychodynamic Psychiatry. (2019) 47:5–26. doi: 10.1521/pdps.2019.47.1.5
36. Leichsenring F, Leibing E, Kruse J, New AS, Leweke F. Borderline personality disorder. Lancet (London England). (2011) 377:74–84. doi: 10.1016/s0140-6736(10)61422-5
37. Kuo JR, Fitzpatrick S, Metcalfe RK, McMain S. A multi-method laboratory investigation of emotional reactivity and emotion regulation abilities in borderline personality disorder. J Behav Ther Exp Psychiatry. (2016) 50:52–60. doi: 10.1016/j.jbtep.2015.05.002
38. Weinberg A, Klonsky ED, Hajcak G. Autonomic impairment in borderline personality disorder: a laboratory investigation. Brain cognition. (2009) 71:279–86. doi: 10.1016/j.bandc.2009.07.014
39. Austin MA, Riniolo TC, Porges SW. Borderline personality disorder and emotion regulation: insights from the Polyvagal Theory. Brain Cognition. (2007) 65:69–76. doi: 10.1016/j.bandc.2006.05.007
40. Zhou X, Dudley SC Jr. Evidence for inflammation as a driver of atrial fibrillation. Front Cardiovasc Med. (2020) 7:62. doi: 10.3389/fcvm.2020.00062
Keywords: borderline personality disorder, atrial fibrillation, casual association, bidirectional, Mendelian randomization
Citation: Zhou W, Wang Z, Hu H, Shi Y, Wang Q and Xue M (2024) Borderline personality disorder and risk of atrial fibrillation: insights from a bidirectional Mendelian randomization study. Front. Psychiatry 15:1392605. doi: 10.3389/fpsyt.2024.1392605
Received: 27 February 2024; Accepted: 24 June 2024;
Published: 10 July 2024.
Edited by:
Massimiliano Beghi, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyReviewed by:
Luigi Francesco Saccaro, University of Geneva, SwitzerlandCopyright © 2024 Zhou, Wang, Hu, Shi, Wang and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Xue, eHVlbWVpMjU3NUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.