
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 25 October 2024
Sec. Psychological Therapy and Psychosomatics
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1391786
 Mitsuhiro Sado1,2,3*
Mitsuhiro Sado1,2,3* Akihiro Koreki2,3,4
Akihiro Koreki2,3,4 Akira Ninomiya2,3
Akira Ninomiya2,3 Chika Kurata2,3
Chika Kurata2,3 Sunre Park3,5
Sunre Park3,5 Daisuke Fujisawa2,3,6
Daisuke Fujisawa2,3,6 Teppei Kosugi2
Teppei Kosugi2 Maki Nagaoka2
Maki Nagaoka2 Atsuo Nakagawa2,7
Atsuo Nakagawa2,7 Masaru Mimura2,3,8
Masaru Mimura2,3,8Introduction: Anxiety disorder is one of the most prevalent mental disorders. Mindfulness-based cognitive therapy (MBCT) is effective for treating anxiety disorders. However, no studies have investigated the cost-effectiveness of MBCT for anxiety disorders. We aimed to conduct a cost-effectiveness analysis alongside a randomized controlled trial (RCT) to clarify the cost-effectiveness of MBCT for anxiety disorders.
Methods: A cost-effectiveness analysis alongside an RCT was conducted for 8 weeks in 40 patients with anxiety disorders at a university hospital. Patients (1) aged 20–75 years; (2) who were diagnosed with panic disorder/agoraphobia or social anxiety disorder based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria; and (3) who provided written consent were analyzed. The participants were allocated randomly (1:1 ratio) to the augmented MBCT group (i.e., MBCT plus treatment as usual [TAU]) or TAU (waitlist control) group. The cost-effectiveness was assessed using the incremental cost-effectiveness ratio (ICER), which is the ratio of the incremental costs divided by the incremental state-trait anxiety inventory- state (STAI-S), state-trait anxiety inventory- trait (STAI-T), and quality-adjusted life years (QALYs). The QALYs were estimated using The Japanese version of EuroQoL five-dimensional 3-level questionnaire. The unit cost data were derived from the government-regulated fees. This study was conducted from a public healthcare insurance perspective. No discount rates were considered.
Results: A total of 38 participants with complete data were included in the analysis. The MBCT was JPY 13,885 more than the cost of TAU and was associated with a STAI-S, STAI-T, and QALY increase of 10.13, 12.00, 0.009 respectively. The ICER were JPY 1,371 (USD13) per STAI-S, JPY 1,157 (USD 11) per STAI-T, and JPY 1,566,357 (USD 14,940) per QALY respectively. MBCT had an 77.5% probability of being cost-effective at a willingness to pay threshold in Japan (JPY 5,000,000 per QALY). The results of the four one-way sensitivity analyses supported the robustness of the base-case analysis findings.
Discussion: Augmented MBCT for anxiety disorders is cost-effective compared with TAU post-treatment from a public healthcare insurance perspective. Future studies should include long-term observations, and analysis from a societal perspective.
Anxiety disorder is one of the most prevalent mental disorders. The 12-month prevalence rates in 2021 were 7.68% in the United States, 7.36% in Europe, and 3.34% in Japan (1). It was more prevalent than depression (6.06%, 5.70%, and 3.15%, respectively), and had a considerable impact on the Global Burden of Disease (2). This burden can be expressed in terms of the monetary costs associated with a given disease. Previous studies revealed that anxiety disorders can impose a substantial economic burden on the afflicted and society as a whole [42.3 billion United States dollars [USD] in 1990 in the US, (3), 8.9 billion British pounds [GBP] in 2007 in England (4), and 2.4 trillion Japanese yen [JPY] in 2008 in Japan (5)].
Clinical guidelines commonly recommend pharmacotherapy and cognitive behavioral therapy (CBT) as the first-line treatments for anxiety disorders (6–8). However, in Japan, the shortage of CBT specialists makes it difficult to perform individual CBT for all suitable patients although most patients prefer this treatment, if available (9). As a result, individual CBT effectively becomes a second-line treatment option. Even so, the number of patients who can receive individual CBT as a second-line treatment is still limited. Therefore, to improve access to psychological treatment, more efficient methods (e.g., group psychotherapy) should be considered before proceeding with more resource-intensive interventions (e.g., individual CBT) as part of a stepped-care approach.
Mindfulness-based cognitive therapy (MBCT) is a promising group psychotherapy for anxiety disorders, which was originally designed to prevent the recurrence of depression (10). Numerous studies have demonstrated its clinical effectiveness in various conditions (e.g., depression, chronic pain, and cancer-associated distress) (11–22), and anxiety is not an exception. A recent meta-analytical review revealed that mindfulness-based intervention (MBI) is effective for anxiety disorders (23). This finding was also observed in a Japanese study; Ninomiya et al. reported that MBCT is effective in patients with anxiety disorders in secondary mental healthcare settings (24).
To expand access to such evidence-based psychological interventions (25), studies investigating the cost-effectiveness of MBCT for anxiety disorders are required. According to recent systematic reviews (26, 27), only one study performed a cost-effectiveness analysis of the MBI associated with anxiety (28). However, as this trial was performed in a patient population with various diagnoses (i.e., depression, anxiety, stress, and adjustment disorders), assessment for anxiety disorders was not possible. To the best of our knowledge, no study has examined the cost-effectiveness of MBIs for anxiety disorders. Therefore, we aimed to conduct a cost-effectiveness analysis alongside a randomized controlled trial (RCT) to clarify the cost-effectiveness of MBCT for anxiety disorders. Among various types of anxiety disorders, we decided to target individuals with panic disorder and social anxiety disorder in this study. Considering that MBCT is designed for the remission state of depression, these disorders, characterized by intermittent symptoms, are appropriate for our focus.
We conducted a cost-effectiveness analysis alongside an RCT. This RCT assessed the effectiveness of MBCT in patients with anxiety disorders in secondary mental healthcare settings. The details of the effectiveness study are described separately (24). This study was approved by the Ethics Review Committee of the Keio University School of Medicine (ID: 20140100) and was conducted in accordance with the Consolidated Standards of Reporting Trials statement (29, 30) and Consolidated Health Economic Evaluation Reporting Standards guidelines (31).
Participants from the outpatient division of the Department of Neuropsychiatry at the Keio University School of Medicine in Tokyo were recruited for the study between September 2014 and May 2015.
The clinical trial was performed as a pragmatic 8-week RCT, comparing augmented MBCT and treatment as usual (TAU) for anxiety disorders with a nested cost-effectiveness analysis.
Patients who (1) were aged between 20 and 75 years; (2) met the diagnostic criteria for panic disorder/agoraphobia or social anxiety disorder specified in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and (3) were able to provide written consent were included in the study. For diagnostic assessment, we used the Japanese version of the Structured Clinical Interview for DSM-IV Axis I Disorders. Meanwhile, patients with (1) a history of substance abuse or dependence, (2) organic brain damage, (3) cognitive dysfunction, (4) current or past episodes of psychosis (including bipolar disorder), (5) antisocial personality disorder, (6) severe physical problems, and (7) suicidal behaviors were excluded. Those who had been previously offered MBIs or who were not expected to attend more than four sessions (e.g. planned relocation) were also excluded. If a participant decided to withdraw from the program and the assessments, he or she was defined as a dropout.
The participants were allocated randomly (1:1 ratio) to either the augmented MBCT group (i.e., MBCT plus TAU) or the waitlist control group (i.e., receiving TAU during the waiting period, and received MBCT after the waiting period completed). A computer-generated random number stratified based on the baseline state-trait anxiety inventory (STAI) state anxiety subscale score (<40 or ≥40) and the anxiety disorder diagnosis (panic disorder/agoraphobia or social anxiety disorder) was used for random allocation to blind the allocation status. The Keio Center for Clinical Research Project Management Office, Tokyo, Japan, performed this process independently of the research team. Owing to the nature of the intervention, we could not conceal the participants or the MBCT therapists’ allocation status.
Participants allocated to the augmented MBCT arm underwent 2-hour MBCT sessions in group-based format (same as the original MBCT) for 8 weeks (eight sessions in total). Each group comprised 10 participants. The MBCT program for depression developed by Segal et al. was applied in this study (10), which consisted of mindfulness practices (e.g., raisin exercise, body scan, sitting meditation, mindful walking, and 3-minute breathing space) and cognitive approaches. Due to the high symptom overlap between depression and anxiety disorders, minimal modifications were made to the original program. The difference from the original program was that we skipped the 1-day silent retreat between weeks 6 and 7 and replaced the lecture related to depression with one relevant to anxiety. The detailed contents of the program can be found in the original report (24).
The participants were requested to practice mindfulness meditation daily and report their records weekly to the research team during the intervention period. The first author conducted the sessions as the primary therapist. He is a qualified mindfulness-based stress reduction (MBSR) teacher at the University of Massachusetts Medical School and has completed all modules of the MBCT teachers’ path at the Oxford Mindfulness Foundation. The third author joined the sessions as a co-therapist.
The TAU program mainly included pharmacotherapy and a brief consultation with a specialized psychiatrist. We did not apply any specific restrictions to drug choice, drug doses, or frequency of psychiatric visits. Specific forms of individual or group psychotherapy (e.g., cognitive behavior therapy, Morita therapy, and interpersonal therapy) were not allowed during the study period. The treatments included in the TAU program differ depending on the country and context. In Japan, a specific form of psychotherapy is only offered in 0.27% of all psychiatrist visits in usual care settings (32). Therefore, the definition of TAU was considered reasonable.
We assessed the clinical conditions at three time points: baseline, during the intervention (4 weeks), and at the end of the intervention (8 weeks).
The primary outcome in the clinical trial was the mean difference in the change in the STAI scores between the groups from baseline to the end of the intervention (8 weeks); this was estimated with a self-report approach. The STAI is a commonly used measure of state and trait anxiety. It can be used in clinical settings to diagnose anxiety and to distinguish it from depressive syndromes. It has 20 items for assessing trait anxiety and 20 items for assessing state anxiety. Higher score indicates higher anxiety status (33). The following events were considered serious adverse consequences: death, life-threatening events, events leading to severe disability or functional impairment, and hospitalization. Adverse events were monitored in each session, and the participants were asked to report them to the researchers.
The health service use data were collected from the clinical reports of patients at each observational time point (i.e., baseline, 4 weeks, and 8 weeks). The participants were requested to report the number/amount of each healthcare service related to the management of psychiatric diseases since the previous assessment. The healthcare services included in the analysis were psychiatrist visits, MBCT cost, and prescribed medications.
The healthcare service use data were converted into cost data by multiplying by the unit cost of each service. The unit cost derived from the data of 2023/2024. The costs were presented in both JPY and USD. The purchasing power parity ratios in 2023 (i.e., one USD) were equal to 104.84 JPY (34). The methods used in estimating each unit cost are as follows.
The cost of a psychiatric visit includes general consultant fees, psychiatric consultation fees, and prescription fees (Table 1). Each unit fee was defined based on a list of government-regulated fees (35).
The unit cost of MBCT was estimated to be JPY 2,700 per session per patient, which is the government-determined reimbursement fee for group psychotherapy (Table 1) (35).
The medication costs consisted of the dispensing and medication costs. The psychotropic drugs were divided into four groups: antidepressants, anxiolytics/hypnotics, antipsychotics, and mood stabilizers. Because the prices of medications in the same category (e.g., paroxetine and sertraline in the antidepressant category) differ, the medication costs are unequal when each patient is prescribed equivalent doses of different drugs in the same category (e.g., paroxetine 40 mg and sertraline 100 mg). To adjust for this difference, the daily dose of each drug was converted into a fraction of the defined daily dose (DDD) based on the World Health Organization-assumed average maintenance daily dose estimated from the dosage recommendations for each drug (36). By multiplying this fraction by the weighted unit cost, the cost of each medicine was estimated using the following formula:
where Cmed-n-d, Dn, DDDn, and Cmed-c represent the cost of medicine n at dose d, the dose of the prescribed medicine n, the DDD of medicine n, and the weighted cost of category C to which the medicine n is attributed, respectively.
We estimated the mean weighted daily medication cost per DDD for each drug category based on the findings of previous studies and government reports (35–38). The results and methods used to estimate them are provided in Table 1 and Supplementary Tables 1A–E.
The total medication costs were estimated by determining the area of the two trapezoids squared by the medication costs per day and the duration of 8 weeks.
In the economic evaluation, we set the primary effectiveness outcome as STAI-S, and STAI-T. We compared the cost-effectiveness of the augmented MBCT and TAU based on the incremental cost-effectiveness ratios (ICERs), calculated as the ratio of the incremental costs divided by the decrease in STAI-S, and STAI-T score. We estimated ICER per quality adjusted life years (QALYs) as well. The QALYs were estimated as the area under the curve of the health-related utilities using the EuroQoL five-dimensional 3 level (EQ-5D-3L) questionnaire (at baseline, 4 weeks, and 8 weeks post-randomization) (39). We used the value set by the Japanese version of the EQ-5D-3L questionnaire (40) to assess health-related utilities. The observational period was set at 8 weeks post-intervention. The analysis was based on a public healthcare insurance perspective. Because the study period was short (8 weeks), the discount rate was not considered.
The t-test for continuous variables were used for testing the treatment engagement. The significance level used in the test was 5% two-sided. Two-sided 95% confidence intervals were used when calculating confidence intervals. The estimated sample size comprised 20 participants for each arm, with one-sided significance level of 5% and statistical power of 80%, allowing for 20% attrition.
Similar to other typical cost-effectiveness analyses alongside clinical trials, the cost-effectiveness was assessed using complete samples because the QALYs required health-related utility scores at all observational points. The ICER was used to compare the augmented MBCT’s cost-effectiveness (MBCT plus TAU) with that of TAU alone. The ICER was estimated as the result of 1,000 resamplings from the original samples using the non-parametric bootstrap method. Uncertainty was assessed by drawing probabilistic acceptability curves for clinical and political decision-making. The acceptability curves represent the probability that the enhanced augmented MBCT is more cost-effective than TAU alone over a range of hypothetical values placed on the incremental outcome (willingness to pay [WTP] by the decision-makers of the healthcare system). The net monetary benefit approach was used to draw the cost-effectiveness acceptability curves. The net monetary benefit is defined as follows:
Where λ is the WTP for an incremental unit of improvement in the outcome measures, and ΔE and ΔC represent the difference in effectiveness and cost, respectively, between the groups.
In order to assess the robustness of the results related to cost-effectiveness, three and four one-way sensitivity analyses for ICER per STAI, and QALY were conducted respectively. In the first analysis, the unit cost of MBCT was set as JPY 32,529 per session regardless of the number of participants. This figure was calculated by multiplying the average hourly wage of physicians in Japan in 2023 (JPY 6,506) (41) by 2 physicians and 2.5 hours (i.e. including 30 minutes for preparation and cleanup and 2 hours for the session). This analysis was aimed at determining the actual human capital cost rather than the revenue from the healthcare insurance: in Japan, the reimbursement is determined according to the “fee for service per patient” schema. In the second analysis, all samples were analyzed including those with missing data. The last observation carry forward method (LOCF) was used for the imputation of missing data. The third analysis was performed using all samples with different unit costs of MBCT (JPY 32,529 per session regardless of the number of participants). The fourth analysis focused exclusively on ICER per QALY to assess the impact of different methodologies for estimating QALYs. Rather than using the base case analysis method, which relies on the absolute area under the curve of health-related utilities, we opted for the incremental area from the baseline to account for baseline differences in the EQ-5D score. Statistical analyses were performed using R (4.0.2) (42).
The final intervention was conducted in July 2015. The process of participant selection from screening to post-intervention is shown in Figure 1. Of the 57 candidate participants, 17 who did not meet the inclusion criteria were excluded. Hence, only 40 participants were randomly allocated to either the MBCT plus TAU group or TAU alone (waitlist group) group. The attrition rates were the same in both groups (one in each group).
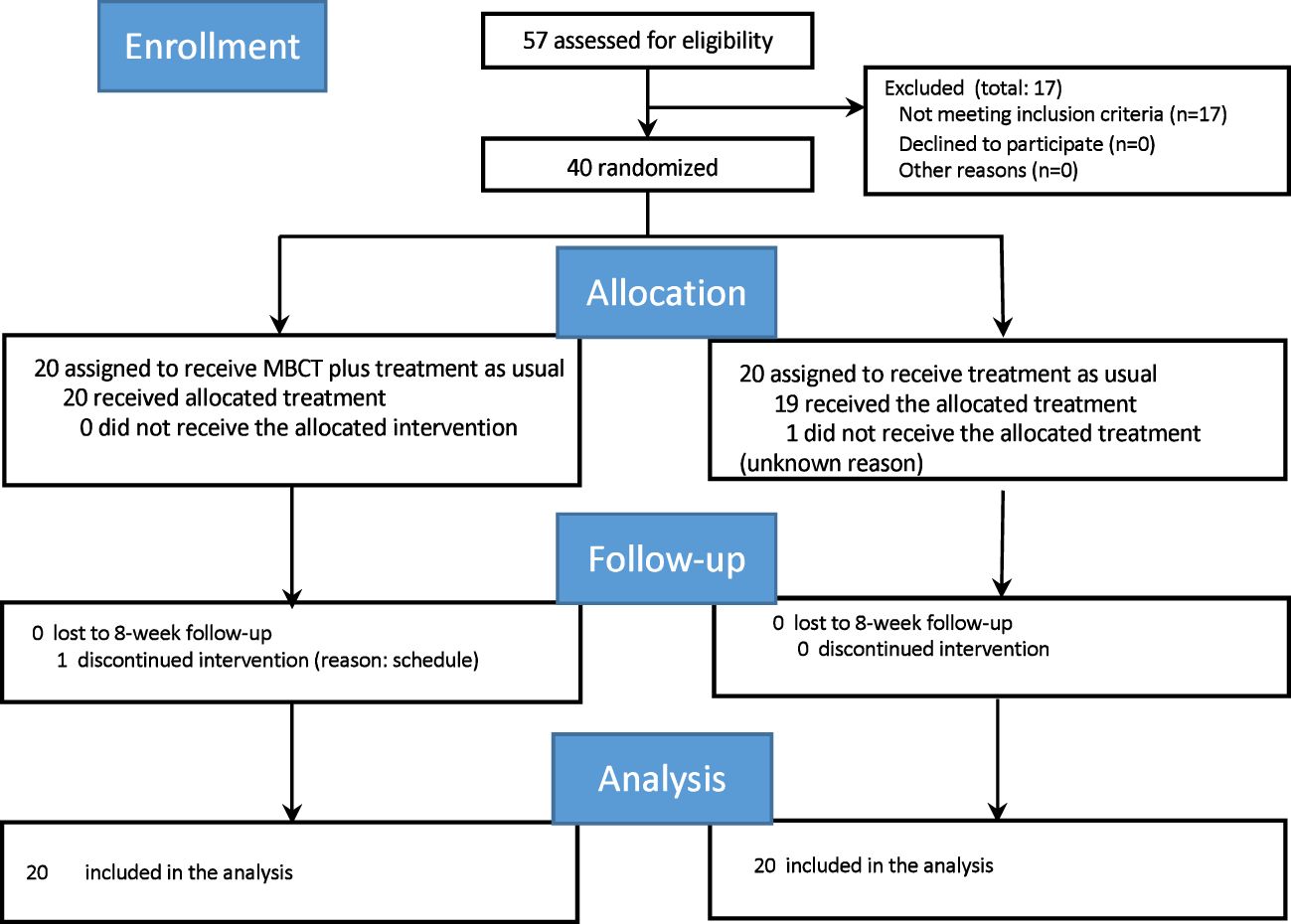
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) diagram of participants flow through the study. MBCT, mindfulness-based cognitive therapy.
As in the original article, the average duration of disorders from onset (mean [standard deviation (SD)]) was 151.2 [123.3] months, and the average treatment duration (mean [SD]) was 110.7 [118.7] months. Approximately 95% of all participants were prescribed at least one psychotropics (2.9 psychotropics on average), while 67.5% were prescribed at least one antidepressant at baseline. No significant differences were observed in any of the variables between the groups, including age, sex, or diagnosis. No significant differences were also observed in the clinical measures and average duration of anxiety disorders from the onset or treatment initiation.
One participant in each arm (two in total) dropped out before the study was completed, one in the augmented MBCT group withdrew from the study during the intervention period (after receiving the first session), and one in the control group discontinued after the baseline assessment. The average number of MBCT sessions attended (mean [SD]) was 6.8 [2.2].
Although the difference was not significant (p=0.262), the number of psychiatrist visits during the study period was slightly higher in the control group (mean [SD]: 3.5 [2.0]) than that in the MBCT group (2.7 [2.4]). No significant difference was observed in the dose of all drug categories between the treatment arms at any point in the study (Table 2). No serious adverse events occurred during the study period. 3.3% of the EQ-5D data were missing.
Table 3 shows the mean costs and clinical outcomes and the difference between the groups (n=38 with complete data). The analysis was conducted with a mixed-effects model repeated-measures approach. The model included intervention group, week, group-by-week interaction, age, and sex as fixed effects.
Although the psychiatrist visit count and medication costs were lower in the augmented MBCT group than in the TAU group, the augmented MBCT group spent JPY 13,894 more than the TAU group because of the additional MBCT cost, which showed statistical significance.
Regarding the clinical outcomes, the scores of STAI-S, and STAI-T in the MBCT significantly reduced (mean [SD])(STAI-S: -10.368 [2.317], STAI-T: -11.684 [2.250]), while those in the TAU was almost constant (STAI-S: -0.316 [2.317], STAI-T: 0.211 [2.250]). Therefore, the difference between the groups at week 8 was significant (STAI-S: −10.053 [3.277] p=0.003, STAI-T: −11.895 [3.182] p<0.001). In the health utility domain of the EQ-5D questionnaire, the score of the augmented MBCT group was 0.061 higher than that of the TAU group; however, the difference was not significant (p=0.23).
A total of 38 participants, with complete data, were included in the base case cost-effectiveness analysis. The MBCT was JPY 13,885 more costly than the cost of TAU and the respective decrements in STAI-state and STAI-trait were 10.13 and 12.00. Then, the ICERs were JPY 1,371 per 1-point decrement in STAI-S and JPY 1,157 per 1-point decrement in STAI-T score, respectively (Table 4). The relevant acceptability curves are shown in Figures 2, 3. Although no established WTP threshold of ICER for STAI available in Japan, the probabilities that MBCT is cost-effective at a WTP of JPY 5,000 per 1-point STAI-S, -T improvement were 98.7% and 99.9% respectively.
Same as the analysis for ICER per STAI, a total of 38 participants, with complete data, were included in the analysis (Table 4). The incremental cost remained the same as in the previous analysis (JPY 13,885) and was associated with a QALY increase of 0.009. Therefore, the ICER was JPY 1,566,357 (USD 14,940) below the threshold adopted in Japan (i.e., JPY 5,000,000 (USD 47,692) per QALY gained) (43). The acceptability curve (Figure 4) demonstrated an 77.5% probability that MBCT is cost-effective at a WTP threshold of JPY 5,000,000 (USD 47,692) per QALY.
In the first scenario with the MBCT cost of JPY 32,529/session (n=38: augmented MBCT 19 vs. TAU 19), The MBCT was JPY 20,618 more costly than the cost of TAU and the respective decrement in STAI-state and STAI-trait were 10.05 and 11.90. Then, the ICERs were JPY 2,052 (USD 20) per STAI-S and JPY 1,733 (USD 17) per STAI-T, respectively (Supplementary Table 2). The relevant acceptability curves are shown in Supplementary Figures 1, 2. The probabilities that MBCT is cost-effective at a WTP of JPY 5,000 per 1-point STAI-S, -T decrement were 96.5% and 99.8%.
In the second scenario, analysis was conducted on all participants, including those with missing values (n=40: augmented MBCT 20 vs. TAU 20). LOCF was performed for the imputation of the missing STAI scores (no imputation was performed for the cost). The MBCT was JPY 13,780 more costly than the cost of TAU and the respective incremental decrement in STAI-state and STAI-trait were 10.22 and 11.83. Then, the ICERs were JPY 1,348 (USD 13) per STAI-S and JPY 1,165 (USD 11) per STAI-T, respectively (Supplementary Table 3). The relevant acceptability curves are shown in Supplementary Figures 3, 4. The probabilities that MBCT is cost-effective at a WTP of JPY 5,000 per STAI decrement were 98.7% and 99.9% respectively.
The third sensitivity analysis with an MBCT unit cost of JPY 32,529/session, including the samples with missing values (n=40: augmented MBCT 20 vs. TAU 20), revealed that the ICER per STAI-S, and per STAI-T were JPY 2,106 (USD 20), and JPY 1,751 (USD 17) respectively (Supplementary Table 4). The augmented MBCT had an 94.6% and 99.5% probability of being cost-effective at a WTP of JPY 5,000 per 1-point STAI improvement (Supplementary Figures 5, 6).
In the first scenario with the MBCT cost of JPY 32,529/session (n=38: augmented MBCT 19 vs. TAU 19), the ICER became JPY 2,364,440 (USD 22,553) per QALY (Supplementary Table 2), which was below the WTP threshold in Japan (JPY 5,000,000 (USD 47,692) per QALY). The acceptability curve indicated that the probability of the augmented MBCT being cost-effective was 72.5% at a WTP threshold of JPY 5,000,000 (USD 47,692) per QALY (Supplementary Figure 7).
In the second scenario, analysis was conducted on all participants, including those with missing values (n=40: augmented MBCT 20 vs. TAU 20). LOCF was performed for the imputation of the missing EQ-5D scores (no imputation was performed for the cost). The incremental QALY remained 0.009, while the incremental cost became JPY 13,780, leading to a slightly preferable ICER (JPY 1,487,395 (USD 14,187) per QALY) (Supplementary Table 3). The augmented MBCT was 79.2% cost-effective at a WTP threshold of JPY 5,000,000 (USD 47,692) per QALY (Supplementary Figure 8).
The third sensitivity analysis with an MBCT unit cost of JPY 32,529/session, including the samples with missing values (n=40: augmented MBCT 20 vs. TAU 20), revealed that the ICER was JPY 2,409,698 (USD 22,985) per QALY (Supplementary Table 4). The augmented MBCT had an 71.3% probability of being cost-effective at a WTP threshold of JPY 5,000,000 (USD 47,692) per QALY (Supplementary Figure 9).
The fourth sensitivity analysis using the incremental area of QALYs from the baseline method, with complete samples (n=38: augmented MBCT 19 vs. TAU 19), revealed that the ICER was JPY 2,408,526 (USD 22,973) per QALY (Supplementary Table 5). The augmented MBCT had an 71.2% probability of being cost-effective at a WTP threshold of JPY 5,000,000 (USD 47,692) per QALY (Supplementary Figure 10).
This study is the first to evaluate the cost-effectiveness of augmented MBCT for anxiety disorders. This study is unique because it 1) was conducted in a secondary mental healthcare setting, where the treatment duration of the participants was quite long, and 2) compared the cost-effectiveness of augmented MBCT with that of TAU alone (i.e., pharmacotherapy: the most prevalent treatment strategy).
Regarding the ICER per STAI, there is no established consensus on a WTP threshold. To the best of our knowledge, no studies have reported the ICER per STAI improvement for anxiety disorders. However, a study evaluating the cost-effectiveness of augmented CBT for treating moderate to severe depression in Japan (44) reported ICERs per 1-point decrement in the GRID-HDRS17, BDI-II, and QIDS scales ranging from JPY 14,262 to 36,166. Although the score ranges of these scales differ, the ICER per STAI (approximately JPY 1,400 per STAI) was notably lower. In the intervention group of this study, the mean STAI scores improved by approximately 10 points for STAI-S and 12 points for STAI-T, approaching the normal range. These findings suggest that such near-remission improvements can be achieved at a cost of approximately JPY14,000, thereby demonstrating the cost-effectiveness of MBCT.
Although the QALY improvement did not reach the statistically significance, the ICER per QALY showed favorable results. The base analysis indicated that MBCT is cost-effective because the ICER [i.e., JPY 1,566,357 (USD 14,940)] is well below the WTP threshold (JPY 5,000,000), and the probability of MBCT being cost-effective is 0.775. Because the results remained the same even after conducting a series of sensitivity analyses (i.e., ICERs were between JPY 1,487,395 (USD 14,187) and JPY 2,409,698 (USD 22,985); and the probabilities of MBCT being cost-effective were between 0.712 and 0.792, the finding that the augmented MBCT is cost-effective is quite robust.
In this study, as previously noted, while both STAI-S and STAI-T showed significant improvement, the incremental QALYs did not reach statistical significance. This may be attributed to the use of the EQ-5D-3L, as previous research suggests that its responsiveness (i.e. the ability to accurately capture change of symptoms overtime) to anxiety disorders is less pronounced compared to depression (45). However, since responsiveness has improved in the EQ-5D-5L (46), the results might have differed had the EQ-5D-5L been used.
Unfortunately, previous systematic reviews (26, 27) were unable to find studies that investigated the cost-effectiveness of MBIs for anxiety disorders. The only relevant study was that conducted by Saha et al. (28), who assessed the cost-effectiveness of MBCT and compared it with that of CBT in the clinical population, including those with anxiety. This study reported that mindfulness group therapy significantly reduced healthcare costs, despite showing no significant difference in effectiveness compared to the control group. However, this trial involved a patient population with various diagnoses (e.g., depression, anxiety, stress, and adjustment disorders), and 76% of the control group participants received individual CBT. Therefore, the results of the present study were difficult to directly compare with those of similar studies.
On the other hand, expanding the scope to other conditions enabled the conduct of further discussions. Shawyer et al. (47) evaluated the cost-effectiveness of the augmented MBCT for preventing new episodes in patients with recurrent major depression. This study was conducted alongside an RCT comparing augmented MBCT (MBCT plus active monitoring) with active monitoring alone for 2 years. Findings showed that MBCT resulted in better outcomes and was less costly (the ICER was AUD 83,744, below the threshold in Australia [AUD 100,000]), highlighting the augmented dominance of MBCT. Similar results were observed in patients with other conditions such as multiple sclerosis (48). In the trial, MBCT was not superior to the waitlist in terms of the QALY gained. However, because healthcare costs were substantially reduced, the probability of MBCT being cost-effective was quite high (87.4% with a WTP threshold of GBP 20,000). These and our findings imply that MBCT is likely to be cost-effective when provided in addition to usual care, irrespective of clinical conditions.
Augmented MBCT is effective and cost-effective compared with TAU for anxiety disorders. However, considering some constraints of the study, such as small sample size, and short study duration combined with the chronic nature of anxiety disorders and concerns raised about the sensitivity of the measurement instruments over such a period, further research over longer period with larger and more representative samples is required to establish more definitive conclusions.
Another policy implication is that a training system for MBCT therapists should be established. The growth of well-trained psychotherapists requires time, cost, and a well-designed system. With the adoption of the “improving access to psychological treatment” program in the UK (49), a well-organized and systematic training program for therapists is essential. In Japan, the Japanese Ministry of Health, Labour and Welfare has provided opportunities for CBT trainees to attend workshops and receive supervision since 2011 (50). A similar system is required to disseminate MBCT to patients.
In addition, a precise estimate of the unit cost for human resources required to provide group psychotherapy is needed. Under the public health insurance system in Japan, group psychotherapy is reimbursed at a uniform rate regardless of the therapist’s degree or level of proficiency. Therefore, from the perspective of insurance-based analysis, these differences in conditions do not affect the results. However, to provide a more accurate estimate, such data is essential.
We found the strength of the study in using multiple effectiveness measures (i.e.STAI, and EQ-5D); having an excellent retention rate (95%); contributing to a rare yet important literature area by using best practices to calculate costs alongside effectiveness in a randomized controlled trial of psychotherapy. Furthermore, by clarifying the cost-effectiveness of MBCT as a group-based psychotherapy, the study offers important insights into expanding access to psychotherapy for patients in need, particularly in settings with limited human resources. However, we also acknowledge some limitations. The first was related to the representativeness of typical patient population. It was constrained due to the study participants being selected only from university hospital patients with a dominance of males over females (70% in the intervention arm and 55% in the control group). Second, Using the wait-list group as a control group may have potentially biased the results in a way that makes them appear larger. Third, the observational period was relatively short (i.e., 8 weeks). One study reported that the MBCT’s effect observed within a short term disappeared in the long term (51); therefore, the assessment of the long-term effect is important. Fourth, the EQ-5D-3L was used in the trial because the EQ-5D-5L was unavailable at the beginning of the study. As the EQ-5D-5L improves the ceiling effect, the difference might have been observed if we had been able to use the EQ-5D-5L instead. Additionally, using other scales such as the SF-6D or HUI might have produced different results. Fifth, we did not conduct an analysis from a societal perspective. This is solely because work productivity was not assessed during the trial. However, economic evaluation from a societal perspective would yield different interpretations than those from a public healthcare insurance perspective. Sixth, inability to conceal the participants or the MBCT therapists’ allocation status might have biased self- reported outcomes, with participants in the MBCT group possibly overestimating benefits and those in the control group underestimating them due to perceived inferiority. Seventh, we collected the data on healthcare service use via self-report. Then, we could not reduce the effects of recall bias. Therefore, we should be aware that these limitations would impact the wider applicability of the findings. Future research should consider these issues to improve the quality of economic evaluations relevant to MBCT for anxiety disorders.
In conclusion, augmented MBCT for anxiety disorders is cost-effective considering WTP threshold for ICER per QALY in Japan (JPY 5,000,000) at 8-week compared with TAU (pharmacotherapy) post-treatment from a public healthcare insurance perspective. Therefore, future studies should conduct a long-term observation, use a larger sample size, and perform an analysis from a societal perspective.
The disclosure of the raw data supporting the conclusions of this article requires the ethical committee’s permission. Requests to access the datasets should be directed to MS, bWl0c3VzYWRvQGtlaW8uanA=.
The studies involving humans were approved by the Ethics Review Committee at Keio University School of Medicine (ID: 20140100). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AK: Data curation, Formal analysis, Writing – review & editing. ANi: Investigation, Validation, Writing – review & editing. CK: Data curation, Writing – review & editing. SP: Investigation, Writing – review & editing. DF: Methodology, Supervision, Writing – review & editing. TK: Investigation, Writing – review & editing. MN: Investigation, Writing – review & editing. ANa: Supervision, Writing – review & editing. MM: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (KAKENHI grant number: 25460629). The funding source had no role in the study design, data collection, analysis, or interpretation; in the writing of the report; or in the decision to submit the paper for publication.
MS received grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology during the conduct of the study; honoraria from Shionogi & Co., Ltd. from Meiji Seika Pharma, Hisamitsu Pharmaceutical, Mochida Pharmaceutical, and Terumo Corporation and grant from Sony Corporate Services Corporation and Meiji Seika Pharma outside of the submitted work. AN received honoraria from Mochida Pharmaceutical Co., Ltd. and grants from Sony Corporate Services Corporation outside the submitted work. DF received research grants from Eisai Pharma and honoraria from Yoshitomiyakuhin Corporation, Mitsubishi Tanabe Pharma Corporation, and Eisai Pharma outside the submitted work. TK received grants from Gunma Hospital outside the submitted work. AN received royalties from Igaku-Shoin and Kongo-Shuppan and honoraria from Dainippon-Sumitomo Pharma, Pfizer, Yoshitomi Yakuhin, Otsuka Pharmaceutical, Meiji Seika, Tsumura & Co., and Mitsubishi Tanabe Pharma outside the submitted work. MM received grants and honoraria from Tsumura, Nishikawa-sangyo, and Teijin Pharma; personal fees from Chugai Pharmaceutical, Yoshitomiyakuhin Corporation, Shionogi &Co., Novartis Pharma K.K, Meiji Seika Pharma Co., Mochida Pharmaceutical Co., Eli Lilly Japan K.K, Janssen Pharmaceutical K.K, Fujifilm RI Pharma Co., Sumitomo Dainippon Pharma Co., Eisai Co., and Asahi Kasei Pharma Corporation; and personal fees from Astellas Pharma Inc., MSD K.K, Otsuka Pharmaceutical Co., Ono Pharmaceutical Co., and Pfizer Inc. outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1391786/full#supplementary-material
Supplementary Figure 1 | Cost-effectiveness acceptability curves (STAI-S) with sensitivity analysis 1.
Supplementary Figure 2 | Cost-effectiveness acceptability curves (STAI-T) with sensitivity analysis 1.
Supplementary Figure 3 | Cost-effectiveness acceptability curves (STAI-S) with sensitivity analysis 2.
Supplementary Figure 4 | Cost-effectiveness acceptability curves (STAI-T) with sensitivity analysis 2.
Supplementary Figure 5 | Cost-effectiveness acceptability curves (STAI-S) with sensitivity analysis 3.
Supplementary Figure 6 | Cost-effectiveness acceptability curves (STAI-T) with sensitivity analysis 3.
Supplementary Figure 7 | Cost-effectiveness acceptability curves (QALY) with sensitivity analysis 1.
Supplementary Figure 8 | Cost-effectiveness acceptability curves (QALY) with sensitivity analysis 2.
Supplementary Figure 9 | Cost-effectiveness acceptability curves (QALY) with sensitivity analysis 3.
Supplementary Figure 10 | Cost-effectiveness acceptability curves (QALY) with sensitivity analysis 4. JPY, Japanese yen; MBCT, mindfulness-based cognitive therapy.
AUD, Australian dollars; CBT, cognitive behavioral therapy; DDD, defined daily dose; EQ-5D, EuroQol-5D; GBP, Great Britain pounds; ICER, incremental cost-effectiveness ratio; JPY, Japanese yen; LOCF, last observation carry forward; MBCT, mindfulness-based cognitive therapy; MBIs, mindfulness-based interventions; MBSR, mindfulness-based stress reduction; QALYs, quality-adjusted life years; RCT, randomized controlled trial; STAI-S, state-trait anxiety inventory-state; STAI-T, state-trait anxiety inventory-trait; TAU, treatment as usual; USD, United States dollars; WTP, willingness to pay.
1. Institute for Health Metrics and Evaluation, University of Washington. Global health data exchange (2024). Available online at: https://vizhub.healthdata.org/gbd-results/ (accessed July 20, 2024).
2. Yang X, Fang Y, Chen H, Zhang T, Yin X, Man J, et al. Global, regional and national burden of anxiety disorders from 1990 to 2019: results from the Global Burden of Disease Study 2019. Epidemiol Psychiatr Sci. (2021) 30:e36. doi: 10.1017/S2045796021000275
3. Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. (1999) 60:427–35. doi: 10.4088/jcp.v60n0702
4. McCrone P. Paying the price: the cost of mental health care in England to 2026. London: King’s Fund. (2008). Available online at: https://www.kingsfund.org.uk/insight-and-analysis/reports/paying-price-mental-health-cost (accessed August 31, 2024).
5. Sado M, Takechi S, Inagaki A, Fujisawa D, Koreki A, Mimura M, et al. Cost of anxiety disorders in Japan in 2008: a prevalence-based approach. BMC Psychiatry. (2013) 13:338. doi: 10.1186/1471-244X-13-338
6. National Institute for Health and Care Excellence. Obsessive-compulsive disorder and body dysmorphic disorder: treatment. London: National Institute for Health and Care Excellence (2005).
7. National Institute for Health and Care Excellence. Generalised anxiety disorder and panic disorder in adults: management. London: National Institute for Health and Care Excellence (2011).
8. National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment and treatment. London: National Institute for Health and Care Excellence (2013).
9. McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. (2013) 74:595–602. doi: 10.4088/JCP.12r07757
10. Segal Z, Williams JM, Teasdale J. Mindfulness-based cognitive therapy for depression. In: A new approach to preventing relapse. Guilford Publications, New York (2002).
11. Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. (2000) 68:615–23. doi: 10.1037//0022-006x.68.4.615
12. Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol. (2008) 76:966–78. doi: 10.1037/a0013786
13. Kuyken W, Warren FC, Taylor RS, Whalley B, Crane C, Bondolfi G, et al. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: an individual patient data meta-analysis from randomized trials. JAMA Psychiatry. (2016) 73:565–74. doi: 10.1001/jamapsychiatry.2016.0076
14. Banth S, Ardebil MD. Effectiveness of mindfulness meditation on pain and quality of life of patients with chronic low back pain. Int J Yoga. (2015) 8:128–33. doi: 10.4103/0973-6131.158476
15. Ljótsson B, Falk L, Vesterlund AW, Hedman E, Lindfors P, Rück C, et al. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome–a randomized controlled trial. Behav Res Ther. (2010) 48:531–9. doi: 10.1016/j.brat.2010.03.003
16. Omidi A, Zargar F. Effect of mindfulness-based stress reduction on pain severity and mindful awareness in patients with tension headache: a randomized controlled clinical trial. Nurs Midwifery Stud. (2014) 3:e21136. doi: 10.17795/nmsjournal21136
17. Rahmani S, Talepasand S. The effect of group mindfulness - based stress reduction program and conscious yoga on the fatigue severity and global and specific life quality in women with breast cancer. Med J Islam Repub Iran. (2015) 29:175.
18. Wells RE, Burch R, Paulsen RH, Wayne PM, Houle TT, Loder E. Meditation for migraines: a pilot randomized controlled trial. Headache. (2014) 54:1484–95. doi: 10.1111/head.12420
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. (2012) 30:1335–42. doi: 10.1200/JCO.2010.34.0331
20. Park S, Sato Y, Takita Y, Tamura N, Ninomiya A, Kosugi T, et al. Mindfulness-based cognitive therapy for psychological distress, fear of cancer recurrence, fatigue, spiritual well-being, and quality of life in patients with breast cancer-A randomized controlled trial. J Pain Symptom Manage. (2020) 60:381–9. doi: 10.1016/j.jpainsymman.2020.02.017
21. Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. (2015) 121:1231–40. doi: 10.1002/cncr.29194
22. Zhang JY, Zhou YQ, Feng ZW, Fan YN, Zeng GC, Wei L. Randomized controlled trial of mindfulness-based stress reduction (MBSR) on posttraumatic growth of Chinese breast cancer survivors. Psychol Health Med. (2017) 22:94–109. doi: 10.1080/13548506.2016.1146405
23. Haller H, Breilmann P, Schröter M, Dobos G, Cramer H. A systematic review and meta-analysis of acceptance- and mindfulness-based interventions for DSM-5 anxiety disorders. Sci Rep. (2021) 11:20385. doi: 10.1038/s41598-021-99882-w
24. Ninomiya A, Sado M, Park S, Fujisawa D, Kosugi T, Nakagawa A, et al. Effectiveness of mindfulness-based cognitive therapy in patients with anxiety disorders in secondary-care settings: A randomized controlled trial. Psychiatry Clin Neurosci. (2020) 74:132–9. doi: 10.1111/pcn.12960
25. Yates BT. Research on improving outcomes and reducing costs of psychological interventions: toward delivering the best to the most for the least. Annu Rev Clin Psychol. (2020) 16:125–50. doi: 10.1146/annurev-clinpsy-071519-110415
26. Feliu-Soler A, Cebolla A, McCracken LM, D’Amico F, Knapp M, López-Montoyo A, et al. Economic impact of third-wave cognitive behavioral therapies: A systematic review and quality assessment of economic evaluations in randomized controlled trials. Behav Ther. (2018) 49:124–47. doi: 10.1016/j.beth.2017.07.001
27. Zhang L, Lopes S, Lavelle T, Jones KO, Chen L, Jindal M, et al. Economic evaluations of mindfulness-based interventions: a systematic review. Mindfulness (NY). (2022) 13:2359–78. doi: 10.1007/s12671-022-01960-1
28. Saha S, Jarl J, Gerdtham UG, Sundquist K, Sundquist J. Economic evaluation of mindfulness group therapy for patients with depression, anxiety, stress and adjustment disorders compared with treatment as usual. Br J Psychiatry. (2020) 216:197–203. doi: 10.1192/bjp.2018.247
29. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. (2008) 148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008
30. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. (2010) 340:c332. doi: 10.1136/bmj.c332
31. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. (2013) 346:f1049. doi: 10.1136/bmj.f1049
32. Japanese Ministry of Health, L, Welfare. Statistics of medical care activities in public health insurance (2020). Available online at: https://iss.ndl.go.jp/books/R100000002-I027835846-00 (accessed July 29, 2021).
33. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. California Consulting Psychologists Press. (1983).
34. OECD Data Explorer. Annual Purchasing Power Parities and exchange rates (2024). Available online at: https://data-explorer.oecd.org/ (accessed August 31, 2024).
35. Japanese Ministry of Health, L, Welfare. Medical fee revision in FY 2024 (2024). Available online at: https://www.mhlw.go.jp/content/12404000/001251499.pdf (accessed August 31, 2024).
36. World Health Organization. Defined daily dose (DDD) (2021). Available online at: https://www.who.int/tools/atc-ddd-toolkit/about-ddd (accessed February 10, 2021).
37. Nakagawa A, Mitsuda D, Sado M, Abe T, Fujisawa D, Kikuchi T, et al. Effectiveness of supplementary cognitive-behavioral therapy for pharmacotherapy-resistant depression: A randomized controlled trial. J Clin Psychiatry. (2017) 78:1126–35. doi: 10.4088/JCP.15m10511
38. Nakagawa A. International comparison study related to the prescription of psychotropics. Tokyo: Japanese Ministry of Health, Labour and Welfare (2011).
39. Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford: Oxford University Press (2007).
40. Tsuchiya A, Ikeda S, Ikegami N, Nishimura S, Sakai I, Fukuda T, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. (2002) 11:341–53. doi: 10.1002/hec.673
41. Japanese Ministry of Health, L, Welfare. Basic survey on wage structure (2023). Available online at: https://www.e-stat.go.jp/stat-search/files?stat_infid=000040163741 (accessed August 31, 2024).
42. R Core Team. R: A language and environment for statistical computing (2016). Available online at: https://www.R-project.org/ (accessed August 31, 2024).
43. Japanese Ministry of Health, L, Welfare. On the establishment of benchmark values in cost-effectiveness evaluation (2018). Available online at: https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000211609.pdf (accessed July 20, 2024).
44. Sado M, Koreki A, Ninomiya A, Kurata C, Mitsuda D, Sato Y, et al. Cost-effectiveness analyses of augmented cognitive behavioral therapy for pharmacotherapy-resistant depression at secondary mental health care settings. Psychiatry Clin Neurosci. (2021) 75(11):341–50. doi: 10.1111/pcn.13298
45. Brazier J, Connell J, Papaioannou D, Mukuria C, Mulhern B, Peasgood T, et al. A systematic review, psychometric analysis and qualitative assessment of generic preference-based measures of health in mental health populations and the estimation of mapping functions from widely used specific measures. Health Technol Assess. (2014) 18:vii–viii, xiii-xxv, 1-188. doi: 10.3310/hta18340
46. Sandin K, Shields G, Gjengedal RGH, Osnes K, Bjorndal MT, Reme SE, et al. Responsiveness to change in health status of the EQ-5D in patients treated for depression and anxiety. Health Qual Life Outcomes. (2023) 21:35. doi: 10.1186/s12955-023-02116-y
47. Shawyer F, Enticott JC, Özmen M, Inder B, Meadows GN. Mindfulness-based cognitive therapy for recurrent major depression: A ‘best buy’ for health care? Aust N Z J Psychiatry. (2016) 50:1001–13. doi: 10.1177/0004867416642847
48. Bogosian A, Chadwick P, Windgassen S, Norton S, McCrone P, Mosweu I, et al. Distress improves after mindfulness training for progressive MS: A pilot randomised trial. Mult Scler. (2015) 21:1184–94. doi: 10.1177/1352458515576261
49. The National Collaborating Centre for Mental Health. The improving access to psychological therapies Manual (2018). Available online at: https://www.england.nhs.uk/wp-content/uploads/2018/06/the-nhs-talking-therapies-manual-v6.pdf (accessed August 19, 2023).
50. National Center of Neurology and Psychiatry.Cognitive Behavior Therapy training program funded by Japan Ministry of Health, Labor, and Welfare (2023). Available online at: https://mhlw-cbt-training.ncnp.go.jp/index.html (accessed August 19, 2023).
Keywords: mindfulness, cost-effectiveness, mindfulness-based cognitive therapy, anxiety disorders, randomized controlled trial
Citation: Sado M, Koreki A, Ninomiya A, Kurata C, Park S, Fujisawa D, Kosugi T, Nagaoka M, Nakagawa A and Mimura M (2024) Cost-effectiveness analysis of mindfulness-based cognitive therapy in patients with anxiety disorders in secondary mental health care settings alongside a randomized controlled trial. Front. Psychiatry 15:1391786. doi: 10.3389/fpsyt.2024.1391786
Received: 26 February 2024; Accepted: 23 September 2024;
Published: 25 October 2024.
Edited by:
Melissa Thong, German Cancer Research Center (DKFZ), GermanyReviewed by:
Muchandifunga Trust Muchadeyi, German Cancer Research Center (DKFZ), GermanyCopyright © 2024 Sado, Koreki, Ninomiya, Kurata, Park, Fujisawa, Kosugi, Nagaoka, Nakagawa and Mimura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mitsuhiro Sado, bWl0c3VzYWRvQGtlaW8uanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.