- Department of Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
Objective: Although the adverse effects of obesity in schizophrenia are documented, there is limited research exists on the implications for untreated initial schizophrenia. Our investigation aimed to explore the connections between BMI and cognitive function in first-episode drug-naïve (FEDN)schizophrenia.
Methods: We enrolled 143 FEDN schizophrenia patients, and collected data on their body mass index, fasting blood glucose and lipid levels. Cognitive function was measured with the MATRICS Consensus Cognitive Battery (MCCB). Using correlation and regression analysis to assess the relationship between BMI and cognitive performance.
Results: The prevalence rate of overweight plus obesity in FEDN schizophrenia patients was 33.57%. Patients with FEDN schizophrenia exhibited extensive cognitive impairment, and those who were overweight/obesity demonstrated more severe impairments in working memory and visual learning when compared to normal/under weight counterparts. Correlation analysis indicated a negative association between working memory and BMI and TG, as well as a link between visual learning and BMI and LDL-C. Multiple linear regression analysis revealed that a higher BMI predicted a decrease in working memory in FEDN schizophrenia patients.
Conclusion: Our results indicate that the rate of overweight plus obesity is high in FEDN schizophrenia patients, and there is an association between BMI and cognitive function in schizophrenia, particularly in relation to working memory.
Introduction
Schizophrenia is a chronic disease with an unclear etiopathogenesis and poor prognosis, representing a heavy economic burden (1). According to epidemiological studies, up to 50% of patients with schizophrenia experience obesity problems (2), and approximately 44% of untreated first-episode schizophrenia patients are overweight or obese (3), which is higher than the rate among general population (2, 3). Furthermore, obesity in individuals with schizophrenia not only increases their vulnerability to physical ailments like cardiovascular disease but also diminishes their life expectancy by approximately a decade (4, 5).
Cognitive impairment is a significant and prevalent issue in schizophrenia (6). A number of studies consistently underscored that cognitive impairment hinders the recovery of social functioning in schizophrenia and is the most challenging core symptom to significantly improve (7–9). Previous research has discovered a connection between obesity and cognitive dysfunction. For instance, obese mice displayed poorer learning and memory capabilities than non-obese counterparts (10, 11). A recent study found that schizophrenia patients had lower working memory, motor speed, and cognitive composite scores as BMI increased (12). Another study pointed out that elevated BMI may detrimentally affect neurocognitive function by disrupting white matter integrity in schizophrenia (13). However, C. W. Wei et al. discovered a positive correlation between BMI and verbal and visuospatial abilities in schizophrenia (14). Furthermore, Rashid et al. suggested that obesity does not exert a direct impact on cognitive function in schizophrenia (15).
However, the aforementioned studies primarily focused on chronic schizophrenia patients, and the results could be influenced by prolonged psychiatric symptoms and the administration of antipsychotic medications (16–18). Antipsychotic medications, especially atypical antipsychotics, have been indisputably linked to obesity-related metabolic disturbances (17, 18). There is also intense debate regarding the impact of antipsychotic drugs on cognitive function (19). Furthermore, there are gender differences in the psychiatric symptoms and cognitive functions of patients with schizophrenia (20). Similarly, Zhu Y et al. found that attentional bias during social information processing also exhibits gender differences (21). There is little research examining the association between obesity and cognition in first-episode drug-naïve (FEDN) schizophrenia patients. Therefore, the insufficient evidence regarding the cognitive implications of obesity in schizophrenia calls for further investigation to establish a clearer understanding.
Considering the sex differences in schizophrenia (20, 21) and the effect of antipsychotic medication (16, 17, 22), we recruited first-episode drug-naïve male patients with schizophrenia to control for these confounding factors. We collected metabolic indicators, including BMI, fasting plasma glucose, and lipids, as well as symptom dimensions and cognitive assessment scores. The primary objective of this study was to investigate the differences in cognitive function between overweight/obesity and normal/under weight FEDN male patients with schizophrenia, followed by an investigation into the correlation between BMI and cognitive function. We postulated that a significant correlation would exist between BMI and cognitive ability in FEDN male schizophrenia. Building upon prior research, we recruited drug-naïve male patients with first-episode schizophrenia to control for these confounding factors.
Materials and methods
Participants
Our study subjects were 143 patients enrolled in the outpatient clinic and inpatient departments of the Affiliated Brain Hospital of Nanjing Medical University from May 2017 to October 2022. All patients were unanimously assessed by two highly experienced associate chief psychiatrists or chief psychiatrists, in accordance with the diagnostic criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5. All study participants were diagnosed with schizophrenia after at least 1 year of follow-up. The inclusion criteria for schizophrenic patients were as follows: (1) the Chinese Han population, right-handed, aged 16-44; (2) Education years ≥ 8 years, intelligence quotient (IQ) ≥ 70; (3) First onset, duration of first psychotic symptoms ≤ 24 months, drug naïve (i.e., no previous exposure to antipsychotics), no prior exposure to physical therapy; (4) Positive and Negative Syndrome Scale (PANSS) total score ≥ 60 points. Exclusion criteria included major somatic disorders, organic mental disorder, dementia/mental retardation, alcohol or substance abuse. Participants provided written consent, and the Medical Research Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University approved this consent procedure.
Clinical and psychological assessments
Age, sex, and years of education were self-reported. Height and weight were measured, and body mass index (BMI) was calculated using the formula BMI = weight (kg)/height squared (m2). Based on the Chinese metabolic abnormality criteria (23), individuals with a BMI≥24 kg/m2 were considered overweight/obesity group, while those with a BMI < 24 kg/m2 were classified as normal/under weight group.
Fasting blood samples were collected between 6:30-7:30 in the morning. The levels of fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured using Beckman AU5821 automatic biochemical analyzer.
We computed duration of illness (DUI) from onset of illness to the date of assessment. Intellectual quotient (IQ) was measured using the Chinese version of the Wechsler Adult Intelligence Scale-Revised (WAIS), which includes four subtests: Knowledge Quiz, Similarity Test, Picture Filling Test, and Block Diagram Test. Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS), which was administered by two experienced psychiatrists who received an intra-class correlation coefficient (ICC) above 0.8 prior to the study inception. All data collection was completed in 5 days.
Measures of cognitive function
The cognitive functions were assessed using the Chinese version of the MATRICS Consensus Cognitive Battery (MCCB) (24). MCCB included 9 sub-items, which were Trail Making Test, Symbol Coding, Hopkins Verbal Learning-Revised, Spatial Span, Mazes, Brief Visuospatial Memory Test-Revised, Fluency, Managing Emotions, and Continuous Performance Test-Identical Pairs. Standardized T scores were calculated for each subtest to assess composite scores and the following seven cognitive domains: speed of processing, attention and vigilance, working memory, visual learning, verbal learning, problem solving, and social cognition (25).
Statistical analyzes
The analysis was conducted using SPSS 27.0. The normality of the data distribution was assessed using the Shapiro-Wilk test. After conducting this test, it was found that all quantitative data adhered to a normal distribution. Independent samples t-test was employed to compare the demographic, clinical characteristics, and cognitive function between groups. The cognitive function of norm and patients was compared using one sample t-test. Pearson correlation analysis was used to explore preliminary associations between BMI and cognitive function. Multiple linear regression analysis was employed to further explore the relationship. In this analysis, cognitive function served as the dependent variable, while meaningful indicators identified through correlation analysis were considered independent variables. Additionally, we controlled for covariates such as age, IQ, and DUI.
Results
Demographic and clinical characteristics
The demographic, metabolic characteristics and PANSS scores are shown in Table 1. The rate of overweight/obesity among FEDN patients with schizophrenia was 33.57% (48/143), which was higher than that of the general population aged 25-33 years (19.10%) (26). Compared to normal/under weight patients, overweight/obesity patients showed significantly elevated levels of BMI, TG and LDL-C (all p <0.05). However, HDL-C is lower in the overweight/obesity group than in the normal/under weight group (p <0.05). There were no significant differences in the remaining demographic variables and PANSS scores between the two groups.
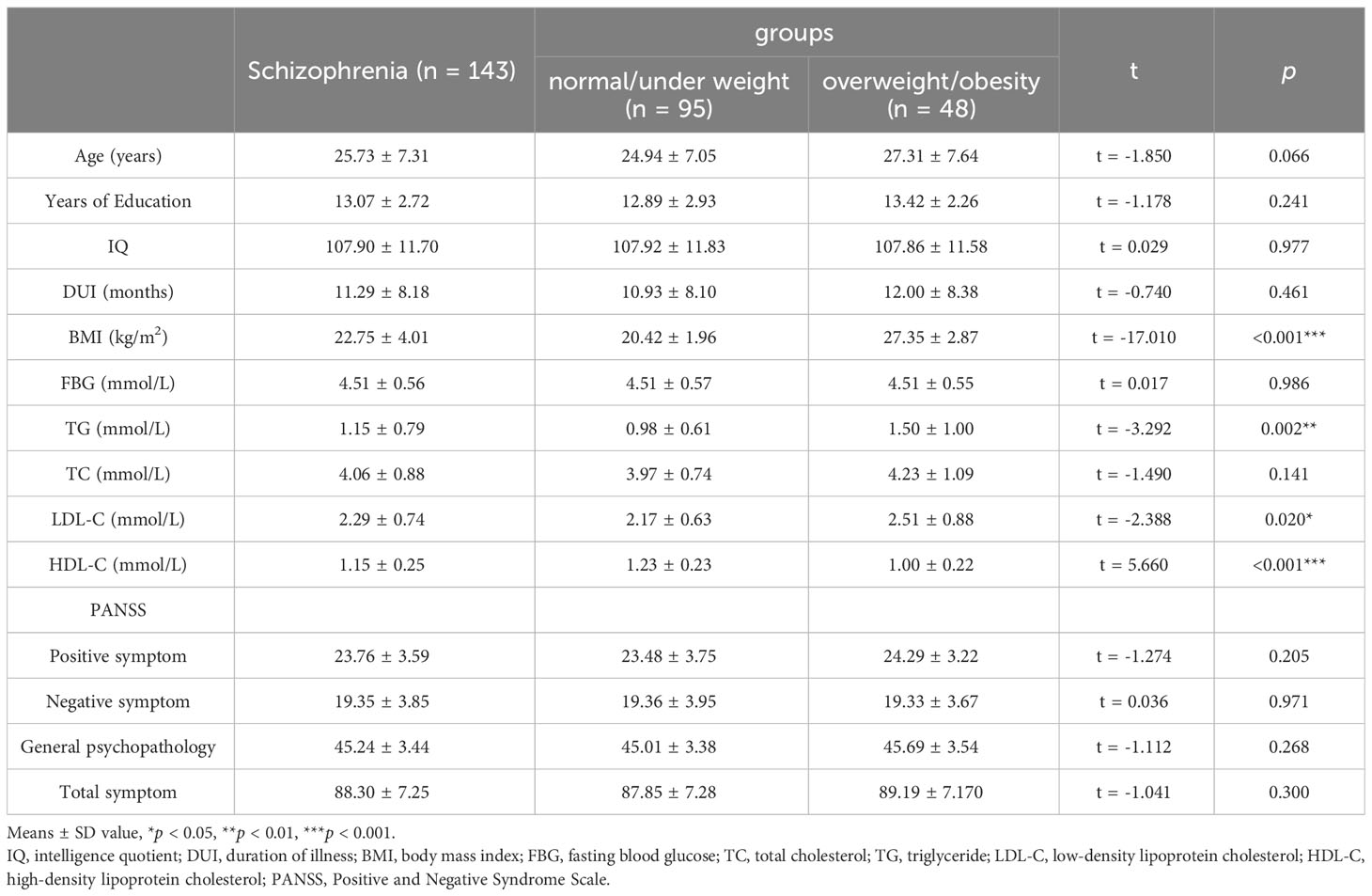
Table 1 Demographic, metabolic characteristics and PANSS scores of first-episode drug-naïve schizophrenia patients.
Cognitive function
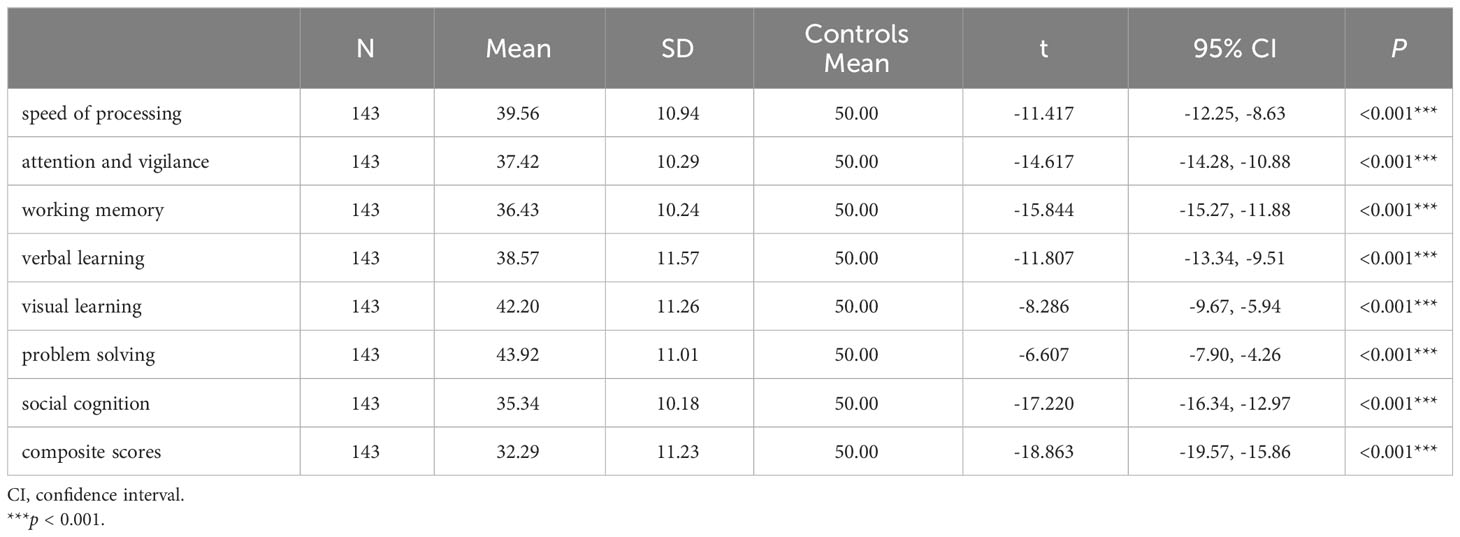
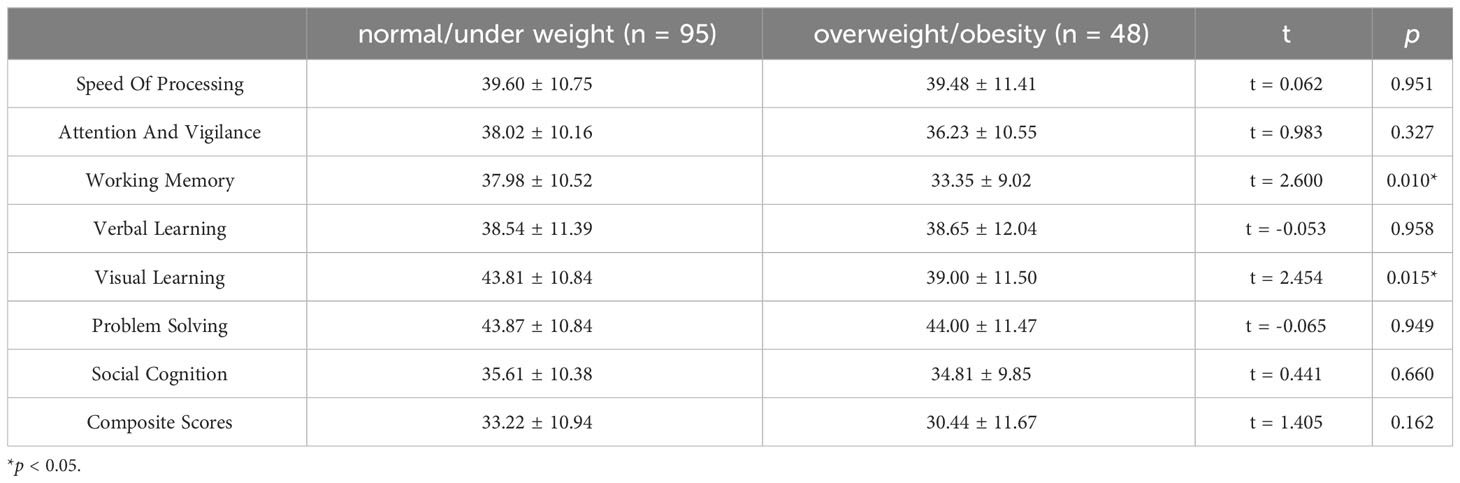
FEDN schizophrenia patients exhibited significant cognitive deficits compared to Chinese MCCB norms (n = 656, mean = 50, SD = 10) (24), as stated in Table 2 (all p < 0.001). And the cognitive differences between overweight/obesity and normal/under weight FEDN schizophrenia patients were demonstrated in Table 3. overweight/obesity patients exhibited markedly inferior performance in working memory and visual learning compared to normal/under weight patients. Box plots depicting significant differences between the two groups are presented in Figure 1. However, no significant differences were observed between the two groups in the remaining cognitive domains.
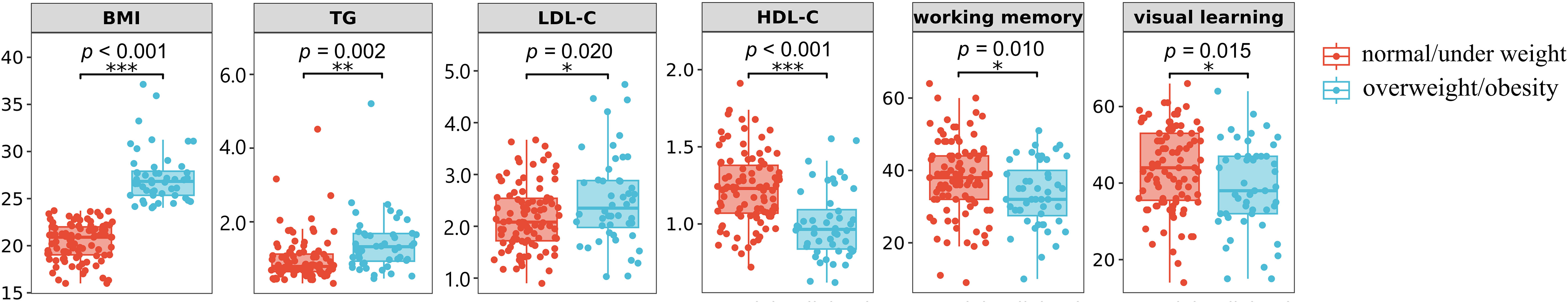
Figure 1 Box plots of significant differences between normal/under weight and overweight/obesity schizophrenia.
Association between BMI and cognitive function
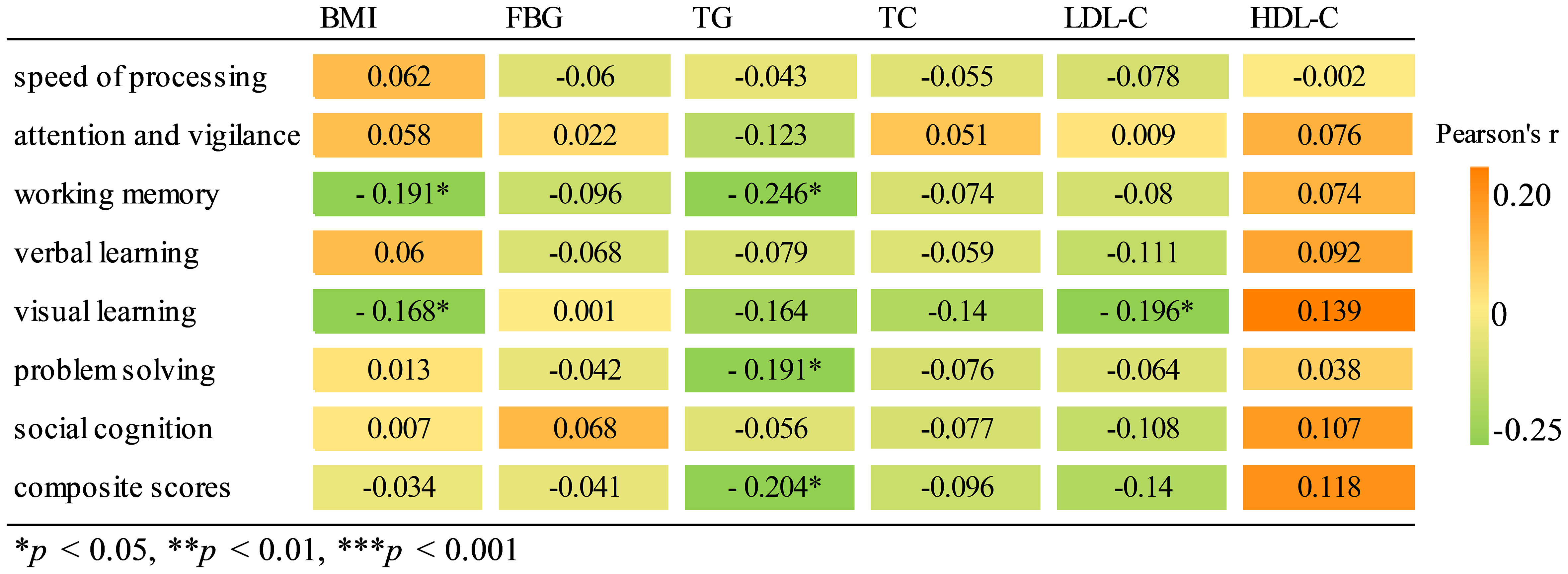
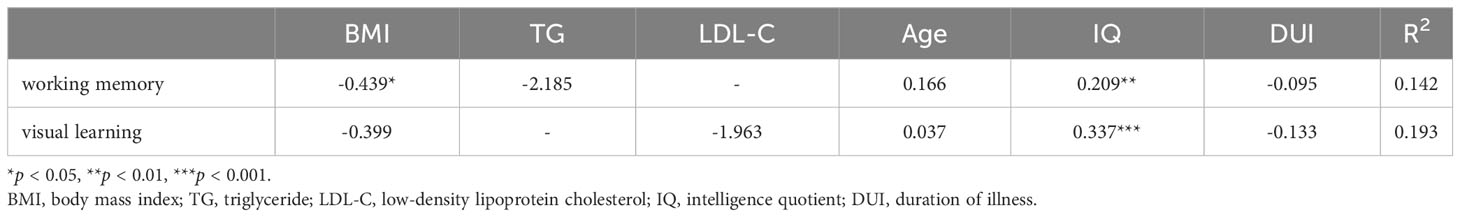
Pearson correlation analysis revealed a significant negative association between working memory and BMI (r = -0.191, p = 0.022) as well as TG (r = -0.246, p = 0.003). Similarly, visual learning exhibited a significant negative correlation with both BMI (r = -0.168, p = 0.045) and LDL-C levels (r = -0196, p = 0.019). There was a negative correlation between TG and problem solving (r = -0191, p = 0.023), as well as composite scores (r = -0204, p = 0.014). Results of correlation analysis are shown in a heatmap in Figure 2. The scatter plot of BMI and cognitive function are shown in Supplementary Figure S2. Further multiple regression analysis revealed that only a higher BMI predicted a decrease in working memory (β = -0.439, p = 0.044, Table 4).
Discussion
The primary findings of this study were as follows: (1) the rate of overweight/obesity among FEDN schizophrenia patients was 33.57%. (2) FEDN schizophrenia patients had extensive cognitive impairment. And overweight/obesity patients exhibited markedly inferior performance in working memory and visual learning compared to normal/under weight patients. (3) Correlation analysis indicated a significant association between lower working memory and higher BMI and TG, as well as a link between poorer visual learning and elevated BMI and LDL-C in FEDN schizophrenia patients. Further regression analysis revealed that BMI significantly and negatively influenced working memory among FEDN schizophrenia patients.
In this study, we observed a higher overweight/obesity rate (33.57%) among FEDN schizophrenia patients (age 25.73 ± 7.31) compared to the general population aged 15-24 years old (7.50%) and 25-33 years old (19.10%) (26). Our findings are consistent with the previous study by Y. Tian et al., which reported an increased prevalence of overweight and obesity among first-episode schizophrenia in contrast to healthy control (3). Moreover, metabolic syndrome is more likely to develop in both drug-naïve schizophrenia patients and their siblings, regardless of antipsychotic effects (27). The findings suggest a greater propensity for overweight and obesity among individuals with schizophrenia, independent of antipsychotic medication impact.
The increased prevalence of overweight/obesity in first-episode schizophrenia could be attributed to a combination of factors, including unhealthy lifestyles, gut microbiota dysbiosis, and genetic susceptibility. Unhealthy lifestyles, such as reduced time spent on complex activities (28) and excessive consumption of nonalcoholic beverages (i.e., high sugar intake) (29), are common among patients with early-stage schizophrenia and contribute to the elevated obesity rate in this population. In addition, dysbiosis of the gut microbiota may provide a common biological basis for the etiology of schizophrenia and obesity. Both conditions are associated with a reduction in anti-inflammatory bacteria and an increase in proinflammatory and pathogenic bacteria (30). The inflammatory signals produced by gut microbiota may serve as a potential link between schizophrenia and obesity.
More interestingly, several studies suggest that the higher obesity rate among first-episode schizophrenia patients may be attributed to genetic susceptibility. The 22q11.2 deletion syndrome, for instance, is the strongest risk factor for schizophrenia (31, 32) and is also associated with a higher risk of developing obesity (33, 34). A study found a strong correlation between schizophrenia and BMI at 18 different genetic loci, with 16q12.11 being the primary locus (35). The duplication or deletion of 16p11.2 can cause various physical and mental symptoms, including schizophrenia and obesity (36–39). The shared genetic loci between BMI and schizophrenia are linked to 20 significantly enriched pathways, with the proton pump inhibitor pathway and AKT phosphorylation targets in the nuclear pathway being particularly significant (40). These pathways have an impact on neurodevelopment (40), providing insights into the biological mechanisms underlying the relationship between schizophrenia and obesity in terms of genetic susceptibility.
In this study, our second finding is that FEDN schizophrenia patients had significantly lower cognitive function than the norm across multiple domains. This is consistent with previous research (41–43), which demonstrated that early-stage schizophrenia patients suffer from widespread cognitive impairment. Moreover, overweight/obesity schizophrenia patients performed significantly worse than normal/under weight patients in working memory and visual learning. This phenomenon may be related to the detrimental effects of obesity on cognitive function, which has also been corroborated by prior studies (10–13).
However, the exact mechanism underlying the association between obesity and cognitive impairments in schizophrenia is complex and not fully understood. Obesity may lead to inflammation, insulin resistance, vascular changes, and alterations in brain structure and function. It can trigger inflammation pathways in microglial cells, causing abnormalities in cerebral blood vessels and potentially leading to cognitive decline (44, 45). Obesity-related insulin resistance can disrupt gene expression in the hippocampus, impairing cognitive functions (46). Obesity is also associated with increased amyloid-β protein deposition and neurofibrillary tangles, hallmarks of Alzheimer’s disease (47). Hormones and cytokines secreted by adipose tissue can affect brain energy metabolism, neuronal survival, emotional states, and cognitive processes, and may cause neuroinflammation and neuronal damage (48–52). Research has linked obesity to gray matter atrophy in various brain regions, notably the prefrontal cortex, which is associated with schizophrenia and cognitive functioning (53, 54). Addressing obesity and its related metabolic effects may be important in managing cognitive impairments in individuals with schizophrenia.
Further correlation analysis revealed a negative association between working memory and BMI and TG, as well as a connection between visual learning and BMI and LDL-C in FEDN schizophrenia patients. Multiple linear regression analysis also revealed that a higher BMI predicted a decrease in working memory in FEDN schizophrenia patients. These findings underscore the potential harm of overweight/obesity on cognitive function in the early stage of schizophrenia, especially in specific cognitive domains such as executive function. However, due to limited relevant studies in FEDN schizophrenia patients, more research is needed to replicate our findings.
Additionally, the research results regarding the association between obesity and cognitive function in patients with schizophrenia who have received antipsychotic medication for a certain period are inconsistent. X. Guo et al. observed a significant association between higher BMI and lower scores on the visual reproduction and digit symbol tests (55). Likewise, S. Hidese et al. detected a negative correlation between BMI and the composite score of Brief Assessment of Cognition in Schizophrenia (BACS) (12). However, C. W. Wei et al.’s study revealed a positive correlation between BMI and language and visuospatial domains (14). Moreover, another study suggests that there is no association between obesity and cognitive impairment in individuals with schizophrenia (56). One speculation is that the more severe cognitive impairments in patients with schizophrenia may obscure the potential correlation between cognitive impairment and other risk factors. For instance, Rashid employed structural equation modeling to unveil the deleterious impact of obesity on the condition of patients with schizophrenia, thereby affecting cognition (15). Another speculation is that the association between obesity and cognition may be related to the ‘obesity paradox’ (57), where higher BMI in younger years is associated with decreased cognitive abilities, but higher BMI in later years is associated with improved cognition. Furthermore, these inconsistent results may also be attributed to the differences in assessment tools, disease progression, frequency of psychiatric episodes, use of antipsychotic medications, and family economic capacity. Therefore, more research is needed to explore the complex and dynamic relationship between obesity and cognitive function in patients with schizophrenia, especially in specific cognitive domains and different stages of the illness.
The strengths of this study lie in the examination of first-episode drug-naïve schizophrenia patients, and the absence of confounding factors, such as antipsychotic intervention and sex effects. However, this study had several limitations. First, due to its cross-sectional design, we were unable to track changes within each patient and determine causal relationships or moderating effects between variables. Therefore, future studies should focus on longitudinal studies of BMI changes in the same individual to more accurately assess the dynamic relationship between BMI and cognitive function, as well as their association with disease progression and treatment outcomes. We hope that such research will provide more evidence and guidance for cognitive rehabilitation in patients with FEDN schizophrenia. Second, our study solely focused on male schizophrenia patients, which limits generalization to the entire population with schizophrenia. To enhance the inclusivity and comprehensiveness of our observation, it is imperative to improve sample diversity by incorporating female patients and healthy individuals. Third, the sample size of this study was relatively small, which could increase the likelihood of obtaining false-negative results. Four, in this study, relying solely on BMI as a measure of obesity has limitations because it does not account for factors such as body fat percentage and differences in body shape. Future research could consider combining multiple methods to measure obesity, such as waist circumference and waist-to-height ratio (WHtR), and conducting more detailed subgroup analyses to comprehensively assess the relationship between the type and severity of obesity and schizophrenia. Finally, the possibility of selection bias cannot be ruled out, since our analysis only included subjects who were tested for all variables. Despite adjusting for various known confounders in our analyzes, there may still exist residual confounding.
Conclusions
In conclusion, our findings indicate that the rate of overweight/obesity among FEDN schizophrenia patients was high. Additionally, overweight/obesity patients exhibited markedly inferior performance in working memory and visual learning compared to normal/under weight patients. Furthermore, regression analysis revealed that BMI significantly and negatively influenced working memory of FEDN schizophrenia patients. These results underscore the potential utility of markers for overweight/obesity as targets for both improvement and prevention strategies in addressing cognitive function in schizophrenia. This study provides more clues for clinical and scientific investigation of this intricate and multifaceted psychiatric disorder.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Research Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XD: Data curation, Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Methodology, Software. SL: Data curation, Writing – original draft, Writing – review & editing, Funding acquisition. YL: Data curation, Writing – review & editing. XF: Conceptualization, Methodology, Software, Writing – review & editing. RZ: Data curation, Investigation, Writing – review & editing. XS: Data curation, Writing – review & editing. JD: Funding acquisition, Project administration, Resources, Validation, Writing – review & editing. SX: Funding acquisition, Project administration, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Medical Science and Technology Development Foundation, Nanjing Municipality Health Bureau (ZKX21033 and YKK23134).
Acknowledgments
We deeply thank the generous contributions of all research participants. We also grateful to our parents for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1362674/full#supplementary-material
References
1. Kadakia A, Catillon M, Fan Q, Williams GR, Marden JR, Anderson A, et al. The economic burden of schizophrenia in the United States. J Clin Psychiatry. (2022) 83(6). doi: 10.4088/JCP.22m14458
2. Annamalai A, Kosir U, Tek C. Prevalence of obesity and diabetes in patients with schizophrenia. World J Diabetes. (2017) 8:390–6. doi: 10.4239/wjd.v8.i8.390
3. Tian Y, Wang D, Wei G, Wang J, Zhou H, Xu H, et al. Prevalence of obesity and clinical and metabolic correlates in first-episode schizophrenia relative to healthy controls. Psychopharmacol (Berl). (2021) 238:745–53. doi: 10.1007/s00213-020-05727-1
4. Rekhi G, Khyne TT, Lee J. Estimating 10-year cardiovascular disease risk in Asian patients with schizophrenia. Gen Hosp Psychiatry. (2016) 43:46–50. doi: 10.1016/j.genhosppsych.2016.09.005
5. Hjorthoj C, Sturup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. (2017) 4:295–301. doi: 10.1016/S2215-0366(17)30078-0
6. Wu JQ, Chen DC, Tan YL, Xiu MH, De Yang F, Soares JC, et al. Cognitive impairments in first-episode drug-naive and chronic medicated schizophrenia: MATRICS consensus cognitive battery in a Chinese Han population. Psychiatry Res. (2016) 238:196–202. doi: 10.1016/j.psychres.2016.02.042
7. Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. (2009) 19:365–84. doi: 10.1007/s11065-009-9109-y
8. Barch DM, Keefe RS. Anticipating DSM-V: opportunities and challenges for cognition and psychosis. Schizophr Bull. (2010) 36:43–7. doi: 10.1093/schbul/sbp139
9. McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. (2023) 28:1902–18. doi: 10.1038/s41380-023-01949-9
10. Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. (2008) 149:2628–36. doi: 10.1210/en.2007-1722
11. Watson LS, Stone TD, Williams D, Williams AS, Sims-Robinson C. High-fat diet impairs tactile discrimination memory in the mouse. Behav Brain Res. (2020) 382:112454. doi: 10.1016/j.bbr.2019.112454
12. Hidese S, Matsuo J, Ishida I, Hiraishi M, Teraishi T, Ota M, et al. Relationship of handgrip strength and body mass index with cognitive function in patients with schizophrenia. Front Psychiatry. (2018) 9:156. doi: 10.3389/fpsyt.2018.00156
13. Spangaro M, Mazza E, Poletti S, Cavallaro R, Benedetti F. Obesity influences white matter integrity in schizophrenia. Psychoneuroendocrinology. (2018) 97:135–42. doi: 10.1016/j.psyneuen.2018.07.017
14. Wei CW, Chen YQ, Ma M, Xiu MH, Zhang XY. Sex differences in the association of body mass index with symptoms and cognitive deficits in Chinese patients with chronic schizophrenia. Transl Psychiatry. (2020) 10:18. doi: 10.1038/s41398-020-0717-x
15. Rashid NA, Lim J, Lam M, Chong SA, Keefe RS, Lee J. Unraveling the relationship between obesity, schizophrenia and cognition. Schizophr Res. (2013) 151:107–12. doi: 10.1016/j.schres.2013.09.020
16. Luther L, Raugh IM, Collins DE, Knippenberg AR, Strauss GP. Negative symptoms in schizophrenia differ across environmental contexts in daily life. J Psychiatr Res. (2023) 161:10–8. doi: 10.1016/j.jpsychires.2023.02.037
17. Olten B, Bloch MH. Meta regression: Relationship between antipsychotic receptor binding profiles and side-effects. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 84:272–81. doi: 10.1016/j.pnpbp.2018.01.023
18. Zhang Y, Tang W, Tang B, Fan K, Zhao K, Fang X, et al. Altered mitochondrial lymphocyte in overweight schizophrenia patients treated with atypical antipsychotics and its association with cognitive function. Front Immunol. (2023) 14:1325495. doi: 10.3389/fimmu.2023.1325495
19. Zhang Y, Fang X, Fan W, Tang W, Cai J, Song L, et al. Brain-derived neurotrophic factor as a biomarker for cognitive recovery in acute schizophrenia: 12-week results from a prospective longitudinal study. Psychopharmacol (Berl). (2018) 235:1191–8. doi: 10.1007/s00213-018-4835-6
20. Liu R, Fang X, Yu L, Wang D, Wu Z, Guo C, et al. Gender differences of schizophrenia patients with and without depressive symptoms in clinical characteristics. Front Psychiatry. (2021) 12:792019. doi: 10.3389/fpsyt.2021.792019
21. Zhu Y, Xu L, Wang W, Guo Q, Chen S, Zhang C, et al. Gender differences in attentive bias during social information processing in schizophrenia: An eye-tracking study. Asian J Psychiatr. (2021) 66:102871. doi: 10.1016/j.ajp.2021.102871
22. Zhou R, He M, Fan J, Li R, Zuo Y, Li B, et al. The role of hypothalamic endoplasmic reticulum stress in schizophrenia and antipsychotic-induced weight gain: A narrative review. Front Neurosci. (2022) 16:947295. doi: 10.3389/fnins.2022.947295
23. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. (2019) 35:e3158. doi: 10.1002/dmrr.3158
24. Shi C, Kang L, Yao S, Ma Y, Li T, Liang Y, et al. The MATRICS consensus cognitive battery (MCCB): co-norming and standardization in China. Schizophr Res. (2015) 169:109–15. doi: 10.1016/j.schres.2015.09.003
25. Zhang H, Wang Y, Hu Y, Zhu Y, Zhang T, Wang J, et al. Meta-analysis of cognitive function in Chinese first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) profile of impairment. Gen Psychiatr. (2019) 32:e100043. doi: 10.1136/gpsych-2018-100043
26. Hu L, Huang X, You C, Li J, Hong K, Li P, et al. Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PloS One. (2017) 12:e0183934. doi: 10.1371/journal.pone.0183934
27. Enez Darcin A, Yalcin Cavus S, Dilbaz N, Kaya H, Dogan E. Metabolic syndrome in drug-naive and drug-free patients with schizophrenia and in their siblings. Schizophr Res. (2015) 166:201–6. doi: 10.1016/j.schres.2015.05.004
28. Hodgekins J, French P, Birchwood M, Mugford M, Christopher R, Marshall M, et al. Comparing time use in individuals at different stages of psychosis and a non-clinical comparison group. Schizophr Res. (2015) 161:188–93. doi: 10.1016/j.schres.2014.12.011
29. Scoriels L, Zimbron J, Garcia-Leon N, Coll-Negre M, Giro M, Perez J, et al. Cross-sectional study of diet patterns in early and chronic schizophrenia. Schizophr Res. (2019) 208:451–3. doi: 10.1016/j.schres.2019.03.029
30. Wu H, Liu Y, Wang J, Chen S, Xie L, Wu X. Schizophrenia and obesity: May the gut microbiota serve as a link for the pathogenesis? iMeta. (2023) 2(2):e99. doi: 10.1002/imt2.99
31. Van L, Boot E, Bassett AS. Update on the 22q11.2 deletion syndrome and its relevance to schizophrenia. Curr Opin Psychiatry. (2017) 30:191–6. doi: 10.1097/YCO.0000000000000324
32. Cleynen I, Engchuan W, Hestand MS, Heung T, Holleman AM, Johnston HR, et al. Genetic contributors to risk of schizophrenia in the presence of a 22q11.2 deletion. Mol Psychiatry. (2021) 26:4496–510. doi: 10.1038/s41380-020-0654-3
33. Voll SL, Boot E, Butcher NJ, Cooper S, Heung T, Chow EW, et al. Obesity in adults with 22q11.2 deletion syndrome. Genet Med. (2017) 19:204–8. doi: 10.1038/gim.2016.98
34. Maurer GW, Malita A, Nagy S, Koyama T, Werge TM, Halberg KA, et al. Analysis of genes within the schizophrenia-linked 22q11.2 deletion identifies interaction of night owl/LZTR1 and NF1 in GABAergic sleep control. PloS Genet. (2020) 16:e1008727. doi: 10.1371/journal.pgen.1008727
35. Yu Y, Fu Y, Yu Y, Tang M, Sun Y, Wang Y, et al. Investigating the shared genetic architecture between schizophrenia and body mass index. Mol Psychiatry. (2023) 28:2312–9. doi: 10.1038/s41380-023-02104-0
36. Guha S, Rees E, Darvasi A, Ivanov D, Ikeda M, Bergen SE, et al. Implication of a rare deletion at distal 16p11.2 in schizophrenia. JAMA Psychiatry. (2013) 70:253–60. doi: 10.1001/2013.jamapsychiatry.71
37. Maillard AM, Hippolyte L, Rodriguez-Herreros B, Chawner SJ, Dremmel D, Aguera Z, et al. 16p11.2 Locus modulates response to satiety before the onset of obesity. Int J Obes (Lond). (2016) 40:870–6. doi: 10.1038/ijo.2015.247
38. Maillard AM, Ruef A, Pizzagalli F, Migliavacca E, Hippolyte L, Adaszewski S, et al. The 16p11.2 locus modulates brain structures common to autism, schizophrenia and obesity. Mol Psychiatry. (2015) 20:140–7. doi: 10.1038/mp.2014.145
39. Peters T, Nullig L, Antel J, Naaresh R, Laabs BH, Tegeler L, et al. The role of genetic variation of BMI, body composition, and fat distribution for mental traits and disorders: A look-up and mendelian randomization study. Front Genet. (2020) 11:373. doi: 10.3389/fgene.2020.00373
40. Bahrami S, Steen NE, Shadrin A, O'Connell K, Frei O, Bettella F, et al. Shared genetic loci between body mass index and major psychiatric disorders: A genome-wide association study. JAMA Psychiatry. (2020) 77:503–12. doi: 10.1001/jamapsychiatry.2019.4188
41. McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry. (2020) 77:201–10. doi: 10.1001/jamapsychiatry.2019.3360
42. Cao X, Chen S, Xu H, Wang Q, Zhang Y, Xie S. Global functioning, cognitive function, psychopathological symptoms in untreated patients with first-episode schizophrenia: A cross-sectional study. Psychiatry Res. (2022) 313:114616. doi: 10.1016/j.psychres.2022.114616
43. Li X, Yuan X, Pang L, Zhang S, Li Y, Huang X, et al. The effect of serum lipids and short-chain fatty acids on cognitive functioning in drug-naive, first episode schizophrenia patients. Psychiatry Res. (2022) 313:114582. doi: 10.1016/j.psychres.2022.114582
44. Shen Q, Chen Z, Zhao F, Pan S, Zhang T, Cheng X, et al. Reversal of prolonged obesity-associated cerebrovascular dysfunction by inhibiting microglial Tak1. Nat Neurosci. (2020) 23:832–41. doi: 10.1038/s41593-020-0642-6
45. Morys F, Dadar M, Dagher A. Association between midlife obesity and its metabolic consequences, cerebrovascular disease, and cognitive decline. J Clin Endocrinol Metab. (2021) 106:e4260–74. doi: 10.1210/clinem/dgab135
46. Hu DH, Li YL, Liang ZJ, Zhong Z, Tang JK, Liao J, et al. [Long-term high-fat diet inhibits hippocampal expression of insulin receptor substrates and accelerates cognitive deterioration in obese rats]. Nan Fang Yi Ke Da Xue Xue Bao. (2018) 38:460–5. doi: 10.3969/j.issn.1673-4254.2018.04.15
47. Tabassum S, Misrani A, Yang L. Exploiting common aspects of obesity and alzheimer's disease. Front Hum Neurosci. (2020) 14:602360. doi: 10.3389/fnhum.2020.602360
48. Ge T, Fan J, Yang W, Cui R, Li B. Leptin in depression: a potential therapeutic target. Cell Death Dis. (2018) 9:1096. doi: 10.1038/s41419-018-1129-1
49. Formolo DA, Cheng T, Yu J, Kranz GS, Yau SY. Central adiponectin signaling - A metabolic regulator in support of brain plasticity. Brain Plast. (2022) 8:79–96. doi: 10.3233/BPL-220138
50. Cisternas P, Martinez M, Ahima RS, William Wong G, Inestrosa NC. Modulation of glucose metabolism in hippocampal neurons by adiponectin and resistin. Mol Neurobiol. (2019) 56:3024–37. doi: 10.1007/s12035-018-1271-x
51. Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol (Lausanne). (2014) 5:74. doi: 10.3389/fendo.2014.00074
52. Ownby RL. Neuroinflammation and cognitive aging. Curr Psychiatry Rep. (2010) 12:39–45. doi: 10.1007/s11920-009-0082-1
53. Lowe CJ, Reichelt AC, Hall PA. The prefrontal cortex and obesity: A health neuroscience perspective. Trends Cognit Sci. (2019) 23:349–61. doi: 10.1016/j.tics.2019.01.005
54. Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/S0140-6736(21)01730-X
55. Guo X, Zhang Z, Wei Q, Lv H, Wu R, Zhao J. The relationship between obesity and neurocognitive function in Chinese patients with schizophrenia. BMC Psychiatry. (2013) 13:109. doi: 10.1186/1471-244X-13-109
56. Depp CA, Strassnig M, Mausbach BT, Bowie CR, Wolyniec P, Thornquist MH, et al. Association of obesity and treated hypertension and diabetes with cognitive ability in bipolar disorder and schizophrenia. Bipolar Disord. (2014) 16:422–31. doi: 10.1111/bdi.12200
Keywords: schizophrenia, cognitive function, BMI, overweight/obesity, normal/under weight
Citation: Deng X, Lu S, Li Y, Fang X, Zhang R, Shen X, Du J and Xie S (2024) Association between increased BMI and cognitive function in first-episode drug-naïve male schizophrenia. Front. Psychiatry 15:1362674. doi: 10.3389/fpsyt.2024.1362674
Received: 28 December 2023; Accepted: 21 February 2024;
Published: 05 March 2024.
Edited by:
Tomiki Sumiyoshi, National Center of Neurology and Psychiatry, JapanReviewed by:
Yuko Higuchi, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, JapanTianhong Zhang, Shanghai Jiao Tong University, China
Copyright © 2024 Deng, Lu, Li, Fang, Zhang, Shen, Du and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinglun Du, ZHVqaW5nbHVuQDEyNi5jb20=; Shiping Xie, eGllc2hpcGluZ0Buam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Xing Deng
Xing Deng Shuiping Lu†
Shuiping Lu† Xinyu Fang
Xinyu Fang Rongrong Zhang
Rongrong Zhang Shiping Xie
Shiping Xie