- 1Affiliated Mental Health Center & Hangzhou Seventh People’s Hospital and School of Brain Science and Brain Medicine, Zhejiang University School of Medicine, Hangzhou, China
- 2Research Center for Healthcare Data Science, Zhejiang Laboratory, Hangzhou, China
- 3Department of Psychology and Behavioral Sciences, Zhejiang University, Hangzhou, Zhejiang, China
- 4Engineering Research Center of Electronic Medical Record (EMR) and Intelligent Expert System, Ministry of Education, College of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China
- 5Liangzhu Laboratory, Ministry of Education (MOE) Frontier Science Center for Brain Science and Brain-machine Integration, State Key Laboratory of Brain-machine Intelligence, Zhejiang University, Hangzhou, China
Background: The relationship between gestational diabetes (GDM) and the risk of depression has been thoroughly investigated in high-income countries on their financial basis, while it is largely unexplored in low- and middle- income countries. This meta-analysis aims to assess how GDM influences the risk of perinatal depression by searching multiple electronic databases for studies measuring the odds ratios between them in low- and middle-income countries.
Methods: Two independent reviewers searched multiple electronic databases for studies that investigated GDM and perinatal mental disorders on August 31, 2023. Pooled odds ratios (ORs) and confidence intervals (CIs) were calculated using the random effect model. Subgroup analyses were further conducted based on the type of study design and country income level.
Results: In total, 16 observational studies met the inclusion criteria. Only the number of studies on depression (n=10) satisfied the conditions to conduct a meta-analysis, showing the relationship between mental illness and GDM has been overlooked in low- and middle-income countries. Evidence shows an elevated risk of perinatal depression in women with GDM (pooled OR 1.92; 95% CI 1.24, 2.97; 10 studies). The increased risk of perinatal depression in patients with GDM was not significantly different between cross-sectional and prospective design. Country income level is a significant factor that adversely influences the risk of perinatal depression in GDM patients.
Conclusion: Our findings suggested that women with GDM are vulnerable to perinatal depressive symptoms, and a deeper understanding of potential risk factors and mechanisms may help inform strategies aimed at prevention of exposure to these complications during pregnancy.
1 Introduction
Gestational diabetes (GDM) is defined as glucose intolerance with onset or first recognition during pregnancy and can affect up to 25% of women during pregnancy globally (1). As one of the most common pregnancy complications, GDM is related to both short- and long-term adverse health outcomes in women and their offspring. Women with GDM are more likely to have gestational hypertension, preeclampsia, emergency Caesarean delivery, and type 2 diabetes mellitus (2–4). Besides, increasing evidence also suggested the close relationship between GDM and the risk of mental disorders, with a predominant focus on the attention drawn to its association with depression (5–7). For instance, a recent meta-analysis in 10 cohort studies with a total population of 2,000,002 identified a significantly increased risk of developing postpartum depressive symptoms in women with GDM (8). The risk of depression in women with GDM is worth emphasizing, as physical health and mental health are tightly connected. When mental health problems coexist with physical health problems, health outcomes, disability, and costs tend to be much worse (9, 10).
However, the relationship between GDM and the risk of perinatal depression in low- and middle-income countries has only recently become the subject of interest. Accumulating evidence shows both the risks of physical and mental health vary based on income levels (11, 12). Moreover, high-income countries tend to have more healthcare budgets and distribute greater proportions of budgets on mental health treatment than low- and middle-income countries. Therefore, previous findings based on high-income countries were insufficient to guide disease treatment in low- and middle- income countries.
Recent research found a mental health-based “poverty trap”: poverty results in poor physical health and early-life conditions, which in turn leads to depression and anxiety disorders that could adversely affect individuals’ childhood development, productivity, women’s empowerment, as well as economic decision-making, and eventually reinforces poverty (9). Hence, understanding the link between physical and mental health, as well as how they interact with income, is an important next step for low- and middle-income countries. It not only allows countries to optimize the distribution of their healthcare budgets, but also reinforces them to escape the poverty trap and enhance economic gains. Therefore, the primary aim of this meta-analysis is to systematically investigate the association between GDM and the risk of perinatal depression in low- and middle-income countries; by doing this, we want to emphasize the importance of caring for depression among the GDM population, especially in low- and middle-income countries.
2 Material and methods
2.1 Literature search
Two investigators independently (YJ and CW) searched databases of Medline, EMBASE, Pubmed, Web of Science, and PsycINFO from inception until August 31, 2023. Search terms such as “gestational diabetes mellitus” and “mental disorders” were adapted from previous systematic reviews in the area (13–15). The complete list of the search terms used is presented in the Supplementary File. Forward and backward citation was also undertaken.
2.2 Study selection
Inclusion criteria were confined to peer-reviewed studies published in English or with sufficiently detailed English abstracts to extract relevant information, measuring both GDM and perinatal mental disorders. Perinatal mental disorders included depression, anxiety, psychotic or eating disorders diagnosed at antenatal (between conception and delivery) or postpartum (up to 1 year following delivery) period, as there were plausible mechanisms for an association between these disorders and GDM. The study type is either cohort (prospective or retrospective) or cross-sectional.
Exclusion criteria included studies conducted in countries classified as high-income by the World Bank. Additionally, studies from high-income regions of Hong Kong, Taiwan, and Macau were excluded from the analysis due to their distinct economic and healthcare conditions compared to mainland China. Furthermore, studies in which mental disorders were diagnosed prior to the onset of GDM were excluded. Finally, studies that did not report unadjusted odds ratios for the relationship between GDM and mental disorders, or did not provide sufficient data for the calculation of odds ratios, were excluded from the meta-analysis.
Following de-duplication, titles and abstracts were screened, followed by full-text screening by two independent reviewers. In total, 16 studies met the study’s inclusion criteria.
2.3 Data extraction
Data extraction was conducted by two independent reviewers (YJ and CW) and the following data were extracted: the last name of the first author, year of publication, country, sample size, study design, diagnostic criteria of exposure and outcome, the timing of outcome assessment (antepartum vs. postpartum), significant risk factors (BMI, age, occupation, etc.), and unadjusted odds ratios with corresponding 95% confidence intervals (CIs).
2.4 Risk of bias assessment
The quality of the selection, comparability, and outcome of the included studies was assessed using a pre-piloted modified Newcastle-Ottawa scale (16) (Supplementary Table S1). Two independent reviewers (YJ and CW) performed the quality assessment and scored the included studies. Scores for selection bias and measurement bias were of particular interest as most of the studies were of observational design. A study with a score of zero in any of the evaluation domains was categorized as high risk of bias. Otherwise studies were categorized as low to moderate risk (17, 18). A lower risk of bias indicates higher quality.
2.5 Data synthesis
Unadjusted ORs with 95% CIs were used as measures of the association as studies were adjusted for different covariates. If ORs for at least three studies were available for one mental disorder, a meta-analysis was performed (19). DerSimonian-Laird random effects model (20) was the most commonly used method in meta-analysis because it is especially useful for providing an overall effect estimate and characterizing the heterogeneity of effects across a series of studies. When the proportion of total variation in study estimates that is due to heterogeneity (denoting as l2), it was decided a-priori such as 90% would preclude meta-analysis as this represents substantial heterogeneity (21). To evaluate the influence of each study, we conduct a sensitivity analysis by omitting each study individually and recalculating the pooled unadjusted ORs for the rest of the studies. All analyses were performed using STATA version 17 (22).
Subgroup analysis was performed for factors that could potentially impact the relationship between GDM and the risk of perinatal mental disorders. Potential factors include study type (prospective or cross-sectional studies), country income level, the timing of diagnostic (symptoms measured in antepartum or postpartum period) and mental disorder type. If ORs for at least three studies were available for each subgroup, a subgroup meta-analysis was additionally performed.
3 Results
3.1 Study characteristics
As shown in Figure 1, we identified 1316 studies from five different electronic databases. During the initial screening by title and abstract, the majority of the articles were excluded for being conducted in high-income countries or intervention studies without baseline data.
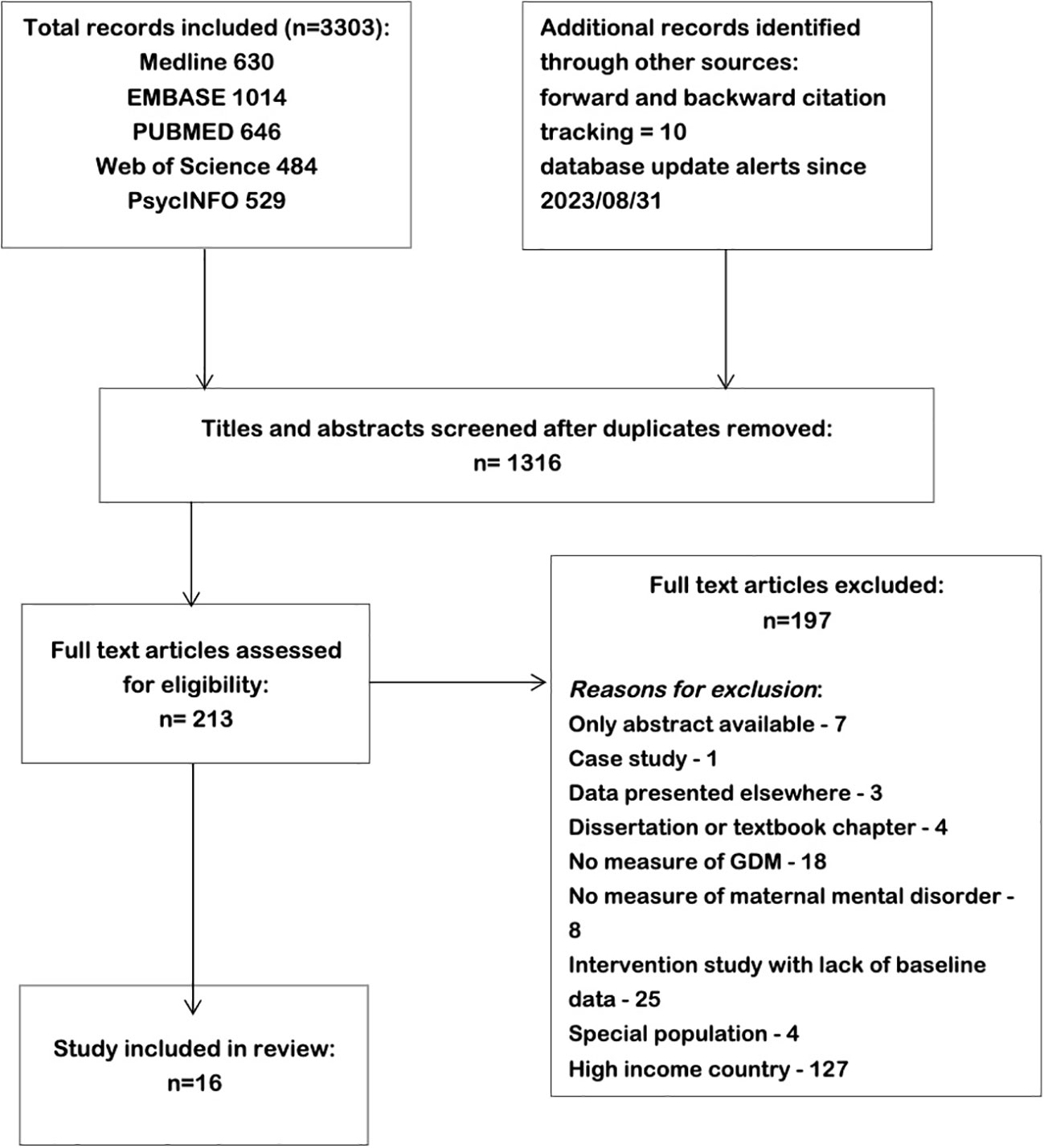
Figure 1 Study selection process for meta-analysis of studies on Gestational diabetes and risk of perinatal mental disorders in low- and middle-income countries.
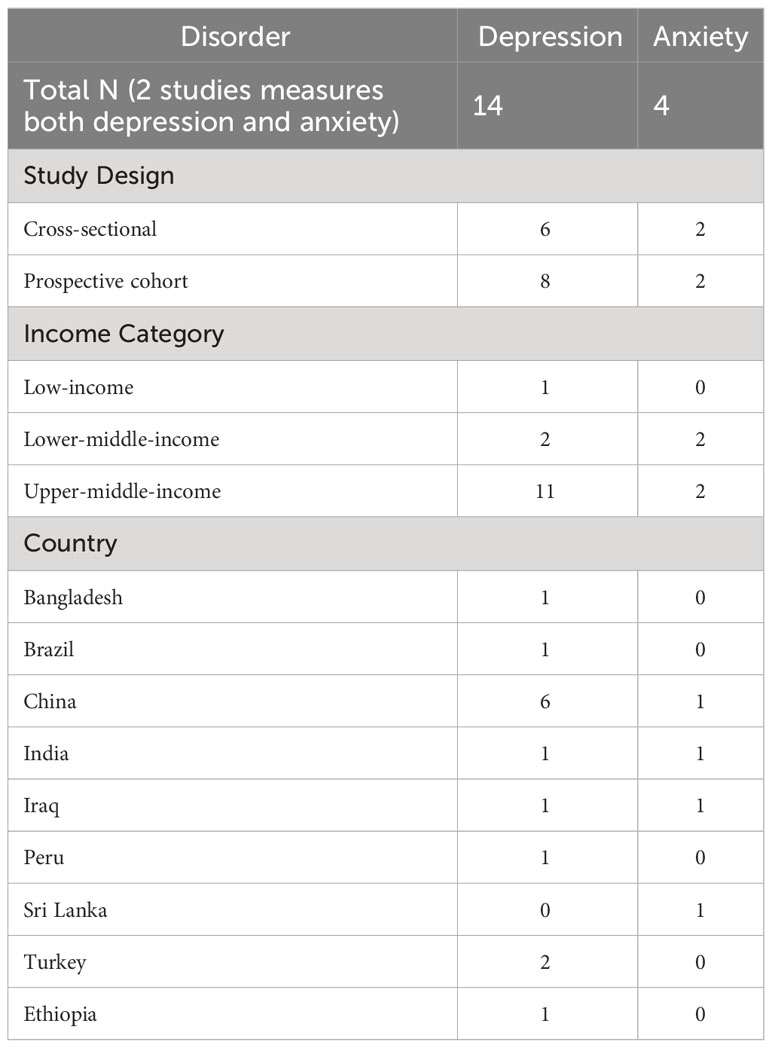
Among the 16 studies included, 10 studies were eligible for meta-analysis, and 6 studies were only used for prevalence and risk factors analysis due to lack of unadjusted ORs. The characteristics of the included studies were summarized in Table 1.
The most prevalent study design was prospective cohort (N=10) and 8 studies were cross-sectional. All of the studies were performed in low- or middle-income countries and 7 studies were from China. Diagnostic criteria for GDM include the International Classification of Diseases (ICD), oral glucose tolerance test, medical records, and self-report. Assessments for depressive symptoms or depression were based on the Edinburgh Postnatal Depression Scale (EDPS), the ICD, the Montogomery and Asberg Depression Rating Scale (MADRS), the Zung Self-Rating Depression Scale (SDS), The Beck Depression Inventory (BDI), and self-report. Assessment for anxiety symptoms or anxiety were based on the Self-Rating Anxiety Scale (SAS), the ICD, and MINI structured interview.
It is worth mentioning that in our original planned analysis, we intended to examine the relationship between GDM and a comprehensive range of perinatal mental disorders, encompassing depression, anxiety, psychotic, and eating disorders. However, the search results suggest current studies from low- and middle-income countries can only be found sufficient when they pertain to either depression or anxiety. Furthermore, among these perinatal mental disorders, only the quantity of literature on depression fulfilled the criteria for meta-analysis. Therefore, this study will primarily concentrate on examining the risk associated with perinatal depression in patients with GDM.
3.2 Risk of depression in patients with GDM
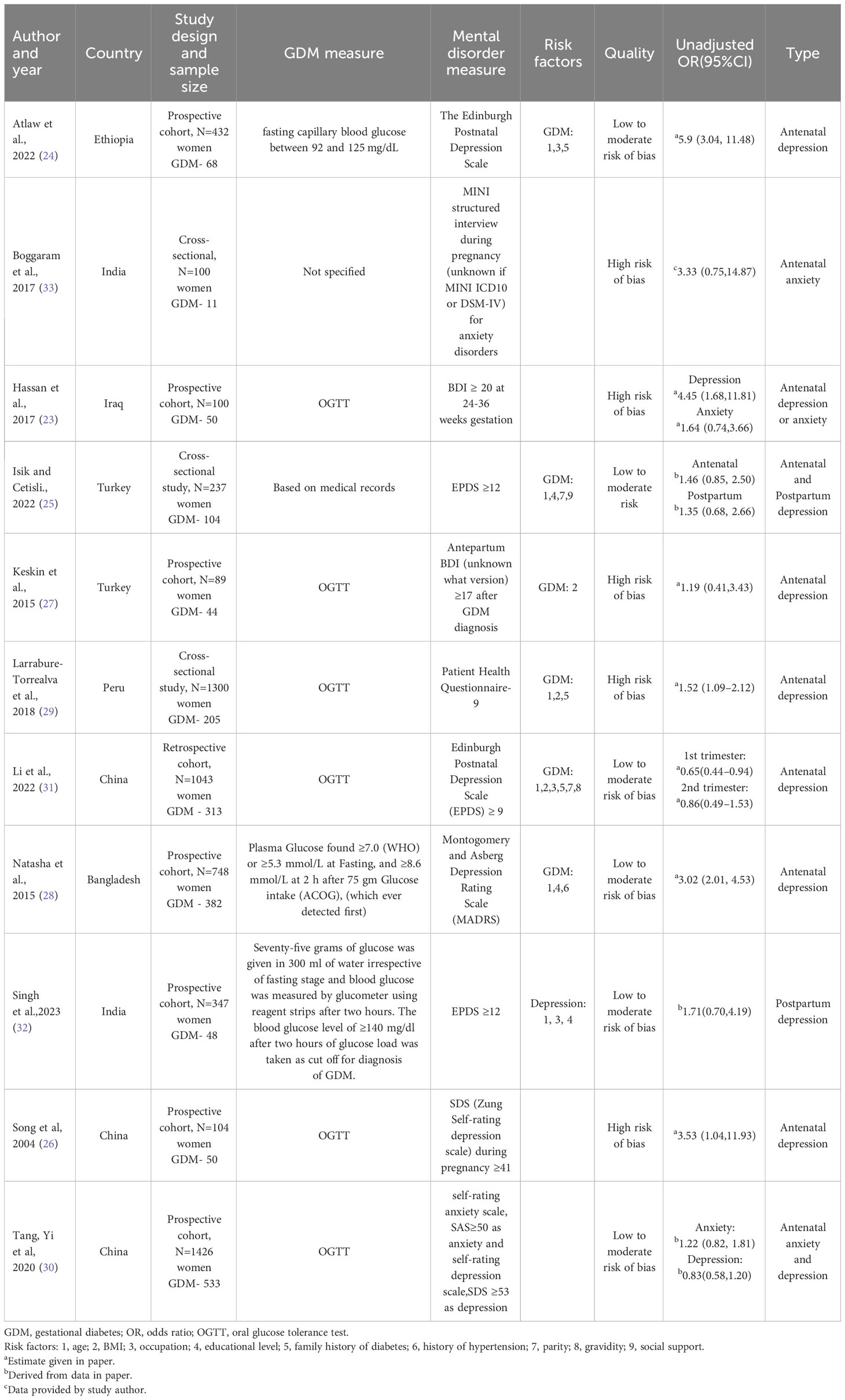
Out of 16 included studies, 10 studies measured diagnoses or symptoms of depression and were eligible for meta-analysis (23–32). Their respective characteristics and relevant findings were presented in Table 2.
The unadjusted ORs varied from 0.83 to 5.90 across studies (Figure 2). Among the 10 studies, 8 studies found a significant increase in risk of depression, while 2 studies reported no association. Pooling together, women with GDM compared with the control group had a notably increased risk of developing perinatal depressive symptoms (pooled unadjusted OR= 1.92, 95% CI 1.24, 2.97). There was a high degree of heterogeneity across studies (l2 = 80.87%, P for heterogeneity = 0.00).
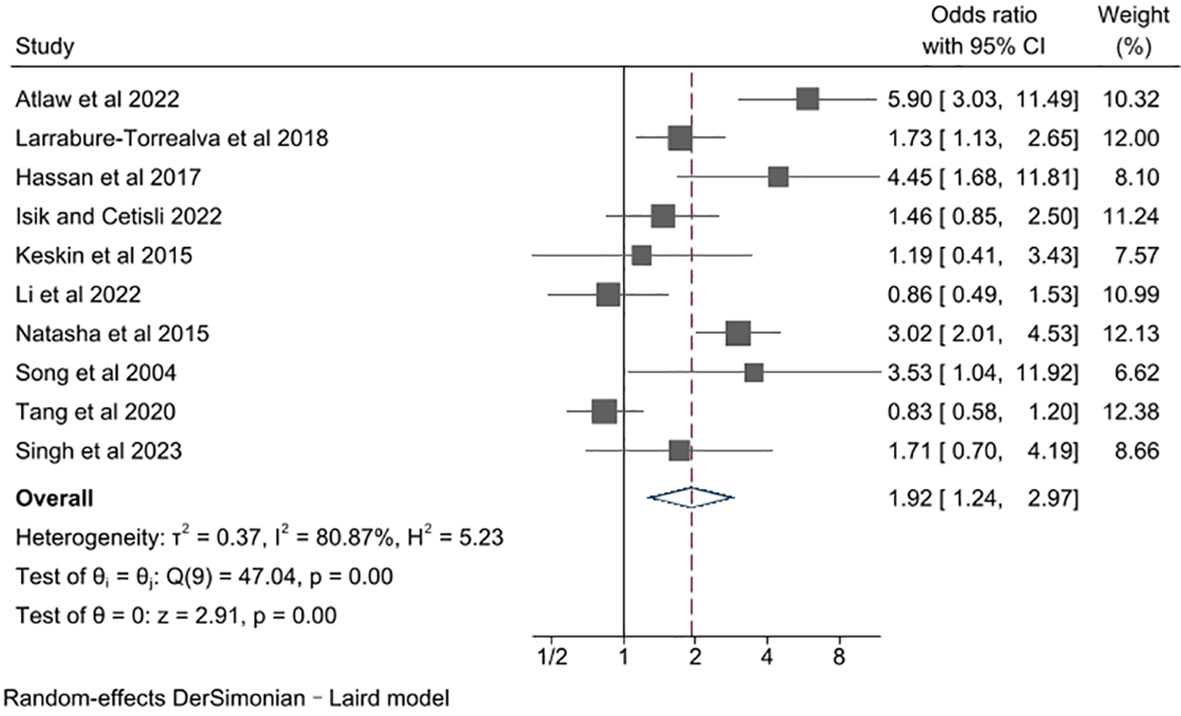
Figure 2 Meta-analysis of studies examining the association between gestational diabetes and risk of perinatal depression.
The following 6 studies were not included in the meta-analysis for having no unadjusted ORs available as effect estimates. Dame et al. reported the proportion of women with antenatal depression among GDM women (proportion = 31%) (34). Mak et al. found that the 3 months postpartum EPDS score was significantly higher in women with GDM than those without GDM (EPDS in GDM group=2.1, EPDS in control group=1.5, p-value <0.001) (35). Chen et al. and Peng et al. provided GDM prevalence and treated depression as exposure (36, 37). Dai et al. aggregated depression, anxiety and obsessive-compulsive disorders into one measure and reports the prevalence of GDM in psychiatric and healthy control group (prevalence in psychiatric group = 20.7%, prevalence in healthy control group=6.1%) (38). Lastly, Levy-Shiff et al. found no association between GDM and depressive symptoms in second trimester (BDI score in GDM group=6.70, BDI score in control group=6.59, p-value=0.42) (39).
3.3 Study type influence in risk of depression in patients with GDM
In this section, we investigated the impact of study type on the reported results of the relationship between GDM and the risk of perinatal depression, as a prior study observed significant variations in associations across different study types (8), by performing a subgroup analysis. In the subgroup analysis, only the difference between cross-sectional and prospective studies was analyzed (Figure 3), as there were not enough retrospective studies presented. The pooled unadjusted ORs for cross-sectional and prospective study design were 1.34 (95% CI 0.90,1.99) and 2.36 (95% CI 1.22, 4.57) respectively. Cross-sectional studies had lower estimates than prospective studies, but the difference in pooled unadjusted ORs across different study design was not substantial (P for group difference = 0.15). There was no evidence of heterogeneity in cross-sectional cohort studies (l2 = 45.36%, P for heterogeneity = 0.16), and a high degree of heterogeneity in prospective cohort studies (l2 = 85.20%, P for heterogeneity = 0.00). Sensitivity analysis did not identify studies that had substantial influences on the overall effect estimate, with pooled unadjusted ORs ranging from 1.66 to 2.14.

Figure 3 Subgroup meta-analysis of studies examining the association between gestational diabetes and risk of perinatal depression according to study design type.
3.4 Income influences in risk of depression in patients with GDM
In this section, we proceeded to conduct a subgroup analysis based on income levels (Figure 4). Specifically, the studies were divided into subgroups of lower-middle-income and upper-middle-income, according to the World Bank’s yearly classification of national income level. That means the studies conducted in a same country, mainly China in our analysis, would be grouped differently due to the income level at their publication year. As a major result, the association between GDM and depression was found to be remarkably influenced by income levels of studied countries (P for group difference = 0.00). The pooled unadjusted ORs for studies performed in lower-middle- and upper-middle-income countries were 3.32 (95% CI 2.07, 5.31) and 1.34 (95% CI 0.89. 2.03), respectively. There was no evidence of heterogeneity in studies from lower-middle-income countries (l2 = 42.18%, P for heterogeneity = 0.16), and a notable degree of heterogeneity in studies from upper-middle-income countries (l2= 67.41%, P for heterogeneity = 0.01). Besides, we found that the risk of depression in women with GDM is significantly higher in lower-middle-income countries compared to that in upper-middle-income countries, suggesting country income level is a significant factor that adversely influences the risk of perinatal depression in middle-income countries. It is unfortunate that data from low-income countries were insufficient to take part in this subgroup analysis, which could have made the analysis result more comprehensive.
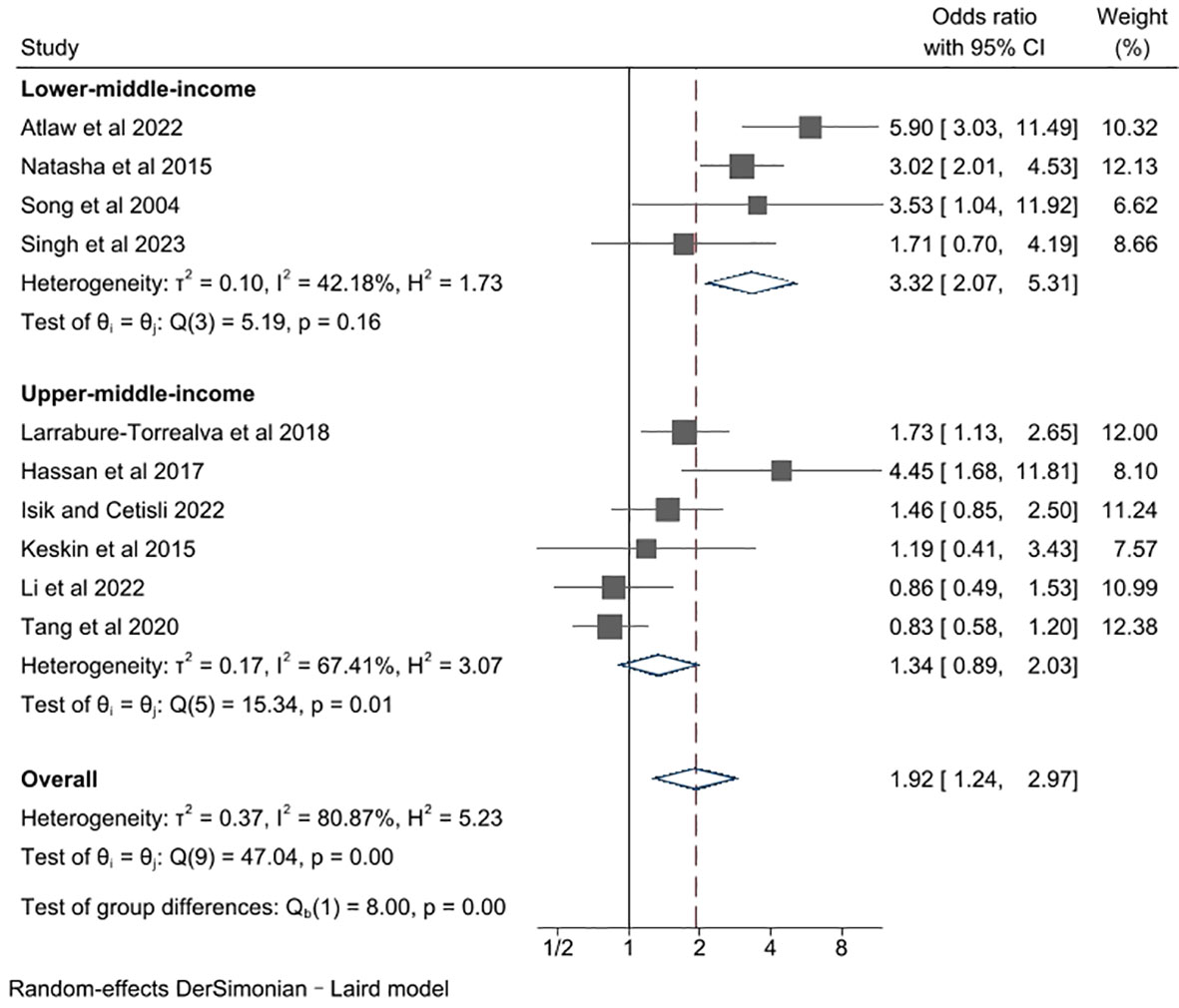
Figure 4 Subgroup meta-analysis of studies examining the association between gestational diabetes and risk of perinatal depression according to country income level.
4 Discussion
4.1 Main findings
Our meta-analysis differed from previous literature with an emphasis on studies conducted in low- and middle-income countries. Pooled unadjusted ORs for risk of perinatal depression was 1.92 (95% CI 1.24, 2.97), indicating that women with GDM have elevated risk of depression than those without GDM. This finding was in accordance with past researches in high income countries (8, 40). Furthermore, the pooled unadjusted ORs was substantially higher in studies conducted in lower-middle-countries than that in upper-middle-income countries, which supports our hypothesis that poverty exposes women to adverse mental and physical conditions. Among the included studies, one study (23) in Iraq and another study in Ethiopia (24) have notably higher unadjusted ORs (OR=4.45, 95% CI 1.68, 11.81 and OR=5.90, 95% CI 3.03, 11.49) compared to other studies in the same country income category. We speculated the elevated risk of depression was linked to constant armed conflicts in the regions. Moreover, it should be pointed out that the number of studies in anxiety disorder and other mental illness did not meet our standard to conduct meta-analysis, leaving opportunities for future research in low- and middle-income countries.
4.2 Potential mechanisms
The mechanism underlying the relationship between GDM and the risk of perinatal depression is unclear. Previous literature on type 2 diabetes speculated that perinatal depression resulted from biochemical changes directly due to GDM or from the psychological factors related to GDM or its treatment (41). There is also evidence suggesting that diabetes and depression may share common biological risk factors. For example, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis has been observed in people with either diabetes or depression (42, 43). Women with GDM are more prone to experience increased inflammation and adipokine concentration, which are also related to depression as well (44, 45). The event of having GDM itself could also result in depressive mood. In addition, we found that GDM and mental disorders shared several common risk factors, including age, education level, and occupation. Women with elder maternal age or unemployed women and housewives are more likely to have GDM and mental disorders (Table 2). Besides, a number of studies found that depressive symptoms were related to difficulties in adaption to diabetic complications and adverse obstetric outcome, including caesarean delivery and preterm delivery (46, 47). Moreover, insufficient nutritional support is also speculated to be associated with mental illness and GDM (48, 49). Studies have indicated a consistent correlation between lower income levels and inferior diet quality. (50, 51). Compared to individuals with higher income, those with lower income consume fewer fruits and vegetables, a greater amount of sugar-sweetened beverages, and have a lower overall diet quality (52, 53). Based on the theory of social causation, the condition of poverty could cause depression through financial stress, decreased social capital and inferior diet (54).
4.3 Strengths and limitations
To our knowledge, this is the first study that has thoroughly reviewed the literature in low- and middle-income countries and meta-analyzed the risk of perinatal depression in women with GDM. Since effect estimate and symptoms of depression may vary across subgroups, our meta-analysis was also grouped by study design and country income level.
Most of the included studies only provided unadjusted ORs, which may inflate the estimates for risk of depression. A few studies indicated that BMI and ethnicity may moderate the impact of perinatal depression, but information related to these confounders were often missing from studies (55, 56). Furthermore, previous literature found that obesity, level of glycemic control and GDM management strategies (insulin vs. diet intervention) may also have an impact on depression (57–59). Despite acknowledging the potential moderating effect of these variables on perinatal depression, the lack of detailed reporting hindered our ability to conduct a robust subgroup analysis.
Nearly half of the studies were identified as high risk of bias. Studies at high risk of bias mostly lack information regarding sample selection process or GDM diagnostic criteria. There was a high degree of heterogeneity among included studies. The source of heterogeneity came from both depression and GDM. Moreover, the screening tools of perinatal depression and GDM varied across studies. For depression evaluation, there were multiple assessment tools including EPDS, BDI, and Patient Health Questionnaire-9, and there is a lack of consensus on the optimal cut-off point in the literature. For instance, the cut-offs for EPDS were 9, 10, and 12 in three included studies. The screening time of postpartum depression include 1-month, 3-months, and 6-months postpartum. Previous studies also have contradictory results regarding 6-months depressive scores (35, 60). For GDM diagnosis, two studies used self-reported data, which may add to the risk of information bias.
4.4 Implications
A future potential and urgent area for research is the investigation of relationships between GDM and the risk of mental disorders other than depression in low- and middle-income countries. Current studies in less common mental disorders, such as eating disorders and bipolar disorder, were mostly performed in high-income countries. Current studies independently found that the prevalence of GDM and mental disorders was both higher in resource-constrained countries (61, 62), but the relationship between them are still relatively unexplored. Research in resource-constrained countries is speculated to have an important impact, as we found in this study on depression that the severity of mental disorders could be significantly negatively correlated to country income level. The research would also be important from both social and healthcare contexts because mental health problems can cause adverse consequences for women, their infants, and even the larger families. Addressing barriers in nutrition education and counselling, diet intervention, antenatal and postpartum care services, as well as emotional support services may contribute to improve health outcomes of pregnant women in low- and middle-income countries. During future investigations, we also emphasize a greater understanding of the underlying mechanism between GDM and depression, for it is essential for interventions to reduce not only the risk of depression but also other complications.
5 Conclusion
In this study, we performed a meta-analysis to examine the risk of perinatal depression among individuals diagnosed with GDM in low- and middle-income countries. We searched for studies on various mental disorders, but only identified sufficient research on depression that met the criteria for inclusion in our meta-analysis. This finding underscores the limited amount of research available on perinatal mental disorders in low- and middle-income countries and emphasizes the urgent need for further studies in this area.
Focusing specifically on perinatal depression, we found a significant increase in the likelihood of experiencing depressive symptoms in individuals with GDM. This finding emphasizes the importance of managing GDM, as doing so can help reduce adverse obstetric outcomes. Additionally, we found that the risk of depression in women with GDM is significantly higher in lower-middle-income countries compared to that in upper-middle-income countries, indicating country income level is a significant factor that adversely impacts the risk of depression in middle-income countries. The implications of this study are particularly relevant for low- and middle-income countries, as depression can directly impact individuals’ economic decision-making and productivity, potentially leading to increased poverty. Therefore, addressing perinatal mental health issues, especially in the context of GDM, is crucial for improving overall well-being and socio-economic outcomes. A deeper understanding of the relation and mechanisms between GDM and depression may help to identify the risk of depression at an early stage and reduce obstetric complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YJ: Writing – original draft, Writing – review & editing, Data curation, Formal analysis. CW: Data curation, Writing – review & editing. WC: Writing – review & editing. JL: Writing – review & editing. HJ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Fundamental Research Funds for the Central Universities (grant number 226-2022-00138 to HJ), Hangzhou Biomedical and Health Industry Special Projects for Science and Technology (grant number 2021WJCY240 to HJ), STI2030-Major Projects (grant number 2022ZD0212400 to HJ) and National Natural Science Foundation of China (grant number 82371453 to HJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1331415/full#supplementary-material
References
1. Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie (2021) 143:112183. doi: 10.1016/j.biopha.2021.112183
2. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J obstetrics gynecology (2004) 191(5):1655–60. doi: 10.1016/j.ajog.2004.03.074
3. Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. Gestational diabetes: A clinical update. World J Diabetes (2015) 6(8):1065–72. doi: 10.4239/wjd.v6.i8.1065
4. Marchetti D, Carrozzino D, Fraticelli F, Fulcheri M, Vitacolonna E. Quality of life in women with gestational diabetes mellitus: A systematic review. J Diabetes Res (2017) 2017:7058082. doi: 10.1155/2017/7058082
5. Byrn MA, Penckofer S. Antenatal depression and gestational diabetes: a review of maternal and fetal outcomes. Nurs women's Health (2013) 17(1):22–33. doi: 10.1111/1751-486X.12003
6. Hinkle SN, Buck Louis GM, Rawal S, Zhu Y, Albert PS, Zhang C. A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia (2016) 59(12):2594–602. doi: 10.1007/s00125-016-4086-1
7. Yamada K, Endo M, Ohashi K. Depression and diet-related distress among Japanese women with gestational diabetes mellitus. Nurs Health Sci (2023) 25(4):609–18. doi: 10.1111/nhs.13054
8. Arafa A, Dong J. Gestational diabetes and risk of postpartum depressive symptoms: A meta-analysis of Cohort studies. J Affect Disord (2019) 253:312–6. doi: 10.1016/j.jad.2019.05.001
9. Ridley M, Rao G, Schilbach F, Patel V. Poverty, depression, and anxiety: Causal evidence and mechanisms. Science (2020) 370(6522):eaay0214. doi: 10.3386/w27157
10. Scott KM, Lim C, Al-Hamzawi A, Alonso J, Bruffaerts R, Caldas-de-Almeida JM, et al. Association of mental disorders with subsequent chronic physical conditions: World mental health surveys from 17 countries. JAMA Psychiatry (2016) 73(2):150–8. doi: 10.1001/jamapsychiatry.2015.2688
11. Lund C, Breen A, Flisher AJ, Kakuma R, Corrigall J, Joska JA, et al. Poverty and common mental disorders in low and middle income countries: A systematic review. Soc Sci Med (2010) 71(3):517–28. doi: 10.1016/j.socscimed.2010.04.027
12. Sareen J, Afifi TO, McMillan KA, Asmundson GJ. Relationship between household income and mental disorders: findings from a population-based longitudinal study. Arch Gen Psychiatry (2011) 68(4):419–27. doi: 10.1001/archgenpsychiatry.2011.15
13. Fisher J, Mello MCD, Patel V, Rahman A, Tran T, Holton S, et al. Prevalence and determinants of common perinatal mental disorders in women in low-and middle-income countries: a systematic review. Bull World Health Organ (2012) 90:139–49. doi: 10.2471/BLT.11.091850
14. Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Obesity and mental disorders during pregnancy and postpartum: a systematic review and meta-analysis. Obstet Gynecol (2014) 123(4):857–67. doi: 10.1097/AOG.0000000000000170
15. Trevillion K, Oram S, Feder G, Howard LM. Experiences of domestic violence and mental disorders: a systematic review and meta-analysis. PloS One (2012) 7(12):e51740. doi: 10.1371/journal.pone.0051740
16. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the Quality of Nonrandomised Studies in Meta-Analyses (2021). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 16 January 2021).
17. Rowland J, Wilson CA. The association between gestational diabetes and ASD and ADHD: a systematic review and meta-analysis. Sci Rep (2021) 11(1):5136. doi: 10.1038/s41598-021-84573-3
18. Wilson CA, Newham J, Rankin J, Ismail K, Simonoff E, Reynolds RM, et al. Systematic review and meta-analysis of risk of gestational diabetes in women with preconception mental disorders. J Psychiatr Res (2022) 149:293–306. doi: 10.1016/j.jpsychires.2022.03.013
19. Deeks J, Higgins J, Altman D. Chapter 10: Analysing data and undertaking meta-analyses, in: Cochrane handbook for Systematic Reviews of Interventions version 6.0 (2021). Available at: https://training.cochrane.org/handbook/current/chapter-10 (Accessed Jan 16, 2021).
20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials (1986) 7(3):177–188. doi: 10.1016/0197-2456(86)90046-2
21. Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
23. Hassan SM, Ejerish MA, Harba U. Effect of depression and anxiety on gestational diabetes in babylon government. Int J Pharm Sci Res (2017) 8(10):4371. doi: 10.13040/IJPSR.0975-8232.8(10).4371-75
24. Atlaw D, Sahiledengle B, Assefa T, Negash W, Tahir A, Regasa T, et al. Incidence and risk factors of gestational diabetes mellitus in Goba town, Southeast Ethiopia: a prospective cohort study. BMJ Open (2022) 12(9):e060694. doi: 10.1136/bmjopen-2021-060694
25. Işik G, Egelioğlu Cerişli N. The effect of gestational diabetes on depression and breastfeeding self-efficacy in pregnancy and postpartum period. Clin Exp Health Sci (2022) 12(2):323–30. doi: 10.33808/clinexphealthsci.770882
26. Song XF, Liu YJ, Wang WH, Liu YL, Xu ZR. Investigation of depressive symptoms and analysis of related factors in patients with gestational diabetes mellitus. Chin J Clin Rehabil (2004) 8(30):6559–61.
27. Keskin FE, Ozyazar M, Pala AS, Elmali AD, Yilmaz B, Uygunoglu U, et al. Evaluation of cognitive functions in gestational diabetes mellitus. Exp Clin Endocrinol Diabetes (2015) 123(04):246–51. doi: 10.1055/s-0034-1395634
28. Natasha K, Hussain A, Khan AA. Prevalence of depression among subjects with and without gestational diabetes mellitus in Bangladesh: a hospital based study. J Diabetes Metab Disord (2015) 14:1–9. doi: 10.1186/s40200-015-0189-3
29. Larrabure-Torrealva GT, Martinez S, Luque-Fernandez MA, Sanchez SE, Mascaro PA, Ingar H, et al. Prevalence and risk factors of gestational diabetes mellitus: findings from a universal screening feasibility program in Lima, Peru. BMC pregnancy childbirth (2018) 18:1–9. doi: 10.1186/s12884-018-1904-0
30. Tang Y, Lan X, Zhang Y, Zhou F, Cai C, Zhang J, et al. Anxiety and depression on gestational diabetes mellitus in early pregnancy. Wei Sheng yan jiu= J Hygiene Res (2020) 49(2):179–84. doi: 10.19813/j.cnki.weishengyanjiu.2020.02.002
31. Li H, Yu X, Qiang W, Lu M, Jiang M, Hou Y, et al. A longitudinal cohort study of gestational diabetes mellitus and perinatal depression. BMC Pregnancy Childbirth (2022) 22(1):1–10. doi: 10.1186/s12884-022-04667-2
32. Singh AK, Palepu S, Saharia GK, Patra S, Singh S, Taywade M, et al. Association between gestational diabetes mellitus and postpartum depression among women in eastern India: A cohort study. Indian J Community Med (2023) 48(2):351–6. doi: 10.4103/ijcm.ijcm_759_22
33. Boggaram SA, Singh H, Manikanta TS, Maheswari E. An exploratory study of identification of psychiatric disorders during pregnancy. Minerva Psychiatry (2017) 58(4):203–8. doi: 10.23736/S0391-1772.17.01945-8
34. Damé P, Cherubini K, Goveia P, Pena G, Galliano L, Façanha C, et al. Depressive symptoms in women with gestational diabetes mellitus: the LINDA-Brazil study. J Diabetes Res (2017) 2017:7341893. doi: 10.1155/2017/7341893
35. Mak JKL, Lee AH, Pham NM, Tang L, Pan XF, Binns CW, et al. Gestational diabetes and postnatal depressive symptoms: a prospective cohort study in Western China. Women and birth : journal of the Australian College of Midwives (2019) 32(3):e427–31. doi: 10.1016/j.wombi.2018.08.171
36. Chen XN, Hu Y, Hu WH, Xia X, Li XT. Risk of adverse perinatal outcomes and antenatal depression based on the zung self-rating depression scale. Reprod Dev Med (2021) 5(01):23–9. doi: 10.4103/2096-2924.313683
37. Peng S, Lai X, Du Y, Meng L, Gan Y, Zhang X. Prevalence and risk factors of postpartum depression in China: A hospital-based cross-sectional study. J Affect Disord (2021) 282:1096–100. doi: 10.1016/j.jad.2021.01.012
38. Dai J, Gui Z, Fan X, Liu J, Han L, Sun Y, et al. Effects of psychiatric disorders on ultrasound measurements and adverse perinatal outcomes in Chinese pregnant women: A ten-year retrospective cohort study. J Psychiatr Res (2022) 156:361–71. doi: 10.1016/j.jpsychires.2022.10.046
39. Levy-Shiff R, Lerman M, Har-Even D, Hod M. Maternal adjustment and infant outcome in medically defined high-risk pregnancy. Dev Psychol (2002) 38(1):93–103. doi: 10.1037/0012-1649.38.1.93
40. Wilson CA, Newham J, Rankin J, Ismail K, Simonoff E, Reynolds RM, et al. Is there an increased risk of perinatal mental disorder in women with gestational diabetes? A systematic Rev meta-analysis. Diabetic medicine: J Br Diabetic Assoc (2020) 37(4):602–22. doi: 10.1111/dme.14170
41. Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care vol (2000) 23(10):1556–62. doi: 10.2337/diacare.23.10.1556
42. Cameron O, Kronfol Z, Greden J, Carroll B. Hypothalamic-pituitary-adrenocortical activity in patients with diabetes mellitus. Arch Gen Psychiatry (1984) 41(11):1090–5. doi: 10.1001/archpsyc.1983.01790220080013
43. Hantsoo L, Jagodnik KM, Novick AM, Baweja R, di Scalea TL, Ozerdem A, et al. The role of the hypothalamic-pituitary-adrenal axis in depression across the female reproductive lifecycle: current knowledge and future directions. Front Endocrinol (2023) 14:1295261. doi: 10.3389/fendo.2023.1295261
44. Carvalho AF, Rocha DQ, McIntyre RS, Mesquita LM, Köhler CA, Hyphantis TN, et al. Adipokines as emerging depression biomarkers: a systematic review and meta-analysis. J Psychiatr Res (2014) 59:28–37. doi: 10.1016/j.jpsychires.2014.08.002
45. Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol (2014) 2(6):488–99. doi: 10.1016/S2213-8587(13)70176-1
46. O'hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol (2013) 9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612
47. Wulsin LR, Jacobson AM, Rand LI. Psychosocial adjustment to advanced proliferative diabetic retinopathy. Diabetes Care (1993) 16(8):1061–6. doi: 10.2337/diacare.16.8.1061
48. Fryers T, Melzer D, Jenkins R. Social inequalities and the common mental disorders: a systematic review of the evidence. Soc Psychiatry Psychiatr Epidemiol. (2003) 38(5):229–37. doi: 10.1007/s00127-003-0627-2
49. Kahneman D, Krueger AB, Schkade D, Schwarz N, Stone AA. Would you be happier if you were richer? A focusing illusion. Science (2006) 312(5782):1908–10. doi: 10.1126/science.1129688
50. Andreyeva T, Luedicke J, Henderson KE, Tripp AS. Grocery store beverage choices by participants in federal food assistance and nutrition programs. Am J Prev Med (2012) 43(4):411–8. doi: 10.1016/j.amepre.2012.06.015
51. French SA, Wall M, Mitchell NR. Household income differences in food sources and food items purchased. Int J Behav Nutr Phys Act. (2010) 7:77. doi: 10.1186/1479-5868-7-77
52. U.S. Department of Health and Human Services. Healthy people 2020 . Washington, DC: U.S. Government Printing Office. Available at: https://www.healthypeople.gov (Accessed 19 Feb 2019).
53. U.S. Department of Agriculture, Food and Nutrition Service, Office of Research, Nutrition and Analysis. Diet quality of americans by food stamp participation status: Data from the national health and nutrition examination survey, 1999–2004 (2008). Alexandria, VA: Project Officer: Jenny Laster Genser. Available at: https://fns-prod.azureedge.net/sites/default/files/NHANES-FSP.pdf (Accessed 5 Feb 2018).
54. Jin Y, Zhu D, He P. Social causation or social selection? The longitudinal interrelationship between poverty and depressive symptoms in China. Soc Sci Med (1982) (2020) 249:112848. doi: 10.1016/j.socscimed.2020.112848
55. Nakku JEM, Nakasi G, Mirembe F. Postpartum major depression at six weeks in primary health care: prevalence and associated factors. Afr Health Sci (2006) 6:207–14. doi: 10.5555/afhs.2006.6.4.207
56. Walmer R, Huynh J, Wenger J, Ankers E, Mantha AB, Ecker J, et al. Mental health disorders subsequent to gestational diabetes mellitus differ by race/ethnicity. Depression Anxiety (2015) 32:774–82. doi: 10.1002/da.22388
57. Bai X, Liu Z, Li Z, Yan D. The association between insulin therapy and depression in patients with type 2 diabetes mellitus: a meta-analysis. BMJ Open (2018) 8(11):e020062. doi: 10.1136/bmjopen-2017-020062
58. Fulton S, Décarie-Spain L, Fioramonti X, Guiard B, Nakajima S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol Metab (2022) 33(1):18–35. doi: 10.1016/j.tem.2021.10.005
59. Kwon M, Lee M, Kim EH, Choi DW, Jung E, Kim KY, et al. Risk of depression and anxiety disorders according to long-term glycemic variability. J Affect Disord (2023) 343:50–8. doi: 10.1016/j.jad.2023.09.017
60. Huang T, Rich-Edwards J, James-Todd T, Gillman MW, Oken E, Rifas-Shiman SL, et al. Pregnancy hyperglycaemia and risk of prenatal and postpartum depressive symptoms. Paediatric Perinatal Epidemiol (2015) 29:281–9. doi: 10.1111/ppe.12199
61. Ngui EM, Khasakhala L, Ndetei D, Roberts LW. Mental disorders, health inequalities and ethics: A global perspective. Int Rev Psychiatry (Abingdon England) (2010) 22(3):235–44. doi: 10.3109/09540261.2010.485273
Keywords: mental disorders, gestational diabetes, meta-analysis, pregnancy, perinatal depression, developing countries
Citation: Jin Y, Wu C, Chen W, Li J and Jiang H (2024) Gestational diabetes and risk of perinatal depression in low- and middle-income countries: a meta-analysis. Front. Psychiatry 15:1331415. doi: 10.3389/fpsyt.2024.1331415
Received: 01 November 2023; Accepted: 24 January 2024;
Published: 12 February 2024.
Edited by:
Mona Nasrallah, American University of Beirut, LebanonReviewed by:
Igor Victorovich Lakhno, Kharkiv National Medical University, UkraineGözde Bacik Yaman, Süleyman Demirel University, Türkiye
Copyright © 2024 Jin, Wu, Chen, Li and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiteng Jiang, aC5qaWFuZ0B6anUuZWR1LmNu; Jingsong Li, bGpzQHpqdS5lZHUuY24=
 Yuqing Jin
Yuqing Jin Chengkai Wu2
Chengkai Wu2 Wanlin Chen
Wanlin Chen Jingsong Li
Jingsong Li Haiteng Jiang
Haiteng Jiang