
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 31 January 2024
Sec. Autism
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1329836
Introduction: Children and adolescents with autism spectrum disorder (ASD) may be particularly vulnerable to the impact of traumatic events, yet the association between ASD and the risk of developing acute stress disorder and post-traumatic stress disorder (PTSD) remains uncertain. This study aims to investigate this association, addressing the gap in large-scale evidence on the subject.
Methods: Conducted as a retrospective and matched cohort study, data was sourced from the National Health Insurance Research Database (NHIRD) in Taiwan, spanning from January 1, 2000, to December 31, 2015. The study included patients aged 18 years or under newly diagnosed with ASD (n=15,200) and compared them with a matched control group (n=45,600). The Cox proportional regression model was employed to assess the risk of acute stress disorder and PTSD.
Results: Over the 15-year follow-up period, a total of 132 participants developed either acute stress disorder or PTSD. Among them, 105 cases (0.691% or 64.90 per 100,000 person-years) were in the ASD group, while 27 cases (0.059% or 5.38 per 100,000 person-years) were in the control group. The adjusted hazard ratio for the ASD group was significantly higher compared to the control group (25.661 with 95% CI = 15.913-41.232; P < .001).
Discussion: This study provides compelling evidence that individuals with ASD face an elevated risk of developing acute stress disorder and PTSD. The findings underscore the importance of clinicians recognizing and addressing this vulnerability in ASD individuals exposed to traumatic events. This emphasizes the need for heightened attention to the risk of PTSD and acute stress disorder in the ASD population.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder, characterized by persistent social communication and interaction deficits, combined with restricted and repetitive behavior. Recent studies have indicated that the worldwide prevalence of ASD was approximately 1% to 2%. Moreover, ASD is considered to be approximately two to three times more prevalent among males than females, with differences among countries (1). In Taiwan, the pooled prevalence of ASD was around 0.26% (2). Higher rates of psychiatric comorbidities in ASD individuals have been reported (3), with attention deficit/hyperactivity disorder (ADHD), anxiety, and depression being the most common comorbidities (4). Children and adolescents with ASD may be vulnerable to psychosocial frustration, traumatic events (5), and increased risks of mood symptoms and suicide ideation (6) or suicide attempt (7, 8).
Post-traumatic stress disorder (PTSD) is characterized by the symptomology of intrusive re-experience, avoidance, hyperarousal, and marked negative change in mood and cognition after exposure to traumatic events, defined as exposure to actual death or threatened death, severe injury, or sexual violence (9, 10). Previous studies conducted in Taiwan, focused mainly on adult groups have revealed that PTSD is mutually associated with diseases such as obstructive sleep apnea (11); asthma (12); hypertension, diabetes, and dyslipidemia (13); osteoporosis (14); epilepsy (7, 8); Parkinson’s disease (15); dementia(16); and substance use disorder (17). The risk of experiencing traumatic events is higher in ASD individuals than in typically developed peers (18). Mood symptomatology, including anxiety and depression, had the strongest link to trauma exposure in ASD youth (19) and also PTSD is often co-exiting with symptoms of depression and anxiety (20). In addition, growing evidence has revealed that the symptomatology of PTSD was observed in ASD individuals with traumatic exposure (21, 22). In children and adolescents with ASD, PTSD seems to develop at a comparable or higher rate when compared to the general population, with prevalence estimated from 0% to approximately 17% in a systemic review (23). On the contrast, in a study with the general population of 1,420 children with trauma exposure, less than 0.5% of the children fulfill the criteria of full-blown PTSD diagnosis (24). To date, the prevalence estimate PTSD in the ASD population remains mostly unknown, without large-scaled population-based studies assessing prevalence (23).
Acute stress disorder is characterized by a cluster of symptoms as the diagnosis of PTSD in the acute phase within one month after trauma exposure (9, 10). Nonetheless, previous studies have claimed that a diagnosis of acute stress disorder may be limited in appropriately predicting individuals who will later develop PTSD (25, 26), including children and adolescents (27, 28). However, the presentation and prevalence of acute stress disorder in the ASD group is yet to be clarified.
Previous studies have revealed that individuals with ASD are at a higher risk of exposure to traumatic life events and of developing PTSD (5, 18, 29). Most previous studies are case reports, case series, cross-sectional observational researches, or systemic reviews (23). A previous cohort study used a questionnaire to define the autistic traits of women with children. The adjusted odds ratio (aOR) of PTSD was significantly higher in the top three quintiles of questionnaires (aOR = 1.4 to 1.9). Furthermore, the association between autistic traits and PTSD was identified (30). Some previous studies have administered questionnaires so as to define autistic traits or various types of structural diagnostic tools of ASD.
Several previous studies have investigated the association of ASD between type 2 diabetes mellitus (31), suicide attempts (7, 8), and substance use disorders (17), and PTSD (23). In addition, some peri-natal or early childhood clinical conditions, such as neonatal hyperbilirubinemia (32), being born prematurely (33), and exposure of general anesthesia (34), are associated with a higher risk of ASD. However, the association among ASD, acute stress disorder, and PTSD is yet to be clarified. We hypothesized that children and adolescents with ASD are at a higher risk of developing acute stress disorder and PTSD. Therefore, we conducted this nationwide population-based cohort study so as to determine the risk of acute stress disorder and PTSD in individuals with ASD in Taiwan.
We used the data from the National Health Insurance Research Database (NHIRD) in Taiwan to investigate the association of ASD with PTSD and acute stress disorder over a 15-year period. The National Health Insurance (NHI) program was begun in 1995, and as of June 2009, the program included contracts with 97% of medical providers in Taiwan. Approximately, 23 million beneficiaries, that is, more than 99% of the entire population have been registered in the NHIRD (35). The information in the NHIRD is stored separately in different sub-datasets, including registry for beneficiaries, registry for medical facilities, registry for board-certified specialists, inpatient claims, ambulatory care claims, and prescriptions dispensed at pharmacies. Demographic features in the patient-level information are provided by the linkage between these datasheets and individual personal identification numbers. The diagnosis recorded in the NHIRD was based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The psychiatric diagnosis was established by certified psychiatrists, according to the Diagnostic Statistical Manual of Mental Disorders, 4th Edition and the revised edition (9, 10) (Supplementary Table S1). The validation of the NHRID has been discussed, and several studies have been investigating the validity of the diagnosis codes, with modest to high sensitivity and positive predictive values. (36–39.). In Taiwan, patients with the catastrophic illness certification, who get care for the illness or it’s related conditions, do not need to pay a co-payment for outpatient or inpatient care, and ASD is one of the catastrophic illnesses (40). Therefore, the patients with ASD, or their parents, could apply for catastrophic illness certification under the NHI program, including our aim of autism spectrum disorder, of the ICD-9-CM code: 299. Since the diagnosis with catastrophic illness certification would have been reviewed by experts and the status of catastrophic illness certification would be with high accuracy for ASD in the NHIRD.
The diagnosis of ASD was used after the publication of the Diagnostic Statistical Manual of Mental Disorders, 5th Edition (DSM-5) in 2013 (41), which included previous diagnoses in the DSM-IV-TR, as Asperger’s disorder, and pervasive developmental disorder, not otherwise specified. This study employed a retrospective, population-based, and matched cohort design, adhering to ICD-9-CM code: 299, autistic disorder in the NHIRD data to represent the ASD.
The subject selection process is presented in Figure 1. The cohort comprised children and adolescents aged 18 years or under who received a new ASD diagnosis (ICD-9-CM codes: 299) between January 1, 2000, and December 31, 2015. A total of 15,200 individuals with ASD were enrolled, and a control group of 45,600 individuals without ASD was selected, matched for sex, age, and index date at a 1:3 ratio. Recognizing the higher prevalence of ASD in males and the diverse age at diagnosis, we chose to match age and gender to address potential confounding factors. The rationale for conducting case/control matching in a 1:3 ratio can be observed in Supplementary Figure S1. Supplementary Figure S1A illustrates that a control/case ratio of 1:3 achieves a test power exceeding 97.5%, acknowledging the impracticality of an unlimited increase in controls. In Supplementary Figure S1B, using a 1:3 ratio results in an estimated power approaching 1.0, prompting our selection of this ratio for optimal statistical power in our study. The index date was defined as the ASD diagnosis event certified by psychiatrists. Participants diagnosed with ASD, acute stress disorder, or PTSD before 2000 were excluded. Follow-up extended from the index date until the diagnosis of PTSD (ICD-9-CM code: 309.81) or acute stress disorder (ICD-9-CM code: 308) by certified psychiatrists.
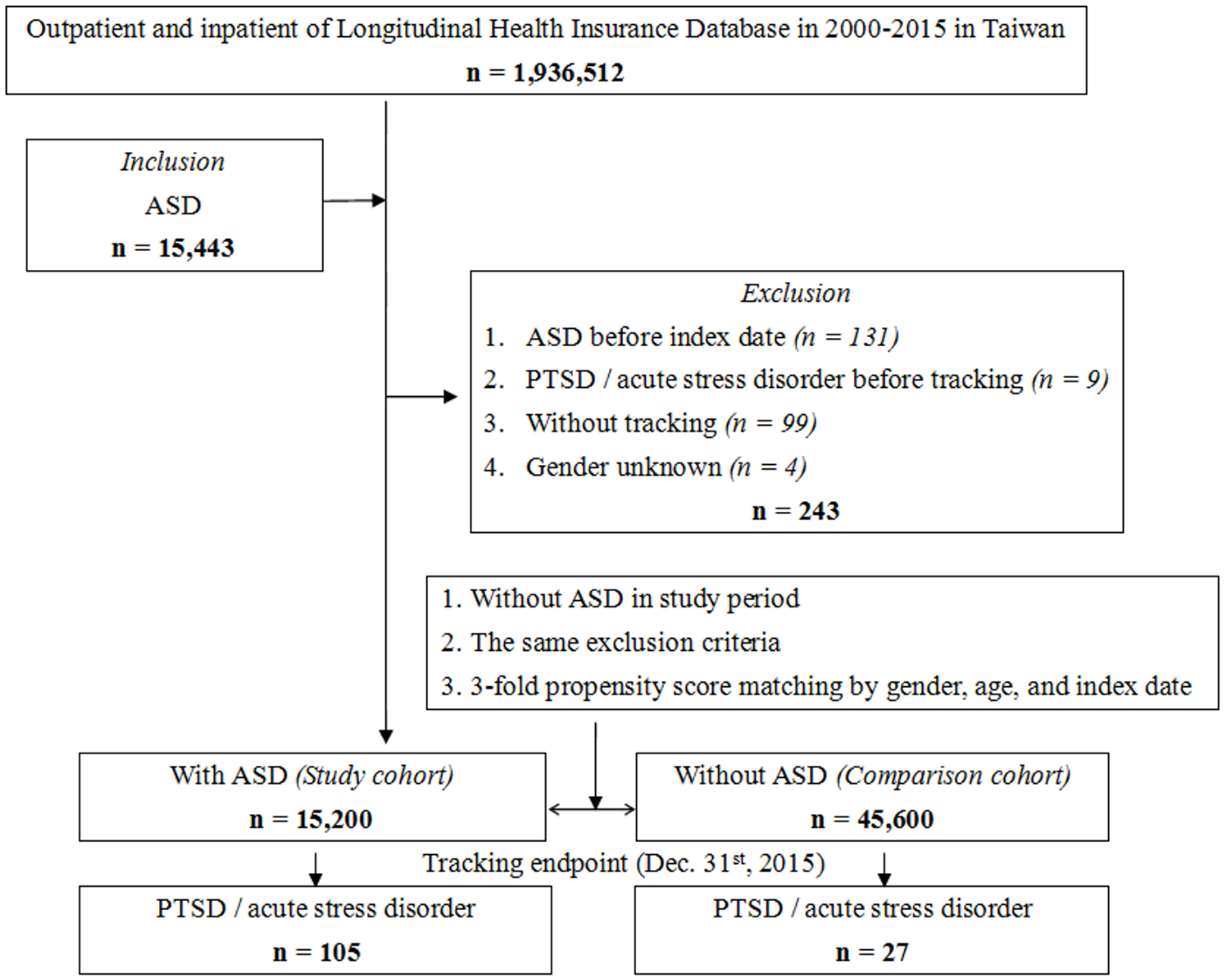
Figure 1 Flowchart of study sample selection from National Health Insurance Research Database in Taiwan.
Urbanization levels were determined based on population size and developmental indicators. Level 1 urbanization included areas with a population exceeding 1,250,000 and specific designations for cultural, economic, political, and metropolitan development. Level 2 comprised areas with a population between 500,000 and 1,249,999, playing significant roles in culture, economy, and politics. Urbanization levels 3 and 4 included populations ranging from 149,999 to 499,999 and less than 149,999, respectively.
Comorbidities were assessed using the Charlson Comorbidity Index (CCI), calculated from ICD-9-CM codes of each individuals in the original data of NHIRD with scores for each comorbidity category. A score of zero denoted the absence of comorbidities, with higher scores indicating a greater comorbidity burden. Additional psychiatric comorbidities considered included intellectual disability (ICD-9-CM code: 317-319, V62.89), childhood emotional disorders (ICD-9-CM code: 313), ADHD (ICD-9-CM code: 314), Tourette syndrome/tics disorders (ICD-9-CM code: 307.2), conduct disorder/oppositional defiant disorder (ICD-9-CM code: 312), other developmental disorders (ICD-9-CM code: 315), enuresis/encopresis (ICD-9-CM code: 307.6-307.7), and injury (ICD-9-CM code: 800-999, E800-E999) (42). The ICD-9-CM codes for diagnoses and clinical situations in this study are presented in Supplementary Table S1.
Complete analysis was performed using the software SPSS version 22.0 (IBM SPSS Statistics for Windows, Version 22.0, IBM Corp, Armonk, NY, USA) χ2 and t tests were applied to evaluate the distributions of the categorical and continuous variables, respectively. The Fisher exact test was used to evaluate the differences in categorical variables between the study and control group. The Cox proportional-hazard regression analysis was applied to determine the risk of PTSD and acute stress disorder, and results are presented as a hazard ratio with a 95% confidence interval. The difference in the risk between the ASD and the control groups were determined using the Kaplan–Meier method with the log-rank test. A 2-tailed P value of <.05 was considered to indicate statistical significance. A sensitivity analysis was performed to assess the persistence of associations following the exclusion of diagnoses for acute stress disorder and/or PTSD within the initial year and the first five years.
Table 1 presents the study population, and Figure 2 of the graphic abstract provides an illustration for the study design and results. Analysis revealed significant differences in age and gender between the ASD case group and the original unmatched group. (Supplementary Table S3) Matching on gender and age facilitated reduced confounding, improved comparability, and increase the power to 0.999 compared to unmatched regression analysis (power= 0.997) (Supplementary Table S4). A total of 15 200 individuals aged 18 years and under were included in the ASD group based on a diagnosis of ASD and 45 600 were included in the control group, with a comparable distribution of sex, age, and insured premiums between both groups. Regarding gender distribution, 81.32% of males in both groups align with the overall gender proportion, while the percentage of females is consistent at 18.68%. The age at diagnosis exhibits a mean of 6.3 years, indicating ASD detection with different symptoms spectrum and severity which lead to a diagnosis on a broad age range (4.27 years standard deviation). Comorbidities such as ADHD, intellectual disability (IQ disability), conduct disorder, oppositional defiant disorder, and other developmental disorders show significant associations with ASD. For instance, 5.85% of individuals with ASD have ADHD, whereas only 0.01% of those without ASD exhibit this comorbidity. The presence of these comorbidities underscores the complex clinical profile of ASD cases. In terms of insured premium, the majority of individuals (99.65%) fall below NT$18,000, emphasizing a uniform socio-economic background. Individuals with ASD are more prevalent in urban areas (40.92%), with a noteworthy concentration in the highest urbanization level. Additionally, a higher proportion of ASD cases is found in hospital centers (47.75%) compared to regional and local hospitals. The study also reveals seasonal and regional variations. However, these differences are minimal, suggesting a relatively homogeneous distribution across seasons and regions in both groups.
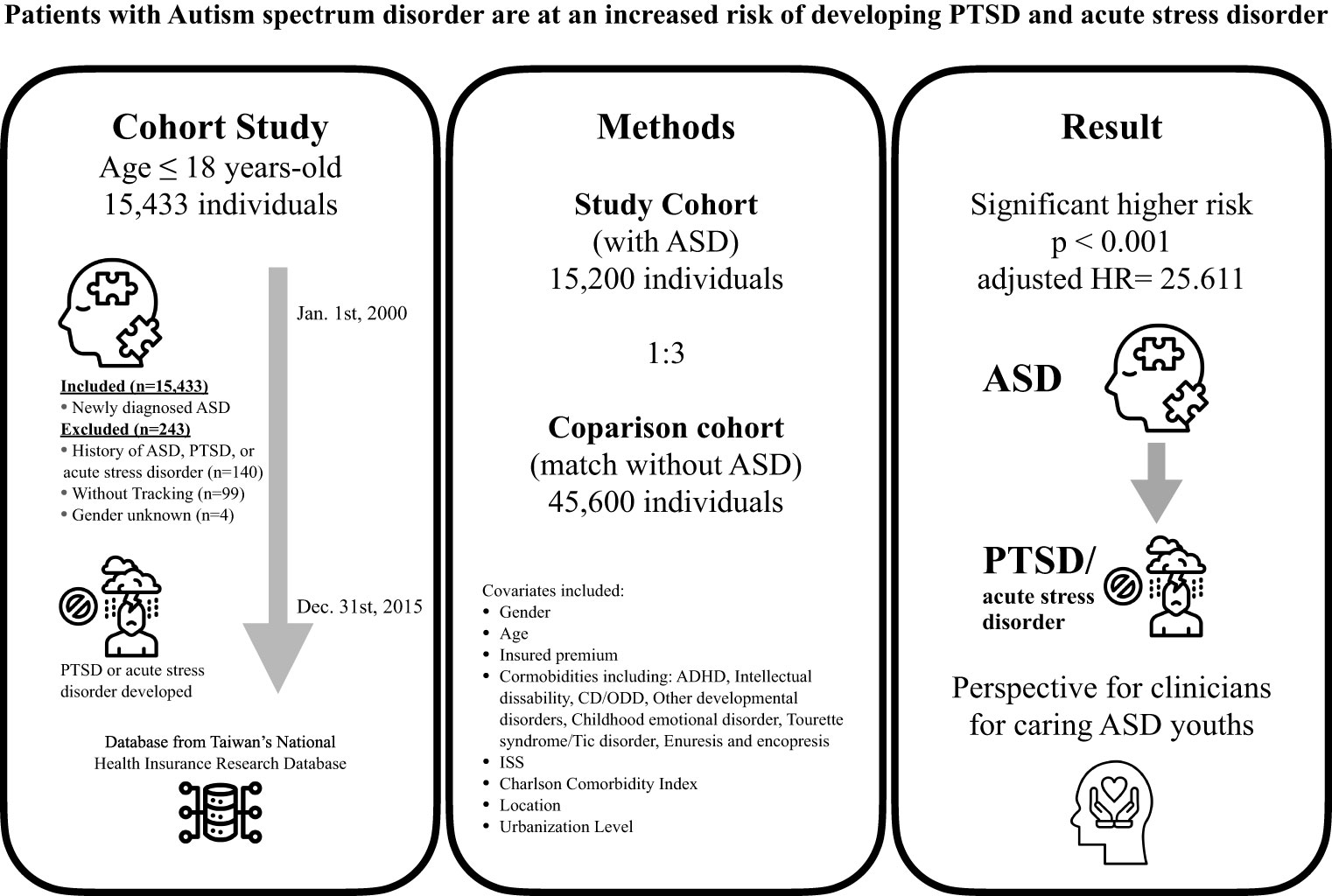
Figure 2 The graphic abstract of study design and results from National Health Insurance Research Database in Taiwan. ASD, Autism spectrum disorder; PTSD, Post-traumatic stress disorder; ADHD, Attention-deficit hyperactivity disorder; CD, Conduct disorder; ODD, Oppositional defiant disorder; ISS, Injury Severity Scale; HR, Hazard ratio. All icons are from the Noun Project.
In the population studied, 132 participants developed either acute stress disorder or PTSD during period of a 15-year timeframe of study: 105 (0.691% or 64.90 per 100 000 person-years) in the ASD group and 27 (0.059% or 5.38 per 100 000 person-years) in the control group. The adjusted hazard ratio for ASD was 25.611 for the studied participants (95% CI =15.913-41.232; P <.001) compared with the control group (Table 2). The Kaplan–Meier analysis revealed that the 15-year cumulative incidence of PTSD and acute stress disorder was significantly higher (log-rank test, P <.001, Figure 3) between the study participants and the controls. In the sensitivity analysis, these significant associations persisted after the exclusion of the diagnosis of acute stress disorder and/or PTSD in the first year and in the first five years (Supplementary Table S2).
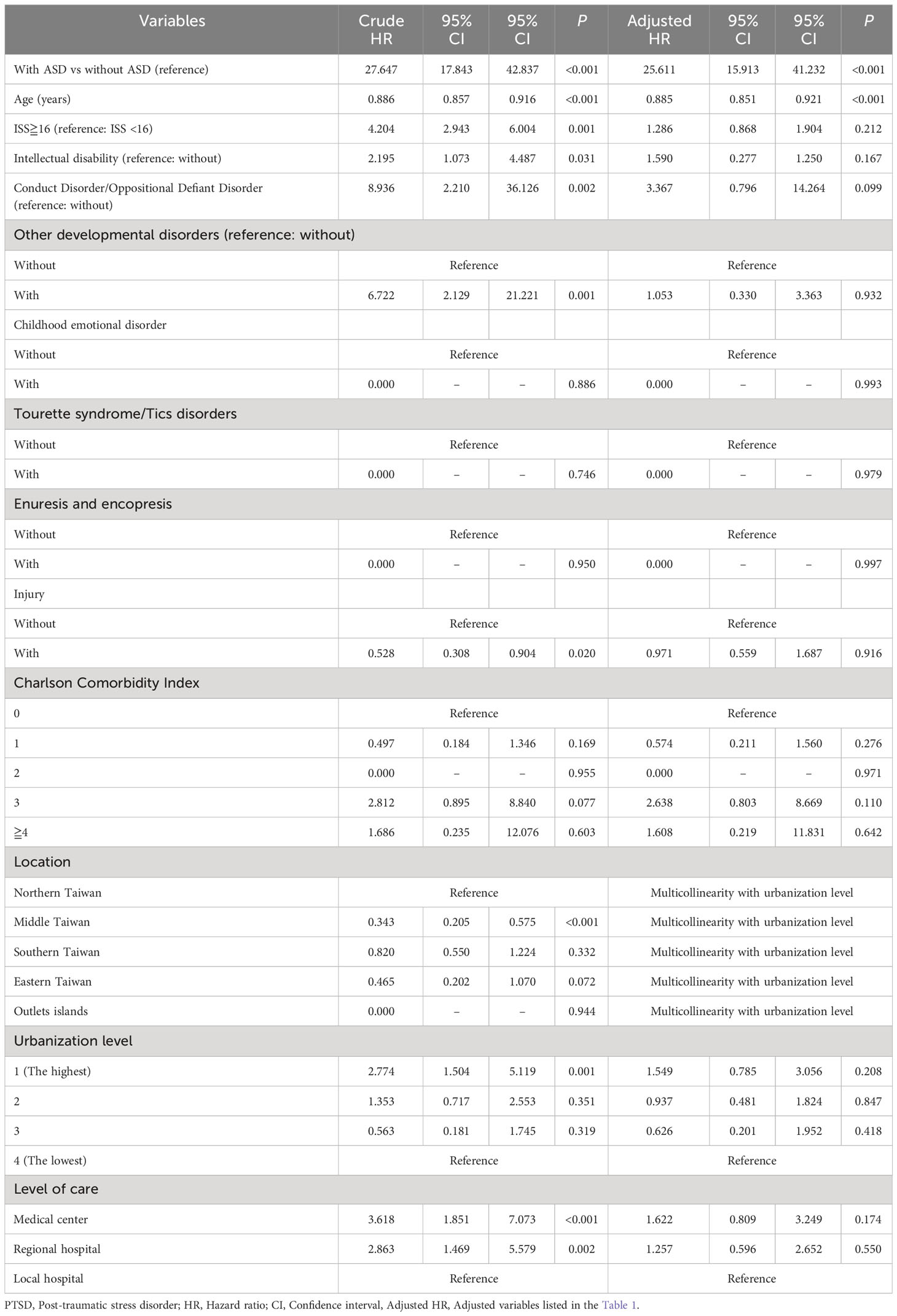
Table 2 Subgroup analysis for risk of PTSD/acute stress disorder in individuals with or without ASD by using Cox regression model.
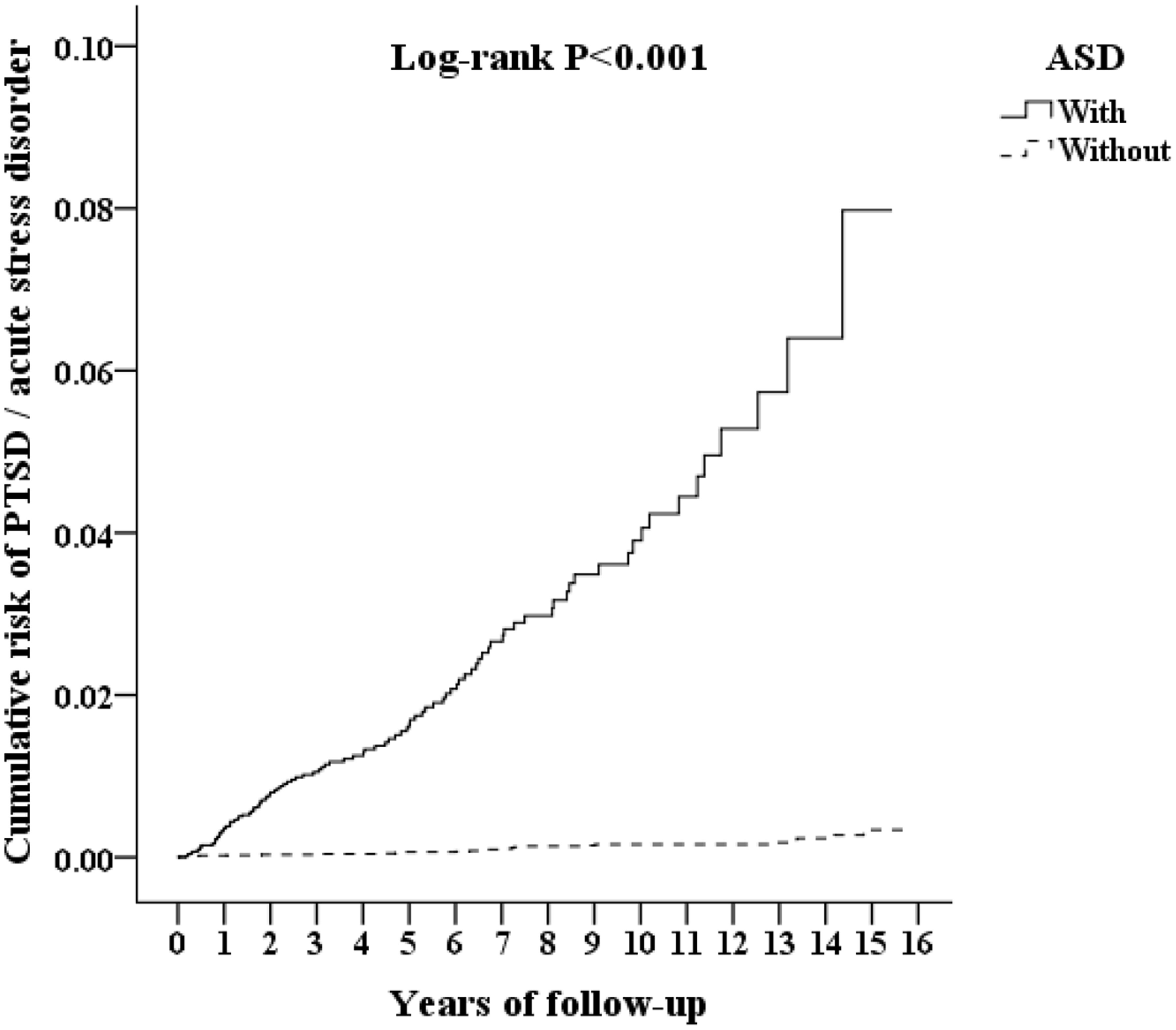
Figure 3 Kaplan-Meier for cumulative risk of PTSD and acute stress disorder aged 18 and less stratified by ASD with log-rank test.
Furthermore, given that past literature has indicated ADHD as one of the factors increasing the risk of PTSD (43), in addition to age and sex, we included ADHD comorbidity in the matching criteria in another regression analysis. (Supplementary Table S4) The results revealed that ASD cases developing PTSD had an adjusted HR of 22.184, with a 95% CI of 12.106~37.195, P < 0.001. This underscores that even after matching for ADHD comorbidity, the significant correlation with a higher risk persists.
The association between ASD and the risk of PTSD or acute stress disorder was analyzed in three subgroups: 1. Acute stress disorder or PTSD, 2. PTSD only, and 3. Acute stress disorder only (Table 3). The adjusted hazard ratio for the <3 visits group is 28.906, for the 3-5 visits group is 15.202, and for the >=6 visits group is 14.532. In each subgroup, ASD was associated with a higher risk of PTSD and/or acute stress disorder. Individuals with ASD who visit more frequently may experience a slight reduction in this risk.
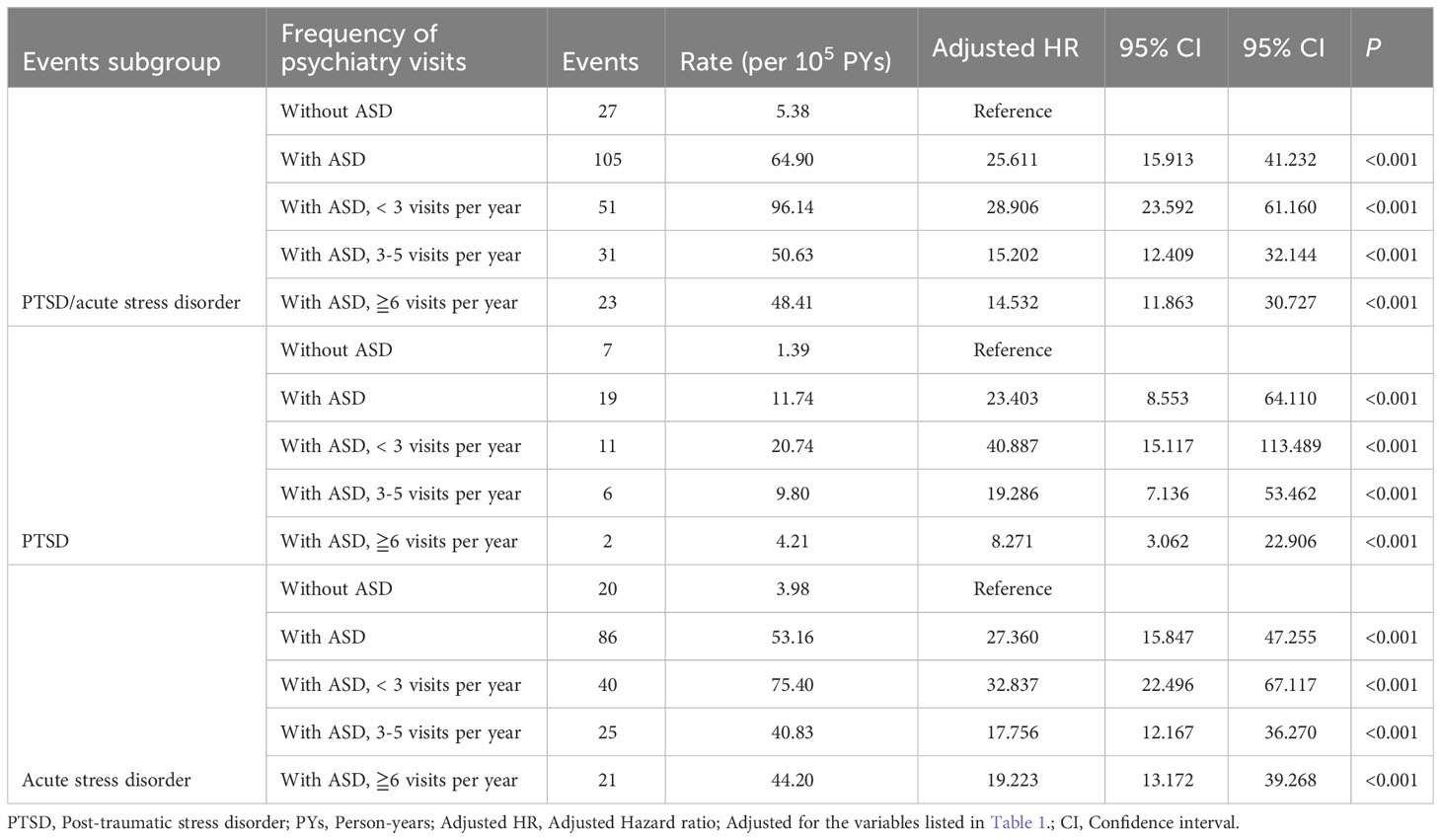
Table 3 Factors of PTSD/acute stress disorder among different frequency of psychiatry visits by using Cox regression.
This study presents several noteworthy findings. First, we established that youths with ASD have a more than 20-fold increased risk of developing acute stress disorder and PTSD. Second, to resolve the influences of protopathic bias, we conducted the sensitivity analysis revealed the association between ASD and acute stress disorder and PTSD even after exclusion of diagnoses within the first year and in the first five years. Third, ASD is associated with acute stress disorder and PTSD for PTSD-alone, acute stress disorder-alone, or combination of PTSD and acute stress disorder. Fourth, increased frequency of psychiatric visits is associated with a less higher risk of acute stress disorder and PTSD. For this finding, we hypothesize that a higher frequency of psychiatric visits might be an indicator of more regular and intensive treatment and care for ASD patients, and these might be associated with better resilience to traumatic events the patients that experienced. Our nationwide cohort study differs from former studies in that we used a wide-ranging national health insurance registry to identify the participants with a formal diagnosis of ASD. Further, our study was across the period of a 15-year timeframe to evaluate the occurrence of acute stress disorder and PTSD. Therefore, this study provides stronger evidence of the relationship between ASD and acute stress disorder and PTSD. To the best of our knowledge, this is the first study investigating the association between ASD and the risk of acute stress disorder and PTSD in a population-based cohort within a 15-year timeframe.
The retrospective design of the study was chosen due to practical considerations and the nature of the data available in the NHIRD in Taiwan. Retrospective studies are often employed when longitudinal data is already accessible, and obtaining prospective data is challenging or resource intensive. In this case, utilizing existing data allowed for a large sample size and an extended follow-up period, providing valuable insights into the association between ASD and the risk of developing PTSD and acute stress disorder. The choice of study design was driven by the feasibility and accessibility of the data, ensuring a comprehensive examination of the research question within the available constraints.
We based our decision to match on age and gender on considerations outlined by Brazauskas and Logan (44), emphasizing the importance of a matched study design in cases requiring reduced individuals or exhibiting a large degree of heterogeneity (44). This choice aimed to optimize the validity and reliability of our study results.
The ASD cohort in our study, in contrast to earlier studies (1, 3), shows lower comorbidity rates. For instance, previous literature indicated higher comorbidity rates for ASD, such as ADHD ranging from 28% to 44%, while our study reports a rate of 5.85%. The comorbidity rate with intellectual disability was 21-45% in previous literature, but our study found a rate of 7.41%. Similarly, the comorbidity rate with learning disabilities was 23.5% in previous literature and 15.73% in this study, showing closer alignment. These lower comorbidity rates might relate to the timeframe of the study, conducted between 2000 and 2015 when the diagnostic system in Taiwan used DSM-IV-TR. During this period, ASD and ADHD diagnoses were mutually exclusive, leading clinicians to preferentially register a more severe ASD diagnosis in NHIRD to enhance access to social welfare or special education resources. This practice may have resulted in an underestimation of ADHD comorbidity rates. Concerning intellectual disability, the DSM-IV-TR required standardized intelligence testing with results falling below two standard deviations for diagnosis. However, due to the severity of ASD or the age of the patients, standardized intelligence testing might not have been feasible, leading to potential underestimation of intellectual disability comorbidity rates. Therefore, the lower comorbidity rates in our study are likely related to the diagnostic system used during the study period.
The diagnostic criteria of PTSD in DSM defined trauma as “exposure to actual or threatened death, serious injury, or sexual violence (9, 10).” However, several studies have attempted to broaden the experience of psychological trauma (non-DSM traumatic events), such as loss, work, relationships, academic achievements, environment, life transitions, or physical struggles, through a questionnaire-based study on participants with ASD (5, 19, 29). Individuals with ASD may develop PTSD symptoms after encountering such non-DSM traumatic events, implying that stressful events in everyday life may result in the development of PTSD in ASD adults (18).
The mechanism between ASD and PTSD as well as the associated risk factors are still under investigation. A hypothetical model assumed that ASD may influence the experience of trauma at different levels, moderating the event encountered, experiences appraised harmful, and the risk of developing PTSD or other negative outcomes such as affective disorders (45).
Children and adolescents with ASD are prone to experiencing bullying or peer victimization (46). An article of systematic review revealed prevalence rates of 33% for physical bullying, 50% for verbal bullying, and 31% for relational bullying in ASD children (47). These encounters may be linked to subsequent manifestations of school refusal (48). Another study identified correlations between bullying victimization among ASD children and ASD symptoms such as social and communication deficits, internalizing behaviors, and condition of participation in integrated inclusive school settings (49). Even in cases of high-functioning ASD children or with Asperger’s syndrome, bullying remains prevalent (50), with over half of such cases reporting instances of victimization in a study (51).
Higher suicidal thoughts or behaviors were observed in children with ASD (6). A review article summarized that ASD individuals, with unique characteristic of sensation, perception, social awareness and cognition, may experience psychosocial events as traumatic compared with general population, such as unusual fears, difficulties with sensory overstimulation, changes in routine, or social demands (52). Various of studies investigated on the possible shared symptomology and mechanism between ASD and PTSD, including memory problems (18), brooding rumination (53), cognitive rigidity (54, 55), emotional dysregulation (56, 57), irritability and aggression (58, 59), and avoidance (60, 61). Furthermore, other studies have indicated that one mediator contributing to increased suicide rates in the PTSD population is the experience of guilt associated with trauma (62, 63). Additionally, individuals with ASD are more prone to experiencing self-conscious emotions such as guilt and shame compared to the typically-developed population (64). It remains to be further explored whether the experience of guilt could potentially serve as a mediating factor in the higher occurrence of PTSD within the ASD youths.
On the aspect of gender difference, a study revealed that female ASD patients are at higher risk of exposure to non-DSM traumatic events and development of PTSD symptoms than males (22). However, another study showed no geneder difference in developing PTSD symptoms in ASD individuals who exposed to non-DSM traumatic events (18). In our study, no significant difference was observed in the occurrence of acute stress disorder or PTSD between males and females. Compared to males, female ASD individuals may be diagnosed in older age, and have tendency of camouflaging their autistic features and using compensatory behaviors (65). The gender may be a potential factor to affect the developing acute stress disorder and PTSD in ASD individuals, but its role is still unclear. The discrepancy on gender difference of previous studies and our work may indicate that a certain group of ASD individual, possibly with trauma experience and PTSD symptoms, has been beyond the scope of PTSD diagnostic criteria of DSM. The undiagnosed group of ASD patients with PTSD symptoms may be potentially neglected (66).
Lastly, the Spencer et al. (43) meta-analysis suggests an elevated risk of individuals with ADHD developing PTSD. Our study, constrained by diagnostic system limitations, reports a relatively low comorbidity rate of ADHD in individuals with ASD, hindering a full representation of this population. However, when including ADHD in matching criteria, the heightened risk persists, affirming that ASD independently associates with an increased development of PTSD/acute stress disorder. Nevertheless, Spencer et al. (43) highlights that the heightened PTSD risk in individuals with ADHD goes beyond trauma exposure, hinting at a potential neurobiological basis. In contrast, our discussion above of ASD and PTSD links ASD symptomatology to an increased likelihood of trauma exposure, potentially elevating PTSD risk. These differing pathways suggest that ASD and ADHD may influence PTSD risk differently, exceeding our study’s scope. Additionally, past studies on ADHD and PTSD often overlooked ASD comorbidity, emphasizing the need for further research to unravel the intricate relationship among ASD, ADHD, and PTSD.
This study has several limitations. First, The incidence of ASD may be underestimated as our study enrolled only medical help-seeking patients. Parents or teachers might observe developmental delays or signs of ASD in children, but not all are brought to hospitals or clinics due to potential social stigma associated with psychiatric evaluation.
Second, the diverse age of ASD diagnosis, ranging from early childhood to adolescence, is influenced by the spectrum nature and severity of symptoms. More severe cases are diagnosed early, while milder cases may receive a diagnosis later. Our large sample reflects this spectrum. Employing age and gender matching, considering higher male prevalence, enhances result power but presents limitations like sample size loss and selection bias. Propensity score matching and sensitivity analysis address these challenges, emphasizing our commitment to accurate and valid results.
Third, in our cohort of youths with ASD, comorbidity rates were notably lower than expected based on previous studies (1, 3). This discrepancy is likely attributed to the use of the DSM-IV-TR diagnostic system during the study period, as discussed in the previous section, resulting in lower rates of comorbidities in our study. Additionally, our study observed a lower comorbidity rate of ADHD in the sample, despite previous literature indicating a higher risk of developing PTSD in individuals with ADHD. By incorporating ADHD comorbidity in our another analysis, we identified a heightened correlation, suggesting that ASD itself is associated with an increased risk of PTSD/acute stress disorder. However, the applicability of these comorbidity rates from our sample to the entire population has certain limitations.
Fourth, the increased number of visits by ASD cases may introduce detection bias and heightened diagnoses and comorbidities. Detection bias persists in this study. In Table 3, individuals with more frequent visits may experience a slightly lower risk. Thus, we interpret that the risk of developing PTSD/acute stress disorder increases regardless of visit frequency, with a slightly lower increase for those with more visits, possibly linked to ASD patients receiving comprehensive medical care during frequent visits, mitigating this risk.
Fifth, this study did not investigate and analyze other factors that could contribute to the development of PTSD, such as domestic violence, peer bullying at school, single-parent households, etc. This limitation is primarily associated with the constraints of the health insurance database’s covered content. Additionally, excessive matching variables may lead to overmatching, potentially obscuring existing risks (67). Sixth, the health insurance database lacks information on whether standardized diagnostic tools, like the Autism Diagnostic Observation Schedule (ADOS) or the Autism Diagnostic Interview-Revised (ADI-R), were used for ASD diagnosis, or data on the severity of ASD and associated psychological assessment results, which could impact the risk of developing PTSD or acute stress disorder.
Finally, our study did not differentiate trauma types or assess the severity of ASD and PTSD adequately within the NHIRD. This lack of specificity may lead to varying risks across different trauma-exposed groups. Additionally, our reliance on DSM-IV-TR criteria for PTSD diagnosis by psychiatrists excluded cases where individuals with ASD experienced non-DSM-defined traumas, potentially displaying PTSD symptoms without meeting diagnostic criteria. We also overlooked individuals with concerns aligning with complex PTSD in ICD-11, emphasizing chronic, repetitive, and prolonged traumas affecting emotional regulation, self-identity, and relational capacities (68). These omissions suggest the need for future research to encompass these considerations.
Through a nationwide population-based cohort study in Taiwan, we assessed the risk of acute stress disorder and PTSD among children and adolescents with ASD. We established that children and adults with ASD have a more than 20-fold increased risk of developing acute stress disorder and PTSD. We sincerely hope this study can be used as a reference to examine patients with ASD and design a therapeutic plan from the perspective of trauma exposure and post-trauma process in daily practice.
The data analyzed in this study is subject to the following licenses/restrictions: Data are available from the National Health Insurance Research Database (NHIRD) published by Taiwan National Health Insurance (NHI) Bureau. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD. Requests to access these datasets should be directed to http://nhird.nhri.org.tw.
The studies involving humans were approved by the institutional review board of the Tri-Service General Hospital (IRB No.2-107-05-026). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because identities of individuals in the NHIRD were completely encrypted to protect privacy.
SL: Visualization, Writing – original draft, Writing – review & editing. WC: Data curation, Investigation, Methodology, Writing – original draft. CC: Formal analysis, Resources, Software, Validation, Visualization, Writing – original draft. NT: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We appreciated the financial support from the Medical Affairs Bureau, Ministry of National Defense (MND-MAB-110-087, MND-MAB-D-111075, and MND-MAB-D-113059), the Tri-Service General Hospital (TSGHE-110240), the Taoyuan Armed Forces General Hospital (TYAFGH-A-110020), and the Cheng Hsin General Hospital-National Defense Medical Center Joint Research Program (CHNDMC-111-9) in Taiwan.
The manuscript is original, and not published, nor under concurrent consideration elsewhere. The authors would like to thank the data from the National Health Insurance Research Database provided by Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1329836/full#supplementary-material
Supplementary Figure 1 | The estimated statistical power of the ratio for the case to control groups (Figure S1a adapted from Woodward 2013*). Figure S1b showed our statistical analysis showed the power was approximate to 1.0 while match ratio was 1:3 . * Woodward M (2013). Epidemiology: Study Design and Data Analysis, 3rd Edition. United Kingdom, Chapman and Hall/CRC.
1. Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet (2014) 383:896–910. doi: 10.1016/s0140-6736(13)61539-1
2. Sun X, Allison C, Matthews FE, Sharp SJ, Auyeung B, Baron-Cohen S, et al. Prevalence of autism in mainland China, Hong Kong and Taiwan: a systematic review and meta-analysis. Mol Autism (2013) 4:7. doi: 10.1186/2040-2392-4-7
3. KhaChadourian V, Mahjani B, Sandin S, Kolevzon A, Buxbaum JD, Reichenberg A, et al. Comorbidities in autism spectrum disorder and their etiologies. Trans Psychiatry (2023) 13(1):71. doi: 10.1038/s41398-023-02374-w
4. Joshi G, Wozniak J, Petty C, Martelon MK, Fried R, Bolfek A, et al. Psychiatric comorbidity and functioning in a clinically referred population of adults with autism spectrum disorders: a comparative study. J Autism Dev Disord (2013) 43:1314–25. doi: 10.1007/s10803-012-1679-5
5. Rumball F, Happe F, Grey N. Experience of trauma and PTSD symptoms in autistic adults: risk of PTSD development following DSM-5 and Non-DSM-5 traumatic life events. Autism Res (2020) 13:2122–32. doi: 10.1002/aur.2306
6. Storch EA, Sulkowski ML, Nadeau J, Lewin AB, Arnold EB, Mutch PJ, et al. The phenomenology and clinical correlates of suicidal thoughts and behaviors in youth with autism spectrum disorders. J Autism Dev Disord (2013) 43:2450–9. doi: 10.1007/s10803-013-1795-x
7. Chen MH, Pan TL, Lan WH, Hsu JW, Huang KL, Su TP, et al. Risk of suicide attempts among adolescents and young adults with autism spectrum disorder: a nationwide longitudinal follow-up Study. J Clin Psychiatry (2017) 78:e1174–9. doi: 10.4088/JCP.16m11100
8. Chen YH, Wei HT, Bai YM, Hsu JW, Huang KL, Su TP, et al. Risk of epilepsy in individuals with posttraumatic stress disorder: a nationwide longitudinal study. Psychosom Med (2017) 79:664–9. doi: 10.1097/psy.0000000000000463
9. American Psychiatric Association. Diagnostic Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). Washington, DC: American Psychiatric Publishing (1994).
10. American Psychiatric Association. Diagnostic Statistical Manual of Mental Disorders, 4th Edition, Text-Revision (DSM-IV-TR). Washington, DC: American Psychiatric Publishing (2000).
11. Lin CE, Chung CH, Chen LF, Chien WC, Chou PH. The impact of antidepressants on the risk of developing obstructive sleep apnea in posttraumatic stress disorder: a nationwide cohort study in Taiwan. J Clin Sleep Med (2019) 15:1233–41. doi: 10.5664/jcsm.7910
12. Hung YH, Cheng CM, Lin WC, Bai YM, Su TP, Li CT, et al. Post-traumatic stress disorder and asthma risk: a nationwide longitudinal study. Psychiatry Res (2019) 276:25–30. doi: 10.1016/j.psychres.2019.04.014
13. Lin CE, Chung CH, Chen LF, You CH, Chien WC, Chou PH. Risk of incident hypertension, diabetes, and dyslipidemia after first posttraumatic stress disorder diagnosis: a nationwide cohort study in Taiwan. Gen Hosp Psychiatry (2019) 58:59–66. doi: 10.1016/j.genhosppsych.2019.03.004
14. Huang WS, Hsu JW, Huang KL, Bai YM, Su TP, Li CT, et al. Post-traumatic stress disorder and risk of osteoporosis: a nationwide longitudinal study. Stress Health (2018) 34:440–5. doi: 10.1002/smi.2806
15. Chan YE, Bai YM, Hsu JW, Huang KL, Su TP, Li CT, et al. Post-traumatic stress disorder and risk of Parkinson disease: a nationwide longitudinal study. Am J Geriatr Psychiatry (2017) 25:917–23. doi: 10.1016/j.jagp.2017.03.012
16. Wang TY, Wei HT, Liou YJ, Su TP, Bai YM, Tsai SJ, et al. Risk for developing dementia among patients with posttraumatic stress disorder: a nationwide longitudinal study. J Affect Disord (2016) 205:306–10. doi: 10.1016/j.jad.2016.08.013
17. Huang JS, Yang FC, Chien WC, Yeh TC, Chung CH, Tsai CK, et al. Risk of substance use disorder and its associations with comorbidities and psychotropic agents in patients with autism. JAMA Pediatr (2021) 175:e205371. doi: 10.1001/jamapediatrics.2020.5371
18. Rumball F, Brook L, Happe F, Karl A. Heightened risk of posttraumatic stress disorder in adults with autism spectrum disorder: the role of cumulative trauma and memory deficits. Res Dev Disabil (2021) 110:103848. doi: 10.1016/j.ridd.2020.103848
19. Taylor JL, Gotham KO. Cumulative life events, traumatic experiences, and psychiatric symptomatology in transition-aged youth with autism spectrum disorder. J Neurodev Disord (2016) 8:28. doi: 10.1186/s11689-016-9160-y
20. Rumball F, Antal K, Happé F, Grey N. Co-occurring mental health symptoms and cognitive processes in trauma-exposed ASD adults. Res Dev Disabil (2021) 110:103836. doi: 10.1016/j.ridd.2020.103836
21. Peterson JL, Earl R, Fox EA, Ma R, Haidar G, Pepper M, et al. Trauma and autism spectrum disorder: review, proposed treatment adaptations and future directions. J Child Adolesc Trauma (2019) 12:529–47. doi: 10.1007/s40653-019-00253-5
22. Haruvi-Lamdan N, Horesh D, Zohar S, Kraus M, Golan O. Autism spectrum disorder and post-traumatic stress disorder: an unexplored co-occurrence of conditions. Autism (2020) 24:884–98. doi: 10.1177/1362361320912143
23. Rumball F. A systematic review of the assessment and treatment of posttraumatic stress disorder in individuals with autism spectrum disorders. Rev J Autism Dev Disord (2019) 6:294–324. doi: 10.1007/s40489-018-0133-9
24. Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Arch Gen Psychiatry (2007) 64:577–84. doi: 10.1001/archpsyc.64.5.577
25. Bryant RA, Creamer M, O’Donnell ML, Silove D, McFarlane AC. A multisite study of the capacity of acute stress disorder diagnosis to predict posttraumatic stress disorder. J Clin Psychiatry (2008) 69:923–9. doi: 10.4088/jcp.v69n0606
26. Bryant RA. Acute stress disorder as a predictor of posttraumatic stress disorder: a systematic review. J Clin Psychiatry (2011) 72:233–9. doi: 10.4088/JCP.09r05072blu
27. Kassam-Adams N, Winston FK. Predicting child PTSD: the relationship between acute stress disorder and PTSD in injured children. J Am Acad Child Adolesc Psychiatry (2004) 43:403–11. doi: 10.1097/00004583-200404000-00006
28. Meiser-Stedman R, McKinnon A, Dixon C, Boyle A, Smith P, Dalgleish T. Acute stress disorder and the transition to posttraumatic stress disorder in children and adolescents: prevalence, course, prognosis, diagnostic suitability, and risk markers. Depress Anxiety (2017) 34:348–55. doi: 10.1002/da.22602
29. Kildahl AN, Helverschou SB, Bakken TL, Oddli HW. “If we do not look for it, we do not see it”: clinicians’ experiences and understanding of identifying post-traumatic stress disorder in adults with autism and intellectual disability. J Appl Res Intell Disabil (2020) 33:1119–32. doi: 10.1111/jar.12734
30. Roberts AL, Koenen KC, Lyall K, Robinson EB, Weisskopf MG. Association of autistic traits in adulthood with childhood abuse, interpersonal victimization, and posttraumatic stress. Child Abuse Negl (2015) 45:135–42. doi: 10.1016/j.chiabu.2015.04.010
31. Chen MH, Lan WH, Hsu JW, Huang KL, Su TP, Li CT, et al. Risk of developing type 2 diabetes in adolescents and young adults with autism spectrum disorder: a nationwide longitudinal study. Diabetes Care (2016) 39:788–93. doi: 10.2337/dc15-1807
32. Hung TW, Tsai JD, Pan HH, Chen HJ, Liao PF, Sheu JN. Is neonatal hyperbilirubinemia exposure associated with a risk of autism spectrum disorder? A nationwide cohort study. Am J Perinatol (2021) 38:1244–53. doi: 10.1055/s-0040-1708033
33. Hwang YS, Weng SF, Cho CY, Tsai WH. Higher prevalence of autism in Taiwanese children born prematurely: a nationwide population-based study. Res Dev Disabil (2013) 34:2462–8. doi: 10.1016/j.ridd.2013.05.019
34. Ko WR, Huang JY, Chiang YC, Nfor ON, Ko PC, Jan SR, et al. Risk of autistic disorder after exposure to general anaesthesia and surgery: a nationwide, retrospective matched cohort study. Eur J Anaesthesiol (2015) 32:303–10. doi: 10.1097/eja.0000000000000130
35. Ho Chan WS. Taiwan’s healthcare report 2010. EPMA J (2010) 1:563–85. doi: 10.1007/s13167-010-0056-8
36. Chou IC, Lin HC, Lin CC, Sung FC, Kao CH. Tourette syndrome and risk of depression: a population-based cohort study in Taiwan. J Dev Behav Pediatrics: JDBP (2013) 34(3):181–5. doi: 10.1097/DBP.0b013e3182829f2b
37. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol (2019) 11:349–58. doi: 10.2147/clep.S196293
38. Wu CS, Kuo CJ, Su CH, Wang SH, Dai HJ. Using text mining to extract depressive symptoms and to validate the diagnosis of major depressive disorder from electronic health records. J Affect Disord (2020) 260:617–23. doi: 10.1016/j.jad.2019.09.044
39. Huang YT, Wei T, Huang YL, Wu YP, Chan KA. Validation of diagnosis codes in healthcare databases in Taiwan, a literature review. Pharmacoepidemiol Drug Saf (2023) 32(7):795–811. doi: 10.1002/pds.5608
40. Ministry of Health and Welfare. Patients with catastrophic illnesses or rare diseases (2016). Available at: https://www.nhi.gov.tw/21nglish/Content_List.aspx?n=F5B8E49CB4548C60&topn=1D1ECC54F86E9050#:~:text=Rare%20diseases%20are%20classified%20as,treatments%20related%20to%20the%20disease (Accessed January 16, 2016).
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5). Washington, DC: American Psychiatric Association (2013).
42. Ogundele MO. Behavioural and emotional disorders in childhood: a brief overview for paediatricians. World J Clin Pediatr (2018) 7:9–26. doi: 10.5409/wjcp.v7.i1.9
43. Spencer AE, Faraone SV, Bogucki OE, Pope AL, Uchida M, Milad MR, et al. Examining the association between posttraumatic stress disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Clin Psychiatry (2016) 77(1):72–83. doi: 10.4088/JCP.14r09479
44. Brazauskas R, Logan BR. Observational studies: matching or regression? Biol Blood Marrow Transplantation: J Am Soc Blood Marrow Transplant (2016) 22(3):557–63. doi: 10.1016/j.bbmt.2015.12.005
45. Kerns CM, Newschaffer CJ, Berkowitz SJ. Traumatic childhood events and autism spectrum disorder. J Autism Dev Disord (2015) 45:3475–86. doi: 10.1007/s10803-015-2392-y
46. Hoover DW, Kaufman J. Adverse childhood experiences in children with autism spectrum disorder. Curr Opin Psychiatry (2018) 31:128–32. doi: 10.1097/YCO.0000000000000390
47. Maïano C, Normand CL, Salvas MC, Moullec G, Aimé A. Prevalence of school bullying among youth with autism spectrum disorders: A systematic review and meta-analysis. Autism Res: Off J Int Soc Autism Res (2016) 9(6):601–15. doi: 10.1002/aur.1568
48. Ochi M, Kawabe K, Ochi S, Miyama T, Horiuchi F, Ueno SI. School refusal and bullying in children with autism spectrum disorder. Child Adolesc Psychiatry Ment Health (2020) 14:17. doi: 10.1186/s13034-020-00325-7
49. Park I, Gong J, Lyons GL, Hirota T, Takahashi M, Kim B, et al. Prevalence of and factors associated with school bullying in students with autism spectrum disorder: A cross-cultural meta-analysis. Yonsei Med J (2020) 61(11):909–22. doi: 10.3349/ymj.2020.61.11.909
50. Carter S. Bullying of students with Asperger syndrome. Issues Compr Pediatr Nurs (2009) 32(3):145–54. doi: 10.1080/01460860903062782
51. van Schalkwyk G, Smith IC, Silverman WK, Volkmar FR. Brief report: bullying and anxiety in high-functioning adolescents with ASD. J Autism Dev Disord (2018) 48(5):1819–24. doi: 10.1007/s10803-017-3378-8
52. Haruvi-Lamdan N, Horesh D, Golan O. PTSD and autism spectrum disorder: Co-morbidity, gaps in research, and potential shared mechanisms. Psychol Trauma (2018) 10:290–9. doi: 10.1037/tra0000298
53. Golan O, Haruvi-Lamdan N, Laor N, Horesh D. The comorbidity between autism spectrum disorder and post-traumatic stress disorder is mediated by brooding rumination. Autism (2022) 26:538–44. doi: 10.1177/13623613211035240
54. Palm KM, Follette VM. The roles of cognitive flexibility and experiential avoidance in explaining psychological distress in survivors of interpersonal victimization. J Psychopathol Behav Assess (2011) 33:79–86. doi: 10.1007/s10862-010-9201-x
55. Leung RC, Zakzanis KK. Brief report: cognitive flexibility in autism spectrum disorders: a quantitative review. J Autism Dev Disord (2014) 44:2628–45. doi: 10.1007/s10803-014-2136-4
56. Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am (2014) 23:15–24. doi: 10.1016/j.chc.2013.07.002
57. Seligowski AV, Lee DJ, Bardeen JR, Orcutt HK. Emotion regulation and posttraumatic stress symptoms: a meta-analysis. Cognit Behav Ther (2015) 44:87–102. doi: 10.1080/16506073.2014.980753
58. Orth U, Wieland E. Anger, hostility, and posttraumatic stress disorder in trauma-exposed adults: a meta-analysis. J Consult Clin Psychol (2006) 74:698–706. doi: 10.1037/0022-006x.74.4.698
59. Matson JL, Adams HL. Characteristics of aggression among persons with autism spectrum disorders. Res Autism Spectr Disord (2014) 8):1578–84. doi: 10.1016/j.rasd.2014.08.004
60. Rieffe C, Camodeca M, Pouw LBC, Lange AMC, Stockmann L. Don’t anger me! Bullying, victimization, and emotion dysregulation in young adolescents with ASD. Eur J Dev Psychol (2012) 9:351–70. doi: 10.1080/17405629.2012.680302
61. Hetzel-Riggin MD, Meads CL. Interrelationships among three avoidant coping styles and their relationship to trauma, peritraumatic distress, and posttraumatic stress disorder. J Nervous Ment Dis (2016) 204:123–31. doi: 10.1097/nmd.0000000000000434
62. Kip A, Diele J, Holling H, Morina N. The relationship of trauma-related guilt with PTSD symptoms in adult trauma survivors: a meta-analysis. psychol Med (2022) 52(12):2201–11. doi: 10.1017/S0033291722001866
63. Chou PH, Wang SC, Wu CS, Ito M. Trauma-related guilt as a mediator between post-traumatic stress disorder and suicidal ideation. Front Psychiatry (2023) 14:1131733. doi: 10.3389/fpsyt.2023.1131733
64. Davidson D, Hilvert E, Misiunaite I, Giordano M. Proneness to guilt, shame, and pride in children with Autism Spectrum Disorders and neurotypical children. Autism Res: Off J Int Soc Autism Res (2018) 11(6):883–92. doi: 10.1002/aur.1937
65. Dean M, Harwood R, Kasari C. The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism (2017) 21:678–89. doi: 10.1177/1362361316671845
66. Brewin CR, Rumball F, Happe F. Neglected causes of post-traumatic stress disorder. BMJ (2019) 365:l2372. doi: 10.1136/bmj.l2372
67. Axelson O. Negative and non-positive epidemiological studies. Int J Occup Med Environ Health (2004) 17(1):115–21. doi: 10.1080/10807030590919981
Keywords: child and adolescent psychiatry, autism spectrum disorder, acute stress disorder, post-traumatic stress disorder, epidemiology
Citation: Li S-T, Chien W-C, Chung C-H and Tzeng N-S (2024) Increased risk of acute stress disorder and post-traumatic stress disorder in children and adolescents with autism spectrum disorder: a nation-wide cohort study in Taiwan. Front. Psychiatry 15:1329836. doi: 10.3389/fpsyt.2024.1329836
Received: 30 October 2023; Accepted: 08 January 2024;
Published: 31 January 2024.
Edited by:
Rosa Calvo Escalona, Hospital Clinic of Barcelona, SpainReviewed by:
Jorge Aguado-Gracia, Hospital Clinic of Barcelona, SpainCopyright © 2024 Li, Chien, Chung and Tzeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nian-Sheng Tzeng, cGllcnJlbnNAbWFpbC5uZG1jdHNnaC5lZHUudHc=; cGllcnJlbnM5MUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.