
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Psychiatry, 04 June 2024
Sec. Psychopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1320780
This article is part of the Research TopicDown the rabbit hole – the psychological and neural mechanisms of psychedelic compounds and their use in treating mental health and medical conditionsView all 14 articles
 Julia Bornemann1*
Julia Bornemann1* James B. Close1
James B. Close1 Kirran Ahmad1
Kirran Ahmad1 Tommaso Barba1
Tommaso Barba1 Kate Godfrey1
Kate Godfrey1 Lauren Macdonald1
Lauren Macdonald1 David Erritzoe1
David Erritzoe1 David Nutt1
David Nutt1 Robin Carhart-Harris1,2
Robin Carhart-Harris1,2Background: Chronic pain is a leading cause of disability worldwide. Fibromyalgia is a particularly debilitating form of widespread chronic pain. Fibromyalgia remains poorly understood, and treatment options are limited or moderately effective at best. Here, we present a protocol for a mechanistic study investigating the effects of psychedelic-assisted-therapy in a fibromyalgia population. The principal focus of this trial is the central mechanism(s) of psilocybin-therapy i.e., in the brain and on associated mental schemata, primarily captured by electroencephalography (EEG) recordings of the acute psychedelic state, plus pre and post Magnetic Resonance Imaging (MRI).
Methods: Twenty participants with fibromyalgia will complete 8 study visits over 8 weeks. This will include two dosing sessions where participants will receive psilocybin at least once, with doses varying up to 25mg. Our primary outcomes are 1) Lempel-Ziv complexity (LZc) recorded acutely using EEG, and the 2) the (Brief Experiential Avoidance Questionnaire (BEAQ) measured at baseline and primary endpoint. Secondary outcomes will aim to capture broad aspects of the pain experience and related features through neuroimaging, self-report measures, behavioural paradigms, and qualitative interviews. Pain Symptomatology will be measured using the Brief Pain Inventory Interference Subscale (BPI-IS), physical and mental health-related function will be measured using the 36-Item Short Form Health Survey (SF-36). Further neurobiological investigations will include functional MRI (fMRI) and diffusion tensor imaging (changes from baseline to primary endpoint), and acute changes in pre- vs post-acute spontaneous brain activity – plus event-related potential functional plasticity markers, captured via EEG.
Discussion: The results of this study will provide valuable insight into the brain mechanisms involved in the action of psilocybin-therapy for fibromyalgia with potential implications for the therapeutic action of psychedelic-therapy more broadly. It will also deliver essential data to inform the design of a potential subsequent RCT.
Chronic pain is a leading cause of disability worldwide (1, 2). Fibromyalgia (FM) is a particularly debilitating form of chronic generalized pain (3–5) with reported prevalence rates varying from between 0.4 and 8% (6–10) FM is characterised by widespread pain, fatigue, sleep difficulties and cognitive disturbance including memory and ability to concentrate e.g., brain fog (5). Frequently reported concomitant symptoms are irritable bowel syndrome (IBS), headache, and temporomandibular disorder (6, 11). Compared with other types of chronic pain, people with FM exhibit disproportionately high rates of psychological comorbidity (3, 9) with 60-80% also experiencing comorbid depression and/or anxiety (12–14). Additionally, FM populations present markedly high rates of lifetime (15), and particularly childhood trauma (16, 17). Women are significantly more likely to be affected than men (8, 11, 18).
The aetiology and pathology of FM remain poorly understood (19), although current hypotheses centre around combined immunological (20), psychological (21), stress (21) and trauma-related (22, 23) mechanisms. Certain physiological changes are regularly observed in FM populations, including a hyperexcitable central nervous system via high levels of glutamate, as well as additional dysregulated monoamine neurotransmitter expression, particularly of serotonin and dopamine (23). Still, this limited understanding has resulted in relatively few effective treatment options. First line medical intervention aims to address these molecular changes and commonly includes off-label anti-depressants [e.g., Tricyclic Antidepressants (TCAs), Selective Serotonin Reuptake Inhibitors (SSRIs), and Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs)], anticonvulsants (e.g., gabapentin, pregabalin), and opioids (e.g., tramadol) (23, 24), while non-pharmacological options involve physiotherapy, pain-management programmes, and cognitive behavioural therapies (24). While these treatment options have shown results in other specific conditions, their efficacy in FM populations is thought to be moderate at best (25–28). Importantly, even if patients report a decrease in pain, their comorbid mental health symptoms are often left insufficiently addressed (25). Neglecting this essential aspect of the chronic pain experience is especially problematic due to the known bi-directional relationship between pain perception and mental health (2, 29, 30). FM is a highly disabling and growing problem worldwide. The lack of adequate treatment is especially problematic for chronic conditions such as FM where quality of life is severely impacted, thus, novel, and integrative treatment options are urgently needed.
The past decade has witnessed a “renaissance” in clinical psychedelic research. Classic psychedelic drugs include LSD (lysergic acid diethylamide), psilocybin (the active compound in “magic mushrooms”), and DMT (dimethyltryptamine, the active compound in the ayahuasca). A growing body of data suggests the safety (31) and efficacy of psychedelics in clinical populations including depression (32–34), addiction (35, 36), obsessive compulsive disorder (37), and end-of-life distress (38–41), as well as in healthy populations (42, 43).
Classic psychedelics act primarily through agonism of excitatory serotonin 2A receptors, though 5-HT 1A, 1C, and 2C receptors agonism is also observed, as well as indirect dopaminergic action (31). Activation of serotonin 2A receptors appears to dysregulate population-level spontaneous neural oscillations (44–46) which may subsequently account for increases in markers of anatomical neuroplasticity (47–51). Increased plasticity via serotonin 2A agonist psychedelics may increase an individual’s sensitivity to extra pharmacological contextual factors known to guide therapeutic outcomes via psychotherapy (52).
In a therapeutic context, as seen in psychedelic-assisted therapy (PAT), it is theorised that psychedelics could open a window of plasticity that might facilitate the reappraisal of deeply entrenched, maladaptive thought patterns towards therapeutically useful outcomes (52–54). Such increases in psychological flexibility make FM is a particularly attractive target for investigation due to its hallmark psychological rigidity (55) and significant cross-over with depression which is potentially positively impacted by PAT (32, 33). Antidepressant action may also be supported by the abovementioned modulation of monoamine neurotransmitters (31).
While psychedelics have not yet been investigated in a FM context, historical studies suggest potential action in chronic pain conditions including cancer pain (56–60) and phantom limb pain (61–63). Interest has recently re-emerged, with modern work suggesting psychedelics’ anti-inflammatory action (63–65) and potential efficacy in treating headache disorders (66–68), phantom limb pain (69), and acute pain (70). Indeed, proposed, and ongoing studies investigating the effects of classic psychedelics in phantom limb pain (71), lower back pain (72), and fibromyalgia (73) are likely to advance our understanding in this area.
A growing body of ‘grey’ literature of case reports and/or protocols pertaining to psychedelic-use has emerged online on forums such as Reddit, Bluelight, and Erowid. A significant portion of these relates to self-medication for chronic pain (74). Specific, crowd-sourced protocols have emerged as a result; organisations such as Clusterbusters boast over 10,000 members who follow published guidance for psychedelic self-medication for cluster headaches (75). Such anecdotal reports may inspire hypotheses for researchers designing research studies and clinical trials. Indeed, Schindler et al. formally investigated the viability of psychedelics on cluster headaches following the Clusterbusters protocol (68). Accordingly, we sought to learn from the lived experience of people who have self-medicated with classical psychedelics for chronic pain to aid in the design of the present study (see Methods section).
This paper presents the protocol for a mechanistic study investigating the effects of PAT in a fibromyalgia population. The principal focus of this trial is the central mechanism(s) of psilocybin i.e., in the brain and on associated mental schemata, primarily captured by electroencephalography (EEG) recordings of the acute psychedelic state. Secondary outcomes will aim to capture broad aspects of the pain experience and related features through Magnetic Resonance Imaging (MRI), self-report measures, behavioural paradigms, and qualitative interviews. This study aims to serve as a preliminary investigation into potential mechanisms of psilocybin in this study population. By publishing our protocol before commencing data collection, we aim to contribute towards a scientific culture of openness and rigor.
Here we describe a single arm, fixed sequence, single-blind, within-subjects study. Our primary outcomes investigate psychological flexibility through potential neurophysiological markers (specifically Lempel-Ziv complexity (LZc) recorded acutely using EEG, and the (Brief Experiential Avoidance Questionnaire (BEAQ) measured at baseline and primary endpoint). Secondary outcomes will provide a context to complement these mechanistic EEG data. Pain symptomatology will be measured using the Brief Pain Inventory Interference Subscale (BPI-IS) and broader aspects of health, including physical and mental health functioning will be measured using the 36-Item Short Form Health Survey (SF-36). We will additionally collect self-reported data on the strength of personally held negative beliefs in line with the relaxed beliefs under psychedelics model (55).
Further neurophysiological investigations will include functional magnetic resonance imaging (fMRI and DTI) (changes from baseline to primary endpoint), and EEG (changes from baseline to in putative resting state and ERP plasticity markers. We believe that a breakthrough on the brain mechanisms involved in therapeutically relevant change process catalysed by psilocybin, will have broad and important scientific and clinical implications.
Patient and public involvement (PPI) was used throughout the process of trial preparation and contributed to protocol development. PPI is an emerging research method across mental health and within psychedelic research (76, 77). The method aims to produce research “with” rather than “for” people with lived experience. Such co-created research fosters empowerment and trust in research produces relevant and transparent outputs (78).
PPI informed our therapeutic protocols, inspired further research questions, and resulted in the development of novel measures investigating the somatic elements of the psychedelic experience [see Bornemann et al. (79)]. Further, our panel of patient contributors has provided input to, and approved all patient facing documents.
We will recruit up to twenty participants with fibromyalgia as defined by the American Rheumatological Society 2016 diagnostic criteria (80). Study completion is set as completion of the final study visit (primary endpoint). Full entry criteria are outlined in Table 1.
Recruitment will take place via flyers, word-of-mouth, and from a pool of self-referrals submitted via a secure centralised e-mail address using a standardised referral form. Participant information sheets will be openly accessible on our study website (81). Primary care providers will be contacted to confirm eligibility.
Participants will attend eight study visits (screening, two preparation sessions, two dosing sessions, and three follow-up sessions) over the eight-week study period (see Figure 1). Participants will attend two dosing sessions and receive psilocybin at least once (up to 25mg). Dosing sessions are separated by four weeks. Long-term follow-ups will be collected for six-months post study completion, with two remote in-depth check-ins at three and six months. Researchers interested in the specific dosing protocol are welcome to contact the communicating authors for additional information.
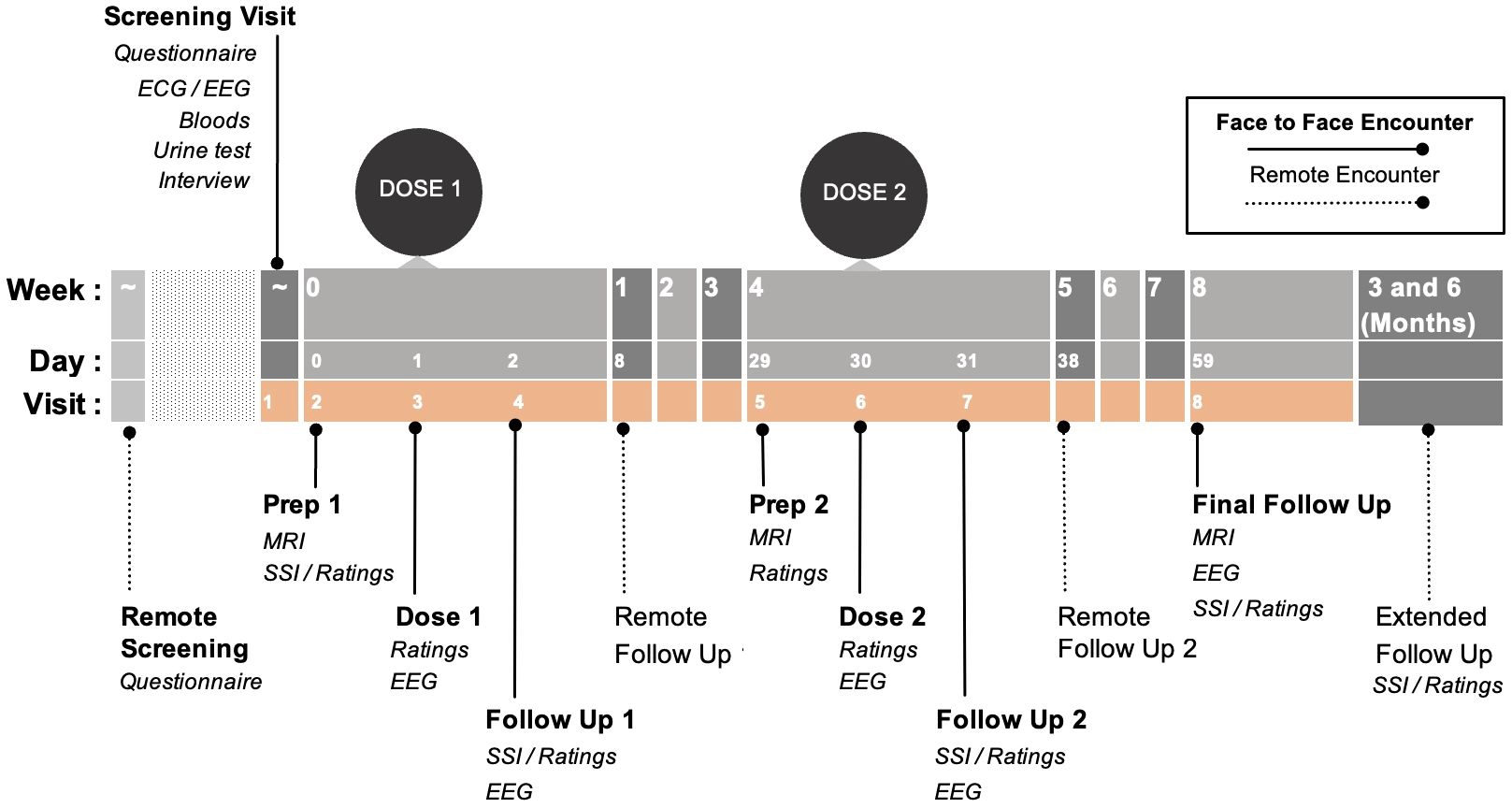
Figure 1 Study timeline: Pink background represents the active study period. Dotted lines represent remote encounters. Please note: this dosing figure is intentionally left blinded. Researchers interested in the specific dosing protocol are welcome to contact the communicating authors for additional information. ECG: Electrocardiogram; EEG: Electroencephalogram; MRI: Magnetic Resonance Imaging; SSI: Semi-structured Interview.
Eligible self-referrals will be invited to a remote screening call where informed consent to begin the screening interviews will be collected. Remote screening calls serve to provide information to participation and determine initial eligibility.
If the participant passes initial eligibility at the remote screening, they are invited to a screening visit. Following a thorough explanation of the study and screening process, patients will have the opportunity to ask questions before providing full written informed consent to participate in the study. They will then have a physical exam, psychiatric assessment including MINI, electrocardiogram (ECG), urine samples and blood sample for routine blood testing. Baseline EEG resting state measures will be collected. This will also serve as an EEG tolerability test.
Information regarding the screening/enrolment process including retention and demographics will be published upon study completion.
This study investigates the effects of PAT. While no single unifying PAT protocol exists, common principles are followed across research and will be employed here. Study visits will take place in a comfortable, low-lit environment (82). As comfort allows, patients will be in semi-reclined positions throughout. Music is known to powerfully affect PAT (83); as such, playlists were carefully co-created with music therapists.
As is standard across psychedelic studies, the therapeutic approach is largely self-directed (84) and is informed by humanistic approaches (85). Of particular interest in this context is Acceptance and Commitment Therapy (ACT), a widely used modality in pain-management (28, 86, 87), and an approach increasingly explored for PAT protocols (88–90). We have integrated various aspects of established pain management practices to accommodate the complex needs of our population. Two “guides” will support each participant for the duration of the trial. Guides will prepare the patient for the experience in remote and in-person preparation sessions. Session objectives will be standardised and include areas such as psychoeducation, trust building, and intention setting. The therapeutic manual for this study will be published once data collection is complete. Patients will also have an MRI scan at each preparation visit with visits lasting approximately 4 hours.
Dosing days will occur the day after preparation sessions. Patients will spend ~8 hours at the research facility, with the drug effect lasting for approximately 4-6 hours. Patients will be wearing eye masks and headphones, as comfort allows. EEGs will be recorded before, and during the acute drug state. An on-site medic will ensure participant safety throughout the day and will approve patients before their discharge at the end of the day. Local participants have the option of spending the night at home if accompanied by a trusted person; all participants may stay at provided on-site accommodation.
Integration days will occur the morning after the dosing day. Participants will meet with their guides to discuss their experience and complete EEG recordings. The visit lasts ~4 hours. Guides will check in with participants at remote follow-ups 1 week later as well. The final follow-up will run as the other integration sessions but will also include one final MRI. Patient-reported outcome measures will be collected remotely after each study visit, as well as remotely after primary study endpoint.
Participant safety was, and will continue to be, the guiding principle for all study related decisions. Participants will always be chaperoned when under the care of the study team, including medical supervision on dosing days where discharge may only occur once the doctor on site has deemed it safe for the participant to leave. Patient wellbeing will additionally be assessed the next morning, and the following week.
Participant mood and pain scores will be monitored continually from enrolment to the end of the 6-month follow up period. Automatic alerts will immediately alert the study team of any reports of thoughts of self-harm or suicide. These will be escalated to therapy and medical teams and followed up with as appropriate. All adverse events will be reported.
We will collect neurophysiological, self-reported, qualitative, and behavioural data. Please see Tables 2–4 for a summary of all outcome measures. Please refer to Table 5 for a breakdown of primary and secondary outcomes.
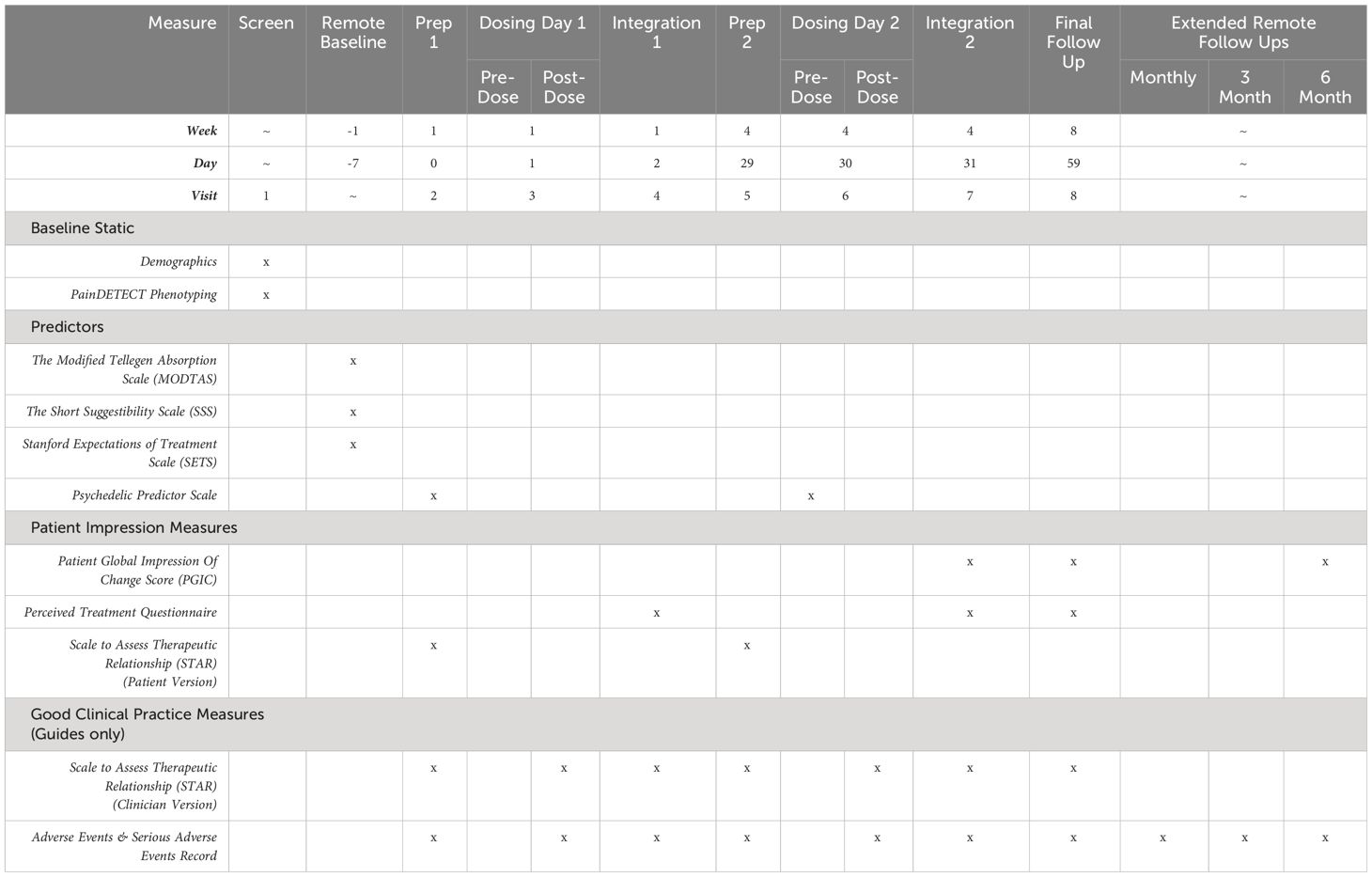
Table 2 Summary of patient-reported predictor, patient impression, and good clinical practice measures.
The present study is conceived as an early-phase mechanistic study, intended to investigate the central action of psychedelics in a population of people living with fibromyalgia. EEG will be recorded at 6 visits: screening, Dose 1, Integration 1, Dose 2, Integration 2, and Final Follow Up (see Table 3).
The primary outcome measure is Lempel-Ziv Complexity (LZc) of the resting state EEG signal during dosing days as detailed above. LZc is a compressibility algorithm used to measure signal diversity and has been previously investigated in psychedelic contexts (91–93). Here we hypothesise an increase in LZc under psilocybin.
In addition to resting state, participants will complete the visual Long-Term Potentiation (vLTP) (94) and roving Mismatch Negativity (rMMN) (95, 96) tasks at integrations to investigate the post-acute neuroplasticity and predictive coding. We also hypothesise reduced alpha power under psilocybin.
We will complement these measures with structural and functional MRI data. We will collect MRI data at 3 timepoints: Prep 1, Prep 2, and Final Follow Up (see Table 3). We will investigate changes in resting-state activity and connectivity, as well as structural changes including diffusion imaging. We hypothesise decreases in brain network modularity after PAT and decreases in diffusivity in prefrontal to subcortical region white-matter tracts, indexed by the diffusion imaging.
While the primary interest of the study is in the neural mechanisms involved in psychedelic-mediated action in a fibromyalgia population, we will also collect efficacy measures at every timepoint of the study. Patient-reported outcome measures (PROMs) will be collected in-person and remotely (via video calls and the online survey platform “Alchemer”). All baseline measurements will be taken at Prep 1, except for Symptom Severity Score and the Widespread Pain Index which will be collected at the Screening Visit. Baseline EEG and MRI will be collected at screening and Prep 1 visits, respectively. All primary endpoint measurements (including EEG and MRI) will be collected at final follow up (8 weeks after prep 1). Please see Tables 2, 3 for a full list of PROMs.
PROMs are grouped as “Core Pain Outcomes,” “Wellbeing/Mental Health Outcomes,” or “Acute mediators” (see table). We will also be collecting “Patient Impression Measures” to assess the effects of expectancy and blinding efficacy. We will assess the therapeutic relationship using the STAR outcome measure, filled in by both patients and their guides. We hypothesise improvements in Core Pain Outcomes and Wellbeing/Mental Health Outcomes.
We will collect 2 other types of behavioural data investigating interoception and physiology (see Table 4). Changes in interoceptive accuracy will be measured using the Heartrate Discrimination Task (HRD) (97) at Integrations 1 and 2.
Physiological data including heart rate variability (HRV), actigraphy, and sleep staging will be collected using a wearable device throughout the study.
Qualitative data will be collected in the form of semi-structured interviews at Prep 1, Integrations 1 and 2, Final Follow Up, and the 6-month Remote follow up (see Table 4). The aims of these interviews are to assess how they lived experience of fibromyalgia change after psychedelic-assisted therapy, as well as its potential therapeutic mechanisms. Patient reports often capture subtle yet powerful changes in personal narratives before and after psychedelic therapy that quantitative data are not able to record.
Our two primary, mechanistic hypotheses relate to EEG and MRI:
1. H1 (EEG): We hypothesise an increase in signal complexity (LZc) at peak drug effects under psilocybin.
2. H2 (fMRI): we hypothesise a decrease in brain network modularity after PAT.
Secondary outcomes include:
1. Reduced alpha power under psilocybin.
2. A relationship between acute increases in LZc and post-PAT changes in psychological flexibility, measured by the BEAQ.
3. Changes in mass univariate functional connectivity after PAT.
4. Changes in PFC-tract diffusivity after PAT.
5. Changes in EEG ERP related markers of functional plasticity after PAT.
6. Changes in pain symptomatology after PAT, measured by the BPI-IS.
All secondary outcomes will be exploratory (see Table 5). Sub-acute EEG analyses will follow previously outlined protocols (91–93). With an assumption of high co-linearity between core outcomes we would explore data reduction approaches (e.g., factor or principal component analyses or canonical correlation analysis) to investigate key contrasts. Two tailed tests will be performed if findings are not aligned with prior hypotheses. Due to prior hypotheses (above) on directionality, one tailed t-tests will be appropriate to perform for H1 & H2. Multiple comparisons corrections and Bayesian analyses will be performed where deemed appropriate. Please see Table 5 for timepoints for each analysis.
A Schedule 1 licence for possession and storage of psilocybin has been obtained from the UK Home Office. Psilocybin supplied by Usona Insitute. Manufacture and encapsulation will be performed by Lonza Pharma and Biotech. Good Manufacturing Practise (GMP) will be maintained at all stages of manufacture. The IMP will be stored in a secure safe at Imperial College London, Hammersmith Campus.
Data will be managed as per the Imperial College Data Management Standard Operating Procedures and a study- specific data management plan. All data collection and management softwares have been meet GDPR standards and have been approved by Imperial College London.
The results of this study will be published in academic journals and presented in both the academic and public domain, including at scientific conferences and in the media in public engagement forums. Patient confidentiality will be maintained in all the above. All publications and presentations relating to the study will be overseen by the P.I and C.I. Authorship of parallel studies initiated outside of the Study Co-ordination Team will be according to the individuals involved in the project but must acknowledge the contribution of the Study Coordination Team.
This study has received a favourable opinion from the London Central Research Ethics Committee and is sponsored by Imperial College London’s Research Governance and Integrity Team. All participants will provide their written informed consent to be screened and, if relevant, participate in this study. The Medicines and Healthcare products Regulatory Agency (MHRA) has confirmed its status as a non-clinical trial and waived the need for MHRA approval. The study has been reviewed and approved by the Health Research Authority (HRA). The study protocol has undergone external peer review and was co-developed with patient advisors. All staff have undergone Good Clinical Practice (GCP) training. The study has been adopted by the National Institute of Health Research (NIHR) Clinical Research Network (CRN) and has been registered on clinicaltrials.gov (NCT05548075). All study sessions will take place at the NIHR-funded Imperial College Research Facility (ICRF) and Imperial Clinical Imaging Facility (CIF).
By publishing the study protocol, we aim to improve methodological transparency, rigour, and accountability and subsequently contribute towards more impactful outcomes. We also aim to highlight the importance of patient involvement in protocol design in generating relevant and equitable research. This study will investigate effect of psilocybin on the neural mechanisms in a fibromyalgia population. The results will provide the first EEG recordings of the acute psychedelic state in a clinical population. Further, they may inform the viability of psilocybin as a potential treatment option and help shape subsequent clinical trials.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
RCH: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing, Funding acquisition. JC: Conceptualization, Methodology, Project administration. Investigation, Writing – review & editing. JB: Conceptualization, Methodology, Project administration, Investigation, Writing – original draft, Writing – review & editing. DJN: Conceptualization, Supervision, Project administration, Writing – review & editing. KA: Conceptualization, Project administration, Investigation, Writing – review & editing. DE: Project administration, Investigation, Writing – review & editing. KG: Investigation, Writing – review & editing. LM: Investigation, Writing – review & editing. TB: Investigation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is primarily funded by the Imperial College London’s Centre for Psychedelic Research. Psilocybin was supplied by Usona Institute.
The authors would like to acknowledge the input from our patient advisors and PPI contributors and steering committee, Yossi Burland and Amy McLachlan, for their time and honesty. We would like to acknowledge Brigitte Brandner, Mick Thacker, Lance McCracken, Tim Read for their guidance; Kenneth Jønck and Nicolai Lassen for creation of the online platform Psychedelic Survey; Albert Busza, Pedro Rente, Matt Wall, Rich Daws, Leevi Kerkela, and Manesh Girn for their advice on MRI sequencing; Fernando Rosas and Jan Vollert for advice of statistical planning; Rachael Sumner, Meg Spriggs, Nicolas Legrand, and Micah Allen for the development of our EEG tasks; and Brian d’Souza and the OpenEar team for developing the music playlist. This paper presents independent research funded by the Centre for Psychedelic Research and supported by the NIHR CRF at Imperial College London Healthcare NHS Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vos T, Afshin A, Aiyar S, Alam T, Allen C, Bannick MS, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London England). (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2.
2. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
3. Duenas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res Vol. (2016) 9:457–67. doi: 10.2147/JPR
4. Verbunt JA, Pernot DH, Smeets RJ. Disability and quality of life in patients with fibromyalgia. Health Qual Life Outcomes. (2008) 6:8. doi: 10.1186/1477-7525-6-8
6. Lee J-W, Lee K-E, Park D-J, Kim S-H, Nah S-S, Lee JH, et al. Determinants of quality of life in patients with fibromyalgia: A structural equation modelling approach. PloS One. (2017) 12:e0171186. doi: 10.1371/journal.pone.0171186
7. Choy E, Perrot S, Leon T, Kaplan J, Petersel D, Ginovker A, et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res. (2010) 10. doi: 10.1186/1472-6963-10-102
8. Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. (2013) 17. doi: 10.1007/s11916-013-0356-5
9. Galvez-Sánchez CM, Reyes del Paso GA. Diagnostic criteria for fibromyalgia: critical review and future perspectives. J Clin Med. (2020) 9:1219. doi: 10.3390/jcm9041219
10. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicentre criteria committee. Arthritis rheumatism. (1990) 33:160–72. doi: 10.1002/art.1780330203
11. Bradley LA. Pathophysiology of fibromyalgia. Am J Med. (2009) 122:S22–30. doi: 10.1016/j.amjmed.2009.09.008
12. Aguglia A, Salvi V, Maina G, Rossetto I, Aguglia E. Fibromyalgia syndrome and depressive symptoms: Comorbidity and clinical correlates. J Affect Disord. (2011) 128:262–6. doi: 10.1016/j.jad.2010.07.004
13. Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression. Pain Res Treat. (2012) 2012:1–9. doi: 10.1155/2012/486590
14. Yepez D, Grandes XA, Talanki Manjunatha R, Habib S, Sangaraju SL. Fibromyalgia and depression: A literature review of their shared aspects. Cureus. (2022) 14(5). doi: 10.7759/cureus.24909
15. Yavne Y, Amital D, Watad A, Tiosano S, Amital H. A systematic review of precipitating physical and psychological traumatic events in the development of fibromyalgia. Semin Arthritis Rheumat. (2018) 48:121–33. doi: 10.1016/j.semarthrit.2017.12.011
16. Gündüz N. Psychiatric comorbidity and childhood trauma in fibromyalgia syndrome. Turkish J Phys Med Rehabil. (2018) 64:91–9. doi: 10.5606/tftrd.2018.1470
17. Bayram K, Erol A. Childhood traumatic experiences, anxiety, and depression levels in fibromyalgia and rheumatoid arthritis. Noro Psikiyatri Arsivi. (2014) 51:344–9. doi: 10.5152/npa.
18. Arout CA, Sofuoglu M, Bastian LA, Rosenheck RA. Gender differences in the prevalence of fibromyalgia and in concomitant medical and psychiatric disorders: A national veterans health administration study. J Women’s Health. (2018) 27:1035–44. doi: 10.1089/jwh.2017.6622
19. Jahan F, Nanji K, Qidwai W, Qasim R. Fibromyalgia syndrome: an overview of pathophysiology, diagnosis and management. Oman Med J. (2012) 27:192–5. doi: 10.5001/omj.2012.44
20. Ryabkova VA, Churilov LP, Shoenfeld Y. Neuroimmunology: what role for autoimmunity, neuroinflammation, and small fiber neuropathy in fibromyalgia, chronic fatigue syndrome, and adverse events after human papillomavirus vaccination? Int J Mol Sci. (2019) 20:5164. doi: 10.3390/ijms20205164
21. Knaster P, Karlsson H, Estlander A-M, Kalso E. Psychiatric disorders as assessed with SCID in chronic pain patients: the anxiety disorders precede the onset of pain. Gen Hosp Psychiatry. (2012) 34:46–52. doi: 10.1016/j.genhosppsych.2011.09.004
22. Bhargava J, Hurley JA. Fibromyalgia (2020). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK540974/.
23. Siracusa R, Paola RD, Cuzzocrea S, Impellizzeri D. Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. Int J Mol Sci. (2021) 22:3891. doi: 10.3390/ijms22083891
24. Chinn S, Caldwell W, Gritsenko K. Fibromyalgia pathogenesis and treatment options update. Curr Pain Headache Rep. (2016) 20. doi: 10.1007/s11916-016-0556-x
25. Atzeni F, Gerardi MC, Masala IF, Alciati A, Batticciotto A, Sarzi-Puttini P, et al. An update on emerging drugs for fibromyalgia treatment. Expert Opin Emerg Drugs. (2017) 22:357–67. doi: 10.1080/14728214.2017.1418323
26. Arnold LM, Clauw DJ. Challenges of implementing fibromyalgia treatment guidelines in current clinical practice. Postgraduate Med. (2017) 129:709–14. doi: 10.1080/00325481.2017.1336417
27. Kwiatek R. Treatment of fibromyalgia. Aust Prescriber. (2017) 40:179–83. doi: 10.18773/austprescr.2017.056
28. Hughes LS, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and commitment therapy (ACT) for chronic pain. Clin J Pain. (2017) 33:552–68. doi: 10.1097/AJP.0000000000000425
29. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural plasticity. (2017) 2017:9724371. doi: 10.1155/2017/9724371
30. Chang M-H, Hsu J-W, Huang K-L, Su T-P, Bai Y-M, Li C-T, et al. Bidirectional association between depression and fibromyalgia syndrome: A nationwide longitudinal study. J Pain. (2015) 16:895–902. doi: 10.1016/j.jpain.2015.06.004
32. Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. Trial of psilocybin versus escitalopram for depression. New Engl J Med. (2021) 384:1402–11. doi: 10.1056/NEJMoa2032994
33. Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. New Engl J Med. (2022) 387:1637–48.
34. Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. psychol Med. (2018) 49:655–63. doi: 10.1017/S0033291718001356
35. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. (2016) 43:55–60. doi: 10.3109/00952990.2016.1170135
36. Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PCR, Strassman RJ, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol (Oxford England). (2015) 29:289–99. doi: 10.1177/0269881114565144
37. Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. (2006) 67:1735–40. doi: 10.4088/JCP.v67n1110
38. Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. (2011) 68:71. doi: 10.1001/archgenpsychiatry.2010.116
39. Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. (2014) 202:513–20. doi: 10.1097/NMD.0000000000000113
40. Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol. (2016) 30:1181–97. doi: 10.1177/0269881116675513
41. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. (2016) 30:1165–80. doi: 10.1177/0269881116675512
42. Rucker JJ, Marwood L, Ajantaival R-LJ, Bird C, Eriksson H, Harrison J, et al. The effects of psilocybin on cognitive and emotional functions in healthy participants: Results from a phase 1, randomised, placebo-controlled trial involving simultaneous psilocybin administration and preparation. J Psychopharmacol. (2022) 36:026988112110647. doi: 10.1177/02698811211064720
43. Griffiths R, Richards W, Johnson M, McCann U, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. (2008) 22:621–32. doi: 10.1177/0269881108094300
44. Barrett FS, Krimmel SR, Griffiths R, Seminowicz DA, Mathur BN. Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. NeuroImage. (2020) 218:116980. doi: 10.1016/j.neuroimage.2020.116980
45. Preller KH, Razi A, Zeidman P, Stämpfli P, Friston KJ, Vollenweider FX, et al. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc Natl Acad Sci. (2019) 116:2743–8. doi: 10.1073/pnas.1815129116
46. Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. (2022) 28:844–51. doi: 10.1038/s41591-022-01744-z
47. Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. (2014) 8. doi: 10.3389/fnhum.2014.00020
48. Lukasiewicz K, Baker JJ, Zuo Y, Lu J. Serotonergic psychedelics in neural plasticity. Front Mol Neurosci. (2021) 14:748359. doi: 10.3389/fnmol.2021.748359
49. Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. (2018) 23:3170–82. doi: 10.1016/j.celrep.2018.05.022
50. Olson DE. Biochemical mechanisms underlying psychedelic-induced neuroplasticity. Biochemistry. (2022) 61:127–36. doi: 10.1021/acs.biochem.1c00812
51. de Vos CMH, Mason NL, Kuypers KPC. Psychedelics and neuroplasticity: A systematic review unravelling the biological underpinnings of psychedelics. Front Psychiatry. (2021) 12. doi: 10.3389/fpsyt.2021.724606
52. Carhart-Harris RL, Chandaria S, Erritzoe DE, Gazzaley A, Girn M, Kettner H, et al. Canalization and plasticity in psychopathology. Neuropharmacology. (2023) 226:109398. doi: 10.1016/j.neuropharm.2022.109398
53. Carhart-Harris RL, Friston KJ. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev. (2019) 71:316–44. doi: 10.1124/pr.118.017160
54. Calder AE, Hasler G. Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology. (2022) 48:104–12. doi: 10.1038/s41386-022-01389-z
55. Aguilera M, Paz C, Compañ V, Medina JC, Feixas G. Cognitive rigidity in patients with depression and fibromyalgia. Int J Clin Health Psychol. (2019) 19:160–4. doi: 10.1016/j.ijchp.2019.02.002
56. Kast EC, Collins VJ. STUDY OF LYSERGIC ACID DIETHYLAMIDE AS AN ANALGESIC AGENT. Anesth Analges. (1964) 43:285–91. doi: 10.1213/00000539-196405000-00013
58. Kast E. Attenuation of anticipation: A therapeutic use of lysergic acid diethylamide. Psychiatr Q. (1967) 41:646–57. doi: 10.1007/BF01575629
59. Pahnke WN, Kurland AA, Goodman LE, Richards WA. LSD-assisted psychotherapy with terminal cancer patients. Curr Psychiatr Therapies. (1969) 9:144–52.
60. Grof S, Goodman LE, Richards WA, Kurland AA. LSD-assisted psychotherapy in patients with terminal cancer. Int Pharmacopsychiat. (1973) 8:129–44. doi: 10.1159/000467984
61. Kuromaru S, Okada S, Hanada M, Kasahara Y, Sakamoto K. The effect of LSD on the phantom limb phenomenon. Journal-Lancet. (1967) 87:22–7.
63. Flanagan TW, Nichols CD. Psychedelics as anti-inflammatory agents. Int Rev Psychiatry. (2018) 30:363–75. doi: 10.1080/09540261.2018.1481827
64. Nardai S, László M, Szabó A, Alpár A, Hanics J, Zahola P, et al. N,N-dimethyltryptamine reduces infarct size and improves functional recovery following transient focal brain ischemia in rats. Exp Neurol. (2020) 327:113245. doi: 10.1016/j.expneurol.2020.113245
65. Szabo A. Psychedelics and immunomodulation: novel approaches and therapeutic opportunities. Front Immunol. (2015) 6. doi: 10.3389/fimmu.2015.00358
66. Sewell RA, Halpern JH, Pope HG. Response of cluster headache to psilocybin and LSD. Neurology. (2006) 66:1920–2. doi: 10.1212/01.wnl.0000219761.05466.43
67. Karst M, Halpern JH, Bernateck M, Passie T. The non-hallucinogen 2-bromo-lysergic acid diethylamide as preventative treatment for cluster headache: An open, non-randomized case series. Cephalalgia. (2010) 30:1140–4. doi: 10.1177/0333102410363490
68. Schindler EAD, Gottschalk CH, Weil MJ, Shapiro RE, Wright DA, Sewell RA, et al. Indoleamine hallucinogens in cluster headache: results of the clusterbusters medication use survey. J Psychoactive Drugs. (2015) 47:372–81. doi: 10.1080/02791072.2015.1107664
69. Ramachandran V, Chunharas C, Marcus Z, Furnish T, Lin A. Relief from intractable phantom pain by combining psilocybin and mirror visual-feedback (MVF). Neurocase. (2018) 24:105–10. doi: 10.1080/13554794.2018.1468469
70. Ramaekers JG, Hutten N, Mason NL, Dolder P, Theunissen EL, Holze F, et al. A low dose of lysergic acid diethylamide decreases pain perception in healthy volunteers. J Psychopharmacol. (2020) 35(4):398–05. doi: 10.1177/0269881120940937
71. Zeidan F. Behavioural and neural mechanisms supporting psilocybin-assisted therapy for phantom limb pain. University of California, San Diego: ClinicalTrials.gov (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05224336?term=psilocybin&cond=Chronic+Pain&draw=2.
72. Woolley J. A double-blind, randomized trial examining the preliminary efficacy of psilocybin therapy for people with chronic low back pain (2022). Available online at: https://clinicaltrials.gov/ct2/show/NCT05351541?term=psilocybin&cond=lower+back+pain&draw=2&rank=1.
73. Hendricks P. Psilocybin-facilitated treatment for chronic pain. University of Alabama at Birmingham: ClinicalTrials.gov (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05068791?term=psilocybin&cond=Chronic+Pain&draw=2.
74. Lyes M, Yang KH, Castellanos J, Furnish T. Microdosing psilocybin for chronic pain: a case series. Pain. (2022) 164:698–702. doi: 10.1097/j.pain.0000000000002778
75. Busting protocol — The dosing method. In: Clusterbusters. Available at: https://clusterbusters.org/resource/the-dosing-method/.
76. Close JB, Bornemann J, Piggin M, Jayacodi S, Luan LX, Carhart-Harris R, et al. A strategy for patient and public involvement in psychedelic research. Psychiatry. (2021) . 12:1696 10. doi: 10.3389/fpsyt.2021.727496
77. Spriggs MJ, Douglass HM, Park RJ, Read T, Danby JL, de Magalhães FJC, et al. Study protocol for ‘Psilocybin as a treatment for anorexia nervosa: A pilot study’. Front Psychiatry. (2021) 12:735523. doi: 10.3389/fpsyt.2021.735523
78. Troya MI, Bartlam B, Chew-Graham C. Involving the public in health research in Latin America: making the case for mental health. Rev Panam Salud Públ. (2018) 42:1–6. doi: 10.26633/RPSP.2018.45
79. Bornemann J, Close JB, Spriggs MJ, Carhart-Harris R, Roseman L. Self-medication for chronic pain using classic psychedelics: A qualitative investigation to inform future research. Front Psychiatry. (2021) 12. doi: 10.3389/fpsyt.2021.735427
80. Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Katz RS, Mease P, et al. The american college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. (2010) 62:600–10. doi: 10.1002/acr.20140
81. PsiloPain. Imperial college london . Available online at: www.imperial.ac.uk/psychedelic-research-centre/participate-in-a-trial/chronic-pain-study/.
82. Grob CS, Bossis AP, Griffiths RR. Use of the classic hallucinogen psilocybin for treatment of existential distress associated with cancer. psychol Aspects Cancer. (2012), 291–308. doi: 10.1007/978-1-4614-4866-2_17
83. Kaelen M, Giribaldi B, Raine J, Evans L, Timmerman C, Rodriguez N, et al. The hidden therapist: evidence for a central role of music in psychedelic therapy. Psychopharmacology. (2018) 235:505–19. doi: 10.1007/s00213-017-4820-5
84. Schenberg EE. Psychedelic-assisted psychotherapy: A paradigm shift in psychiatric research and development. Front Pharmacol. (2018) 9. doi: 10.3389/fphar.2018.00733
85. Phelps J. Developing guidelines and competencies for the training of psychedelic therapists. J Humanistic Psychol. (2017) 57:450–87. doi: 10.1177/0022167817711304
86. McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: Model, process, and progress. Am Psychol. (2014) 69:178–87. doi: 10.1037/a0035623
87. NICE. Overview | Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain | Guidance | NICE (2021). Available online at: www.nice.org.ukhttps://www.nice.org.uk/guidance/NG193.
88. Luoma JB, Sabucedo P, Eriksson J, Gates N, Pilecki BC. Toward a contextual psychedelic-assisted therapy: Perspectives from Acceptance and Commitment Therapy and contextual behavioural science. J Contextual Behav Sci. (2019) 14:136–45. doi: 10.1016/j.jcbs.2019.10.003
89. Watts R, Luoma JB. The use of the psychological flexibility model to support psychedelic assisted therapy. J Contextual Behav Sci. (2020) 15:92–102. doi: 10.1016/j.jcbs.2019.12.004
90. Sloshower J, Guss JR, Krause R, Wallace RM. Psilocybin-assisted therapy of major depressive disorder using Acceptance and Commitment Therapy as a therapeutic frame. J Contextual Behav Sci. (2020) 15:12–9. doi: 10.1016/j.jcbs.2019.11.002
91. Scott G, Carhart-Harris RL. Psychedelics as a treatment for disorders of consciousness. Neurosci Consciousness. (2019) 2019. doi: 10.1093/nc/niz003
92. Mediano PAM, Rosas FE, Timmermann C, Roseman L, Nutt DJ, Feilding A, et al. Effects of external stimulation on psychedelic state neurodynamics. bioRXiv. (2020). doi: 10.1101/2020.11.01.356071
93. Timmermann C, Roseman L, Schartner M, Milliere R, Williams LTJ, Erritzoe D, et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep. (2019) 9:1–13. doi: 10.1038/s41598-019-51974-4
94. Sumner RL, McMillan R, Spriggs MJ, Campbell D, Malpas G, Maxwell E, et al. Ketamine improves short-term plasticity in depression by enhancing sensitivity to prediction errors. Eur Neuropsychopharmacol. (2020) 38:73–85. doi: 10.1016/j.euroneuro.2020.07.009
95. Spriggs MJ, Sumner RL, McMillan RL, Moran RJ, Kirk IJ, Muthukumaraswamy SD. Indexing sensory plasticity: Evidence for distinct Predictive Coding and Hebbian learning mechanisms in the cerebral cortex. NeuroImage. (2018) 176:290–300. doi: 10.1016/j.neuroimage.2018.04.060
96. Sumner RL, Spriggs MJ, Muthukumaraswamy SD, Kirk IJ. The role of Hebbian learning in human perception: a methodological and theoretical review of the human Visual Long-Term Potentiation paradigm. Neurosci Biobehavioural Rev. (2020) 115:220–37. doi: 10.1016/j.neubiorev.2020.03.013
Keywords: psilocybin, psychedelic therapy, chronic pain, fibromyalgia, EEG
Citation: Bornemann J, Close JB, Ahmad K, Barba T, Godfrey K, Macdonald L, Erritzoe D, Nutt D and Carhart-Harris R (2024) Study protocol for “Psilocybin in patients with fibromyalgia: brain biomarkers of action”. Front. Psychiatry 15:1320780. doi: 10.3389/fpsyt.2024.1320780
Received: 12 October 2023; Accepted: 04 March 2024;
Published: 04 June 2024.
Edited by:
Leehe Peled-Avron, Bar-Ilan University, IsraelReviewed by:
Emeline Maillet, New York University, United StatesCopyright © 2024 Bornemann, Close, Ahmad, Barba, Godfrey, Macdonald, Erritzoe, Nutt and Carhart-Harris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia Bornemann, ai5ib3JuZW1hbm4xOUBpbXBlcmlhbC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.