- 1Lev Hasharon Mental Health Center, Tzur Moshe, Israel
- 2Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 3Abarbanel Mental Health Center, Bat Yam, Israel
Introduction: Chloral hydrate (CH), a medication dating back to 1832, is tranquilizer and sleep promoter still used today. It remains an option for short-term insomnia therapy and sedation before medical procedures, despite its controversial safety profile.
Methods: This study investigated the potential benefits of chloral hydrate addition for increasing sleep duration and reducing agitation and violence in inpatients with treatment-resistant schizophrenia (TRS). A retrospective, observational case series design was utilized, analyzing data from fourteen patients diagnosed with TRS disorders.
Results: CH addition increased the rate of full night sleep and decreased the rates of agitation and verbal and physical violence events. Notably, no adverse events including falls were reported during CH addition.
Discussion: CH shows some short-term benefits in improving sleep disorders and reducing violent and agitated behavior in patients with TRS. Our study has limitations due to its small sample size, retrospective design and lack of a control group. A large-scale, double-blind, randomized trial is needed to further explore the efficacy and safety of CH in psychiatric populations with TRS accompanied by agitation, violence and disturbed sleep.
Introduction
Chloral hydrate (CH) is a medication developed in 1832 by Justus von Liebig (1). Its main use is as sedative and hypnotic medication. Early in the 20th century, evidence showed that regular CH use could result in addiction (2, 3). Consequently, benzodiazepines were introduced as a replacement for CH (4).
CH is readily absorbed by the digestive system and rapidly metabolized by the liver into the metabolite trichloroethanol, which has a duration of action lasting eight to eleven hours in adults. Through its interactions with GABA-A and AMPA receptors, this drug acts as an antagonist to ion channels and calcium flow, ultimately suppressing the central nervous system (5).
The safety of CH is considered relatively high when used in the correct dosage, making it suitable for use in children as a pre-medication drug before medical procedures such as echocardiograph (6). CH is also used for management of chronic insomnia in elderly persons with a recommended dosage for sleep disorders of 500-1000mg (7, 8).
There are currently no FDA-approved drug products that contain CH in the USA, however CH is an approved product currently in use in Australia, Canada, Hong Kong and the UK. CH administration carries the risk of respirational depression and may also result in prolonged sedation, ventricular dysrhythmias, severe hypotension and irritating gastric effects (9, 10). The main side effects are digestive, cardiologic (risk of rhythm disorder), dermatologic, neuropsychiatric (withdrawn, delusions, hallucination, dependence) and ophthalmologic. Death could occur after absorption of overdoses of around 10 g of chloral hydrate (5).
According to the product monograph, reviewed by the Odan Laboratories in 2022, CH can lead to abuse, misuse, addiction, physical dependence and withdrawal reaction (11). Nowadays, CH is prescribed as a hypnotic drug with very limited use in psychiatry, mainly due to the widespread availability of benzodiazepines, which have undergone extensive research and have a better safety profile. CH 500mg has been found effective in inducing and maintaining sleep in a small sample of elderly inpatients with mild dementia (12). The authors were unable to find any studies on the efficacy of CH for inducing sleep, in patients with treatment resistant schizophrenia (TRS). The present study investigated the short-term (10 days) efficacy and safety of CH in patients with TRS.
Methods
Participants
This retrospective observational case series study was conducted in a regional mental health center in Israel between October 1st, 2021 and March 31st, 2022. The study protocol was approved by the local Institutional Review Board.
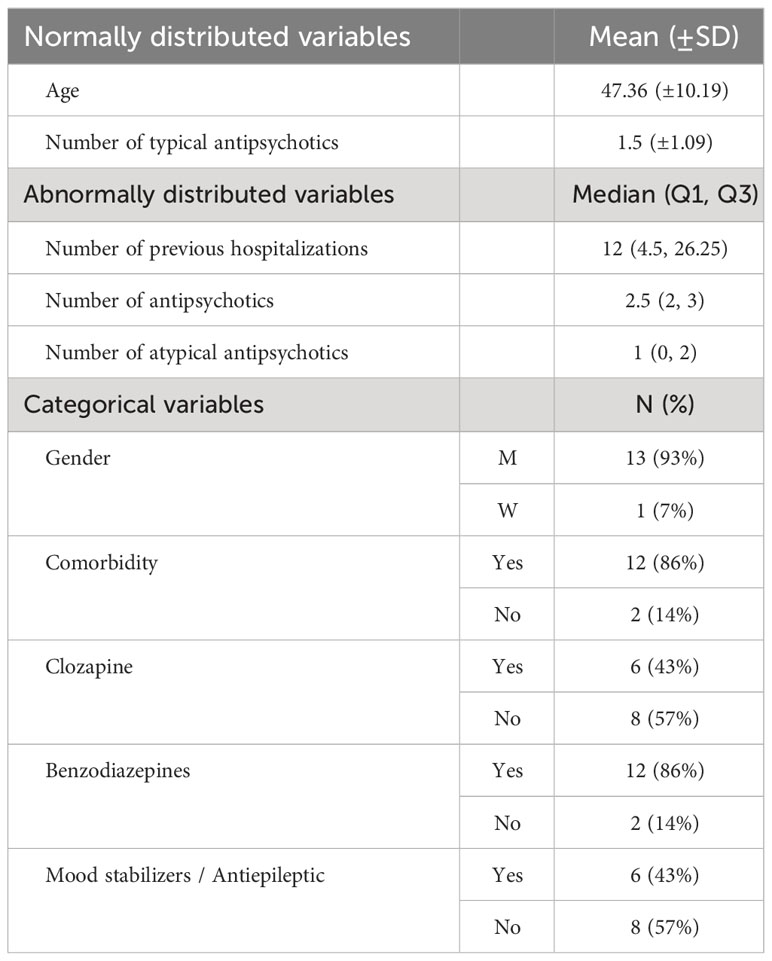
Data were extracted from the medical records of 14 inpatients (13 men and 1 woman) aged 32-60 years (µ 47.36, SD ± 10.19). The study population consisted of chronic inpatients diagnosed with TRS, hospitalized in a closed ward for at least six months. The patients received 1000 mg. CH oral solution, once a day, 30 minutes before bedtime, as recommended in the Chloral Hydrate Summary Report (13). The reason for CH add-on on those specific difficult patients was the need to find a solution for their sleep difficulties.
Measures
Demographic and clinical data were extracted from the medical records for the day CH was started and included age, gender, main psychiatric diagnosis, comorbidities, previous hospitalizations and medications such as antipsychotics, mood stabilizers, and benzodiazepines.
The extracted clinical data were for the ten days prior to the CH treatment initiation and the ten days following its commencement. These data included: number of nights with uninterrupted sleep, of instances of isolation or restriction, of agitation events, of violence (verbal and physical), of reported adverse events (such as headaches or changes in blood pressure), of falls and of referrals to a general hospital.
Statistical analyses
Due to the abnormal distribution of the data, Related-Samples Wilcoxon Signed Rank Tests were utilized for the analysis. All results are expressed as mean ± SD, median (Q1, Q3), or ratios (%) as appropriate. Statistical analyses were performed using SPSS ver. 28.0 (SPSS Inc., Armonk, NY). Statistical significance was set at p <0.05.
Results
Table 1 displays the participants’ demographic and clinical data.
During the initial ten days of CH treatment, a statistically significant increase in the rate of nights with uninterrupted sleep was observed compared to the ten days before treatment.
Verbal and physical violence showed a significant decrease (Table 2).
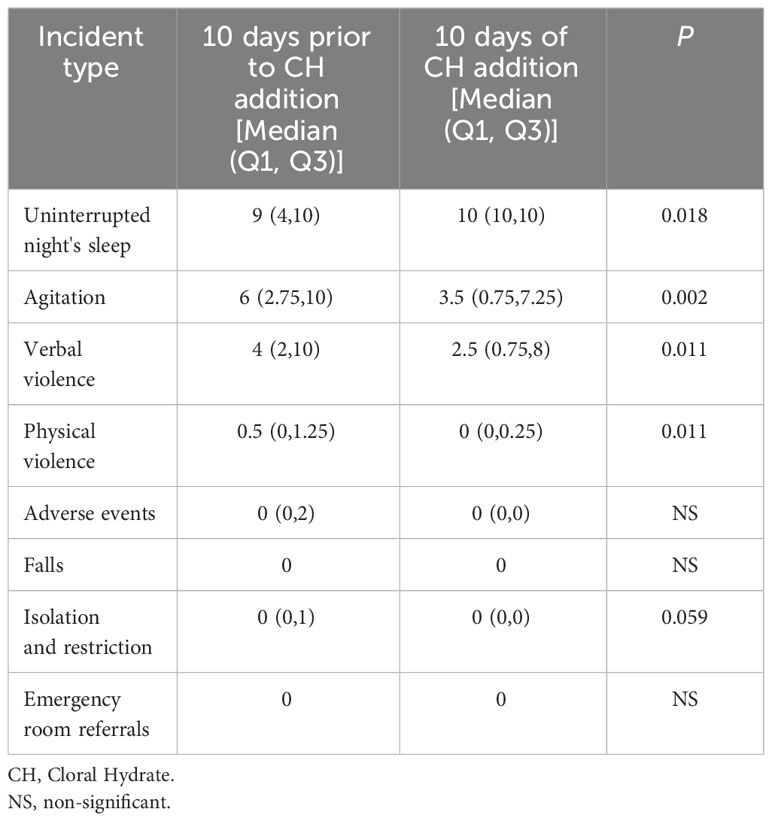
Table 2 Comparison between the 10 Days Prior to Initiation of Chloral Hydrate Treatment and during 10 Days of Chloral Hydrate Addition.
Adverse events, as abnormal laboratory results, falls or emergency room referrals, did not differ after ten days of CH treatment, compared to the then days before treatment (Table 2).
No significant differences were found in other variables.
Discussion
Our study aimed to investigate whether CH as an add-on therapy for TRS inpatients improves sleep quality, agitation, and violence.
The population of inpatients with TRS often experiences disrupted sleep patterns, possibly linked to disturbances in their circadian rhythm. The authors were unable to find a treatment that has been developed specifically to address sleep disturbances in schizophrenia patients. When necessary, cognitive-behavioral therapy for insomnia is the preferred treatment choice (14). However, TRS inpatients tend to be affected by severe cognitive impairments with difficulties to express themselves, rendering cognitive-behavioral therapy challenging and difficult.
Our findings revealed significant improvements in night’s sleep, along with a notable decrease in agitation and in verbal and physical violence, following the initiation of CH treatment. Sleep disturbances in schizophrenia have been associated with dysregulation in calcium channel activity and alterations in GABA and glutamate neurotransmission (15). The interaction of CH with GABA receptors (16) may contribute to its beneficial effect on sleep quality. Agonistic modulators and agonists of GABA receptors are known to be positively involved in the induction and consolidation of NREM sleep (17). CH might have the same agonist effect on sleep regulation. No specific recent studies have been found in humans on the possible effect of CH on GABA receptors resulting in sleep improvement.
Although various side effects of CH have been reported and its safety questioned, as this drug toxic profile might be lethal at 4 to 10 times (4-10g) the recommended dosage (18, 19), we did not encounter any adverse events as abnormal laboratory results or falls in our study. Our results show that CH might be safe when used at the right dosage for inpatients suffering from TRS.
In conclusion, CH shows some short-term benefits in improving sleep disorders and reducing violent and agitated behavior in patients with TRS.
Our study was constrained, however, by its small sample size, lack of control group, selection bias and retrospective open-trial design. We suggest conducting a large-scale, double-blind, randomized trial to gain a more comprehensive understanding of CH’s efficacy and safety in psychiatric populations with severe mental illnesses. Such research would provide more robust evidence and valuable insights into the potential benefits of CH as a treatment option in TRS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Abarbanel Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Due to the retrospective study design, the study was approved as exempt from the need for written informed consent by the Abarbanel Institutional Review Board.
Author contributions
AS: Methodology, Supervision, Writing – original draft. HA: Investigation, Methodology, Writing – original draft. EB: Formal Analysis, Methodology, Project administration, Software, Supervision, Writing – original draft. DH: Writing – review & editing. SW: Writing – review & editing. RS: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liebig J. Ueber die verbindungen, welche durch die einwirkung des chlors auf alkohol, aether, ölbildendes gas und essiggeist entstehen. Annalen Der Physik. (1832) 100(2):243–95. doi: 10.1002/andp.18321000206
2. Chopra R, Chopra GS. Chloral hydrate and paraldehyde as drugs of addiction. Indian Med Gazette. (1932) 67(9):481.
3. Robinson J. A case of chloral hydrate addiction. Int J Soc Psychiatry. (1966) 12(1):66–71. doi: 10.1177/002076406601200110
4. Tariq SH, Pulisetty S. Pharmacotherapy for insomnia. Clinics Geriatric Med. (2008) 24(1):93–105. doi: 10.1016/j.cger.2007.08.009
5. Gauillard J, Cheref S, Vacherontrystram MN, Martin JC. Chloral hydrate: a hypnotic best forgotten? L'encephale. (2002) 28(3 Pt 1):200–4.
6. Napoli KL, Ingall CG, Martin GR. Safety and efficacy of chloral hydrate sedation in children undergoing echocardiography. J Pediatr. (1996) 129(2):287–91. doi: 10.1016/S0022-3476(96)70256-1
7. Bain KT. Management of chronic insomnia in elderly persons. Am J Geriatric Pharmacother. (2006) 4(2):168–92. doi: 10.1016/j.amjopharm.2006.06.006
8. National Institute of Diabetes and Digestive and Kidney Diseases (US). LiverTox: clinical and research information on drug-induced liver injury. Natl Institute Diabetes Digestive Kidney Dis. (2012).
9. Bowyer K, Glasser SP. Chloral hydrate overdose and cardiac arrhythmias. Chest. (1980) 77(2):232–5. doi: 10.1378/chest.77.2.232
10. Dallman JA, Ignelzi MA Jr, Briskie DM. Comparing the safety, efficacy and recovery of intransal midazolam vs. oral chloral hydrate and promethazine. Pediatr Dent. (2001) 23(5):424–30.
11. Odan Laboratories. Product monograph including patient medication information: Chloral Hydrate Syrup Odan. (2022).
12. Linnoila M, Viukari M, Numminen A, Auvinen J. Efficacy and side effects of chloral hydrate and tryptophan as sleeping aids in psychogeriatric patients. Int Pharmacopsychiatry. (1980) 15(2):124–8. doi: 10.1159/000468423
13. Yuen MV, Gianturco SL, Pavlech LL, Storm KD, Yoon S, Mattingly AN. Chloral Hydrate: Summary Report. (2020).
14. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Internal Med. (2015) 163(3):191–204. doi: 10.7326/M14-2841
15. Ferrarelli F. Sleep abnormalities in schizophrenia: state of the ar and next steps. Am J Psychiatry. (2021) 178(9):903–13. doi: 10.1176/appi.ajp.2020.20070968
16. Peoples RW, Weight FF. Trichloroethanol potentiation of gamma-aminobutyric acid-activated chloride current in mouse hippocampal neurones. Br J Pharmacol. (1994) 113(2):555. doi: 10.1111/j.1476-5381.1994.tb17025.x
17. Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. (1999) 22(1):33–42. doi: 10.1093/sleep/22.1.33
Keywords: chloral hydrate, treatment resistant schizophrenia (TRS), sleep, violence, agitation
Citation: Shelef A, Alaa H, Bloemhof-Bris E, Halperin D, Weizman S and Stryjer R (2024) Short-term chloral hydrate as an add-on treatment may improve sleep and alleviate agitation in inpatients with treatment resistant schizophrenia: a retrospective case series study. Front. Psychiatry 15:1293676. doi: 10.3389/fpsyt.2024.1293676
Received: 13 September 2023; Accepted: 26 January 2024;
Published: 29 February 2024.
Edited by:
Massimo Tusconi, University of Cagliari, ItalyReviewed by:
Serdar M Dursun, University of Alberta, CanadaTakashi Kanbayashi, University of Tsukuba, Japan
Copyright © 2024 Shelef, Alaa, Bloemhof-Bris, Halperin, Weizman and Stryjer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esther Bloemhof-Bris, ZXN0aGVyYmwuYnJAZ21haWwuY29t
†These authors have contributed equally to this work
 Assaf Shelef
Assaf Shelef Habashi Alaa
Habashi Alaa Esther Bloemhof-Bris
Esther Bloemhof-Bris Dania Halperin
Dania Halperin Shira Weizman2,3
Shira Weizman2,3