- 1Institute for Mental and Physical Health and Clinical Translation, School of Medicine, Deakin University, Geelong, VIC, Australia
- 2Institute of Clinical Medicine, Psychiatry, University of Eastern Finland, Kuopio, Finland
- 3Kuopio Musculoskeletal Research Unit (KMRU), Institute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland
- 4Biostatistics Unit, Faculty of Health, Deakin University, Geelong, VIC, Australia
- 5Barwon Health, University Hospital Geelong, Geelong, VIC, Australia
Introduction: We conducted a systematic review to evaluate the quality and extent of evidence on associations between personality disorders (PDs) and musculoskeletal disorders (MSDs) in population-based studies, since these disorders are leading causes of disease burden worldwide.
Methods: A search strategy of published, peer-reviewed and gray literature was developed in consultation with a liaison librarian and implemented for Embase, CINAHL Complete, Medline Complete, and PsycINFO via the EBSCOhost platform from 1990 to the present and CORDIS and ProQuest Dissertations & Theses Global, respectively. The inclusion criteria were as follows: I) general population participants aged ≥15 years; II) self-report, probable PD based on positive screen, or threshold PD according to the DSM-IV/5 (groupings: any, Clusters A/B/C, specific PD) or ICD-10/11; III) MSDs identified by self-report or ICD criteria (arthritis, back/neck conditions, fibromyalgia, osteopenia/osteoporosis) and III) cohort, case-control, and cross-sectional study designs. Two reviewers independently screened articles and extracted the data. Critical appraisal was undertaken using the Joanna Briggs Institute checklists for systematic reviews of etiology and risk. A descriptive synthesis presents the characteristics of included studies, critical appraisal results, and descriptions of the main findings. This review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Results: There were 11 peer-reviewed, published articles included in this review (n = 9 cross-sectional and n = 2 case-control studies); participants were ≥18 years in these studies. No published gray literature was identified. Semi-structured interviews were the most common method to ascertain PDs; all studies utilized self-reported measures to identify MSDs. Overall, we detected limited and conflicting evidence for associations between PDs and MSDs.
Discussion: The main result may be explained by lack of population-based longitudinal evidence, heterogenous groupings of PD, and few comparable cross-sectional and case-control studies. Strengths of the review include a comprehensive search strategy and a discussion of mechanisms underlying possible associations between PDs and MSDs.
Conclusions: The quality of most studies included in this review that examined associations between PD and MSDs in general population adults was high. However, the results demonstrated limited and conflicting evidence for these associations, in part, due to lack of comparable evidence, which should be addressed in future research.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021243094.
1 Introduction
Mental disorders and musculoskeletal disorders (MSDs) are each leading contributors to years lived with disability (YLD) (1). Personality disorders (PDs) are a common form of mental disorders, with a worldwide prevalence estimate of approximately 8% (2). PDs manifest as difficulties with emotion regulation, interpersonal relating, and adaptive functioning such as coping with daily life stressors and demands (3, 4). Existing classifications characterize PD as 10 distinct categorical disorders that are organized into Clusters A (“odd-eccentric” features), B (“dramatic/emotional/erratic” features), and C (“anxious/fearful” features) (5). Contemporary approaches to the classification of PD have resulted in the International Classification of Diseases (ICD-11) adopting a dimensional approach to classification focusing on patterns of traits that contribute to impairment in global personality functioning (6). Separately, MSDs are a group of conditions or consequences from injury that affect bones (i.e., osteoporosis, osteopenia), joints (i.e., types of arthritis such as osteoarthritis, rheumatoid arthritis, and psoriatic arthritis), muscles, and other soft tissues, as well as those that implicate multiple body areas or systems (i.e., chronic back/neck pain and fibromyalgia) (7). These MSDs can result in courses of acute or chronic painful symptoms, significantly restricting mobility and functioning, and leading to increased morbidity and mortality [see literature review by Briggs et al. (8)]. There is growing evidence of high occurrences of comorbid PD with common types of MSDs in clinical populations (9). However, awareness of these associations in the general population is still limited.
Among patients with chronic back/neck pain, the proportion of PD is reported to range between 43.6% and 69.6% (clinically based studies) (10–12). Longitudinally, the occurrence of chronic back pain in patients with remitted and non-remitted borderline PD is 47.8% and 57.7%, respectively (at 16 years of follow-up) (13). In addition, PD is common among patients with fibromyalgia with frequencies ranging from 8.7% to 65.0% in clinical studies (14–18). Moreover, in a retrospective cohort study using hospitalization/physician claim data, patients with rheumatoid arthritis had increased incidence of PD (incident rate ratio = 1.61; 95% CI: 1.29–1.99) compared to controls (matched 5:1 on sex, age, and region of residence in Manitoba, Canada) as well as other types of immune-mediated inflammatory diseases (19). Moreover, there is longitudinal evidence that remission status among patients with borderline PD is associated with osteoarthritis over the long term with 4.0% (remitted) and 15.5% (non-remitted) having osteoarthritis at the study baseline and 11.9% (remitted) and 26.8% (non-remitted) after 16 years (13, 20). However there was no population-based control group. There is also clinical cross-sectional evidence to suggest patients (women only) with borderline PD may have reduced bone mineral density (BMD) and be at risk of osteoporosis (21, 22).
Improved recognition of these associations in a population-based setting is warranted; evidence from a large epidemiological study showed that as separate categories of disorders, PDs and MSDs, such as arthritis, are associated with significant population-level quality-adjusted life year (QALY) losses, which suggests a high overall burden to individuals and society (23).
Recently, we undertook a scoping review of the comorbidity of PD and MSDs, which included existing reviews (9). It revealed that there were no existing systematic reviews that incorporated critical appraisal of the evidence, or meta-analyses, and none that focused on population-based associations between PD and MSDs. Therefore, we developed and published a protocol, which outlines the methodological approach to conduct a systematic review, and address this gap in the literature (24). Prior to commencing the conduct, we confirmed no existing systematic review on this topic via a search of PROSPERO, Open Registries, and Medline Complete, CINAHL Complete, and PsycInfo databases (EbscoHost platform). The systematic review is needed to assess the quality of existing evidence and quantify associations to provide directions for future research and practice. In addition, evaluating the evidence will enable inferences to be made about these associations in the population, along with potential associated needs (23).
Therefore, a systematic review was undertaken, with the objective of evaluating population-based epidemiological associations between PD and burdensome MSDs including arthritis, back/neck pain, fibromyalgia/muscular pain, and osteopenia/osteoporosis. These MSDs were selected as outcomes for this review, as we recently scoped the evidence on this topic (9) and identified these MSDs as possibly highly comorbid with PD in clinical and/or general population settings. In addition, we identified emerging evidence of associations with poorer bone health (25). In accordance, the research questions were as follows:
1. Is there an association between PDs and arthritis, back/neck pain, fibromyalgia/muscular pain, and osteopenia/osteoporosis and/or “any” of these conditions?
1.1. What methodological characteristics explain the heterogeneity in results?
2. For the question above, what is the quality and levels of evidence for these associations?
2 Methods
The protocol for this review is published (24), registered with PROPSERO (CRD42021243094), and was conducted in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) (26). It was also guided by the Joanna Briggs Institute resources for conducting systematic reviews of etiology and risk (27).
2.1 Inclusion criteria
The Population, Exposure, Outcome (PEO) framework (27) was used to characterize the inclusion and exclusion criteria for this review and is presented in Table 1. Eligible study designs were population based, observational cross-sectional (analytical), case-control, or cohort studies. Therefore, intervention, qualitative, and descriptive study designs were not considered eligible. No restrictions on participant characteristics or language of publications were applied.
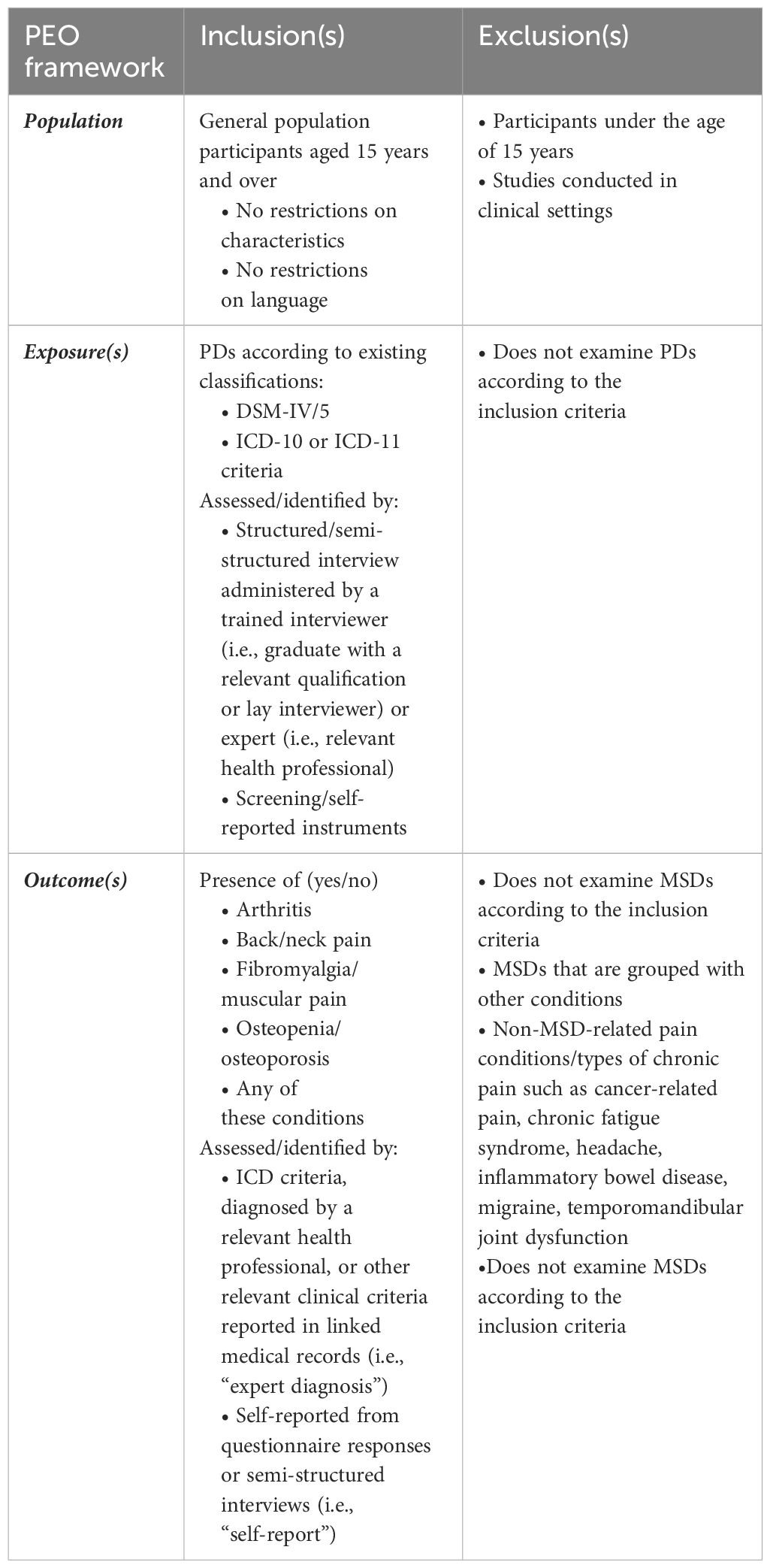
Table 1 Inclusion and exclusion criteria according to the Population, Exposure, Outcome (PEO) framework.
2.2 Evidence sources
Peer-reviewed and published gray literature evidence sources were restricted to those published in or after 1990. For this review, published gray literature included dissertations published in ProQuest Dissertations & Theses Global or reports/publications deriving from relevant projects/programs housed in the European Commission’s Community Research and Development Information Service (CORDIS). These evidence sources were considered due to the potential to extract relevant epidemiological data. Additional information sources were identified by screening and reviewing the reference lists of eligible studies. Further details are presented below (see section on search strategy).
2.3 Study identification and selection
2.3.1 Search strategy
A comprehensive search strategy was developed to identify eligible evidence sources. First, in consultation with a liaison librarian (LG), a pilot search was developed and implemented in Medline Complete using the EBSCOhost platform on 26 August 2021 as part of the protocol development (24). Text words contained in the titles and abstracts of relevant articles were identified, and the search was developed using index terms (MeSH) and keywords, Boolean operators, truncations, and explode functions. A “gold set” list (25, 28–34) of relevant articles were identified from a recent scoping review (9) and used to test the preliminary search. All gold set articles were returned in the pilot search yield. Next, the search for Medline Complete was translated for CINAHL Complete and APA PsycINFO (via EBSCOhost), and Embase. Gray literature was searched using an adapted search for ProQuest Dissertations & Theses Global databases and CORDIS. The complete search was implemented on 16 September 2021 and updated on 20 February 2024 (see Supplementary Tables 1-6). Finally, the reference lists of included studies were exported using SCOPUS.
Assisted by the liaison librarian (LG), one reviewer (SEQ) implemented the search strategy and managed the records. The records from the combined searches were exported to Mendeley reference management software and Covidence (35) with duplicates removed. The flow of citations and reasons for exclusion at the full-text review are presented in Figure 1 and in the results (see Section 3).
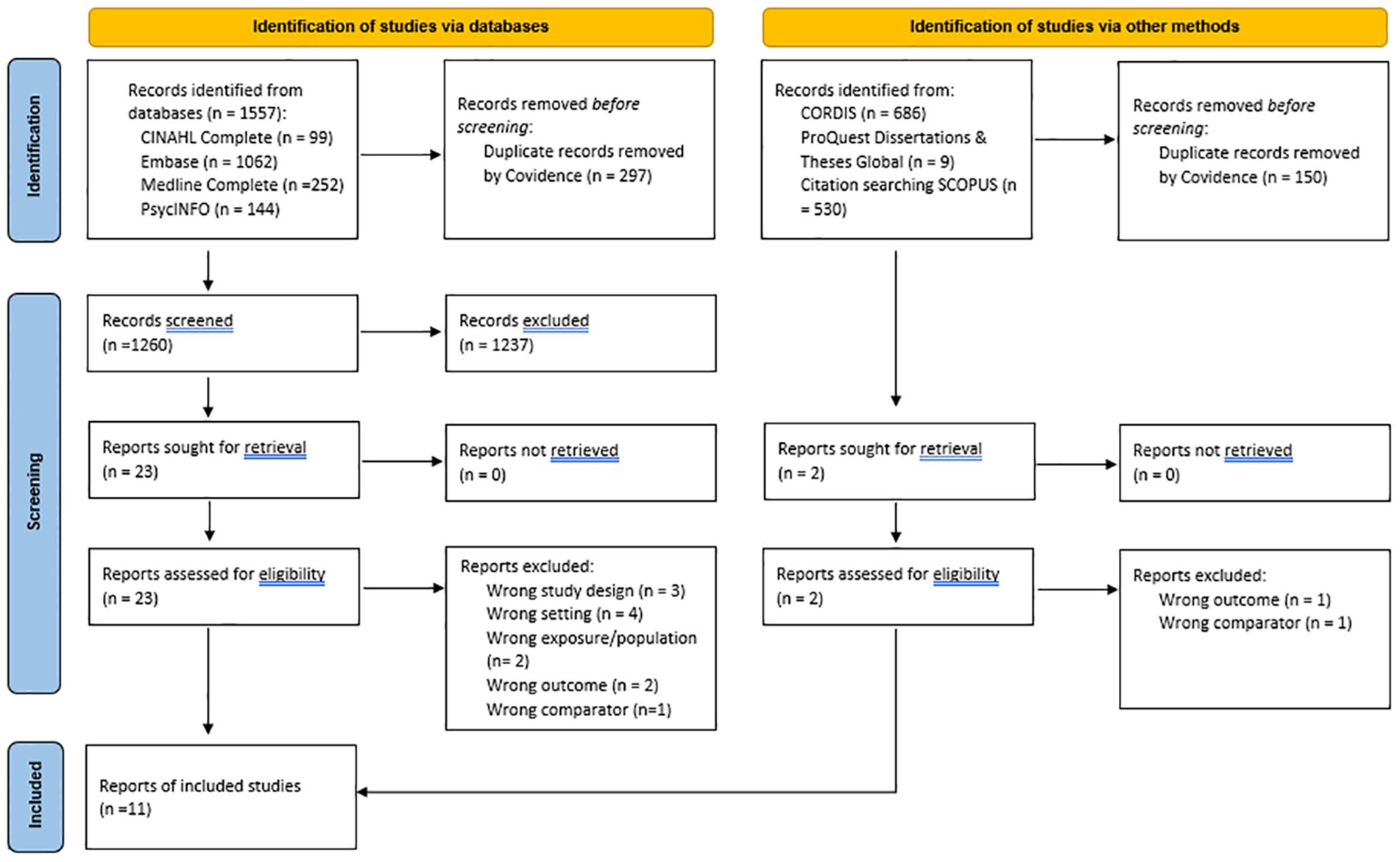
Figure 1 PRISMA 2020 flow diagram adapted from “Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.
2.3.2 Search strategy
Two reviewers (LJW and SEQ) conducted pilot testing of the inclusion and exclusion on a sample of 25 randomly selected articles from the search yield prior to screening. Next, the same two reviewers independently undertook screening of titles/abstracts and review full-text review of articles in Covidence. Discrepancies at the screening and full-text review phase were resolved in one consensus meeting each. One reviewer (SEQ) screened the records identified from the other methods. The final list of published peer-reviewed and gray literature was confirmed by the supervising author (LJW).
2.4 Data collection, extraction, and reporting
2.4.1 Critical appraisal of individual studies
Two reviewers (ALS and SEQ) independently assessed for risk of bias of the included studies using an adapted version of the critical appraisals tools by the Joanna Briggs Institute (36). Disagreements were resolved between the reviewers in one consensus meeting.
The critical appraisal tools assess pertinent biases in observational studies, including potential selection bias, information bias, and confounding, according to the relevant study design (36). Specifically, for the cross-sectional studies, we rated seven items concerning a) the inclusion/exclusion criteria, b) the description of the sample/representativeness, c) reliability and validity of the exposure(s), d) whether confounding factors were identified and measured, e) whether confounding factors were accounted for in study design/analysis, f) reliability and validity of the outcome(s), and g) appropriateness of statistical analyses. For the case-control studies identified, nine items were rated regarding a) comparison group representativeness (of source population), b) appropriateness of recruitment/matching of cases and controls, c) appropriateness of criteria to identify cases and controls, d) reliability and validity of the exposure(s), e) measurement of exposure(s) for cases and controls, f) whether confounding factors were identified and measured, g) whether confounding factors were accounted for in study design/analysis, h) reliability and validity of the outcome(s), and i) appropriateness of statistical analyses (36). The items were rated as follows: 0) did not satisfy the criteria (i.e., no) and 1) satisfied the criteria (i.e., yes). We summed the ratings to derive a critical appraisal score for each study (0–7 for cross-sectional studies; 0–9 for case-control studies.) This method was similarly applied in a separate published review on the prevalence of PDs in the community (2). The results of the critical appraisal are presented in Table 2.
2.4.2 Data extraction
A data extraction form was developed in consultation with a statistician (MM) using Microsoft Excel. One reviewer (SEQ) extracted the data, and another (ALS) validated the data with discrepancies resolved in one meeting. The primary outcome(s) were the presence of each MSD (categorical: yes/no). Models with the highest number of confounding adjustments were extracted. Where analyses addressed the reverse association, the outcome was presence of PD or probable PD (any, Clusters, and/or specific PDs). The odds ratio (OR) and 95% confidence interval (95% CI) were the principal summary measures. For regression analyses, b values were used as the summary measure.
2.4.3 Reporting of results
The MOOSE and PRISMA guidelines were considered for the reporting of results (40). The characteristics and results from individual studies, including critical appraisal scores, are presented in Tables 2, 3, respectively. We determined levels of evidence for associations based on an adapted method previously published by Lievense et al. in the rheumatological setting (41) and in other published reviews (42, 43). This is presented in Box 1 (below). There were too few studies with appropriately comparable study populations and groupings of PD to undertake meta-analyses.
Box 1. Criteria for levels of evidence derived from Lievense et al. (41).
Strong evidence
Generally consistent findings in:
Multiple high-quality cohort studies
Moderate evidence
Generally consistent findings in:
1 high-quality cohort study and ≥2 high quality case-control studies
≥3 high-quality case-control studies
Limited evidence
Generally consistent findings in:
1 high-quality cohort study
<2 high-quality cross-sectional, case-control studies
Inconsistent evidence
If ≤75% studies reported consistent findings
No evidence
No evidence was identified
3 Results
The results of the study identification and selection process are presented in Figure 1. Via the EBSCOhost platform, the searches from APA PsycINFO yielded 143 citations (additional n = 1 from the updated search), 94 from CINAHL Complete (additional n = 5 from updated search), 948 from Embase (additional n = 114 from updated search), and 236 from Medline Complete (additional n = 16 from updated search). After duplicates (n = 297 total) were removed, the total search yield included 1,260 citations. Of the 1,260 citations screened, 23 underwent full-text review, and 12 were excluded with reasons. No further citations were yielded from searching the other evidence sources. Thus, 11 peer-reviewed published articles were included in this review.
3.1 Characteristics
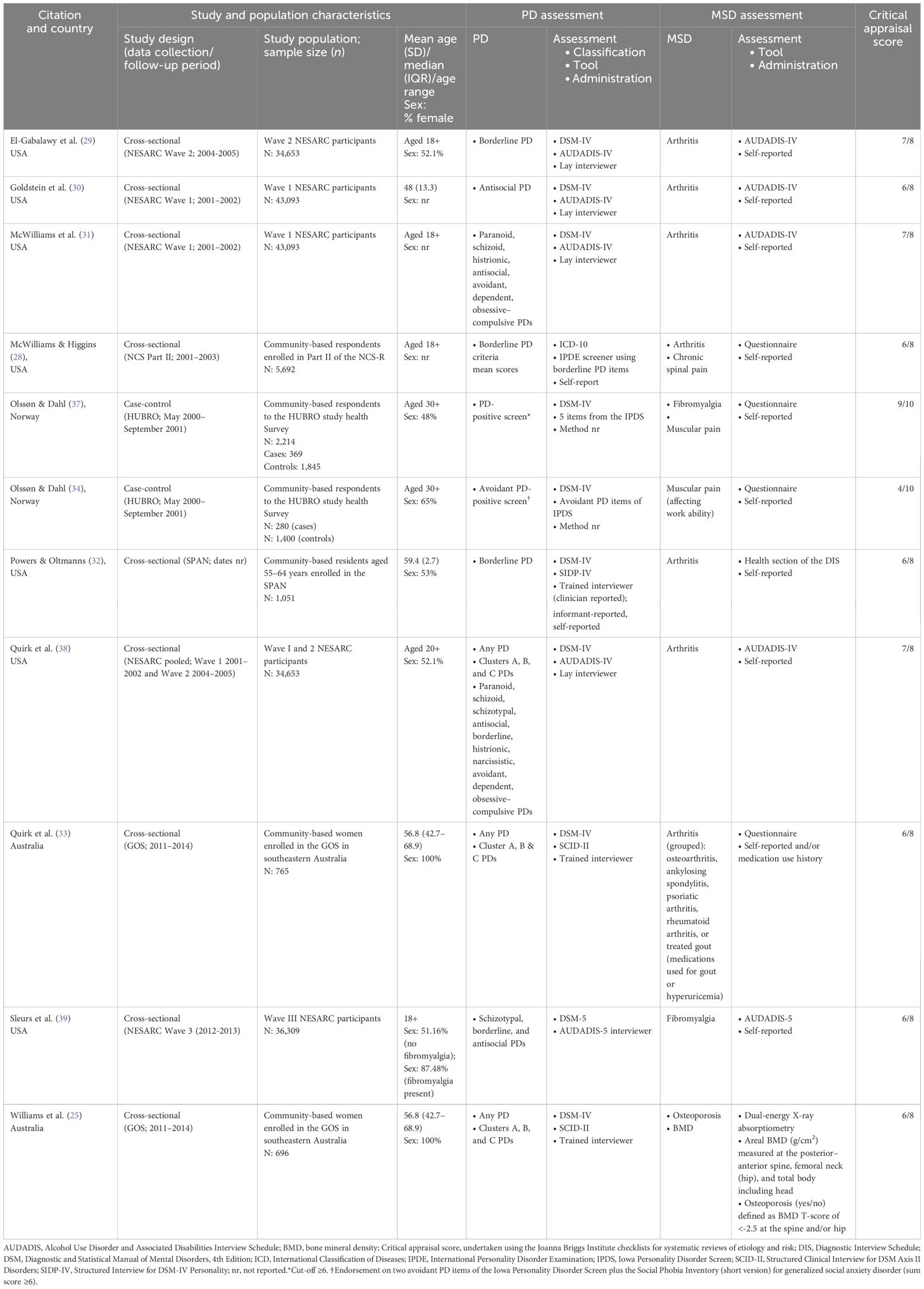
The characteristics of the included studies and summary of results are presented in Tables 2, 3. The reports of the identified studies were published between 2008 and 2020. Of the 11 studies included, nine were cross-sectional and two were case control. Seven out of the 11 studies were conducted in the United States (US) (28–32, 38, 39), with two studies each from Norway (34, 37) and Australia (25, 33). Epidemiological data sources included Waves I/II/III of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) (29–31, 38, 39), the National Comorbidity Survey—Part II (28), and The St. Louis Personality and Aging Network (SPAN) study (32) each from the US; the Oslo Health Study (HUBRO) from Norway (34, 37), and the Geelong Osteoporosis Study (GOS) located in Australia (25, 33). Participant samples ranged from n = 696 in the GOS study from Australia (25) to n = 43,093 in two studies utilizing data from the NESARC (30, 31); two studies utilized samples comprising only women (25, 33).
Eight studies used semi-structured interviews to identify PDs including the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV)/AUDADIS-5 (29–31, 38, 39) performed by trained lay interviewers, the Structured Interview for DSM-IV Personality (SIDP-IV) (32) and the Structured Clinical Interview for DSM-IV (SCID-II) by trained interviewers (25, 33). Two studies identified probable PDs using The Iowa Personality Disorder Screen (IPDS) (34, 37) with another using the borderline PD scale from the International Personality Disorder Examination (IPDE) Screening Questionnaire (28). Majority (10/11) of studies utilized self-report of MSDs including arthritis (28, 29, 31–33, 38), fibromyalgia/muscular pain (37, 39), and spinal pain (28). One study determined osteoporosis (yes/no) by a BMD T-score of <−2.5 at the spine and/or hip using dual-energy X-ray absorptiometry (Prodigy; GE Lunar, Madison, WI, USA) (25). There were no studies identified that classified PD using measures based on the ICD-11 classification system.
The median critical appraisals score was 6, and thus, the reporting quality of most studies were considered high. In terms of the cross-sectional studies, the most common reason for not achieving a total sum score of 7 concerned the quality of describing the study population and setting, and/or the use of a reliable and valid measure of the study exposure (i.e., use of an adapted measure to assess PD). Regarding the case-control studies (whereby a total possible score is 9), a lack of information or unclear descriptions of the methodology (i.e., appropriate matching of cases and controls, validity and reliability of the exposure measure, and details concerning the appropriateness of the statistical analyses) resulted in a lower critical appraisal score.
3.2 Descriptive synthesis
The following sections present the findings from the descriptive synthesis and evidence gap analysis in text and tables. Specifically, Table 3 presents the results from all individuals studies/analyses with the evidence gap analysis shown in Supplementary Tables 7-14.
3.2.1 Personality disorders and arthritis
In the literature, associations between PDs and arthritis were examined in seven cross-sectional studies (28–33, 38). Of these, four utilized data from the NESARC (29–31, 38); the remaining three presented analyses from Part II of the NCS (28), the SPAN (32), and the GOS (33).
In Wave I of the NESARC, Goldstein et al. examined antisocial PD, antisocial PD features, and history of conduct disorder and the association with past-year arthritis, compared to people with no history of these PDs/features, and according to sex (30). Men and women with antisocial PD had increased odds of arthritis compared to those without a history (30). Separately, in Wave II of the NESARC, El-Gabalawy et al. reported an association between borderline PD and increased odds of past-year arthritis (29). In a further study using data from both waves I and II, Quirk et al., examined all PDs and PD Clusters in relation to past-year arthritis considering the role of age in these associations (38). Across all specific PDs and PD groupings, individuals with grouped Cluster B PDs had the highest odds of arthritis compared to those without these PDs. In addition, for those in the younger age group (under 55 years), any PD, schizoid, schizotypal, and obsessive–compulsive PDs were each associated with increased odds of arthritis compared to younger individuals without these PDs (38). Of the analyses deriving from the NESARC, all accounted for pertinent sociodemographics and at least mood, anxiety, and substance use disorders (29, 30, 38). In addition, the separate analyses presented in the reports by El-Gabalawy et al. and Goldstein et al. also adjusted for PDs other than the exposure (29, 30). Finally, in the GOS, Quirk et al. reported increased odds of a history of arthritis among women with Cluster B PDs compared to women without these PDs after adjustment for sociodemographic information, lifestyle behaviors, lifetime history of any mood, anxiety, substance use, or eating disorders (and the interactions of these) (33).
In terms of the reverse association, in Wave I of the NESARC, McWilliams et al. examined associations between past-year arthritis and odds of several specific PDs (avoidant, dependent, obsessive–compulsive, paranoid, schizoid, histrionic, and antisocial PDs) (31). Compared to people without, people with arthritis had increased odds of paranoid, schizoid, histrionic, antisocial, avoidant, and obsessive–compulsive PDs—analyses accounted for pertinent sociodemographic factors (31). In addition, in Part-II of the NCS, McWilliams reported that, compared to people without, those with lifetime arthritis had higher BPD symptomatology scores after accounting for sociodemographics, past-year mood disorders, anxiety disorders, and externalizing disorders (28). Separately, in the SPAN, Powers et al., reported increased odds of arthritis among people with interviewer- and informant-rated borderline PD features, but not self-reported borderline PD features, among adults aged 55 to 64 years. However, the association between all three types of assessment modes (of borderline PD features) and arthritis was fully mediated by BMI (32).
Also, non-significant findings for associations between PDs and arthritis were also observed. Analyses deriving from Wave I of the NESARC revealed that dependent PD was not significantly associated with greater odds of arthritis (31) with further analyses from pooled Waves I and II of the NESARC showing non-significant results for associations between histrionic and narcissistic PDs and arthritis (38). In subgroup analyses deriving from Wave I of the NESARC, antisocial behavioral syndromes were not significantly associated with arthritis among women, nor was a history of conduct disorder for both women and men (30). Also in the NESARC (Waves I and II pooled), those aged over 55 years, having any grouped Cluster A PDs, or the specific PDs of schizoid, schizotypal, or obsessive–compulsive were not significantly associated with greater odds of arthritis; being in the younger age group (under 55 years) and having any C Cluster PD was not significantly associated with arthritis (38). Finally, in the GOS (women only), any PD grouped, Cluster A PDs, and Cluster C PDs were not significantly associated with arthritis (25).
3.2.2 Personality disorders and spinal pain
In analyses utilizing data from Part-II of the NCS, McWilliams et al. examined associations between a history of spinal pain, as the exposure, and borderline PD symptomatology (28). Compared to people without, people with past-year spinal pain had higher borderline PD symptomatology following adjustment for pertinent sociodemographics, past-year mood disorders, anxiety disorders, and externalizing disorders (28). This association was similarly observed among people with remitted spinal pain compared to people without remitted spinal pain (28). However, in further analyses, people with past-year spinal pain did not report significantly different borderline PD symptomatology compared to people who had remitted (28).
3.2.3 Personality disorders and fibromyalgia/muscular pain
Two separate reports of studies utilizing data from the HUBRO (34, 37) and one from Wave III of the NESARC examined associations between PDs and fibromyalgia/muscular pain (39). Olssøn and Dahl examined both directions of associations between personality problems and fibromyalgia and muscular pain, respectively. First, a higher proportion of people with probable PD had past-year muscular pain and lifetime fibromyalgia compared to controls (age–gender matched), respectively (37). Moreover, muscular pain, but not fibromyalgia, was associated with increased odds of probable PD after additional adjustments for education and employment status (37). Next, Olssøn and Dahl examined avoidant personality problems specifically and found that a higher proportion of people with probable avoidant PD had muscular pain compared to controls (age and gender matched). In reverse, muscular pain was not significantly associated with probable avoidant PD (adjusted further for education and employment status) (34). Elsewhere, in Wave 3 of the NESARC, Sleurs et al. reported that compared to those without, people with fibromyalgia have increased odds of “any” PD as well as each schizotypal, borderline, and antisocial PDs (39); analyses accounted for sociodemographic information.
3.2.4 Personality disorders and bone health
In one study using the GOS data, associations between any PD and osteoporosis among women were not statistically significant (25). However, in analyses examining associations between PD Clusters and BMD, women with Cluster A PDs, but not Cluster B or C PDs, had lower hip BMD (i.e., poorer bone health) compared to women without these PDs (25). Women with Cluster A, B, or C PDs did not have significantly reduced total body BMD or spine BMD.
3.2.5 Levels of evidence
Overall, our evidence gap analysis (see Supplementary Tables 7-14) revealed that there was either conflicting or limited evidence for associations between PDs and arthritis, spinal pain, fibromyalgia, and BMD. In addition, there was currently no evidence (i.e., these studies have not yet been conducted) for associations between a range of specific PDs in regard to these MSDs especially concerning BMD.
4 Discussion
This review had two aims: 1) to evaluate the evidence for associations between PDs and MSDs including arthritis, back/spinal pain, fibromyalgia/muscular pain, and aspects of bone health; to explore potential sources of heterogeneity in the results including study and population characteristics and the assessment of PD and 2) ascertain the quality and levels of evidence for these associations.
Overall, our descriptive synthesis revealed that there is currently limited and conflicting levels of evidence for associations between PDs and each of the MSDs. While 10 of the 11 studies reviewed were considered of “high quality,” we identified several methodological factors that might contribute to the limited evidence base for these associations. First, the evidence identified was derived primarily from cross-sectional studies, and thus, evidence from case-control and cohort studies are needed to ascertain moderate or strong evidence for these associations if evident. In addition, we identified heterogenous groupings of PD as the exposure of interest—particularly for “any” PD—and the extent to which the full range of specific PDs were examined also varied. Considering the relatively small number of studies identified, the numerous PD groupings, and number of participants, the comparisons of alike studies were limited.
In terms of specific MSD outcomes, we identified conflicting evidence for associations between Cluster A PDs and arthritis. To illustrate, in the NESARC, paranoid PD among all ages was associated with increased odds of arthritis with schizoid and schizotypal PDs each being associated with arthritis among those under 55 years (not among those over 55 years.) When examined as a “Cluster” in relation to arthritis, the association with these PDs were attenuated (although remained significant). Separately, in the GOS, these PDs were examined as a Cluster among women with an average age of approximately 57 years; in this study, Cluster A PDs were not significantly associated with increased odds of arthritis. Similarly, in pooled Waves 1 and 2 of the NESARC, Quirk et al. reported no significant associations between Cluster C PDs (grouped) and increased odds of arthritis among individuals of all ages or those aged under 55 years. However, in separate analyses from the same report, people over the age of 55 years with Cluster C PDs were found to have significantly increased odds of arthritis; in the GOS, Cluster C PDs were not significantly associated with arthritis. Thus, the conflicting evidence for associations between Cluster A and C PDs and arthritis appears to be influenced by how these PDs are grouped and the nature of the study populations including age and sex factors.
There is a need to improve the evidence base on population-based associations between PD and MSDs. This expansion would contribute to a more comprehensive understanding of the interplay between these conditions and encourage investigation into potential shared etiological pathways, common risk factors, and consideration of therapeutic implications.
4.1 Mechanisms
In a recent scoping review, we highlighted that the mechanisms underlying potential associations between PDs and MSDs could be understood from the perspective of the biopsychosocial model (9). To date, biopsychosocial models have been employed to understand how the interaction of psychological, social, and biological factors operate in the etiology and maintenance of pain specifically (44–46). Thus, the biopsychosocial models provides a transdiagnostic lens to conceptualize how mechanisms might operate in concert across PD and MSDs.
To illustrate, exposure to early stress, trauma, and other forms of adversities in childhood and adolescence—critical periods for personality development and the acquisition of adaptive self-regulatory processes—are understood to alter stress mechanisms. These mechanisms are understood to have a role in vulnerability to PD via alterations in the hypothalamic–pituitary–adrenal (HPA) axis and morphological changes in brain areas involved in the stress response (47). Childhood adversity and types of trauma have been separately linked to PD (48–50) and to MSDs including arthritis (51) and chronic back pain (52). Alterations in the stress response can also lead to increased vulnerability of a range of physical disorders (45, 46). Moreover, a recent study reported associations between childhood adversity/trauma and borderline PD symptomatology among patients with chronic pain including pain related to MSDs (53).
Of interest, we observed several analyses showing associations between Cluster B PDs and arthritis specifically. There is evidence that stress is associated with emotional dysregulation, which often presents as hypersensitivity and reactivity and difficulty coping among people with these PDs (54). Stress is also associated with immune dysregulation and inflammation in the body (55). Acute stress arising as a symptom or consequence of PD may “trigger” or exacerbate MSDs that have inflammatory origins such as conditions of the joints through immune dysregulation and inflammatory responses. Separately, Turk and Monarch explained that intense emotions, such as anger, may interfere with help seeking and willingness to engage with treatment recommendations among people with psychiatric disorders and thus minimize the opportunity for effective rehabilitation (44). Others have highlighted the complexity of the experience of pain among people with borderline PD. For example, it is suggested that, as a consequence of self-regulation difficulties, the experience of endogenous pain may be felt as more intense and less tolerable, compared to self-inflicted pain; pain is also understood to serve an affect-regulating function for people with borderline PD, which can, in turn, lead to pain attenuation and tolerance (56, 57). To substantiate, previous experimental research has uncovered that modulating pain can regulate affect in patients with PD (58), which is reduced following treatment with DBT (59).
In this review, there was mixed evidence for associations between “Cluster C” PDs and MSDs. “Cluster C” PDs are traditionally conceptualized to manifest the behaviors of extreme avoidance of social interaction, which are motivated by feelings and fears of perceived inadequacy and rejection (5). People with features of these disorders may experience anxiety associated with pain leading to types of avoidance coping or safety behaviors. These may manifest externally through the avoidance of social interactions/situations or internally to avoid experiencing distressing thoughts, feelings, or sensations concerning pain (60). In addition, a recent review has summarized that features of these disorders tend to be more stable over time (61), and thus, avoidance styles of coping may became more entrenched and operate in the maintenance of comorbidities.
Given the plausible biopsychosocial factors linking PDs and MSDs, we have previously suggested that a multidisciplinary approach to identifying and treating comorbidities in clinicals settings may be needed (9). However, there is a lack of research into, and targeted measures and interventions for, each psychiatric and musculoskeletal medicine settings to identify and concurrently manage PDs and MSDs.
4.2 Strengths and limitations of this review
In terms of strengths, we developed and implemented a comprehensive search strategy including a thorough search for evidence from gray literature sources, which enabled a complete synthesis of the existing literature. We reported on the current levels of evidence for associations between PDs and revealed where evidence gaps exist, which will prompt and guide further research on this topic. We also provided a conceptual description of the possible mechanisms that might underly associations between PDs and MSDs—ideas to be further investigated and empirically tested in clinical settings.
In terms of the limitations, most notably was the paucity of data for each outcome. The evidence gap analysis revealed “no evidence” for a number of specific PDs and MSDs particularly in relation to BMD. Thus, the authors suggest that a call to action is needed to address the evidence gap and understand if people with PDs may be more susceptible to MSDs for which clinical monitoring and management may be required. In addition, the outcomes were chiefly assessed using self-reported measures (except for one study, which also utilized clinical measures of BMD). Recent evidence from a population-based study comparing self-report to register data in Finland (women only) reported that self-reported measures may be insufficient for accurately identifying MSDs (62). Thus, MSDs may be underrepresented in the reviewed studies.
A significant proportion of the studies utilized data from Waves of the NESARC (5 of the 11 studies), and there were few opportunities to synthesize the evidence examining associations between PDs and MSDs from varied studies and settings. In addition, this review included eligible analyses, which involved multiple comparisons of the same data source, which may be considered a limitation. Similarly, in terms of the assessment of PD, we note that multiple comparisons, that is, the number of possible PD groupings (i.e., 3 PD Clusters, 10 specific PDs, and pooled PDs) in relation to MSDs within and across studies, has limited what conclusions can be drawn about associations between PDs and MSDs. It is suggested that derivation of an “any PD” may assist in overcoming this methodological issue in future research. However, it is acknowledged that traditionally, these specific PDs vary in clinical presentation and severity, and thus, meaningful, and sensitive data may be lost if any one or more PDs are grouped as a unitary variable.
In terms of study designs, the available evidence was derived chiefly from cross-sectional studies, and no longitudinal studies were identified. Therefore, it is not presently possible to determine the direction of causality among associations between PDs and MSDs. There was also insufficient evidence sources to complete planned meta-analyses on associations between PDs and MSDs.
Therefore, considerably expanding the evidence base on the longitudinal course of PD and MSDs would facilitate a thorough exploration of the underlying mechanisms and understanding of potential clinical implications. For example, a burgeoning evidence base might lead to a greater focus in both psychiatric and musculoskeletal medicine settings to identify and concurrently manage PDs and MSDs. In addition, an evolving evidence base would allow for a deeper and more nuanced interpretation of synthesized evidence in future reviews on this important topic. Future research should also consider the application of the ICD-11 classification of PD in relation to these comorbidities.
5 Conclusions
To conclude, the quality of most studies included in this review that examined associations between PD and MSDs in general population adults was high. However, the results demonstrated limited and conflicting evidence for these associations—in part due to a lack of comparable cross-sectional studies and no detected longitudinal evidence, which is now needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SQ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. RH: Writing – original draft, Writing – review & editing. MM: Methodology, Writing – original draft, Writing – review & editing. AS: Methodology, Writing – original draft, Writing – review & editing. JH: Writing – original draft, Writing – review & editing. LW: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. LW is supported by a National Health and Medical Research Council (NHMRC) Emerging Leadership Fellowship (1174060). HK-H and SEQ are supported by the Päivikki and Sakari Sohlberg Foundation (grants 7679, 230035) and Ane and Signe Gyllenberg Foundation (grants 5525, 5799).
Acknowledgments
The authors acknowledge and thank Lisa Grbin, Liaison Librarian at Deakin University, for expert advice and input into the search strategy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1288874/full#supplementary-material
References
1. Institute for Health Metrics and Evaluation (IHME). GBD Results. Seattle, WA: IHME, University of Washington. (2020). Available at: https://vizhub.healthdata.org/gbd-results/.
2. Winsper C, Bilgin A, Thompson A, Marwaha S, Chanen AM, Singh SP, et al. The prevalence of personality disorders in the community: A global systematic review and meta-analysis. Br J Psychiatry. (2020) 216:69–78. doi: 10.1192/bjp.2019.166
3. Chanen AM, Jovev M, Jackson HJ. Adaptive functioning and psychiatric symptoms in adolescents with borderline personality disorder. J Clin Psychiatry. (2007) 68:297–306. doi: 10.4088/JCP.v68n0217
4. Chanen AM, Thompson KN. The age of onset of personality disorders. In: Age of Onset of Mental Disorders: Etiopathogenetic and Treatment Implications. Springer International Publishing (2018). p. 183–201. doi: 10.1007/978-3-319-72619-9_10
5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (5th ed). Washington, DC: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
6. Bach B, First MB. Application of the ICD-11 classification of personality disorders. BMC Psychiatry. (2018) 18:351. doi: 10.1186/s12888-018-1908-3
7. World Health Organization. Musculoskeletal conditions. Available online at: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions.
8. Briggs AM, Cross MJ, Hoy DG, Sànchez-Riera L, Blyth FM, Woolf AD, et al. Musculoskeletal health conditions represent a global threat to healthy aging: A report for the 2015 world health organization world report on ageing and health. Gerontologist. (2016) 56:S243–55. doi: 10.1093/geront/gnw002
9. Quirk SE, Koivumaa-Honkanen H, Honkanen R, Kavanagh BE, Heikkinen J, Williams LJ. Exploring the comorbidity between personality and musculoskeletal disorders among adults: A scoping review. Front Psychiatry. (2023) 13:1079106. doi: 10.1186/s13643-021-01721-6
10. Dersh J, Gatchel RJ, Mayer T, Polatin P, Temple OR. Prevalence of psychiatric disorders in patients with chronic disabling occupational spinal disorders. Spine (Phila Pa 1976). (2006) 31:1156–62. doi: 10.1097/01.brs.0000216441.83135.6f
11. Long DM, Filtzer DL, BenDebba M, Hendler NH. Clinical features of the failed-back syndrome. J Neurosurg. (1988) 69:61–71. doi: 10.3171/jns.1988.69.1.0061
12. Polatin PB, Kinney RK, Gatchel RJ, Lillo E, Mayer TG. Psychiatric illness and chronic low-back pain. The mind and the spine–which goes first? Spine (Phila Pa 1976). (1993) 18:66–71. doi: 10.1097/00007632-199301000-00011
13. Keuroghlian AS, Frankenburg FR, Zanarini MC. The relationship of chronic medical illnesses, poor health-related lifestyle choices, and health care utilization to recovery status in borderline patients over a decade of prospective follow-up. J Psychiatr Res. (2013) 47:1499–506. doi: 10.1016/j.jpsychires.2013.06.012
14. Fu T, Gamble H, Siddiqui U. Psychiatric and Personality Disorder Survey of Patients with Fibromyalgia. Ann Depress Anxiety. (2015) 2(6):1064.
15. Gumà-Uriel L, Peñarrubia-María MT, Cerdà-Lafont M, Cunillera-Puertolas O, Almeda-Ortega J, Fernández-Vergel R, et al. Impact of IPDE-SQ personality disorders on the healthcare and societal costs of fibromyalgia patients: A cross-sectional study. BMC Fam Pract. (2016) 17:1–11. doi: 10.1186/s12875-016-0464-5
16. Thieme K, Turk DC, Flor H. Comorbid Depression and anxiety in fibromyalgia syndrome: Relationship to somatic and psychosocial variables. Psychosom Med. (2004) 66:837–44. doi: 10.1097/01.psy.0000146329.63158.40
17. Uguz F, Çiçek E, Salli A, Karahan AY, Albayrak I, Kaya N, et al. Axis I and Axis II psychiatric disorders in patients with fibromyalgia. Gen Hosp Psychiatry. (2010) 32:105–7. doi: 10.1016/j.genhosppsych.2009.07.002
18. Kayhan F, Küçük A, Satan Y, İlgün E, Arslan Ş, İlik F. Sexual dysfunction, mood, anxiety, and personality disorders in female patients with fibromyalgia. Neuropsychiatr Dis Treat. (2016) 12:349–55. doi: 10.2147/NDT.S99160
19. Blaney C, Sommer J, El-Gabalawy R, Bernstein C, Walld R, Hitchon C, et al. Incidence and temporal trends of co-occurring personality disorder diagnoses in immune-mediated inflammatory diseases. Epidemiol Psychiatr Sci. (2020) 29:e84. doi: 10.1017/S2045796019000854
20. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. (2004) 65:1660–5. doi: 10.4088/jcp.v65n1211
21. Kahl KG, Greggersen W, Rudolf S, Stoeckelhuber BM, Bergmann-Koester CU, Dibbelt L, et al. Bone mineral density, bone turnover, and osteoprotegerin in depressed women with and without borderline personality disorder. Psychosom Med. (2006) 68:669–74. doi: 10.1097/01.psy.0000237858.76880.3d
22. Kahl KG, Rudolf S, Stoeckelhuber BM, Dibbelt L, Gehl HB, Markhof K, et al. Bone mineral density, markers of bone turnover, and cytokines in young women with borderline personality disorder with and without comorbid major depressive disorder. Am J Psychiatry. (2005) 162:168–74. doi: 10.1176/appi.ajp.162.1.168
23. Penner-Goeke K, Henriksen CA, Chateau D, Latimer E, Sareen J, Katz LY. Reductions in quality of life associated with common mental disorders: results from a nationally representative sample. J Clin Psychiatry. (2015) 76:1506–12. doi: 10.4088/JCP.14m09271
24. Quirk SE, Koivumaa-honkanen H, Honkanen RJ, Mohebbi M, Kavanagh BE, Heikkinen J, et al. Associations between personality and musculoskeletal disorders in the general population: A systematic review protocol. Front Psychiatry. (2023) 13:1079162. doi: 10.3389/fpsyt.2022.1079162
25. Williams LJ, Quirk SE, Koivumaa-Honkanen H, Honkanen R, Pasco JA, Stuart AL, et al. Personality disorder and physical health comorbidities: A link with bone health? Front Psychiatry. (2020) 11:602342. doi: 10.3389/fpsyt.2020.602342
26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
27. Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z. JBI manual for Evidence Synthesis. (2014). doi: 10.46658/JBIMES-24-01
28. McWilliams LA, Higgins KS. Associations between pain conditions and borderline personality disorder symptoms: Findings from the national comorbidity survey replication. Clin J Pain. (2013) 29:527–32. doi: 10.1097/AJP.0b013e31826ab5d0
29. El-Gabalawy R, Katz LY, Sareen J. Comorbidity and associated severity of borderline personality disorder and physical health conditions in a nationally representative sample. Psychosom Med. (2010) 72:641–7. doi: 10.1097/PSY.0b013e3181e10c7b
30. Goldstein RB, Dawson DA, Chou SP, Ruan WJ, Saha TD, Pickering RP, et al. Antisocial behavioral syndromes and past-year physical health among adults in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. (2008) 69:368–80. doi: 10.4088/JCP.v69n0305
31. McWilliams LA, Clara IP, Murphy PD, Cox BJ, Sareen J. Associations between arthritis and a broad range of psychiatric disorders: findings from a nationally representative sample. J Pain. (2008) 9(1):37–44. doi: 10.1016/j.jpain.2007.08.002
32. Powers AD, Oltmanns TF. Borderline personality pathology and chronic health problems in later adulthood: the mediating role of obesity. Pers Disord. (2013) 4:152–9. doi: 10.1037/a0028709
33. Quirk SE, Stuart AL, Brennan-Olsen SL, Pasco JA, Berk M, Chanen AM, et al. Physical health comorbidities in women with personality disorder: Data from the Geelong Osteoporosis Study. Eur Psychiatry. (2016) 34:29–35. doi: 10.1016/j.eurpsy.2015.12.007
34. Olssøn I, Dahl AA. Avoidant personality problems - Their association with somatic and mental health, lifestyle, and social network. A community-based study. Compr Psychiatry. (2012) 53(6):813–21. doi: 10.1016/j.comppsych.2011.10.007
35. Covidence systematic review software. (2023) Melbourne, Australia: Veritas Health Innovation. Available at: https://www.covidence.org/.
36. Joanna Briggs Institute. Critical Appraisal tools for use in JBI Systematic Reviews. Available online at: https://jbi.global/critical-appraisal-tools.
37. Olssøn I, Dahl AA. Personality problems are considerably associated with somatic morbidity and health care utilisation. Eur Psychiatry. (2009) 24:442–9. doi: 10.1016/j.eurpsy.2009.05.004
38. Quirk SE, El-Gabalawy R, Brennan SL, Bolton JM, Sareen J, Berk M, et al. Personality disorders and physical comorbidities in adults from the United States: data from the National Epidemiologic Survey on Alcohol and Related Conditions. Soc Psychiatry Psychiatr Epidemiol. (2015) 50:807–20. doi: 10.1007/s00127-014-0974-1
39. Sleurs D, Tebeka S, Scognamiglio C, Dubertret C, Le Strat Y. Comorbidities of self-reported fibromyalgia in United States adults: A cross-sectional study from The National Epidemiological Survey on Alcohol and Related Conditions (NESARC-III). Eur J Pain (United Kingdom). (2020) 24:1471–83. doi: 10.1002/ejp.1585
40. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. J Am Med Assoc. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
41. Lievense AM, Bierma-Zeinstra SMA, Verhagen AP, Verhaar JAN, Koes BW. Influence of hip dysplasia on the development of osteoarthritis of the hip. Ann Rheum Dis. (2004) 63:621–6. doi: 10.1136/ard.2003.009860
42. Chandrasekaran V, Brennan-Olsen SL, Stuart AL, Pasco JA, Berk M, Hodge JM, et al. Bipolar disorder and bone health: A systematic review. J Affect Disord. (2019) 249:262–9. doi: 10.1016/j.jad.2019.02.013
43. Quirk SE, Williams LJ, O’Neil A, Pasco JA, Jacka FN, Housden S, et al. The association between diet quality, dietary patterns and depression in adults: A systematic review. BMC Psychiatry. (2013) 13:175. doi: 10.1186/1471-244X-13-175
44. Turk DC, Monarch ES. Biopsychosocial Perspective on Chronic Pain. 3rd Editio. Gatchel RJ, editor. Turk DC: Guilford Publications (2018). doi: 10.1176/jnp.9.4.623
45. Weisberg JN, Vittengl JR, Clark L, Gatchel RJ, Gorin AA. Personality and pain: Summary and future perspectives. In: Personality Characteristics of Patients with Pain. American Psychological Association (2004). p. 259–82. doi: 10.1037/10376-012
46. Weisberg JN, Vittengl JR, Clark LA, Gatchel RJ, Gorin AA. Personality and personality disorders in chronic pain. Curr Rev Pain. (2000) 4:60–70. doi: 10.1007/s11916-000-0011-9
47. Cattane N, Rossi R, Lanfredi M, Cattaneo A. Borderline personality disorder and childhood trauma: Exploring the affected biological systems and mechanisms. BMC Psychiatry. (2017) 17(1):221. doi: 10.1186/s12888-017-1383-2
48. Afifi TO, Mather A, Boman J, Fleisher W, Enns MW, Macmillan H, et al. Childhood adversity and personality disorders: results from a nationally representative population-based study. J Psychiatr Res. (2011) 45:814–22. doi: 10.1016/j.jpsychires.2010.11.008
49. Raposo SM, Mackenzie CS, Henriksen CA, Afifi TO. Time does not heal all wounds: older adults who experienced childhood adversities have higher odds of mood, anxiety, and personality disorders. Am J Geriatr Psychiatry. (2014) 22:1241–50. doi: 10.1016/j.jagp.2013.04.009
50. Björkenstam E, Ekselius L, Burström B, Kosidou K, Björkenstam C. Association between childhood adversity and a diagnosis of personality disorder in young adulthood: a cohort study of 107,287 individuals in Stockholm County. Eur J Epidemiol. (2017) 32:721–31. doi: 10.1007/s10654-017-0264-9
51. Afifi TO, Mota N, MacMillan HL, Sareen J. Harsh physical punishment in childhood and adult physical health. Pediatrics. (2013) 132:e333–40. doi: 10.1542/peds.2012-4021
52. You DS, Albu S, Lisenbardt H, Meagher MW. Cumulative childhood adversity as a risk factor for common chronic pain conditions in young adults. Pain Med (United States). (2019) 20:486–94. doi: 10.1093/pm/pny106
53. Johnson BN, Lumley MA, Cheavens JS, McKernan LC. Exploring the links among borderline personality disorder symptoms, trauma, and pain in patients with chronic pain disorders. J Psychosom Res. (2020) 135:110164. doi: 10.1016/j.jpsychores.2020.110164
54. Crowell SE, Beauchaine TP, Linehan MM. A biosocial developmental model of borderline personality: elaborating and extending Linehan’s theory. Psychol Bull. (2009) 135:495. doi: 10.1037/A0015616
55. Saccaro LF, Schilliger Z, Dayer A, Perroud N, Piguet C. Inflammation, anxiety, and stress in bipolar disorder and borderline personality disorder: A narrative review. Neurosci Biobehav Rev. (2021) 127:184–92. doi: 10.1016/j.neubiorev.2021.04.017
56. Sansone RA, Sansone LA. Borderline personality and the pain paradox. Psychiatry (Edgmont). (2007) 4(4):40–6.
57. Sansone RA, Sansone LA. Chronic pain syndromes and borderline personality. Innov Clin Neurosci. (2012) 9:10–4.
58. Schmahl C, Bohus M, Esposito F, Treede RD, Di Salle F, Greffrath W, et al. Neural correlates of antinociception in borderline personality disorder. Arch Gen Psychiatry. (2006) 63(6):659–67. doi: 10.1001/archpsyc.63.6.659
59. Niedtfeld I, Schmitt R, Winter D, Bohus M, Schmahl C, Herpertz SC. Pain-mediated affect regulation is reduced after dialectical behavior therapy in borderline personality disorder: a longitudinal fMRI study. Soc Cognit Affect Neurosci. (2017) 12:739. doi: 10.1093/SCAN/NSW183
60. Gatchel RJ. Comorbidity of chronic pain and mental health disorders: The biopsychosocial perspective. Am Psychol. (2004) 59:795–805. doi: 10.1037/0003-066X.59.8.795
61. Wu A, Francois D. Personality disorders in late life: an update. GeroPsych J Gerontopsychology Geriatr Psychiatry. (2022) 35:167–75. doi: 10.1024/1662-9647/a000261
Keywords: personality disorder, PD, musculoskeletal disorders, musculoskeletal diseases, review, systematic review, comorbidity
Citation: Quirk SE, Koivumaa-Honkanen H, Honkanen RJ, Mohebbi M, Stuart AL, Heikkinen J and Williams LJ (2024) A systematic review of personality and musculoskeletal disorders: evidence from general population studies. Front. Psychiatry 15:1288874. doi: 10.3389/fpsyt.2024.1288874
Received: 05 September 2023; Accepted: 15 April 2024;
Published: 21 May 2024.
Edited by:
Michele Sanza, University of Bologna, ItalyReviewed by:
Jorge Magalhães Rodrigues, Atlântico Business School, PortugalCarmen María Galvez-Sánchez, University of Jaén, Spain
Copyright © 2024 Quirk, Koivumaa-Honkanen, Honkanen, Mohebbi, Stuart, Heikkinen and Williams. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shae E. Quirk, c2hhZS5xdWlya0BkZWFraW4uZWR1LmF1
 Shae E. Quirk
Shae E. Quirk Heli Koivumaa-Honkanen2,3
Heli Koivumaa-Honkanen2,3 Risto J. Honkanen
Risto J. Honkanen Amanda L. Stuart
Amanda L. Stuart Lana J. Williams
Lana J. Williams