- Department of Gastroenterology, Peking University Third Hospital, Beijing, China
Objective: Potential causal associations between psychiatric disorders and irritable bowel syndrome have been demonstrated in observational studies; however, these studies are susceptible to underlying confounding and reverse causation biases. We aimed to assess the causal effects of psychiatric disorders on irritable bowel syndrome (IBS) and the potential mediators from a genetic perspective by conducting a Mendelian randomization (MR) study with mediation analysis.
Method: Genetic instruments associated with psychiatric disorders, potential mediators, and IBS were obtained from large-scale genome-wide association studies (GWAS). Three MR methods - the inverse-variance weighted (IVW) method, MR-Egger method, and weighted median method, were used to investigate causal association estimates. Heterogeneity among different genetic instrumental variables (IVs) was assessed using Q tests. Additionally, the MR-PRESSO and MR-Pleiotropy methods were used to verify horizontal pleiotropy and detect outliers that might bias the results, which were removed from further analysis. Consequently, we used MR mediation analysis to investigate potential mediators in the causal associations between psychiatric disorders and IBS.
Results: MR provided evidence of the causal effects of genetically predicted broad depression, major depressive disorder (MDD), anxiety disorder, post-traumatic stress disorder (PTSD), and schizophrenia on IBS. The results of MR mediation analysis demonstrated that the reduction in acetate levels mediated 12.6% of the effects of broad depression on IBS; insomnia mediated 16.00%, 16.20%, and 27.14% of the effects of broad depression, MDD, and PTSD on IBS, respectively; and the increase in blood β-hydroxybutyrate levels mediated 50.76% of the effects of schizophrenia on IBS.
Conclusion: Our study confirmed the brain-gut axis involvement and potential modulators in the pathophysiology of psychiatric disorder-induced IBS from a genetic perspective, and suggests potential therapeutic targets for the disrupted brain-gut axis.
1 Introduction
Irritable bowel syndrome (IBS) is a common and chronic gut-brain interaction disorder, characterized by a combination of abdominal symptoms such as pain, bloating, and changes in bowel habits and stool form, with an abnormal mental state (1, 2). IBS affects people across the world, with a reported prevalence of 7.0% in Southeast Asian and Middle Eastern studies, 11.8–14.0% in North American, Northern European, and Australian studies, and 15.0–21.0% in Southern European, African, and South American studies (3). Although IBS is not a life-threatening condition, it reduces the quality of life and imposes a high socioeconomic burden on patients, especially women and young individuals (4, 5). The pathophysiology of IBS is complex and not completely understood; however, genetics, diet, infectious gastroenteritis, and the gut microbiome are known risk factors for IBS (6). Genetic variants in genes encoding serotonin transporter (SLC6A4), sucrase-isomaltase or SCN5A (a voltage-gated sodium channel), and corticotropin-releasing hormone (CRH) receptors 1 and 2 may be associated with IBS (6).
Numerous studies have shown that psychiatric disorders frequently accompany IBS, particularly depressive and anxiety disorders, which occur in up to 23% of IBS cases (6, 7). Comorbidities of post-traumatic stress disorder (PTSD) and schizophrenia have also been identified in IBS patients; for instance, 36% of IBS patients met the lifetime diagnostic criteria of PTSD (8, 9), and 13.6% of PTSD patients met the Rome III criteria for IBS (9, 10). The prevalence of IBS in schizophrenia and attention-deficit/hyperactivity disorder (ADHD) was significantly higher than that in the control group without schizophrenia and ADHD (8, 11, 12). Interestingly, a recent genome-wide pleiotropic association study revealed a shared genetic etiology between IBS and PTSD, ADHD, bipolar disorder, major depressive disorder (MDD), and anorexia nervosa (AN), indicating that IBS and psychiatric disorders may share a common or linked pathogenic pathway in the brain-gut axis (13). According to previous studies, psychological symptoms and disorders may have developed as a result of the effect of IBS on an individual or may have existed before the onset of digestive symptoms (6, 14). All evidence suggests that the brain–gut axis (the bidirectional interaction between the central and enteric nervous systems) plays a pivotal role in the pathophysiology of IBS (15). However, despite strong epidemiological evidence on the association between IBS and psychiatric disorders, most studies have been observational, with limitations of being susceptible to potential confounding and reverse causation biases, which can lead to inconsistent and biased results. Consequently, the current understanding of the association between IBS and psychiatric disorders remains tenuous.
In this study, we aimed to explore whether psychiatric disorders are risk factors for IBS by conducting a Mendelian randomization (MR) study. MR is a widely used genetic epidemiological method that utilizes single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to investigate the potential causal effects of risk factors on health outcomes. Accumulating evidence has proven the reliability of MR studies. Alleles are randomly separated during meiosis; therefore, MR is less likely to be influenced by confounders (e.g., environmental exposure, socioeconomic status, and behavior). MR can also prevent the reverse causation bias that exists in observational findings because genetic variation occurs before the disease, and the order of the two cannot be reversed (16–18).
In difficult cases, such as the above-mentioned causal associations between psychiatric disorders and IBS, when MR findings are similar with those of observational studies and consistently demonstrate the associations, it provides a higher level of confidence in establishing the causal relationships between these two pathologies, and the interplay in the brain-gut axis can be further confirmed from a genetic perspective. This will prompt physicians to pay more attention to the digestive symptoms of patients with psychological distress at an earlier stage than before. It will facilitate a more proactive approach to managing and treating these disorders adequately to prevent psychotic episodes, exacerbation of digestive symptoms, and subsequent development of IBS. Ultimately, this approach will help to improve patients’ quality of life. In this study, we performed an MR mediation analysis to investigate the potential mediators between psychiatric disorders and IBS to explore the underlying pathophysiological modulators of brain-gut interactions.
2 Materials and methods
2.1 Data sources
2.1.1 Psychiatric disorders
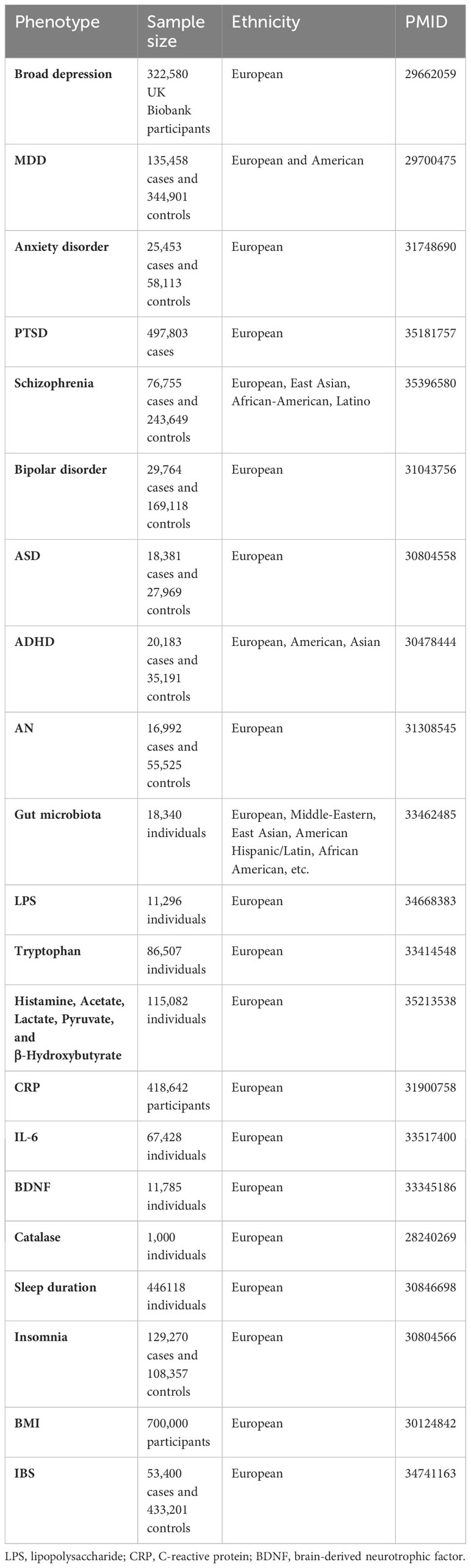
Two sets of genetic instruments were used to determine the cause of depression in patients with IBS. Fourteen independent variants significantly associated with broad depression (defined as self-reported past help-seeking for mental health difficulties such as nerves, anxiety, tension, or depression) were identified through a genome-wide association study (GWAS) meta-analysis conducted in 2018 with a participant pool of 322,580 UK Biobank participants (19). Genetic instruments for MDD were also derived from a GWAS meta-analysis performed in 2018, which contained seven cohorts with 135,458 MDD cases and 344,901 controls (20), in which 44 SNPs were reported to be significantly related to MDD.
Only five genome-wide SNPs significantly associated with lifetime anxiety disorders were selected from the 2019 GWAS (21), including 25,453 cases and 58,113 controls (21). Genetic instruments for PTSD were taken from the most recent GWAS by Wendt et al. (22), who reported 32 independent SNPs that showed genome-wide significance and found to be associated with PTSD when meta-analyzed using the 6-item PTSD trait. Genetic instruments for schizophrenia were obtained from a GWAS meta-analysis conducted in 2022 by Trubetskoy et al. (23), who identified 343 independent SNPs located at 287 loci in an extended GWAS. Thirty SNPs associated with bipolar disorder were obtained from a GWAS by Stahl et al. (24), who used data from 198,882 individuals of European ancestry collected from 39 cohorts across Europe, North America, and Australia.
In addition to the above-mentioned psychiatric disorders that are usually associated with IBS, we further explored several other psychiatric disorders, including Autism Spectrum Disorder (ASD) (25), ADHD (26), and AN (27), that show microbiota-gut-brain axis dysfunction. All sources of the SNPs are listed in Table 1. Furthermore, the inclusion and exclusion criteria of patients with psychiatric disorders were important to identify genome-wide-significant loci and can be found in these GWAS study or GWAS meta-analysis, especially the prescribed medication was considered as one of the key indicators, for example, the patients who reported having a prescribed medications for any psychiatric disorders were excluded when selecting healthy/control individuals (19, 21, 23, 24).
2.1.2 Potential mediators
The term “brain–gut–microbiome axis” has long been proposed, whereby the composition and function of gut microbiota are affected by the abnormal central nervous system, resulting in gastrointestinal symptoms due to the related dysbiosis intestinal homeostasis, such as inflammation, oxidative stress, as well as changes in microbiome-derived neurotransmitters, metabolites, neuroendocrine factors, and enzymes (28). However, details of the microbiome-related pathophysiology of IBS remain elusive. Additionally, sleep disorders and an increase in body mass index (BMI) are triggered by psychiatric stressors (29, 30) and are also risk factors for IBS (31, 32); however, whether BMI and sleep disorders mediate the causal effects between psychiatric disorders and IBS remains unclear. In this study, we suspected that these factors are potential mediators of the causal effects of psychiatric disorders on IBS. Genetic instruments of the gut microbiota were acquired from a large-scale association study (33) containing 24 cohorts of 18,340 participants from different ancestries, most of whom were European. The sources of the IVs for the other potential mediators are shown in Table 1 (34–43).
2.1.3 IBS
Genetic instruments associated with IBS were derived from the latest GWAS conducted by Eijsbouts et al. (44) encompassing 53,400 European cases and 433,201 controls. These IBS cases should meet at least one of the following four conditions: 1) meet the digestive health questionnaire (DHQ) Rome III symptom criteria without other diagnostic explanations for these symptoms; 2) have a DHQ self-report of previous medical IBS diagnosis or electronic medical records; 3) provide an unprompted ‘self-report’; and 4) a self-reported IBS diagnosis in response to the question ‘Has a doctor ever told you that you have any … serious medical conditions?’ Linked hospital episode statistics indicated that hospital admission due to IBS was the primary or secondary ICD-10 diagnosis. The DHQ also asked about previous IBS diagnosis, environmental exposures and associated conditions (including anxiety or depression, based on treatment sought or offered). Full GWAS summary statistics are available at https://www.ebi.ac.uk/gwas/publications/34741163.
2.2 Mendelian randomization design and SNP selection
The MR is established based on three core assumptions, as shown in Figure 1A: (i) relevance: IVs such as SNPs are strongly associated with exposures (p < 5 × 10–8); (ii) independence: instrumental SNPs should be independent from the potential confounders that might influence the outcome and exposures; and (iii) exclusion hypothesis: SNPs are only associated with outcome through the exposures without other alternative ways, that is, if SNPs can directly affect the outcomes without through the exposure, the results of MR have horizontal pleiotropic effects and violated the third assumption (18). The most important step in MR analysis is the selection of the appropriate SNPs to be used as instruments (Supplementary Figure 1), which must be strongly linked to exposures with low linkage disequilibrium (LD) (r2 < 0.001, window size = 10000 kb). To meet the second assumption and reduce interference from confounders in Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/), each instrumental SNP was estimated for possible connections with confounders, including BMI, white matter microstructure, sleep duration, insomnia, household income, and smoking. SNPs associated with these confounders were subsequently excluded from MR analysis. For harmonization, palindromic SNPs were excluded because the directions of the positive and negative chains could not be determined for the same alleles on both strands.
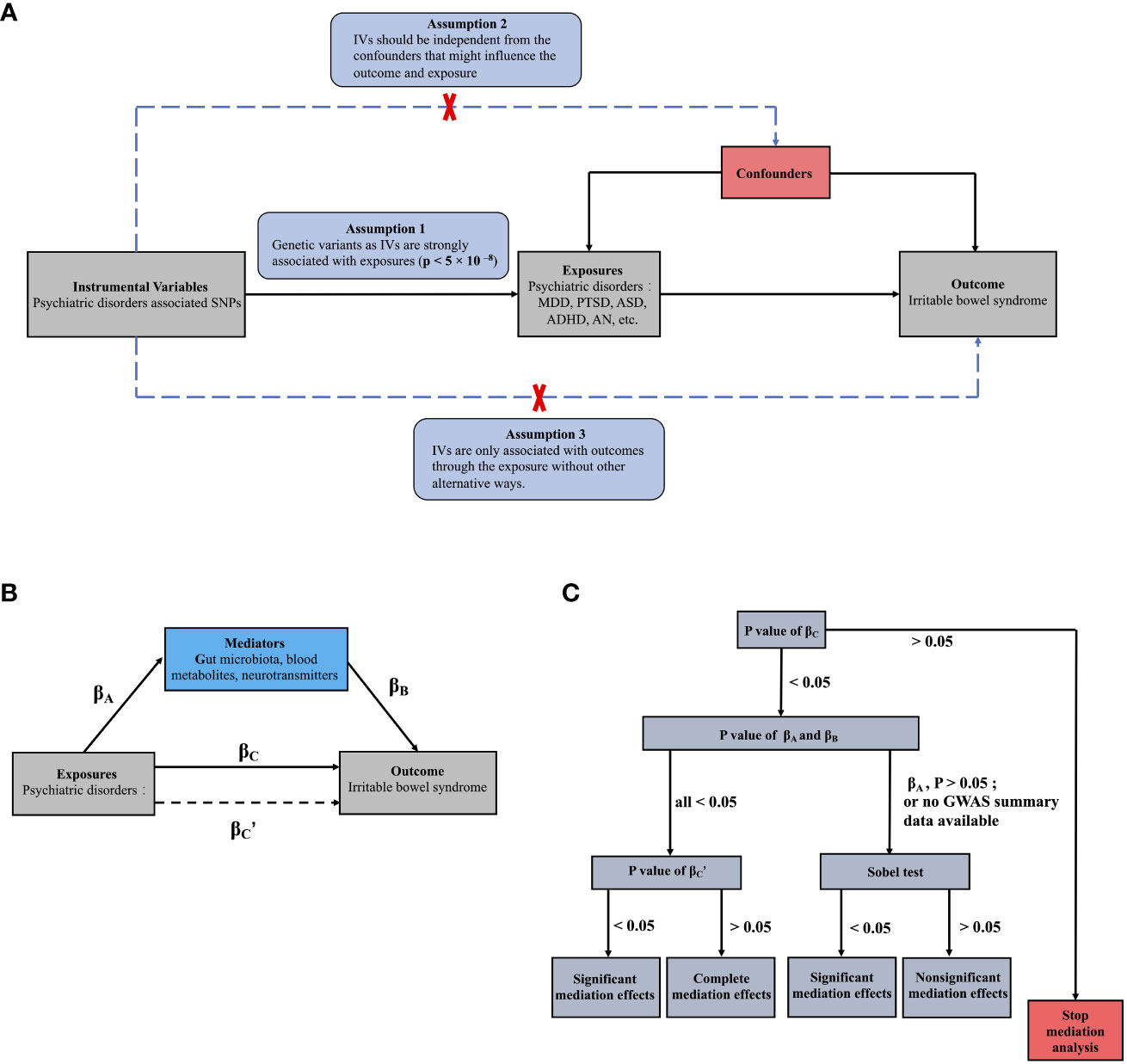
Figure 1 The main design of this MR study: (A) Three assumptions of the MR study; (B) Basic schematic of mediation analysis; (C) The process of MR mediation analysis in the present study. (B) βA represents the regression coefficients for the association between psychiatric disorders and potential mediators; βB represents the regression coefficients for the association between potential mediators and IBS; βC represents the total effect between psychiatric disorders and IBS, without the adjustment for mediator; and βC’ represents the direct effect between psychiatric disorders and IBS, taking into account adjustment for potential mediators.
We used univariable two-sample MR study to investigate the causal associations between psychiatric disorders and IBS. Then we further investigated potential pathways from psychiatric disorders to IBS by using univariable and multivariable MR studies: First, the causal effects of the potential mediators proposed in Part 2.1.2 on IBS were investigated through a univariable two-sample MR study (βB). Second, the causal associations between psychiatric disorders and these mediators that showed significant causal effects on IBS were also acquired (βA). Lastly, MR mediation analysis was utilized to investigate whether the mediators associated with both psychiatric disorders (βA; P < 0.05) and IBS (βB; P < 0.05) mediated any causal effects of psychiatric disorders on IBS (45). For the mediation analysis, multivariable MR was performed as previously described (46). Briefly, IVs for both psychiatric disorders and mediators were included in the same MR model to investigate their direct effects on IBS after mutual adjustment. Any attenuation of the direct MR effects (βC’; P<0.05) of psychiatric disorders, after adjusting for mediators, would support mediation through mediators (Figures 1B, C) compared with those observed in the two-sample MR measuring the total effects (βC) of psychiatric disorders on IBS (47, 48). Additionally, if the βA showed nonsignificant P values or the GWAS summary data for psychiatric disorders or mediators were not fully available, limiting multivariable MR analysis, the Sobel test was used to infer mediation effects (49).
2.3 Statistical methods
All statistical analyses were performed using R 4.1.0 with the R packages “TwoSampleMR”, “MR-PRESSO”, and “MRlap”. In the MR estimation, we chose the inverse-variance weighted (IVW) method as the primary analysis, which can obtain an unbiased result only when all IVs are valid with no directional pleiotropy effects. MR analysis was also conducted using other methods to check the robustness of the results, such as the weighted median method and MR-Egger method, which can estimate the causal effect in cases where some IVs are invalid (31). The weighted median method estimates allow up to 50% of the information in the analysis to come from invalid IVs; thus, it provides consistent effect estimates even in the presence of IVs with heterogeneous effects (50). Considering pleiotropic effects, the MR-Egger method provides a valid causal estimate if the SNP-exposure associations are not correlated with the direct effects of IVs on the outcome (51). If the P values of MR estimation were less than 0.05, it indicates significant causal associations. Additionally, the MR-pleiotropy method was used to verify the horizontal pleiotropy, and the MR-Pleiotropy Residual Sum and Outlier method (MR-PRESSO) was used to detect outliers that might introduce bias into the results. Subsequently, the outliers were removed from further analyses. In addition, the presence of heterogeneity among different IVs was assessed using Cochran’s (IVW) and Rücker’s (MR-Egger) Q tests.
Given that our GWAS summary statistics are mostly drawn from European populations; the possibility of sample overlap cannot be ignored. To mitigate potential bias arising from sample overlap, we applied the MRlap function to correct the IVW results (52). If the corrected effects are closely consistent with the observed effects and do not exhibit significant differences, then we can be reasonably confident in the IVW-MR estimates. Conversely, if substantial differences occur, the corrected effect should be given priority because it is independent of sample overlap. GWAS summary data on exposures and outcomes were needed in MRlap analysis, and if it was not available, we assessed the bias and Type 1 error rates caused by sample overlap in MR (53).
3 Results
3.1 Step 1: Associations between genetically predicted psychiatric disorders and IBS
After removing the SNPs in LD or palindromes, the outliers identified in the MR-PRESSO method, as well as the SNPs associated with confounders between psychiatric disorders and IBS (i.e., broad depression: white matter abnormalities (54, 55); MDD: sleep disorders (56–58); PTSD: household income (59, 60); schizophrenia: insomnia, smoking, and white matter abnormalities (61–64); bipolar disorder: BMI (65, 66); ADHD: insomnia (67)), the remaining SNPs utilized as IVs are shown in Supplementary Tables S1-S9. Both the IVW MR method and weighted median method showed statistically significant associations between genetically predicted broad depression (IVW OR: 3.92, P: 0.007; weighted median OR: 3.11, P:0.023), MDD (IVW OR: 2.53, P: 3.20×10-10; weighted median OR: 1.65, P: 0.006), anxiety disorder (IVW OR: 1.12, P: 0.006; weighted median OR: 1.09, P: 0.032), PTSD (IVW OR: 1.92, P: 5.02×10-5; weighted median OR: 1.74, P: 0.001), schizophrenia (IVW OR: 1.08, P: 0.016; weighted median OR: 1.10, P: 0.023) and risk of IBS, although the MR-Egger method showed no statistical significance. The MR pleiotropy method identified non-significant horizontal pleiotropy in these associations, while the Q tests showed significant heterogeneity except for anxiety disorders (Figures 2A–E, 3). Moreover, non-significant associations were found between genetically predicted bipolar disorder, bipolar I disorder, ASD, ADHD, AN, and the risk of IBS using all three methods (P > 0.05), and neither heterogeneity nor horizontal pleiotropy was identified in these associations, suggesting that these results were consistent (Figures 2F–I, 3, 4).
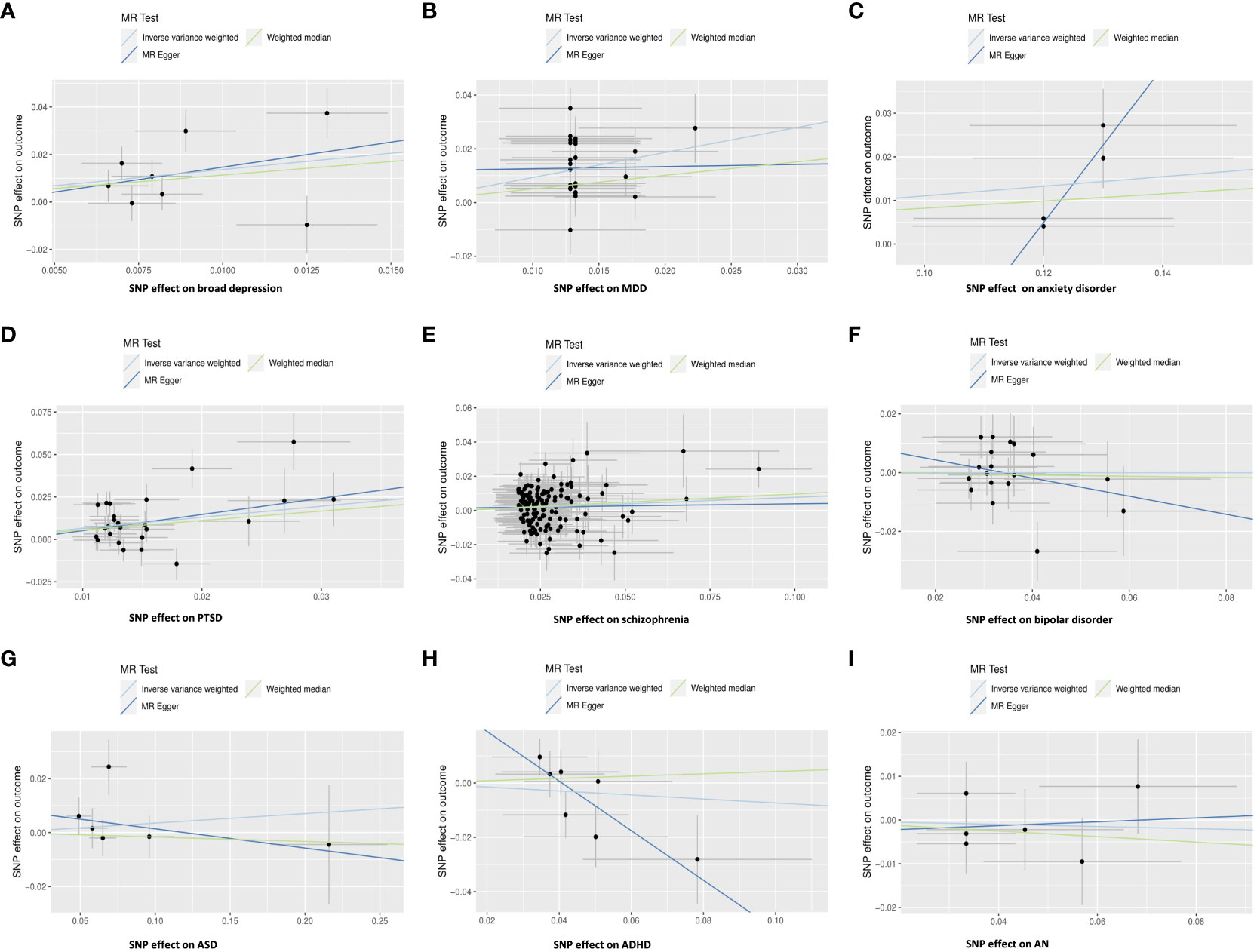
Figure 2 Scatter plot: Relationships between genetically predicted psychiatric disorders and IBS using the IVW method, weighted median method, and MR-Egger method. (A) broad depression; (B) MDD; (C) anxiety disorder; (D) PTSD; (E) schizophrenia; (F) bipolar disorder; (G) ASD; (H) ADHD; (I) AN.
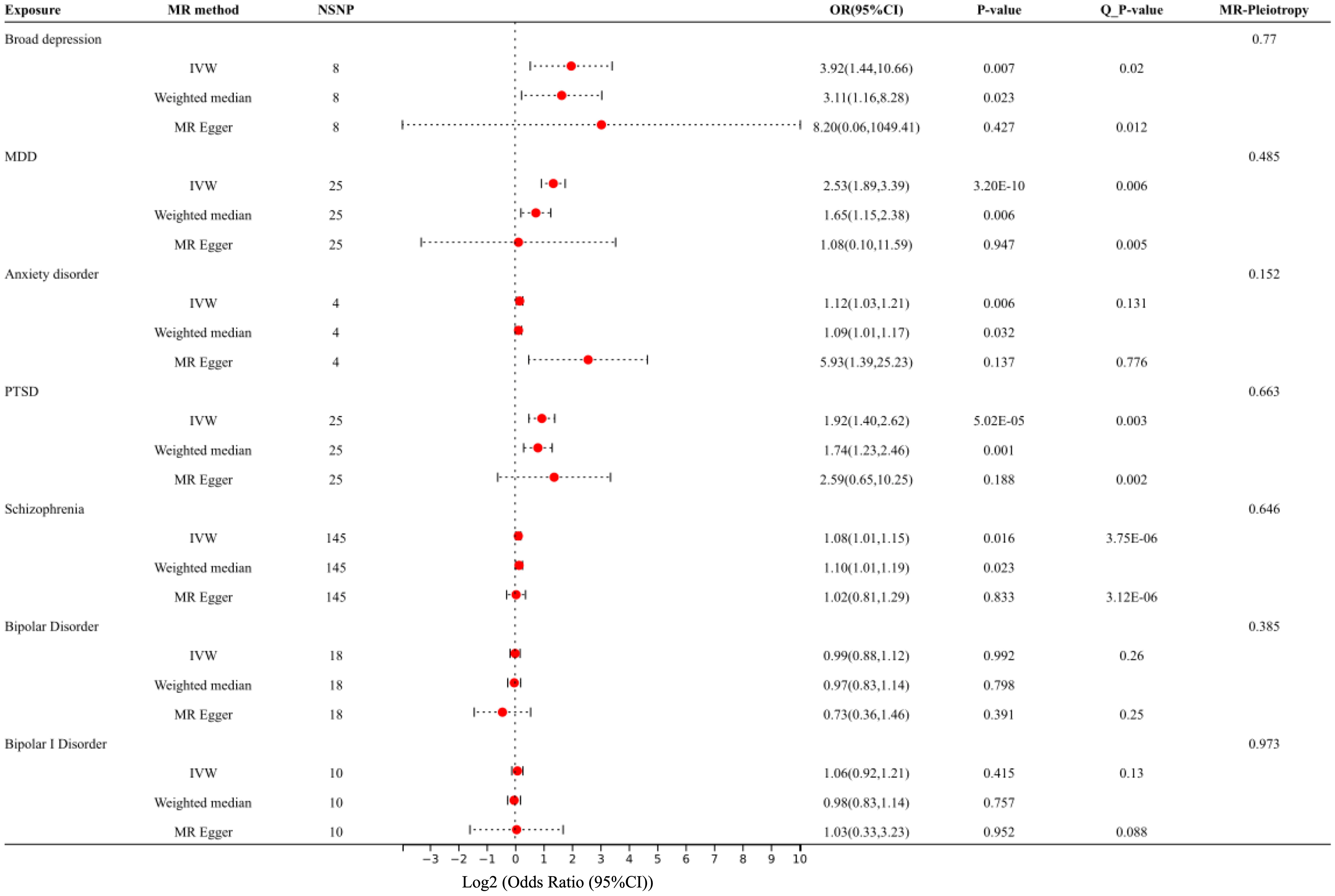
Figure 3 Forest plot: Association of genetically predicted broad depression, MDD, anxiety, PTSD, schizophrenia, bipolar disorder, and IBS, using the IVW method, weighted median method, and MR-Egger method. The effect estimates are presented as odds ratios (OR, odds ratios; NSNP, the number of single nucleotide polymorphisms; 95% CI, 95% confidence interval; Q_P value, the results of the Q test).
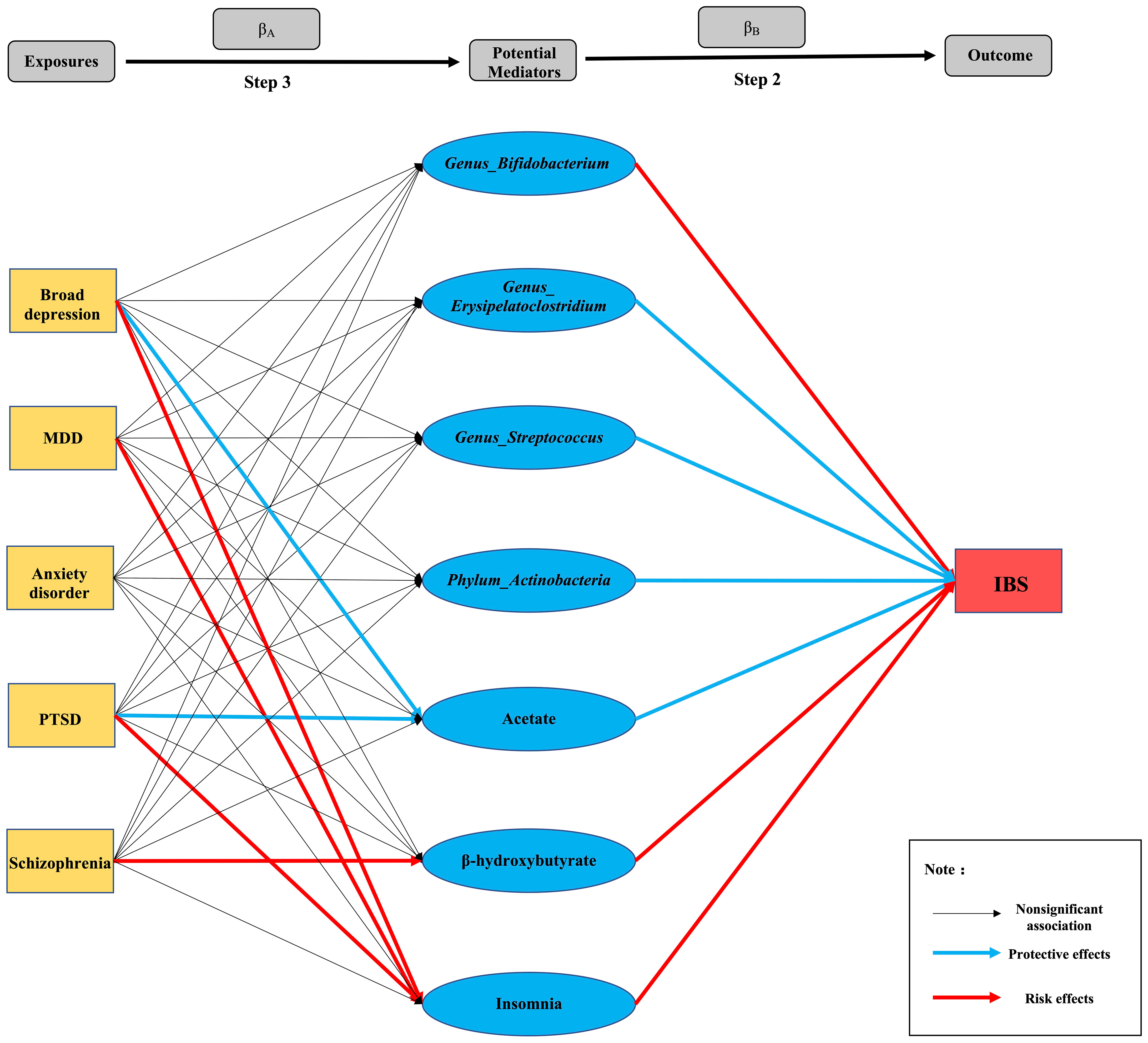
Figure 4 Summary diagram of Step 2 and Step 3: Each line segment between exposures and outcomes represents a connection, and the arrow represents the direction of the association.
3.2 Step 2: Associations between genetically predicted potential mediators and IBS
We investigated 24 potential mediators of the causal effects of psychiatric disorders on IBS after a literature review. As shown in Supplementary Table S10, the gut microbiota genera, Erysipelatoclostridium (IVW OR: 0.895, P =0.028) and Streptococcus (IVW OR: 0.958, P: 0.002) had protective effects on IBS. However, the causal effects of the Actinobacteria phylum and its genus Bifidobacterium on IBS showed inconsistent results, i.e., the estimates of the IVW method and weighted median method showed the protective effects of the Actinobacteria phylum (IVW OR:0.944, P: 1.826E-5) and Bifidobacterium genus (IVW OR: 0.982, 0.045) on IBS, while the MR-Egger method showed anti-protective effects (Actinobacteria phylum, MR-Egger OR: 1.348, P: 0.046; Bifidobacterium genus, MR-Egger OR: 1.179, P: 0.002). No causal associations were found between IBS and the genera Allisonella, Enterorhabdus, Ruminococcus1, Ruminococcustorques, and Tyzzerella3.
Regarding blood metabolites, inflammatory biomarkers, neurotransmitters, and acetate showed protective effects on IBS (IVW OR: 0.719, P: 0.048); however, β-hydroxybutyrate showed anti-protective effects (weighted median, OR: 1.294, P: 0.004). Simultaneously, inconsistent results were also observed in the association between IL-6 and IBS, in which the IVW (OR: 1.137, P: 6.923E-16) and weighted median method (OR: 1.069, P: 0.009) showed that increasing levels of IL-6 had a causal effect on IBS, whereas, the MR-Egger method showed that IL-6 had a protective effect on IBS (OR:0.883, P =0.023). Additionally, there was no evidence supporting the causal associations among genetically predicted lipopolysaccharide (LPS), C-reactive protein (CRP), tryptophan, histamine, lactate, pyruvate, catalase, or brain-derived neurotrophic factor (BDNF) and IBS in any of the three methods of our MR study.
Insomnia was a significant risk factor for IBS (IVW OR: 2.051; P: 1.548E-5), as shown by all three MR methods. However, there was no significant causal relationship between sleep duration and IBS (IVW OR: 0.916; P: 0.740). Furthermore, we found no significant association between genetically predicted BMI and IBS using the three MR methods (IVW OR: 1.056, P:0.994) (Supplementary Tables S11-S34).
3.3 Step 3: Associations between genetically predicted psychiatric disorders and potential mediators significantly associated with IBS
Here, we assumed that psychiatric disorders with significant causal associations with IBS were exposures (broad depression, MDD, anxiety disorder, PTSD, and schizophrenia), and potential mediators with significant causal associations with IBS were outcomes to explore their causal associations (βA). We found that broad depression (IVW OR: 0.598, P: 0.031) and PTSD (IVW OR: 0.867, P: 0.021) causally led to lower levels of acetate and an increased risk of insomnia (broad depression, IVW OR: 1.356, P: 0.001; PTSD, IVW OR: 1.279, P: 4.085E-10). In addition to broad depression and PTSD, an increased risk of insomnia was found in MDD (IVW OR: 1.234, P: 1.397E-07); however, all showed significant heterogeneity. Furthermore, the MR-Egger method showed a significant causal effect of genetically predicted schizophrenia on increased blood β-hydroxybutyrate levels (IVW OR: 1.164, P:0.007), with significant horizontal pleiotropy (P = 0.015). Unfortunately, since the GWAS summary data for IL-6 were not available, we were unable to study the causal effects of psychiatric disorders on IL-6 levels, and we did not find any potential mediators affected by anxiety disorders, which requires further exploration (Supplementary Table S35).
3.4 Step 4: MR mediation analysis
Based on the results of step 2 and step 3 (Figure 4), we conjectured that 1) the reduction in acetate might mediate the effects of broad depression and PTSD on IBS; 2) insomnia might mediate broad depression, MDD, and PTSD-induced IBS; and 3) the increase in β-hydroxybutyrate levels in the blood might mediate schizophrenia-induced IBS (P values of βA, βB, and βC were all lower than 0.05). Thus, we performed a multivariable MR to investigate βC’. We found a significant attenuation in the effect of genetically predicted broad depression on IBS after adjusting for acetate or insomnia (βC’, P < 0.05), which mediated 12.60% and 16.00% causal effects of broad depression on IBS, respectively. Unfortunately, since the GWAS summary data for MDD, PTSD, and schizophrenia were not fully available, we were unable to perform multivariate MR analysis to identify their mediators. Therefore, Sobel tests were performed instead, in which we verified that insomnia mediated 16.20% and 27.14% of the causal effect of genetically predicted MDD and PTSD on IBS, respectively, and the increase in β-hydroxybutyrate levels in the blood mediated 50.76% of the causal effect of genetically predicted schizophrenia on IBS (Table 2).
3.5 MRlap analysis
We found that the samples in GWAS on broad depression, MDD, anxiety disorder, PTSD, AN, blood metabolites, CRP, sleep duration, insomnia, and BMI might be overlapped with the samples from GWAS on IBS, because they all contained the participants from UK Biobank. The GWAS summary data on broad depression, blood metabolites (acetate, lactate, pyruvate, and β-hydroxybutyrate), sleep durations, insomnia, and BMI were available, thus the MRlap analysis was performed, which showed that the causal effects of these indicators on IBS, as determined by the MRlap correction, align with those obtained through the primary MR analyses (Supplementary Table S36). The GWAS summary data on MDD, anxiety disorder, PTSD, AN, histamine, and CRP were not available in our included studies, however, low bias and Type 1 error rates were found (Supplementary Table S37). These outcomes reaffirmed the robustness of the IVW method.
4 Discussion
4.1 MR study confirmed the causal associations between psychiatric disorders and IBS
To the best of our knowledge, this is the first study to illustrate the causal associations and potential mediators between psychiatric disorders and IBS from a genetic perspective. In this two-sample MR study, we found that broad depression, MDD, lifetime anxiety disorder, schizophrenia, and PTSD were risk factors for IBS.
Depression and anxiety are common psychiatric symptoms of IBS. In a Chinese population, diarrhea-predominant IBS (IBS-D) and depressive disorder showed two shared genetic variants (SYT8 rs3741231 G allele and SSPO rs12536873 TT genotype) associated with neurogenesis and neurotransmission, providing a genetic basis for the high comorbidity of IBS-D and depressive disorder (68). A recent meta-analysis showed that patients with IBS have an eight-fold greater risk of anxiety and a seven-fold greater risk of depression than controls in the Indian population (69). Two prospective studies have found that higher levels of anxiety and depression at baseline were significant predictors of IBS at the 1-year and 12-year follow-ups; conversely, IBS patients without anxiety and depression at baseline reported significantly higher levels of anxiety and depression at the 1-year follow-up, suggesting that independent gut-brain and brain-gut pathways operate in the pathophysiology of IBS (15, 70). However, it is likely that underlying confounders, such as changes in medication, life stressors, environmental exposure, socioeconomic burden, lifestyle, or other conditions unrelated to gastroenterology, will affect these data over a long or even a short period of time, leading to biased results (70). MR studies can methodologically exclude interference from these confounders. The results of our study agree with those of these longitudinal studies, further confirming the above evidence.
The association between PTSD and IBS remains controversial. A meta-analysis (10) comprising eight studies showed that PTSD was a significant risk factor for IBS. This finding is consistent with another study, which revealed a correlation between early-life abuse and trauma and a higher risk for the development of IBS in adulthood (8). However, some studies have disputed this association, as they found that a history of severe sexual/physical abuse was associated with higher pain thresholds for rectal distension in women with IBS (71). Additionally, a single-arm study showed that the prevalence of PTSD was not higher among patients with IBS than in the general population (72). Interestingly, our study confirmed that genetically predicted PTSD is a risk factor for IBS, opening new directions for future mechanistic studies.
Schizophrenia is a complex and debilitating brain disorder characterized by behavioral abnormalities, including cognitive dysfunction, psychosis, delusions, apathy, and withdrawal (73). Some risk factors for schizophrenia, such as inflammation, food intolerance, and Toxoplasma gondii exposure, partly involve the biological pathways of the gut, indicating that the gut-brain interaction may play a pivotal role in the pathophysiology of schizophrenia (73). To date, only a few studies have explored the association between schizophrenia and IBS, and meta-analyses have been conducted to verify this relationship. A cohort study by Lee et al. (74) showed no statistically significant difference in the incidence rate of schizophrenia between the IBS cohort and the cohort without IBS, even after 5 years of follow-up. Another small-scale retrospective study in 1997 suggested that the schizophrenia group exhibited a higher risk of IBS than the control group (11). After over 25 years, this result was reconfirmed by our MR study.
4.2 MR study suggests no causal link between bipolar disorder and IBS.
Abnormal levels of cytokines, including TNF-α, IL-8, and IL-10, are significantly associated with IBS and bipolar disorder symptoms, indicating that IBS and bipolar disorder share similar pathophysiological mechanisms (75–79). Thus, the comorbidity of both disorders was hypothesized. However, our MR study suggests no causality between bipolar disorder (including bipolar I disorder) and IBS incidence, which is consistent with the results of a meta-analysis of 11 studies that provided data on IBS (80). Conversely, IBS was found to increase the incidence of subsequent bipolar disorder in a nationwide cohort study; however, this finding needs to be further confirmed in future studies (74).
4.3 Possible factors mediating psychiatric disorder-induced IBS
Prior to the mediation analysis, we explored the causal associations between genetically predicted psychiatric disorders and potential mediators that are significantly associated with IBS. The findings indicated that broad depression and PTSD causally led to lower levels of acetate, as well as an increased risk of insomnia. Additionally, MDD was found to causally increase the risk of insomnia, while schizophrenia causally increased blood β-hydroxybutyrate levels. Therefore, acetate, insomnia, and β-hydroxybutyrate might play crucial roles in psychiatric disorders-induced IBS.
Acetate, the most abundant short chain fatty acid (SCFA) produced by the gut microbiota and a precursor used by many gut commensals to produce propionate and butyrate, affects the metabolic pathway through the G protein-coupled receptor and free fatty acid receptor 2 in colonic cells to improve bowel function (81, 82). A reduction in acetate, propionate, and butyrate levels was found in patients with IBS and linked to specific IBS symptoms such as colonic hyperalgesia and hypersensitivity (83). Additionally, a study showed that acetate supplementation produces antidepressant-like effects by increasing histone acetylation and improving synaptic plasticity in the hippocampus (84). Interestingly, in our MR mediation analysis, we found that the reduction in blood acetate mediated 12.6% of the causal effects of broad depression on IBS, which further supports the key beneficial role of acetate in the brain-gut axis. Unfortunately, in our MR study, although PTSD was causally associated with lower levels of acetate, which was a risk factor for IBS, we found no mediating effects of acetate on PTSD-induced IBS in the mediation analysis.
Another blood metabolite, β-hydroxybutyrate, a ketone body that serves as an energy source during starvation or exercise, shows several beneficial effects in the treatment of seizures, hypertension, NLRP3-mediated inflammation, and neurodegenerative diseases (85). The correlation between β-hydroxybutyrate and IBS has not been documented; however, in the present MR study, β-hydroxybutyrate showed anti-protective effects on IBS. Huang et al. (86) found that patients with schizophrenia had significantly higher serum levels of β-hydroxybutyrate than healthy controls, which was significantly correlated with fasting glucose and triglycerides, thus speculating that serum levels of β-hydroxybutyrate may act as a potential indicator of energy utilization impairment in schizophrenia. Consistently, our study showed that genetically predicted schizophrenia was a risk factor for increasing the β-hydroxybutyrate level in the blood, which further mediated 50.76% of total effects of schizophrenia on IBS.
Emerging evidence suggests that insomnia is positively correlated with many psychiatric disorders, such as ADHD, bipolar disorder, PTSD, MDD, OCD, and schizophrenia; bidirectional causal associations between insomnia and psychiatric disorders have also been observed (87), which might be explained by shared common abnormalities in hypothalamic–pituitary–adrenal (HPA) axis activation, serotonin system dysfunction, and overexpression of immune system peptides (88). A Korean population-based cohort study (56) found that subjects with insomnia also showed a higher prevalence of IBS than those without insomnia, which further highlights the pivotal role of insomnia in brain-gut axis disturbances. In the present study, we found significant causal effects of genetically predicted broad depression, MDD, and PTSD on insomnia, which is consistent with the results of a previous study (87). Insomnia was also a risk factor for IBS, mediating proportion of 16.00%, 16.20%, and 27.4% of the total effects of broad depression, MDD, and PTSD on IBS, respectively.
Overall, our study revealed that acetate, β-hydroxybutyrate, and insomnia were important mediators in psychiatric disorders-induced IBS, supporting their key roles in brain-gut axis. Discovering the key mediators in the causal association between two diseases can not only help to reveal the mechanisms, but also identify markers of disease evolution or exacerbation, so as to facilitate early intervention. For instance, our study found blood β-hydroxybutyrate level mediated schizophrenia-induced IBS, suggesting β-hydroxybutyrate as a potential biomarker. If the blood β-hydroxybutyrate level is elevated in patients with schizophrenia, IBS is likely to be induced, which will worsen abdominal symptoms and further reduce the patients’ quality of life. Therefore, early intervention to improve blood β-hydroxybutyrate level and mental health is important. Of course, these outcomes need further validations by more studies.
4.4 Strengths of this study
Overall, our study has several strengths. First, in observational settings, MR studies can simulate randomized controlled trials, which are costly, laborious, and time-consuming. In contrast, MR studies can technically reduce cost and effort and efficiently avoid confounding bias for SNPs that are randomly assigned at conception (89). Second, compared to other observational studies, MR studies can avoid the reverse causal effect and are less likely to be affected by confounders between exposure and outcome. Third, we selected significant genome-wide SNPs for diseases in the GWAS, which provided large and repeatedly checked samples. After a rigorous process of removing outliers, SNPs in LP or palindromes, and SNPs associated with underlying confounders, our study was more reliable. Fourth, we focused on nine psychiatric disorders with a high or low probability of comorbidity with IBS and further verified the causal association between them at the genetic level, filling the gap of insufficient research on the relationship between IBS and certain psychiatric disorders, such as bipolar disorder, schizophrenia, ASD, ADHD, and AN. Fifth, the utilization of mediation analysis and the multivariable MR method to identify potential mediators between psychiatric disorders and IBS is conducive to identifying the mechanisms of gut-brain axis abnormalities, which may improve health policies for managing these patients. Lastly, although sample overlap between GWAS summary data on exposures and outcomes in the analysis was inevitable, the results of MRlap analysis as well as low bias and Type 1 error rates in MR indicated the bias induced by sample overlap should be minimal in these causal estimates.
4.5 Limitations of this study
Although the present MR study was rigorously performed and had several strengths, some limitations remain. First, to minimize spurious causal relationships induced by individuals with diverse genetic backgrounds, the IVs in our study were selected from sources of European ancestry and genomic control research; thus, our results may not be generalizable to other races. Second, the heterogeneity in some results was challenging to eliminate; thus, we used three methods in addition to the IVW analysis to test the robustness of the results. Third, owing to the lack of a GWAS exploring the subtypes of IBS, we were unable to study the causal associations of these mental disorders with different types of IBS. Forth, the prevalence rate of IBS was higher in women than in men (5.2% vs. 2.9% in Rome-IV) and increasing evidence suggested the pathogenesis of IBS might differed by genders (90). However, since there were no GWAS summary data on IBS by genders, exploring the causal association of psychiatric disorders with IBS in different genders cannot be achieved through MR study. Furthermore, many gut microbiota, blood metabolites, and neurotransmitters are essential in mediating brain-gut axis communication. However, due to limited GWAS summary data, we could only explore the mediation role of some of them in psychiatric disorder-induced IBS; thus, larger, higher-quality, and more detailed GWASs are needed.
5 Conclusions
In the present MR study, we further verified that psychiatric disorders such as broad depression, MDD, lifetime anxiety disorder, schizophrenia, and PTSD could increase the risk of IBS. Furthermore, the MR mediation analysis demonstrated that the reduction in acetate levels mediated the effects of broad depression on IBS; insomnia mediated broad depression, MDD, and PTSD-induced IBS; and the increase in blood β-hydroxybutyrate levels mediated schizophrenia-induced IBS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
TZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. YC: Conceptualization, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. XL: Conceptualization, Methodology, Software, Validation, Writing – review & editing. JZ: Conceptualization, Methodology, Validation, Visualization, Writing – review & editing. LD: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82170557) and the National Key R&D Program of China (2019YFA0905604).
Acknowledgments
We would like to thank all investigators for making genetic association summary data openly available. We thank Wenwei Ying, Department of Urology, Peking University First Hospital, for his technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1279266/full#supplementary-material
Supplementary Figure 1 | The screening and validation process of SNPs: Before initial MR analysis, the candidate SNPs that strongly associated with exposures, not associated with confounders, and with low linkage disequilibrium, were included. In the process of initial MR analysis, for harmonization, the palindromic SNPs were excluded. Subsequently, the horizontal pleiotropic effects were examined to validate whether SNPs can directly affect the outcomes without through the exposure so as to violate the third assumption. Lastly, the outliers that might introduce bias into the results were detected and excluded. After first round of exclusion of ineligible SNPs, the remained SNPs were included for a new round of MR analysis until all ineligible SNPs were excluded, and ultimately the final MR study were performed.
Abbreviations
MR, Mendelian randomization; GWAS, genome-wide association studies; IVW, inverse-variance weighted; IBS, irritable bowel syndrome; MDD, major depressive disorder; PTSD, posttraumatic stress disorder; ASD, Autism Spectrum Disorder; ADHD, Attention deficit/hyperactivity disorder; AN, anorexia nervosa; BMI, body mass index; LD, linkage disequilibrium; MR-PRESSO, MR-Pleiotropy Residual Sum and Outlier methods.
References
1. Yan R, Andrew L, Marlow E, Kunaratnam K, Devine A, Dunican IC, et al. Dietary fibre intervention for gut microbiota, sleep, and mental health in adults with irritable bowel syndrome: A scoping review. Nutrients (2021) 13(7):2159. doi: 10.3390/nu13072159
2. Zhang T, Zhang C, Zhang J, Sun F, Duan L. Efficacy of probiotics for irritable bowel syndrome: A systematic review and network meta-analysis. Front Cell infection Microbiol (2022) 12:859967. doi: 10.3389/fcimb.2022.859967
3. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol (2012) 10(7):712–721.e714. doi: 10.1016/j.cgh.2012.02.029
4. Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet (London England) (2020) 396(10263):1675–88. doi: 10.1016/S0140-6736(20)31548-8
5. Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA clinical practice guideline on the pharmacological management of irritable bowel syndrome with constipation. Gastroenterology (2022) 163(1):118–36. doi: 10.1053/j.gastro.2022.04.016
6. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol (2020) 17(8):473–86. doi: 10.1038/s41575-020-0286-8
7. Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Alimentary Pharmacol Ther (2019) 50(2):132–43. doi: 10.1111/apt.15325
8. Irwin C, Falsetti SA, Lydiard RB, Ballenger JC, Brock CD, Brener W. Comorbidity of posttraumatic stress disorder and irritable bowel syndrome. J Clin Psychiatry (1996) 57(12):576–8. doi: 10.4088/JCP.v57n1204
9. Kearney DJ, Kamp KJ, Storms M, Simpson TL. Prevalence of gastrointestinal symptoms and irritable bowel syndrome among individuals with symptomatic posttraumatic stress disorder. J Clin Gastroenterol (2022) 56(7):592–6. doi: 10.1097/MCG.0000000000001670
10. Ng QX, Soh AYS, Loke W, Venkatanarayanan N, Lim DY, Yeo WS. Systematic review with meta-analysis: The association between post-traumatic stress disorder and irritable bowel syndrome. J Gastroenterol Hepatol (2019) 34(1):68–73. doi: 10.1111/jgh.14446
11. Gupta S, Masand PS, Kaplan D, Bhandary A, Hendricks S. The relationship between schizophrenia and irritable bowel syndrome (IBS). Schizophr Res (1997) 23(3):265–8. doi: 10.1016/S0920-9964(96)00099-0
12. Kedem S, Yust-Katz S, Carter D, Levi Z, Kedem R, Dickstein A, et al. Attention deficit hyperactivity disorder and gastrointestinal morbidity in a large cohort of young adults. World J Gastroenterol (2020) 26(42):6626–37. doi: 10.3748/wjg.v26.i42.6626
13. Gong W, Guo P, Li Y, Liu L, Yan R, Liu S, et al. Role of the gut-brain axis in the shared genetic etiology between gastrointestinal tract diseases and psychiatric disorders: A genome-wide pleiotropic analysis. JAMA Psychiatry (2023) 80(4):360–70. doi: 10.1001/jamapsychiatry.2022.4974
14. Lee HS. Irritable bowel syndrome or psychiatric disorders: which comes first? J Neurogastroenterol Motil (2022) 28(3):335–6. doi: 10.5056/jnm22065
15. Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut (2012) 61(9):1284–90. doi: 10.1136/gutjnl-2011-300474
16. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med (2008) 27(8):1133–63. doi: 10.1002/sim.3034
17. Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol (2004) 33(1):30–42. doi: 10.1093/ije/dyh132
18. Chen J, Chen X, Xie Y, Sun Y, Wang X, Hesketh T. Irritable bowel syndrome and migraine: evidence from Mendelian randomization analysis in the UK Biobank. Expert Rev Gastroenterol Hepatol (2021) 15(10):1233–9. doi: 10.1080/17474124.2021.1949290
19. Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun (2018) 9(1):1470. doi: 10.1038/s41467-018-03819-3
20. Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet (2018) 50(5):668–81. doi: 10.1038/s41588-018-0090-3
21. Purves KL, Coleman JRI, Meier SM, Rayner C, Davis KAS, Cheesman R, et al. A major role for common genetic variation in anxiety disorders. Mol Psychiatry (2020) 25(12):3292–303. doi: 10.1038/s41380-019-0559-1
22. Wendt FR, Pathak GA, Deak JD, De Angelis F, Koller D, Cabrera-Mendoza B, et al. Using phenotype risk scores to enhance gene discovery for generalized anxiety disorder and posttraumatic stress disorder. Mol Psychiatry (2022) 27(4):2206–15. doi: 10.1038/s41380-022-01469-y
23. Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature (2022) 604(7906):502–8. doi: 10.1038/s41586-022-04434-5
24. Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet (2019) 51(5):793–803. doi: 10.1038/s41588-019-0397-8
25. Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet (2019) 51(3):431–44. doi: 10.1038/s41588-019-0344-8
26. Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet (2019) 51(1):63–75. doi: 10.1038/s41588-018-0269-7
27. Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JRI, Gaspar HA, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet (2019) 51(8):1207–14. doi: 10.1038/s41588-019-0439-2
28. Mishima Y, Ishihara S. Molecular mechanisms of microbiota-mediated pathology in irritable bowel syndrome. Int J Mol Sci (2020) 21(22):8664. doi: 10.3390/ijms21228664
29. Abramovitch A, Anholt GE, Cooperman A, van Balkom A, Giltay EJ, Penninx BW, et al. Body mass index in obsessive-compulsive disorder. J Affect Disord (2019) 245:145–51. doi: 10.1016/j.jad.2018.10.116
30. Riemann D, Krone LB, Wulff K, Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology (2020) 45(1):74–89. doi: 10.1038/s41386-019-0411-y
31. Bao W, Qi L, Bao Y, Wang S, Li W. Alleviating insomnia should decrease the risk of irritable bowel syndrome: Evidence from Mendelian randomization. Front Pharmacol (2022) 13:900788. doi: 10.3389/fphar.2022.900788
32. Dong Y, Berens S, Eich W, Schaefert R, Tesarz J. Is body mass index associated with symptom severity and health-related quality of life in irritable bowel syndrome? A cross-sectional study. BMJ Open (2018) 8(10):e019453. doi: 10.1136/bmjopen-2017-019453
33. Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet (2021) 53(2):156–65. doi: 10.1038/s41588-020-00763-1
34. Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet (2018) 27(20):3641–9. doi: 10.1093/hmg/ddy271
35. Lane JM, Jones SE, Dashti HS, Wood AR, Aragam KG, van Hees VT, et al. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet (2019) 51(3):387–93. doi: 10.1038/s41588-019-0361-7
36. Han X, Ong JS, An J, Hewitt AW, Gharahkhani P, MacGregor S. Using Mendelian randomization to evaluate the causal relationship between serum C-reactive protein levels and age-related macular degeneration. Eur J Epidemiol (2020) 35(2):139–46. doi: 10.1007/s10654-019-00598-z
37. Ahluwalia TS, Prins BP, Abdollahi M, Armstrong NJ, Aslibekyan S, Bain L, et al. Genome-wide association study of circulating interleukin 6 levels identifies novel loci. Hum Mol Genet (2021) 30(5):393–409. doi: 10.1093/hmg/ddab023
38. Li S, Weinstein G, Zare H, Teumer A, Völker U, Friedrich N, et al. The genetics of circulating BDNF: towards understanding the role of BDNF in brain structure and function in middle and old ages. Brain Commun (2020) 2(2):fcaa176. doi: 10.1093/braincomms/fcaa176
39. Leskelä J, Toppila I, Härma MA, Palviainen T, Salminen A, Sandholm N, et al. Genetic profile of endotoxemia reveals an association with thromboembolism and stroke. J Am Heart Assoc (2021) 10(21):e022482. doi: 10.1161/JAHA.121.022482
40. Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, Wang H, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun (2019) 10(1):1100. doi: 10.1038/s41467-019-08917-4
41. Richardson TG, Leyden GM, Wang Q, Bell JA, Elsworth B, Davey Smith G, et al. Characterising metabolomic signatures of lipid-modifying therapies through drug target mendelian randomisation. PloS Biol (2022) 20(2):e3001547. doi: 10.1371/journal.pbio.3001547
42. Lotta LA, Pietzner M, Stewart ID, Wittemans LBL, Li C, Bonelli R, et al. A cross-platform approach identifies genetic regulators of human metabolism and health. Nat Genet (2021) 53(1):54–64. doi: 10.1038/s41588-020-00751-5
43. Suhre K, Arnold M, Bhagwat AM, Cotton RJ, Engelke R, Raffler J, et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun (2017) 8:14357. doi: 10.1038/ncomms14357
44. Eijsbouts C, Zheng T, Kennedy NA, Bonfiglio F, Anderson CA, Moutsianas L, et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat Genet (2021) 53(11):1543–52. doi: 10.1038/s41588-021-00950-8
45. Huang C, Shi M, Wu H, Luk AOY, Chan JCN, Ma RCW. Human serum metabolites as potential mediators from type 2 diabetes and obesity to COVID-19 severity and susceptibility: evidence from mendelian randomization study. Metabolites (2022) 12(7):598. doi: 10.3390/metabo12070598
46. Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK. Dissecting causal pathways using mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Genetics (2017) 207(2):481–7. doi: 10.1534/genetics.117.300191
47. Palaniswamy S, Gill D, De Silva NM, Lowry E, Jokelainen J, Karhu T, et al. Could vitamin D reduce obesity-associated inflammation? Observational and Mendelian randomization study. Am J Clin Nutr (2020) 111(5):1036–47. doi: 10.1093/ajcn/nqaa056
48. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol (2015) 181(4):251–60. doi: 10.1093/aje/kwu283
49. Chong RS, Li H, Cheong AJ, Fan Q, Koh V, Raghavan L, et al. Mendelian randomization implicates bidirectional association between myopia and primary open angle glaucoma or intraocular pressure. Ophthalmology (2023) 130(4):394–403. doi: 10.1016/j.ophtha.2022.11.030
50. Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory biomarkers and risk of schizophrenia: A 2-sample mendelian randomization study. JAMA Psychiatry (2017) 74(12):1226–33. doi: 10.1001/jamapsychiatry.2017.3191
51. Jones HJ, Martin D, Lewis SJ, Davey Smith G, O'Donovan MC, Owen MJ, et al. A Mendelian randomization study of the causal association between anxiety phenotypes and schizophrenia. Am J Med Genet Part B Neuropsychiatr Genet (2020) 183(6):360–9. doi: 10.1002/ajmg.b.32808
52. Mounier N, Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization. Genet Epidemiol (2023) 47(4):314–31. doi: 10.1002/gepi.22522
53. Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol (2016) 40(7):597–608. doi: 10.1002/gepi.21998
54. Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res (2011) 1392:121–31. doi: 10.1016/j.brainres.2011.03.069
55. Wang L, Leonards CO, Sterzer P, Ebinger M. White matter lesions and depression: a systematic review and meta-analysis. J Psychiatr Res (2014) 56:56–64. doi: 10.1016/j.jpsychires.2014.05.005
56. Lee SK, Yoon DW, Lee S, Kim J, Choi KM, Shin C. The association between irritable bowel syndrome and the coexistence of depression and insomnia. J psychosomatic Res (2017) 93:1–5. doi: 10.1016/j.jpsychores.2016.12.007
57. Wang B, Duan R, Duan L. Prevalence of sleep disorder in irritable bowel syndrome: A systematic review with meta-analysis. Saudi J Gastroenterol (2018) 24(3):141–50. doi: 10.4103/sjg.SJG_603_17
58. Cai L, Bao Y, Fu X, Cao H, Baranova A, Zhang X, et al. Causal links between major depressive disorder and insomnia: A Mendelian randomisation study. Gene (2021) 768:145271. doi: 10.1016/j.gene.2020.145271
59. Costanian C, Tamim H, Assaad S. Prevalence and factors associated with irritable bowel syndrome among university students in Lebanon: findings from a cross-sectional study. World J Gastroenterol (2015) 21(12):3628–35. doi: 10.3748/wjg.v21.i12.3628
60. Dorahy MJ, Rowlands A, Renouf C, Hanna D, Britt E, Carter JD. Impact of average household income and damage exposure on post-earthquake distress and functioning: A community study following the February 2011 Christchurch earthquake. Br J Psychol (London Engl 1953) (2015) 106(3):526–43. doi: 10.1111/bjop.12097
61. Batalla-Martín D, Belzunegui-Eraso A, Miralles Garijo E, Martínez Martín E, Romaní Garcia R, Heras JSM, et al. Insomnia in schizophrenia patients: prevalence and quality of life. Int J Environ Res Public Health (2020) 17(4):1350. doi: 10.3390/ijerph17041350
62. Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. psychol Med (2020) 50(14):2435–43. doi: 10.1017/S0033291719002678
63. Stauffer EM, Bethlehem RAI, Warrier V, Murray GK, Romero-Garcia R, Seidlitz J, et al. Grey and white matter microstructure is associated with polygenic risk for schizophrenia. Mol Psychiatry (2021) 26(12):7709–18. doi: 10.1038/s41380-021-01260-5
64. Talley NJ, Powell N, Walker MM, Jones MP, Ronkainen J, Forsberg A, et al. Role of smoking in functional dyspepsia and irritable bowel syndrome: three random population-based studies. Alimentary Pharmacol Ther (2021) 54(1):32–42. doi: 10.1111/apt.16372
65. Liu CS, Carvalho AF, Mansur RB, McIntyre RS. Obesity and bipolar disorder: synergistic neurotoxic effects? Adv Ther (2013) 30(11):987–1006. doi: 10.1007/s12325-013-0067-7
66. Akhondi N, Memar Montazerin S, Soltani S, Saneei P, Hassanzadeh Keshteli A, Esmaillzadeh A, et al. General and abdominal obesity in relation to the prevalence of irritable bowel syndrome. Neurogastroenterol Motil (2019) 31(4):e13549. doi: 10.1111/nmo.13549
67. Fadeuilhe C, Daigre C, Richarte V, Grau-López L, Palma-Álvarez RF, Corrales M, et al. Insomnia disorder in adult attention-deficit/hyperactivity disorder patients: clinical, comorbidity, and treatment correlates. Front Psychiatry (2021) 12:663889. doi: 10.3389/fpsyt.2021.663889
68. Zhu S, He M, Liu Z, Qin Z, Wang Z, Duan L. Shared genetic susceptibilities for irritable bowel syndrome and depressive disorder in Chinese patients uncovered by pooled whole-exome sequencing. J advanced Res (2020) 23:113–21. doi: 10.1016/j.jare.2020.01.016
69. Ghoshal U, Biswas SN, Dixit VK, Yadav JS. Anxiety and depression in Indian patients with irritable bowel syndrome: A meta-analysis. Indian J Gastroenterol (2023) 42(1):32–9. doi: 10.1007/s12664-022-01300-0
70. Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Alimentary Pharmacol Ther (2016) 44(6):592–600. doi: 10.1111/apt.13738
71. Ringel Y, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, et al. Sexual and physical abuse are not associated with rectal hypersensitivity in patients with irritable bowel syndrome. Gut (2004) 53(6):838–42. doi: 10.1136/gut.2003.021725
72. Cohen H, Jotkowitz A, Buskila D, Pelles-Avraham S, Kaplan Z, Neumann L, et al. Post-traumatic stress disorder and other co-morbidities in a sample population of patients with irritable bowel syndrome. Eur J Internal Med (2006) 17(8):567–71. doi: 10.1016/j.ejim.2006.07.011
73. Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep (2015) 17(5):27. doi: 10.1007/s11920-015-0574-0
74. Lee YT, Hu LY, Shen CC, Huang MW, Tsai SJ, Yang AC, et al. Risk of psychiatric disorders following irritable bowel syndrome: A nationwide population-based cohort study. PloS One (2015) 10(7):e0133283. doi: 10.1371/journal.pone.0133283
75. Zhen Y, Chu C, Zhou S, Qi M, Shu R. Imbalance of tumor necrosis factor-α, interleukin-8 and interleukin-10 production evokes barrier dysfunction, severe abdominal symptoms and psychological disorders in patients with irritable bowel syndrome-associated diarrhea. Mol Med Rep (2015) 12(4):5239–45. doi: 10.3892/mmr.2015.4079
76. Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, et al. The effects of add-on low-dose memantine on cytokine levels in bipolar II depression: a 12-week double-blind, randomized controlled trial. J Clin Psychopharmacol (2014) 34(3):337–43. doi: 10.1097/JCP.0000000000000109
77. Fiedorowicz JG, Prossin AR, Johnson CP, Christensen GE, Magnotta VA, Wemmie JA. Peripheral inflammation during abnormal mood states in bipolar I disorder. J Affect Disord (2015) 187:172–8. doi: 10.1016/j.jad.2015.08.036
78. Zhu S, Wang B, Jia Q, Duan L. Candidate single nucleotide polymorphisms of irritable bowel syndrome: a systemic review and meta-analysis. BMC Gastroenterol (2019) 19(1):165. doi: 10.1186/s12876-019-1084-z
79. Zhu SW, Liu ZJ, Sun QH, Duan LP. Effect of the interleukin 10 polymorphisms on interleukin 10 production and visceral hypersensitivity in Chinese patients with diarrhea-predominant irritable bowel syndrome. Chin Med J (2019) 132(13):1524–32. doi: 10.1097/CM9.0000000000000306
80. Nikolova VL, Pelton L, Moulton CD, Zorzato D, Cleare AJ, Young AH, et al. The prevalence and incidence of irritable bowel syndrome and inflammatory bowel disease in depression and bipolar disorder: A systematic review and meta-analysis. Psychosomatic Med (2022) 84(3):313–24. doi: 10.1097/PSY.0000000000001046
81. Kumar J, Rani K, Datt C. Molecular link between dietary fibre, gut microbiota and health. Mol Biol Rep (2020) 47(8):6229–37. doi: 10.1007/s11033-020-05611-3
82. Calderon G, Patel C, Camilleri M, James-Stevenson T, Bohm M, Siwiec R, et al. Associations of habitual dietary intake with fecal short-Chain fatty acids and bowel functions in irritable bowel syndrome. J Clin Gastroenterol (2022) 56(3):234–42. doi: 10.1097/MCG.0000000000001521
83. Fredericks E, Theunissen R, Roux S. Short chain fatty acids and monocarboxylate transporters in irritable bowel syndrome. Turkish J Gastroenterol (2020) 31(12):840–7. doi: 10.5152/tjg.2020.19856
84. Huang W, Hu W, Cai L, Zeng G, Fang W, Dai X, et al. Acetate supplementation produces antidepressant-like effect via enhanced histone acetylation. J Affect Disord (2021) 281:51–60. doi: 10.1016/j.jad.2020.11.121
85. Yan X, Liu XY, Zhang D, Zhang YD, Li ZH, Liu X, et al. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell Mol Immunol (2021) 18(10):2344–57. doi: 10.1038/s41423-021-00760-2
86. Huang YC, Lin PY, Lee Y, Wu CC, Hsu ST, Hung CF, et al. β-hydroxybutyrate, pyruvate and metabolic profiles in patients with schizophrenia: A case control study. Psychoneuroendocrinology (2016) 73:1–8. doi: 10.1016/j.psyneuen.2016.07.209
87. Sun X, Liu B, Liu S, Wu DJH, Wang J, Qian Y, et al. Sleep disturbance and psychiatric disorders: a bidirectional Mendelian randomisation study. Epidemiol Psychiatr Sci (2022) 31:e26. doi: 10.1017/S2045796021000810
88. Gao X, Meng LX, Ma KL, Liang J, Wang H, Gao Q, et al. The bidirectional causal relationships of insomnia with five major psychiatric disorders: A Mendelian randomization study. Eur Psychiatry (2019) 60:79–85. doi: 10.1016/j.eurpsy.2019.05.004
89. Chen X, Kong J, Diao X, Cai J, Zheng J, Xie W, et al. Depression and prostate cancer risk: A Mendelian randomization study. Cancer Med (2020) 9(23):9160–7. doi: 10.1002/cam4.3493
Keywords: psychiatric disorders, irritable bowel syndrome, Mendelian randomization, mediation analysis, causal effect
Citation: Zhang T, Chen Y, Li X, Zhang J and Duan L (2024) Genetic associations and potential mediators between psychiatric disorders and irritable bowel syndrome: a Mendelian randomization study with mediation analysis. Front. Psychiatry 15:1279266. doi: 10.3389/fpsyt.2024.1279266
Received: 18 August 2023; Accepted: 15 January 2024;
Published: 30 January 2024.
Edited by:
Oliver Tüscher, Johannes Gutenberg University Mainz, GermanyReviewed by:
Akiyoshi Saitoh, Tokyo University of Science, JapanZheng-kun Hou, Guangzhou University of Chinese Medicine, China
Copyright © 2024 Zhang, Chen, Li, Zhang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Duan, ZHVhbmxwQGJqbXUuZWR1LmNu
 Tao Zhang
Tao Zhang Yuzhu Chen
Yuzhu Chen Xiaoang Li
Xiaoang Li Jindong Zhang
Jindong Zhang Liping Duan
Liping Duan